Introduction
Cancer metastasis is the major cause of death in
most cancer patients. It is a complex cascade process, in which
cancer cells detach from the primary tumor, migrate, adhere and
invade through the basement membrane or extracellular matrix (ECM),
survive in the circulatory system, invade into distant secondary
organs or tissues, and start to proliferate (1). During the metastatic process,
proteolytic enzymes play critical roles in helping cancer cells to
enter into the vascular and lymphatic systems and invading tissues
at ectopic sites. Among all proteolytic enzymes, the members of
matrix metallopro-teinases (MMPs) are the main group of proteolytic
enzyme that is involved in the tumor invasion, metastasis and
angiogenesis. MMPs or matrixins are a group of zinc-dependent
endopeptidase enzymes, that respond to ECM degradation and tumor
cell invasion, metastasis and angiogenesis (2). MMP-2 (gelatinase A, 72 kDa) and MMP-9
(gelatinase B, 92 kDa) are the key enzymes in degradation of the
ECM components of basal membrane and type IV collagen, a major
component of the basement membrane. Activities of MMPs are
controlled by their endogenous inhibitors, tissue inhibitors of
metalloproteinases (TIMPs) such as TIMP-1 and TIMP-2, in cancer
cells (3,4). It was reported that when the balance
of MMPs and TIMPs was disrupted, direct inhibition of MMPs and
increase of TIMPs in cancer may be a particular attractive target
for therapeutic intervention in tumor invasion and metastasis
(5). Therefore, inhibition of MMP
activity and expression is important for inhibiting cancer
metastasis, which affects mortality in patients.
Mitogen-activated protein kinases (MAPKs) include
extracellular signal-regulated kinase 1 and 2 (ERK1/2), c-Jun
N-terminal kinase/stress-activated protein kinase (JNK/SAPK), and
p38 MAPK, play an important regulatory role in cell growth,
differentiation, apoptosis, and metastasis (6). AKT (also known as PKB) is also
involved in multiple cellular processes such as cell growth, cell
proliferation, angiogenesis and metastasis in various cancers
(7). They have a central role in
regulating the expression of MMPs (8–11).
In addition, the expression of MMPs is also regulated by
nuclear-factor-κB (NF-κB) and the activator protein 1 (AP-1) as the
MMP gene has an NF-κB and AP-1 binding site in its promoter region
(12,13). Inhibition of the MAPK and PI3K/Akt
pathways as well as NF-κB and AP-1 activities may lead to potential
prevention of cancer cell proliferation, invasion, and
metastasis.
Traditionally, Rhodomyrtus tomentosa (Aiton)
Hassk., the family myrtaceae, has been used for anti-inflamation,
to treat diarrhea, gastrointestinal, urinary tract infections and
antiseptic wash for wounds (14,15).
It is native to Southeast Asia and is a troublesome invader of
native plant communities in Florida (16). Rhodomyrtone is a pure compound,
isolated from Rhodomyrtus tomentosa leaves. Previous studies
have shown that rhodomyrtone displays antibacterial activity
against a wide range of gram-positive bacteria such as Bacillus
subtilis, Enterococcus faecalis, Staphylococcus
aureus, Staphylococcus epidermidis, Streptococcus
spp., and methicillin-resistant Staphylococcus aureus (MRSA)
(17–21). Moreover, rhodomyrtone stimulated
pro- and anti-inflammatory cytokine responses (22) and reduced hyperproliferation and
abnormal differentiation of HaCaT cells (23). However, the anti-metastatic
activity of rhodomyrtone on cancer cells has not yet been
reported.
Non-melanoma skin cancer (NMSC) is the most common
cancer affecting white-skinned individuals and the incidence is
increasing worldwide. There are two main types of NMSC including
the basal cell carcinomas (BCCs) and squamous cell carcinomas
(SCCs) (24,25). SCC is the second most common skin
cancer, accounting for ~20% of NMSC cases. It is more common in
older people. The main risk factor for skin cancer is exposure to
UV radiation, which causes cellular damage (26,27).
Current treatments of SCCs consist of surgery, photodynamic
therapy, radiation therapy, chemotherapy or combination therapy,
but these treatments are unsatisfactory. Thus, it is necessary to
search for a new effective therapeutic agent to treat SCCs.
In this study, we investigated the inhibitory effect
of rhomyrtone on cancer metastasis in A431 cells. It was
demonstrated that rhodomyrtone effectively inhibits cell migration,
invasion and adhesion in human epidermoid carcinoma A431 cells.
Materials and methods
Chemical and antibodies
Rhodomyrtone was obtained from Dr Wilawan
Mahabusarakum, Department of Chemistry, Faculty of Science, Prince
of Songkla University, Songkhla, Thailand. It was dissolved in
dimethylsulfoxide (DMSO). MTT (3-(4,5-dimethyl-2,5-diphenyl
tetrazolium bromide), DMSO and trypan blue were purchased from
Sigma-Aldrich Corp. (St. Louis, MO, USA). Dulbecco's modified
Eagle's medium (DMEM) was purchased from Gibco/BRL (Gaithersburg,
MD, USA). Matrigel was purchased from BD Biosciences (Bedford, MA,
USA). Immobilon Western Chemiluminescent HRP substrate was
purchased from Merck Millipore Corp. (Merck KGA, Darmstadt,
Germany). The protein assay kit and Coomassie Brilliant Blue R-250
were obtained from Bio-Rad Labs (Hercules, CA, USA). Antibodies
(Abs) for immunoblotting analysis including rabbit monoclonal Abs
against matrix metalloproteinase-2 (MMP-2), MMP-9, tissue inhibitor
of metalloproteinase-1 (TIMP-1), TIMP-2, RAS, growth factor
receptor-bound protein-2 (GRB2), focal adhesion kinase (FAK), p-FAK
(Try397), pPDK1 (Ser241), p-cRaf, extracellular signal regulation
kinase 1/2 (ERK1/2), p-ERK1/2, p38, p-p38, c-Jun N-terminal kinase
1/2 (JNK1/2), pJNK1/2, AKT, pAKT (Ser473) pAKT (Thr308), c-Fos,
c-Jun, p-cJun, nuclear factor κB (NF-κB), p-NF-κB and anti-mouse
immunoglobulin G and anti-rabbit immunoglobulin G horseradish
peroxidase-conjugated secondary antibodies were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA), and mouse monoclonal
Abs against β-actin was obtained from Merck Millipore Corp. (Merck
KGaA).
Cell line and cell culture
The human epidermoid carcinoma cell line (A431) was
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). A431 cells were maintained as a monolayer in DMEM (Gibco
Life Technologies, Carlbad, CA, USA) supplemented with 10% FBS (GE
Healthcare Life Science, Little Chalfont, UK), 100 U/ml penicillin
and 100 µg/ml streptomycin (GE Healthcare Life Science,
Inc.) at 37°C in a humidified 5% CO2.
Cell viability analysis
A431 cells (7×103 cell/well) were seeded
in a 96-well plate for 24 h. Then cells were treated with
rhodomyrtone at various concentrations (0, 0.5, 1.5, 3, 5, 10 and
15 µg/ml) for 24 h. After treatment 0.5 mg/ml of MTT
solution was added to each well and incubated for 2 h at 37°C. The
supernatant was removed and DMSO was added to each well to
solubilize water insoluble purple formazan crystals. The absorbance
was measured using a Epoch™ Microplate Spectrophotometer at 570 nm
and survival percentage (%) was calculated relative to the
control.
In vitro migration and invasion
assay
Cells were pretreated with 0, 0.5, 1.5 and 3
µg/ml rhodomyrtone for 24 h. The cells were harvested and
seeded to the upper chamber of the Transwell insert [polyethylene
terephthalate (PET) filters, Merck Millipore Corp.] at
104 cell/well in serum-free medium. The lower chambers
were filled with FBS medium as chemoattractant, and thereafter
these Transwell inserts were incubated for 24 h at 37°C in 5% (v/v)
CO2. For invasion assay, the Transwell insert was coated
with 30 µg Matrigel (BD Biosciences, MA, USA) and lower
chambers were filled with FBS medium. After incubation, the cells
were removed with a cotton swab and those on the lower surface of
the membrane were fixed with methanol and stained with 0.5% crystal
violet. Cells that migrate through the membrane were viewed and
photographed under an inverted microscope (Olympus). The percentage
of the migratory cells for each treatment was calculated by NIH
ImageJ software, version 1.46r.
Cell-matrix adhesion assay
Cells at 2×105 cells/well were pretreated
with various concentrations of rhodomyrtone (0, 0.5, 1.5 and 3
µg/ml) for 24 h, then 1×104 cells/well were
seeded into the Matrigel-coated 96-well plate for 1 h. The
non-adherent cells were then removed with PBS and the adherent
cells were reacted with 0.5 mg/ml of MTT solution at 37°C for 2 h.
