Introduction
The polyphenolic phytoalexin, resveratrol
(3,5,4′-trihydroxy-trans-stilbene), is a naturally occurring
phytochemical that is produced by more than 70 plant species and is
highly enriched in grape skins, raspberries, mulberries, peanuts,
and red wine (1,2). This natural product has been shown to
possess many biological activities, including anti-inflammatory,
neuroprotective, antiviral, and antifungal properties (3,4). In
addition, resveratrol may be a potential anticancer agent in the
humans (5,6). Resveratrol is likely to exert
anticancer properties by inhibiting three major stages of
carcinogenesis, namely tumor initiation, promotion, and progression
(2). Dozens of reports have
documented that resveratrol can inhibit the proliferation of
several kinds of tumors, such as leukemia, pancreas, breast,
prostate, bladder, and colon cancers (7–12).
However, the molecular mechanisms that contribute to the anticancer
activity of resveratrol are not completely understood.
It has been shown that the resveratrol-induced
growth inhibition is associated with cell-cycle arrest and
induction of apoptotic cell death in cancer cell lines derived from
different origins (13–18). Alterations in expression of the
anti-apoptotic protein Bcl-2, loss of mitochondrial function,
release of cytochrome c, and activation of caspases may be
involved in resveratrol-induced apoptotic cell death (19–22).
Furthermore, resveratrol has been reported to induce
p53-independent apoptosis in human HCT116 colon carcinoma cells
(23), although p53 appears to be
required for resveratrol-induced apoptosis in other cancer cell
lines (24,25). In those previous reports, the
concentrations of resveratrol used are varied. Then, it is possible
that the signaling cascades for the resveratrol-induced apoptotic
cell death are different depending on the types of cancer cells.
Resveratrol is also known to act as an antioxidant and to activate
adenosine-monophosphate-activated protein kinase (AMPK) and sirtuin
1 (SIRT1) (26), but whether these
properties are responsible for the mechanisms through which
resveratrol induces cancer cell death has not been fully
defined.
In the present study, we examined the effects of
resveratrol on cell viability in a variety of human cancer cell
lines. We found that they display quite different sensitivity to
resveratrol's anti-viability activity, depending on the type of
cancer cells. Further studies were then undertaken to focus on the
elucidation and analysis of the cell death-initiating signaling
mechanisms that can be activated by resveratrol in different cancer
cell lines. We also addressed whether several cellular events
stimulated with resveratrol, including SIRT1 and AMPK activation,
are involved in its anti-viability effect on cancer cells.
Materials and methods
Cell culture
Human leukemic monocyte lymphoma cell line, U937,
and human acute T lymphoblastic leukemia cell line, MOLT-4, were
obtained from Health Science Research Resources Bank (Osaka,
Japan). The two leukemic cell lines were cultured in suspension
using RPMI-1640 medium (Nissui Pharmaceutical, Tokyo, Japan) with
10% heat inactivated fetal bovine serum at 37°C in a humidified air
with 5% CO2. The human breast cancer cell line MCF-7,
and human colorectal cancer cell line SW480, were obtained from
American Type Culture Collection (Manassas, VA, USA). The human
hepatocellular liver carcinoma cell line HepG2 (RCB1886), human
lung adenocarcinoma epithelial cell line A549 (RCB0098), and human
colon cancer cell lines Caco-2 (RCB0988) and HCT116 (RCB2979), were
provided by the RIKEN BRC through the National Bio-Resource Project
of the MEXT, Japan. These solid tumor cell lines were grown in
Dulbecco's minimal essential medium (DMEM) (Nacalai Tesque, Kyoto,
Japan), supplemented 10% fetal bovine serum, and maintained at 37°C
in a humidified atmosphere containing 5% CO2.
Cell viability assay
Cell viability was assessed using the colorimetric
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-nyltetrazolium bromide)
tetrazolium reduction assay (27).
Cells were seeded in 96-well plates at 20,000 per well and
cultivated for 24–48 h before the onset of treatment with
resveratrol, SIRT1720, or their vehicle DMSO. An equal volume of
DMSO (≤0.5%, v/v) was used as solvent control, which was confirmed
to have little or no effect on cell proliferation. At the end of
each treatment time, MTT was added to each well at a final
concentration of 0.5 mg/ml, followed by incubation at 37°C for 3 h.
The absorbance of each sample was measured with a FilterMax F5
multi-mode microplate reader (Molecular Devices, Sunnyvale, CA,
USA) at 595 nm. Cell-free wells containing only medium were used
for background subtraction. The number of viable cells was
calculated from the number of untreated cells, and the data were
expressed as percent cell viability. All experiments were performed
at least in triplicate.
Cell death detection using flow
cytometry
Cell death was detected by flow cytometry after
Annexin V-FITC/propidium iodide (PI) double staining as specified
in the protocol provided with the FITC Annexin V Apoptosis
Detection kit II (BD Pharmingen, Erembodegen, Belgium). Cells were
seeded in 6-well plates, cultivated for 24-48 h, and treated with
resveratrol or DMSO. Then, cells were washed with PBS, harvested by
trypsinization, centrifuged, and resuspended in binding buffer from
the kit containing Annexin V-FITC and PI. The samples were analyzed
using a flow cytometer (FACSCanto II, Becton-Dickinson, Franklin
Lakes, NJ, USA), as reported earlier (28).
TUNEL staining
The terminal deoxynucleotide transferase-mediated
dUTP nick end labeling (TUNEL) technique was conducted to detect
apoptotic cancer cells. Cytospin preparations of cancer cells were
stained with Takara In Situ Apoptosis Detection kit (Takara
Bio, Ohtsu, Japan) according to the manufacturer's instructions.
Briefly, cells were fixed with 10 N mildform solution (Wako Pure
Chemical, Osaka, Japan) for 30 min at room temperature.
Subsequently, cells were incubated with terminal deoxynucleotidyl
transferase (TdT) enzyme and anti-fluorescein
isothiocyanate-horseradish peroxidase. Labeled DNA was visualized
by chromogenic staining with 3′3-diaminobenzidine. Counterstaining
was performed with methyl green.
DNA agarose gel electrophoresis
Genomic DNA was isolated from cancer cells by the
standard method. After precipitation by ethanol, DNA was dissolved
in TE buffer (pH 8.0). DNA (500 ng) was pipetted onto a 1% agarose
gel containing 100 ng/ml ethidium bromide, and electrophoresis was
performed.
Western blot analysis
Cells were harvested and lysed in 300 µl of
RIPA buffer (25 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 1% sodium
deoxycholate, 0.1% SDS, pH 7.4; Thermo Fisher Scientific, Rockford,
IL, USA) containing protease inhibitor cocktail on ice. The lysates
were centrifuged at 18,000 × g for 10 min at 4°C and the resulting
supernatants were collected. The proteins in the supernatant were
measured using BCA Protein assay kit (Thermo Fisher Scientific).
When required, the membrane fractions were prepared as described
previously (29). Mitochondrial
and cytosolic fractionation was performed using K-assay®
Cytochrome c Releasing Apoptosis assay (Kamiya Biochemical
Co., Seattle, WA, USA). Immunoblotting was performed as described
in our previous reports (29,30).
Samples (30–100 µg of protein) were run on 10–16%
SDS-polyacrylamide gel and electrotransferred to polyvinylidine
difluoride filter membrane. The membrane was blocked for 60 min at
room temperature in Odyssey blocking buffer, followed by overnight
incubation with primary antibody at 4°C. Primary antibody detection
was performed with horseradish peroxidase-conjugated or
IRDye®-labeled secondary antibodies. Binding of the
antibody was detected by an ECL Plus chemiluminescent system (GE
Healthcare, Tokyo, Japan) and levels of protein expression were
quantitated by a luminoimage LAS-3000 analyzer (Fuji Film, Tokyo,
Japan). Fluorescent of IR-Dye was analyzed by Odyssey CLx Infrared
Imaging System (LI-COR Bioscience, Lincoln, NE, USA).
