Introduction
Malignant mesothelioma, highly aggressive fatal
cancer, is associated with occupational and environmental asbestos
exposure (1). Its incidence is
increasing in Japan, Western European countries and is expected to
increase in other developing countries (2). The molecular mechanism of its
carcinogenesis is not yet fully understood. Previously, we studied
microRNA expression profile of mesothelioma cell lines and found
that significant numbers of microRNA were differentially expressed
in malignant mesothelioma. In addition to various upregulated
microRNAs in mesothelioma cell lines, we found no expression of
many microRNAs, namely miR-1 and miR-214. By microRNA mimic
transfection study, we reported the role of miR-1, and miR-214 in
proliferation and invasion of mesothelioma cells. Many genes are
co-targeted by miR-1 and miR-214. Out of these genes, we paid
attention to PIM1 gene, a proto-oncogene, for its high expression
in mesothelioma cell lines (3).
Provirus integration site for Moloney murine leukemia virus 1
(PIM1) is a serine/threonine kinase that acts as protooncogene to
mediate cell survival. PIM1 has been reported to be upregulated in
various human cancers such as prostate (4), pancreatic (5), gastric cancer (6) and glioblastoma (7). The overexpression of PIM1 was well
correlated to carcinogenesis by promoting tumor cell proliferation
and inhibiting apoptosis (8). In
the present study, we analyzed the proliferation, the cell cycle
regulation, invasion and the migration of mesothelioma cells by
PIM1 downregulation with siRNA transfection.
Materials and methods
Mesothelioma cell lines
Mesothelioma cell lines, CRL-5915 (purchased from
the American Type Culture Collection, ATCC; Manassas, VA, USA) and
ACC-MESO-1 (RIKEN BioResearch Center, Tsukuba, Japan) (9) were maintained in Roswell Park
Memorial Institute 1640 medium with GlutaMAX (RPMI-1640) with 1%
kanamycin, 1% fungizone and 10% fetal bovine serum (FBS; all
purchased from Gibco/Life Technologies, Tokyo, Japan).
Transient transfections of mesothelioma
cells with small interfering RNA (siRNA) after siRNA
optimization
To optimize IC50 (half-maximum inhibitory
concentration) of siRNA, we used different concentrations of siRNA
for both cell lines which not causing cytotoxic effect on
mesothelioma cell lines (20, 30 and 40 nM). The cells were cultured
3×103 with the different concentrations of siRNA in
96-well plate for three days then adding PrestoBlue reagent
(PrestoBlue™ Cell Viability reagent; Invitrogen) every day and cell
viability was measured by GloMax® Explorer. All
transfection studies were carried out in triplicate with 20 nM of
PIM1-siRNA or negative control-siRNA with 5 μl of
Lipofectamine RNAiMax reagent in Opti-MEM media (Gibco) in 6-well
plates for 24 h before harvesting the treated cells. Details of
PIM1-siRNA were (siGENOME SMARTpool PIM-siRNA, GAUAUGGUGUGUGGAGAUA,
CAGAUAGGCCAACCUUCGA, GUGGAGAAGGACCGGAUUU, AGAUAUUCCUUUCGAGCAU) and
negative control-siRNA (UAGCGACUAAACACAUCAA) (Dharmacon, Lafayette,
CO, USA). After transfection with siRNAs, the cells were utilized
for various cell based assays, cell proliferation, cell cycle,
invasion and migration assays.
RNA isolation
Total RNA was extracted from the mesothelioma cell
lines using CellAmp™ Direct RNA Prep kit (Takara/Clontech
Laboratories) according to the manufacturer's recommended protocol.
The extracted RNA was quantified with a NanoVue Plus
spectrophotometer (GE Healthcare BioSciences, Tokyo, Japan) and
Qubit 2.0 (Life Technologies) using fluorometer-based RNA assay
kit.
Real-time reverse transcription
polymerase chain reaction
A total of 200 ng of total RNA was
reverse-transcribed and amplified using One Step SYBR®
PrimeScript® RT-PCR kit (Takara Bio) with the Mx3000P
real-time PCR (Stratagene, La Jolla, CA, USA) system. Relative
expression was calculated by the comparative CT (ΔΔCT) method.
Western blot analysis
Cells (5×105) were seeded and transfected
in 6-well plates for 3 days and cell lysates were obtained from
siRNA transfected cells using M-PER (mammalian protein extraction
reagent) with Halt Protease Inhibitor Cocktail and Phosphatase
inhibitor (Thermo Fisher Scientific, Waltham, MA, USA). Total
protein (20–30 μg) was electrophoresed on TGX acrylamide Gel
(Bio-Rad Laboratories, Tokyo, Japan) at 75 V for 90 min and
transferred onto a polyvinylidene difluoride (PVDF) membrane using
Mini Blot Module (Thermo Fisher Scientific) at 20 V for 60 min. The
protein-transferred membrane was processed with primary antibodies,
anti-PIM1 antibody (1:2000, rabbit monoclonal, ab75776; Abcam) and
anti-GAPDH antibody (1:1000, rabbit polyclonal sc-25778; Santa Cruz
Biotechnology, Dallas, TX, USA) and biotin labeled secondary
antibody (HRP-linked goat anti-rabbit IgG, 7074S; Cell Signaling
Technology, Danvers, MA, USA) and all were diluted in Can Get
Signal buffer (Toyobo Life Science, Osaka, Japan). The membrane was
washed and stained with ImmunoCruz™ Western Blotting Luminol
reagent (Santa Cruz Biotechnology) and images were captured using
C-DiGit Blot scanner (LI-COR Biosciences, Lincoln, NE, USA) with
Image Studio software.