After that 100 µl of DMSO was added to each well to
solubilize water insoluble purple formazan crystals. The absorbance
was measured at 570 nm using a microplate reader. The percentage
(%) of cell adhesion was calculated relative to the control.
Gelatin zymography
Cells were treated with various concentrations of
rhodomyrtone (0, 0.5, 1.5 and 3 µg/ml) for 24 h, the
conditioned media were collected and mixed with non-reducing sample
buffer. Samples were separated by SDS-PAGE containing 0.1% gelatin.
After electrophoresis, gels were washed with 2.5% Triton X-100 for
30 min, 3 times and incubated in zymogram incubation buffer (50 mM
Tris-HCl, pH 7.6, 10 mM CaCl2, 50 mM NaCl, 0.05% Brij35)
for 48 h at 37°C. Then the gels were rinsed with distilled water
and stained with Coomassie Brilliant Blue R-250. The bands of
gelatinolytic activity were quantified using NIH ImageJ software,
version 1.46r.
Western blot analysis
Cells were lysed in RIPA buffer (50 mM Tris-HCl, pH
7.5, 5 mM EDTA, 250 mM NaCl, 0.5% Triton X-100), after treatment
with 0, 0.5, 1.5 and 3 µg/ml of rhodomyrtone. Total proteins
were separated by SDS-PAGE and transferred onto a PVDF membrane
(Millipore Corp., Billerica, MA, USA). After protein transferring,
the membranes were subsequently blocked with 5% non-fat milk for 1
h at room temperature to block non-specific binding. Then membranes
were incubated with specific primary antibody against MMP-2, MMP-9,
TIMP-1, TIMP-2, RAS, GRB2, FAK, p-FAK (Try397), pPDK1 (Ser241),
p-cRaf, ERK1/2, p-ERK1/2, p38, p-p38, JNK1/2, pJNK1/2, AKT, pAKT
(Ser473) pAKT (Thr308), c-Fos, c-Jun, p-cJun, NF-κB, p-NF-κB and
β-actin at 4°C, overnight. Subsequently, membranes were incubated
with anti-mouse or anti-rabbit antibody conjugated with horseradish
peroxidase (Cell Signaling Technology) for 1 h at room temperature.
The protein bands were detected by chemiluminescence using enhanced
chemiluminescence reagent (ECL) (Millipore) and exposed to CCD
camera (Biotek Instruments, Winooski, VT, USA). The quantitative
results for the protein of interest were expressed as relative to
an internal housekeeping control such as β-actin.
Statistical analysis
To compare the data from different treatments,
one-way ANOVA was used. All data presented were obtained from at
