Introduction
Glioblastoma (GBM) is the most aggressive primary
brain tumor in adults. The overall survival, even at best therapy
and standard care, is normally less than 15 months, also long-term
survival is rare (1). Surgical
resection followed by irradiation and chemotherapy using
temozolomide is currently still the standard treatment for patients
with GBM. However, despite novel therapy approaches, the outcome
remains poor. GBM are characterized by a diffuse and infiltrative
growth into the surrounding healthy brain parenchyma (2). Even if GBM does not metastasize
outside the brain, the expression of many proteins involved in
metastatic processes like MMPs that destroy the extracellular
matrix (ECM), cell adhesion molecules (CAM) such as integrins,
cadherins or selectins that are necessary for cell-cell or cell-ECM
interactions, members of the TGF-β signaling cascade which
regulates cell migration and proteins involved in cell metabolism
or transcription factors involved in the epithelial to mesenchymal
transition (EMT) are dysregulated in GBM cells (3–8).
Today, Viscum album extracts are known under
several trade names, such as Iscador, AbnobaVISCUM, Helixor and
others which are mainly available in European countries. Dependent
on the host tree the mistletoe is growing on, the time the plant is
harvested as well as on the technique how the drug is prepared, the
compounds in different Viscum album extracts vary. Important
compounds of mistletoe extracts providing anticancer effects are
ML-1, ML-2, ML-3 and viscotoxins (VT). VT are small peptides
inducing cytotoxicity whereas MLs are glycosylated type II
ribosomal inhibitory proteins (RIP II) of two subunits of which the
α-chain serves as a toxic 28 S rRNA N-glycosidase. Other
ingredients of Viscum album extracts with known effects on
mammalian cells are oligo- and polysaccharides as well as
flavonoids and triterpenes. ML-1 is known to be an important
anticancer compound within the MLs (9,10).
We and others have shown that in cancer cells ML
containing drugs not only block protein translation as predicted by
the ML function such as RIPs, but also provide multimodal
anticancer functions. At higher concentration these drugs induce
cytotoxicity in cancer cells, potentiate cellular anticancer immune
responses, alter the expression of cancer associated genes and
reduce tumor growth in mice (11–15).
Until today it is not completely understood how these plant
extracts transmit their function as regulators of gene expression.
Are VTs or other minor compounds like triterpenoids or flavonoids
present in the extracts, beside the MLs, involved in the observed
alteration in gene expression? Do MLs have to be glycosylated to be
efficiently taken up by tumor cells and to transmit their function?
To determine whether different ML containing drugs alter the
expression of genes involved in cancer progression such as
metastasis/motility associated genes and if the glycosylation of
MLs, or minor additional compounds present in some preparations,
are involved in gene regulation, we used three different ML
containing drugs: Iscador Qu, a Viscum album extract,
Aviscumine, a recombinant ML-1 and purified ML-1 from mistletoe
plants growing on ash trees. We could demonstrate that these three
drugs, even to a different level, modulate gene expression of
motility associated genes and reduce glioma cell motility in a
ML-dependent way.
Materials and methods
Mistletoe preparations
Iscador Qu, kindly provided by Iscador AG (Lörrach,
Germany), is a fermented extract prepared from the mistletoe
growing on oaks. It contains high amounts of MLs as well as VTs and
other minor compounds such as oligo- and polysaccharides,
triterpenes, flavonoids and others. ML and VT contents were as
follows: Iscador Qu20 (Charge 4080/3: total ML 1095
ng/ml as determined by the provider using an ELISA assay detecting
ML1-3, VT 48 μg/ml). Aviscumine (ME-503), a recombinant, but
non-glycosylated ML-1 produced in E. coli (16) and purified to GMP quality, was a
kind gift of Hans Lentzen (Melema Pharma GmbH, Hamburg Germany).
Purified glycosylated ML-1 isolated from mistletoes growing on ash
trees was a kind gift of Christoph Heyder (Abnoba GmbH, Pforzheim,
Germany) (17–21).
Cell culture
LNT-229 human malignant glioma cells, kindly
provided by Nicolas de Tribolet (Lausanne, Switzerland), were
maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco-Life
Technologies, Eggenstein, Germany) containing 10% fetal calf serum
(FCS; Gibco), penicillin (100 U/ml), streptomycin (100
μg/ml) in a humidified atmosphere containing 5%
CO2. Cell viability was measured using crystal violet
staining as previously described (15).
Microarray analysis and quantitative
RT-PCR
For microarray analysis RNA of treated and untreated
cells was isolated using the RNApure isolation kit (Macherey-Nagel
GmbH, Düren, Germany) and transcribed into cDNA using SuperScript
II (Invitrogen, Karlsruhe, Germany). For each sample 10 μg
reverse transcribed total RNA in a volume of 1080 μl in
DNase-free water was mixed with 1080 μl TaqMan Gene
Expression Master Mix (Thermo Fisher Scientific, Waltham, MA, USA),
dispensed in 20 μl/well into the TaqMan Array Human Tumor
Metastasis 96-well plate (cat. no. 4414098; Thermo Fisher
Scientfic) and run on an ABI 7500 system.
For quantitative reverse transcribed polymerase
chain reaction (RT-qPCR) cDNA was prepared as for the microarray
analysis. Quantitative PCR was determined using SYBR-Green Master
Mix (Thermo Fisher Scientific) on a Roche LightCycler 480. Relative
mRNA expression was quantified ([EΔCT (gene of
interest)/EΔCT (housekeeping gene)]). The following
primers were used: EPHB2 forward, 5′-CCACTCATC ATCGGCTCCTC-3′ and
EPHB2 reverse, 5′-GCTCAAACCCCCGTCTGTTA-3′; GAPDH forward,
5′-TGCACCACCAACTGCTTAGC-3′ and GAPDH reverse,
5′-GGCATGGACTGTGGTCATGAG-3′; IL1B forward,
5′-GCTCGCCAGTGAAATGATGG-3′ and IL1B reverse,
5′-GGTGGTCGGAGATTCGTAGC-3′; MMP-2 forward,
5′-CCAGAGACAGTGGATGATGCC-3′ and MMP-2 reverse,
5′-GGAGTCCGTCCTTACCGTCAA-3′; MMP-14 forward,
5′-CGGCCCTTTCCAGCCTCTG-3′ and MMP-14 reverse,
5′-GAGGTCTGAGGGTCCTGCC-3′; MTA1 forward, 5′-CCCAGTAGGGGTCTGGCAAA-3′
and MTA1 reverse, 5′-GGTAGGACTTCCCGTTGAGC-3′; NME forward,
5′-AGCCGGAGTTCAAACCTAAGC-3′ and NME reverse,
5′-TTTGTGTGTCTGCCTCCCCT-3′; PTGS2 forward,
5′-GTTCCACCCGCAGTACAGAA-3′ and PTGS2 reverse,
5′-AGGGCTTCAGCATAAAGCGT-3′; SERPINB5 forward,
5′-CATCCAGGTCTTTGTGCTCCT-3′ and SERPINB5 reverse,
5′-GGGCCTGGAGTCACAGTTATC-3′; TGFB1 forward, 5′-GCCCTGGACACCAACTAT
TG-3′ and TGFB1 reverse, 5′-CGTGTCCAGGCTCCAAATG-3′; TGFB2 forward,
5′-CAAAAGCCAGAGTGCCTGAA-3′ and TGFB2 reverse,
5′-CAGTTACATCGAAGGAGAGC-3′; TGFBR2 forward,
5′-GGAGTTTCCTGTTTCCCCCG-3′ and TGFBR2 reverse,
5′-AGGGAAGCTGCACAGGAGTC-3′; TIMP-2 forward,
5′-GTTTATCTACACGGCCCCCT-3′ and TIMP-2 reverse,
5′-TCGGCCTTTCCTGCAATGAG-3′. RT-qPCR cycling conditions were 95°C
for 10 min, followed by 45 cycles at 95°C for 15 sec, 60°C for 1
min and 72°C for 20 sec.
Immunoblot analysis
The general procedure has been previously described
(22). Supernatants were generated
by cultivating cells in serum deprived medium for 48 h, followed by
a centrifugation step to avoid contamination. Protein contents were
analyzed according to Bradford (BCA; Thermo Fisher Scientific).
Following antibodies were used: anti-MMP-2 (Merck Millipore,
Darmstadt, Germany), anti-MMP-14 (Epitomics, Burlingame, CA, USA),
anti-GAPDH (Santa Cruz Biotechnology, Dallas, TX, USA),
anti-Smad2/3 (Cell Signaling Technology, Danvers, MA, USA) and
anti-TIMP-2 (R&D Systems GmbH, Wiesbaden, Germany). Protein
contents were quantified using the ChemiDoc MP system and ImageLab
software (Bio-Rad Laboratories GmbH, Munich, Germany).
Immunofluorescence
LNT-229 cells (1×105) were seeded on
poly-L-lysin coated glass coverslips in 12-well plates and treated
after attachment for different time-points with 8 ng/ml of ML
Iscador Qu, Aviscumine, ML-1 or were left untreated. The cells were
fixed in 4% paraformaldehyde for 10 min, washed three times with
phosphate-buffered saline (PBS) and blocked in 10% goat serum with
0.3% Triton X-100 in PBS for 1 h followed by overnight incubation
with 2 μg/ml anti-ML antibody or isotype rabbit IgG (Santa
Cruz Biotechnology) at 4°C. The polyclonal rabbit anti-ML antibody
was a kind gift of the Iscador AG. To visualize the bound antibody
the cells were stained with a goat anti-rabbit Alexa Fluor 488
antibody (Thermo Fisher Scientific) for 1 h, mounted with
Vectashield HardSet Mounting medium with DAPI (Vector Laboratories
Inc., Burlingame, CA, USA) and examined by confocal microscopy
using a Zeiss LSM 510. Images were analyzed with the software
ImageJ.