Cell proliferation assay
siRNA transfected cells (3×103) were
incubated in RPMI-1640 media with 1% FBS in 96-well plates in
triplicate for 3 days. Total number of viable cells (based on
quantitation of ATP present, which indicate the presence of
metabolically active cells) were determined every 24, 48 and 72 h
by CellTiter-Glo 2.0 reagent (Promega) according to the
manufacturer's recommended protocol using GloMax Explorer.
Cell cycle phase analysis
siRNA transfected cells (5×104) were
incubated in RPMI-1640 media with 1% FBS in collagen-coated 12-well
plates in triplicate for 48 and 72 h. Cells were harvested after
trypsinization and slowly fixed in 70% ethanol for more than 1 h.
Ethanol fixed cells were washed and stained with Guava Cell Cycle
reagent and data were collected with flow cytometer using Cytosoft
cell cycle module software (Guava Technologies, Hayward, CA, USA).
Guava EasyCyte Mini flow cytometric raw data were analyzed using
FCS Express 5 Pro software (De Novo Software, Glendale, CA,
USA).
Cell invasion assay
siRNA transfected cells (1×104) for
ACC-MESO-1 and 1.5×105 siRNA transfected cells for
CRL-5915 were incubated in BD FluoroBlok culture inserts with
8-μm pores (BD Biosciences, Franklin Lakes, NJ, USA) coated
with matrix Matrigel (Life Technologies) according to the
manufacturer's protocol. Invaded cells were stained with Hoechst
33342 (Life Technologies) and imaged area of insert membrane was
captured with fluorescent microscope (IX81, inverted microscope
with DP80 CCD camera; Olympus, Tokyo, Japan). The total numbers of
invading cells were calculated by analyzing fluorescent images with
CellProfiler cell imaging software (10).
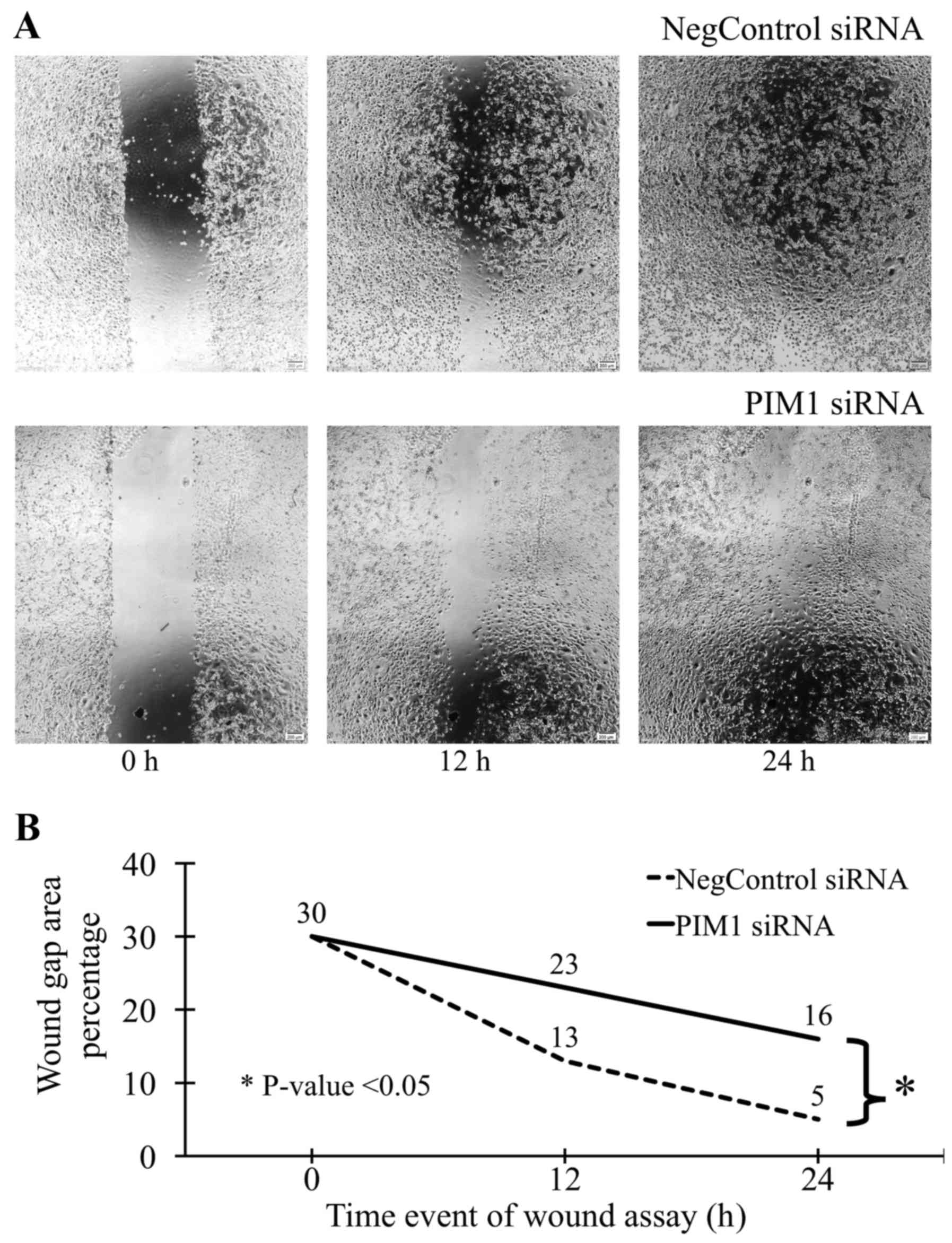
Cell migration assay
Cell migration was analyzed using a wound/scratch
assay. siRNA transfected cells were incubated in RPMI-1640 media
with 10% FBS in collagen-coated 24-well plates overnight. The wound
was created in cell monolayer by scratching the cells with 1 ml
micropipette tips and floating cells were removed by washing with
fresh media. Real-time microscopic pictures were taken at 0, 12 and
24 h post scratching by incubating the cells in stage-top incubator
on inverted microscope with automatic picture acquisition at given
time interval (Olympus IX81 with cellSens software). The area of
the wound gap that was not covered by cell migration (percentage)
was determined using TScrach software (11).
