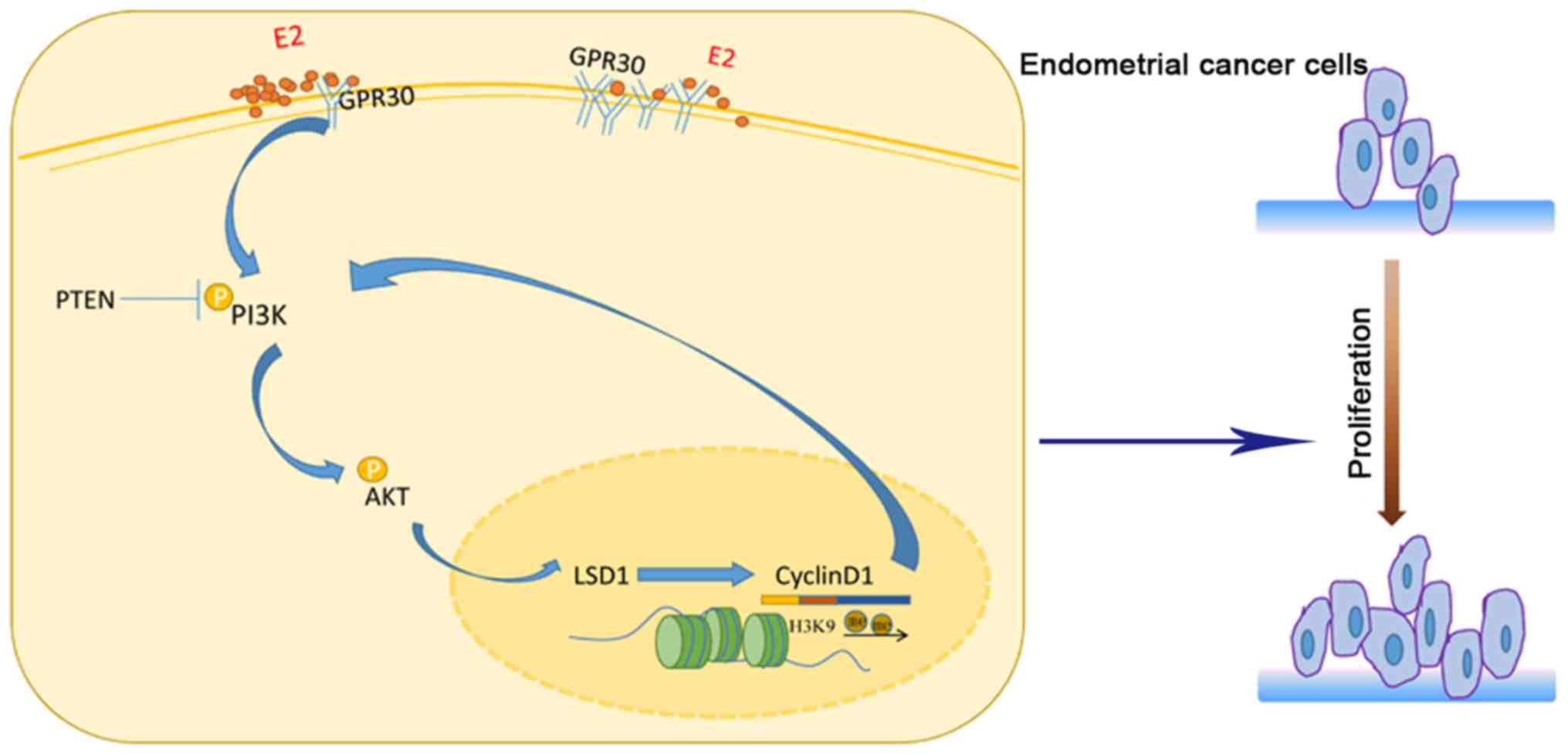
Introduction
Endometrial cancer (EC) is the most commonly
diagnosed gynecological cancer. The National Cancer Institute
estimates ~60,050 new cancer cases and 10,470 deaths in the United
States in 2016 (1). Endometrial
cancer consists of two types: estrogen dependent (type I) and
non-estrogen dependent (type II) (2); type I accounts for >70–80% of all
endometrial cancer types (3,4). In
recent years, estrogen has been considered to be the classic
etiologic factor for type I endometrial cancer tumorigenesis, which
is directly related to PTEN loss or mutation, PI3K/AKT, MAPK/ERK
and Wnt/B-catenin pathway activation (3,5–8).
Although many studies have been conducted, the modulation
mechanisms of estrogen-driven endometrioid endometrial carcinoma
remain poorly understood.
Histone lysine methylation is a crucial regulator of
transcription, and its dysregulation is associated with oncogenesis
(9–11). As the first demethylase detected by
Shi and coworkers in 2004 (11),
histone lysine specific demethylase 1 (LSD1, KDM1A) is an amine
oxidase that functions as a histone demethylase that specifically
mono- or di-demethylates histone 3 lysine 4 (H3K4) or lysine 9
(H3K9) and a transcriptional co-repressor included in the
REST/Co-Rest complex (12,13). LSD1 expression is elevated in many
human malignancies, such as breast cancer (14), prostate cancer (15), pancreatic cancer (16), ovarian cancer (17), small cell lung carcinoma (18) and leukemia (19). Theisen et al (20) demonstrated that the LSD1 inhibitor
HCI2509 inhibited the proliferation of type II endometrial
carcinoma cell lines by disturbing cell cycle progression and
inducing apoptotic cell death. Recently, Liu et al (21) reported that LSD1 was overexpressed
in endometrioid endometrial adenocarcinoma (EEA) and is related to
overall survival (OS) and disease-free survival (DFS) of EEA
patients. However, the underlying biological function and molecular
mechanism of LSD1 in EEA remains largely unknown.
Increasing evidence suggested that LSD1 play a major
role in hormone-dependent gene expression and proliferation
processes of cell growth and is related to transcriptional
regulation of estrogen- or androgen-responsive genes (22,23).
Estrogen stimulation increases LSD1 level in the EREs of pS2 and PR
genes in breast cancer (24). It
had been demonstated that LSD1 can be recruited by nuclear receptor
TLX to the promoter of PTEN to downregulate it (25,26),
which is a negative regulator of the PI3K/AKT pathway (27). Inactivating PI3K/AKT signaling
could block EGF-induced expression of LSD1 in ovarian cancer
(28). These findings imply that
LSD1 may be involved in PI3K/AKT signaling and might play a
critical role in EEC occurrence and development. As expected, this
study found remarkable upregulation of LSD1 after E2 treatment in
endometrial cancer. In addition, we found that LSD1/cyclin
D1/PI3K/AKT established a feedback loop in the regulation of the
proliferation of endometrial cancer Ishikawa and HEC-1-A cells. Our
findings provide a novel insight into the mechanism of LSD1 in
carcinogenesis.
Materials and methods
Ethics statement
Our study was permitted by the Ethics Committee of
both Shanghai General Hospital (Shanghai, China) and Shanghai Jiao
Tong University School of Medicine, and written consent was
obtained from all participants.