TGF-β ELISA
LNT-229 cells were seeded and treated with Iscador
Qu, Aviscumine, ML-1 (8 ng/ml of ML) for 24 h or were left
untreated. To block the effect of the ML treatment, the polyclonal
rabbit anti-ML antibody (4.8 μg/ml) was added to ML
containing medium 30 min before adding the drugs to LNT-229 cells.
After addition of MLs, cell supernatants were generated and
concentrated using 4 kDa Amicon centrifugal filters (Merck
Millipore). Protein concentrations for normalization were
determined using the Bradford array. The ELISA was performed
according to instruction of the manufacturer (RayBiotech, Norcross,
GA, USA).
Boyden chamber migration assay
LNT-229 cells were seeded in 12-well plates, were
allowed to attach and treated with Iscador Qu, Aviscumine, ML-1 (8
ng/ml ML) or were left untreated. To block the effect of ML, a
pan-specific polyclonal rabbit anti-ML antibody (Iscador AG)
neutralizing the effects of ML-1, -2 and -3 was added 30 min prior
to the addition of ML containing drugs to glioma cells. After 24 h
of ML treatment, the cells were washed to remove residual ML and
2×104 cells were seeded in doublets in membrane inserts
of transwell migration chambers (8 μm pores; Corning
Incorp., Corning, NY, USA). Migrated cells were fixed 24 h later
with methanol, stained with hematoxylin and counted as previously
described (15). To avoid errors
in the quantification of cell migration derived from differences in
cell proliferation between ML treated and untreated cells, in
parallel 1×104 of the cells were seeded in triplets in
microwell plates, stained with crystal violet as described 24 h
later (22) and were used for
normalization of cell migration.
Statistical analysis
If not mentioned otherwise, the figures show the
mean or one representative experiment out of at least three
independent experiments as indicated. Quantitative data were
assessed for significance by unpaired Student's t-test (P<0.05;
P<0.01; P<0.001).
Results
Different effects of Iscador Qu,
Aviscumine and ML-1 on the expression of cell motility associated
genes in LNT-229 glioma cells
In our previous experiments we have observed that
ML-rich Iscador Qu, but not ML-poor Iscador P, reduces the
expression of genes involved in tumor development and progression
such as genes regulating proliferation, cell survival or immune
surveillance. Genes found to be upregulated in glioma were mainly
downregulated by Iscador Qu whereas genes found to be downregulated
in GBM specimen were upregulated (15 and data not shown). To
analyze the influence of different ML preparations on the
expression of motility associated genes and to evaluate whether the
glycosylation of MLs influences gene expression, we used three
different ML containing drugs (Table
I). To avoid cytotoxicity-related changes in gene expression we
first determined the EC50 values (24 h) for all
preparations in LNT-229 cells and found them to be 37 ng/ml of ML
(Iscador Qu), 95 ng/ml (Aviscumine) and >240 ng/ml (ML-1). For
further experiments we used ML concentrations that were far below
EC50 values (up to 8 ng/ml ML for Iscador Qu, up to 8
ng/ml Aviscumine and up to 16 ng/ml ML-1). To analyze differences
in gene expression induced by the three preparations we treated
LNT-229 glioma cells for 24 h with 8 ng/ml of ML (Iscador Qu). This
concentration induces <15% of cell number reduction in LNT-229
(15). A concentration of 8 ng/ml
of ML in Iscador Qu counts for 0.35 μg/ml of VT, a
concentration at which we have never observed cytotoxicity if
treating glioma cells with a comparable VT concentration present in
ML poor Iscador P (15). For
Aviscumine or ML-1, we used concentrations up to 16 ng/ml ML, which
is also far below the determined EC50 value. All
concentrations used in the experiments are below those that are
necessary to kill non-tumor brain cells (15 and data not
shown).
 | Table IML preparations used in the present
study. |
Table I
ML preparations used in the present
study.
| Preparation | Manufacturer | Host tree |
Characteristics |
|---|
| Iscador Qu | Iscador AG | Oak | Fermented extract,
high ML-content, contains glycosylated MLs, and minor compounds
such as viscotoxins, flavonoids and triterpenes, oligo- and
polysaccharides |
| Aviscumine | Melema Pharma
GmbH | – | Recombinant,
unglycosylated ML-1 produced in E. coli |
| ML-1 | Abnoba AG | Ash | Purified ML-1,
glycosylated protein |
Using a PCR based microarray measuring the
expression of 92 metastasis associated genes, quantitative RT-PCR
of well-known genes involved in the regulation of cell motility, as
well as RT-PCR based validation of differentially expressed genes