The following antibodies, which are commercially
available, were used: anti-human AMPK rabbit polyclonal antibody
(Cell Signaling, Danvers, MA, USA), anti-human phospho-AMPK
(Thr-172) rabbit polyclonal antibody (Cell Signaling), anti-mouse
Akt (pan) rabbit monoclonal antibody (Cell Signaling), anti-human
phospho-Akt (Ser-473) rabbit monoclonal antibody (Cell Signaling),
and anti-human Bax rabbit polyclonal antibody (Santa Cruz
Biotechnology, Santa Cruz, CA, USA). Anti-human β-actin rabbit
polyclonal antibody (Bioss, Woburn, MA, USA) was used as a loading
control. Anti-human VDAC mouse monoclonal antibody (Cell Signaling)
was used as a marker of the mitochondrial outer membrane.
RNA extraction and quantitative real-time
PCR
Total RNA was isolated from cells with Sepazol-RNA I
Super G (Nacalai Tesque). ReverTra Ace qPCR RT Master Mix (Toyobo,
Osaka, Japan) was used for the reverse transcription reaction, and
real-time PCR analyses were performed using SYBR Premix Ex Taq (Tli
RNaseH Plus), ROX plus (Takara Bio). Values were normalized to
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) according to the
manufacturer's protocol (MX3000P real-time PCR system; Agilent
Technologies Inc., Santa Clara, CA, USA).
Transient transfection of H-Ras in
U937
An expression plasmid encoding the human H-Ras,
pcDNA3-H-Ras_wt (#39503), was purchased from Addgene (Cambridge,
MA, USA). Transfection into the cells was performed using HVJ
Envelope Vector Kit GenomONE (Ishihara Sangyo, Osaka, Japan)
according to the manufacture's instructions. Briefly, 80 µg
of plasmid DNA was encapsulated into HVJ-Es vector and added to
2×106 of U937 cells. The cells were centrifuged (2000 g)
at 4°C for 10 min. The efficacy of the transfection was evaluated
by qPCR analysis. This approach led to successful overexpression
(>60-fold) of the H-Ras gene.
Statistical analysis
Using GraphPad Prism® version 6.0
software (San Diego, CA, USA), statistical analysis was performed
and graphs were drawn. Mean values and standard error of mean were
calculated and all values are expressed as means ± SEM. Comparisons
of more than three data were determined by one-way ANOVA followed
by the Tukey's multiple comparison test. Student's t-test was used
when two means were compared. The level of statistical significance
was set at P<0.05.
Results
Effects of resveratrol on cell viability
in a variety of cancer cell lines
Cell viability was examined by MTT assay following
treatment with different concentrations (1–100 µM) of
resveratrol for 24 and 48 h in a variety of human cancer cell
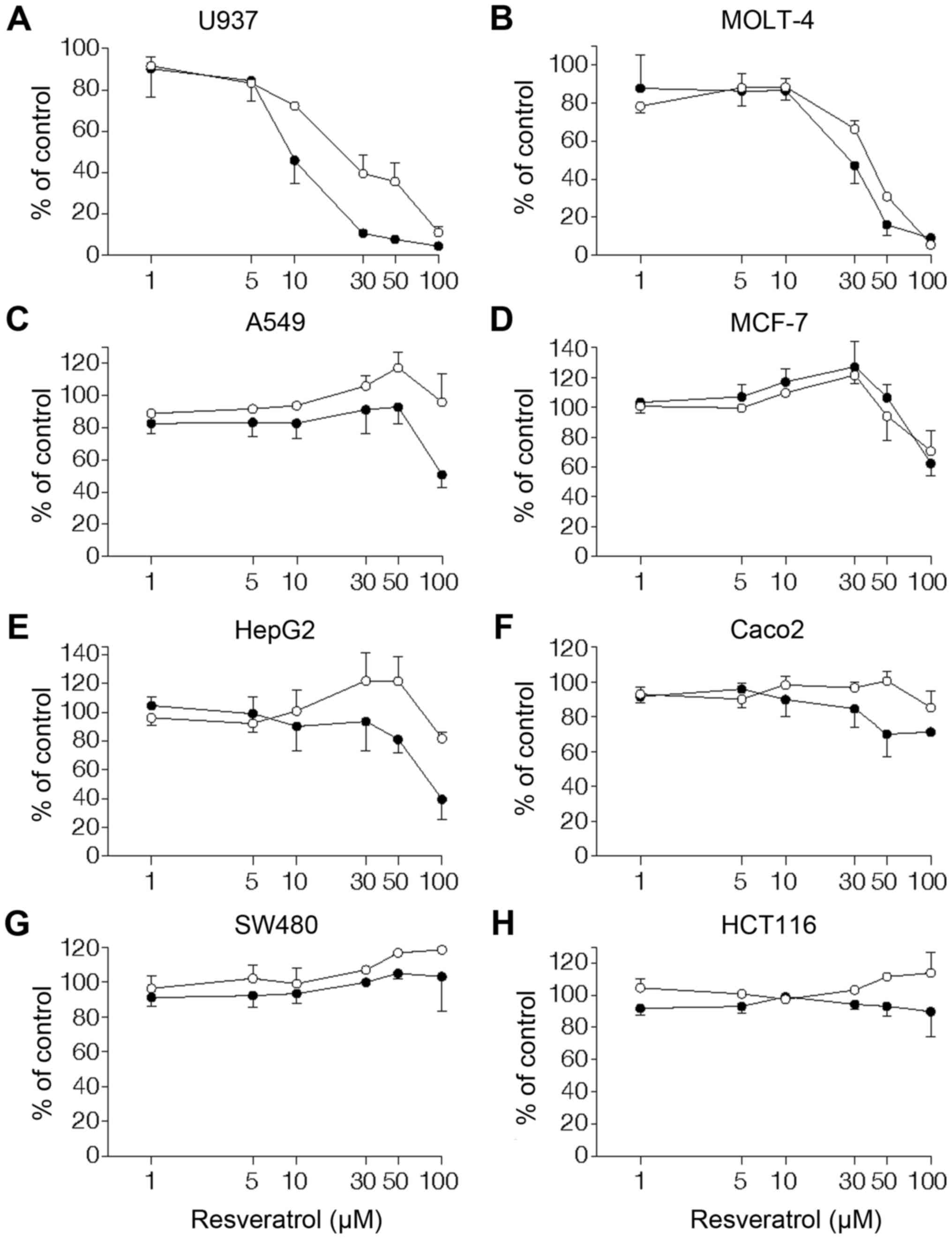
lines. In leukemic monocyte lymphoma U937, resveratrol inhibited
cell viability in a concentration- and time-dependent manner, with
10 µM being the lowest effective concentration (Fig. 1A). Similarly, a great inhibition by
resveratrol of cell viability was observed in the leukemic cell
line MOLT-4 (Fig. 1B). As for U937
and MOLT-4, treatment with 100 µM resveratrol for 24 h
resulted in a 90±4% (n=3) and 82±9% (n=3) decrease in cell
viability, respectively. Resveratrol also affected cell viability
in MCF-7 breast cancer cells (Fig.
1C) and HepG2 liver cancer cells (Fig. 1D). However, the anti-viability
effect of resveratrol on MCF-7 and HepG2 was less pronounced than
that on human leukemic cells. In the A549 lung cancer cell line, a
significant inhibition of cell viability (51±14%, n=3) was detected
only by long-term treatment with resveratrol at a high
concentration (100 µM, 48 h) (Fig. 1E). When the cytocidality of
resveratrol was assessed using colorectal carcinoma cell lines,
Caco-2, HCT116, and SW480, these cancer cells were resistant to
resveratrol. Thus, resveratrol showed only a marginal
anti-viability effect on Caco-2 (Fig.
1F) and was without effect on viability of HCT116 and SW480
(Fig. 1G and H).