least three independent experiments and were presented as mean ±
standard deviation (SD). A p-value of 0.05 was taken as minimum
basis for assigning significance. The statistical analyses were
performed using SPSS 17.0 software.
Results
Effect of rhodomyrtone on A431 cell
proliferation
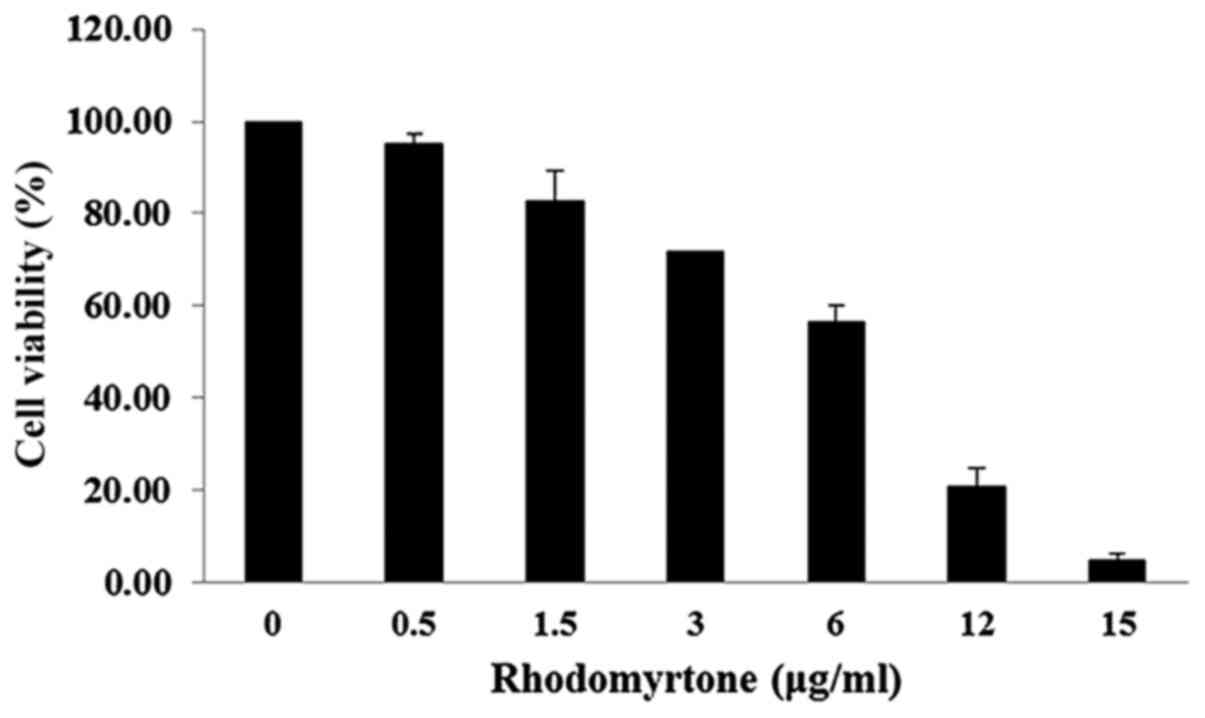
The effect of rhodomyrtone on A431 cell viability
was analyzed by MTT assay. The viability of A431 cells treated with
rhodomyrtone under different concentrations (0, 0.5, 1.5, 3, 5, 10
and 15 µg/ml) for 24 h as shown in Fig. 1. The result demonstrated that
rhodomyrtone inhibited A431 cell viability in a time- and
dose-dependent manner. At the high concentrations, rhodomyrtone
significantly inhibited cell proliferation of A431 cells while at
lower concentrations no significant effect was observed. The
non-cytotoxic concentration and subcytotoxic concentration (<1.5
µg/ml showing >80% cell proliferation) was selected for
the subsequent experiment.
Effect of rhodomyrtone on A431 cell
migration and invasion
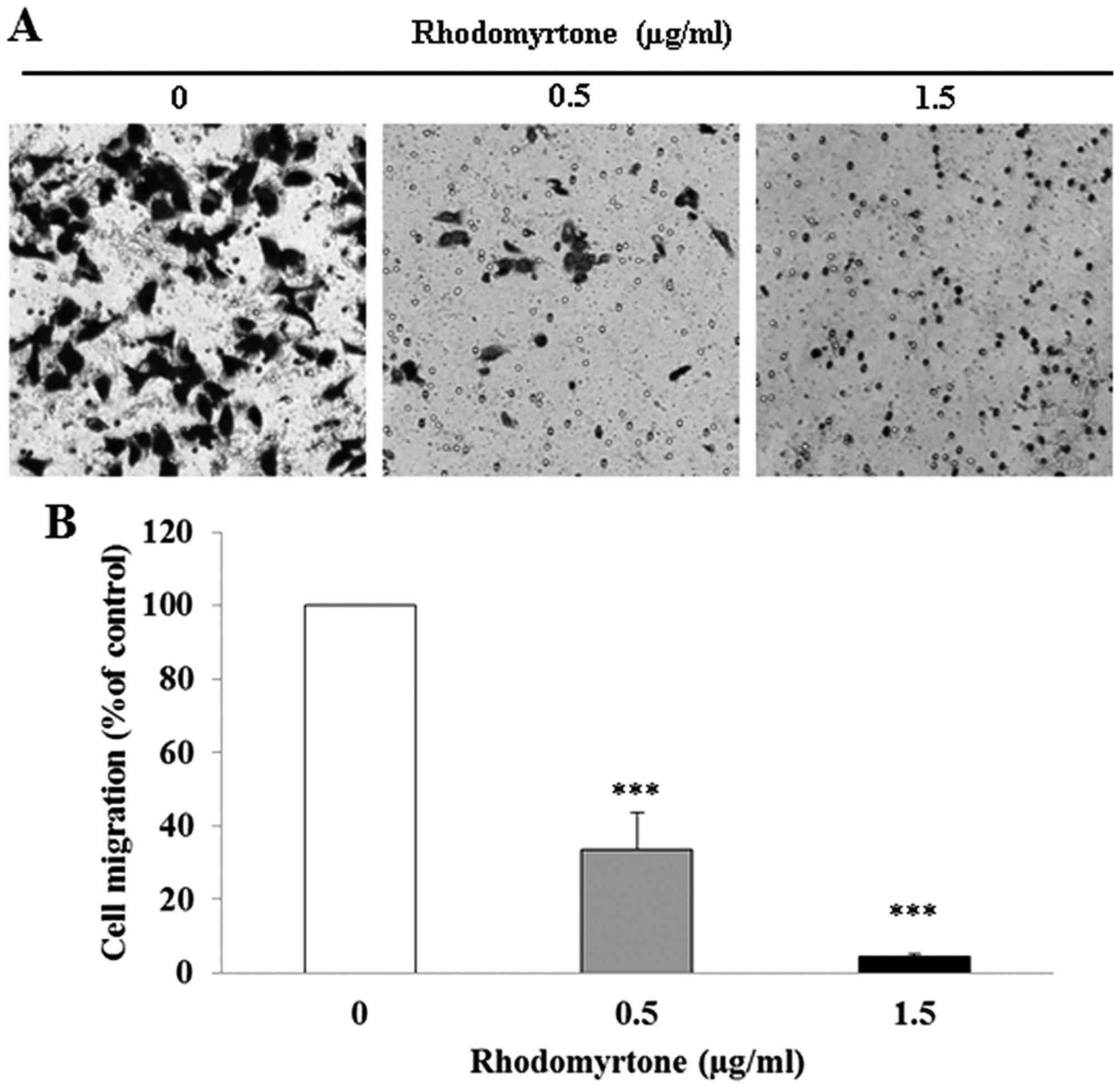
The effect of rhodomyrtone on A431 cell migration
and invasion was determined by Transwell chamber assay. After A431
cells wre treated with rhodomyrtone at 0.5 and 1.5 µg/ml for
24 h, rhodomyrtone significantly reduced cell migration in a
dose-dependent manner (Fig. 2;
p<0.001). The percentage of cell migration was 33.6±9.9 and
4.4±0.8% after treatment with 0.5 and 1.5 µg/ml
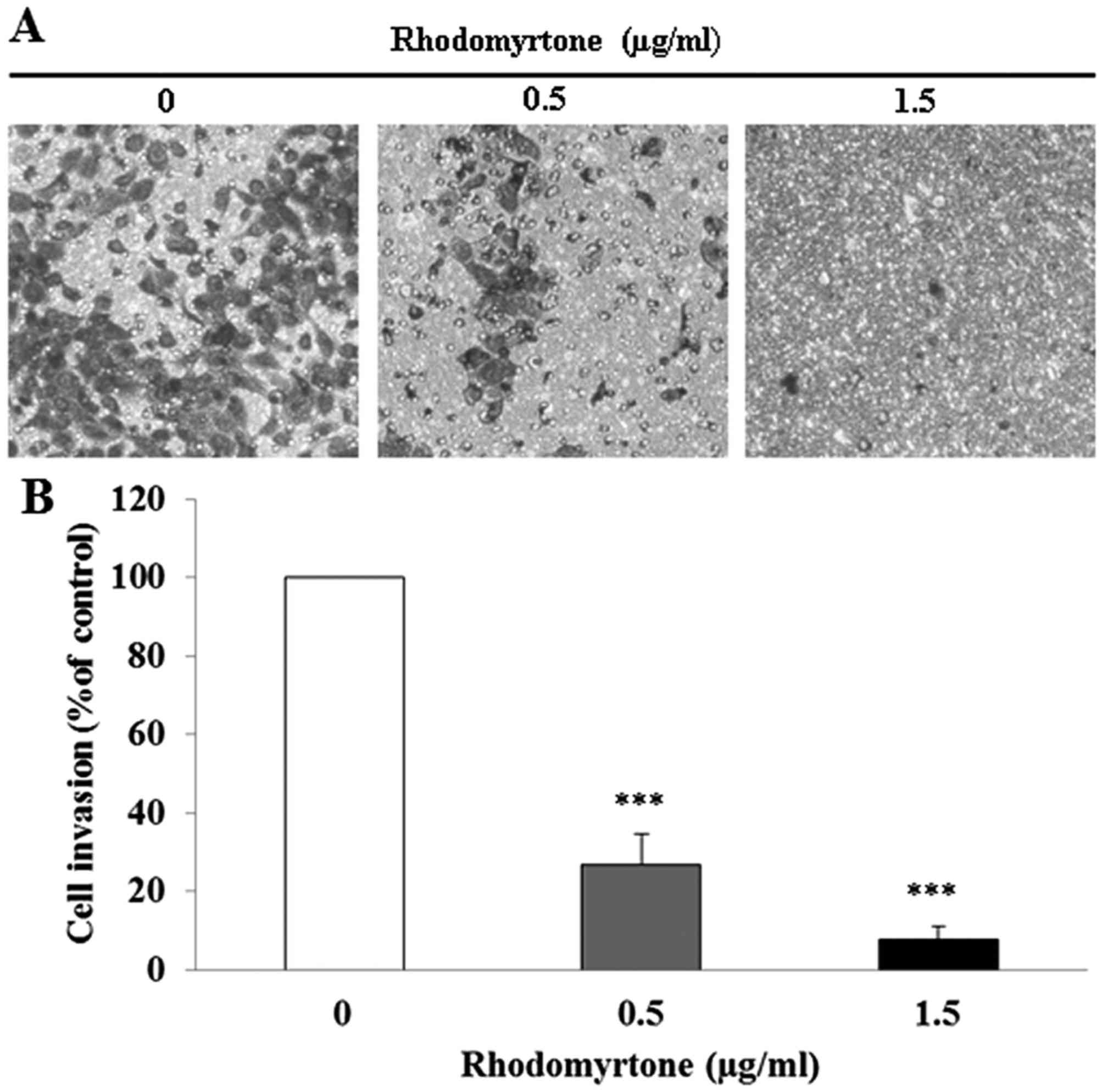
rhodomyrtone, respectively. Moreover, we found that rhodomyrtone
significantly inhibited the invasion of A431 cell through
Matrigel-coated filter in a dose-dependent manner (p<0.001).
Exposure of A431 cells to 0.5 and 1.5 µg/ml rhodomyrtone
inhibited 73.2 and 92.3% of cell invasion, respectively (Fig. 3). These results revealed that
rhodomyrtone markedly inhibits migration and invasion of A431
cells.
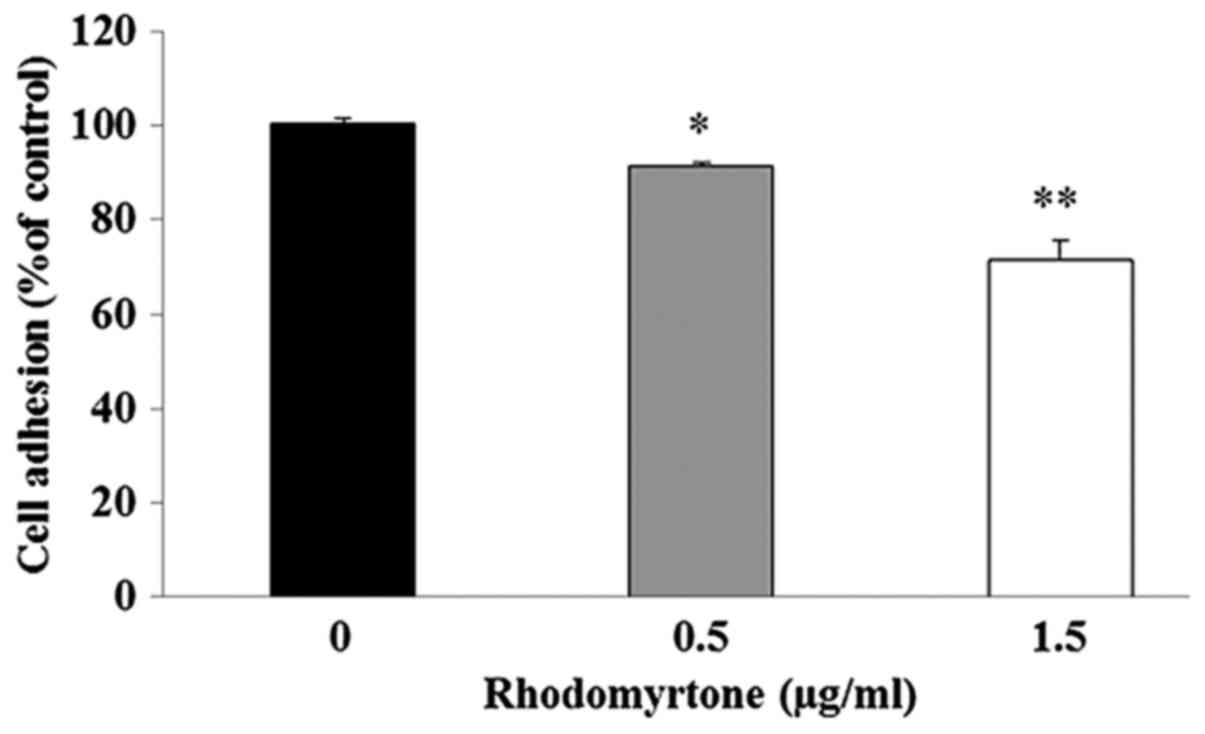
Effect of rhodomyrtone on adhesion
ability of A431 cells
We examined the effect of rhodomyrtone on adhesion
ability of A431 cells to Matrigel. The result demonstrated that the
adhesive capacities of A431 cells to Matrigel were significantly
decreased after treatment with 0.5 and 1.5 µg/ml
rhodomyrtone when compared to the untreated control group as shown
in Fig. 4.
