Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of cancer-related mortality among
females worldwide (1). Currently,
standard therapies for breast cancer patients include surgery,
radiotherapy and chemotherapy, which plays an irreplaceable role
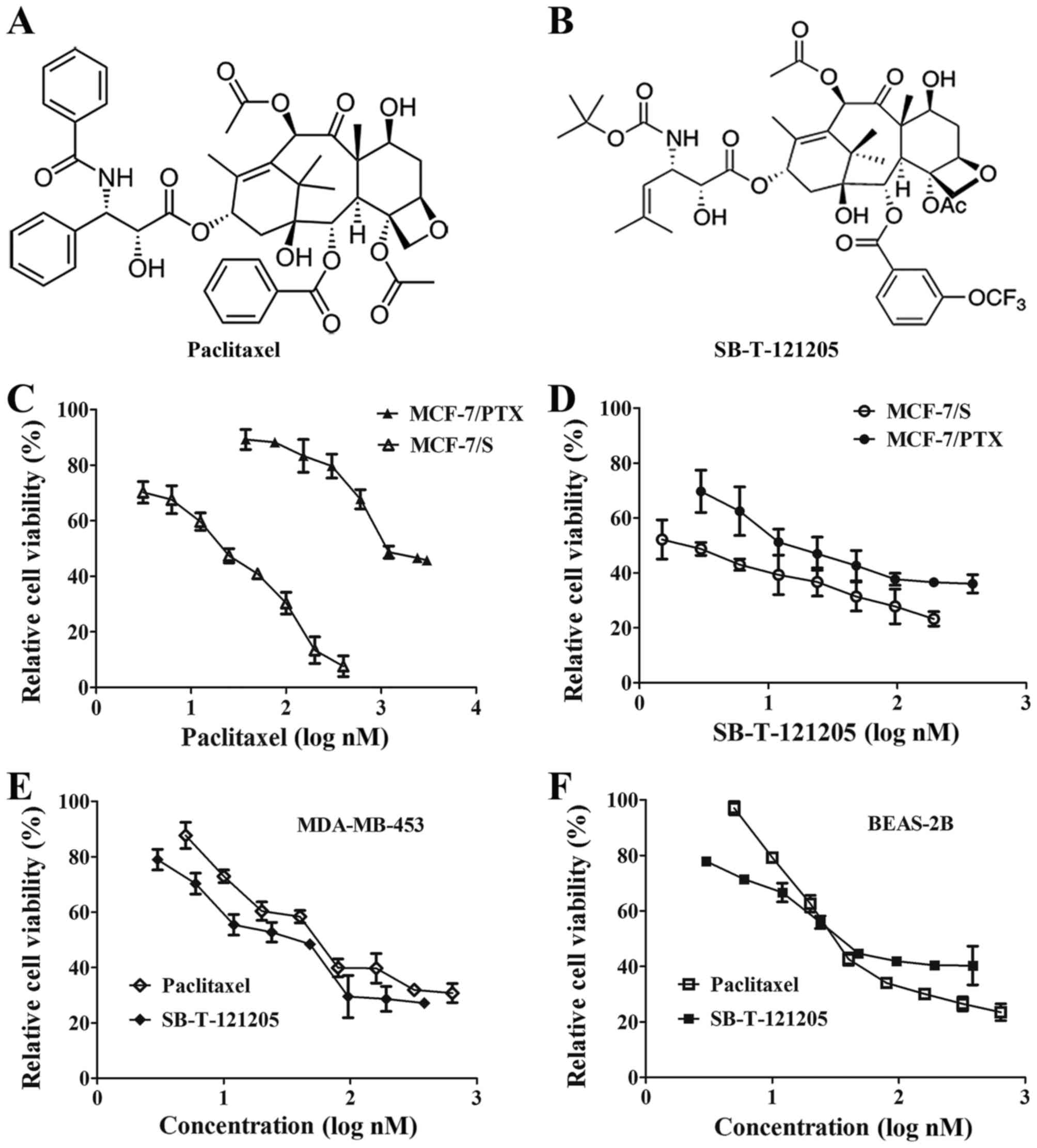
(2). Paclitaxel (PTX; Fig. 1A), a first-line therapeutic agent
used clinically to treat breast cancer, exerts its antitumor
activity by promoting the polymerization of tubulin and stabilizing
the resulting micro-tubules, causing cell cycle arrest at the G2/M
phase that leads to apoptotic death of cancer cells (3). However, paclitaxel resistance often
occurs after a period of treatment, causing a serious problem in
chemotherapy (4). Accumulated
studies manifest that there are several major mechanisms for
paclitaxel resistance, including point mutations in β-tubulin,
alterations in the expression of β-tubulin isotypes, particularly
the β-III tubulin isoform, overexpression of ATP-binding cassette
transporters and suppression of apoptosis. More importantly, the
drug-resistance to chemotherapy may eventually result in mortality
of cancer patients due to tumor metastasis (5,6). It
is therefore highly desirable to develop novel agents with minimum
side-effects and improved activity against various tumors,
especially against drug-resistant human breast cancer.
In the last decades, a number of taxanes have been
designed and synthesized based on the structure of the
first-generation taxanes, paclitaxel and docetaxel. Many of these
new taxanes exhibited strong antitumor activity against
paclitaxel-resistant tumor xenografts. For instance, Roh et
al (7) found two of the
3′-N-acyl-paclitaxel analogues, in which the phenyl group of
3′-N-benzoyl was replaced with 1-cyclopentenyl (1k) and
1-cyclohexenyl (1n), displayed seven times higher cytotoxicity than
paclitaxel against doxorubicin-resistant breast cancer cells. It
was also reported that another taxane, Lx2-32c, was active against
paclitaxel-resistant breast cancer cells (MX-1/T) through intrinsic
apoptosis signaling pathway, and exhibited efficacy against its
tumor xenografts in nude mice, which suggests the potential of
Lx2-32c to be a promising drug candidate (8). TPI-287, a new microtubule stabilizer,
showed cytotoxicity similar to that of paclitaxel in breast cancer
cells and efficacy against primary breast tumor xenografts in an
animal model. Also, it was found to significantly decrease
metastatic colonization of breast cancer in the brain (9). Moreover, Ojima et al (10) developed a series of novel
second-generation taxanes with systematic modifications at the C2,
C10, C3′ and C3′N positions. For example, among these
new-generation taxanes synthesized and assayed, SB-T-1214 and
SB-T-121303, exhibited significantly lower IC50 values,
9.00±0.77 nM and 3.65±0.21 nM, respectively for
paclitaxel-resistant ovarian cancer cells than paclitaxel
(532.95±3.18 nM). Such results clearly warrant further exploitation
of next-generation taxanes with superior potency, efficacy and
pharmacological properties against breast cancer.
Transgelin 2 is reported to be implicated in
tumorigenesis, boosting tumor progression and promoting metastases
(11). Additionally, abnormal
expression of transgelin 2 was discovered in lung, gastric and
colorectal cancer (12–14). We previously reported that
transgelin 2 expression was extremely high in paclitaxel-resistant
human breast cancer cells (MCF-7/PTX) compared to breast cancer
drug-sensitive cells by proteomics analysis (15). Knockdown of transgelin 2 via small
interfering RNA sensitized MCF-7/PTX cells to paclitaxel, and
suppressed their migration/invasion abilities, suggesting that
transgelin 2 might be a new biomarker for breast cancer (16). On the other hand, aberrant
activation of the phosphatidylinositol 3 kinase/serine-threonine
kinase (PI3K/Akt) pathway contributes to chemo-resistance, tumor
metastasis and poor prognosis (17,18).
Notably, we reported that the PI3K/Akt pathway was activated in
MCF-7/PTX cells and the TAGLN2-knockdown inhibited the
PI3K/Akt pathway, suggesting that the PI3K/Akt pathway would be
critical to breast cancer progression (19).
We confirmed that the MCF-7/PTX cells, developed by
our laboratory for the assay used, were highly resistant to
paclitaxel and exhibited strong migration/invasion capacities
(20,21). In the present study, eight novel
next-generation taxanes were screened by MTT assay. Among these
taxanes, SB-T-121205 (Fig. 1B) was
found to be highly potent against the paclitaxel-resistant
MCF-7/PTX cells. Subsequently, the effect of SB-T-121205 on cell
apoptosis, epithelial-mesenchymal transition (EMT) property,
migration and invasion was assessed in MCF-7/PTX cells, along with
the underlying molecular mechanisms. Our data indicated that
SB-T-121205 exerted its high potency against MCF-7/PTX cells
through activation of the transgelin 2 and PI3K/Akt pathway, which
suggests that SB-T-121205 would serve as an efficacious drug
candidate for breast cancer treatment.
