Introduction
Rac1, an important member of the small G protein
family, influences cell migration and invasion by generating
endogenous reactive oxygen species (ROS) (1,2).
ROS, mainly generated from the NADPH oxidase, the cytochrome p450
and the mitochondrial electron transport system, are well known to
regulate a variety of intracellular signal transduction pathways to
mediate various biological responses, including cell proliferation,
invasion and angiogenesis (3).
Previous studies have reported NADPH oxidase catalytic subunit
gp91phox and its homologues, the NOx family proteins, occur a
series of changes and then transfer electrons through the plasma
membrane to produce ROS, when the resting cell is stimulated by any
of the variety of stimuli (4).
Excessive ROS activate downstream signaling pathways to regulate
expression of MMPs (5). Invasion
and metastasis are fundamental properties of various malignant
cancer cells. MMPs, a family of zinc-dependent endopeptidases,
induce cancer cell invasion and spread through the degradation of
the extracellular matrix (ECM) and the basal membrane (6). A balance between extracellular matrix
(ECM) deposition and matrix destruction is important for
maintaining normal tissues architecture and integrity. This balance
is modulated by MMPs and their tissue inhibitors (TIMPs). MMP-2
(gelatinase-A) and MMP-9 (gelatinase-B) are essentially associated
with elevated malignant cancer presence (7,8).
MMP-3 plays a critical role in glioma invasiveness via degradation
of hyaluronic acid-rich matrix of the brain (8). Research has shown that MMP-7 is
overexpressed in epithelial ovarian cancer and triptolide inhibited
the migration and invasion by repression of the expression of MMP-7
and MMP-19 in ovarian cancer SKOV3 cells (9). Based on these findings, controlling
MMP expression has been proposed as an important therapeutic means
for malignant tumor treatment and Rac1 has a number of downstream
effectors including MMPS, ROS and mitogen-activated protein kinases
(MAPK) (10,11). Therefore, RAC1 as a target is
essential to control the migration and invasion of tumors.
Rhein (4,5-dihydroxyanthraquinone-2-carboxylic
acid), extracted from rhubarb (Rheum palmatum L), has been
found to possess various pharmacological effects, including
anti-fibrosis, antioxidant (12,13),
anti-inflammatory in LPS-activated macrophages (14) and antimicrobial activities
(15). In addition, rhein exerts
its anticancer effects via the modulation of processes of cellular
proliferation (13), apoptosis,
migration, and invasion (16).
Earlier studies have demonstrated that rhein inhibited metastasis
and invasion in human tongue cancer SCC-4 cells through
downregulation of expression level of MMP-2 protein, and restrain
the phosphorylation of c-Jun protein and c-Jun NH2-terminal kinase
(JNK) and p38 kinase (17). Rhein
in mouse skin JB6 epithelial cells inhibited
12-O-tetra-decanoylphorbol-13-acetate (TPA)-induced AP-1
activity and cell transformation by blocking JNK-dependent pathway,
but did not restrain the phosphorylation of ERK and p38 MAPK
(18). These data indicate that
rhein may be another important component in rhubarb possessing
antitumor activity.
Our research group found earlier (19) that rhein possess obvious growth
inhibition on SKOV3-PM4 cells with directional highly lymphatic
metastasis, but the underlying mechanisms of cell migration and
invasion involved in the signal transduction pathway have not been
elucidated. In this study, we demonstrated that rhein shows great
inhibition of PMA-stimulated SKOV3-PM4 cell proliferation,
migration and invasion by inhibiting the activity of Rac1 and its
downstream ROS-dependent signaling pathway p38/JNK MAPK and
decrease the expression level of transcriptional factor activator
protein-1 (AP-1).
Materials and methods
Reagents
Rhein was purchased from Langze (Nanjing, China);
phorbol 12-myristate 13-acetate (PMA, Cayman, MI, USA) and NSC
23766 (Selleck, Shanghai, China) were used as Rac1 activator and
Rac1 inhibitor, respectively.
Cell culture and drug treatments
Human SKOV3-PM4 cell line was provided by the
Oncology Laboratory at the Experimental Center of Guangxi Medical
University (Nanning, China) and maintained in RPMI-1640 (Gibco;
Life Technologies, Gaithersburg, MA, USA) supplemented with 10%
fetal calf serum (Gibco) and 1% penicillin-streptomycin and then
incubated at 37°C with 5% CO2 humidified atmosphere
(20). The medium was changed
every 2 days. PMA can activate the NADPH oxidase activity and
NSC23766 inhibits its activity. No significant cytotoxicity to
SKOV3-PM4 cells was found when the concentration of PMA and
NSC23766 was 100 nM and 12.5 µM, respectively (21). Considering that the inhibitory rate
of SKOV3-PM4 cells treated with rhein alone was <20%, the
concentration of rhein was chosen as ≤11.3 µM. In exponential
growth phase, SKOV3-PM4s were divided into nine treatment groups:
group I (control); group II (7.7 μM rhein); group III (11.3
μM rhein); group IV (100 nM PMA alone); group V (PMA plus
7.7 μM rhein) group VI (PMA plus 11.3 μM rhein);
group VII (12.5 μM NSC23766 alone); group VIII (NSC23766
plus 7.7 μM rhein); group IX (NSC23766 plus 11.3 μM
rhein). Rhein was dissolved in dimethyl sulfoxide (DMSO) at a stock
concentration of 176 mM. The final concentration of rhein was 0–20
μM with a maximal DMSO concentration <0.05% (DMSO has no
effect on cell growth at a concentration <2%).
Cell proliferation assay
SKOV3-PM4 cells were allowed to grow in 96-well
plates at 5,000 cells/well for 24 h and then treated with different
concentrations of rhein, respectively. Forty-eight hours later, 20
μl of 5 mg/ml MTT solution was added to each well. The
plates were incubated at 37°C in 5% CO2 for 4 h, then
the supernatant was discarded and 150 μl DMSO was added to
dissolve the formazan. The absorbance at a wavelength of 490 nm was
measured using a microplate reader (Thermo Fisher Scientific Inc.,
Waltham, MA, USA). The percentage of inhibition was calculated as
follows: % inhibition = [1−(mean A of sample/mean A of control)]
×100%.
Wound healing assay
SKOV3-PM4 cells were seeded in the plates at a
density of 1×105/well and incubated for 24 h to form a
monolayer with confluence. Cell motility was performed by wound
healing assay. Briefly, the cells in the different groups, as
described above, were scraped with a 10-μl pipette tip
running through the center of the well and washed twice with PBS.
RPMI-1640 medium with no serum treated in the different groups as
described previously was added to the wells. SKOV3-PM4 cells were
grown at 37°C for indicated times and images were taken.