identified by the microarray analysis, we identified 33 cell
motility/invasion modulating genes that were regulated by at least
one ML containing drug in LNT-229 glioma cells. The levels of 18
mRNAs decreased, whereas the levels of 15 mRNAs rose upon
treatment. For Iscador Qu, 15 mRNAs were downregulated and 7
upregulated. For Aviscumine, 8 mRNAs were downregulated and 14
upregulated. For ML-1, 8 mRNAs were downregulated and 10
upregulated suggesting that minor compounds present in the
mistletoe extract Iscador Qu might enhance downregulation of mRNA
expression (Fig. 1 and Table I). Besides this, we detected
differences in mRNA expression after the treatment of LNT-229 cells
with either non-glycosylated Aviscumine or glycosylated ML-1,
especially in the group of upregulated mRNAs (Fig. 1). In the group of the 18
downregulated mRNAs, 16 mRNAs code for genes harboring
pro-migratory or pro-invasive function, the function of two genes
(RBL1 and KISS1R) is controversially discussed. In the group of 15
upregulated mRNAs, 4 mRNAs are known to provide anti-motility
effects (BRMS1, FGF2, NME and SERPINB5), 7 are described to be
pro-migratory (FAT1, IL-18, KRAS, MMP1, PTGS2, S100A4 and SERPIN1)
and 4 mRNAs provide either pro- or anti-migratory effects dependent
on the micro-milieu in which they are expressed (HGF, IL-1B, MET
and MYC) (Fig. 1).
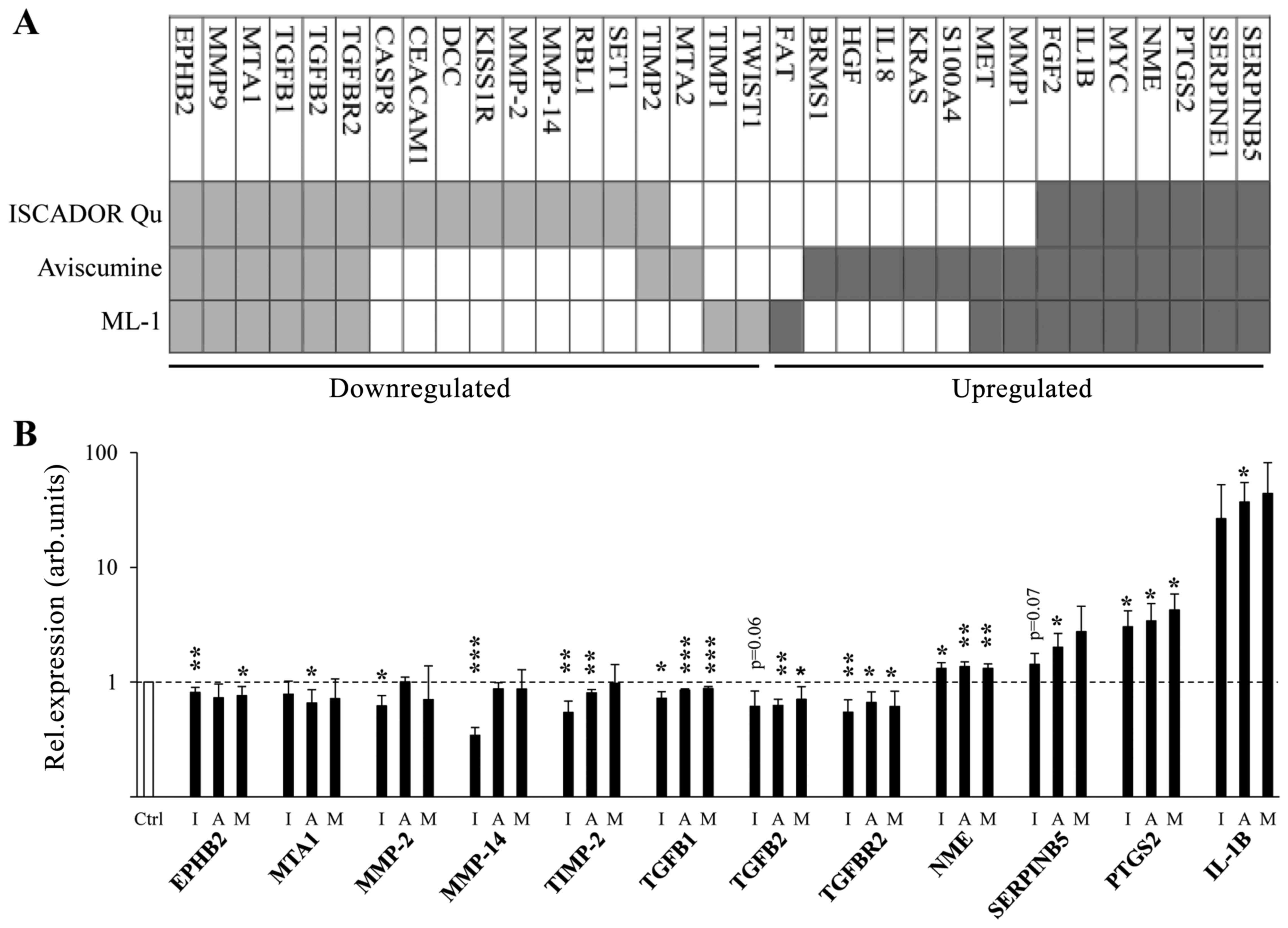 | Figure 1ML containing drugs induce changes in
the expression of motility associated genes. (A) Heat map depicting
gene expression changes in LNT-229 glioma cells treated for 24 h
with Iscador Qu, Aviscumine or ML-1 at a concentration of 8 ng/ml
of ML. (B) Modified expression of 12 genes (EPHB2, MTA1, MMP-2,
MMP-14, TIMP-2, TGFB1, TGFB2, TGFBR2, NME, SERPINB5, PTGS2 and
IL1B) determined by microarray data was validated in ML treated
cells (8 ng/ml, 24 h) cells in comparison to untreated cells by
RT-qPCR, using GAPDH as reference gene (n=3, SD,
*P<0.05; **P<0.01;
***P<0.001, significance level compared to control
treated cells). I, Iscador Qu; A, Aviscumine; M, ML-1. |
Differential expression of motility
associated genes is independent from variations in the lectin
uptake by glioma cells
To analyze whether the differences in gene
expression induced by Iscador Qu, Aviscumine or ML-1 might be
caused by differences in the uptake of MLs present in these drugs
and might also depend on the glycosylation of MLs, this influencing
the uptake, we treated the cells with a concentration that counts
for a subtoxic concentration of 8 ng/ml of ML and performed
immunofluorescence experiments using a pan-specific ML antibody. As
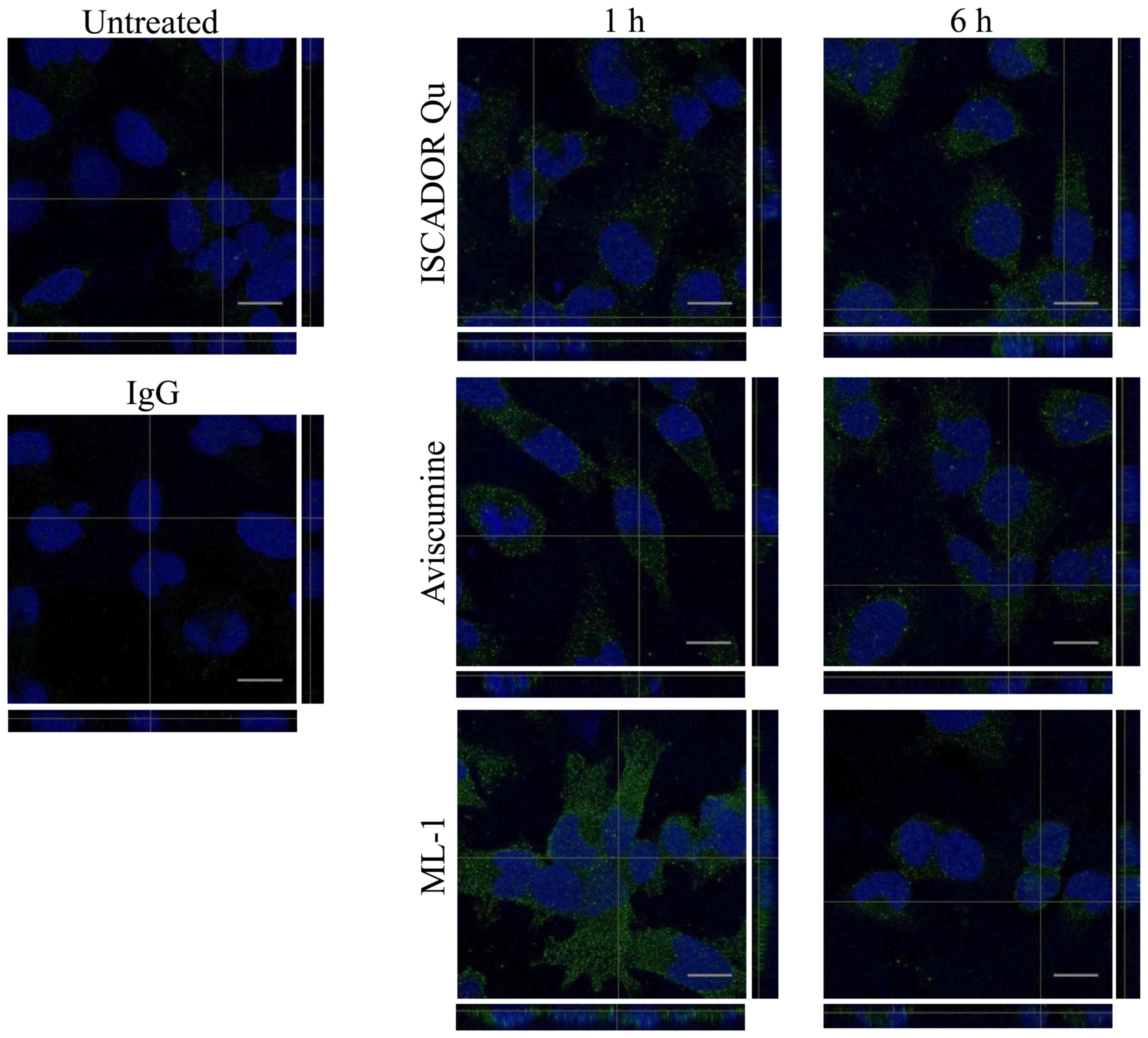
demonstrated in Fig. 2, MLs from
all three preparations were incorporated by the cells 1 h after
treatment. At this time-point, the intensity of ML staining was
approximately equal for Iscador Qu and Aviscumine, whereas ML-1
uptake was enhanced. At a later time-point (6 h), MLs were taken up
equally independent of their glycosylation or from additional
compounds being present in Iscador Qu.
ML containing drugs influence the
expression of proteins involved in the TGF-β signaling pathway in
glioma cells
TGF-β is one of the most important tumor promoting
cytokines in GBM since in glioma cells TGF-β provides
immunosuppressive function and induces a more migratory phenotype
(23). We have demonstrated
previously that TGF-β was downregulated by Iscador Qu (15). We now demonstrate that both TGF-β1
and -β2 mRNAs were also downregulated by Aviscumine and ML-1, and
co-incubation of the preparations with a ML-specific antibody prior
to the treatment of glioma cells reversed this effect (Fig. 3A). In addition to TGF-β, also TGF-β
receptor type II (TGFBR2) mRNA is reduced (Fig. 1B). The downregulation of TGF-β mRNA
in ML treated LNT-229 glioma cells also leads to a downregulation
of the TGF-β1 protein by at least Iscador Qu and ML-1 (Fig. 3B). Notably, even if SMAD2, a
prominent intracellular transducer protein of the TGF-β signaling,
was not modulated on its mRNA level (data not shown), SMAD2 protein
is reduced in ML treated glioma cells ML-dependently (Fig. 3C and D) suggesting that this effect
might be induced by the function of MLs as RIPs.
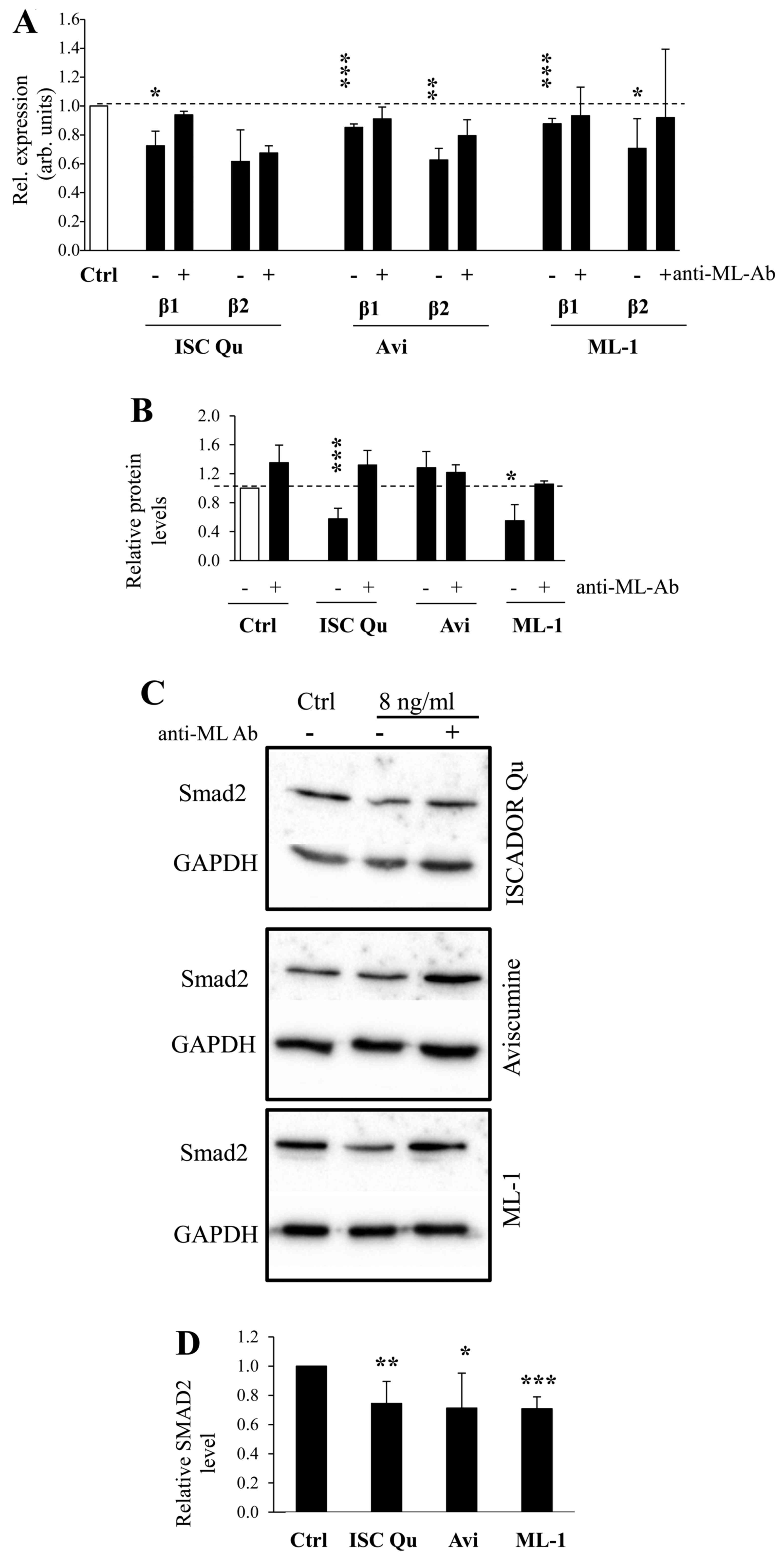 | Figure 3ML containing drugs modulate the
expression of TGF-β and its intracellular transducer SMAD2. (A)
Downregulated expression of TGF-β1 and TGF-β2 in Iscador Qu,
Aviscumine or ML-1 treated LNT-229 cells (8 ng/ml ML, 24 h) in
comparison to untreated cells by RT-qPCR, using GAPDH as reference.