Cell lines and cell culture
Endometrial cancer cell lines Ishikawa, RL-95-2,
HEC-1-A, and HEC-1-B were maintained in our laboratory and were
initially obtained from the ATCC. Cells were cultured in 1:1
DMEM/F12 (Gibco, Auckland, New Zealand) with 5%
penicillin-streptomycin and 10% fetal bovine serum (Gibco,
Gaithersburg, MD, USA).
Gene silence and plasmid
transfection
Ishikawa and HEC-1-A cells were seeded in a 6-well
plate. The LSD1, cyclin D1 and GPR30 small interfering RNAs (siRNA)
were designed and synthesized by RiboBio Inc. (RiboBio, Guangzhou,
China) to knock down the LSD1, cyclin D1, GPR30 genes at a 50 nM
concentration, respectively. The sequences are as follows: siLSD1:
5′-CCACGAGUCAAACCUUUAUTT-3′ (F), 5′-AUAAAGGUUUGACUCGUGGTT-3′ (R);
siGPR30: 5′-GCUGUACAUUGAGCAGAAATT-3′ (F),
5′-UUUCUGCUCAAUGUACAGCTT-3′ (R); sicyclin D1:
5′-UGGAAUAGCUUCUGGAAUUdTdT-3′ (F), 5′-dTdAACCUUAUCGAAGACCUUAA-3′
(R); siCtrl served as a transfection control:
5′-UUCUCCGAACGUGUCACGUTT-3′ (F), 5′-ACGUGACACGUUCGGAGAATT-3′ (R).
The cyclin D1 plasmid was purchased from the Public Protein/Plasmid
Library (Genecopoeia, Nanjing, China).
Hormone and drug treatments
To investigate the effects of β-estradiol on LSD1
expression, Ishikawa and HEC-1-A cells were treated with different
concentrations of E2 (Sigma-Aldrich, St. Louis, MO, USA) for 24 h,
followed by western blotting to determine LSD1 protein changes.
Subsequently, cells were treated with E2 (1 nM) for various times
to examine the effect of time. To further analyze whether the
PI3K/AKT pathway is essential for E2 induced LSD1 accumulation,
LY294002 (10 mM, Sigma-Aldrich), a specific inhibitor for PI3K/AKT,
was used to pre-treat cells for 1 h prior to E2 treatment for 48 h,
and the changes in relevant protein levels were examined by western
blotting. Cells were transfected with two sequences of siLSD1 or
siControl prior to ethanol or E2 (1 nM) treatment for another 48 h
to investigate their roles in cell proliferation, cell cycle and
cell apoptosis.
Immunoblotting and antibodies
Whole cells were lysed in lysis buffer on ice. Then,
total protein was loaded onto SDS-PAGE and transferred onto
polyvinylidene fluoride membranes (PVDF). Then, the membranes were
blocked with 5% BSA (Roche, Mannheim, Germany) for 1 h prior to
incubation overnight with primary antibodies at 4°C on a shaking
table. Antibodies against human LSD1, PTEN, AKT, phosphorylated AKT
(Ser473), ERK, phosphorylated ERK (Thr202/Tyr204), and cleaved
caspase-3 (1:1,000 diluted in 5% BSA) were purchased from Cell
Signaling Technology Inc. Antibodies against human cyclin D1, P21,
and histone H3 (1:1,000 diluted in 5% BSA) were purchased from
Abcam Inc. Antibodies against human β-actin and GAPDH (1:2,000
diluted in 5% BSA) were purchased from Abzoom Biolabs. Inc.
Immunodetection was achieved after incubation with the secondary
antibodies (1:5,000, West Grove, PA, USA) in BSA for 1 h at room
temperature. ECL chemiluminescent reagents were used to reveal the
target signal on the membranes.
Tissue samples and
immunohistochemistry
Tissues samples were obtained from 48 endometrial
cancer patients and 25 patients with normal endometrium who
underwent hysterectomy operation at Shanghai General Hospital from
2008 to 2015. IHC analyses of LSD1 protein levels were implemented
as previously described (29). The
sections were incubated with rabbit anti-human LSD1 and cyclin D1
antibody (diluted to 1:500; CST). Expression of LSD1 protein was
assessed by the following method: the index of LSD1 expression was
calculated as the intensity of the staining (0–3) × the percentage
of positively stained cells (0–3). The final histological staining
scores (HSS) were divided into two groups as followed:
low-expression group (HSS <4) and high-expression group (HSS
≥4). In 48 endometrial tissues, the expression of LSD1 and the
cyclin D1 level were analyzed by Pearson correlation test.
CCK-8 assay
Cells were plated into 96-well plates (2,000 cells
per well), and incubated for 24 h. CCK-8 solution (Signalway
Antibody Co., Ltd. MD, USA) was added for another 2 h and then
incubated for 12, 24 and 48 h. Then, the absorbance was measured at
450 nM with a GENios multifunction reader (Tecan, Zurich,
Switzerland).
Clonogenic assays
Cells were seeded in capsules at a density of 1,000
cells/plate after treatment. After 3 weeks of incubation, colonies
of >50 cells were produced, which were photographed.
Cell apoptosis and cell cycle
analysis
Ishikawa and HEC-1-A cells were cultured, treated
with or without 1 nM estradiol and LSD1 siRNA, and harvested at the
indicated times. To analyze cell apoptosis, cells were collected
and washed with ice-cold PBS three times 24 h after transfection.
Cells were incubated with PE Annexin V and propidium iodide (PI)
according to the PE Annexin V Apoptosis Detection Kit I (BD
Pharmingen, CA, USA) protocol and analyzed using a BD FACSCalibur.
For cell cycle analysis, cells were fixed in ice-cold ethanol (75%)
and incubated at 4°C overnight. Then, the cells were stained with
10 µl of PI (10 mg/ml) in the presence of 10 µg/ml
RNase A and analyzed using a BD Biosciences FACS Aria flow
cytometer.
Chromatin immunoprecipitation
Cells were fixed in formaldehyde, lysed, and
sonicated to break the chromatin into ~500-bp fragments. A
ChIP-grade antibody against H3K9me2 was used to precipitate
chromatin fragments from cell extracts. Isotype-specific IgG was
used as a negative control. We used real-time quantitative PCR to
amplify the DNA fragments in the antibody precipitated DNA and the
unprecipitated input DNA was used to calculate the percentage. The
PCR primer set used for amplification of the precipitated fragments
was F, ACGAAGTTCCTAGTCGAGAT; R, CGCGTGCGCCCTGGCCCAG.
Statistical analysis
All values are expressed as the mean ± standard
error. The results were analyzed by two-way analysis of variance or
t-test as appropriate with SAS Release 8.02 (SAS Institute Inc.,
Cary, NC, USA) or GraphPad Prism v5.0 (GraphPad, San Diego, CA,
USA). P<0.05 was considered statistically significant. All
experiments were performed in triplicate.