Statistical analysis
All statistical analysis was performed with GraphPad
QuickCalcs t-test calculator (https://www.graphpad.com/quickcalcs/).
Results
Validation of PIM1 knockdown by siRNA
transfection
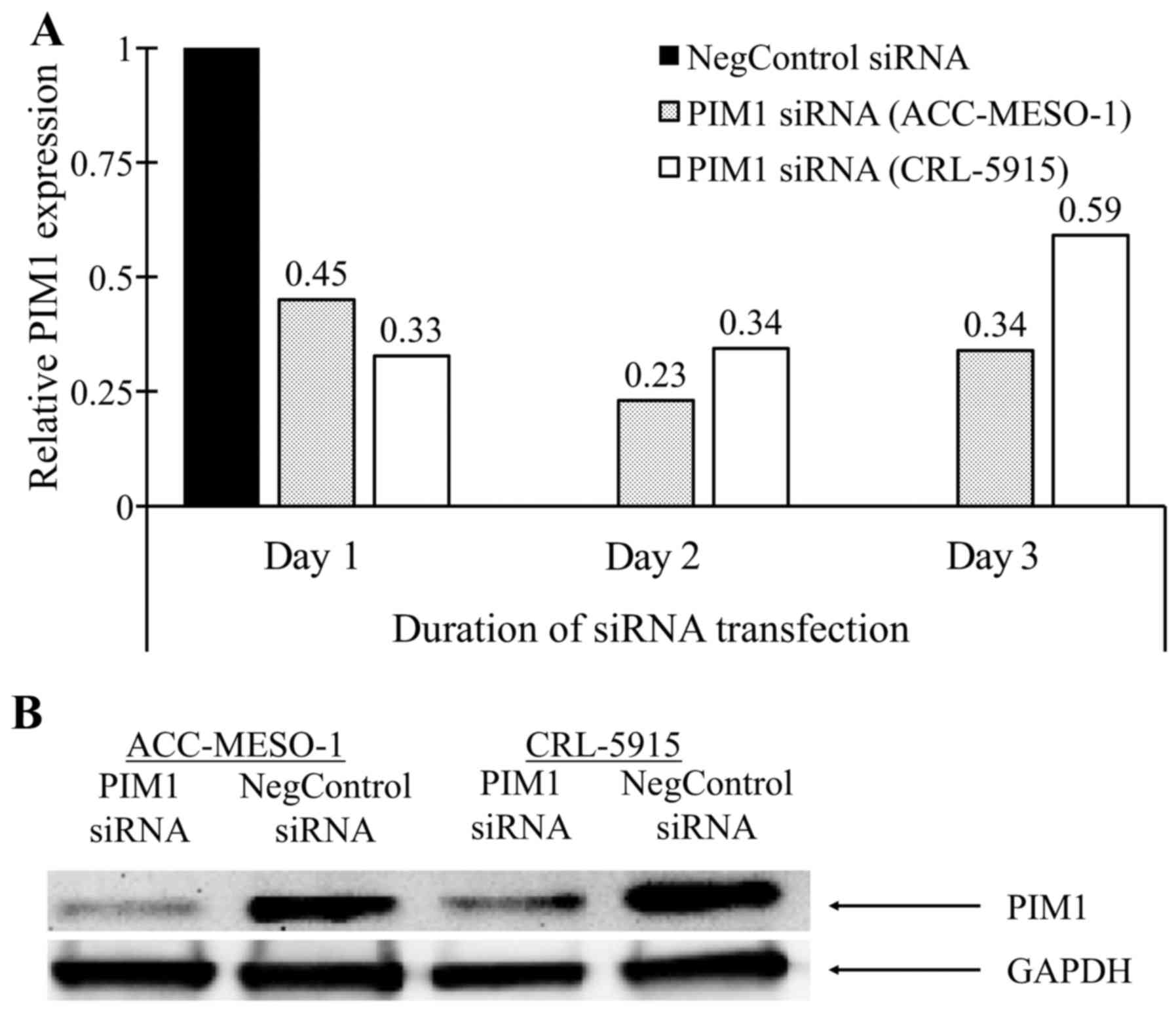
After optimization of IC50 of siRNA, we
found that all the different concentration of PIM1-siRNA and
negative control-siRNA have less cytotoxic effect on both cell
lines. With 20 nM of PIM1-siRNA, we found that the downregulation
of PIM1 mRNA was from 50 to 70% (Fig.
1A). Significant down-regulation of PIM1 was found at protein
level by western blot analysis (Fig.
1B).