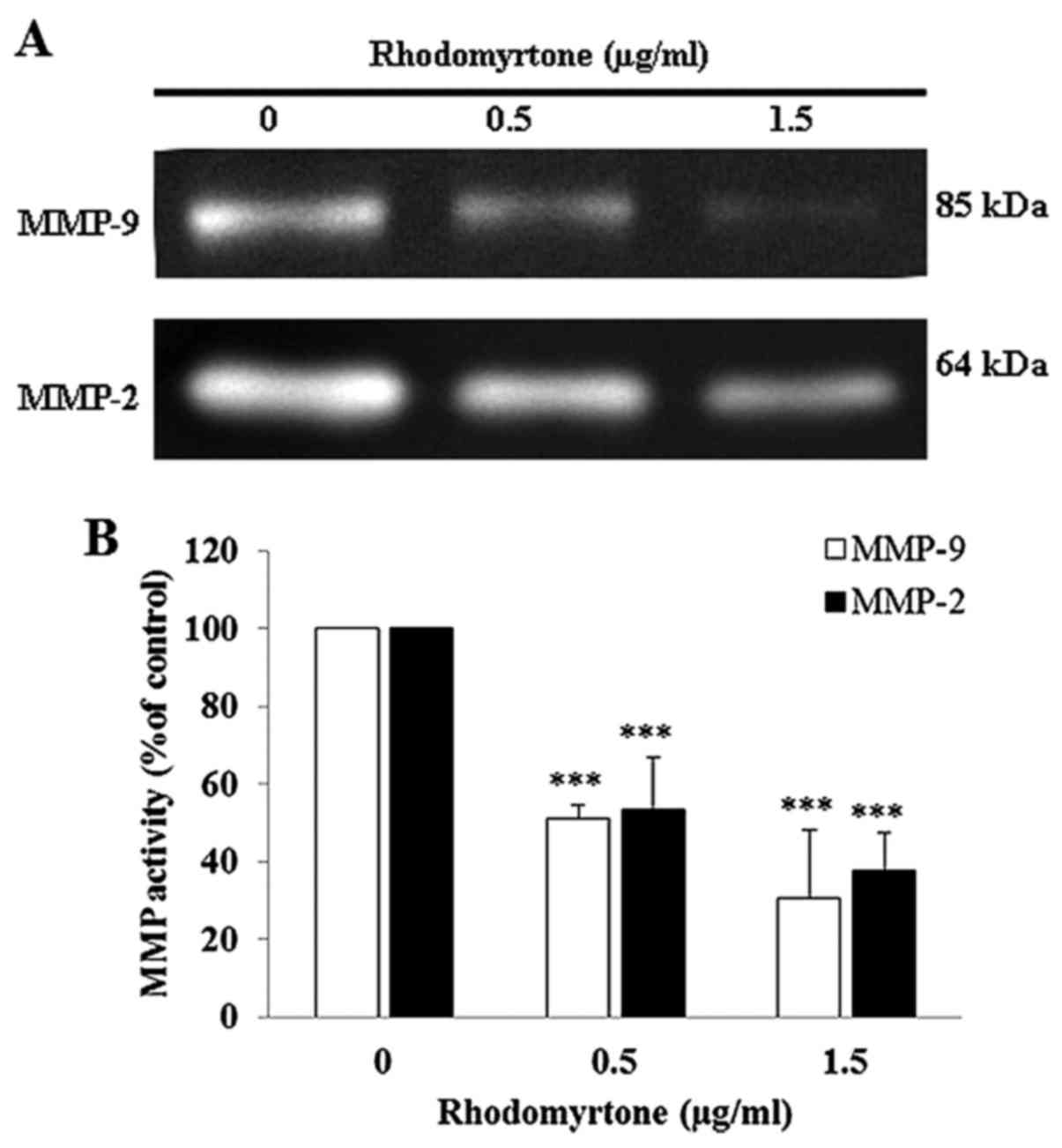
Effect of rhodomyrtone on MMP-9 and MMP-2
activities
To determine the possible mechanism of rhodomyrtone
to inhibit cell migration and invasion, we investigated the
activity of MMP-2 and MMP-9 in culture media of A431 cells using
gelatin zymography. After treatment with 0.5 and 1.5 µg/ml
of rhodomyrtone for 24 h, the conditioned medium was collected and
MMP activity was estimated from densitometric analysis. The result
showed rhodomyrtone reduced MMP-2 and MMP-9 activities in a
dose-dependent manner (Fig. 5).
MMP-2 activity was reduced by 53.5 and 37.8% and MMP-9 activity was
reduced by 51.1 and 30.3% upon treatment with 0.5 and 1.5
µg/ml of rhodomyrtone, respectively. The results indicated
that rhodomyrtone inhibited MM-2 and MMP-9 activities in A431
cells.
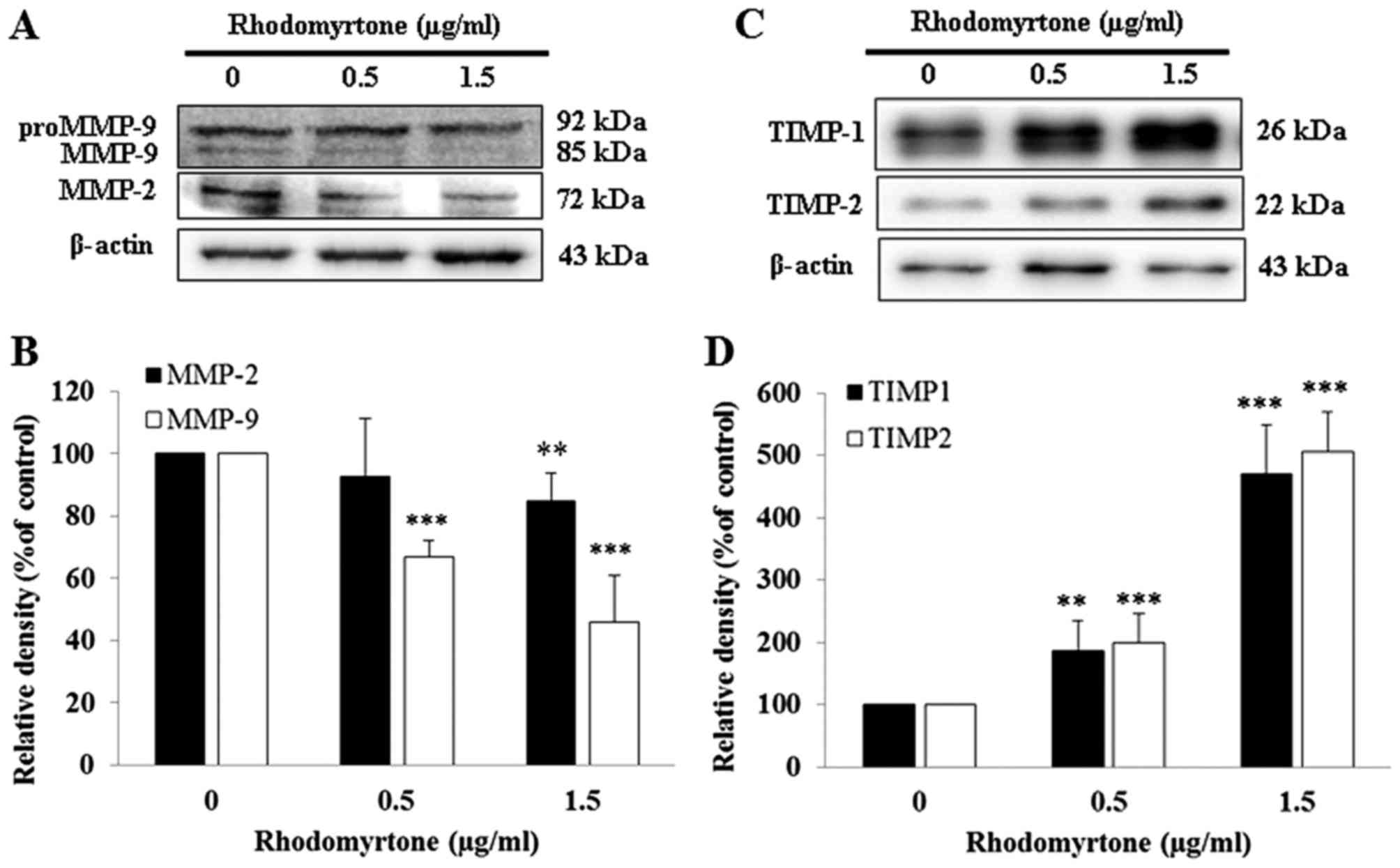
Effects of rhodomyrtone on MMP-2, MMP-9,
TIMP-1 and TIMP-2 expression in A431 cells
MMP-2 and MMP-9 are involved in the degradation of
ECM and are essential to the cell migration and invasion during
metastasis. The effects of rhodomyrtone on MMP-2 and MMP-9
expression were detected by western blot analysis. As shown in
Fig. 6A and B, rhodomyrtone
suppressed the expression of MMP-2 and MMP-9 in a dose-dependent
manner. Inhibition of MMP-2 was ~15.3% upon treatment with 1.5
µg/ml rhodomyrtone and MMP-9 was ~33.1 and 54.2% with 0.5
and 1.5 µg/ml rhodomyrtone, respectively. Furthermore, we
demonstrated that rhodomyrtone markedly increased TIMP-1 and TIMP-2
protein expression upon treatment with 0.5 and 1.5 µg/ml
rhodomyrtone for 24 h (Fig. 6C and
D). TIMP-1 and TIMP-2 are known to be the specific endogenous
inhibitors of MMPs.