Materials and methods
Chemicals and antibodies
In the present study, the anticancer activity of
SB-T-121205 was assessed using paclitaxel as the positive control.
Paclitaxel was purchased from Nanjing Sike Pharmaceutical, Co.,
Ltd. (Nanjing, China). All taxanes were kindly provided by Dr
Changwei Wang, the laboratory of Professor Iwao Ojima at Stony
Brook University. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) was obtained from Sigma-Aldrich (St.
Louis, MO, USA). Annexin-V-FLUOS staining kit was obtained from
Invitrogen (Waltham, MA, USA). The primary rabbit monoclonal
antibodies against N-cadherin, and GSK-3β were acquired from Abcam
(Cambridge, MA, USA). The primary rabbit monoclonal antibodies
against E-cadherin, phosphatase and tensin homologue deleted on
chromosome ten (PTEN), Akt, phospho-Akt (p-Akt), p-GSK-3β and Snail
were purchased from Cell Signaling Technology (Danvers, MA, USA).
The rabbit polyclonal antibodies against caspase-3, caspase-9 and
poly(ADP-ribose) polymerase (PARP) were also obtained from Cell
Signaling Technology. The rabbit polyclonal antibodies against
vimentin and transgelin 2 were from GeneTex, Inc. (Irvine, CA,
USA). The rabbit monoclonal antibodies against Bcl-2 and Bax were
acquired from Epitomics (Burlingame, CA, USA). The rabbit
polyclonal anti-β-actin antibody was obtained from Beijing Bo Aosen
Biotechnology Co., Ltd. (Beijing, China).
Horseradish-peroxidase-conjugated goat anti-rabbit IgG was from CW
Biotech (Beijing, China).
Cell lines and cell culture
The human breast cancer cell lines, MCF-7/S and
MDA-MB-453, and non-tumorigenic human bronchial epithelial cell
line (BEAS-2B) were obtained from the Cell Bank of Shanghai,
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences. MCF-7/PTX cell line was successfully established as
previously described (16) with
the concentration of 30 nM paclitaxel. The cells were grown in
RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA,
USA) and 1% penicillin (Harbin Pharmaceutical Group, Co., Ltd.,
Harbin, China)/streptomycin (North China Pharmaceutical Group, Co.,
Ltd., Shijiazhuang, China) at 37°C in a humidified atmosphere of 5%
CO2. Cells in exponential phase growth were observed
under inverted light microscope (Olympus Corp., Tokyo, Japan).
MTT assay
Cells at 5×105/ml density were seeded
into 96-well plates (Corning, Inc., Corning, NY, USA) with 100
µl medium for the duration indicated. After 72 h, 20
µl of MTT (5 mg/ml) was added into each well and incubated
at 37°C for 4 h. Then, 150 µl of dimethyl sulfoxide (DMSO)
was added into each well for dissolving the formazan for 15 min.
The absorbance was tested at 490 nm on a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). The 50% growth inhibitory
concentration (IC50) of drug was calculated to evaluate
the drug sensitivity. Each experiment was repeated three times.
Flow cytometry assay
For cell cycle assay, cells were exposed to
paclitaxel (600 nM) or SB-T-121205 (10 or 20 nM) for 48 h and then
harvested. After three washes with cold phosphate-buffered saline
(PBS), cells were fixed with 70% cold ethanol at 4°C overnight. The
next day, cells were washed with cold PBS and suspended in a 500
µl staining solution of propidium iodide (PI; Sigma-Aldric)
staining solution at 37°C for 30 min without light. The samples
were tested using FACSCanto™ II flow cytometry (Becton Dickinson,
Franklin Lakes, NJ, USA).
For cell apoptosis analysis, cells with different
treatments after 48 h were collected, washed and were resuspended
with cold PBS. Then, the cells were double stained with Annexin
V-FITC and PI at 37°C for 20 min from light with an Annexin-V-FLUOS
staining kit in accordance with the manufacturer's instructions.
The stained cells were analyzed using FACSCanto™ II flow cytometry.
Annexin V+/PI− were regarded as early
apoptotic cells and Annexin V+/PI+ were late
apoptotic cells. Experiments were repeated in triplicate,
independently.
Western blot assay
Cells with different treatments were lysed in RIPA
buffer containing protease inhibitor (Roche, Basel, Switzerland) on
ice. Then, equal amount of protein lysates were electrophoretically
separated by 10% sodium dodecyl sulfate polyacrylamide
gelelectrophoresis (SDS-PAGE; Beyotime Institute of Biotechnology,
Beijing, China) and transferred to polyvinylidene fluoride (PVDF)
membranes (Millipore, Billerica, MA, USA). After blocking with 5%
non-fat dried milk for 2 h, the membranes were incubated with
diluted antibodies N-cadherin (1:1,000, ab76011), E-cadherin
(1:1,000, #3195), vimentin (1:500, GTX100619), PTEN (1:1,000,
#9188), Akt (1:1,000, #4691), p-Akt (1:800, #4060), GSK-3β
(1:1,000, ab32391), p-GSK-3β (1:1,000, #9322), Bcl-2 (1:2,500,
#1017-1), Bax (1:2,500, #1063-1), caspase-3 (1:800, #9662),
caspase-9 (1:800, #9502), PARP (1:800, #9542), Snail (1:1,000,
#3879), transgelin 2 (1:2,000, GTX115082) and β-actin (1:800,
bs-0061R) overnight at 4°C. After incubation with a horseradish
peroxidase-conjugated secondary antibody (1:25,000, cat. no.
CW0103), for 2 h at 37°C, the protein bands were detected using the
SuperSignal West Pico kit (Thermo Fisher Scientific). All western
blot experiments were repeated at least three times.
Mammosphere formation assay
Mammosphere culture was carried out in a serum-free
DMEM/F-12 (Gibco) supplemented with 2% B27 (Gibco), 20 µg/l
human epidermal growth factor (PeproTech, Rocky Hill, NJ, USA), 10
µg/l human basic fibroblast growth factor (PeproTech), and 5
mg/l insulin (Jiangsu Wanbang Biochemical Pharmaceutical, Co.,
Ltd., Xuzhou, China). Single cells prepared from mechanical and
enzymatic dissociation were plated in 6-well ultralow attachment
plates (Corning) at 1×104/ml density in culture. Single
cell status was confirmed under microscope. After 14 days, the
number of mammospheres was counted under an inverted light
microscope. Experiments were repeated in triplicate,
independently.
Wound healing scratch assay
Cells (5×105/ml) were seeded in 6-well
plate until confluent. Cells were serum-starved overnight and an
artificial scratch wound was created. The cells were then
maintained in serum-free culture at 37°C in a humidified atmosphere
of 5% CO2. Migration photos were captured at 0, 24 and
48 h after scratching. Experiments were repeated in triplicate
independently. Percent wound closure was calculated using the
following equation: percent wound closure (%) =
[1−(Lt/L0)] × 100%.
Transwell invasion assay
The invasiveness of cells was evaluated by a Boyden
chamber method. The polycarbonate filters (8 µm pore size;
Corning) were coated with Matrigel Matrix (BD Biosciences, San
Jose, CA, USA) and incubated at 37°C for 5 h. Next,
5×105 cells suspended in 200 µl serum-free
RPMI-1640 were added into the upper chamber, while 800 µl of
complete media was added to the lower chamber. After 48 h, cells
migrated through Matrigel and adhered onto the lower chamber was
fixed in 4% paraformaldehyde for 30 min, stained with 0.1% crystal
violet (Beyotime Institute of Biotechnology) and counted by
fluorescent microscopy (Olympus). Each invasion assay was repeated
in three independent experiments.
Statistical analysis
Statistical analysis was performed using one-way
ANOVA. All values were expressed as mean ± standard deviation (SD)
from triplicate experiments performed in a parallel manner.
P<0.05 were considered statistically significant.