Cell invasion assay
The invasion ability of the SKOV3-PM4 was assessed
using the Matrigel Invasion assay (Sigma-Aldrich, St. Louis, MO,
USA). Briefly, cells were adjusted to a density of 5×103
cells/ml with a series of drug solutions, as described above.
Subsequently, 200 μl of cell suspensions in serum-free
RPMI-1640 medium from different groups was seeded into the upper
chamber of the Transwell insert (8-μm pore size; Costar,
Acton, MA, USA), and the RPMI-1640 medium containing 20% FBS was
added to the lower chamber. Cells were removed following incubation
for 48 h at 37°C in 5% CO2. The Matrigel was wiped with
a cotton swab and the cells were fixed with 95% ethanol for 20 min
prior to staining with 2% crystal violet. Also, cells were imaged
and counted under a light microscope.
Nitroblue tetrazolium assay
The SKOV3-PM4 cells were seeded in 6-well plates and
treated with drugs as previously described. In brief, cells in
different groups were harvested by centrifugation and incubated in
nitroblue tetrazolium (NBT) solution (2 mg/ml, Solarbio, Beijing,
China) for 20 min at 37°C. Subsequently, 200 μl HCl (1
mol/l) was added at 4°C to terminate the reaction. After
centrifugation, the supernatant was discarded and 100 μl
DMSO was added to solubilize the formazan crystals. The solute was
transferred to a 96-well plate and then mixed thoroughly before
reading on a microplate reader at 570 nm (Thermo Fisher Scientific,
Inc.). The NADPH oxidase activity was expressed as: (mean A of
sample/mean A of control) ×100%.
ROS production
The level of oxygen-free radical was determined by
flow cytometry using 2′,7′-dichlorodihydrofluorescein diacetate
(DCFH-DA, Sigma). SKOV3-PM4 cells in different groups were
incubated with DCFH-DA in RPMI-1640 media (1:1,000) for 20 min,
respectively. The cells were then washed with phosphate-buffered
saline (PBS) and cellular fluorescence was measured with a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Assays were performed three times independently.
Western blot analysis
After 48-h treatment with different drugs, the
proteins of SKOV3-PM4 cells were collected and separated on 10%
SDS-PAGE in Tris-glycine buffer. Electrophoresis was performed at a
constant voltage of 100 V. The proteins were then transferred to NC
membranes (Millipore, MA, USA) in a semi-dry blotter (Hoeffer,
Canada). The membranes were incubated in blocking buffer [0.1%
Tween-20 in phosphate-buffered solution (PBST) solution containing
5% skim milk] for 2 h, incubated with a primary antibody in a
blocking solution overnight at 4°C. Primary antibodies against
p-p38 (1:200, Abcam, USA); p38 (1:200, Abcam); p-JNK (1:200,
Abcam); JNK (1:200, Abcam USA); p-AP-1 (1:200, Abcam); AP-1 (1:200,
Abcam); MMP-2 (1:200, Abcam); MMP-3 (1:200, Abcam); MMP-7 (1:200,
Abcam); MMP-19 (1:200, Abcam); TIMP-1 (1:500, Abcam), TIMP-2
(1:600, Abcam), NM23-H1 (1:200, Abcam) and GAPDH (1:2,000, Abcam)
were used. After washing three times at 10-min intervals with PBST,
anti-rabbit antibody solution (1:5,000 Abcam) was added to the
membrane for 2 h. The secondary antibody solution was washed three
times with PBST (each wash for 10 min at room temperature). The
PBST was discarded and the membranes were scanned using the Odyssey
infrared imaging system (LI-COR, Lincoln, NE, USA). The intensity
of protein staining was determined with Quantity One software.
Statistical analysis
All experiments were performed at least in
triplicate and repeated three times. Unless otherwise indicated,
the results are expressed as the mean ± standard deviation (SD).
Statistical analysis was performed by SPSS 16.0 (IBM Corp., Armonk,
NY, USA). The results from the nine groups were compared by one-way
analysis of variance (ANOVA) and two-way ANOVA was used for
comparison of two independent variances among groups. For all
statistical analyses, differences were considered to be
statistically significant at P<0.05.
Results
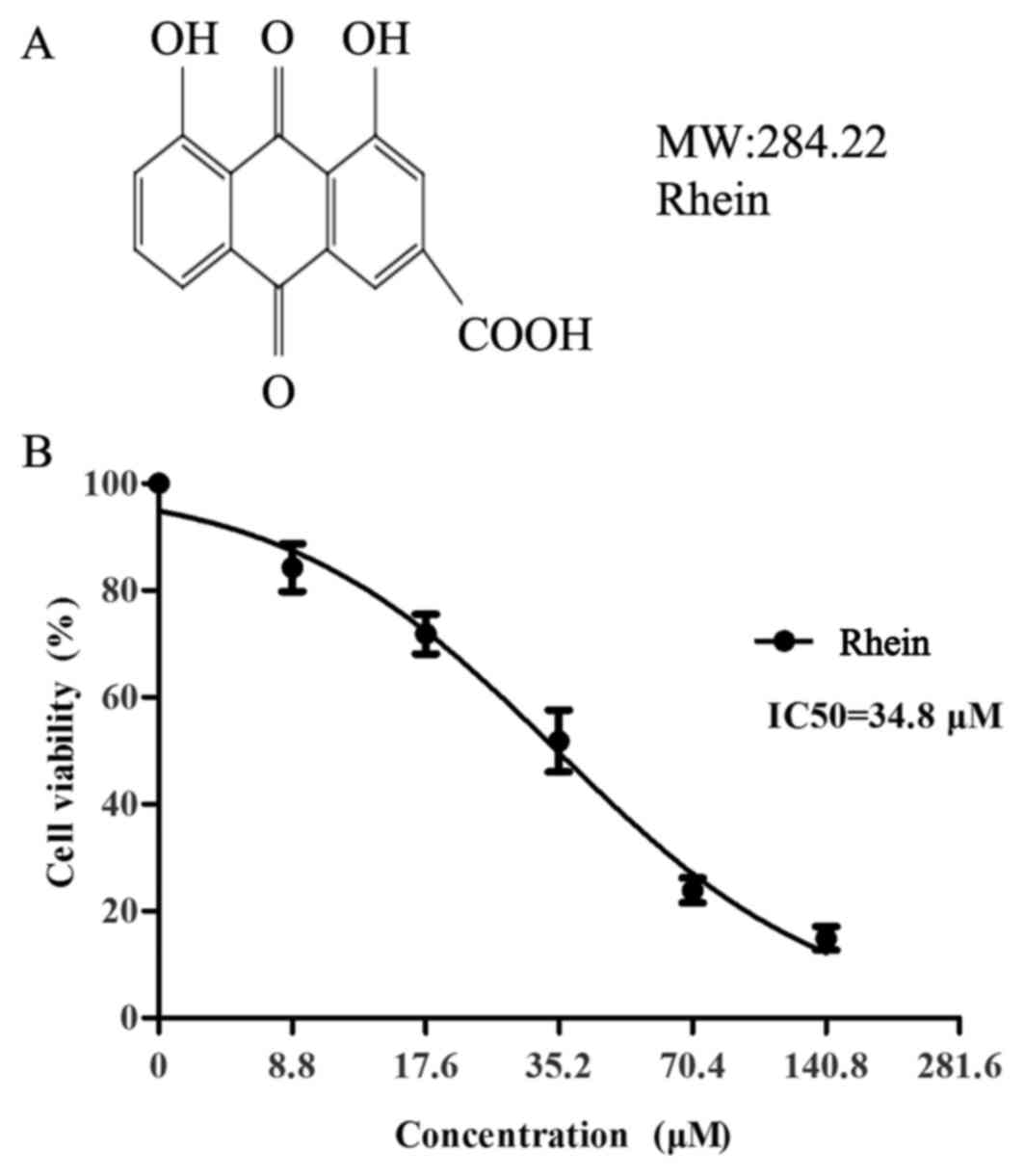
Proliferation of SKOV3-PM4 cells was
suppressed by rhein
The effect of rhein on the proliferation of
SKOV3-PM4 cells was investigated by MTT assay. The inhibition ratio
of the SKOV3-PM4 cells exposed to different concentrations of rhein
is shown in Fig. 1. The results
showed that rhein could significantly inhibit the proliferation of
SKOV3-PM4 cells in a dose-dependent manner, with IC50
values of 35.2 μM. Non-toxic concentration of rhein was
<12 μM. So we selected 7.7 and 11.3 μM rhein as
the experimental concentration.