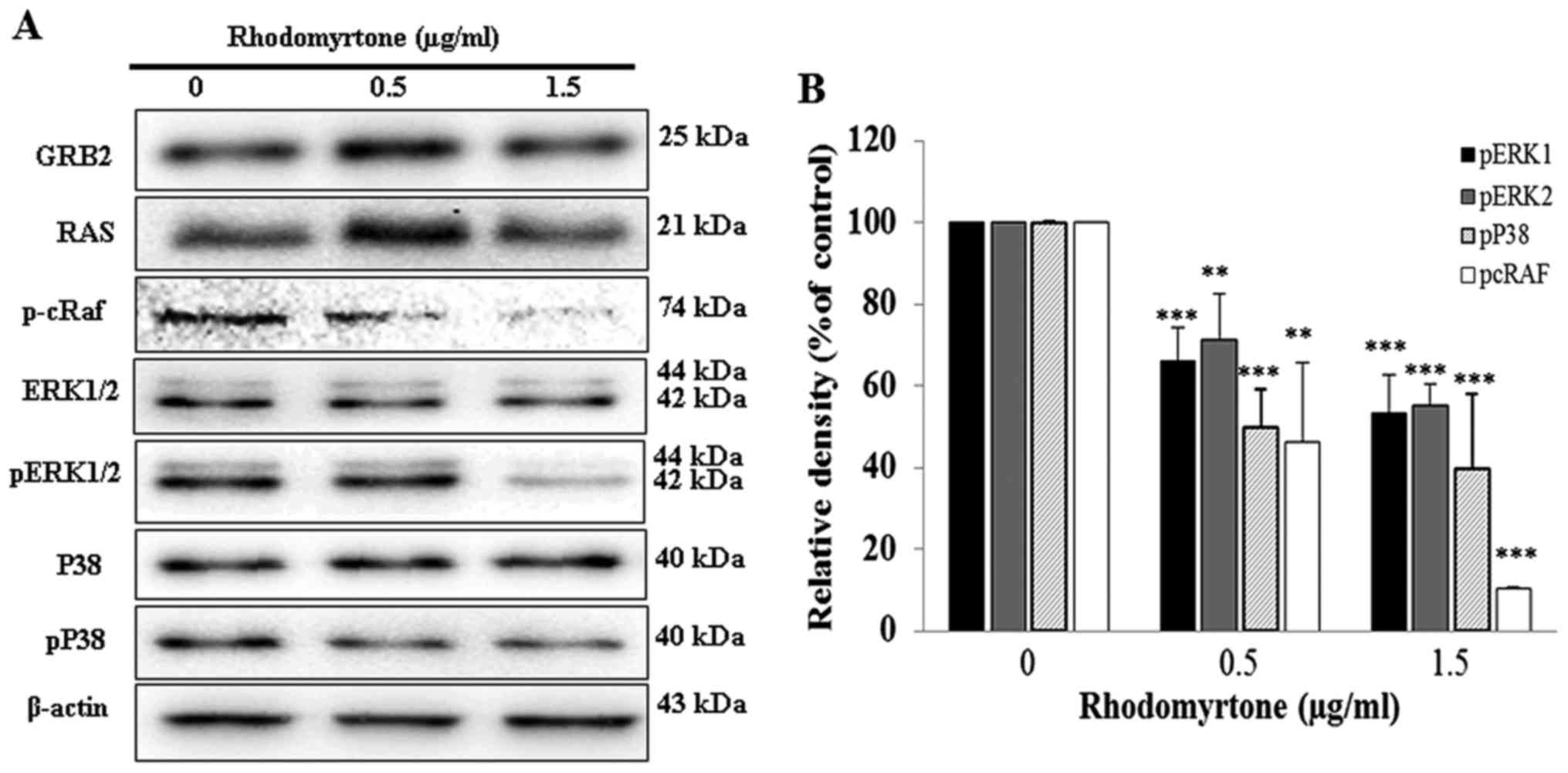
Effect of rhodomyrtone on GRB2, RAS, MAPK
signaling pathway and p-cRaf expression
We determined the mechanisms of rhodomyrtone for
anti-metastatic effects on A431 cells. GRB2, RAS, p-cRaf and MAPK
expression were investigated in A431 cells. The results showed that
rhodomyrtone significantly reduced the phosphorylation of cRaf,
ERK1/2 and p38 in a dose-dependent manner, but no significant
alterations were observed in GRB2, RAS, ERK1/2 and p38 as shown in
Fig. 7.
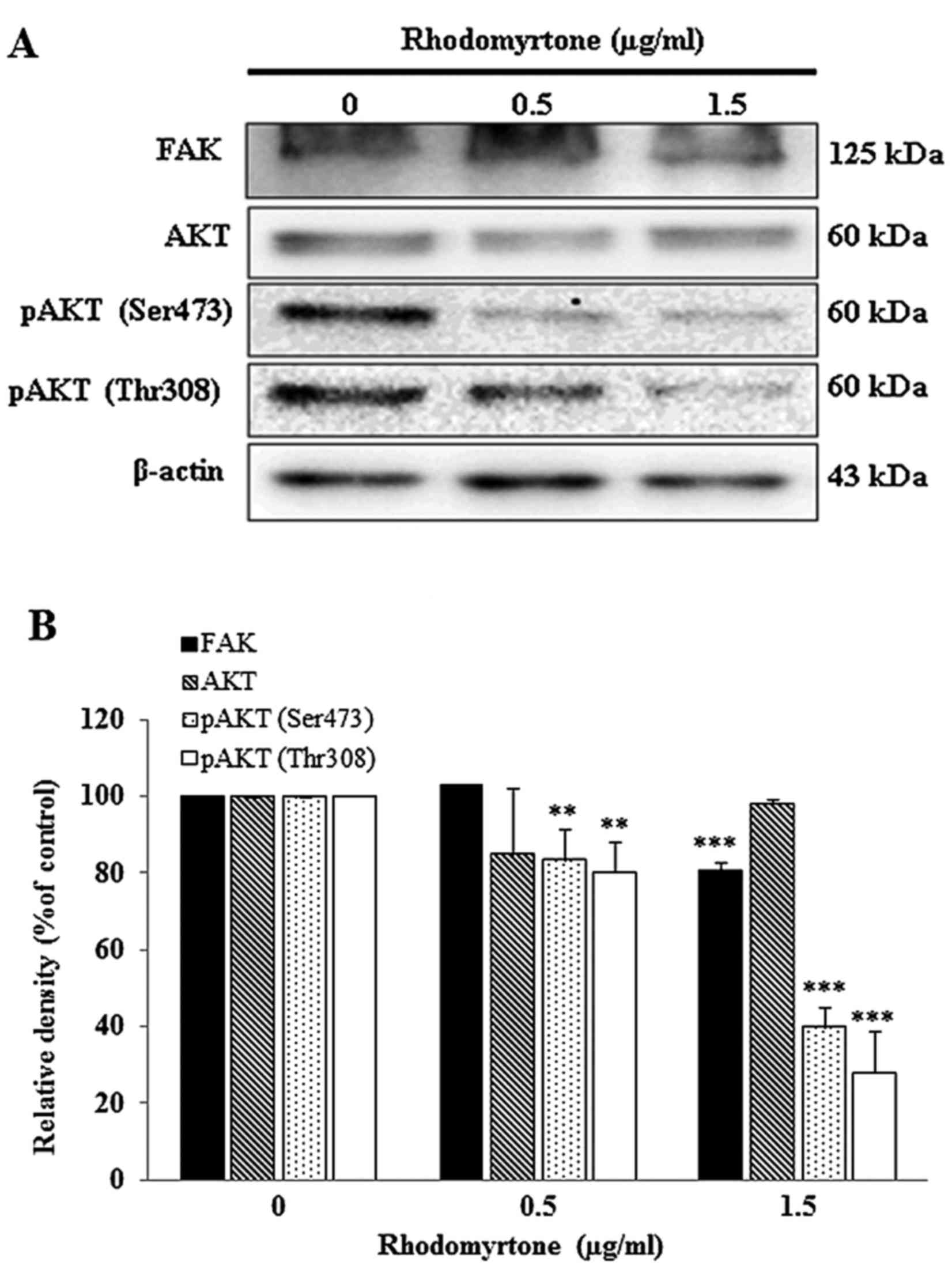
Effect of rhodomyrtone on FAK and AKT
signaling pathway
To determine whether rhodomyrtone inhibits A431
cells migration and invasion via FAK/PI3K/Akt, cells were treated
with 0.5 and 1.5 µg/ml rhodomyrtone for 24 h. FAK, AKT, pAKT
(Ser473) and pAKT (Thr308) were detected by western blot analysis.