Results
The intrinsic cytotoxicity of SB-T-121205
on cancer and normal cells
The intrinsic cytotoxicity of the eight novel
taxanes against MCF-7/PTX cells were measured by MTT assay.
Treating cells with these taxanes for 72 h significantly inhibited
cell growth. Among these taxanes examined, SB-T-121205 showed the
highest inhibition. We therefore, chose SB-T-121205 for further
study. After 72 h of exposure, SB-T-121205 obviously restrained
growth of MCF-7/PTX cells with an IC50 value of
19.01±2.03 nM, which was two orders of magnitude lower than
paclitaxel (2290.87±125.18 nM) (16) (Table
I and Fig. 1C and D). The
molecular formula of SB-T-121205 is
C44H56F3NO16 with
molecular weight 911.91. Moreover, the effect of SB-T-121205 on the
proliferation of parental MCF-7/S cells was examined and the result
showed SB-T-121205 inhibited the cell growth with the
IC50 value of 2.14±0.32 nM, which was ~10 times lower
than that of paclitaxel (20.0±0.9 nM) (16) (Fig. 1C
and D).
 | Table IThe effect of paclitaxel and
new-generation taxanes on cell viability in MCF-7/PTX
cells.a |
Table I
The effect of paclitaxel and
new-generation taxanes on cell viability in MCF-7/PTX
cells.a
| Taxane | IC50
(nM) | Taxane | IC50
(nM) |
|---|
| SB-T-1214 | 80.50±7.62 | SB-T-121303 | 21.67±2.25 |
| SB-T-101141 | 66.66±5.59 | SB-T-12301 | 54.59±4.61 |
| SB-T-121205 | 19.01±2.03 | SB-T-121405 | 34.90±2.97 |
| SB-T-121605 | 31.43±2.84 | SB-T-1230105 | 119.05±9.68 |
| Paclitaxel | 2290.87±125.18 | | |
We examined the cell growth inhibitory effect of
SB-T-121205 on MDA-MB-453 cells. SB-T-121205 was found to have
slightly lower IC50 value (38.67±3.58 nM) than
paclitaxel (IC50 54.62±3.28 nM) (Fig. 1E). We also examined the
cytotoxicity of paclitaxel and SB-T-121205 against non-tumorigenic
BEAS-2B human bronchial epithelial cells. Treating BEAS-2B cells
with different concentration of paclitaxel or SB-T-121205 revealed
that BEAS-2B cells are slightly less sensitive to SB-T-121205
(IC50 59.80±1.89 nM) than paclitaxel (IC50
54.57±2.10 nM) (Fig. 1F). Thus,
for the treatment of MCF7/S and MCF7/PTX and MDA-MB-453 cancer
cells, SB-T-121205 has much wider therapeutic index than
paclitaxel.
SB-T-121205 induces apoptosis and G2/M
arrest in MCF-7/PTX cells
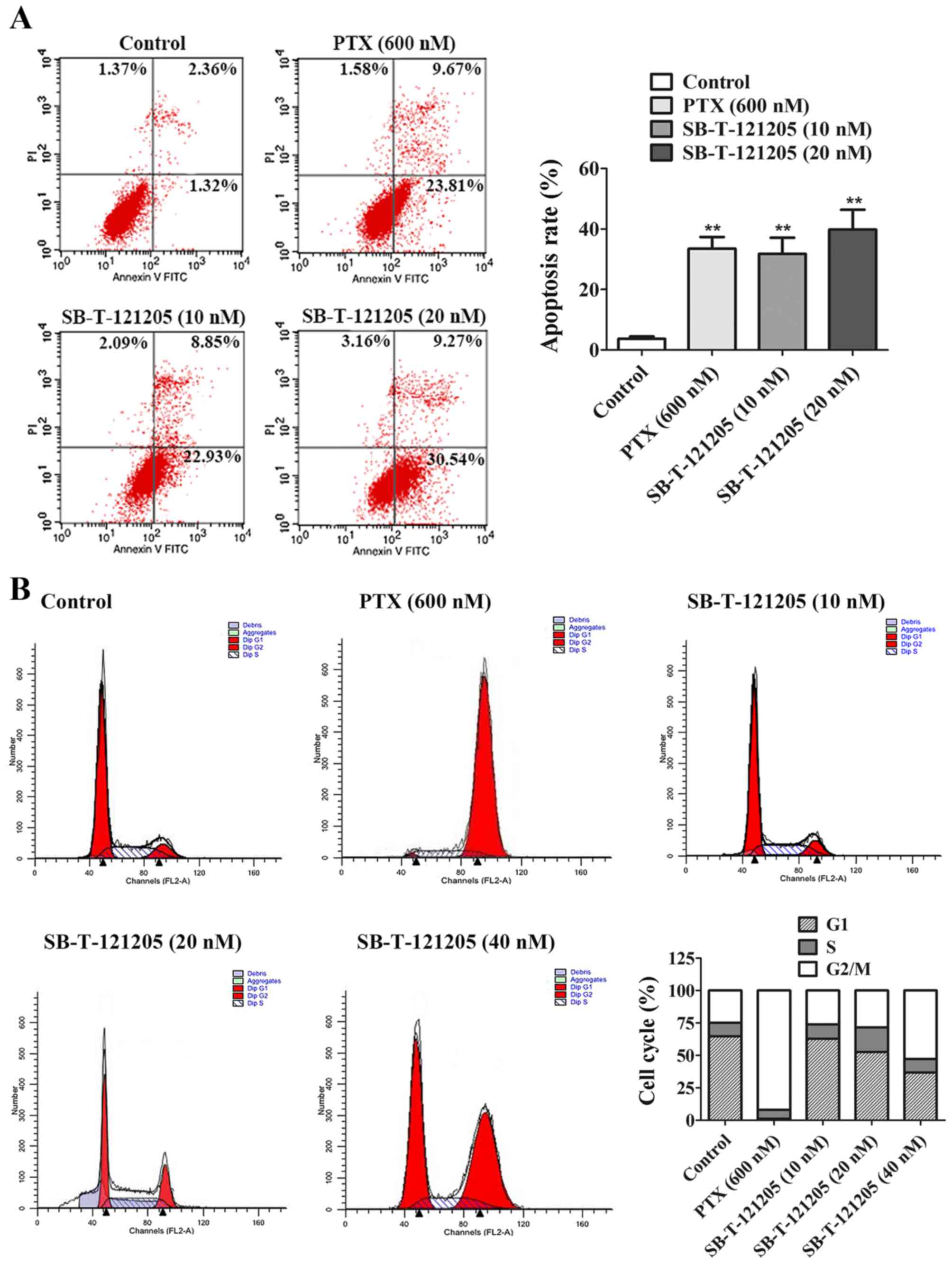
To confirm whether SB-T-121205 triggered apoptosis
of MCF-7/PTX cells, we carried out Annexin V and PI double staining
followed by flow cytometric analysis. MCF-7/PTX cells were
incubated with paclitaxel alone or SB-T-121205 alone for 48 h. As
shown in Fig 2A, paclitaxel (600
nM) and SB-T-121205 (10 or 20 nM) indeed increased cell apoptosis
rates that reached 33.48, 31.78 and 39.81%, respectively (Fig. 2A). Since the concentration used for
paclitaxel is 30–60 times higher than that of SB-T-121205,
SB-T-121205 is far better than paclitaxel in promoting cell
apoptosis. In brief, it is confirmed that SB-T-121205 has the
ability to induce apoptosis in MCF-7/PTX cells.
To further investigate the mechanisms that
SB-T-121205 inhibits the cancer cell growth, the MCF-7/PTX cells
were exposed to various concentrations of SB-T-121205 for 48 h, and
then cell cycle analysis was performed. Compared with the control
group, paclitaxel (600 nM) markedly elevated the number of cells in
G2/M phase in MCF-7/PTX cells and the proportion of MCF-7/PTX cells
in G2/M phase increased from 24.84 to 92.06%. SB-T-121205 at low
concentrations (10, 20 and 40 nM) also increased the percentage of
cells in G2/M phase in a dose-dependent manner from 26.23% (10 nM)
to 52.77% (40 nM) (Fig. 2B). The
results confirmed that SB-T-121205 arrests the mitosis of MCF-7/PTX
cells at the G2/M phase of the cell cycle in a manner similar to
other known taxanes.