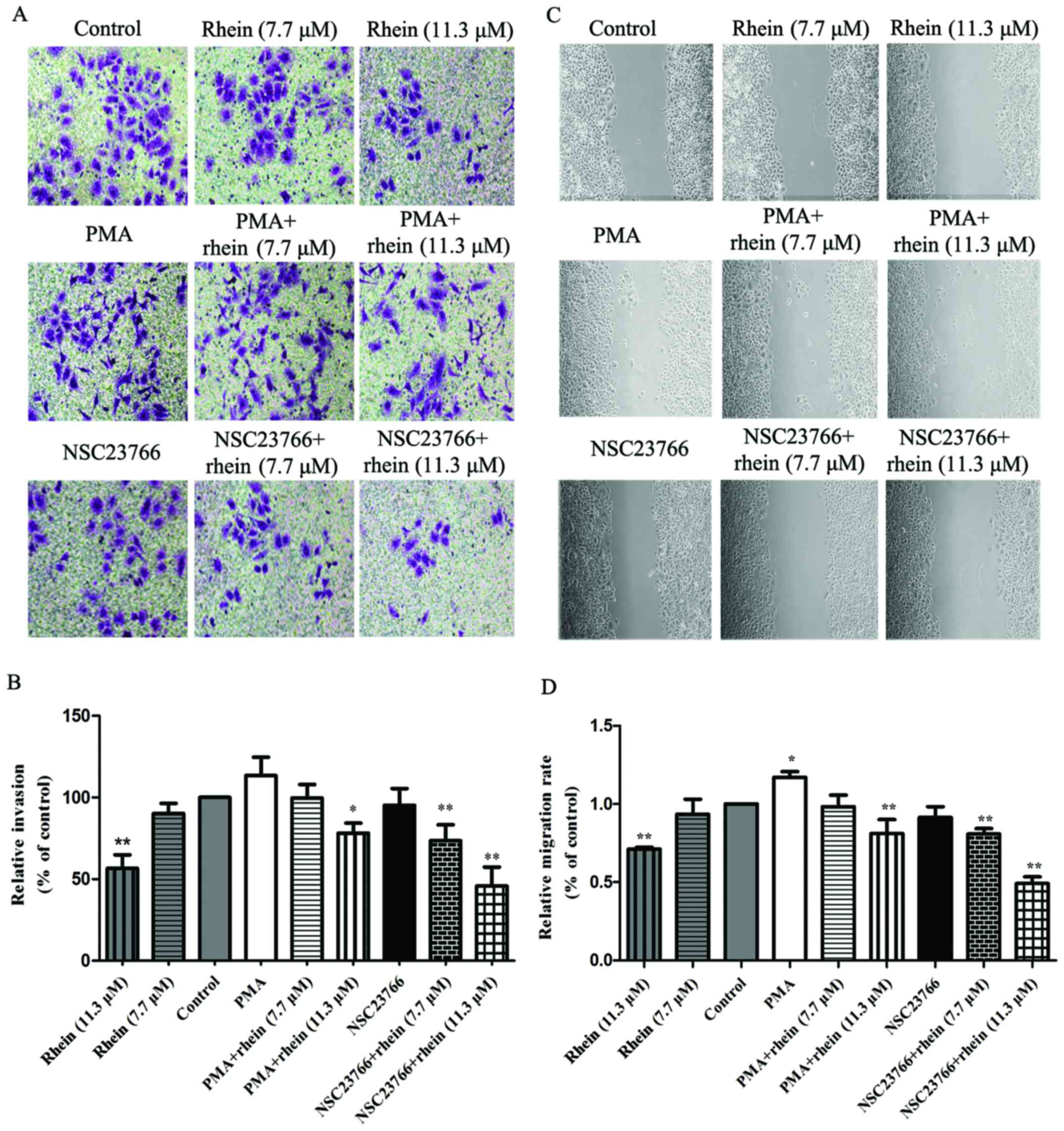
Rhein suppresses migration and invasion
in SKOV3-PM4 cells
To investigate the effects of rhein on SKOV3-PM4
cell migration and invasion, the wound healing assay and the
Matrigel-based Transwell invasion assay were performed. As shown in
Fig. 2A and B, compared with
control cells, PMA can enhance the ability of SKOV3-PM4 cell
migration, but NSC23766 has no effect. Rhein significantly
inhibited SKOV3-PM4 cell migration (P<0.01). The ability of
SKOV3-PM4 cell migration was suppressed by treatment with PMA
combined with rhein. NSC23766 plus rhein significantly inhibited
the migration of SKOV3-PM4 cells compared to the control
(P<0.01). In addition, the results of the wound healing assay
were consistent with the Matrigel-based Transwell invasion assay
(Fig. 2C and D). Taken together,
rhein markedly inhibited cell migration and invasion, suggesting
that rhein is an effective inhibitor for ovarian carcinoma cell
invasion, migration and metastasis.