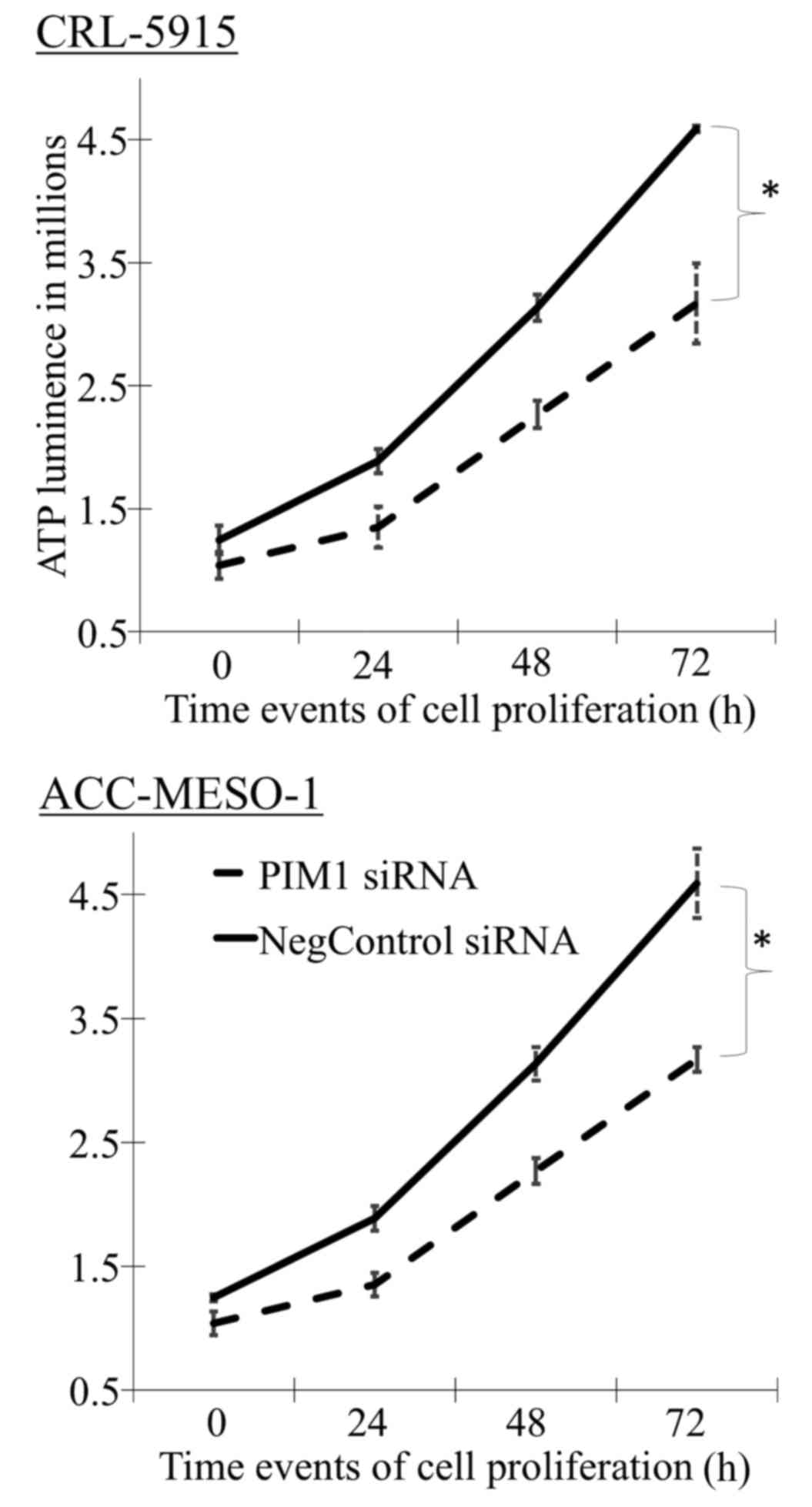
PIM1 knockdown reduces cell proliferation
with G1 arrest
Mesothelioma cell lines transfected with PIM1-siRNA
showed ~20% reduced proliferation rate compared to that with
negative control-siRNA in a time-dependent manner (Fig. 2 and Table I). In addition, we also noted
difference in the proliferation rates between cell lines.
 | Table ICell proliferation analysis. |
Table I
Cell proliferation analysis.