Fig. 8A showed rhodomyrtone could
suppress FAK and the phosphorylation of AKT. The quantitative
results showed that rhodomyrtone significantly inhibited the FAK
and the phosphorylation of AKT in a dose- and time-dependent manner
(Fig. 8B).
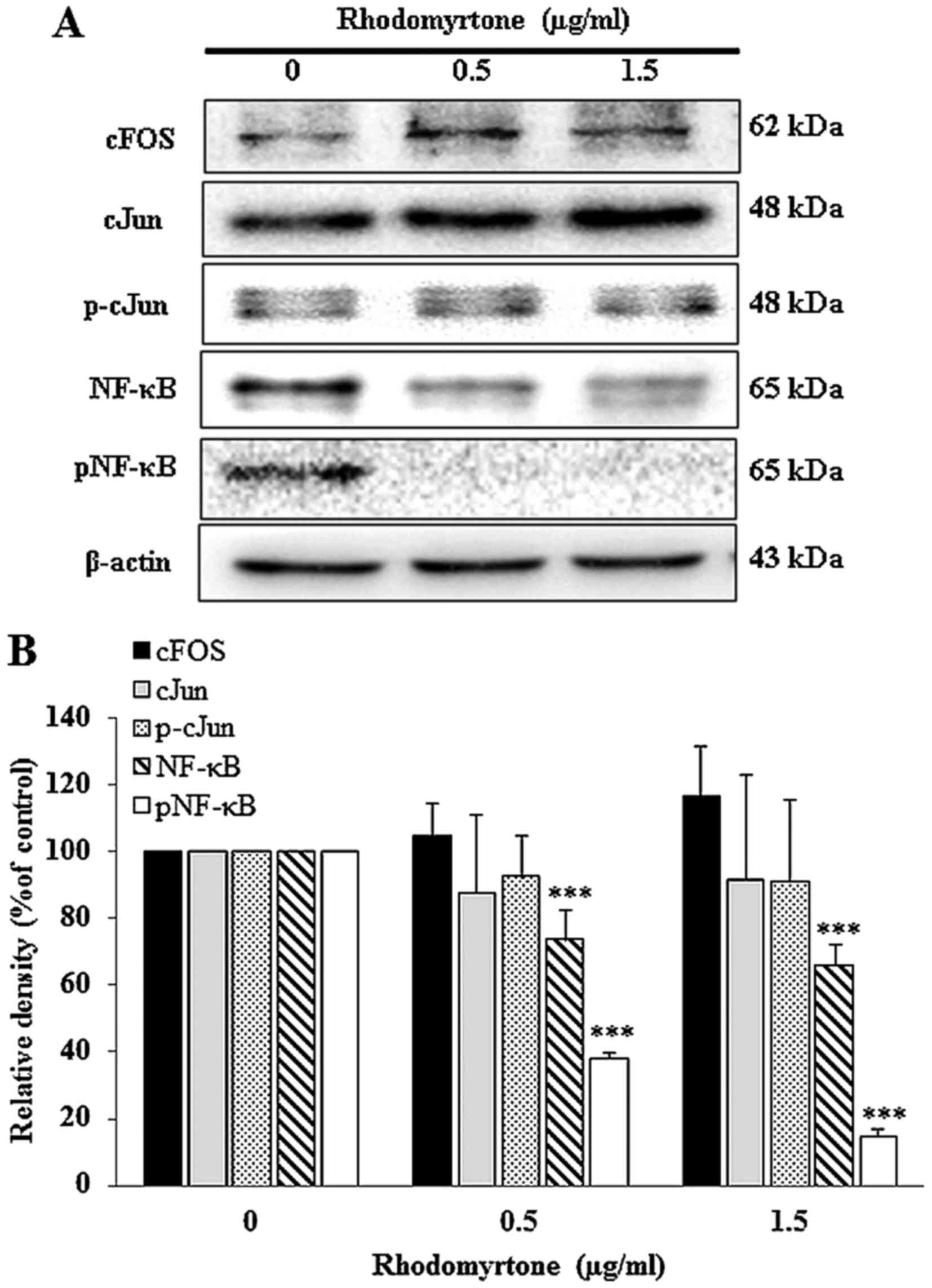
Effect of rhodomyrtone on AP-1 and NF-κB
protein expression
The inhibitory effect of rhodomyrtone on
transcription factor NF-κB and AP-1 in A431 cells were investigated
by western blotting. Data demonstrated that A431 cells treated with
rhodomyrtone significantly decreased the level of NF-κB and pNF-κB
expression in a dose-dependent manner (Fig. 9), but, there was no change in the
expression of cFos, cJun and p-cJun (components of transcription
factor AP-1) under the same conditions. The results indicated that
rhodomyrtone significantly inhibited NF-κB protein expression.
Discussion
Therapeutic agents to prevent development of
metastases are an urgent therapeutic need. Present cancer
chemotherapy is mainly targeted on primary tumors but the late
stage patient survival has improved very little. We demonstrated
that rhodomyrtone could inhibit A431 cell proliferation in a
dose-dependent manner (Fig. 1).
Rhodomyrtone at non-cytotoxic concentration and subcytotoxic
concentration (0–1.5 µg/ml) significantly reduced A431 cell
migration and cell invasion by Transwell chamber (Fig. 2) and the Matrigel-coated Boyden
chamber assay in a dose-dependent manner (Fig. 3). Rhodomyrtone also exhibited the
anti-adhesion in A431 cells on Matrigel as shown in Fig. 4, These results indicated that
rhodomyrtone inhibits cell metastasis of A431 human skin cancer
independent of cell cytotoxicity. Consistent with Lee et al
who showed andrographolide at low-cytotoxic concentration inhibits
the invasion and migration of human non-small cell lung cancer A549
cells (28). Thus, rhodomyrtone
might be used as a chemotherapeutic agent for cancer treatment in
skin cancer in the future.
This study showed that rhodomyrtone significantly
inhibited MMP-2 and MMP-9 protein expression as well as MMP-2 and
MMP-9 enzyme activity but increased TIMP-1 and TIMP-2 expression.
Overexpression of MMP-2 and MMP-9 are involved in cancer
angiogenesis, cancer invasion and metastasis. Thus inhibition of
MMP-2 and MMP-9 expression or enzyme activity provide early targets
of cancer metastasis prevention (29–31).
Reduction of MMP-2 and MMP-9 activities and protein expression have
been shown to inhibit cell migration and invasion in various types
of cancer cells (32–38). Moreover, Wang et al reported
that upregulation of TIMP-1 could inhibit activity of MMP-2 and
suppressed HepG2 and MHCC97L metastasis (34). In addition, invasion of
hepatocellular carcinoma was inhibited by Chrysanthemum
indicum ethanolic extract via the imbalance of MMPs and TIMPs
(35). This finding supported
possible anti-metastatic mechanism of rhodomyrtone in skin
cancer.
Several studies have demonstrated the role of the
MAPK and PI3K/AKT pathway in regulating MMPs expression (9,39).
In this study, we found that rhodomyrtone significantly inhibited
the cRaf, ERK1/2 and p38 phosphorylation in A431 cells in a
dose-dependent manner (Fig. 7).
Likewise, previous reports showed the inhibition of MMP-2 and MMP-9
expression in cancer cells via ERK1/2 pathway (9,32,39,40).
Chen et al and Chien et al showed the expression of
MMP-2 and MMP-9 were regulated by p38α MAPK pathway (41,42).
In addition, we showed that rhodomyrtone inhibited FAK and the
phosphorylation of AKT (Ser473) and AKT (Thr308) in A431 cells.