Results
LSD1 is highly expressed in human
endometrial cancer tissues and cell lines
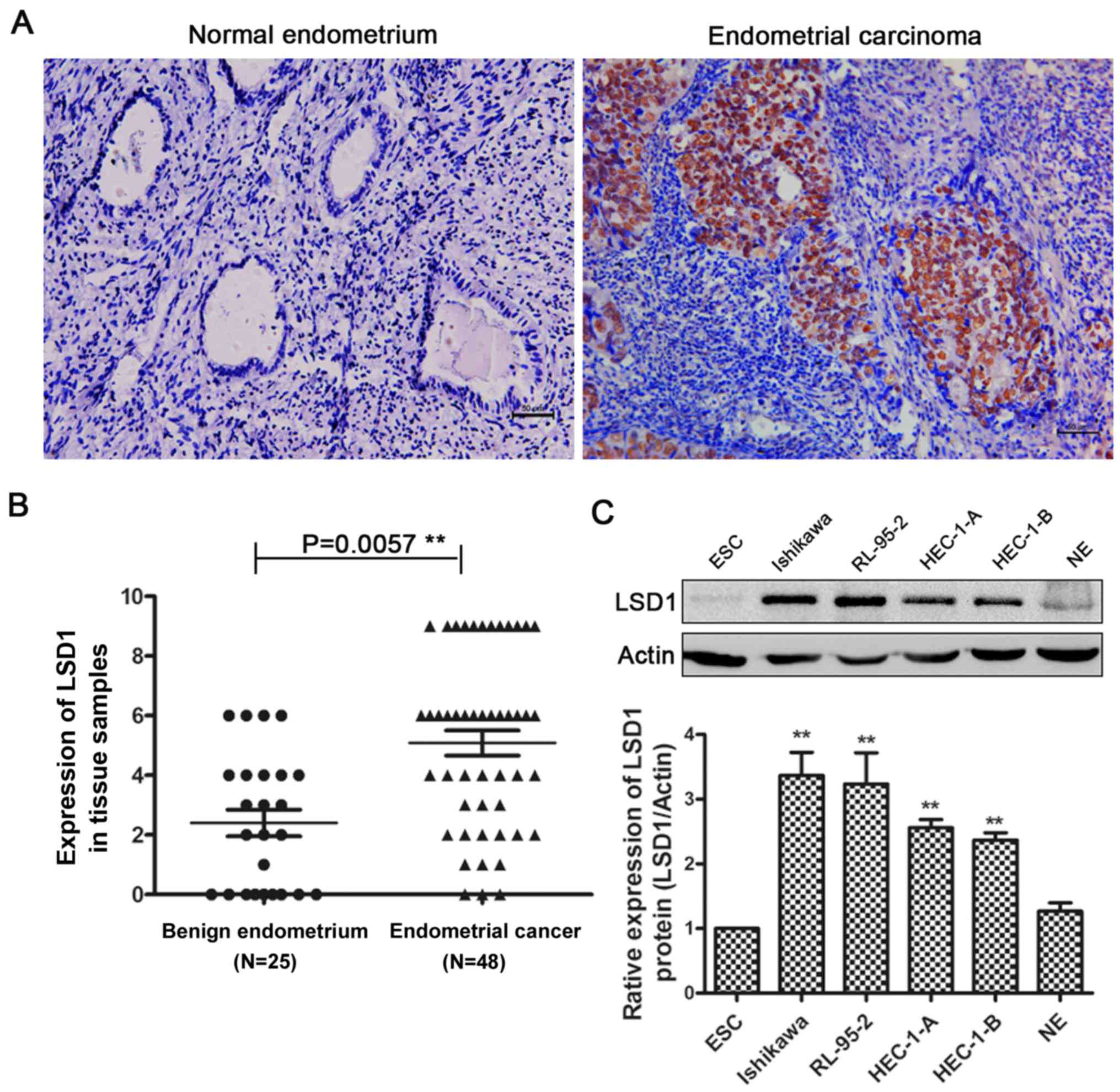
We performed immunohistochemistry (IHC) in normal
endometrium and endometrial cancer tissues. Compared with normal
endometrial tissues, endometrial carcinoma tissues had more
positive IHC staining of LSD1 in the nucleus (Fig. 1A), and IHC scoring confirmed the
significantly higher LSD1 protein expression in carcinoma tissues
(Fig. 1B). LSD1 expression was
showed a positive result with the FIGO stage (P=0.015) and tumor
grade (P=0.031), but no relationship was observed between LSD1
expression and the age, nodal metastasis or the depth of tumor
myometrial invasion (Table I).
Furthermore, we examined the expression of LSD1 in several human
endometrial cancer cell lines, with protein from primary cultured
normal endometrial cells (NE), and endometrial stromal cells (ESC)
as controls (Fig. 1C). The results
suggested that LSD1 expression is high in these human endometrial
cancer cell lines, with the highest levels observed in Ishikawa
cells. We chose Ishikawa and HEC-1-A cells in the following
investigation.
 | Table IAssociation between clinical
characteristics of LSD1 expression in endometrial cancer
patients. |
Table I
Association between clinical
characteristics of LSD1 expression in endometrial cancer
patients.
| Parameters | N=48 | LSD1 expression
| P-value |
|---|
Low
(N=15)
(%) | High
(N=33)
(%) |
|---|
| Age | | | | 0.809 |
| <50 | 18 | 6 (40.0) | 12 (36.3) | |
| ≥50 | | | | |
| FIGO stage | 30 | 9 (60.0) | 21 (63.6) | |
| Stage I-II | 26 | 12 (80.0) | 14 (42.4) | 0.015 |
| Stage III-IV | 22 | 3 (20.0) | 19 (57.6) | |
| Grade |
| G1–G2 | 31 | 13 (86.7) | 18 (54.5) | 0.031 |
| G3 | 17 | 2 (13.3) | 15 (45.5) | |
| Nodal
metastasis |
| Positive | 6 | 0 (0.0) | 6 (18.2) | 0.078 |
| Negative | 42 | 15 (100.0) | 27 (81.8) | |
| Invasion |
| <1/2 | 31 | 10 (66.7) | 21 (63.6) | 0.839 |
| ≥1/2 | 17 | 5 (33.3) | 12 (36.4) | |
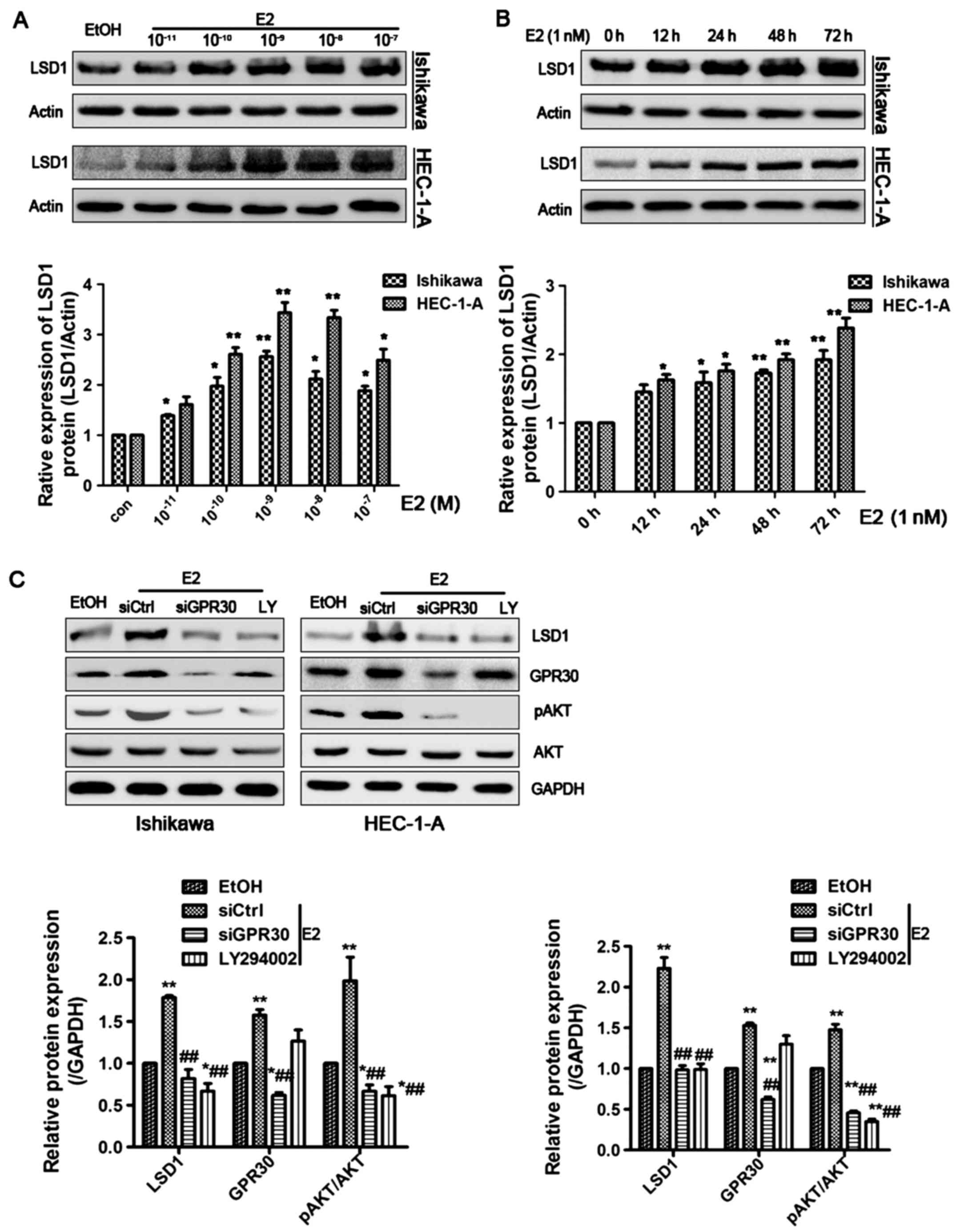
E2 induces LSD1 expression through the