| 0 h | 24 h | 48 h | 72 h |
|---|
| CRL-5915 | | | | |
| PIM1 siRNA | 1.04±0.10 | 1.35±0.10 | 2.27±0.10 | 3.17±0.10 |
| Negative control
siRNA | 1.25±0.03 | 1.89±0.10 | 3.13±0.14 | 4.59±0.28 |
| ACC-MESO-1 | | | | |
| PIM1 siRNA | 1.81±0.11 | 2.14±0.17 | 2.88±0.11 | 3.29±0.33 |
| Negative control
siRNA | 2.02±0.12 | 2.44±0.10 | 3.47±0.11 | 4.70±0.03 |
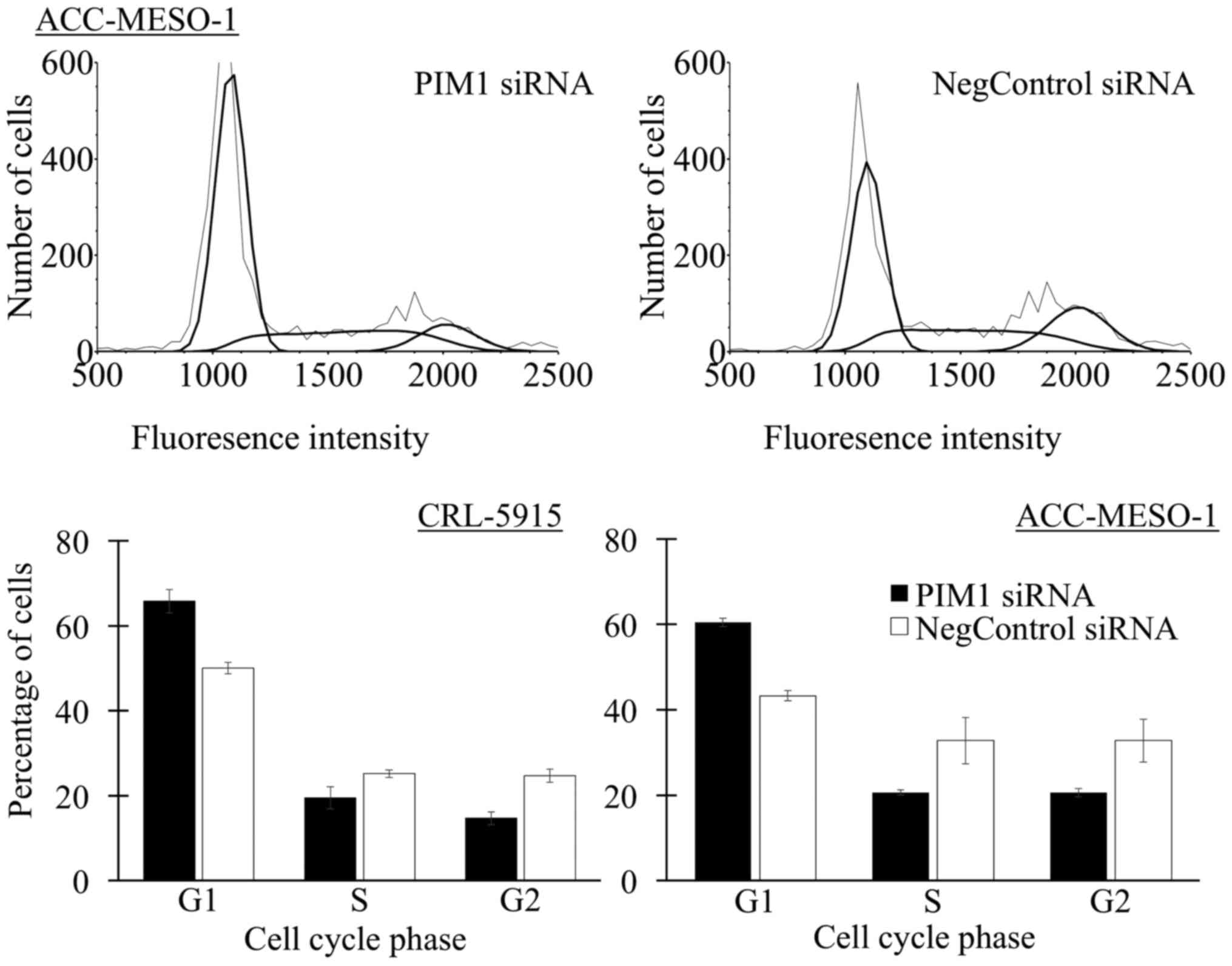
With siRNA transfection of mesothelioma cells for 2
days, the proportion of G1 phase of cell cycle in ACC-MESO-1 and
CRL-5915 with PIM1 transfection were 60.5 and 65.8% and that with
negative control was 43.3 and 50% (Fig. 3 and Table II). With siRNA transfection of
mesothelioma cells for 3 days, the proportion of G1 phase of cell
cycle in ACC-MESO-1 and CRL-5915 with PIM1 transfection were 84 and
78.6% and that with negative control was 74.5 and 74.1%.
 | Table IICell cycle phase analysis. |
Table II
Cell cycle phase analysis.
| G1 | S | G2 | P-value |
|---|
| ACC-MESO-1 | | | | |
| PIM1 siRNA | 60.5±1.0 | 20.6±0.6 | 20.6±1.0 | 0.0001 |
| Negative control
siRNA | 43.3±1.2 | 32.8±5.4 | 32.8±5.0 | |
| CRL-5915 | | | | |
| PIM1 siRNA | 65.8±2.7 | 19.5±2.6 | 14.7±1.5 | 0.0009 |
| Negative control
siRNA | 50.0±1.4 | 25.2±0.9 | 24.7±1.6 | |
These results suggest that PIM1-siRNA transfected
cells showed ~10% increase in G1 phase compared to negative
control-siRNA. In addition, the ratio of G1 arrest was higher on
day 2 than day 3 in both cell lines.