Coincubation of ML drugs with a ML-neutralizing antibody (4.8
μg/ml, 30 min) was used to block ML specific effects. (B)
Protein levels of TGF-β1 in LNT-229 supernatants were quantified by
ELISA. The cells were treated as in A, and afterwards cultivated in
serum-free medium for 48 h to generate supernatants. (C) SMAD2
protein expression in ML treated LNT-229 cells. The cells were
treated as in A and protein analysis was done by immunoblot, one of
three independent experiments is shown. (D) Quantification of SMAD2
protein was prepared from three independent experiments. (A/B/D,
n=3, SD, *P<0.05; **P<0.01;
***P<0.001, significance level compared to control
treated cells). |
Matrix metalloproteinases are major players in the
destruction of the ECM, a process necessary for invasive tumor cell
growth. It is well known that TGF-β modulates the expression and
activity of MMPs and also of MMP inhibitors and activators in
invasive cancer cells and also in gliomas (3,24).
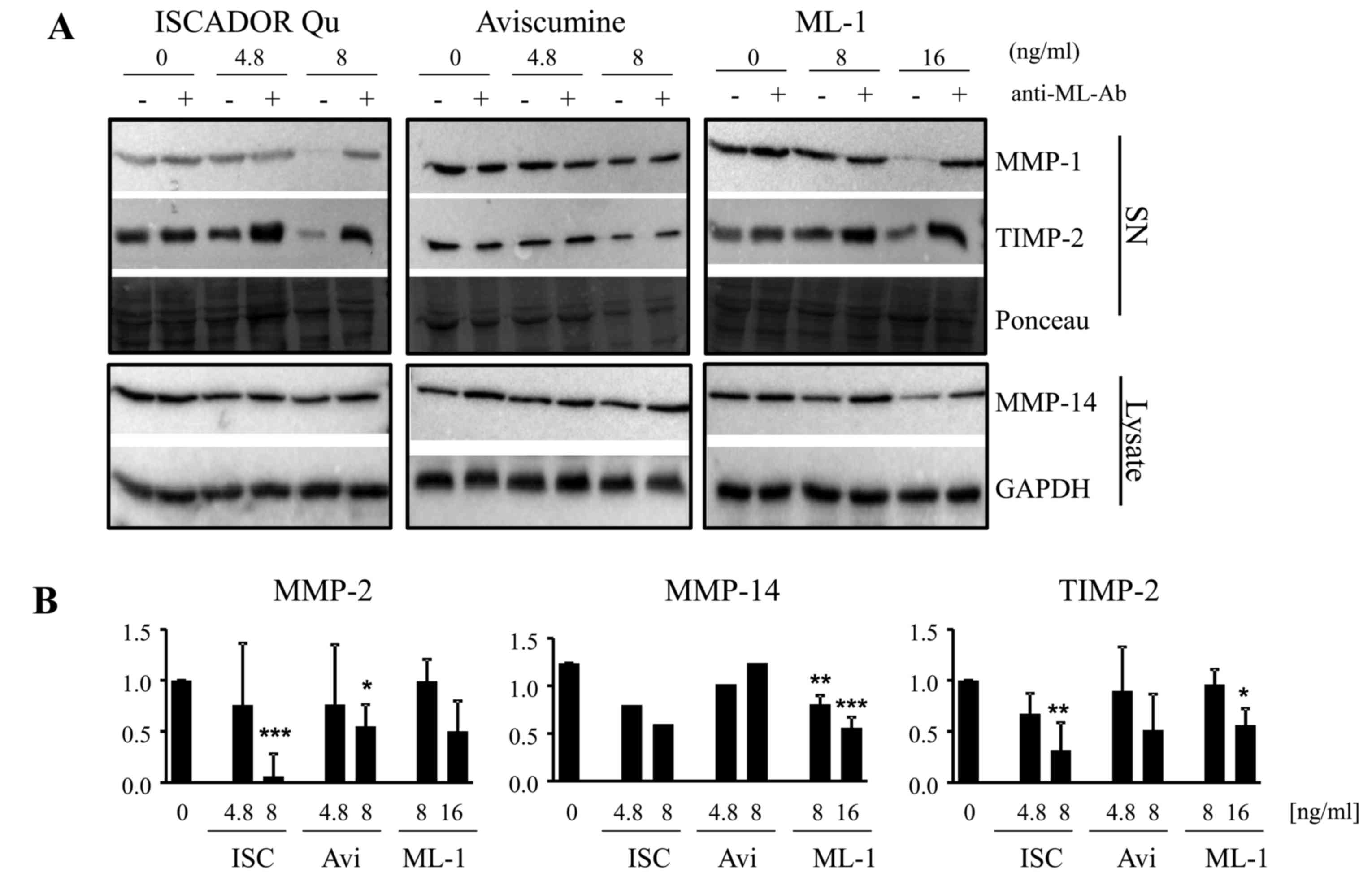
We have also shown in a recent publication that Iscador Qu
downregulates MMP expression (15). By microarray, qPCR and immunoblot
analyses we now demonstrate that this, but to a different extent,
is also true for the other ML containing drugs. In LNT-229 cells,
the treatment with Iscador Qu significantly reduced the expression
of MMP-2, of the tissue inhibitor of metalloproteinase (TIMP)-2 and
of MT-1MMP/MMP-14 mRNA at a concentration of 8 ng/ml of ML, whereas
this effect was minor for ML-1 and not detectable for Aviscumine.
For MMP-14, a significant downregulation of MMP-14 mRNA was
observed exclusively in Iscador Qu treated cells whereas TIMP-2
mRNA was reduced by both Iscador Qu and Aviscumine (Fig. 1B). Notably, the differences in
MMP-2, MMP-14 and TIMP-2 mRNA downregulation by the different
preparations are not directly translated into protein levels.
Whereas Iscador Qu downregulates MMP-2 and TIMP-2 at a
concentration of 8 ng/ml of ML in glioma cell supernatants, at this
ML concentration lesser effects were detectable for Aviscumine and
not detectable for ML-1. MT1-MMP/MMP-14, that converts inactive
pro-MMP-2 into its active form, was slightly reduced by Iscador Qu
and ML-1, but not by Aviscumine at 8 ng/ml. A concentration of 16
ng/ml of ML-1 has to be used to achieve the same effect as
determined for Iscador Qu. In contrast to changes in the mRNA
expression observed by ML treatment, the downregulation in MMP and
TIMP protein levels was dependent on the presence of MLs since
addition of a ML-specific antibody reversed the observed effect
almost completely (Fig. 4).
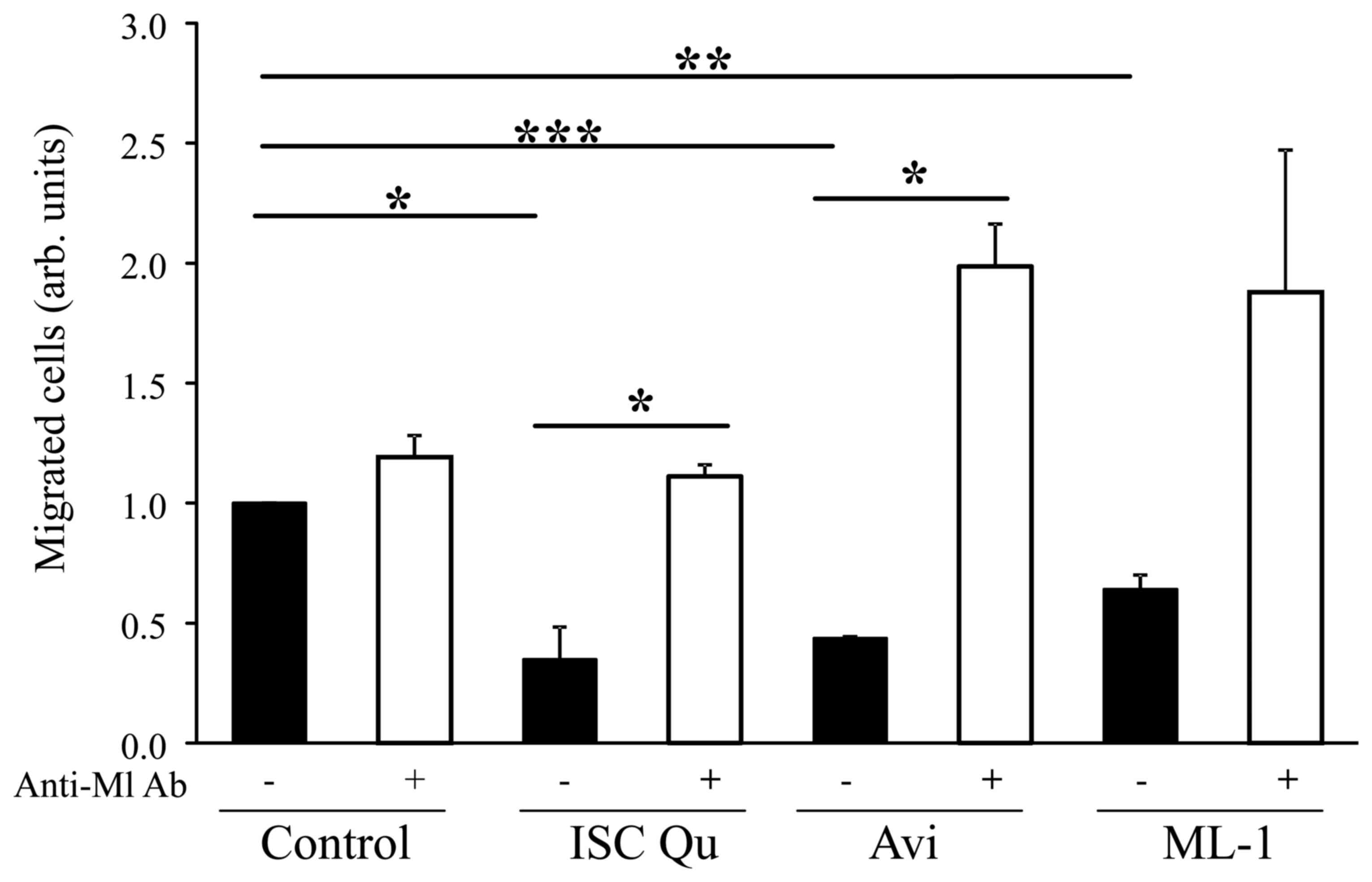
Iscador Qu, Aviscumine and ML-1 reduce
glioma cell migration
Since a variety of genes associated with glioma cell
invasion and migration are differentially regulated by ML treatment
and even by different ML containing drugs, we analyzed whether the
regulation of motility associated gene expression induced by ML
functionally affects glioma cell motility. As demonstrated by
Podlech et al (15) for
Iscador Qu and now validated for Iscador Qu, but also shown for
Aviscumine and ML-1, ML containing drugs significantly reduce
glioma cell migration. The reduction of cell migration is dependent
on the presence of MLs since addition of a ML-binding antibody
completely abrogates the migration inhibitory effect (Fig. 5).