SB-T-121205 suppresses the EMT property
of MCF-7/PTX cells
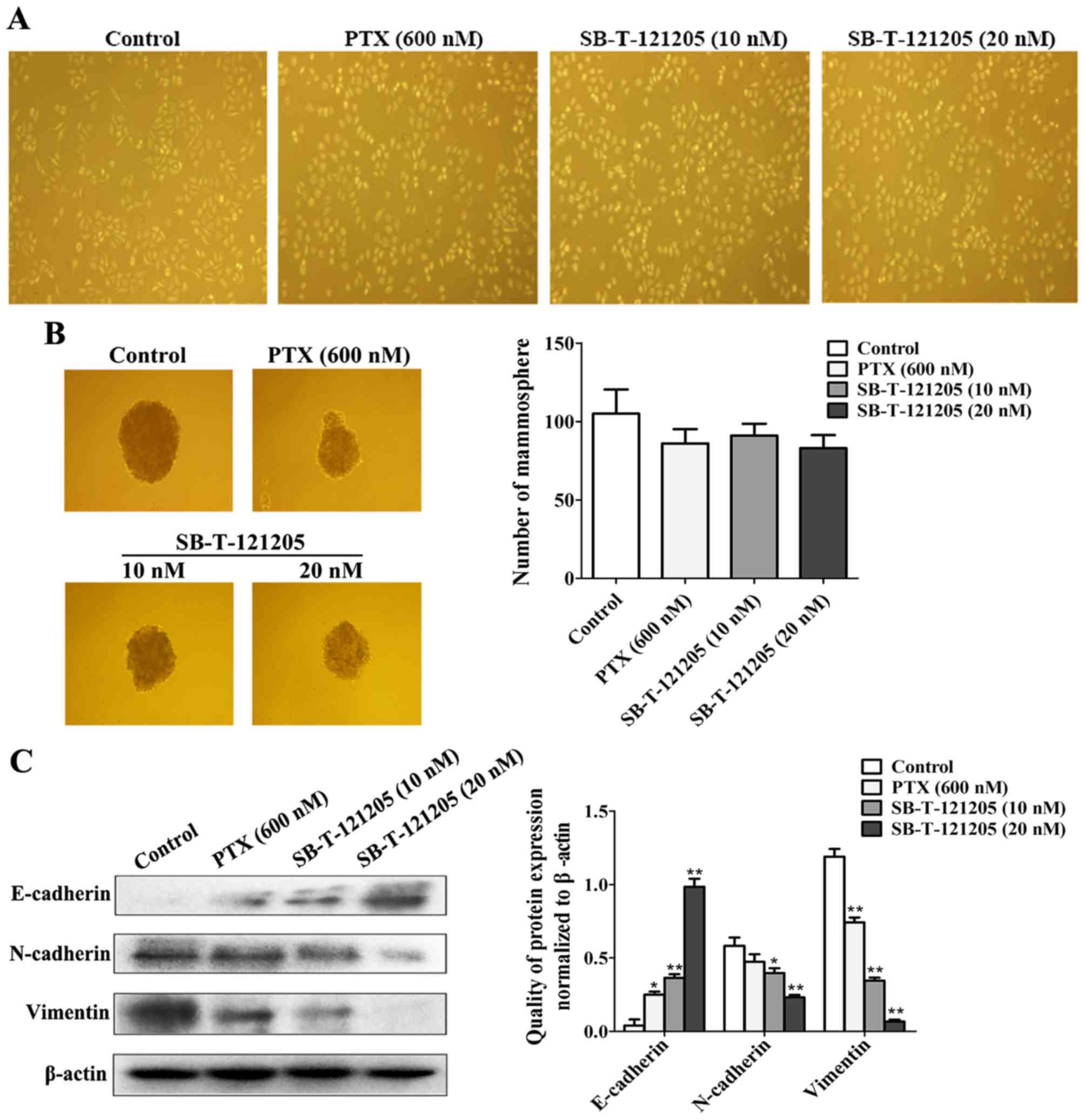
It is well known that EMT is a critical event in the
development of cancers (22). To
further examine whether SB-T-121205 could alter the EMT property of
MCF-7/PTX cells, mammosphere formation and western blot assays were
performed. As Fig. 3A shows,
SB-T-121205 exposure triggered morphological changes in the
MCF-7/PTX cells from elongated shape to cobblestone shape (Fig. 3A), which is a characteristic EMT
morphology. After treated with SB-T-121205, the mammosphere forming
ability of MCF-7/PTX cells was decreased (Fig. 3B). In addition, a low concentration
(20 nM) of SB-T-121205 distinctly increased the expression of
epithelial marker E-cadherin, whereas the levels of mesenchymal
markers N-cadherin and vimentin were reduced (Fig. 3C). However, the effect of high
concentration (600 nM) of paclitaxel was inferior to that of the
low concentration of SB-T-121205. Thus, SB-T-121205 possesses much
higher potency than paclitaxel for suppression of EMT in MCF-7/PTX
cells.
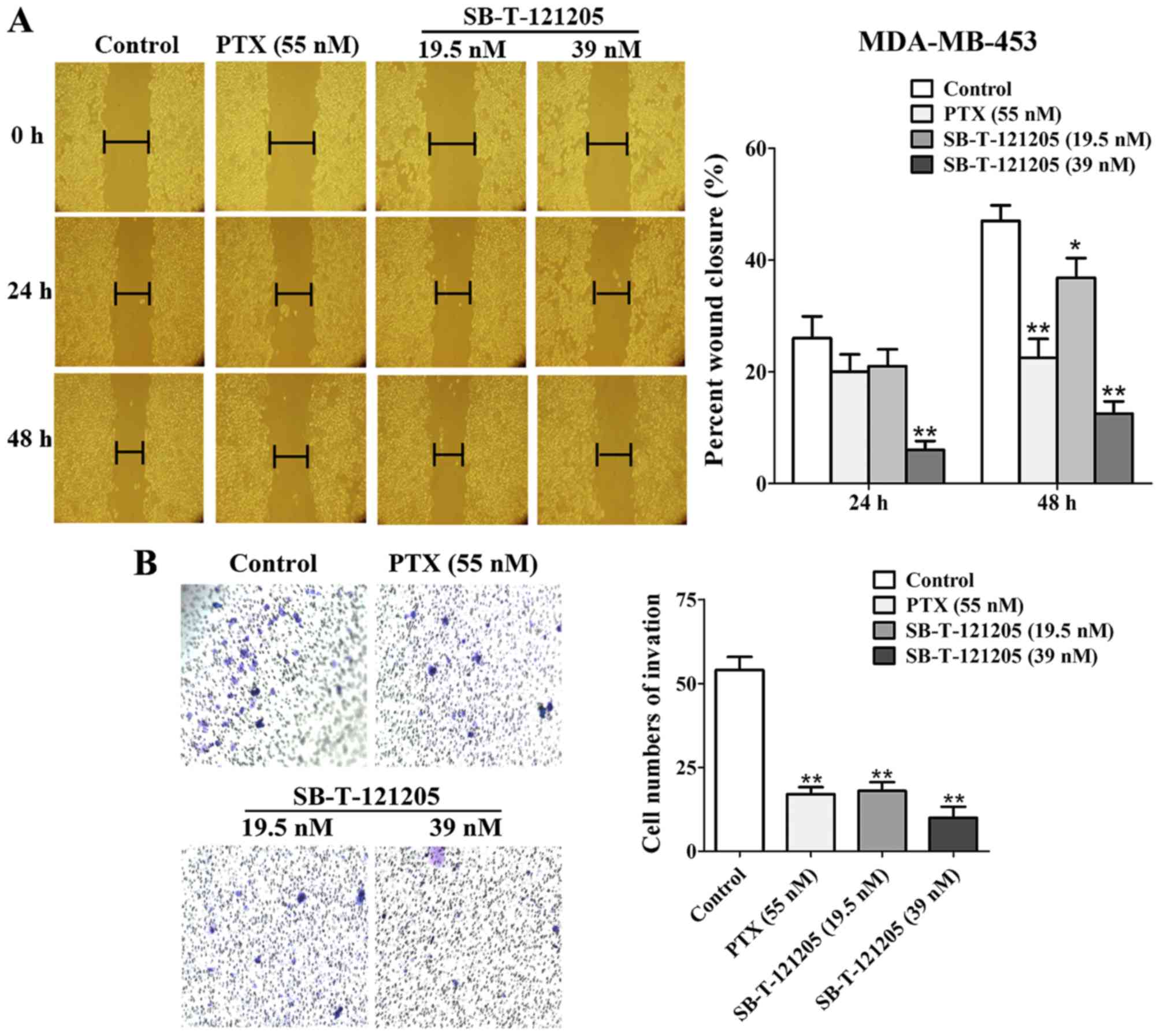
SB-T-121205 inhibits migration and
invasion in MCF-7/PTX and MDA-MB-453 cells
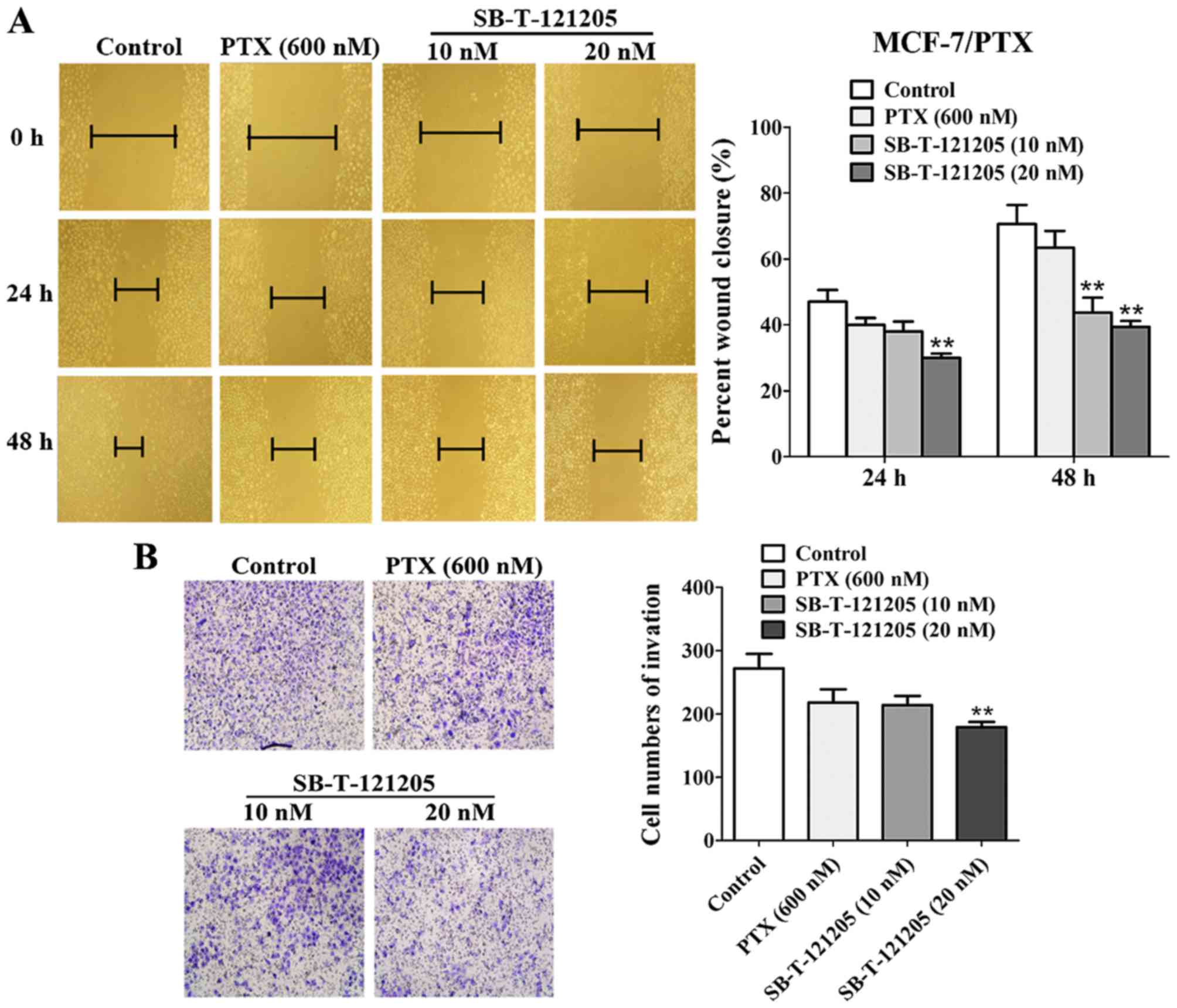
Tumor migration and invasion are major obstacles for
successful chemotherapy (23).
Accordingly, the effects of SB-T-121205 on the migration and
invasion of MCF-7/PTX and MDA-MB-453 cells were evaluated by wound
healing scratch and Transwell invasion methods, respectively.
MCF-7/PTX cells treated with SB-T-121205 displayed decreased
migratory abilities at 24 and 48 h (Fig. 4A). The percent wound closure of