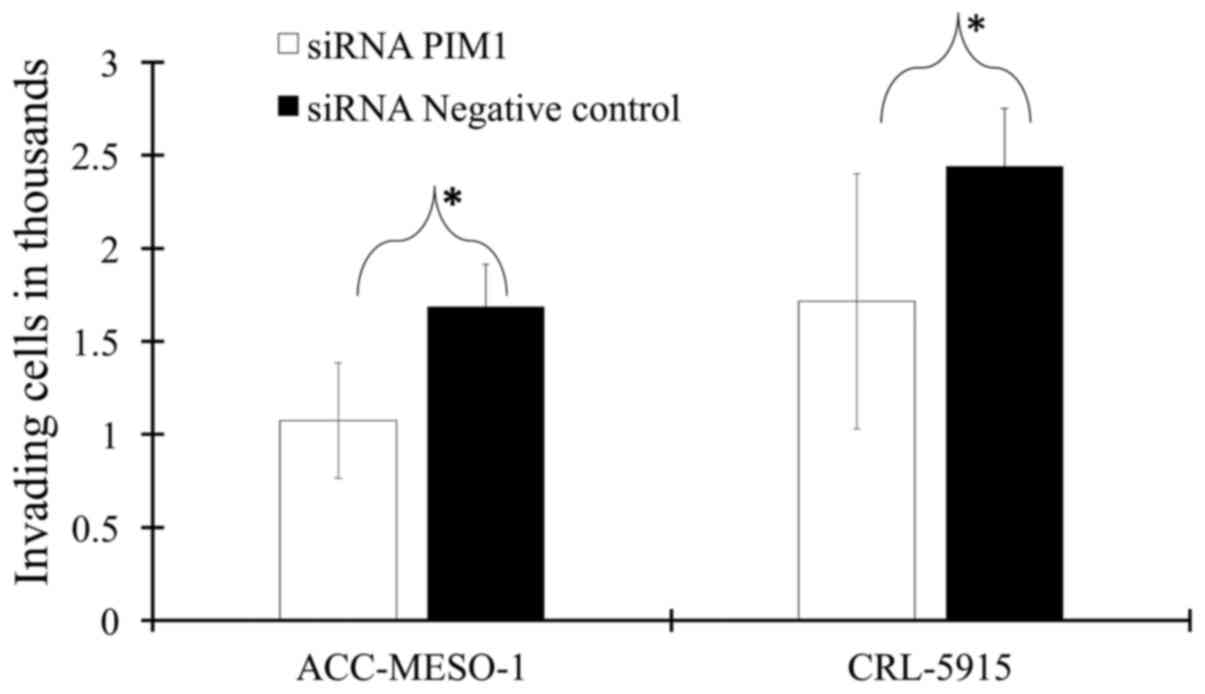
PIM1 knockdown reduces cell invasion and
cell migration
We found that the ability of ACC-MESO-1 for invasion
is faster than CRL-5915 as ACC-MESO-1 invade via the Matrigel layer
in 2 days while CRL-5915 need 3–4 days for its invasion, thus, we
used reduced number of cells and increased thickness of the
Matrigel layer in case of ACC-MESO-1.
Mesothelioma cells with PIM1 transfection showed
~30% less invasiveness compared to that with negative control-siRNA
transfection (Fig. 4 and Table III). Similarly, the mesothelioma
cells with PIM1 transfection showed less migration than that with
negative control-siRNA (Fig.
5).
 | Table IIIInvasion assay. |
Table III
Invasion assay.
| No. of invading
cells/one quadrant of insert
|
|---|
| Mean | SD | P-value |
|---|
| CRL-5915 | | | |
| PIM1 siRNA | 1716 | 313 | 0.0168 |
| Negative control
siRNA | 2439 | 686 | |
| ACC-MESO-1 | | | |
| PIM1 siRNA | 1074 | 230 | 0.0005 |
| Negative control
siRNA | 1684 | 309 | |
Discussion
The conventional management of mesothelioma by
either surgery or radiotherapy and chemotherapy are not very
effective, and even the recent clinical trials of trimodal therapy
with neoadjuvant chemotherapy followed by extra-pleural
pneumonectomy and radiotherapy have not shown significant advantage
over conventional therapy (12–14).
In population- based studies, survival times ranged from 5 months
to 13.2 months (15). Although
surgery is an option for patients with early-stage mesothelioma,
most patients present with advanced locally invasive disease, not
amenable to surgical resection. For these patients, the current
best regime is palliative chemotherapy with cisplatin and
pemetrexed. However, this regimen has been shown a modest survival
benefit (16). Therefore, the
novel strategies for management of mesothelioma patients have to be
developed urgently in the coming years. There are various trials
ongoing for the treatment of malignant mesothelioma. A recent study
has previously reported the high expression and prognostic value of
CD26 in malignant mesothelioma (17,18),
humanized anti-CD26 monoclonal antibody, YS-110, having inhibitory
effect on malignant mesothelioma cell growth in vitro and
in vivo experiments (19),
and one of the promising new developments for the antibody-based
treatment of mesothelioma (19,20).
Other trials such as pembrolizumab against programmed death 1
(PD-1) antibody, PF-03446962 against activin receptor-like kinase 1
(ALK-1), defactinib against focal adhesion kinase (FAK) are ongoing
(21–23).
RNA interference (RNAi) has broad potential as a
therapeutic role to reversibly silence a gene. Recently delivery of
siRNA to the targeted tissue has been improved to achieve the
clinical potential of RNAi (24,25).
We have previously reported the high expression of nuclear PIM1 in
mesothelioma by immunohistochemical staining and mesothelioma cell
lines by western blot analysis (3). Upregulation of PIM1 has been reported
in prostate (4), pancreatic
(5) and non-small cell lung
cancers (6) with good prognostic
significance. However, its high expression in stomach (6), bladder (26), esophagus (26) and head and neck carcinoma (27) was correlated with poor prognosis.
Therefore, PIM1 may have different functions in tumorigenesis
depending on the tumor type and underlying signaling pathways. We
investigated the functional role of PIM1 in mesothelioma cell lines
by siRNA-based knockdown and confirmed its potential role on cell
survival, migration and invasion. PIM1 overexpression has been
shown to play a major role in the inhibition of apoptosis and the
regulation of cell proliferation (28) as well as in promotion of cell
survival through phosphorylation of other substrate proteins as a
proapoptotic factor FOXO3a (8).