Discussion
Until today no effective therapy regimens are
available for malignant GBM. One important characteristic issue for
the treatment failure in glioma is its highly invasive growth that
makes a complete resection impossible. A therapeutic drug that
inhibits GBM cell motility, does not provide severe side-effects
and that can be used as an adjuvant during radiation and
chemotherapy might therefore further improve the prognosis of GBM
patients. Viscum album extracts or ML-1 containing drugs
show, at least in vitro and in cancer mouse models,
therapeutic impact such as induction of cell death, inhibition of
tumor cell proliferation and stimulation of the anticancer immune
response (21,25–31).
Besides this, several clinical trials have demonstrated benefits of
this therapy for cancer patients such as enhancement of the quality
of life, lesser side-effects of chemotherapy and, for certain
cancer entities, also slower tumor growth and a prolongation of
survival (9,32–35).
We have previously shown that Iscador Qu, a ML rich extract, also
mitigates the motility of glioma cells (15). The focus of this project was to
identify motility associated genes regulated by ML containing drugs
to determine if different preparations provide comparable effects
in gene regulation and whether changes in motility associated gene
expression by ML containing drugs also lead to the inhibition of
glioma cell motility.
Working concentrations of the different ML
preparations were chosen for three reasons: i) Since no further
information was available about the concentration of ML-1 in
Iscador Qu, we chose a concentration that counts for maximal 8
ng/ml of total ML and a non-toxic concentration of 0.35 ng/ml VT.
These concentrations do not induce cell death or caspase activity
in LNT-229 cells and provide <15% reduction in cell growth
(15). ii) There is a direct
comparison possible regarding the effects of Aviscumine and ML-1
since these agents only differ in their gylcosylation status: ML-1
being naturally glycosylated and Aviscumine not. iii) Due to the
lower toxic effect of ML-1 (EC50 >240 ng/ml) higher
concentrations of ML-1 might be used during cancer therapy. For
this we also tested the effect of 16 ng/ml of ML-1 on gene
expression in glioma cells. On the level of reduced expression in
treated glioma cells, all tested ML containing drugs downregulate
the pro-migratory genes ephrin B2 (EPHB2), metastasis-associated
protein 1 (MTA1), TGFBR2, its ligands TGF-β1 and -β2 and the TGF-β
intracellular transducer protein SMAD2, however to different levels
(Figs. 1 and 3). TGF-β is one of the most important
pro-tumorigenic cytokines in glioma. Besides its immunosuppressive
function, it enhances glioma cell invasion and migration via
triggering the activation of MMPs and therefore, destruction of the
ECM (3,23). SMAD2 is a central transducer
protein in the TGF-β signaling cascade and its activity is
regulated by phosphorylation (36). However, if the ML-induced reduction
of TGF-β alone is sufficient for the reduction of the intracellular
TGF-β signaling and the downregulation of TGF-β target genes, or if
the downregulation of TGFBR2 and SMAD2 further enhances these
effects, remains unclear. Overexpression of the TGF-β target EPHB2
in glioma cells reduces cell adhesion and induces cell migration
and invasion in vivo and in vitro, whereas silencing
of EPHB2 decreases tumor cell migration and is connected to the
proto-oncogene Ras (37,38), which we have also found to be
downregulated by Iscador Qu and ML-1. MTA1, also a target of TGF-β,
has been described to promote motility and invasiveness of pancreas
carcinoma cells and is amplified in highly invasively growing cells
developed from recurrent GBM (39). Whether the downregulation of EPHB2
mRNA or other TGF-β target genes like MMP-2 is a direct effect of
MLs or whether it is mediated by reducing the intracellular TGF-β
signal has not been examined so far. Iscador Qu, that besides MLs,
also contains VTs and minor ingredients such as triterpenes,
flavonoids and others, provides superior effects in mRNA
downregulation compared to Aviscumine and ML-1. In Iscador Qu
treated glioma cells, 15 mRNAs were downregulated, whereas only 8
mRNAs were downregulated by Aviscumine or ML-1, respectively. The
differences in the mRNA regulatory effects do not seem to be
dependent on the uptake of MLs since after 6 h, intracellular ML
was equally detectable for Iscador Qu, Aviscumine and ML-1
(Fig. 2). Even if mRNA
downregulation was dependent on at least ML-1 present in all
preparations as demonstrated by the addition of the ML-specific
antibody (Figs. 3 and 4), the glycosylation of ML-1 does not
seem to be important for its inhibitory function since nearly the
same profile of downregulated genes were identified in Aviscumine
and ML-1 treated glioma cells (Fig.
1). The superior effects of Iscador Qu are potentially caused
by additional compounds in this extract. It has been recently
published that triterpenes as well as flavonoids provide antitumor
activity, can influence mRNA expression involving the expression,
secretion and activation of MMP-2, a mRNA we solely found to be
significantly downregulated in Iscador Qu treated LNT-229 cells
(Fig. 1) (40,41).
In the panel of mRNAs that were only downregulated by Iscador Qu we
found genes with prominent pro-migratory function. In glioma cells,
a reduction of caspase-8 (CASP8) below basal levels results in a
reduction of cell migration (42),
CEACAM1 is an adhesion molecule that promotes migration and
invasion of several cancers (43,44)
and the netrin-1 receptor DCC promotes filopodia formation and cell
spreading by activating CDC42 and Rac1 (45). The TGF-β responsive genes MMP-2,
MMP-14 and TIMP-2, the latter also serving as an activator for
MMP-2, are important proteins of glioma cell motility (46) and the nuclear proto-oncogene SET1
stimulates cell migration in a Rac1-dependent manner, its knockdown
inhibits cell migration and invasion of breast cancer cells
(47). The function of the KISS1
receptor (KISS1R) and the retinoblastoma like protein (RBL1) are
controversially discussed. KISS1R can enhance tumor cell spreading
but has been also described to stimulate invadopodia formation and
cancer cell invasion (48). In GBM
and metastatic colon cancer cells, RBL1/p107 showed enhanced
expression compared to normal tissue, but its level falls during
invasion (49).
In the group of 15 upregulated mRNAs, 4 mRNAs are
known to provide anti-motility or anti-invasive function (BRMS1,
FGF2, NME and SERPINB5), 7 are described to be pro-migratory (FAT1,
IL-18, KRAS, MMP1, PTGS2, S100A4 and SERPIN1) and 4 mRNAs provide
either pro- or anti-migratory effects dependent on the
circumstances under which they are expressed in tumor cells (HGF,
IL1B, MET and MYC; Table II). In
the panel of upregulated anti-migratory/anti-invasive mRNAs, the
breast-cancer metastasis suppressor 1 (BRMS1) has been described to
suppress glioma progression by regulating invasion, migration and
adhesion (50). Higher expression
of fibroblast growth factor 2 (FGF2) was observed in the lesser
invasive proneural GBM subtype compared to invasively growing
mesenchymal subtype GBMs. Besides this, GBM patients with a lower
expression of the FGF2-dependent PDGF receptor A (PDGFRA) have a
better prognosis than patients with high amounts of PDGFRA
(51,52). Nucleoside diphosphate kinase A
(NME) has been suggested to play an important role in the
suppression of glioma invasion and migration (53,54)
and the serpin peptidase inhibitor, member 5 (SERPIN5B/MASPIN)
which is often silenced in GBM by promoter hypermethylation,
effectively suppresses migration and invasiveness of malignant
cancer cells (55,56). However, some genes upregulated by
mistletoe treatment harbor pro-migratory or pro-invasive function,
but this does not lead to functional consequences (Fig. 5). Even if the expression of genes
involved in metastatic, pro-migratory or pro-invasive processes
differs between the three ML preparations we tested, the
anti-migratory effect of all substances was nearly equal. This
might be a result not only by changes in gene expression induced by
MLs, but also an effect transmitted by the function of MLs as
inhibitors of ribosomal translation, or a secondary effect by
modulating the transcription or translation of certain
transcription or transcriptional cofactors. Nevertheless, ML
containing compounds like Iscador Qu, Aviscumine or even ML-1 might
provide clinical benefit as adjuvant therapeutics in the treatment
of patients with invasively growing tumors.
 | Table IIList of cell motility regulating
genes differentially expressed by ML containing drugs. |
Table II
List of cell motility regulating
genes differentially expressed by ML containing drugs.