four groups at 48 h were 70.59±5.80% (control), 63.43±5.13%
(paclitaxel, 600 nM), 43.75±4.52% (SB-T-121205, 10 nM) and
39.39±1.80% (SB-T-121205, 20 nM), respectively. Also, SB-T-121205
(10 and 20 nM) reduced the number of invasive cells in a
dose-dependent manner (Fig. 4B),
wherein 10 nM of SB-T-121205 showed an equivalent effect to 600 nM
of paclitaxel.
Similarly, treating MDA-MB-453 cells with paclitaxel
(55 nM, a concentration close to the IC50 value) or
SB-T-121205 (19.5 nM, a concentration close to the
1/2IC50 value) or SB-T-121205 (39 nM, a concentration
close to the IC50 value) inhibited the migration and
invasion of MDA-MB-453 cells (Fig. 5A
and B). SB-T-121205 exhibited its effects on migration and
invasion of MDA-MB-435 cells in a dose-dependent manner, and was
clearly more effective than paclitaxel. Thus, we have found that
SB-T-121205 not only suppresses the EMT process, but also inhibits
migratory and invasive abilities of MCF-7/PTX and MDA-MB-435 human
breast cancer cells much more effectively than paclitaxel.
SB-T-121205 represses transgelin 2
expression and activation of the PI3K/Akt pathway in MCF-7/PTX
cells
To understand molecular mechanism by which
SB-T-121205 receded cell proliferation and migratory/invasive
abilities of MCF-7/PTX cells, the protein levels of transgelin 2
and the PI3K/Akt pathway were analyzed. After the treatment of
MCF-7/PTX cells with SB-T-121205, the expression of transgelin 2,
p-Akt and p-GSK-3β was downregulated with a concomitant augment
expression of PTEN, whereas the levels of Akt and GSK-3β were
unaffected (Fig. 6A).