The level of PIM1 has been shown to increase during the progression
of the cell cycle from G1 and remain high throughout G1/S and G2
phases, thus, PIM1 protein level changes during cell cycle stages
appear to be related to the onset of DNA synthesis and commitment
to proliferation (29) and high
level of PIM1 activate cell cycle inhibitors as p21 and p27 thereby
promote cell cycle phases (30).
We found that the downregulation of PIM1 caused decrease in
proliferation rates of mesothelioma cells and cell cycle analysis
have shown the accumulation of mesothelioma cells in G1 phase. We
also analyzed the potential role of PIM1 to influence the invasion
of mesothelioma cell lines by Transwell invasion assay and wound
assay and found significant decrease in invasion through Matrigel
and decrease in migration of mesothelioma cells. Based on our
preliminary results, PIM1 expression in human mesothelioma tissue
may be useful in differential diagnosis of malignant mesothelioma
from the reactive mesothelial hyperplasia (data not shown).
Increasing evidence has shown that PIM1 would be a
novel and essential drug target in numerous types of cancer
(31), in particular prostate
cancer (4). Novel molecules
inhibiting PIM kinases have been evaluated in preclinical studies,
demonstrating to be effective and with a favorable toxicity
profile. Given the promising results, some of these compounds are
currently under investigation in clinical trials (32). We analyzed in this study the
possibility for application of therapeutic potential in
mesothelioma.
In conclusion, the downregulation of PIM1 in
mesothelioma cell have shown to reduce the proliferation, invasion
and migration of mesothelioma cells. Considering that mesothelioma
is a rapidly growing invasive cancer, downregulation of PIM1 has
potential for therapeutic management of mesothelioma. However,
further mechanism of regulation of its downstream genes is needed
to understand how its regulation modulates the mesothelioma
cells.
Acknowledgments
The authors thank Ms. Naomi Fukuhara for her
administrative assistance. Part of the present study was carried
out at the Analysis Center of Life Science, Hiroshima
University.
References
|
1
|
Delgermaa V, Takahashi K, Park EK, Le GV,
Hara T and Sorahan T: Global mesothelioma deaths reported to the
World Health Organization between 1994 and 2008. Bull World Health
Organ. 89:716–724. 724A–724C. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McDonald JC and McDonald AD: The
epidemiology of mesothelioma in historical context. Eur Respir J.
9:1932–1942. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amatya VJ, Mawas AS, Kushitani K, Mohi
El-Din MM and Takeshima Y: Differential microRNA expression
profiling of mesothelioma and expression analysis of miR-1 and
miR-214 in mesothelioma. Int J Oncol. 48:1599–1607. 2016.PubMed/NCBI
|
|
4
|
Dhanasekaran SM, Barrette TR, Ghosh D,
Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA and Chinnaiyan
AM: Delineation of prognostic biomarkers in prostate cancer.
Nature. 412:822–826. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reiser-Erkan C, Erkan M, Pan Z, Bekasi S,
Giese NA, Streit S, Michalski CW, Friess H and Kleeff J:
Hypoxia-inducible protooncogene Pim-1 is a prognostic marker in
pancreatic ductal adenocarcinoma. Cancer Biol Ther. 7:1352–1359.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warnecke-Eberz U, Bollschweiler E, Drebber
U, Metzger R, Baldus SE, Hölscher AH and Mönig S: Prognostic impact
of protein overexpression of the proto-oncogene PIM-1 in gastric
cancer. Anticancer Res. 29:4451–4455. 2009.PubMed/NCBI
|
|
7
|
Herzog S, Fink MA, Weitmann K, Friedel C,
Hadlich S, Langner S, Kindermann K, Holm T, Böhm A, Eskilsson E, et
al: Pim1 kinase is upregulated in glioblastoma multiforme and
mediates tumor cell survival. Neuro Oncol. 17:223–242. 2015.
View Article : Google Scholar :
|
|
8
|
Magnuson NS, Wang Z, Ding G and Reeves R:
Why target PIM1 for cancer diagnosis and treatment? Future Oncol.
6:1461–1478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Usami N, Fukui T, Kondo M, Taniguchi T,
Yokoyama T, Mori S, Yokoi K, Horio Y, Shimokata K, Sekido Y, et al:
Establishment and characterization of four malignant pleural
mesothelioma cell lines from Japanese patients. Cancer Sci.
97:387–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lamprecht MR, Sabatini DM and Carpenter
AE: CellProfiler: Free, versatile software for automated biological
image analysis. Biotechniques. 42:71–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gebäck T, Schulz MM, Koumoutsakos P and
Detmar M: TScratch: A novel and simple software tool for automated
analysis of monolayer wound healing assays. Biotechniques.
46:265–274. 2009.PubMed/NCBI
|
|
12
|
Hasegawa S, Okada M, Tanaka F, Yamanaka T,
Soejima T, Kamikonya N, Tsujimura T, Fukuoka K, Yokoi K and Nakano
T: Trimodality strategy for treating malignant pleural
mesothelioma: Results of a feasibility study of induction
pemetrexed plus cisplatin followed by extrapleural pneumonectomy
and post- operative hemithoracic radiation (Japan Mesothelioma
Interest Group 0601 Trial). Int J Clin Oncol. 21:523–530. 2016.