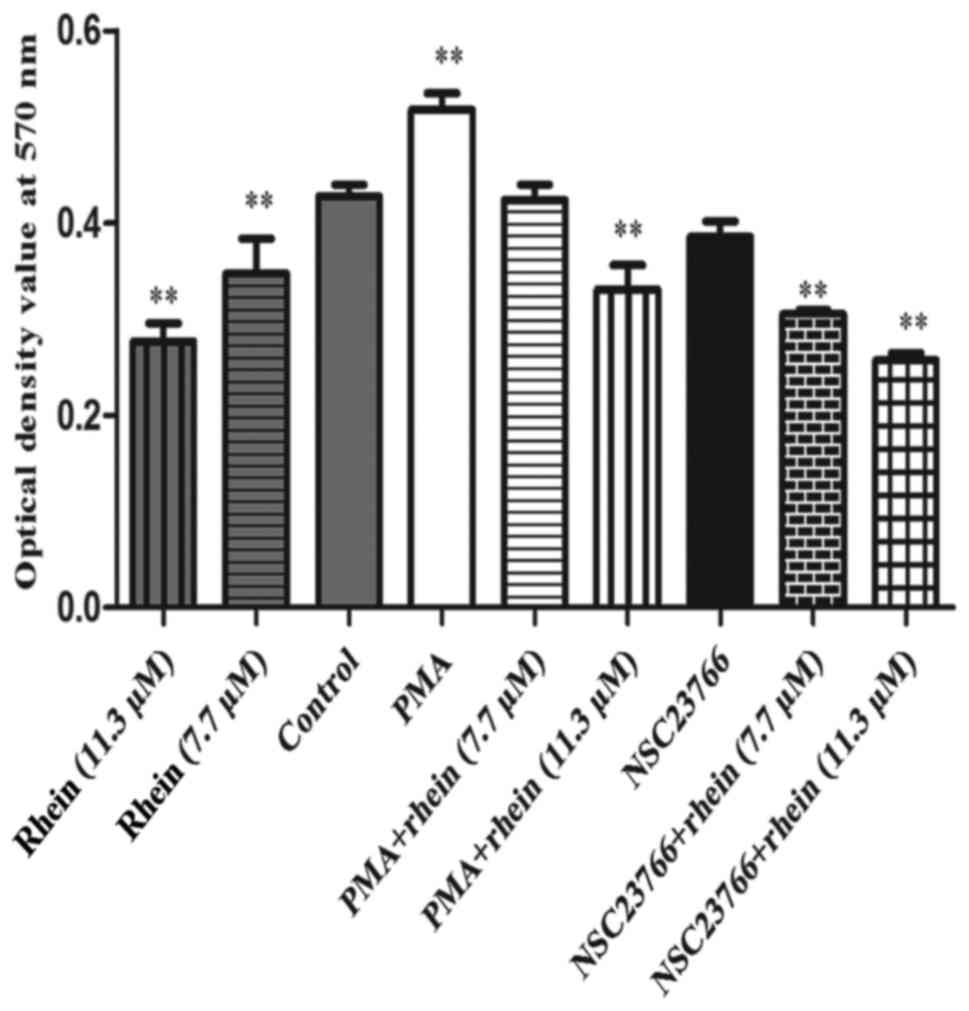
Rhein participates in NADPH oxidase
deactivation
It is known that NADPH oxidase plays an important
role in activating the generation of ROS (3,4).
Rac1 can activate NADPH oxidase which results in corresponding
cellular effects (2-4). An NBT assay (Fig. 3) demonstrated that Rac1 activator
PMA remarkably increased the activity of NADPH oxidase (P<0.01),
but in the cells treated with rhein (7.7/11.3 μM) alone, PMA
plus rhein (11.3 μM) and NSC23766 plus rhein (7.7/11.3
μM), the activity of NADPH oxidase significantly decreased
compared to the control (P<0.01). PMA significantly increased
the activity of NADPH oxidase and this effect was also attenuated
by rhein. Thus, NADPH oxidase induced by PMA can be suppressed by
rhein.
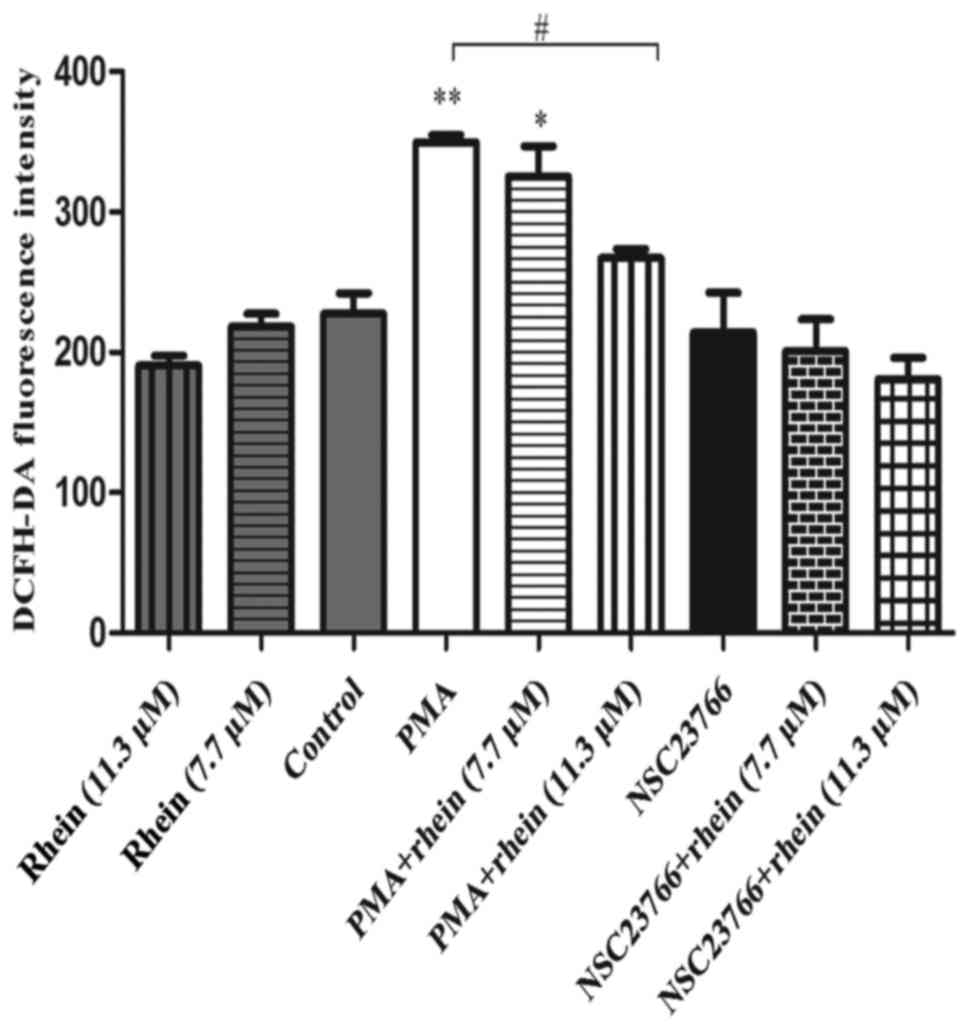
Rhein downregulates migration and
invasion of SKOV3-PM4 cells by scavenging intracellular reactive
oxygen species (ROS)
ROS contribution to cell migration and invasion has
been well documented (22). We
therefore, speculated that rhein might suppress SKOV3-PM4 cell
migration via scavenging ROS. Here, the generation of intracellular
ROS was examined using a DCFH-DA probe. As shown in Fig. 4, the level of ROS did not change
significantly after cells treated with rhein alone, NSC23766 alone
or with NSC23766 combined with rhein. However, PMA can remarkably
increase the intracellular ROS production as compared to control
cells (P<0.01). In cells treated with PMA combined with rhein,
the ROS levels were downregulated clearly compared to cells treated
with PMA alone (P<0.05).