| Gene | Protein |
Pro-/anti-migratory | Array
| RT-qPCR
| Function | Connected to TGF-β
signaling | Ref. |
|---|
| I | A | M | I | A | M |
|---|
| Downregulated
genes |
| EPHB2 | Ephrin type-B
receptor 2 | Pro-migratory | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | EPHB2 is a TGF-β
target important for TGF-β-mediated invasion and migration in
breast cancer. EphB2 expression stimulates glioma neurosphere cell
migration and invasion. Its overexpression in glioma cells results
in reduced cell adhesion and increased cell invasion. | Yes | (34,38,57) |
| CASP8 | Caspase-8 | Pro-migratory | ↓ | | | | n.d. | | Downregulation of
caspase-8 results in an inhibition of the migratory potential of
glioma cells. | Yes | (42) |
| CEACAM1 | Carcinoembryonic
antigen related cell adhesion molecule 1 | Pro-migratory | ↓ | | | | n.d. | | CEACAM1 inhibits
cell-matrix adhesion and promotes cell migration and promotes
invasiveness of thyroid cancer. | Yes | (43,44) |
| DCC | Deleted in colon
cancer | Pro-migratory | ↓ | | | | n.d. | | The netrin-1
receptor DCC promotes filopodia formation and cell spreading by
activating Cdc42 and Rac1. | No | (45) |
| KISS1R | KISS-1
receptor | Controversial | ↓ | | | | n.d. | | KISS1R signaling
promotes invadopodia formation in human breast cancer. | Yes | (48) |
| MMP-2 | Matrix
metalloproteinase-2 | Pro-migratory | No change | ↓ | | | MMP-2 expression is
elevated by the degree of malignancy of glioma. MMP-2 and -9
together play an important role in the invasiveness of gliomas by
modulating the degradation of the ECM. | Yes | (58) |
| MMP-14/MT1-MMP | Matrix
metalloproteinase-14 | Pro-migratory | ↓ | ↓ | ↓ | ↓ | | | MMP14 promotes
glioma invasion, proliferation and angiogenesis. Coexpression of
MMP-14 and MMP-19 predicts poor survival in human glioma. | Yes | (59,60) |
| MMP-9 | Matrix
metalloproteinase-9 | Pro-migratory | ↓ | ↓ | ↓ | Very low
expression | MMP-9 expression
strongly associates with poor prognosis in p53+ GBM.
Epigenetic regulation of miRNA-211 by MMP-9 governs glioma cell
malignant features. | Yes | (61,62) |
| MTA1 |
Metastasis-associated protein 1 | Pro-migratory | ↓ | | | ↓ | ↓ | ↓ | MTA1 is upregulated
in glioma tissue and amplified in aggressive stem cells during
glioma recurrence. Its expression promotes motility and
invasiveness of pancreatic carcinoma cells. | Yes | (39,63) |
| MTA2 | Metastasis
associated protein 2 | Pro-migratory | | ↓ | | | n.d. | | MTA2 knockdown
suppresses proliferation and invasion of human glioma cells. | Yes | (64) |
| RBL1 | Retinoblastoma-like
1 | Controversial | ↓ | | | | n.d. | | RBL-1 expression is
enhanced in glioma compared to normal tissue In colon cancer, RBL-1
expression rises towards carcinogenesis and falls during
invasion. | ? | (49) |
| SET1 | SET nuclear
proto-oncogene | Pro-migratory | ↓ | ↑ | | | n.d | | SET stimulates cell
migration in a Rac1-dependent manner, its knockdown inhibits cell
migration and invasion in breast carcinoma cells. | No | (65,66) |
| TIMP1 | Tissue inhibitor of
metalloproteinase 1 | Anti-migratory | ↓ | | | | | ↓ | TIMP-1 is capable
of inhibiting the activities of all known MMPs. | Yes | (67) |
| TIMP2 | Tissue inhibitor of
metalloproteinase 2 | Controversial | No change | ↓ | ↓ | | Upregulation TIMP-2
promotes MMP-2 activation and cell invasion in a human GBM cell
line. | No | (68) |
| TGFB1 | Transforming growth
factor β 1 | Pro-migratory | ↓ | | ↓ | ↓ | ↓ | ↓ | Pro-migratory
cytokine in GBM. | | (7) |
| TGFB2 | Transforming growth
factor β 2 | Pro-migratory | Not present | ↓ | ↓ | ↓ | Pro-migratory
cytokine in GBM. | | (7) |
| TGFBR2 | Transforming growth
factor β receptor type 2 | Pro-migratory | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | Receptor for the
pro-migratory cytokine TGF-β. | Yes | (7) |
| TWIST1 | Twist-related
protein 1 | Pro-migratory | | | ↓ | | n.d. | | TWIST1 promotes
invasion of human GBM cells through mesenchymal change. | Yes | (69) |
| Upregulated
genes |
| BRMS1 | Breast cancer
metastasis suppressor 1 | Anti-migratory | | ↑ | | | n.d | | BRMS1 suppresses
glioma progression by regulating invasion, migration and adhesion
of glioma cells. | Yes | (50) |
| FAT1 | FAT atypical
cadherin 1 | Pro-migratory | | | ↑ | | n.d. | | FAT1 knockdown
results in decreased migration and invasion in glioma cells. | ? | (71) |
| FGF2 | Fibroblast growth
factor 2 | Anti-migratory | ↑ | ↑ | ↑ | | n.d. | | Enhanced expression
of FGF2 in the lesser aggressive and invasive proneural subtype of
GBM compared to invasively growing mesenchymal subtype of GBM.
Better prognosis of glioma patients expressing the FGF2-dependent
PDGF receptor A. | Yes | (51,52) |
| HGF | Hepatocyte growth
factor | Controversial | | ↑ | | | n.d. | | HGF is the ligand
of the pro-invasive receptor MET. MET can also bind glioma secreted
VEGF instead of HGF, this leading to lesser glioma cell
invasion. | Yes | (72) |
| IL-18 | Interleukin 18 | Pro-migratory | | ↑ | | | n.d. | | IL-18 is a driver
of GBM cell migration. | Yes | (73) |
| IL-1B | Interleukin 1
β | Controversial | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | Expression of IL-1β
in the tumor micro-environment significantly increases migration
and invasion of glioma cells. Non-toxic concentrations of ML induce
the release of pro-inflammatory cytokines such as IL-1α, IL-1β,
IL-6, IL-8 and IFNγ. | Yes | (74,75) |
| K-Ras | Kirsten Ras
protooncogene | Pro-migratory | | ↑ | | | n.d. | | KRAS-induced
interleukin-8 overexpression promotes cell growth and migration in
non-small cell lung cancer. | Yes | (76) |
| MET | Hepatocyte growth
factor receptor | Controversial | | ↑ | ↑ | | n.d | | MET is a
pro-invasive receptor, it can also bind glioma secreted VEGF
instead of its natural ligand HGF, this leading to lesser glioma
cell invasion. | ? | (72) |
| MMP-1 | Matrix
metalloproteinase 1 | Pro-migratory | | ↑ | ↑ | | n.d. | | EGF induces MMP-1
expression and invasion in glioma cell lines. | Yes | (77,78) |
| MYC | c-Myc | Controversial | ↑ | ↑ | ↑ | | n.d. | | Myc is a
pro-proliferative proto-oncogene. Increased c-myc activity was
found in migration-restricted proliferative glioma cells. Besides
this, myc suppresses the activation of TGFβ-induced genes. | Yes | (79) |
| NME1/NM23/ | Nucleoside
diphosphate kinase A | Anti-migratory | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | NME1/NM23 is
described as a metastasis suppressor and plays an important role in
the suppression of glioma invasion and migration. | Yes | (53,80) |
| PTGS2 |
Prostaglandin-endoperoxide synthase 2 | Pro-migratory | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | PTGS2 is associated
with recurrence and metastatic disease in multiple cancers. In GBM
cells with COX-2 or ID1 overexpression, PTGS2 demonstrates greater
migration/invasine potential. | Yes | (81) |
| S100A4 | S100 Calcium
binding protein A4 | Pro-migratory | | ↑ | | | n.d. | | Extracellular
S100A4 stimulates the migration rate of astrocytic tumor
cells. | Yes | (82) |
| SERPINE1 | Serpine
1/plasminogen activator inhibitor-1 (PAI1) | Pro-migratory | ↑ | ↑ | ↑ | | n.d. | | SERPINE 1 is
described as a marker protein for metastatic melanoma, it is highly
expressed in GBM. | Yes | (83) |
|
SERPINB5/MASPIN | Serpin peptidase
inhibitor, member 5/Maspin | Anti-migratory | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | Overexpression of
SERPIN B5 effectively suppresses the invasiveness and motility of
malignant cancer cells. SERPIN 5 is silenced in glioma cells
promoter hypermethylation. Its overexpression inhibits cell growth
of glioma cells. | Yes | (55) |
Acknowledgments
The authors would like to thank the ISUS Foundation,
the Software AG Foundation and Iscador AG for funding of the
present study. They also thank the Iscador AG, Melema Pharma GmbH
and Abnoba GmbH for providing us with the appropriate material.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Claes A, Idema AJ and Wesseling P: Diffuse
glioma growth: A guerilla war. Acta Neuropathol. 114:443–458. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wick W, Platten M and Weller M: Glioma
cell invasion: Regulation of metalloproteinase activity by
TGF-beta. J Neurooncol. 53:177–185. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wild-Bode C, Weller M and Wick W:
Molecular determinants of glioma cell migration and invasion. J
Neurosurg. 94:978–984. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wick W, Naumann U and Weller M:
Transforming growth factor-beta: A molecular target for the future
therapy of glioblastoma. Curr Pharm Des. 12:341–349. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kahlert UD, Nikkhah G and Maciaczyk J:
Epithelial-to-mesenchymal(-like) transition as a relevant molecular
event in malignant gliomas. Cancer Lett. 331:131–138. 2013.