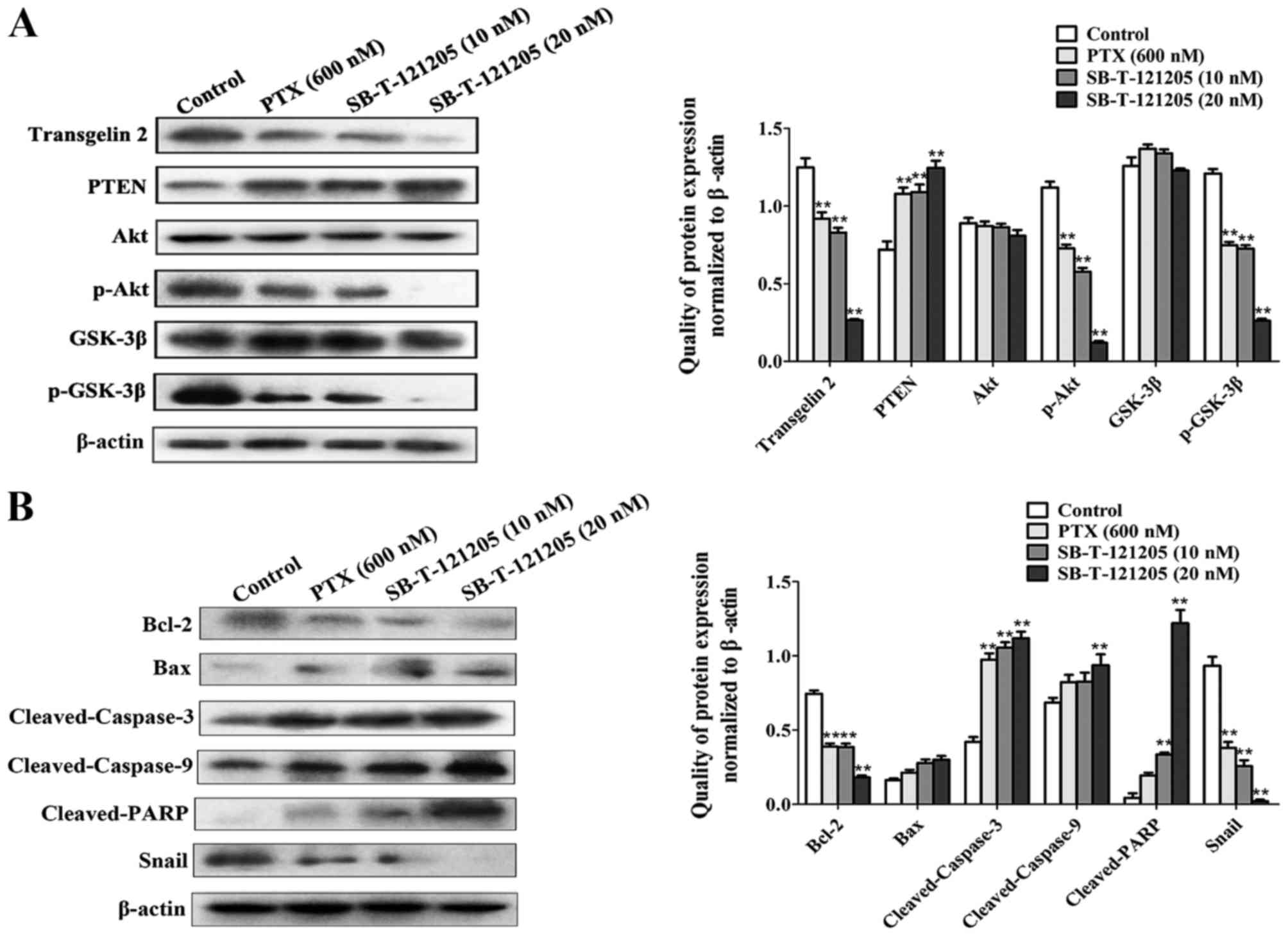 | Figure 6SB-T-121205 suppresses the PI3K/Akt
pathway in MCF-7/PTX cells. (A) The levels of transgelin 2, PTEN,
Akt, p-Akt, GSK-3β, and p-GSK-3β were tested in MCF-7/PTX cells
treated with paclitaxel alone or SB-T-121205 alone for 48 h. (B)
Expression of Bcl-2, Bax, cleaved-caspase-3, cleaved-caspase-9,
cleaved-PARP, and Snail was examined in MCF-7/PTX cells treated
with paclitaxel or SB-T-121205. Data are presented as mean ± SD
from three experiments, and β-actin was used as loading control,
**P<0.01 vs. control group. |
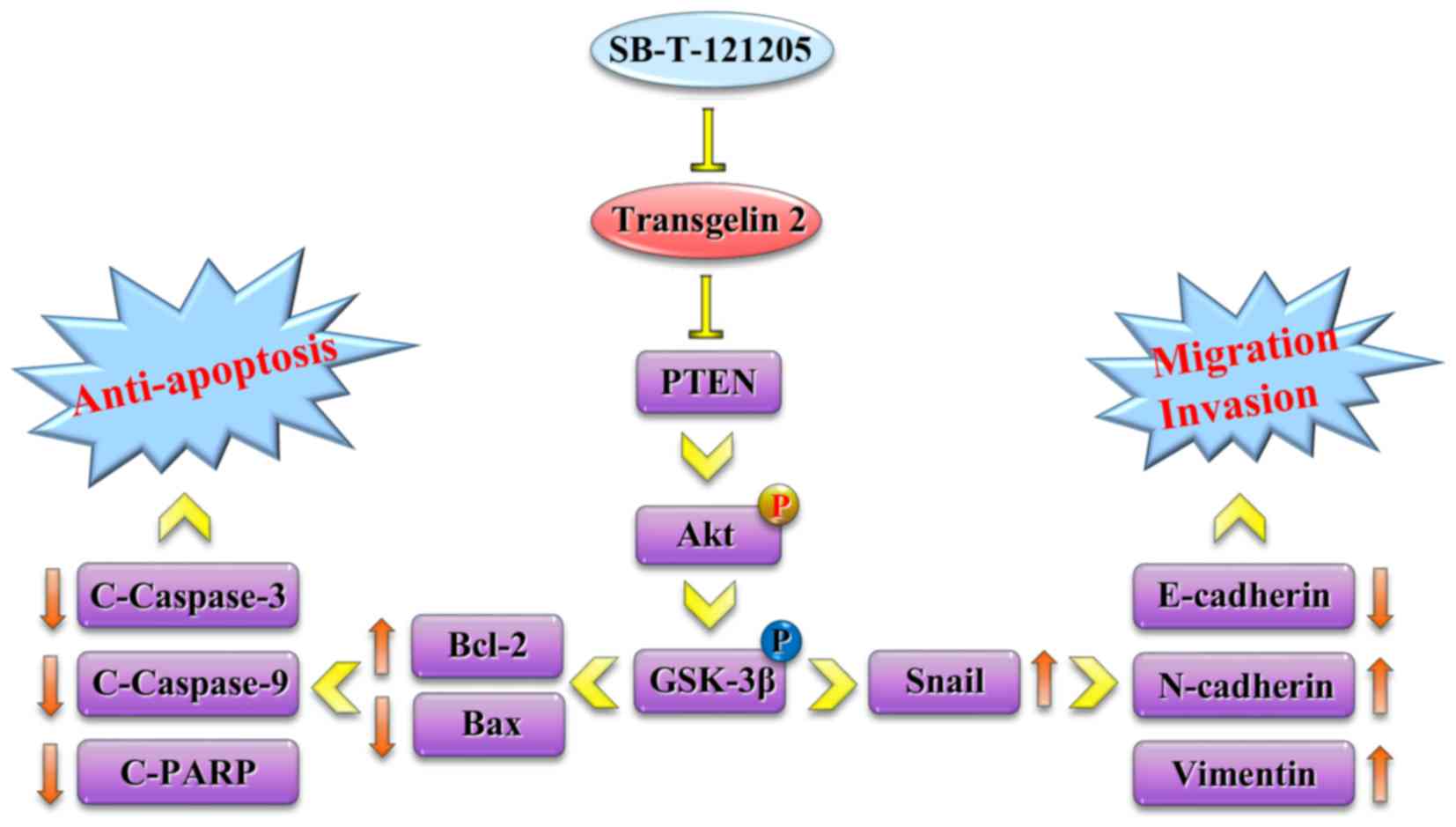
Furthermore, the deactivation of Akt signaling
pathway augmented the expression of pro-apoptotic factor Bax and
the cleavage of caspase-3, caspase-9, PARP significantly, while
suppressed pro-survival factor Bcl-2, ultimately leading to
apoptosis. On the other hand, the downstream molecule Snail was
strongly downregulated (Fig. 6B).
In short, these observations suggest that SB-T-121205 is an
antitumor agent that affects cell behavior by modulating transgelin
2 and the PI3K/Akt pathway.
Discussion
Paclitaxel was first approved in April 1992 for the
treatment of platinum-resistant breast cancer by the Food and Drug
Administration, USA. It exerts vital anticancer effect through its
distinct antimitotic mechanism of action (24). However, a number of studies since
then have revealed that treatment with paclitaxel brought about
undesirable adverse effects, including drug-resistance, hence
seriously restricting its therapeutic effects and clinical
applications (25). For this
reason, it is essential to deal with the problem of paclitaxel
resistance to enhance the survival of patients with breast
cancer.
There are two main ways to solve the problem of drug
resistance. The first is to develop resistance reversal agents.
Scientists in the past few decades have screened many compounds to
identify compounds with multidrug resistance reversal activities
against various drug-resistant cell lines. Compounds such as
verapamil (26) and metformin
(27) were discovered through
these efforts and developed. These compounds sensitize tumor cells
to chemotherapeutic drugs, and this line of research is continuing.
The second is to discover novel compounds with antitumor activities
that may have high efficacy against drug-resistant targets and then
directly kill cancer cells, which would also provide new insights
into prevention and treatment of breast cancer.