GPR30/PI3K/AKT signal pathway
17β-estradiol (E2) was shown to increase the protein
level of LSD1 in a dose-dependent manner, with the greatest effect
at a dose of 1 nM in both Ishikawa and HEC-1-A cells (Fig. 2A). Moreover, we found that E2 (1
nM) markedly elevated LSD1 protein expression in a time-dependent
manner (Fig. 2B). To further
investigate the underlying molecular mechanisms, we treated
Ishikawa and HEC-1-A cells with the membrane-associated estrogen
receptor, GPR30 siRNA, and LY294002, a specific inhibitor for
PI3K/AKT pathway for 1 h prior to E2 (1 nM) treatment for 48 h.
E2-induced activation of LSD1 was attenuated by siGPR30 or LY294002
treatment (Fig. 2C). These data
indicate that E2 enhances LSD1 expression through the activation of
the GPR30/PI3K/AKT signaling pathways.
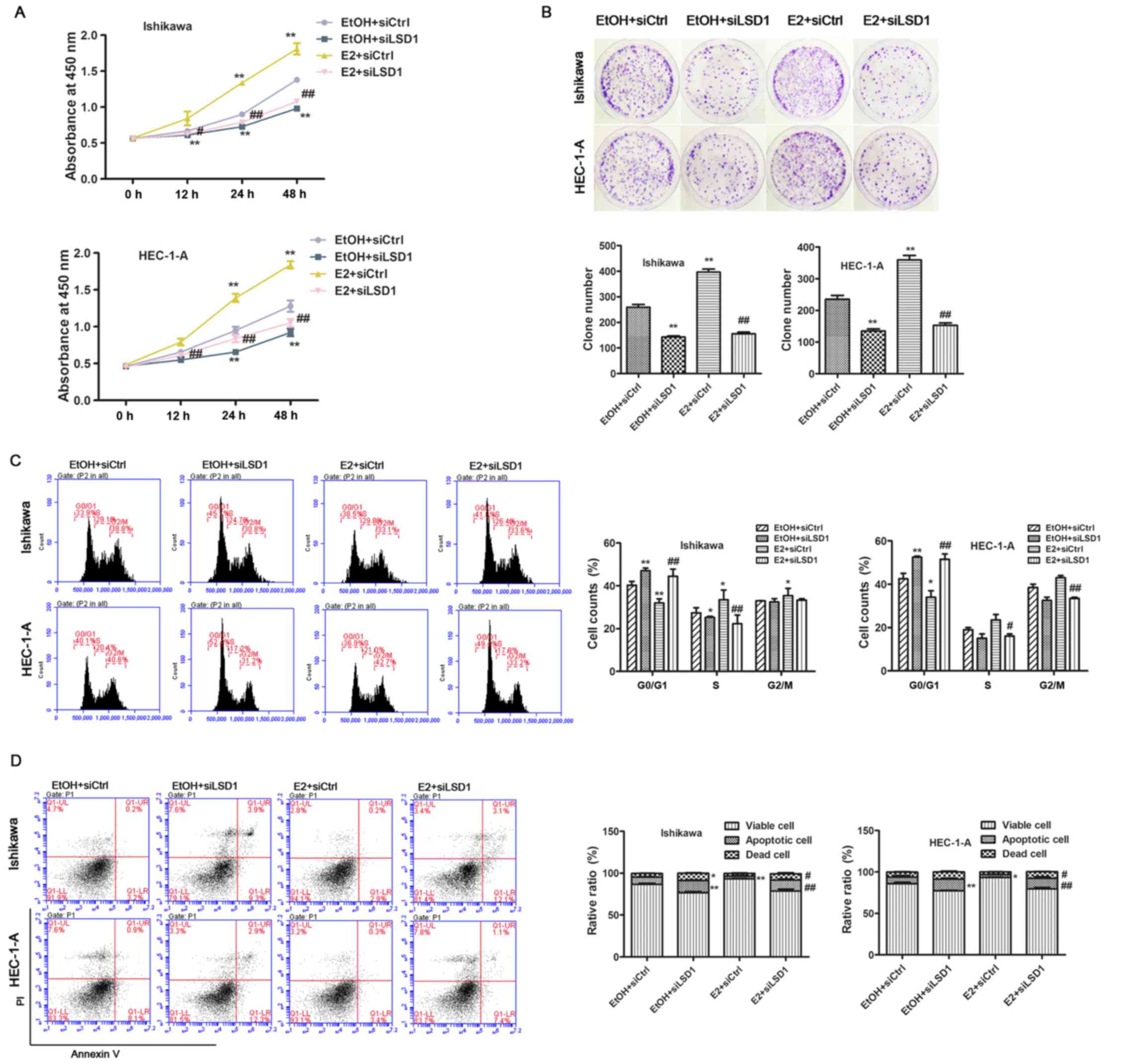
Upregulation of LSD1 is required for
estrogen-induced ECC proliferation
Since the observation of significant accumulation of
LSD1 in endometrial carcinoma, we speculated that LSD1 might play a
crucial role in estrogen-driven cellular proliferation. To confirm
this hypothesis, we first explored the biological function of LSD1
in endometrial cancer cells, we knocked down LSD1 prior to ethanol
(EtOH) or E2 (1 nM) treatment in both Ishikawa and HEC-1-A cells. A
CCK-8 assay revealed that cellular proliferation was decreased in
LSD1-depleted cells at 24 and 48 h compared to negative control
(siCtrl) transfected cells. Estrogen addition greatly promoted cell
growth post E2 treatment for 24 h, while the knockdown of LSD1
significantly abolished E2-enhanced proliferation (Fig. 3A). In addition, the attenuation of
LSD1 caused significant inhibition of cell growth compared to
siCtrl cells as measured by clonogenic assays (Fig. 3B). Because cell cycle and cell
apoptosis are closely associated with cell growth, we performed
cell apoptosis detection and flow cytometric analysis of the cell
cycle. As a result, we found that siLSD1 treatment led to acute
arrest in the G0/G1 phase (Fig.