Inhibitory effect of rhein on Rac1
activation stimulates expression of MMPs
Previous studies reported that MMPs participate in
the degradation of the extracellular matrix, which is believed to
involve in the invasion and metastasis processes (23,24).
Using western blot analysis, we found that in the cells treated
with PMA, a Rac1 activator, the expression of MMP-9 in SKOV3-PM4
cells was upregulated, whereas the MMP-7 expression level was
downregulated as compared to the control. The protein expression
levels of MMP-2, -3, -7, -9 and MMP-19 in the cells treated with
NSC23766 or rhein (11.3 μM) alone were remarkably reduced in
comparison with the control, and their expression levels in the
cells treated with PMA plus rhein (11.3 μM) also
significantly reduced as compared to the cells treated with PMA
alone. MMP-2, -3, -7, -9 and MMP-19 expression levels in the cells
induced by NSC23766 combined with rhein were greatly lower than
those in the cells induced by NSC23766 alone. NM23-H1 expression
level was downregulated in the cells treated with PMA, whereas
NM23-H1, TIMP-1 and TIMP-2 expression levels had no significant
change in the cells treated with NSC23766 as compared to the
control. In the cells induced by rhein, whether high or low
concentration of rhein, the protein expression levels of NM23-H1,
TIMP-2 and TIMP-1 were significantly enhanced as compared to the
control. Similar results were also observed in the cells treated
with rhein combined with NSC23766.
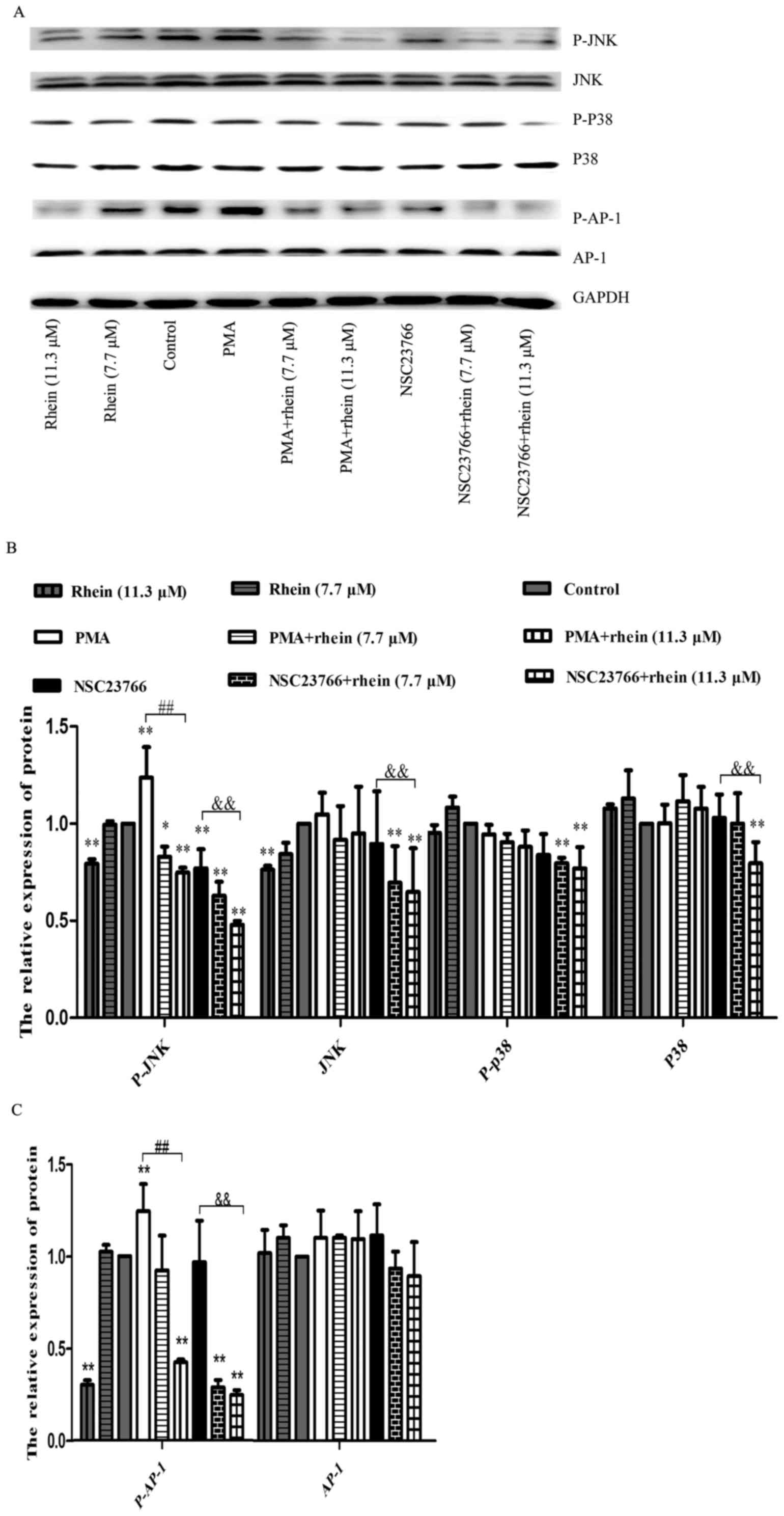
Rhein attenuates the increase of JNK and
p38 expression and phosphorylation induced by ROS
It was reported that transcription factor AP-1 is
regulated by MAPK and AP-1 is also known to be a key transcription
factor of MMPs (22,25,26).