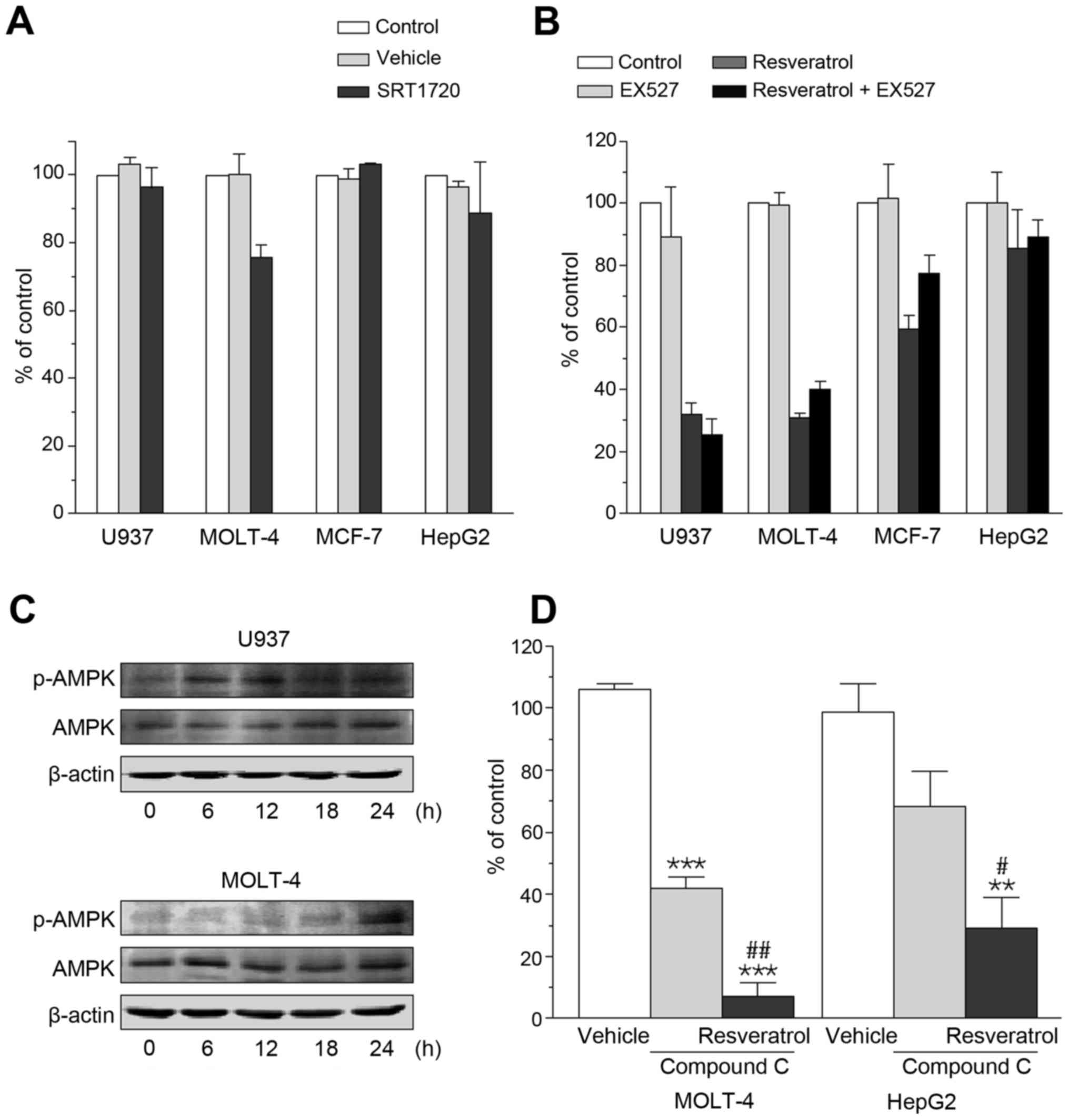
The benefits of resveratrol are considered to be
derived, in part, from activation of SIRT1 (26), and SRT1720 is a selective activator
of SIRT1 that is 1,000-fold more potent than resveratrol (31). In sharp contrast with resveratrol,
however, SIRT1720 even at a concentration of 1 µM has no
effect on cell viability in U937, MOLT-4, MCF-7, and HepG2
(Fig. 2A). Furthermore, even
though these cells were treated with the SIRT1 inhibitor EX527 (1
µM), the anti-viability effect of resveratrol remained
unaffected (Fig. 2B).
Resveratrol is also known to activate AMPK (31), and AMPK has been widely shown to be
involved in cell death induction in conditions of its sustained
activation (32). Increased
phosphorylation of AMPK was detectable in U937 at 6 and 12 h and in
MOLT-4 at 24 h after treatment with 100 µM resveratrol
(Fig. 2C). However, the presence
of the AMPK inhibitor compound C (5 µM) failed to negate the
anti-viability effect of resveratrol observed in U937, MOLT-4,
MCF-7, and HepG2. Fig. 2D shows
that compound C by itself caused a decrease in cell viability but a
further cell viability reduction was detected when MOLT-4 and HepG2
were treated with resveratrol in the presence of compound C.
Assessment of resveratrol-induced
apoptotic cell death
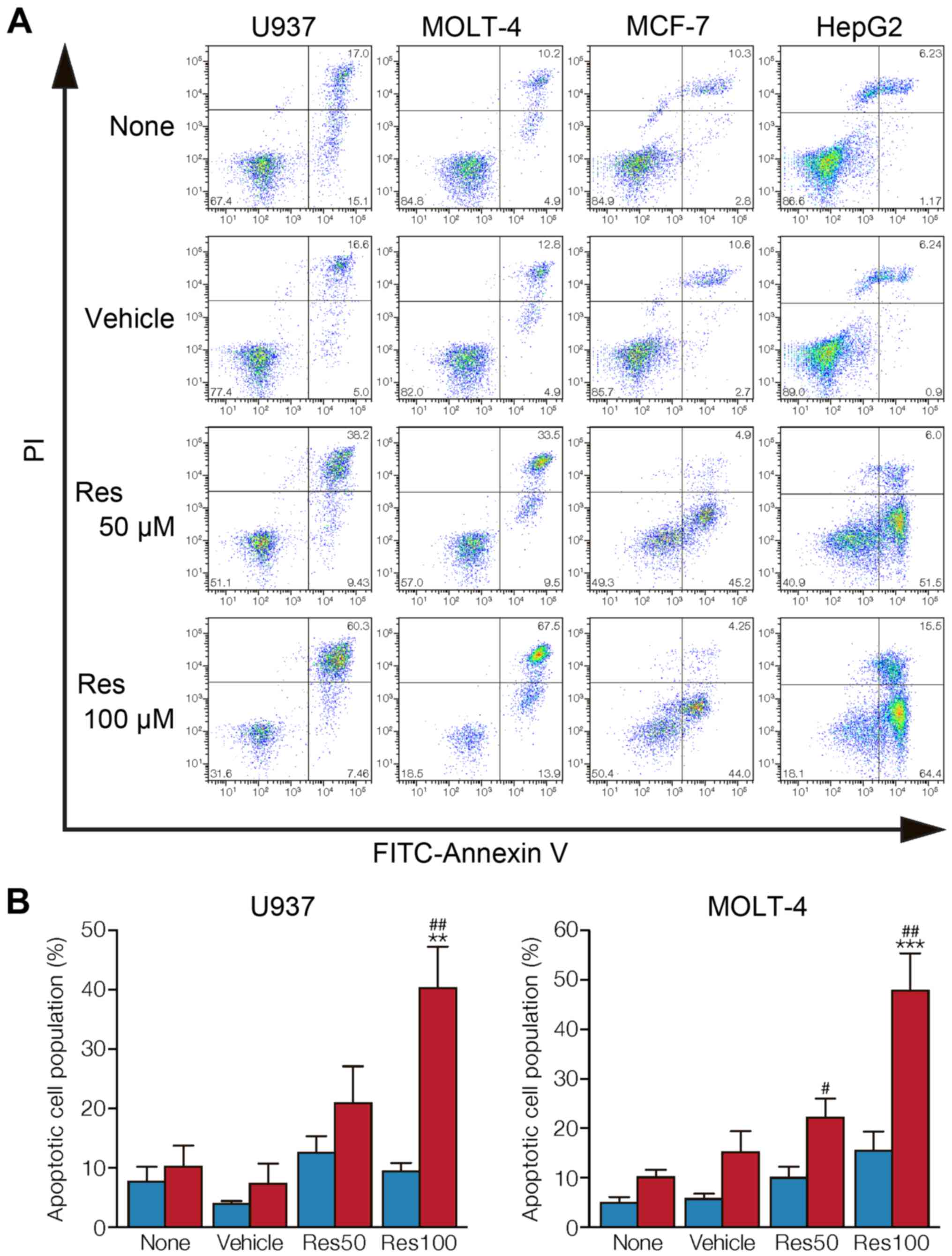
To examine whether cancer cells undergo apoptosis,
untreated, vehicle-treated, or resveratrol-treated U937, MOLT-4,
MCF-7, and HepG2 were stained with Annexin V and PI. Flow cytometry
analysis of stained cells can distinguish cells into four
quadrants, viable (Annexin V-negative, PI-negative), early
apoptosis (Annexin V-positive, PI-negative), late apoptosis
(annexin-positive, PI-positive), and necrotic (Annexin V-negative,
PI-positive) cells. When treated with 50 and 100 µM
resveratrol for 24 h, the leukemic cells U937 and MOLT-4, showed a
significant increase in the population of late apoptotic cells in a
concentration-dependent manner (Fig.