Considerable efforts have been made to develop
new-generation taxoids with outstanding antitumor activity, much
better than paclitaxel. For example, Nikolakakis et al
(28) reported that
7β-O-glycosylated taxanes (9 and 15) were more potent
(IC50 12–15 nM) than docetaxel (IC50 40 nM)
and much more potent than paclitaxel (IC50 >5
µM) against adriamycin-resistant human breast cancer cells
(MCF-7/ADR). It was also documented that
2′-(N-methylpyridinium acetate) derivative of paclitaxel
showed excellent potency in lung cancer cells and breast cancer
cells (29). Based on
structure-activity relationships (SAR) study, a series of
new-generation taxanes have been developed by Ojima et al
(10,30), which exhibited 2–3 orders of
magnitude higher potency than paclitaxel or docetaxel against
multidrug-resistant breast, ovarian, colon, pancreatic and prostate
cancer cell lines (31). These
new-generation taxanes have modifications at C10, C3′, C3′N
and/or C2. A newly developed next-generation taxane, SB-T-121205
possesses a 3-trifluoromethoxylbenzoyl group at C2 on the top of
modifications in the new-generation taxanes mentioned above. The
present study disclosed, for the first time, the excellent
activities of SB-T-121205 in inhibiting the growth of MCF-7/S,
MCF-7/PTX and MDA-MB-453 human breast cancer cells. An interesting
observation in this study was that BEAS-2B normal human cells were
relatively insensitive to SB-T-121205, which means that SB-T-121205
has a good therapeutic index. It was observed that the apoptosis
induced at 20 nM SB-T-121205 in MCF-7/PTX cells was more powerful
than 600 nM paclitaxel, suggesting SB-T-121205 possesses an
extremely strong anti-proliferative activity. SB-T-121205 induced
G2/M phase arrest in MCF-7/PTX cells in a manner similar to
paclitaxel. In addition, SB-T-121205 changed cell morphology,
modulated EMT marker expression and weakened the mammosphere
forming ability, then mitigated the EMT process in MCF-7/PTX cells.
Importantly, SB-T-121205 exhibited its ability to restrain the
migration and invasion capacities of MCF-7/PTX cells and MDA-MB-453
cells. Consequently, as a novel next-generation taxane, SB-T-121205
appears to be a very promising lead compound for drug
development.
Transgelin 2, located at chromosome 1q21–q25, is an
important actin-binding protein responsible for the actin
cytoskeleton dynamics (12).
Abundant evidence has indicated that transgelin 2 exerts oncogenic
activity. Transgelin 2 has been shown to be involved in lymph node
metastasis, distant metastasis as well as tumor-lymph
node-metastasis (TNM) staging system in colorectal cancer (CRC).
Transgelin 2 may serve as a new biomarker for predicting
progression and prognosis of CRC (14). Nohata et al (32) revealed that transgelin 2, directly
regulated by miR-1, was downregulated by a siRNA and then decreased
cell proliferation and invasion in human neck squamous cell
carcinoma cells. In our models of paclitaxel-resistant breast
cancer, we found that SB-T-121205 suppressed the transgelin 2
protein expression, which can explain the observed altered
biological behavior of MCF-7/PTX cells. It has been generally
accepted that the PI3K/Akt pathway participates in drug resistance,
tumor migration, differentiation and apoptosis. Suppression of the
PI3K/Akt pathway has been proven to be an efficient way to
attenuate cell growth and migration (33,34).
Wang et al (35) verified
that the PI3K/Akt pathway was activated in cisplatin-resistant lung
cancer cells (A549/CDDP), and deactivation of Akt signaling pathway
significantly suppressed Snail expression and subsequently induced
a substantial decrease in migratory ability and invasiveness of
A549/CDDP cells. Snail, a zinc-finger transcription factor, is
known as a crucial regulator in the aggressive phenotype of EMT
(36). It was supported that
PI3K/Akt signal played an essential function during
miR-519a-induced hepatocellular carcinoma cell proliferation and
cell cycle progression (37). We
found that the downregulation of transgelin 2 by SB-T-121205 caused
repression of the PI3K/Akt pathway. Deactivation of Akt signal led
to revitalization of the mitochondria apoptosis pathway and
downregulation of Snail in MCF-7/PTX cells (Fig. 7), which appears to be an important
contributor to the unique mechanism of action of SB-T-121205.
In conclusion, we found that SB-T-121205 enhances
cell apoptosis, as well as inhibits the migration and invasion
abilities of MCF-7/PTX cells, partly by targeting transgelin 2 and
the PI3K/Akt pathway. We also found that SB-T-121205 downregulates
the transgelin 2 expression. Deactivation of transgelin 2 can be
further explored as a basis for new strategies for breast cancer
treatment. These findings strongly indicate that SB-T-121205 is a
highly promising lead compound for the development of
next-generation chemotherapeutic agents in breast cancer
treatment.
Abbreviations:
|
PTX
|
paclitaxel
|
|
PI3K/Akt
|
phosphatidylinositol 3
kinase/serine-threonine kinase
|
|
EMT
|
epithelial-mesenchymal transition
|
Acknowledgments
The present study is supported by grants from the
National Natural Science Foundation of China (nos. 81473177,
81502616 and 81672954) and the Science Foundation of Guangdong
Province, China (no. 2015B020211012), as well as a grant from the
National Institutes of Health, USA (CA103314 to I.O.).
References
|
1
|
DeSantis CE, Bray F, Ferlay J,
Lortet-TiNeulent J, Anderson BO and Jemal A: International
Variation in Female Breast Cancer Incidence and Mortality Rates.
Cancer Epidemiol Biomarkers Prev. 24:1495–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zdenkowski N, Butow P, Tesson S and Boyle
F: A systematic review of decision aids for patients making a
decision about treatment for early breast cancer. Breast. 26:31–45.
2016. View Article : Google Scholar
|
|
3
|
Maushagen R, Reers S, Pfannerstill AC,
Hahlbrock A, Stauber R, Rahmanzadeh R, Rades D, Pries R and
Wollenberg B: Effects of paclitaxel on permanent head and neck
squamous cell carcinoma cell lines and identification of
anti-apoptotic caspase 9b. J Cancer Res Clin Oncol. 142:1261–1271.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khongkow P, Gomes AR, Gong C, Man EP,
Tsang JW, Zhao F, Monteiro LJ, Coombes RC, Medema RH, Khoo US, et
al: Paclitaxel targets FOXM1 to regulate KIF20A in mitotic
catastrophe and breast cancer paclitaxel resistance. Oncogene.
35:990–1002. 2016. View Article : Google Scholar
|
|
5
|
Wang L, Li H, Ren Y, Zou S, Fang W, Jiang
X, Jia L, Li M, Liu X, Yuan X, et al: Targeting HDAC with a novel
inhibitor effectively reverses paclitaxel resistance in non-small
cell lung cancer via multiple mechanisms. Cell Death Dis.
7:e20632016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Zhao J, Zhang W, Liu G, Yin D, Li
J, Zhang S and Li H: Establishment of paclitaxel-resistant cell
line and the underlying mechanism on drug resistance. Int J Gynecol
Cancer. 22:1450–1456. 2012.PubMed/NCBI
|
|
7
|
Roh EJ, Kim D, Lee CO, Choi SU and Song
CE: Structure-activity relationship study at the 3′-N-position of
paclitaxel: Synthesis and biological evaluation of
3′-N-acyl-paclitaxel analogues. Bioorg Med Chem. 10:3145–3151.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Q, Li Y, Jin J, Lang L, Zhu Z, Fang W
and Chen X: Lx2-32c a novel taxane derivative, exerts
anti-resistance activity by initiating intrinsic apoptosis pathway
in vitro and inhibits the growth of resistant tumor in vivo. Biol
Pharm Bull. 35:2170–2179. 2012. View Article : Google Scholar
|
|
9
|
Fitzgerald DP, Emerson DL, Qian Y, Anwar
T, Liewehr DJ, Steinberg SM, Silberman S, Palmieri D and Steeg PS:
TPI-287, a new taxane family member, reduces the brain metastatic
colonization of breast cancer cells. Mol Cancer Ther. 11:1959–1967.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ojima I, Chen J, Sun L, Borella CP, Wang
T, Miller ML, Lin S, Geng X, Kuznetsova L, Qu C, et al: Design,
synthesis, and biological evaluation of new-generation taxoids. J
Med Chem. 51:3203–3221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yakabe K, Murakami A, Kajimura T,
Nishimoto Y, Sueoka K, Sato S, Nawata S and Sugino N: Functional
significance of transgelin-2 in uterine cervical squamous cell
carcinoma. J Obstet Gynaecol Res. 42:566–572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin H, Cheng X, Pei Y, Fu J, Lyu Z, Peng
H, Yao Q, Jiang Y, Luo L and Zhuo H: Identification and
verification of transgelin-2 as a potential biomarker of
tumor-derived lung-cancer endothelial cells by comparative
proteomics. J Proteomics. 136:77–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu XC, Zhang YH, Zhang WB, Li T, Gao H and
Wang YH: MicroRNA-133a functions as a tumor suppressor in gastric
cancer. J Biol Regul Homeost Agents. 28:615–624. 2014.
|
|
14
|
Zhang Y, Ye Y, Shen D, Jiang K, Zhang H,
Sun W, Zhang J, Xu F, Cui Z and Wang S: Identification of
transgelin-2 as a biomarker of colorectal cancer by laser capture
microdissection and quantitative proteome analysis. Cancer Sci.