View Article : Google Scholar
|
|
7
|
Naumann U, Harter PN, Rubel J, Ilina E,
Blank AE, Esteban H and Mittelbronn M: Glioma cell migration and
invasion as potential target for novel treatment strategies. Transl
Neurosci. 4:314–329. 2013. View Article : Google Scholar
|
|
8
|
Kathagen A, Schulte A, Balcke G, Phillips
HS, Martens T, Matschke J, Günther HS, Soriano R, Modrusan Z,
Sandmann T, et al: Hypoxia and oxygenation induce a metabolic
switch between pentose phosphate pathway and glycolysis in glioma
stem-like cells. Acta Neuropathol. 126:763–780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mistletoe Extracts :(PDQ®): Health
Professional Version. PDQ Cancer Information Summaries (Internet)
Bethesda (MD): 2016, Available from: https://www.ncbi.nlm.nih.gov/books/NBK66054/.
|
|
10
|
Yau T, Dan X, Ng CC and Ng TB: Lectins
with potential for anti-cancer therapy. Molecules. 20:3791–3810.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elluru S, Duong Van Huyen JP, Delignat S,
Prost F, Bayry J, Kazatchkine MD and Kaveri SV: Molecular
mechanisms underlying the immunomodulatory effects of mistletoe
(Viscum album L.) extracts Iscador. Arzneimittelforschung. 56(6A):
461–466. 2006.PubMed/NCBI
|
|
12
|
Büssing A and Schietzel M:
Apoptosis-inducing properties of Viscum album L. extracts from
different host trees, correlate with their content of toxic
mistletoe lectins. Anticancer Res. 19(1A): 23–28. 1999.PubMed/NCBI
|
|
13
|
Pryme IF, Bardocz S, Pusztai A and Ewen
SW: Suppression of growth of tumour cell lines in vitro and tumours
in vivo by mistletoe lectins. Histol Histopathol. 21:285–299.
2006.
|
|
14
|
Hajtó T, Fodor K, Perjési P and Németh P:
Difficulties and perspectives of immunomodulatory therapy with
mistletoe lectins and standardized mistletoe extracts in
evidence-based medicine. Evid Based Complement Alternat Med.
2011:2989722011. View Article : Google Scholar :
|
|
15
|
Podlech O, Harter PN, Mittelbronn M,
Pöschel S and Naumann U: Fermented mistletoe extract as a
multimodal antitumoral agent in gliomas. Evid Based Complement
Alternat Med. 2012:5017962012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schöffski P, Riggert S, Fumoleau P,
Campone M, Bolte O, Marreaud S, Lacombe D, Baron B, Herold M,
Zwierzina H, et al European Organization for Research and Treatment
of Cancer New Drug Development Group: Phase I trial of intravenous
aviscumine (rViscumin) in patients with solid tumors: A study of
the European Organization for Research and Treatment of Cancer New
Drug Development Group. Ann Oncol. 15:1816–1824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Urech K, Schaller G and Jäggy C:
Viscotoxins, mistletoe lectins and their isoforms in mistletoe
(Viscum album L.) extracts Iscador. Arzneimittelforschung. 56(6A):
428–434. 2006.PubMed/NCBI
|
|
18
|
Jung ML, Baudino S, Ribéreau-Gayon G and
Beck JP: Characterization of cytotoxic proteins from mistletoe
(Viscum album L.). Cancer Lett. 51:103–108. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eck J, Langer M, Möckel B, Witthohn K,
Zinke H and Lentzen H: Characterization of recombinant and
plant-derived mistletoe lectin and their B-chains. Eur J Biochem.
265:788–797. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eck J, Langer M, Möckel B, Baur A, Rothe
M, Zinke H and Lentzen H: Cloning of the mistletoe lectin gene and
characterization of the recombinant A-chain. Eur J Biochem.
264:775–784. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zwierzina H, Bergmann L, Fiebig H, Aamdal
S, Schöffski P, Witthohn K and Lentzen H: The preclinical and
clinical activity of aviscumine: A potential anticancer drug. Eur J
Cancer. 47:1450–1457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naumann U, Kügler S, Wolburg H, Wick W,
Rascher G, Schulz JB, Conseiller E, Bähr M and Weller M: Chimeric
tumor suppressor 1, a p53-derived chimeric tumor suppressor gene,
kills p53 mutant and p53 wild-type glioma cells in synergy with
irradiation and CD95 ligand. Cancer Res. 61:5833–5842.
2001.PubMed/NCBI
|
|
23
|
Platten M, Wick W and Weller M: Malignant
glioma biology: Role for TGF-beta in growth, motility,
angiogenesis, and immune escape. Microsc Res Tech. 52:401–410.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakano A, Tani E, Miyazaki K, Yamamoto Y
and Furuyama J: Matrix metalloproteinases and tissue inhibitors of
metalloproteinases in human gliomas. J Neurosurg. 83:298–307. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nikolai G, Friedl P, Werner M, Niggemann B
and Zänker KS: Effect of a mistletoe extract (Iscador QuFrF) on
viability and migratory behavior of human peripheral
CD4+ and CD8+ T lymphocytes in
three-dimensional collagen lattices. In Vitro Cell Dev Biol Anim.
33:710–716. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gren A: Effects of Iscador preparations on
the reactivity of mouse immune system. Neuro Endocrinol Lett.
30:530–534. 2009.PubMed/NCBI
|
|
27
|
Kuttan G and Kuttan R: Immunological
mechanism of action of the tumor reducing peptide from mistletoe
extract (NSC 635089) cellular proliferation. Cancer Lett.
66:123–130. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Braedel-Ruoff S: Immunomodulatory effects
of Viscum album extracts on natural killer cells: Review of
clinical trials. Forsch Komplement Med. 17:63–73. 2010. View Article : Google Scholar
|
|
29
|
Schink M, Tröger W, Dabidian A, Goyert A,
Scheuerecker H, Meyer J, Fischer IU and Glaser F: Mistletoe extract
reduces the surgical suppression of natural killer cell activity in
cancer patients. a randomized phase III trial. Forsch Komplement
Med. 14:9–17. 2007. View Article : Google Scholar
|
|
30
|
Antony S, Kuttan R and Kuttan G: Role of
natural killer cells in iscador mediated inhibition of metastasis
by adoptive immunotherapy. Immunol Invest. 29:219–231. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thies A, Dautel P, Meyer A, Pfüller U and
Schumacher U: Low-dose mistletoe lectin-I reduces melanoma growth
and spread in a scid mouse xenograft model. Br J Cancer.
98:106–112. 2008. View Article : Google Scholar
|
|
32
|
Tröger W, Galun D, Reif M, Schumann A,
Stanković N and Milićević M: Viscum album [L.] extract therapy in
patients with locally advanced or metastatic pancreatic cancer: A
randomised clinical trial on overall survival. Eur J Cancer.
49:3788–3797. 2013. View Article : Google Scholar
|
|
33
|
Büssing A, Raak C and Ostermann T: Quality
of life and related dimensions in cancer patients treated with
mistletoe extract (iscador): A meta-analysis. Evid Based Complement
Alternat Med. 2012:2194022012. View Article : Google Scholar
|
|
34
|
Ostermann T, Raak C and Büssing A:
Survival of cancer patients treated with mistletoe extract
(Iscador): A systematic literature review. BMC Cancer. 9:4512009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trefzer U, Gutzmer R, Wilhelm T, Schenck
F, Kähler KC, Jacobi V, Witthohn K, Lentzen H and Mohr P: Treatment
of unresectable stage IV metastatic melanoma with aviscumine after
anti-neoplastic treatment failure: A phase II, multi-centre study.
J Immunother Cancer. 2:272014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naumann U, Maass P, Gleske AK, Aulwurm S,
Weller M and Eisele G: Glioma gene therapy with soluble
transforming growth factor-beta receptors II and III. Int J Oncol.
33:759–765. 2008.PubMed/NCBI
|
|
37
|
Nakada M, Niska JA, Miyamori H, McDonough
WS, Wu J, Sato H and Berens ME: The phosphorylation of EphB2
receptor regulates migration and invasion of human glioma cells.
Cancer Res. 64:3179–3185. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang SD, Rath P, Lal B, Richard JP, Li Y,
Goodwin CR, Laterra J and Xia S: EphB2 receptor controls
proliferation/migration dichotomy of glioblastoma by interacting
with focal adhesion kinase. Oncogene. 31:5132–5143. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang Q, Zhang QB, Dong J, Wu YY, Shen YT,
Zhao YD, Zhu YD, Diao Y, Wang AD and Lan Q: Glioma stem cells are
more aggressive in recurrent tumors with malignant progression than
in the primary tumor, and both can be maintained long-term in
vitro. BMC Cancer. 8:3042008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang W, Men X and Lei P: Review on
anti-tumor effect of triterpene acid compounds. J Cancer Res Ther.
10(Suppl 1): 14–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke
FC, Huang YT and Lee MT: The antitumor activities of flavonoids. In
Vivo. 19:895–909. 2005.PubMed/NCBI
|
|
42
|
Gdynia G, Grund K, Eckert A, Böck BC,
Funke B, Macher-Goeppinger S, Sieber S, Herold-Mende C, Wiestler B,
Wiestler OD, et al: Basal caspase activity promotes migration and
invasiveness in glioblastoma cells. Mol Cancer Res. 5:1232–1240.
2007. View Article : Google Scholar
|
|
43
|
Liu W, Wei W, Winer D, Bamberger AM,
Bamberger C, Wagener C, Ezzat S and Asa SL: CEACAM1 impedes thyroid
cancer growth but promotes invasiveness: A putative mechanism for
early metastases. Oncogene. 26:2747–2758. 2007. View Article : Google Scholar
|
|
44
|
Ebrahimnejad A, Streichert T, Nollau P,
Horst AK, Wagener C, Bamberger AM and Brümmer J: CEACAM1 enhances
invasion and migration of melanocytic and melanoma cells. Am J
Pathol. 165:1781–1787. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shekarabi M and Kennedy TE: The netrin-1
receptor DCC promotes filopodia formation and cell spreading by
activating Cdc42 and Rac1. Mol Cell Neurosci. 19:1–17. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakada M, Kita D, Futami K, Yamashita J,
Fujimoto N, Sato H and Okada Y: Roles of membrane type 1 matrix
metalloproteinase and tissue inhibitor of metalloproteinases 2 in
invasion and dissemination of human malignant glioma. J Neurosurg.