3A and B). However, MCF-7 and HepG2 treated with resveratrol
for 24 h underwent apoptosis with an increased population of early
apoptosis (Fig. 3A).
Resveratrol-induced chromosomal DNA
fragmentation
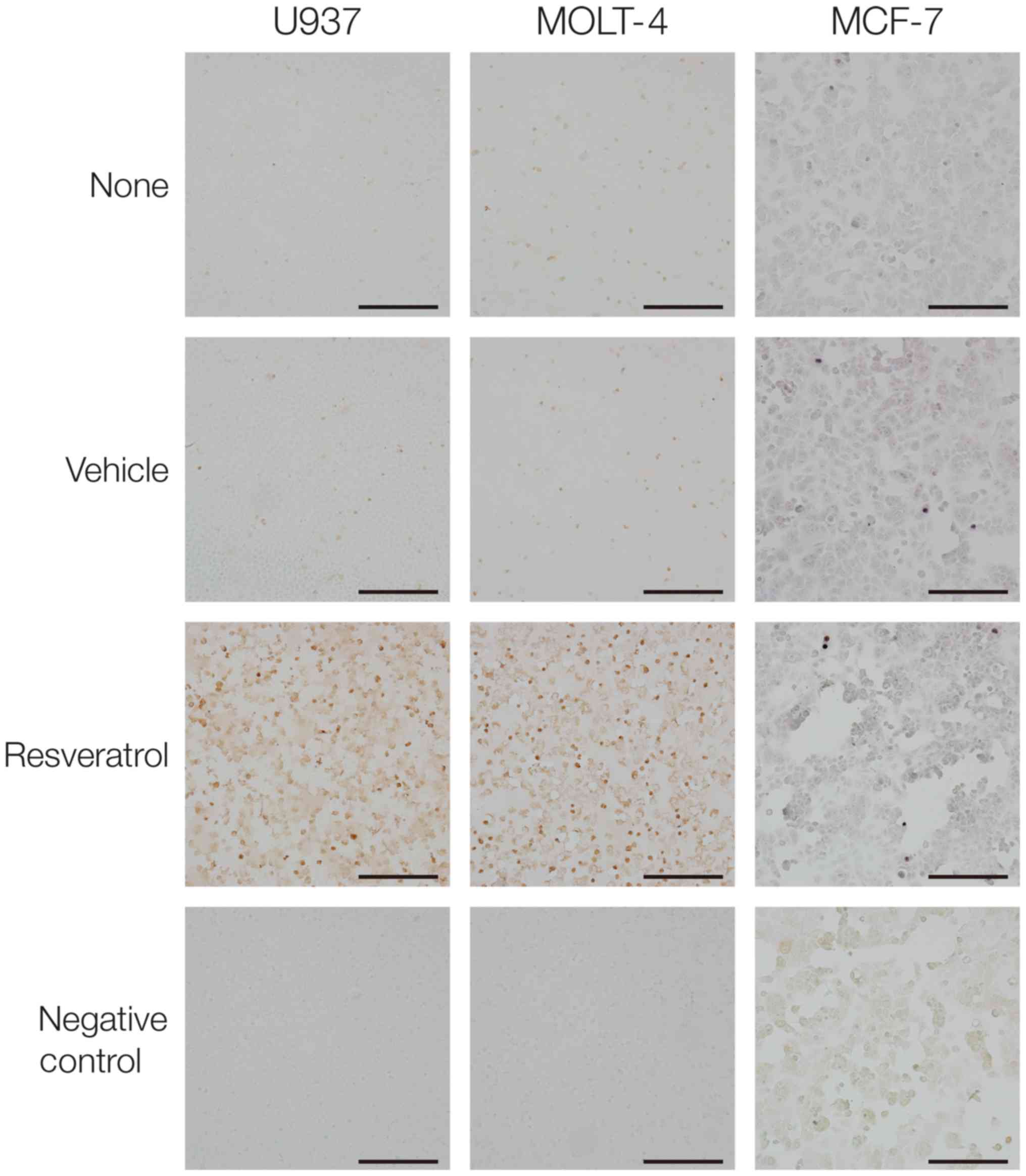
DNA fragmentation represents a characteristic
hallmark of late apoptosis. The TUNEL staining was used for the
detection of chromosomal DNA fragmentation (Fig. 4). Treatment with 100 µM
resveratrol for 24 h led to a marked increase in TUNEL-positive
cells in U937 and MOLT-4. In the face of resveratrol treatment,
however, the proportion of TUNEL-positive cells was much less in
MCF-7 as compared to the two leukemic cells.
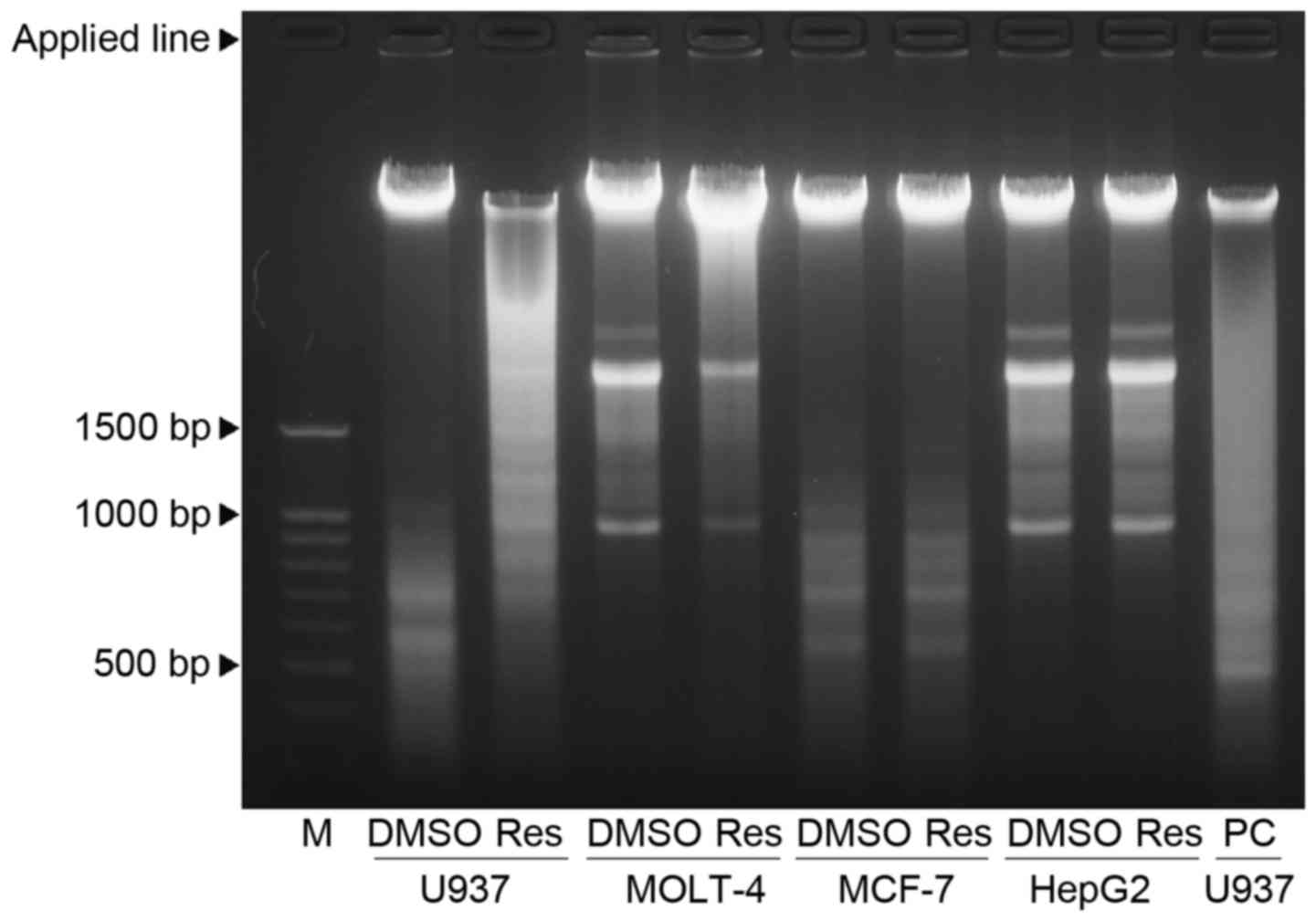
DNA fragmentation after resveratrol treatment was
also assessed by DNA agarose gels (Fig. 5). Both U937 and MOLT-4 cells
treated with 100 µM resveratrol for 24 h displayed a smear
pattern of DNA fragmentation, although ladder formation was more
pronounced in U937 than in MOLT-4. In contrast, MCF-7 showed a
relatively clear band of intact DNA regardless of resveratrol
treatment.