View Article : Google Scholar
|
|
13
|
Kapeles M, Gensheimer MF, Mart DA, Sottero
TL, Kusano AS, Truong A, Farjah F, Laramore GE, Stelzer KJ and
Patel SA: Trimodality treatment of malignant pleural mesothelioma:
An Institutional Review. Am J Clin Oncol. Aug 27–2015.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krug LM, Pass HI, Rusch VW, Kindler HL,
Sugarbaker DJ, Rosenzweig KE, Flores R, Friedberg JS, Pisters K,
Monberg M, et al: Multicenter phase II trial of neoadjuvant
pemetrexed plus cisplatin followed by extrapleural pneumonectomy
and radiation for malignant pleural mesothelioma. J Clin Oncol.
27:3007–3013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montanaro F, Rosato R, Gangemi M, Roberti
S, Ricceri F, Merler E, Gennaro V, Romanelli A, Chellini E,
Pascucci C, et al: Survival of pleural malignant mesothelioma in
Italy: A population-based study. Int J Cancer. 124:201–207. 2009.
View Article : Google Scholar
|
|
16
|
Marinaccio A, Montanaro F, Mastrantonio M,
Uccelli R, Altavista P, Nesti M, Costantini AS and Gorini G:
Predictions of mortality from pleural mesothelioma in Italy: A
model based on asbestos consumption figures supports results from
age-period- cohort models. Int J Cancer. 115:142–147. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amatya VJ, Takeshima Y, Kushitani K,
Yamada T, Morimoto C and Inai K: Overexpression of CD26/DPPIV in
mesothelioma tissue and mesothelioma cell lines. Oncol Rep.
26:1369–1375. 2011.PubMed/NCBI
|
|
18
|
Aoe K, Amatya VJ, Fujimoto N, Ohnuma K,
Hosono O, Hiraki A, Fujii M, Yamada T, Dang NH, Takeshima Y, et al:
CD26 overexpression is associated with prolonged survival and
enhanced chemosensitivity in malignant pleural mesothelioma. Clin
Cancer Res. 18:1447–1456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inamoto T, Yamada T, Ohnuma K, Kina S,
Takahashi N, Yamochi T, Inamoto S, Katsuoka Y, Hosono O, Tanaka H,
et al: Humanized anti-CD26 monoclonal antibody as a treatment for
malignant mesothelioma tumors. Clin Cancer Res. 13:4191–4200. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thompson MA, Ohnuma K, Abe M, Morimoto C
and Dang NH: CD26/dipeptidyl peptidase IV as a novel therapeutic
target for cancer and immune disorders. Mini Rev Med Chem.
7:253–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bickel A, Koneth I, Enzler-Tschudy A,
Neuweiler J, Flatz L and Früh M: Pembrolizumab-associated minimal
change disease in a patient with malignant pleural mesothelioma.
BMC Cancer. 16:6562016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimizu T, Fukuoka K, Takeda M, Iwasa T,
Yoshida T, Horobin J, Keegan M, Vaickus L, Chavan A, Padval M, et
al: A first-in-Asian phase 1 study to evaluate safety,
pharmacokinetics and clinical activity of VS-6063, a focal adhesion
kinase (FAK) inhibitor in Japanese patients with advanced solid
tumors. Cancer Chemother Pharmacol. 77:997–1003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wheatley-Price P, Chu Q, Bonomi M, Seely
J, Gupta A, Goss G, Hilton J, Feld R, Lee CW, Goffin JR, et al: A
Phase II study of PF-03446962 in patients with advanced malignant
pleural mesothelioma. CCTG Trial IND207. J Thorac Oncol.
11:2018–2021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao J and Feng SS: Nanocarriers for
delivery of siRNA and co-delivery of siRNA and other therapeutic
agents. Nanomedicine (Lond). 10:2199–2228. 2015. View Article : Google Scholar
|
|
25
|
Kanasty R, Dorkin JR, Vegas A and Anderson
D: Delivery materials for siRNA therapeutics. Nat Mater.
12:967–977. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo S, Mao X, Chen J, Huang B, Jin C, Xu Z
and Qiu S: Overexpression of Pim-1 in bladder cancer. J Exp Clin
Cancer Res. 29:1612010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peltola K, Hollmen M, Maula SM, Rainio E,
Ristamäki R, Luukkaa M, Sandholm J, Sundvall M, Elenius K, Koskinen
PJ, et al: Pim-1 kinase expression predicts radiation response in
squamocellular carcinoma of head and neck and is under the control
of epidermal growth factor receptor. Neoplasia. 11:629–636. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amaravadi R and Thompson CB: The survival
kinases Akt and Pim as potential pharmacological targets. J Clin
Invest. 115:2618–2624. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang H, Hittelman W and Nagarajan L:
Ubiquitous expression and cell cycle regulation of the protein
kinase PIM-1. Arch Biochem Biophys. 330:259–265. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morishita D, Katayama R, Sekimizu K,
Tsuruo T and Fujita N: Pim kinases promote cell cycle progression
by phosphorylating and down-regulating p27Kip1 at the
transcriptional and posttranscriptional levels. Cancer Res.
68:5076–5085. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Block KM, Hanke NT, Maine EA and Baker AF:
IL-6 stimulates STAT3 and Pim-1 kinase in pancreatic cancer cell
lines. Pancreas. 41:773–781. 2012.PubMed/NCBI
|
|
32
|
Mondello P, Cuzzocrea S and Mian M: Pim
kinases in hematological malignancies: Where are we now and where
are we going? J Hematol Oncol. 7:952014. View Article : Google Scholar : PubMed/NCBI
|