94:464–473. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lam BD, Anthony EC and Hordijk PL:
Analysis of nucleocytoplasmic shuttling of the proto-oncogene
SET/I2PP2A. Cytometry A. 81:81–89. 2012.
|
|
48
|
Goertzen CG, Dragan M, Turley E, Babwah AV
and Bhattacharya M: KISS1R signaling promotes invadopodia formation
in human breast cancer cell via β-arrestin2/ERK. Cell Signal.
28:165–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu F, Li JQ, Miki H, Nishioka M, Fujita J,
Ohmori M, Imaida K and Kuriyama S: p107 Expression in colorectal
tumours rises during carcinogenesis and falls during invasion. Eur
J Cancer. 38:1838–1848. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mei P, Bai J, Shi M, Liu Q, Li Z, Fan Y
and Zheng J: BRMS1 suppresses glioma progression by regulating
invasion, migration and adhesion of glioma cells. PLoS One.
9:e985442014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sooman L, Freyhult E, Jaiswal A, Navani S,
Edqvist PH, Pontén F, Tchougounova E, Smits A, Elsir T, Gullbo J,
et al: FGF2 as a potential prognostic biomarker for proneural
glioma patients. Acta Oncol. 54:385–394. 2015. View Article : Google Scholar
|
|
52
|
Chen D, Persson A, Sun Y, Salford LG, Nord
DG, Englund E, Jiang T and Fan X: Better prognosis of patients with
glioma expressing FGF2-dependent PDGFRA irrespective of
morphological diagnosis. PLoS One. 8:e615562013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Boissan M, Poupon MF and Lacombe ML: NM23
and metastasis suppressor genes: Update. Med Sci (Paris).
23:1115–1123. 2007.In French. View Article : Google Scholar
|
|
54
|
McDermott WG, Boissan M, Lacombe ML, Steeg
PS and Horak CE: Nm23-H1 homologs suppress tumor cell motility and
anchorage independent growth. Clin Exp Metastasis. 25:131–138.
2008. View Article : Google Scholar
|
|
55
|
Chou RH, Wen HC, Liang WG, Lin SC, Yuan
HW, Wu CW and Chang WS: Suppression of the invasion and migration
of cancer cells by SERPINB family genes and their derived peptides.
Oncol Rep. 27:238–245. 2012.
|
|
56
|
Xu L, Liu H, Yu J, Wang Z, Zhu Q, Li Z,
Zhong Q, Zhang S, Qu M and Lan Q: Methylation-induced silencing of
maspin contributes to the proliferation of human glioma cells.
Oncol Rep. 36:57–64. 2016.PubMed/NCBI
|
|
57
|
Lam S, Wiercinska E, Teunisse AF, Lodder
K, ten Dijke P and Jochemsen AG: Wild-type p53 inhibits
pro-invasive properties of TGF-β3 in breast cancer, in part through
regulation of EPHB2, a new TGF-β target gene. Breast Cancer Res
Treat. 148:7–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang M, Wang T, Liu S, Yoshida D and
Teramoto A: The expression of matrix metalloproteinase-2 and -9 in
human gliomas of different pathological grades. Brain Tumor Pathol.
20:65–72. 2003. View Article : Google Scholar
|
|
59
|
Wang L, Yuan J, Tu Y, Mao X, He S, Fu G,
Zong J and Zhang Y: Co-expression of MMP-14 and MMP-19 predicts
poor survival in human glioma. Clin Transl Oncol. 15:139–145. 2013.
View Article : Google Scholar
|
|
60
|
Ulasov I, Yi R, Guo D, Sarvaiya P and
Cobbs C: The emerging role of MMP14 in brain tumorigenesis and
future therapeutics. Biochim Biophys Acta. 1846:113–120.
2014.PubMed/NCBI
|
|
61
|
Shastry AH, Thota B, Arimappamagan A and
Santosh V: P53 stratification reveals the prognostic utility of
matrix metalloproteinase-9 protein expression in glioblastoma.
Neurol India. 63:399–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Asuthkar S, Velpula KK, Chetty C, Gorantla
B and Rao JS: Epigenetic regulation of miRNA-211 by MMP-9 governs
glioma cell apoptosis, chemosensitivity and radiosensitivity.
Oncotarget. 3:1439–1454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hofer MD, Menke A, Genze F, Gierschik P
and Giehl K: Expression of MTA1 promotes motility and invasiveness
of PANC-1 pancreatic carcinoma cells. Br J Cancer. 90:455–462.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cheng CY, Chou YE, Ko CP, Yang SF, Hsieh
SC, Lin CL, Hsieh YH and Chen KC: Metastasis tumor-associated
protein-2 knockdown suppresses the proliferation and invasion of
human glioma cells in vitro and in vivo. J Neurooncol. 120:273–281.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
ten Klooster JP, Leeuwen I, Scheres N,
Anthony EC and Hordijk PL: Rac1-induced cell migration requires
membrane recruitment of the nuclear oncogene SET. EMBO J.
26:336–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li J, Yang XF, Ren XH, Meng XJ, Huang HY,
Zhao QH, Yuan JH, Hong WX, Xia B, Huang XF, et al: Stable SET
knockdown in breast cell carcinoma inhibits cell migration and
invasion. Biochem Biophys Res Commun. 453:7–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gomez DE, Alonso DF, Yoshiji H and
Thorgeirsson UP: Tissue inhibitors of metalloproteinases:
Structure, regulation and biological functions. Eur J Cell Biol.
74:111–122. 1997.PubMed/NCBI
|
|
68
|
Lu KV, Jong KA, Rajasekaran AK, Cloughesy
TF and Mischel PS: Upregulation of tissue inhibitor of
metalloproteinases (TIMP)-2 promotes matrix metalloproteinase
(MMP)-2 activation and cell invasion in a human glioblastoma cell
line. Lab Invest. 84:8–20. 2004. View Article : Google Scholar
|
|
69
|
Mikheeva SA, Mikheev AM, Petit A, Beyer R,
Oxford RG, Khorasani L, Maxwell JP, Glackin CA, Wakimoto H,
González-Herrero I, et al: TWIST1 promotes invasion through
mesenchymal change in human glioblastoma. Mol Cancer. 9:1942010.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Elias MC, Tozer KR, Silber JR, Mikheeva S,
Deng M, Morrison RS, Manning TC, Silbergeld DL, Glackin CA, Reh TA,
et al: TWIST is expressed in human gliomas and promotes invasion.
Neoplasia. 7:824–837. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Madan E, Dikshit B, Gowda SH, Srivastava
C, Sarkar C, Chattopadhyay P, Sinha S and Chosdol K: FAT1 is a
novel upstream regulator of HIF1α and invasion of high grade
glioma. Int J Cancer. 139:2570–2582. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lu KV, Chang JP, Parachoniak CA, Pandika
MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ,
Cheresh DA, et al: VEGF inhibits tumor cell invasion and
mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell.
22:21–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kast RE: The role of interleukin-18 in
glioblastoma pathology implies therapeutic potential of two old
drugs-disulfiram and ritonavir. Chin J Cancer. 34:161–165. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fathima Hurmath K, Ramaswamy P and
Nandakumar DN: IL-1β microenvironment promotes proliferation,
migration, and invasion of human glioma cells. Cell Biol Int.
38:1415–1422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lyu SY and Park WB: Effects of Korean
mistletoe lectin (Viscum album coloratum) on proliferation and
cytokine expression in human peripheral blood mononuclear cells and
T-lymphocytes. Arch Pharm Res. 30:1252–1264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sunaga N, Imai H, Shimizu K, Shames DS,
Kakegawa S, Girard L, Sato M, Kaira K, Ishizuka T, Gazdar AF, et
al: Oncogenic KRAS-induced interleukin-8 overexpression promotes
cell growth and migration and contributes to aggressive phenotypes
of non-small cell lung cancer. Int J Cancer. 130:1733–1744. 2012.
View Article : Google Scholar :
|
|
77
|
Pullen NA, Anand M, Cooper PS and Fillmore
HL: Matrix metalloproteinase-1 expression enhances tumorigenicity
as well as tumor-related angiogenesis and is inversely associated
with TIMP-4 expression in a model of glioblastoma. J Neurooncol.
106:461–471. 2012. View Article : Google Scholar
|
|
78
|
Anand M, Van Meter TE and Fillmore HL:
Epidermal growth factor induces matrix metalloproteinase-1 (MMP-1)
expression and invasion in glioma cell lines via the MAPK pathway.
J Neurooncol. 104:679–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Dhruv HD, McDonough Winslow WS, Armstrong
B, Tuncali S, Eschbacher J, Kislin K, Loftus JC, Tran NL and Berens
ME: Reciprocal activation of transcription factors underlies the
dichotomy between proliferation and invasion of glioma cells. PLoS
One. 8:e721342013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jung S, Paek YW, Moon KS, Wee SC, Ryu HH,
Jeong YI, Sun HS, Jin YH, Kim KK and Ahn KY: Expression of Nm23 in
gliomas and its effect on migration and invasion in vitro.
Anticancer Res. 26:249–258. 2006.PubMed/NCBI
|
|
81
|
Xu K, Wang L and Shu HK: COX-2
overexpression increases malignant potential of human glioma cells
through Id1. Oncotarget. 5:1241–1252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Belot N, Pochet R, Heizmann CW, Kiss R and
Decaestecker C: Extracellular S100A4 stimulates the migration rate
of astrocytic tumor cells by modifying the organization of their
actin cytoskeleton. Biochim Biophys Acta. 1600:74–83. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Klein RM, Bernstein D, Higgins SP, Higgins
CE and Higgins PJ: SERPINE1 expression discriminates site-specific
metastasis in human melanoma. Exp Dermatol. 21:551–554. 2012.
View Article : Google Scholar : PubMed/NCBI
|