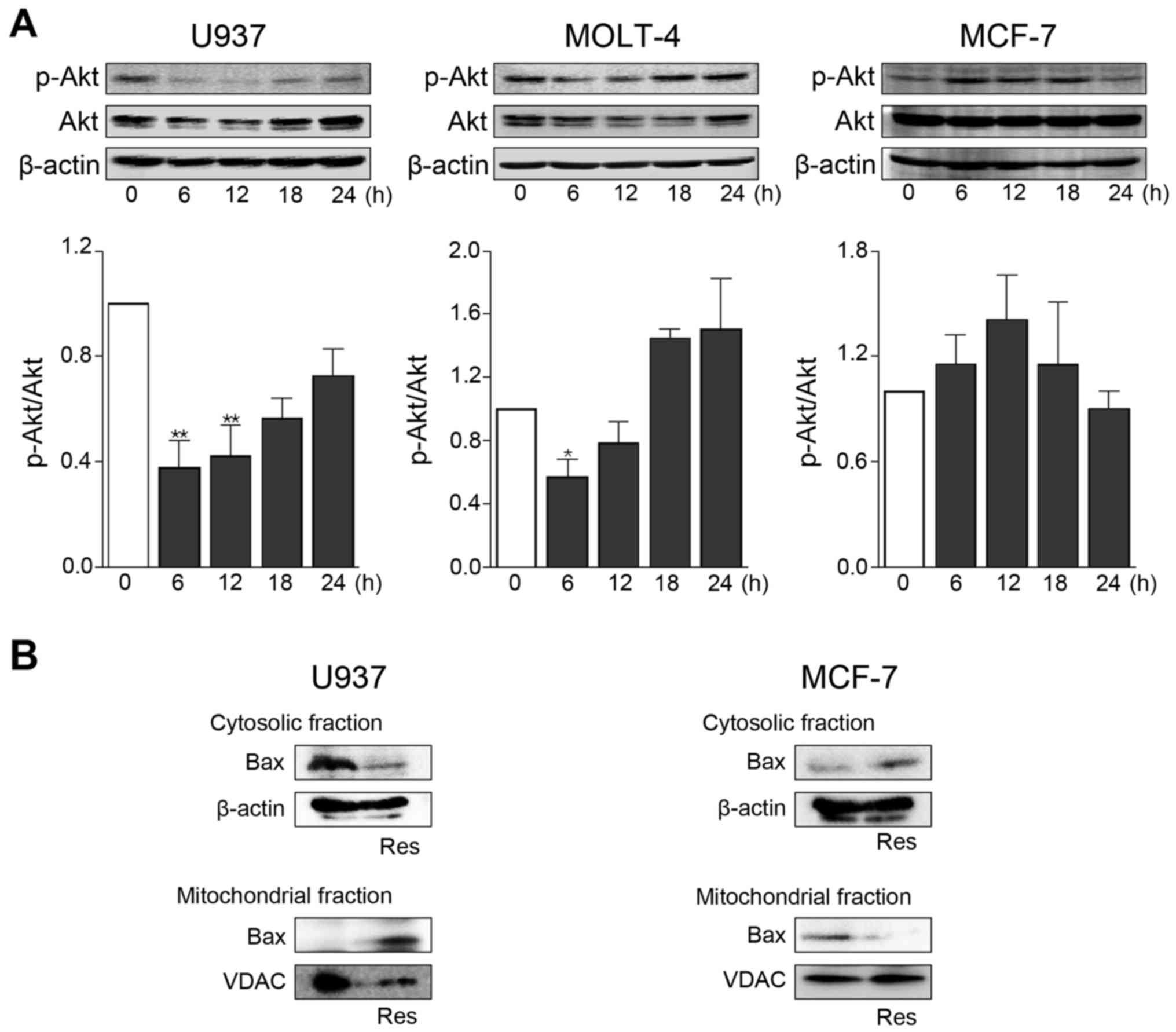
Effects of resveratrol on Akt activation
and Bax mitochondrial translocation
Activation of the serine/threonine kinase Akt was
assessed by western blot analysis for phosphorylated Akt at Ser-473
(Fig. 6A). In U937 and MOLT-4, Akt
phosphorylation showed a transient but significant decrease after
treatment with 100 µM resveratrol. The total expression
level of Akt was constantly unaltered by resveratrol, thus
demonstrating that the reduced amount of phosphorylated Akt was not
due to decreased expression of Akt. However, Akt phosphorylation
levels were substantially unchanged in MCF-7 treated with
resveratrol.
Pro-apoptotic protein Bax plays a critical role as
an executioner of mitochondrial outer membrane permeabilization
during apoptosis (33). Akt kinase
can directly prevent Bax translocation to mitochondria via
phosphorylation (34,35). Translocation of Bax to mitochondria
was evidently increased when U937 cells were treated with 100
µM resveratrol for 24 h (Fig.
6B). Such an effect of resveratrol was not observed in MCF-7
cells (Fig. 6B).