Woo et al reported that activation of one or more MAPK
pathways was often associated with the MMP expression in various
cell types (26). In order to
explore the relationship among Rac1, MAPK and AP-1, we determined
the relevant protein expression levels using western blot analysis.
As shown in Fig. 6, p-JNK and
p-AP-1 expression levels were decreased in SKOV3-PM4 cells treated
with rhein, whereas their expression levels were significantly
enhanced in the cells treated with PMA. In the cells treated with
NSC23766 alone, p-JNK and p-AP-1 expression levels decreased while
JNK and AP-1 expression levels had no change. The p-JNK and p-AP-1
protein expression levels were both decreased in the cells induced
by PMA combined with rhein as compared to PMA treatment alone.
Similar results were also obtained in the cells treated with
NSC23766 combined with rhein as compared to NSC23766 treatment
alone. The protein expression levels of p38, p-p38 and JNK did not
significantly change in any treatment group, except for rhein plus
NSC23766 group. AP-1 expression level did not change in any
treatment group. A stronger protein phosphorylation of JNK and AP-1
could be induced by a high concentration of rhein (11.3 μM),
whether or not combined with NSC2376, as compared to the control
group.
Discussion
Ovarian cancer is one of the most common malignant
tumors of female reproductive system. Ovarian cancer is a highly
metastatic disease with a lack of early clinical symptoms and
effective diagnosis measures. Therefore, the 5-year survival rate
is about 30–40% and the survival rate of patients with advanced
stage ovarian cancer is still less than 20% (27). Ovarian cancer metastasis and
diffusion include direct spread, lymph node metastasis,
hematogenous metastasis and implantation metastasis (20), while lymph node metastasis is one
of the most important indicators of biological characteristics in
ovarian cancer patients (28,29).
Ovarian cancer remains a formidable treatment challenge, as most
patients with ovarian carcinoma are diagnosed at advanced stage of
disease (30). Therefore, it is
important to study the molecular mechanism of invasion and
metastasis of ovarian cancer cells and there is an urgent need to
develop effective therapeutic drugs for advanced stage ovarian
cancer to inhibit lymph node metastasis of the ovarian cancer
cells, which is of great significance to improve the quality of
life of patients with advanced cancer.
Many studies have shown that rhein possess potent
antioxidant and antiproliferative effects on various cancer cells
(15). Furthermore, it has been
shown that rhein induced apoptosis in SCC-4 cells (17). In our study, the results also
showed that rhein significantly inhibited the migration and
invasion of SKOV3-PM4 cells (Fig.
2). These findings suggest that rhein may be a powerful
candidate agent to prevent ovarian cancer metastasis. However, the
underlying mechanisms of the inhibition of SKOV3-PM4 cell invasion
by rhein are not yet completely elucidated. Therefore, further
studies are required.
The migration of cancer cells is pivotal for cancer
invasion and metastasis (10,11,22).
Cancer malignancy is proportional to the ability of cancer cell
migration. Initially, we measured the effect of rhein on cell
proliferation. As shown in Figs.
1B and 2, rhein can markedly
inhibit not only the growth of SKOV3-PM4 cells but also the
invasive and migration ability of these cells with a low-toxic
concentration (12 μM) in vitro. Our findings also
showed that rhein could decrease the expression levels of tumor
metastasis-associated proteins such as MMPS family (Fig. 5), suggesting that the inhibitory
effect of rhein on SKOV3-PM4 cell invasiveness is via decreased
production of tumor metastasis-related proteins.
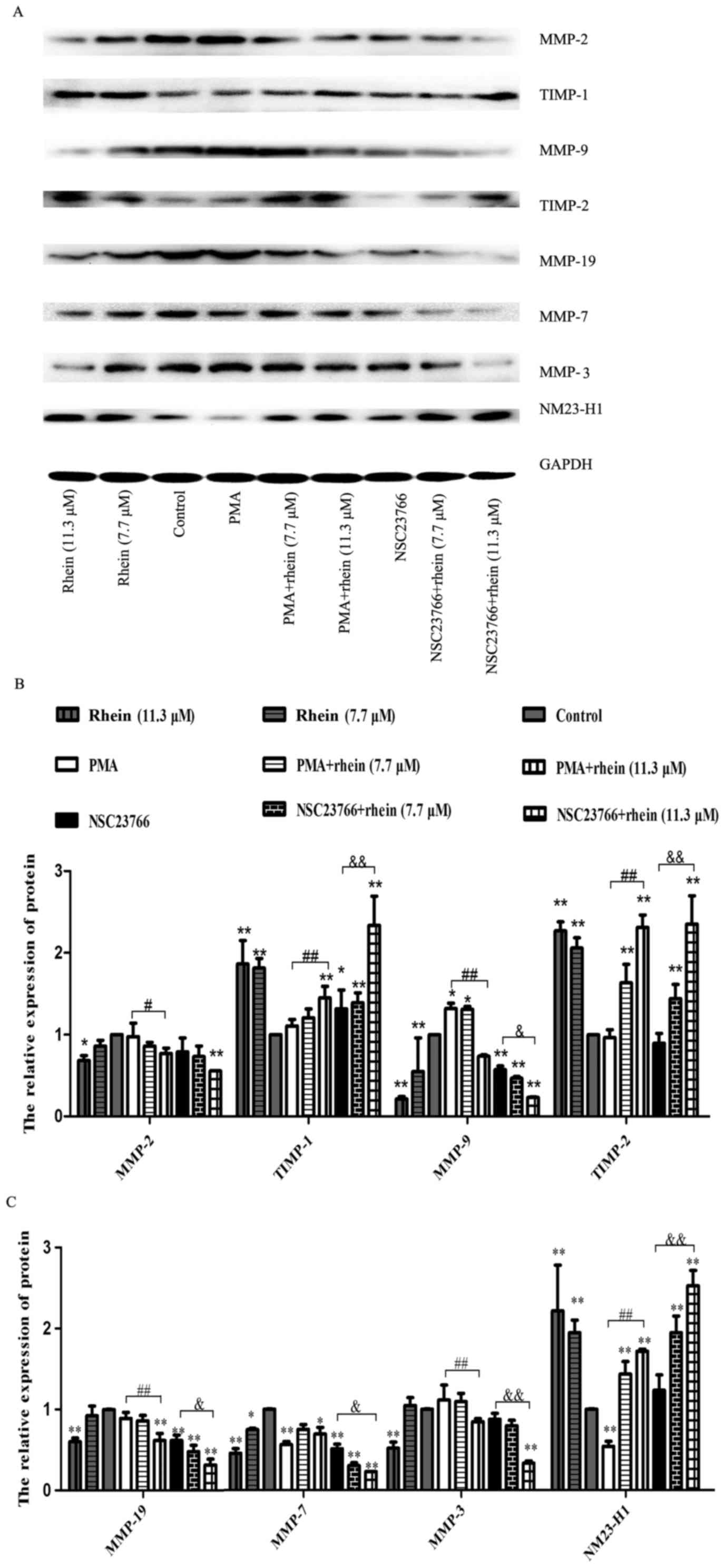 | Figure 5Rhein affects the expression levels
of associated proteins in SKOV3-PM4 cells. The SKOV3-PM4 cells
(1×107 cells/bottle; 75-cm culture bottles) in the
different groups were incubated with drugs for 48 h, then the total
proteins were collected. (A) Protein expressions of MMP-2, MMP-9,
MMP-3, MMP-7, MMP-19, TIMP-1, TIMP-2 and NM23-H1 were measured by
western blotting. (B and C) Statistical analysis of MMP-2, MMP-9,
TIMP-1, TIMP-2, MMP-3, MMP-7, MMP-19 and NM23-H1 expression levels.