3C) and induction of apoptosis (Fig. 3D) under both EtOH and E2
treatments.
LSD1 establishes a feedback loop in
PI3K/AKT/cyclin D1 axis by demethylating H3K9me2 at the promoter of
cyclin D1
To further assess the molecular mechanisms
responsible for the growth inhibition function of LSD1 in
estrogen-driven endometrial cancer cells, given that PI3K/AKT and
MAPK-ERK signaling pathways are critical in type I endometrial
cancer development and that hyperactivation of the two pathways
contributed to enhanced tumorigenic proliferation, we examined
PI3K/AKT, ERK1/2 using western blotting in treated Ishikawa and
HEC-1-A cells. The levels of phospho-AKT were notably reduced, as
expected, by LSD1 silencing in the EtOH and E2 treated groups,
while phospho-ERK expression had little change (Fig. 4A). This implied that LSD1 could
promote ECC proliferation via the activation of PI3K/AKT signaling
but not the MAPK-ERK pathway. Interestingly, no PTEN upregulation
was found in both Ishikawa cells (PTEN−) or HEC-1-A
cells (PTEN+) (30)
when LSD1 was knocked down, which suggested LSD1 affects PI3K/AKT
signaling via other pathways rather than regulating PTEN. We then
detected PI3K/AKT downstream cell cycle and apoptosis related
proteins. The attenuation of LSD1 by siRNA caused downregulation of
cyclin D1, but did not change Bcl-2 (data not shown) compared to
siCtrl groups with EtOH treatment. In contrast, P21
(cyclin-dependent kinase inhibitor 21) and cleaved caspase-3 were
upregulated. E2 stimulated the expression of phospho-AKT,
phospho-ERK, cyclin D1, Bcl-2, and weakened the expression of P21
and cleaved caspase-3. When LSD1 was silenced, we found
phospho-AKT, cyclin D1 and Bcl-2 were ablated. Moreover, P21 and
cleaved caspase-3 were upregulated (Fig. 4B). Because there was a dramatic
change in cyclin D1 expression after treatment with LSD1-specific
RNAi, and a recent study reported that cyclin D1 interference
inhibits PI3K/AKT level in colon cancer cells (31), we speculated cyclin D1 may play a
similar role in ECCs. To verify this, we interfered with cyclin D1
using siRNA, and a western blotting confirmed that the
phosphorylation levels of p-AKT and Bcl-2 were reduced as expected
(Fig. 4C). Cleaved caspase-3
expression was elevated. When we re-overexpressed cyclin D1 in
Ishikawa and HEC-1-A cells, levels of p-AKT, Bcl-2 and cleaved
caspase-3 were restored. As expected, CCK-8 assays illustrated that
the overexpression of cyclin D1 could counteract the LSD1 effects
on cellular proliferation inhibition in ECCs while LY294002
addition led to extreme proliferation inhibition in ECCs (Fig. 4D). An increase in total H3K9me2 was
observed by western blotting when LSD1 was silenced in E2 treated
HEC-1-A cells. We did not detect obvious H3K4me2 changes (Fig. 4E). To test whether LSD1 contributes
to the direct increases of cyclin D1 gene expression by
demethylation in EEC, we performed chromatin immunoprecipitation
(ChIP) analyses, H3K9 dimethylation levels were markedly increased
compared to siControl treated HEC-1-A cells (Fig. 4F).
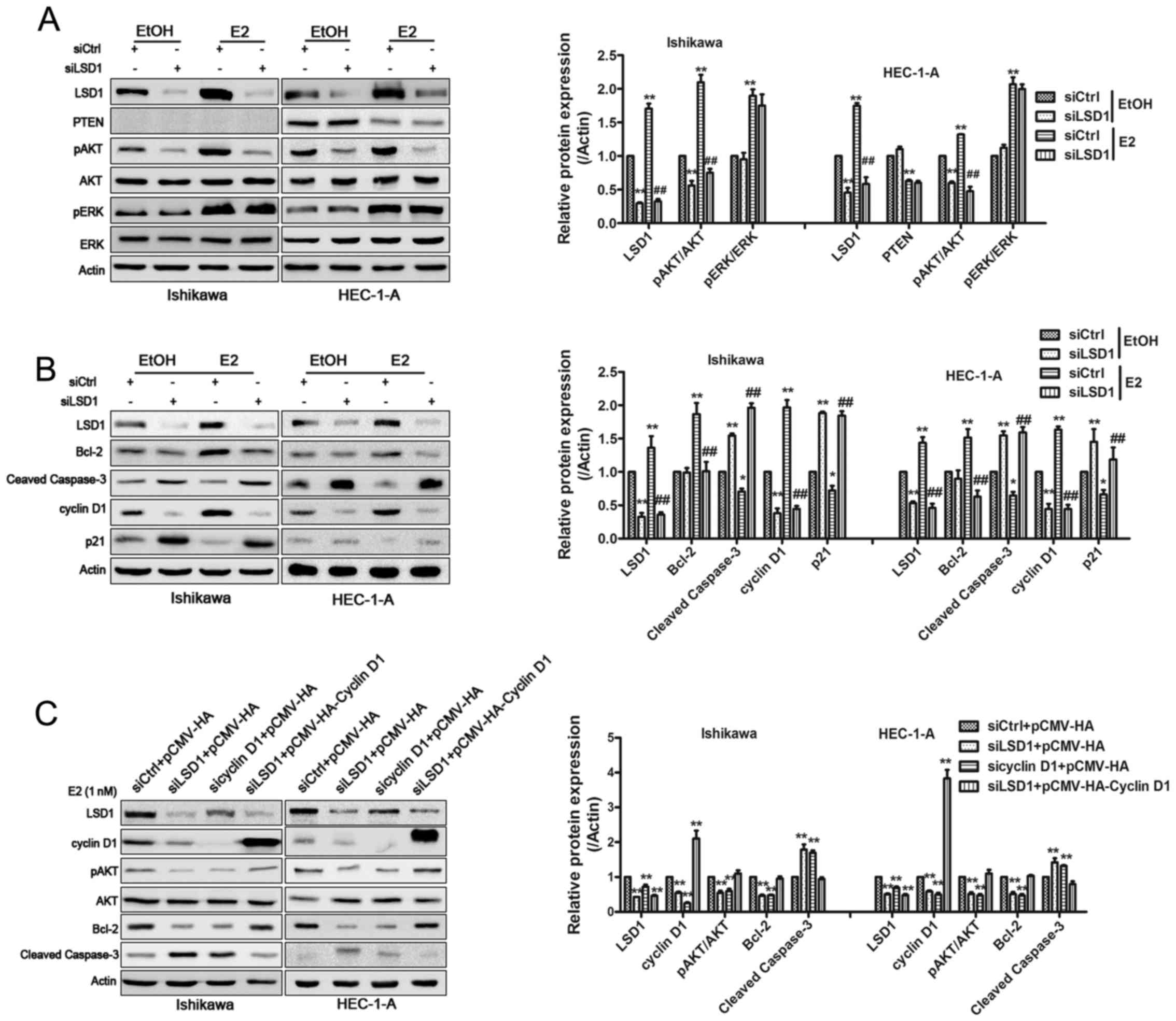 | Figure 4LSD1 establishes a positive-feedback
loop in PI3K/AKT signaling. Western blotting was performed to
analyze the expression of AKT, p-AKT, ERK, p-ERK, PTEN (A) and
cyclin D1, P21, Bcl-2 and cleaved caspase-3 (B).