Previous studies showed the inhibition of cell invasion and
migration of human non-small cell lung cancer through FAK/PI3K/AKT
signaling pathway (43). Qin et
al also showed the inhibition of breast cancer cell invasion by
suppressing the expression of MMP-2 and MMP-9 through the integrin
β1/FAK/PI3K/AKT/β-catenin signaling by excisanin A (44). The promoter regions of MMP genes
show remarkable conservation of regulatory elements, including AP-1
and NF-κB (12,13). NF-κB is constitutively activated in
various types of cancer, including breast cancer and has been shown
to contribute to the development and progression of tumors
including HCC cells (45). Herein,
we found that rhodomyrtone significantly inhibited NF-κB protein
expression (Fig. 9). Similarly to
the previous result demonstrated that tomatidine inhibited the
invasion of A549 cells by reducing MMPs expression via ERK and AKT
signaling pathways and NF-κB activity (46). Consistent with Lu et al
showed the inhibition of migration and invasion in melanoma cells
by α-solanine via JNK, PI3K/AKT and NF-κB pathway (47).
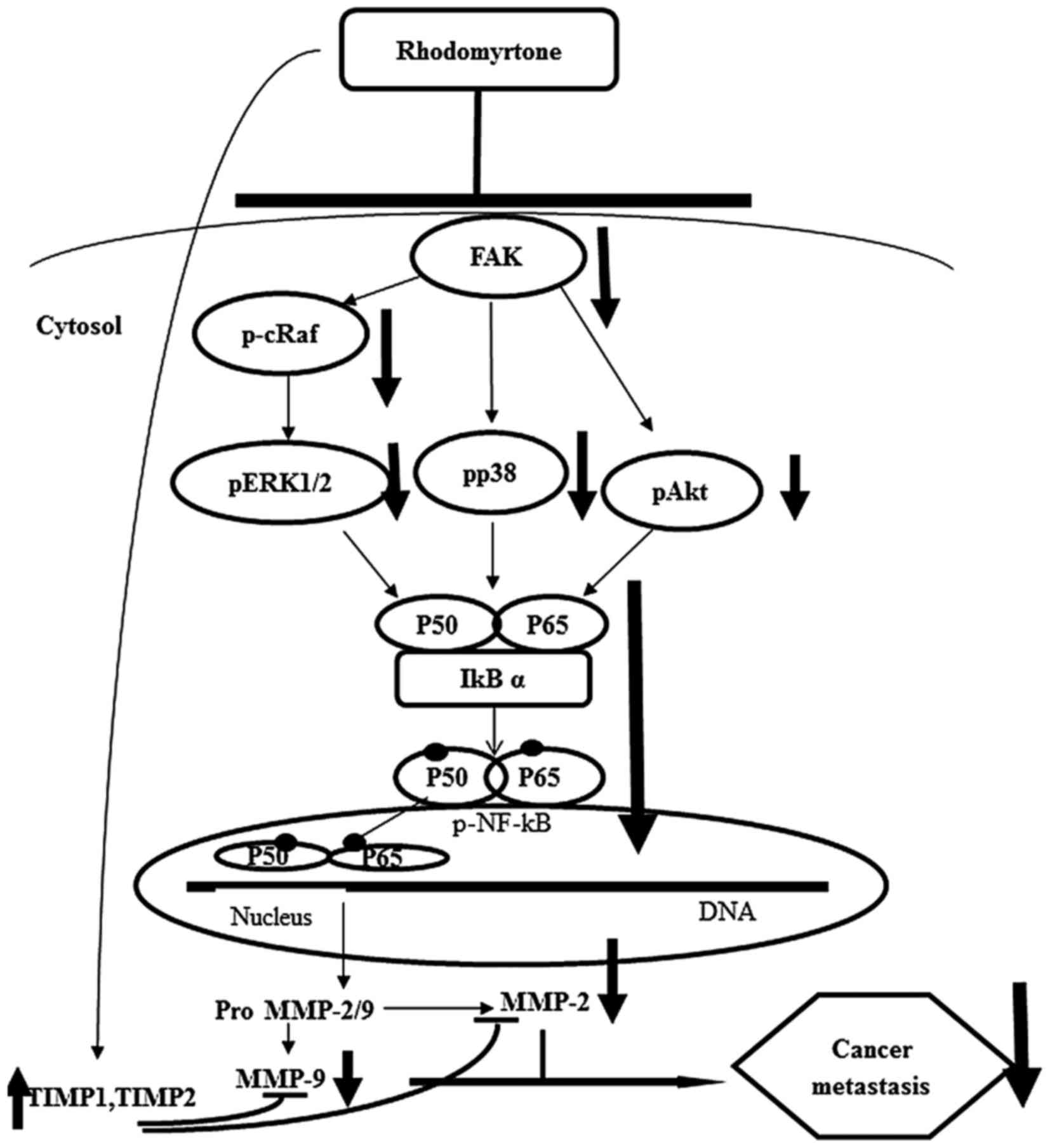
In conclusion, we demonstrated that rhodomyrtone
inhibited cell migration, adhesion and invasion of A431 cells by
suppressing MMP-2 and MMP-9 activities and MMP-2 and MMP-9 protein
expression. Furthermore, we showed the mechanism of anti-metastatic
effect of rhodomyrtone on A431 cells through the inhibition of
Raf/ERK, p38 MAPK and FAK/Akt signaling pathways via NF-κB
activities (Fig. 10). These
findings reveal that rhodomyrtone is a new therapeutic agent
preventing cancer metastasis.
Acknowledgments
We would like to thank the Agricultural Research
Development Agency (Public Organization), and Office of the Higher
Education Commission Thailand.
References
|
1
|
Bravo-Cordero JJ, Hodgson L and Condeelis
J: Directed cell invasion and migration during metastasis. Curr
Opin Cell Biol. 24:277–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu YH, Yu LJ, Shao ED, Wu JL and Ji JW:
The regulating role of mutant IkappaBalpha in expression of TIMP-2
and MMP-9 in human glioblastoma multiform. Chin Med J (Engl).
122:205–211. 2009.
|
|
3
|
Figueira RC, Gomes LR, Neto JS, Silva FC,
Silva ID and Sogayar MC: Correlation between MMPs and their
inhibitors in breast cancer tumor tissue specimens and in cell
lines with different metastatic potential. BMC Cancer. 9:202009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giannelli G, Bergamini C, Marinosci F,
Fransvea E, Quaranta M, Lupo L, Schiraldi O and Antonaci S:
Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular
carcinoma. Int J Cancer. 97:425–431. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang JH, Han IH, Sung MK, Yoo H, Kim YG,
Kim JS, Kawada T and Yu R: Soybean saponin inhibits tumor cell
metastasis by modulating expressions of MMP-2, MMP-9 and TIMP-2.
Cancer Lett. 261:84–92. 2008. View Article : Google Scholar
|
|
6
|
Lopez-Bergami P, Huang C, Goydos JS, Yip
D, Bar-Eli M, Herlyn M, Smalley KS, Mahale A, Eroshkin A, Aaronson
S, et al: Rewired ERK-JNK signaling pathways in melanoma. Cancer
Cell. 11:447–460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crowe DL, Tsang KJ and Shemirani B: Jun
N-terminal kinase 1 mediates transcriptional induction of matrix
metalloproteinase 9 expression. Neoplasia. 3:27–32. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shukla S, Maclennan GT, Hartman DJ, Fu P,
Resnick MI and Gupta S: Activation of PI3K-Akt signaling pathway
promotes prostate cancer cell invasion. Int J Cancer.
121:1424–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen PN, Hsieh YS, Chiou HL and Chu SC:
Silibinin inhibits cell invasion through inactivation of both
PI3K-Akt and MAPK signaling pathways. Chem Biol Interact.
156:141–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwon GT, Cho HJ, Chung WY, Park KK, Moon A
and Park JH: Isoliquiritigenin inhibits migration and invasion of
prostate cancer cells: Possible mediation by decreased JNK/AP-1
signaling. J Nutr Biochem. 20:663–676. 2009. View Article : Google Scholar
|
|
11
|
Lee SJ, Park SS, Lee US, Kim WJ and Moon
SK: Signaling pathway for TNF-alpha-induced MMP-9 expression:
Mediation through p38 MAP kinase, and inhibition by anti-cancer
molecule magnolol in human urinary bladder cancer 5637 cells. Int
Immunopharmacol. 8:1821–1826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Westermarck J and Kähäri VM: Regulation of
matrix metallo-proteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
13
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panthong A, Kanjanapothi D and Taylor WC:
Ethnobotanical review of medicinal plants from Thai traditional
books, Part I: Plants with anti-inflammatory, anti-asthmatic and
antihypertensive properties. J Ethnopharmacol. 18:213–228. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Panthong A, Kanjanapothi D, Taesotikul T
and Taylor WC: Ethnobotanical review of medicinal plants from Thai
traditional books, Part II: Plants with antidiarrheal, laxative and
carminative properties. J Ethnopharmacol. 31:121–156. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shankar S, Kumar D and Srivastava RK:
Epigenetic modifications by dietary phytochemicals: Implications
for personalized nutrition. Pharmacol Ther. 138:1–17. 2013.
View Article : Google Scholar
|
|
17
|
Dachriyanus S, Sargent MV, Skelton BW,
Soediro I, Sutisna M, White AH and Yulinah E: Rhodomyrtone, an
antibiotic from Rhodomyrtus tomentosa. Aust J Chem. 55:229–232.