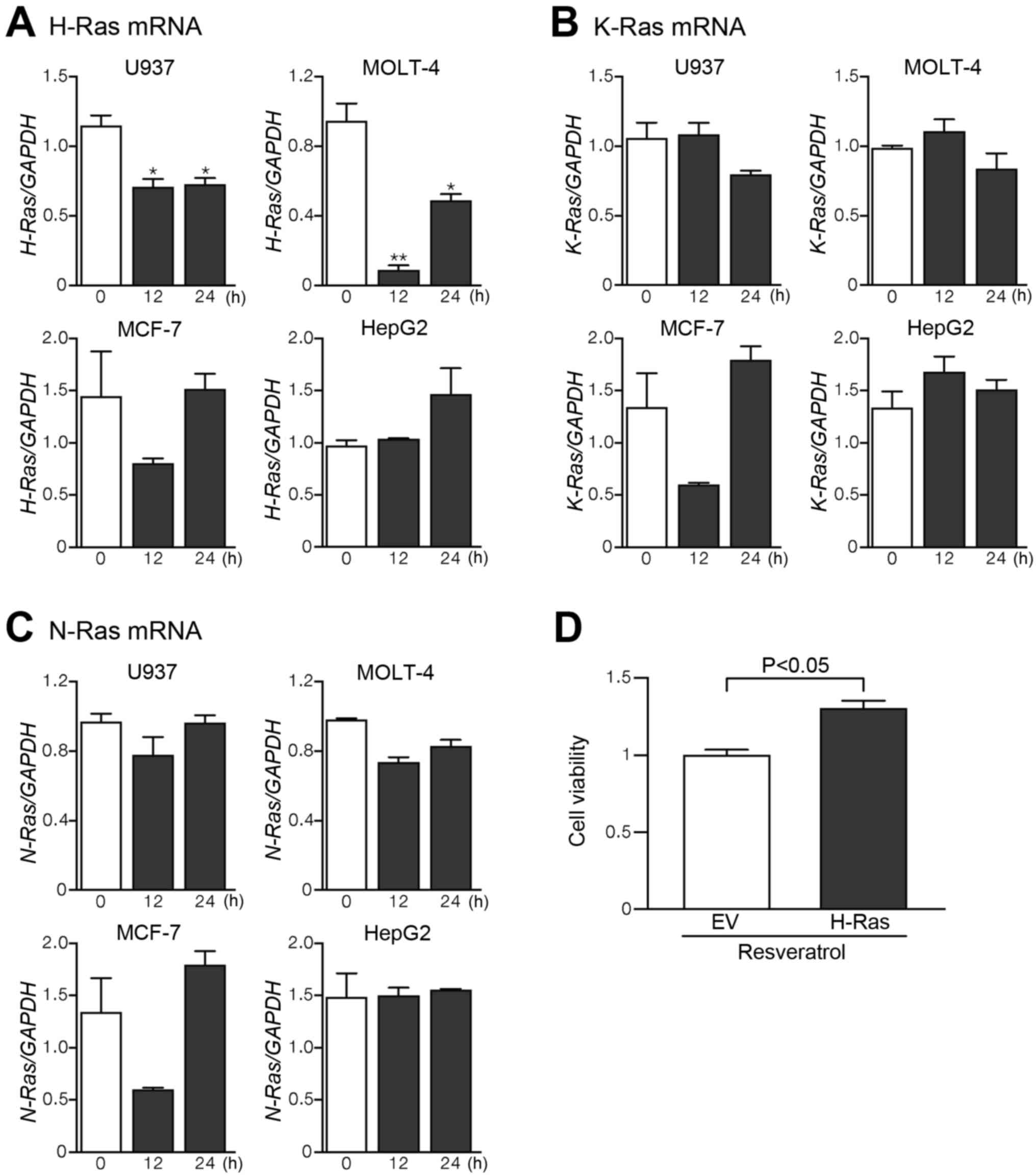
Akt is a direct downstream effector of
phosphatidylinositol 3-kinase (PI3K) (36,37),
and PI3K can be activated by Ras, a protein superfamily of small
GTPase (38). The three canonical
members of the Ras gene family (H-Ras, N-Ras, and K-ras) were
identified in humans (39). In
leukemic cells U937 and MOLT-4, treatment with 100 µM
resveratrol for 12-24 h resulted in a significant decrease in mRNA
expression levels of H-Ras (Fig.
7A). However, the mRNA levels of H-Ras were marginally affected
by resveratrol treatment in MCF-7 and HepG2 cells. Resveratrol had
no appreciable effect on the mRNA levels of K-Ras and N-Ras in
leukemic cells as well as MCF-7 and HepG2 cells (Fig. 7B and C). In order to ascertain the
impact of H-Ras on resveratrol-induced apoptotic death in leukemic
cells, whether overexpression of H-Ras can modify the inhibition by
resveratrol of cell viability was examined by MTT assay. The
anti-viability effect of 100 µM resveratrol was
significantly attenuated in H-Ras-overexpressed U937 cells
(Fig. 7D).
Discussion
Substantial evidence has shown that resveratrol, a
polyphenol that is present in a variety of plant species, can
prevent or slow the progression of a wide variety of diseases,
including cardiovascular disorders (40), and extend the lifespan of various
organisms from yeast to vertebrates (41). Resveratrol is currently being
documented as a potential cancer chemo-prevention agent (5,6). It
has gained much attention for its anticancer activities in
vitro, ex vivo, and in cancer models in vivo. In
this study, we used the MTT assay to assess the viability of
various human cancer cells treated with resveratrol. We noted great
differences in the anti-viability effect of resveratrol between
different types of human cancer cells. We found that, while the
inhibition of cell viability by resveratrol was marked in U937 and
MOLT-4 leukemia cells, it moderately inhibited cell viability in
MCF-7 breast cancer cells, HepG2 liver cancer cells, and A549 lung
cancer cells. Furthermore, resveratrol had a negligible effect on
cell viability in Caco-2, HCT116, and SW480 colon cancer cells.
Flow cytometric analysis of cancer cells by means of
Annexin V and PI showed that resveratrol increased late apoptosis
of leukemic cells, U937 and MOLT-4, whereas treatment of MCF-7 and
HepG2 with this natural stilbenoid resulted in an increase in early
apoptotic cell populations. Early stage apoptosis is represented by
disruption of the mitochondrial membrane potential, but cells
retain membrane integrity. As mitochondrial dysfunction is an early
event in the process of apoptosis, early apoptotic cells would
result from a declined mitochondrial transmembrane potential that
can be preceded by the release of cytochrome c from
mitochondria. On the other hand, late stages of apoptosis are
characterized by DNA fragmentation and loss of cell membrane
permeability. Thus, a hallmark of late apoptosis is extensive
genomic DNA fragmentation that generates a multitude of DNA
double-stranded breaks with accessible 3′-hydroxyl groups. TUNEL is
an established method for detecting DNA strand breaks produced by
DNA fragmentation that results from apoptotic signaling cascades.
Our TUNEL staining showed a high proportion of TUNEL-positive cells
in the two leukemic cells, U937 and MOLT-4, but not in MCF-7 breast
cancer cells. When agarose gel electrophoresis, alternative method
for detecting DNA fragmentation, was employed, U937 and MOLT-4
treated with resveratrol displayed a significant ladder formation
but MCF-7 showed a relatively clear band of intact DNA regardless
of whether resveratrol was given. We thus demonstrated that a large
number of cells in human leukemic cells, but not in MCF-7, when
treated with resveratrol, were characterized by a high incidence of
DNA fragmentation, suggesting that MCF-7 was resistant to DNA
fragmentation induced by resveratrol. These results suggest that
human leukemic cells are more sensitive to resveratrol in terms of
apoptotic cell death when compared with human solid tumor cells
such as breast cancer cells.
Resveratrol is well known to activate SIRT1 and AMPK
(26). However, the ability of
resveratrol to activate these targets is unlikely to contribute to
its anti-viability effect on human cancer cells. The potent SIRT1
activator SRT1720 did not mimic the effect of resveratrol on cancer
cell viability. Moreover, the anti-viability effect of resveratrol
was not impaired in human cancer cells treated with the SIRT1
inhibitor EX527 and the AMPK inhibitor compound C. A previous
report has shown that the SIRT1 inhibitor sirtinol serves as a
growth arrest inducer with reduced Ras/mitogen-activated protein
kinase signaling in human cancer cells (42).
The serine/threonine kinase Akt regulates multiple
biological processes including cell survival, proliferation,
growth, and glycogen metabolism, and aberrant regulation of these
processes are considered hallmarks of cancer (43). In this study, treatment with
resveratrol was found to lead to a transient but significant
reduction in Akt phosphorylation in U937 and MOLT-4 leukemic cells
without any change in the total amount of Akt. Akt inhibits a
conformational change in the pro-apoptotic Bax protein and its
translocation to mitochondria, thus preventing the disruption of
the mitochondrial inner membrane potential (34,35).
We showed that resveratrol evidently promoted translocation of Bax
to mitochondria in leukemic cells. This could result in allowing
permeabilization of the mitochondrial outer membrane
extraordinarily releasing cytochrome c and other proteins to
activate caspases and induce cell demolition. In MCF-7 breast
cancer cells, resveratrol did not reduce Akt phosphorylation and
was without increasing effect on Bax translocation to mitochondria
accordingly. This finding could account in part for less
effectiveness of resveratrol on apoptotic cell death in MCF-7 as
compared with leukemic cells.
Akt is a direct downstream effector of PI3K
(36,37), and PI3K can be activated by the
protein superfamily of small GTPase Ras (38). The three canonical members of the
Ras gene family (H-Ras, N-Ras, and K-ras) were identified in humans
more than a quarter century ago, and they encode four highly
related Ras protein isoforms (39). H-Ras preferentially activates the
PI3K/Akt pathway, while K-Ras activates the RAF/MEK/ERK pathway and
N-Ras may activate both pathways (44-46).