*P<0.05, **P<0.01 compared with
siControl groups. #P<0.05, ##P<0.01
compared with the siControl groups treated with E2. (C) Plasmid
encoding cyclin D1 to LSD1-knockdown cells rescued the inhibitory
effects in pAKT expression and changes of cyclin D1, Bcl-2 and
cleaved caspase-3. *P<0.05, **P<0.01
compared with control groups. (D) CCK-8 assays were conducted to
quantify cell viability for relevant treated Ishikawa and HEC-1-A
cells. *P<0.05, **P<0.01 compared with
siControl+pCMV-HA groups. #P<0.05,
##P<0.01 compared with the siLSD1+pCMV-HA-cyclin D1
groups. (E) Total H3K4me2 and H3K9me2 levels were assessed by
western blotting after knockdown of LSD1 in E2 treated HEC-1-A
cells. **P<0.01 compared with control groups. (F)
ChIP analysis using an H3K9me2 antibody showed that the knockdown
of LSD1 induced accumulation of H3K9me2 at the promoter region of
the cyclin D1. IgG is used as a negative control.
**P<0.01 compared with control groups. |
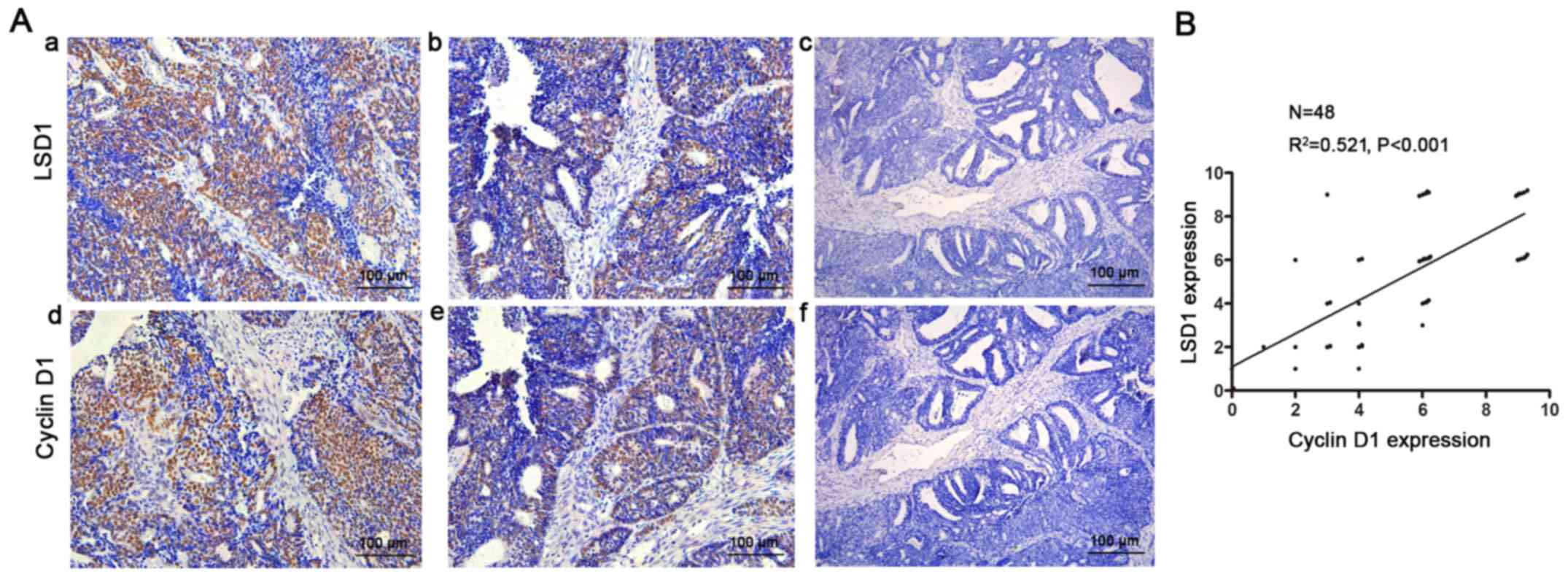
LSD1 is positively correlated with
cyclinD1 in EEC tissues
Furthermore, we confirmed this phenomenon in
endometrial cancer tissues. We detected LSD1 and cyclin D1 using
IHC staining in EEC tissue specimens (Fig. 5A). Consistently, correlation
analysis showed that the expression of LSD1 was positively
correlated with the level of cyclin D1 (R2=0.521,
P<0.001, Fig. 5B).
Discussion
Modification of histone is not only associated with
the chromosome remodeling and function, but also important in
determining the cell fate, cell growth, as well as carcinogenesis
(32). Lysine-specific demethylase
1, the first found histone demethylase, has been implicated in the
process of tumor progression at various stages. Recently, it has
been reported that LSD1 is involved in endometrioid endometrial
carcinoma, but the underlying mechanisms have yet to be elucidated.
In this study, we investigated how LSD1 functions in
estrogen-induced endometrial cancer cells.
As an estrogen-related carcinoma, type I endometrial
cancer occurs and evolves under continuous estrogen stimulation
(4). Mechanistically, estrogen
activates estrogen receptors through a genomic pattern by nuclear
receptors (ERα/ERβ) and a non-genomic pattern by transmembrane ER
(GPR30) (33). Pollock et
al (24) found that estrogen
signaling increases LSD1 level in the EREs of pS2 and PR genes and
inhibiting LSD1 activity attenuated E2 signaling in breast cancer.
In this study, we showed that β-estradiol induced the upregulation
of LSD1 in a dose- and time-dependent manner. The GPR30/PI3K/AKT
pathway leads to cellular growth in ECCs (34,35),
and estrogen stimulation upregulated GPR30/PI3K/AKT signaling
(36), our results similarly
confirmed this finding. Shao et al (28) reported that inactivating the
PI3K/AKT pathway but not the ERK pathway could block EGF-induced
expression of LSD1 in ovarian cancer cells. When GPR30 is knocked
down or PI3K/AKT is inactivated by LY294002, LSD1 expression
previously activated by estrogen disappeared, which indicated that
GPR30/PI3K/AKT signaling is a determinant for estrogen-induced LSD1
elevation.