Data represent mean ± SD, n=3. *P<0.05 and
**P<0.01 compared to control; #P<0.05
and ##P<0.01 compared to PMA;
&P<0.05 and &&P<0.01
compared to NSC23766. |
Rac1 is well recognized to play a key role in
malignant tumor progression including migration, lamellipodia
formation and cytoskeleton organization (10). Rac1 has a large number of
downstream effectors and so it is critical to inhibit the invasion
of the tumors as important targets. It is well known that Rac1 is
the upstream signaling protein for NADPH oxidase-mediated ROS
production (31). Of note,
non-mitochondrial NADPH oxidase is the major source of PMA-induced
ROS in these tumor cells and has regulating roles in various
physiological and pathological processes (3,4,32,33).
Here, we found that after PMA, activating Rac1 increased the levels
of intracellular ROS indirectly, which induced activation of MAPK
that contributes to cell migration and invasion. We also used
NSC23766 to perform the same experiments. NSC23766, Rac1 inhibitor,
inhibited Rac1 expression and disrupted the microfilament skeleton
rearrangement (34), as well as in
cell functioning, including cell growth, adhesion, migration and
gene transcription. Consistent with our findings rhein and NSC23766
suppress the MAPK signaling responsible for migration and invasion
of SKOV3-PM4 cells through the reduction of Rac1-mediated ROS.
Here, incubation with NSC23766 demonstrated that ROS are also
fundamental for MMP-mediated invasion by SKOV3-PM4 cells (Fig. 5).
There is substantial evidence showing that the ROS
mediated MMP gene expression and regulated downstream signal
molecules, which are involved in tumor invasion of mitogen
activated protein kinase (MAPK) including serine/threonine kinases
consisting of the c-Jun N-terminal kinases (JNKs), the
extracellular signal-regulated kinases (ERKs) and the p38 kinase
(22,35). It was reported that the
transcription of MMP gene is regulated by upstream regulatory
elements, including NF-κB and AP-1 binding sites (26,36).
However, AP-1 is an essential transcriptional factor for the
regulation of the MMP gene expression (36,37).
It has been reported that AP-1 transcription modulate the
expression of c-Fos in the gastric cancer cells (37). Some previous literature also
reported in gastric cancer AGS cells that JNK is critically
required for c-Jun activation, while ERK is the critical kinase for
c-Fos activation (23). Since we
discovered that JNK1/2 and P38 played essential roles in MMP
expression via AP-1, we hypothesized that rhein may work through an
inhibition of JNK1/2 or p38 to suppress MMP expression via AP-1. To
verify our hypothesis, western blotting was performed to determine
the effect of rhein on JNK1/2 or p38 phosphorylation. As shown in
Fig. 6, our data showed that the
PMA treatment led to a remarkable increase in JNK1/2 and AP-1
phosphorylation, while in the presence of PMA plus rhein JNK1/2 and
AP-1 phosphorylation was clearly inhibited. Rhein and NSC23766
significantly reduced the phosphorylation of JNK and AP-1
expression in the cells treated with PMA. Based on the above
conclusion, rhein suppressed mechanism of MMPs through the
inhibition of the Rac1-ROS-p38/JNK MAPK-AP-1 signaling axis.
In conclusion, this study demonstrates that the
reduction in the metastasis ability of rhein is at least partly due
to the inhibition of the Rac1/ROS/MAPK/AP-1 pathway signaling and
the decreased MMP expression. The critical role that we found for
Rac1 in MMP activation, makes Rac1 attractive as a potential
pharmacological target for antitumoral therapy in ovarian cancer.
Therefore, our data collectively suggest that rhein may be a
potential therapeutic agent for controlling ovarian cancer
metastasis.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81360502) and Guangxi
Natural Science Foundation (no. 2014GXNSFAA118225).
References
|
1
|
Park SJ and Jeon YJ: Dieckol from Ecklonia
cava suppresses the migration and invasion of HT1080 cells by
inhibiting the focal adhesion kinase pathway downstream of Rac1-ROS
signaling. Mol Cells. 33:141–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park SJ, Kim YT and Jeon YJ: Antioxidant
dieckol downregulates the Rac1/ROS signaling pathway and inhibits
Wiskott-Aldrich syndrome protein (WASP)-family verprolin-homologous
protein 2 (WAVE2)-mediated invasive migration of B16 mouse melanoma
cells. Mol Cells. 33:363–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Babior BM: NADPH oxidase: An update.
Blood. 93:1464–1476. 1999.PubMed/NCBI
|
|
5
|
Nelson KK and Melendez JA: Mitochondrial
redox control of matrix metalloproteinases. Free Radic Biol Med.
37:768–784. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Velinov N, Poptodorov G, Gabrovski N and
Gabrovski S: The role of matrixmetalloproteinases in the tumor
growth and metastasis. Khirurgiia (Sofiia). 1:44–49. 2010.In
Bulgarian.
|
|
7
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Niwa H, Tsuneyama K and Takano Y: Expressions of MMP-2,
MMP-9 and VEGF are closely linked to growth, invasion, metastasis
and angiogenesis of gastric carcinoma. Anticancer Res.
26A:3579–3583. 2006.
|
|
8
|
Wang FQ, So J, Reierstad S and Fishman DA:
Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by
activation of progelatinase. Int J Cancer. 114:19–31. 2005.
View Article : Google Scholar
|
|
9
|
Zhao H, Yang Z, Wang X, Zhang X, Wang M,
Wang Y, Mei Q and Wang Z: Triptolide inhibits ovarian cancer cell
invasion by repression of matrix metalloproteinase 7 and 19 and
upregulation of E-cadherin. Exp Mol Med. 44:633–641. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nomura N, Nomura M, Mizuki N and Hamada J:
Rac1 mediates phorbol 12-myristate 13-acetate-induced migration of
glioblastoma cells via paxillin. Oncol Rep. 20:705–711.