2002. View
Article : Google Scholar
|
|
18
|
Saising J, Hiranrat A, Mahabusarakam W,
Ongsakul M and Voravuthikunchai SP: Rhodomyrtone from Rhodomyrtus
tomentosa (Aiton) Hassk. as a natural antibiotic for staphylococcal
cutaneous infections. J Health Sci. 54:589–595. 2008. View Article : Google Scholar
|
|
19
|
Limsuwan S and Voravuthikunchai SP:
Boesenbergia pandurata (Roxb.) Schltr., Eleutherine americana Merr.
and Rhodomyrtus tomentosa (Aiton) Hassk. as antibiofilm producing
and anti-quorum sensing in Streptococcus pyogenes. FEMS Immunol Med
Microbiol. 53:429–436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Limsuwan S, Trip EN, Kouwen TR, Piersma S,
Hiranrat A, Mahabusarakam W, Voravuthikunchai SP, van Dijl JM and
Kayser O: Rhodomyrtone: A new candidate as natural antibacterial
drug from Rhodomyrtus tomentosa. Phytomedicine. 16:645–651. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sianglum W, Srimanote P, Wonglumsom W,
Kittiniyom K and Voravuthikunchai SP: Proteome analyses of cellular
proteins in methicillin-resistant Staphylococcus aureus treated
with rhodomyrtone, a novel antibiotic candidate. PLoS One.
6:e166282011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Srisuwan S, Tongtawe P, Srimanote P and
Voravuthikunchai SP: Rhodomyrtone modulates innate immune responses
of THP-1 monocytes to assist in clearing methicillin-resistant
Staphylococcus aureus. PLoS One. 9:e1103212014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chorachoo J, Saeloh D, Srichana T,
Amnuaikit T, Musthafa KS, Sretrirutchai S and Voravuthikunchai SP:
Rhodomyrtone as a potential anti-proliferative and apoptosis
inducing agent in HaCaT keratinocyte cells. Eur J Pharmacol.
772:144–151. 2016. View Article : Google Scholar
|
|
24
|
Scherer D and Kumar R: Genetics of
pigmentation in skin cancer - a review. Mutat Res. 705:141–153.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rigel DS: Cutaneous ultraviolet exposure
and its relationship to the development of skin cancer. J Am Acad
Dermatol. 58(Suppl 2): S129–S132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Afaq F: Natural agents: Cellular and
molecular mechanisms of photoprotection. Arch Biochem Biophys.
508:144–151. 2011. View Article : Google Scholar :
|
|
27
|
Bowden GT: Prevention of non-melanoma skin
cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer.
4:23–35. 2004. View
Article : Google Scholar
|
|
28
|
Lee YC, Lin HH, Hsu CH, Wang CJ, Chiang TA
and Chen JH: Inhibitory effects of andrographolide on migration and
invasion in human non-small cell lung cancer A549 cells via
down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol.
632:23–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okada N, Ishida H, Murata N, Hashimoto D,
Seyama Y and Kubota S: Matrix metalloproteinase-2 and -9 in bile as
a marker of liver metastasis in colorectal cancer. Biochem Biophys
Res Commun. 288:212–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Waas ET, Wobbes T, Lomme RM, DeGroot J,
Ruers T and Hendriks T: Matrix metalloproteinase 2 and 9 activity
in patients with colorectal cancer liver metastasis. Br J Surg.
90:1556–1564. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guruvayoorappan C and Kuttan G:
Amentoflavone inhibits experimental tumor metastasis through a
regulatory mechanism involving MMP-2, MMP-9, prolyl hydroxylase,
lysyl oxidase, VEGF, ERK-1, ERK-2, STAT-1, NM23 and cytokines in
lung tissues of C57BL/6 mice. Immunopharmacol Immunotoxicol.
30:711–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao YC, Shih YW, Chao CH, Lee XY and
Chiang TA: Involvement of the ERK signaling pathway in fisetin
reduces invasion and migration in the human lung cancer cell line
A549. J Agric Food Chem. 57:8933–8941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liew K, Yong PV, Lim YM, Navaratnam V and
Ho AS: 2-Methoxy-1,4-Naphthoquinone (MNQ) suppresses the invasion
and migration of a human metastatic breast cancer cell line
(MDA-MB-231). Toxicol In Vitro. 28:335–339. 2014. View Article : Google Scholar
|
|
34
|
Wang N, Zhu M, Tsao SW, Man K, Zhang Z and
Feng Y: Up-regulation of TIMP-1 by genipin inhibits MMP-2
activities and suppresses the metastatic potential of human
hepatocellular carcinoma. PLoS One. 7:e463182012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang ZD, Huang C, Li ZF, Yang J, Li BH,
Liang RR, Dai ZJ and Liu ZW: Chrysanthemum indicum ethanolic
extract inhibits invasion of hepatocellular carcinoma via
regulation of MMP/TIMP balance as therapeutic target. Oncol Rep.
23:413–421. 2010.PubMed/NCBI
|
|
36
|
Liao CL, Lai KC, Huang AC, Yang JS, Lin
JJ, Wu SH, Gibson Wood W, Lin JG and Chung JG: Gallic acid inhibits
migration and invasion in human osteosarcoma U-2 OS cells through
suppressing the matrix metalloproteinase-2/-9, protein kinase B
(PKB) and PKC signaling pathways. Food Chem Toxicol. 50:1734–1740.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu CC, Yang JS, Chiang JH, Hour MJ,
Amagaya S, Lu KW, Lin JP, Tang NY, Lee TH and Chung JG: Inhibition
of invasion and migration by newly synthesized quinazolinone MJ-29
in human oral cancer CAL 27 cells through suppression of MMP-2/9
expression and combined down-regulation of MAPK and AKT signaling.
Anticancer Res. 32:2895–2903. 2012.PubMed/NCBI
|
|
38
|
Hwang ES and Lee HJ: Allyl isothiocyanate
and its N-acetyl-cysteine conjugate suppress metastasis via
inhibition of invasion, migration, and matrix
metalloproteinase-2/-9 activities in SK-Hep 1 human hepatoma cells.
Exp Biol Med (Maywood). 231:421–430. 2006.
|
|
39
|
Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC
and Lu KH: Silibinin suppresses human osteosarcoma MG-63 cell
invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of
MMP-2. Carcinogenesis. 28:977–987. 2007. View Article : Google Scholar
|
|
40
|
Weng CJ, Chau CF, Hsieh YS, Yang SF and
Yen GC: Lucidenic acid inhibits PMA-induced invasion of human
hepatoma cells through inactivating MAPK/ERK signal transduction
pathway and reducing binding activities of NF-kappaB and AP-1.
Carcinogenesis. 29:147–156. 2008. View Article : Google Scholar
|
|
41
|
Chen YY, Liu FC, Chou PY, Chien YC, Chang
WS, Huang GJ, Wu CH and Sheu MJ: Ethanol extracts of fruiting
bodies of Antrodia cinnamomea suppress CL1–5 human lung
adenocarcinoma cells migration by inhibiting matrix
metalloproteinase-2/9 through ERK, JNK, p38, and PI3K/Akt signaling
pathways. Evid Based Complement Alternat Med. 2012:3784152012.
|
|
42
|
Chien ST, Lin SS, Wang CK, Lee YB, Chen
KS, Fong Y and Shih YW: Acacetin inhibits the invasion and
migration of human non-small cell lung cancer A549 cells by
suppressing the p38α MAPK signaling pathway. Mol Cell Biochem.
350:135–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shieh JM, Cheng TH, Shi MD, Wu PF, Chen Y,
Ko SC and Shih YW: α-Tomatine suppresses invasion and migration of
human non-small cell lung cancer NCI-H460 cells through
inactivating FAK/PI3K/Akt signaling pathway and reducing binding
activity of NF-κB. Cell Biochem Biophys. 60:297–310. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qin J, Tang J, Jiao L, Ji J, Chen WD, Feng
GK, Gao YH, Zhu XF and Deng R: A diterpenoid compound, excisanin A,
inhibits the invasive behavior of breast cancer cells by modulating
the integrin β1/FAK/PI3K/AKT/β-catenin signaling. Life Sci.
93:655–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nakagawa H and Maeda S: Inflammation- and
stress-related signaling pathways in hepatocarcinogenesis. World J
Gastroenterol. 18:4071–4081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yan KH, Lee LM, Yan SH, Huang HC, Li CC,
Lin HT and Chen PS: Tomatidine inhibits invasion of human lung
adenocarcinoma cell A549 by reducing matrix metalloproteinases
expression. Chem Biol Interact. 203:580–587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lu MK, Shih YW, Chang Chien TT, Fang LH,
Huang HC and Chen PS: α-Solanine inhibits human melanoma cell
migration and invasion by reducing matrix metalloproteinase-2/9
activities. Biol Pharm Bull. 33:1685–1691. 2010. View Article : Google Scholar
|