In leukemic cells, resveratrol treatment resulted in a significant
decrease in mRNA expression levels of H-Ras, whereas the mRNA
levels of H-Ras were marginally affected by resveratrol treatment
in breast and liver cancer cells. Resveratrol had no appreciable
effect on the mRNA levels of K-Ras and N-Ras in leukemic cells or
breast and liver cancer cells. These results suggest that the
resveratrol-induced reduction in Akt activity in leukemic cells
could be the result of the downregulation of H-Ras gene expression.
This can be supported by the finding that the inhibitory effect of
resveratrol on cell viability was significantly attenuated in U937
cells overexpressing H-Ras. We thus interpret our present results
to indicate that resveratrol can lead to apoptotic death in
leukemic cells through a mechanism that involves reduced Akt
activation resulting from the downregulation of Ras.
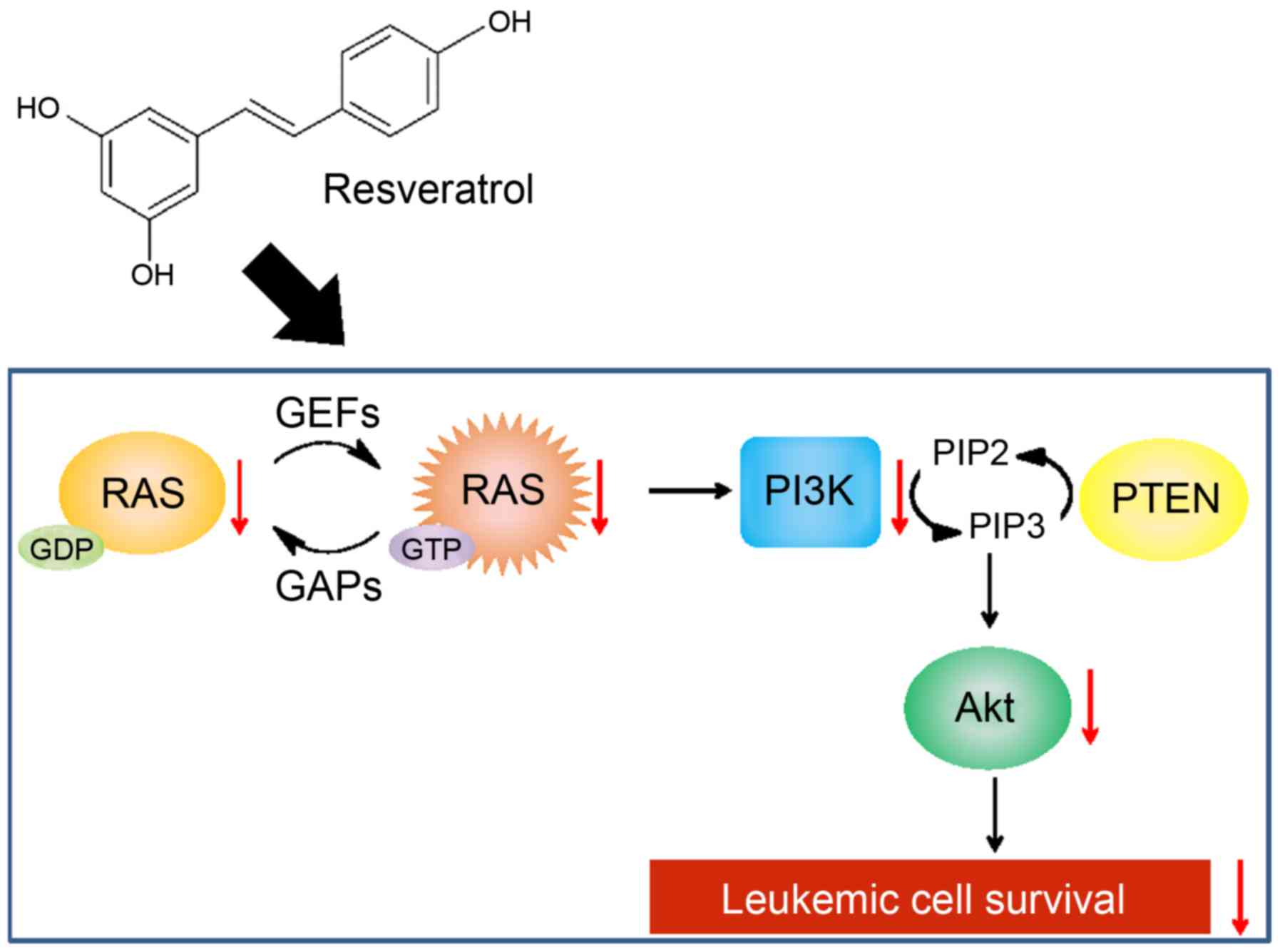
In conclusion, we demonstrated that resveratrol can
induce apoptosis in human leukemic cells to a greater extent than
in human solid tumor cells via reducing Akt activation due to the
downregulation of Ras (Fig. 8).
Resveratrol is not overtly toxic to animals when administered at
doses high enough to achieve pharmacological effects (3). Thus, this natural product that is
common constituent of human diet may offer greater safety, both
through its inherently lower toxicity and through allowing
reductions in harmful and unintended effects as compared with
synthetic drugs. Although one report suggested only weak potential
anti-leukemic activity of resveratrol in experiments on mice in
vivo implanted with a mouse myeloid leukemia cells (21), further study with leukemia
xenograft models using immunodeficient mice will be required to
verify in vivo anti-leukemic effects of resveratrol. Our
present results will likely aid in identifying that resveratrol may
have a potential benefit for leukemia prevention and may be a
useful adjunct to conventional chemotherapy for human leukemia.
Abbreviations:
|
AMPK
|
adenosine-monophosphate-activated
protein kinase
|
|
PI
|
propidium iodide
|
|
SIRT1
|
sirtuin 1
|
|
TdT
|
terminal deoxynucleotidyl
transferase
|
|
TUNEL
|
terminal deoxynucleotide
transferase-mediated dUTP nick end labeling
|
Acknowledgments
This study was supported in part by Joint Research
Project of the Institute of Medical Science, the University of
Tokyo (W.O.). We are thankful to Professor Takashi Kondo,
University of Toyama, for giving valuable suggestions and support
during this research.
References
|
1
|
Kopp P: Resveratrol, a phytoestrogen found
in red wine. A possible explanation for the conundrum of the
'French paradox'? Eur J Endocrinol. 138:619–620. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhat KPL, Kosmeder JW II and Pezzuto JM:
Biological effects of resveratrol. Antioxid Redox Signal.
3:1041–1064. 2001. View Article : Google Scholar
|
|
4
|
Virgili M and Contestabile A: Partial
neuroprotection of in vivo excitotoxic brain damage by chronic
administration of the red wine antioxidant agent, trans-resveratrol
in rats. Neurosci Lett. 281:123–126. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Athar M, Back JH, Tang X, Kim KH,
Kopelovich L, Bickers DR and Kim AL: Resveratrol: A review of
preclinical studies for human cancer prevention. Toxicol Appl
Pharmacol. 224:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carter LG, D'Orazio JA and Pearson KJ:
Resveratrol and cancer: Focus on in vivo evidence. Endocr Relat
Cancer. 21:R209–R225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clément MV, Hirpara JL, Chawdhury SH and
Pervaiz S: Chemopreventive agent resveratrol, a natural product
derived from grapes, triggers CD95 signaling-dependent apoptosis in
human tumor cells. Blood. 92:996–1002. 1998.PubMed/NCBI
|
|
8
|
Tessitore L, Davit A, Sarotto I and
Caderni G: Resveratrol depresses the growth of colorectal aberrant
crypt foci by affecting bax and p21(CIP) expression.
Carcinogenesis. 21:1619–1622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding XZ and Adrian TE: Resveratrol
inhibits proliferation and induces apoptosis in human pancreatic
cancer cells. Pancreas. 25:e71–e76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aziz MH, Nihal M, Fu VX, Jarrard DF and
Ahmad N: Resveratrol-caused apoptosis of human prostate carcinoma
LNCaP cells is mediated via modulation of phosphatidylinositol
3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther.