LSD1 has been implicated as an oncogene in several
types of cancer and tracks cellular growth pathways (37,38).
We first investigated the biological behavior of LSD1 in ECCs. Our
results demonstrated that LSD1 silencing inhibited cellular
proliferation, enhanced cell cycle G1/S arrest and induced
apoptosis under both EtOH and E2 treatment. This evidence suggests
that LSD1 acts as a tumor promoter in endometrial cancer that
promotes tumor proliferation by inducing various aggressive
physiological behaviors. Lin et al (39) recently found that LSD1 can be
recruited by Snail to the promoter of PTEN, where it demethylates
histone H3 lysine 4 and contributes to transcriptional repression.
Yokoyama et al also reported that the LSD1-CoREST complex
can be recruited by nuclear receptor TLX to the promoter of PTEN to
downregulate PTEN expression (25,26).
Plenty of research work has suggested that the most frequently
altered signaling cascade in EEC is the PI3K/AKT pathway, which is
dysregulated by oncogenic mutations and PTEN dysfunction, resulting
in uncontrolled cell proliferation (40). As a tumor suppressor gene, PTEN
activity can be reduced by subcutaneous estradiol in vivo
and estradiol treatment in vitro (41,42).
This prompted us to examine the role of LSD1 in estrogen-driven
cell growth and influence on PI3K/AKT signaling pathway.
Intriguingly, we found that knocking down LSD1 decreased
estrogen-induced pAKT and altered the expression of certain
downstream genes related to the cell cycle (cyclin D1 and P21) and
cell apoptosis (Bcl-2, cleaved caspase-3), but did not upregulate
PTEN expression in either PTEN-mutated Ishikawa or wtPTEN HEC-1-A
cells as expected. This result indicated that LSD1 plays a critical
role in the estrogen-regulated PI3K/AKT pathway but without causing
significant changes in PTEN expression in EEC.
As a marker of poor prognosis and an important
promoter of the cell cycle, cyclin D1 is overexpressed in various
cancer types, and acts as a sensor in response to a number of
extracellular stimuli (43–45).
Our previous study showed that estrogen enhances ECC proliferation
via promotion of cyclin D1 expression (36). Surprisingly, the depletion of LSD1
tremendously suppressed estrogen-induced cyclin D1 levels, which
lead us to hypothesize that LSD1 carcinogenesis is associated with
cyclin D1 variation. Zhong et al reported that both cyclin
D1 and cyclin D1-CDK4/6 kinase activity can decrease cell motility
and reduce invasion and migration in breast cancer cells (46). Moreover, cyclin D1 was demonstrated
to regulate DICER, a critical component in microRNA biogenesis and
mature microRNA production, and subsequent miRNA expression
(47). Recently, Chen et al
found that cyclin D1 interference inhibits proliferation, invasion
and migration by reducing PI3K/AKT levels in colon cancer cells
(31). We speculated that cyclin
D1 also plays an important role in LSD1-regulated estrogen-induced
endometrial cancer cell proliferation. Our study confirmed this
role, knockdown of cyclin D1 reduced LSD1 expression, inhibited
p-AKT and its downstream gene Bcl-2 while upregulating cleaved
caspase-3, consistent with the siLSD1 groups. Overexpression of
cyclin D1 reversed the effects of the PI3K/AKT pathway in LSD1
silencing Ishikawa and HEC-1-A cells. Additional PI3K/AKT
inhibition led to the blocking of proliferation, which indicated
the existence of a LSD1/cyclin D1/PI3K/AKT feedback loop in EEC. By
ChIP assay, we showed that LSD1 removes transcriptionally
repressive di-methyl marks from H3K9 in the cyclin D1 promoter
region. The results presented here demonstrate that LSD1 induces
EEC growth through downregulating the expression of cyclin D1 via
demethylating H3K9me2, which can be reversed when cyclin D1 is
simultaneously upregulated. Correlation analysis by
immunohistochemistry also verified the positive correlation between
LSD1 and cyclin D1 in endometrial cancer tissues.
In conclusion, our findings present the first
evidence that LSD1 plays an essential role in estrogen-regulated
type I endometrial cancer and establishes a crucial LSD1/cyclin
D1/PI3K/AKT feedback loop in endometrial cancer cells (Fig. 6), which supplements the epigenetic
characteristics of endometrial cancer. As a novel agent in
endometrial cancer, LSD1 will be a potent therapeutic target in the
future. In recent years, the enzymatic activity of LSD1 and its
overexpression in many human malignancies has become a significant
focus for the development of pharmacologic inhibitors (48). Based on these results, further
studies are underway to elucidate the molecular mechanisms of LSD1
and to seek effective LSD1 inhibitors in order to better understand
the molecular basis of endometrial cancer.
Abbreviations:
|
EC
|
endometrial cancer
|
|
EEC
|
endometrioid endometrial carcinoma
|
|
LSD1
|
histone lysine specific demethylase
1
|
|
ATCC
|
American Type Culture Collection
|
|
Bcl-2
|
B cell leukemia lymphoma-2
|
|
H3K4m2
|
dimethylation of histone H3 lysine
4
|
|
H3K9m2
|
dimethylation of histone H3 lysine
9
|
|
E2
|
17β-estradiol
|
|
HSS
|
histological staining scores
|
|
EtOH
|
ethanol
|
|
P21
|
cyclin-dependent kinase inhibitor
21
|
|
caspase-3
|
cysteinyl aspartate-specific
proteinase-3
|
|
ChIP
|
chromatin immunoprecipitation
|
Acknowledgments
This study was supported by the National Key
Clinical Specialist Construction Programs of China, the National
Natural Science Foundation of China (NSFC nos. 81201541, 81502230
and 81402134) and the Shanghai Pu Jiang Talent Program
(12PJD002).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deligdisch L and Holinka CF: Endometrial
carcinoma: Two diseases? Cancer Detect Prev. 10:237–246.
1987.PubMed/NCBI
|
|
3
|
Makker A and Goel MM: Tumor progression,
metastasis, and modulators of epithelial-mesenchymal transition in
endometrioid endometrial carcinoma: An update. Endocr Relat Cancer.
23:R85–R111. 2016. View Article : Google Scholar
|
|
4
|
Gibson WJ, Hoivik EA, Halle MK,
Taylor-Weiner A, Cherniack AD, Berg A, Holst F, Zack TI, Werner HM,
Staby KM, et al: The genomic landscape and evolution of endometrial
carcinoma progression and abdominopelvic metastasis. Nat Genet.
48:848–855. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Westin SN, Ju Z, Broaddus RR, Krakstad C,
Li J, Pal N, Lu KH, Coleman RL, Hennessy BT, Klempner SJ, et al:
PTEN loss is a context-dependent outcome determinant in obese and
non-obese endometrioid endometrial cancer patients. Mol Oncol.