2008.PubMed/NCBI
|
|
11
|
Kim Y, Lee YS, Choe J, Lee H, Kim YM and
Jeoung D: CD44-epidermal growth factor receptor interaction
mediates hyaluronic acid-promoted cell motility by activating
protein kinase C signaling involving Akt, Rac1, Phox, reactive
oxygen species, focal adhesion kinase, and MMP-2. J Biol Chem.
283:22513–22528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo MZ, Li XS, Xu HR, Mei ZC, Shen W and
Ye XF: Rhein inhibits liver fibrosis induced by carbon
tetrachloride in rats. Acta Pharmacol Sin. 23:739–744.
2002.PubMed/NCBI
|
|
13
|
Aviello G, Rowland I, Gill CI, Acquaviva
AM, Capasso F, McCann M, Capasso R, Izzo AA and Borrelli F:
Anti-proliferative effect of rhein, an anthraquinone isolated from
Cassia species, on Caco-2 human adenocarcinoma cells. J Cell Mol
Med. 14:2006–2014. 2010. View Article : Google Scholar
|
|
14
|
Gao Y, Chen X, Fang L, Liu F, Cai R, Peng
C and Qi Y: Rhein exerts pro- and anti-inflammatory actions by
targeting IKKβ inhibition in LPS-activated macrophages. Free Radic
Biol Med. 72:104–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou YX, Xia W, Yue W, Peng C, Rahman K
and Zhang H: Rhein: A review of pharmacological activities. Evid
Based Complement Alternat Med. 2015:5781072015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang CY, Chan HL, Lin HY, Way TD, Kao MC,
Song MZ, Lin YJ and Lin CW: Rhein induces apoptosis in human breast
cancer cells. Evid Based Complement Alternat Med. 2012:9525042012.
View Article : Google Scholar
|
|
17
|
Chen YY, Chiang SY, Lin JG, Ma YS, Liao
CL, Weng SW, Lai TY and Chung JG: Emodin, aloe-emodin and rhein
inhibit migration and invasion in human tongue cancer SCC-4 cells
through the inhibition of gene expression of matrix
metalloproteinase-9. Int J Oncol. 36:1113–1120. 2010.PubMed/NCBI
|
|
18
|
Lin S, Li JJ, Fujii M and Hou DX: Rhein
inhibits TPA-induced activator protein-1 activation and cell
transformation by blocking the JNK-dependent pathway. Int J Oncol.
22:829–833. 2003.PubMed/NCBI
|
|
19
|
Tang M, Li H, Zhou G, Xie Y, Ruan H and Li
D: Rhein inhibits the movement and invasion of human ovarian
carcinoma cells through Rac1/LIMK1/cofilin signaling pathway. Chin
Pharmacol Bull. 32:366–372. 2016.
|
|
20
|
Xie Y, Zhong Y, Gao T, Zhang X, Li LI,
Ruan H and Li D: Human lymphatic endothelial cells contribute to
epithelial ovarian carcinoma metastasis by promoting
lymphangiogenesis and tumour cell invasion. Exp Ther Med.
11:1587–1594. 2016.PubMed/NCBI
|
|
21
|
Wang C, Pan Z, Hou H, Li D, Mo Y, Mo C and
Li J: The enhancement of radiation sensitivity in nasopharyngeal
carcinoma cells via activation of the Rac1/NADPH signaling pathway.
Radiat Res. 185:638–646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jung JS, Ahn YH, Moon BI and Kim HS:
Exogenous C2 ceramide suppresses matrix metalloproteinase gene
expression by inhibiting ROS production and MAPK signaling pathways
in PMA-stimulated human astroglioma cells. Int J Mol Sci.
17:4772016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia Y, Lian S, Khoi PN, Yoon HJ, Joo YE,
Chay KO, Kim KK and Do Jung Y: Chrysin inhibits tumor
promoter-induced MMP-9 expression by blocking AP-1 via suppression
of ERK and JNK pathways in gastric cancer cells. PLoS One.
10:e01240072015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shuman Moss LA, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: Changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Kuang L, Pan X, Liu J, Wang Q, Du
B, Li D, Luo J, Liu M, Hou A, et al: Isoalvaxanthone inhibits colon
cancer cell proliferation, migration and invasion through
inactivating Rac1 and AP-1. Int J Cancer. 127:1220–1229. 2010.
View Article : Google Scholar
|
|
26
|
Woo JH, Lim JH, Kim YH, Suh SI, Min DS,
Chang JS, Lee YH, Park JW and Kwon TK: Resveratrol inhibits phorbol
myristate acetate-induced matrix metalloproteinase-9 expression by
inhibiting JNK and PKC delta signal transduction. Oncogene.
23:1845–1853. 2004. View Article : Google Scholar
|
|
27
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pepper MS: Lymphangiogenesis and tumor
metastasis: Myth or reality? Clin Cancer Res. 7:462–468.
2001.PubMed/NCBI
|
|
29
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olivier RI, Lubsen-Brandsma MA, Verhoef S
and van Beurden M: CA125 and transvaginal ultrasound monitoring in
high-risk women cannot prevent the diagnosis of advanced ovarian
cancer. Gynecol Oncol. 100:20–26. 2006. View Article : Google Scholar
|
|
31
|
Wu WS: The signaling mechanism of ROS in
tumor progression. Cancer Metastasis Rev. 25:695–705. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Storz P: Reactive oxygen species in tumor
progression. Front Biosci. 10:1881–1896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ushio-Fukai M and Nakamura Y: Reactive
oxygen species and angiogenesis: NADPH oxidase as target for cancer
therapy. Cancer Lett. 266:37–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen QY, Zheng Y, Jiao DM, Chen FY, Hu HZ,
Wu YQ, Song J, Yan J, Wu LJ and Lv GY: Curcumin inhibits lung
cancer cell migration and invasion through Rac1-dependent signaling
pathway. J Nutr Biochem. 25:177–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shin I, Kim S, Song H, Kim HR and Moon A:
H-Ras-specific activation of Rac-MKK3/6-p38 pathway: Its critical
role in invasion and migration of breast epithelial cells. J Biol
Chem. 280:14675–14683. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee HJ, Hwang E, Park B, Zhang M, Sun ZW,
Lee DG, Park SY and Yi TH: Methanol extract of bitter melon
alleviates UVB-induced MMPs expression via MAP kinase and AP-1
signaling in human dermal fibroblasts in vitro. Phytother Res.
30:1519–1526. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pulverer BJ, Kyriakis JM, Avruch J,
Nikolakaki E and Woodgett JR: Phosphorylation of c-jun mediated by
MAP kinases. Nature 3. 53:670–674. 1991. View Article : Google Scholar
|