101:523–529. 2010. View Article : Google Scholar
|
|
15
|
Chen S, Dong Q, Hu S, Cai J, Zhang W, Sun
J, Wang T, Xie J, He H, Xing J, et al: Proteomic analysis of the
proteins that are associated with the resistance to paclitaxel in
human breast cancer cells. Mol Biosyst. 10:294–303. 2014.
View Article : Google Scholar
|
|
16
|
Cai J, Chen S, Zhang W, Hu S, Lu J, Xing J
and Dong Y: Paeonol reverses paclitaxel resistance in human breast
cancer cells by regulating the expression of transgelin 2.
Phytomedicine. 21:984–991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Cai J, Chen S, Zheng X, Hu S,
Dong W, Lu J, Xing J and Dong Y: Paclitaxel resistance in MCF-7/PTX
cells is reversed by paeonol through suppression of the
SET/phosphatidylinositol 3-kinase/Akt pathway. Mol Med Rep.
12:1506–1514. 2015.PubMed/NCBI
|
|
18
|
Shu YJ, Weng H, Ye YY, Hu YP, Bao RF, Cao
Y, Wang XA, Zhang F, Xiang SS, Li HF, et al: SPOCK1 as a potential
cancer prognostic marker promotes the proliferation and metastasis
of gallbladder cancer cells by activating the I3K/AKT pathway. Mol
Cancer. 14:122015. View Article : Google Scholar
|
|
19
|
Cai J, Chen S, Zhang W, Zheng X, Hu S,
Pang C, Lu J, Xing J and Dong Y: Salvianolic acid A reverses
paclitaxel resistance in human breast cancer MCF-7 cells via
targeting the expression of transgelin 2 and attenuating I3K/Akt
pathway. Phytomedicine. 21:1725–1732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen SY, Hu SS, Dong Q, Cai JX, Zhang WP,
Sun JY, Wang TT, Xie J, He HR, Xing JF, et al: Establishment of
paclitaxel-resistant breast cancer cell line and nude mice models,
and underlying multidrug resistance mechanisms in vitro and in
vivo. Asian Pac J Cancer Prev. 14:6135–6140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng X, Chen S, Yang Q, Cai J, Zhang W,
You H, Xing J and Dong Y: Salvianolic acid A reverses the
paclitaxel resistance and inhibits the migration and invasion
abilities of human breast cancer cells by inactivating transgelin
2. Cancer Biol Ther. 16:1407–1414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu F, Li G, Gao J, Sun Y, Liu P, Gao H, Li
P, Lei T, Chen Y, Cheng Y, et al: SPOCK1 is upregulated in
recurrent glioblastoma and contributes to metastasis and
Temozolomide resistance. Cell Prolif. 49:195–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu X, Li D, Yu F, Jia C, Xie J, Ma Y, Fan
S, Cai H, Luo Q, Lv Z, et al: miR-194 inhibits the proliferation,
invasion, migration, and enhances the chemosensitivity of non-small
cell lung cancer cells by targeting forkhead box A1 protein.
Oncotarget. 7:13139–13152. 2016.PubMed/NCBI
|
|
24
|
Sakamoto J, Matsui T and Kodera Y:
Paclitaxel chemotherapy for the treatment of gastric cancer.
Gastric Cancer. 12:69–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ge X, Cao Z, Gu Y, Wang F, Li J, Han M,
Xia W, Yu Z and Lyu P: FKFB3 potentially contributes to paclitaxel
resistance in breast cancer cells through TLR4 activation by
stimulating lactate production. Cell Mol Biol (Noisy-le-grand).
62:119–125. 2016.
|
|
26
|
Lin ST, May EW, Chang JF, Hu RY, Wang LH
and Chan HL: GRMC1 contributes to doxorubicin-induced
chemoresistance in MES-SA uterine sarcoma. Cell Mol Life Sci.
72:2395–2409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Han R, Xiao H, Lin C, Wang Y, Liu H,
Li K, Chen H, Sun F, Yang Z, et al: Metformin sensitizes
EGFR-TKI-resistant human lung cancer cells in vitro and in vivo
through inhibition of IL-6 signaling and EMT reversal. Clin Cancer
Res. 20:2714–2726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nikolakakis A, Haidara K, Sauriol F, Mamer
O and Zamir LO: Semi-synthesis of an O-glycosylated docetaxel
analogue. Bioorg Med Chem. 11:1551–1556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wrasidlo W, Gaedicke G, Guy RK, Renaud J,
Pitsinos E, Nicolaou KC, Reisfeld RA and Lode HN: A novel
2′-(N-methylpyridinium acetate) prodrug of paclitaxel induces
superior antitumor responses in preclinical cancer models.
Bioconjug Chem. 13:1093–1099. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ojima I, Slater JC, Michaud E, Kuduk SD,
Bounaud PY, Vrignaud P, Bissery MC, Veith JM, Pera P and Bernacki
RJ: Syntheses and structure-activity relationships of the
second-generation antitumor taxoids: Exceptional activity against
drug-resistant cancer cells. J Med Chem. 39:3889–3896. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuznetsova L, Chen J, Sun L, Wu X, Pepe A,
Veith JM, Pera P, Bernacki RJ and Ojima I: Syntheses and evaluation
of novel fatty acid-second-generation taxoid conjugates as
promising anticancer agents. Bioorg Med Chem Lett. 16:974–977.
2006. View Article : Google Scholar
|
|
32
|
Nohata N, Sone Y, Hanazawa T, Fuse M,
Kikkawa N, Yoshino H, Chiyomaru T, Kawakami K, Enokida H, Nakagawa
M, et al: miR-1 as a tumor suppressive microRNA targeting TAGLN2 in
head and neck squamous cell carcinoma. Oncotarget. 2:29–42.
2011.PubMed/NCBI
|
|
33
|
Zhu Y, Cheng Y, Guo Y, Chen J, Chen F, Luo
R and Li A: Protein kinase D2 contributes to TNF-α-induced
epithelial mesenchymal transition and invasion via the
I3K/GSK-3β/β-catenin pathway in hepatocellular carcinoma.
Oncotarget. 7:5327–5341. 2016.
|
|
34
|
Zhou XM, Sun R, Luo DH, Sun J, Zhang MY,
Wang MH, Yang Y, Wang HY and Mai SJ: Upregulated TRIM29 promotes
proliferation and metastasis of nasopharyngeal carcinoma via
PTEN/AKT/mTOR signal pathway. Oncotarget. 7:13634–13650.
2016.PubMed/NCBI
|
|
35
|
Wang H, Zhang G, Zhang H, Zhang F, Zhou B,
Ning F, Wang HS, Cai SH and Du J: Acquisition of
epithelial-mesenchymal transition phenotype and cancer stem
cell-like properties in cisplatin-resistant lung cancer cells
through AKT/β-catenin/Snail signaling pathway. Eur J Pharmacol.
723:156–166. 2014. View Article : Google Scholar
|
|
36
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tu K, Liu Z, Yao B, Han S and Yang W:
MicroRNA-519a promotes tumor growth by targeting PTEN/I3K/AKT
signaling in hepatocellular carcinoma. Int J Oncol. 48:965–974.
2016.
|