5:1335–1341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang HY, Shih A, Cao HJ, Davis FB, Davis
PJ and Lin HY: Resveratrol-induced cyclooxygenase-2 facilitates
p53-dependent apoptosis in human breast cancer cells. Mol Cancer
Ther. 5:2034–2042. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB,
Yang K, Shen HF and Xie LP: Resveratrol induces apoptosis and cell
cycle arrest of human T24 bladder cancer cells in vitro and
inhibits tumor growth in vivo. Cancer Sci. 101:488–493. 2010.
View Article : Google Scholar
|
|
13
|
Nakagawa H, Kiyozuka Y, Uemura Y, Senzaki
H, Shikata N, Hioki K and Tsubura A: Resveratrol inhibits human
breast cancer cell growth and may mitigate the effect of linoleic
acid, a potent breast cancer cell stimulator. J Cancer Res Clin
Oncol. 127:258–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joe AK, Liu H, Suzui M, Vural ME, Xiao D
and Weinstein IB: Resveratrol induces growth inhibition, S-phase
arrest, apoptosis, and changes in biomarker expression in several
human cancer cell lines. Clin Cancer Res. 8:893–903.
2002.PubMed/NCBI
|
|
15
|
Shih A, Davis FB, Lin HY and Davis PJ:
Resveratrol induces apoptosis in thyroid cancer cell lines via a
MAPK- and p53-dependent mechanism. J Clin Endocrinol Metab.
87:1223–1232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roman V, Billard C, Kern C, Ferry-Dumazet
H, Izard JC, Mohammad R, Mossalayi DM and Kolb JP: Analysis of
resveratrol-induced apoptosis in human B-cell chronic leukaemia. Br
J Haematol. 117:842–851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang YC, Tsai SH, Chen L, Lin-Shiau SY
and Lin JK: Resveratrol-induced G2 arrest through the inhibition of
CDK7 and p34CDC2 kinases in colon carcinoma HT29 cells. Biochem
Pharmacol. 65:1053–1060. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao PC, Ng LT, Lin LT, Richardson CD,
Wang GH and Lin CC: Resveratrol arrests cell cycle and induces
apoptosis in human hepatocellular carcinoma Huh-7 cells. J Med
Food. 13:1415–1423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JW, Choi YJ, Suh SI, Baek WK, Suh MH,
Jin IN, Min DS, Woo JH, Chang JS, Passaniti A, et al: Bcl-2
overexpression attenuates resveratrol-induced apoptosis in U937
cells by inhibition of caspase-3 activity. Carcinogenesis.
22:1633–1639. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wolter F, Akoglu B, Clausnitzer A and
Stein J: Downregulation of the cyclin D1/Cdk4 complex occurs during
resveratrol-induced cell cycle arrest in colon cancer cell lines. J
Nutr. 131:2197–2203. 2001.PubMed/NCBI
|
|
21
|
Gao X, Xu YX, Divine G, Janakiraman N,
Chapman RA and Gautam SC: Disparate in vitro and in vivo
antileukemic effects of resveratrol, a natural polyphenolic
compound found in grapes. J Nutr. 132:2076–2081. 2002.PubMed/NCBI
|
|
22
|
Mouria M, Gukovskaya AS, Jung Y, Buechler
P, Hines OJ, Reber HA and Pandol SJ: Food-derived polyphenols
inhibit pancreatic cancer growth through mitochondrial cytochrome c
release and apoptosis. Int J Cancer. 98:761–769. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mahyar-Roemer M, Katsen A, Mestres P and
Roemer K: Resveratrol induces colon tumor cell apoptosis
independently of p53 and precede by epithelial differentiation,
mitochondrial proliferation and membrane potential collapse. Int J
Cancer. 94:615–622. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang C, Ma WY, Goranson A and Dong Z:
Resveratrol suppresses cell transformation and induces apoptosis
through a p53-dependent pathway. Carcinogenesis. 20:237–242. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuo PL, Chiang LC and Lin CC: Resveratrol-
induced apoptosis is mediated by p53-dependent pathway in Hep G2
cells. Life Sci. 72:23–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baur JA: Biochemical effects of SIRT1
activators. Biochim Biophys Acta. 1804:1626–1634. 2010. View Article : Google Scholar :
|
|
27
|
Sakata K, Kondo T, Mizuno N, Shoji M,
Yasui H, Yamamori T, Inanami O, Yokoo H, Yoshimura N and Hattori Y:
Roles of ROS and PKC-βII in ionizing radiation-induced eNOS
activation in human vascular endothelial cells. Vascul Pharmacol.
70:55–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Inoue S, Arai N, Tomihara K, Takashina M,
Hattori Y and Noguchi M: Extracellular Ca(2+)-dependent enhancement
of cytocidal potency of zoledronic acid in human oral cancer cells.
Eur J Pharmacol. 761:44–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taguchi K, Sakata K, Ohashi W, Imaizumi T,
Imura J and Hattori Y: Tonic inhibition by G protein-coupled
receptor kinase 2 of Akt/endothelial nitric-oxide synthase
signaling in human vascular endothelial cells under conditions of
hyperglycemia with high insulin levels. J Pharmacol Exp Ther.
349:199–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tomita K, Takashina M, Mizuno N, Sakata K,
Hattori K, Imura J, Ohashi W and Hattori Y: Cardiac fibroblasts:
Contributory role in septic cardiac dysfunction. J Surg Res.
193:874–887. 2015. View Article : Google Scholar
|
|
31
|
Milne JC, Lambert PD, Schenk S, Carney DP,
Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al: Small
molecule activators of SIRT1 as therapeutics for the treatment of
type 2 diabetes. Nature. 450:712–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cardaci S, Filomeni G and Ciriolo MR:
Redox implications of AMPK-mediated signal transduction beyond
energetic clues. J Cell Sci. 125:2115–2125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gillies LA and Kuwana T: Apoptosis
regulation at the mitochondrial outer membrane. J Cell Biochem.
115:632–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsuruta F, Masuyama N and Gotoh Y: The
phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax
translocation to mitochondria. J Biol Chem. 277:14040–14047. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gardai SJ, Hildeman DA, Frankel SK,
Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL and
Henson PM: Phosphorylation of Bax Ser184 by Akt regulates its
activity and apoptosis in neutrophils. J Biol Chem.
279:21085–21095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Franke TF, Yang SI, Chan TO, Datta K,
Kazlauskas A, Morrison DK, Kaplan DR and Tsichlis PN: The protein
kinase encoded by the Akt proto-oncogene is a target of the
PDGF-activated phosphatidylinositol 3-kinase. Cell. 81:727–736.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Franke TF, Kaplan DR, Cantley LC and Toker
A: Direct regulation of the Akt proto-oncogene product by
phosphati-dylinositol-3,4-bisphosphate. Science. 275:665–668. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Castellano E and Downward J: RAS
interaction with PI3K: More than just another effector pathway.
Genes Cancer. 2:261–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Castellano E and Santos E: Functional
specificity of ras isoforms: So similar but so different. Genes
Cancer. 2:216–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bradamante S, Barenghi L and Villa A:
Cardiovascular protective effects of resveratrol. Cardiovasc Drug
Rev. 22:169–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ota H, Tokunaga E, Chang K, Hikasa M,
Iijima K, Eto M, Kozaki K, Akishita M, Ouchi Y and Kaneki M: Sirt1
inhibitor, Sirtinol, induces senescence-like growth arrest with
attenuated Ras-MAPK signaling in human cancer cells. Oncogene.
25:176–185. 2006.
|
|
43
|
Testa JR and Tsichlis PN: AKT signaling in
normal and malignant cells. Oncogene. 24:7391–7393. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yan J, Roy S, Apolloni A, Lane A and
Hancock JF: Ras isoforms vary in their ability to activate Raf-1
and phosphoinositide 3-kinase. J Biol Chem. 273:24052–24056. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Voice JK, Klemke RL, Le A and Jackson JH:
Four human ras homologs differ in their abilities to activate
Raf-1, induce transformation, and stimulate cell motility. J Biol
Chem. 274:17164–17170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eisfeld AK, Schwind S, Hoag KW, Walker CJ,
Liyanarachchi S, Patel R, Huang X, Markowitz J, Duan W, Otterson
GA, et al: NRAS isoforms differentially affect downstream pathways,
cell growth, and cell transformation. Proc Natl Acad Sci USA.
111:4179–4184. 2014. View Article : Google Scholar : PubMed/NCBI
|