9:1694–1703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slomovitz BM and Coleman RL: The
PI3K/AKT/mTOR pathway as a therapeutic target in endometrial
cancer. Clin Cancer Res. 18:5856–5864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Jia Y, Che Q, Zhou Q, Wang K and Wan
XP: AMF/PGI-mediated tumorigenesis through MAPK-ERK signaling in
endometrial carcinoma. Oncotarget. 6:26373–26387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, van der Zee M, Fodde R and Blok
LJ: Wnt/β-catenin and sex hormone signaling in endometrial
homeostasis and cancer. Oncotarget. 1:674–684. 2010. View Article : Google Scholar
|
|
9
|
Strahl BD and Allis CD: The language of
covalent histone modifications. Nature. 403:41–45. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elsheikh SE, Green AR, Rakha EA, Powe DG,
Ahmed RA, Collins HM, Soria D, Garibaldi JM, Paish CE, Ammar AA, et
al: Global histone modifications in breast cancer correlate with
tumor phenotypes, prognostic factors, and patient outcome. Cancer
Res. 69:3802–3809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y, Lan F, Matson C, Mulligan P,
Whetstine JR, Cole PA, Casero RA and Shi Y: Histone demethylation
mediated by the nuclear amine oxidase homolog LSD1. Cell.
119:941–953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zuchegna C, Aceto F, Bertoni A, Romano A,
Perillo B, Laccetti P, Gottesman ME, Avvedimento EV and Porcellini
A: Mechanism of retinoic acid-induced transcription: Histone code,
DNA oxidation and formation of chromatin loops. Nucleic Acids Res.
42:11040–11055. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lim S, Janzer A, Becker A, Zimmer A,
Schüle R, Buettner R and Kirfel J: Lysine-specific demethylase 1
(LSD1) is highly expressed in ER-negative breast cancers and a
biomarker predicting aggressive biology. Carcinogenesis.
31:512–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagasawa S, Sedukhina AS, Nakagawa Y,
Maeda I, Kubota M, Ohnuma S, Tsugawa K, Ohta T, Roche-Molina M,
Bernal JA, et al: LSD1 overexpression is associated with poor
prognosis in basal-like breast cancer, and sensitivity to PARP
inhibition. PLoS One. 10:e01180022015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kashyap V, Ahmad S, Nilsson EM, Helczynski
L, Kenna S, Persson JL, Gudas LJ and Mongan NP: The lysine specific
demethylase-1 (LSD1/KDM1A) regulates VEGF-A expression in prostate
cancer. Mol Oncol. 7:555–566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qin Y, Zhu W, Xu W, Zhang B, Shi S, Ji S,
Liu J, Long J, Liu C, Liu L, et al: LSD1 sustains pancreatic cancer
growth via maintaining HIF1α-dependent glycolytic process. Cancer
Lett. 347:225–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen C, Ge J, Lu Q, Ping G, Yang C and
Fang X: Expression of lysine-specific demethylase 1 in human
epithelial ovarian cancer. J Ovarian Res. 8:282015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohammad HP, Smitheman KN, Kamat CD, Soong
D, Federowicz KE, Van Aller GS, Schneck JL, Carson JD, Liu Y,
Butticello M, et al: A DNA hypomethylation signature predicts
antitumor activity of LSD1 inhibitors in SCLC. Cancer Cell.
28:57–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fiskus W, Sharma S, Shah B, Portier BP,
Devaraj SG, Liu K, Iyer SP, Bearss D and Bhalla KN: Highly
effective combination of LSD1 (KDM1A) antagonist and pan-histone
deacetylase inhibitor against human AML cells. Leukemia.
28:2155–2164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Theisen ER, Gajiwala S, Bearss J, Sorna V,
Sharma S and Janat-Amsbury M: Reversible inhibition of lysine
specific demethylase 1 is a novel anti-tumor strategy for poorly
differentiated endometrial carcinoma. BMC Cancer. 14:7522014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YD, Dai M, Yang SS, Xiao M, Meng FL
and Chen XW: Overexpression of lysine-specific demethylase 1 is
associated with tumor progression and unfavorable prognosis in
Chinese patients with endometrioid endometrial adenocarcinoma. Int
J Gynecol Cancer. 25:1453–1460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai C, He HH, Gao S, Chen S, Yu Z, Gao Y,
Chen S, Chen MW, Zhang J, Ahmed M, et al: Lysine-specific
demethylase 1 has dual functions as a major regulator of androgen
receptor transcriptional activity. Cell Rep. 9:1618–1627. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bennesch MA, Segala G, Wider D and Picard
D: LSD1 engages a corepressor complex for the activation of the
estrogen receptor α by estrogen and cAMP. Nucleic Acids Res.
44:8655–8670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pollock JA, Larrea MD, Jasper JS,
McDonnell DP and McCafferty DG: Lysine-specific histone demethylase
1 inhibitors control breast cancer proliferation in ERα-dependent
and -independent manners. ACS Chem Biol. 7:1221–1231. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun G, Alzayady K, Stewart R, Ye P, Yang
S, Li W and Shi Y: Histone demethylase LSD1 regulates neural stem
cell proliferation. Mol Cell Biol. 30:1997–2005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yokoyama A, Takezawa S, Schüle R, Kitagawa
H and Kato S: Transrepressive function of TLX requires the histone
demethylase LSD1. Mol Cell Biol. 28:3995–4003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Bai M, Ning C, Xie B, Zhang J,
Liao H, Xiong J, Tao X, Yan D, Xi X, et al: Gankyrin facilitates
follicle-stimulating hormone-driven ovarian cancer cell
proliferation through the PI3K/AKT/HIF-1α/cyclin D1 pathway.
Oncogene. 35:2506–2517. 2016. View Article : Google Scholar
|
|
28
|
Shao G, Wang J, Li Y, Liu X, Xie X, Wan X,
Yan M, Jin J, Lin Q, Zhu H, et al: Lysine-specific demethylase 1
mediates epidermal growth factor signaling to promote cell
migration in ovarian cancer cells. Sci Rep. 5:153442015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Zhang J, Qian W, Dong Y, Yang Y,
Liu Z, Feng Y, Ma D, Zhang Z and Wu S: Gankyrin is frequently
overexpressed in cervical high grade disease and is associated with
cervical carcinogenesis and metastasis. PLoS One. 9:e950432014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin X, Gossett DR, Wang S, Yang D, Cao Y,
Chen J, Guo R, Reynolds RK and Lin J: Inhibition of AKT survival
pathway by a small molecule inhibitor in human endometrial cancer
cells. Br J Cancer. 91:1808–1812. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Jiang J, Zhao M, Luo X, Liang Z,
Zhen Y, Fu Q, Deng X, Lin X, Li L, et al: microRNA-374a suppresses
colon cancer progression by directly reducing CCND1 to inactivate
the PI3K/AKT pathway. Oncotarget. 7:41306–41319. 2016.PubMed/NCBI
|
|
32
|
Torres-Padilla ME, Parfitt DE, Kouzarides
T and Zernicka-Goetz M: Histone arginine methylation regulates
pluripotency in the early mouse embryo. Nature. 445:214–218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Prossnitz ER, Arterburn JB, Smith HO,
Oprea TI, Sklar LA and Hathaway HJ: Estrogen signaling through the
transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol.
70:165–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ge X, Guo R, Qiao Y, Zhang Y, Lei J, Wang
X, Li L and Hu D: The G protein-coupled receptor GPR30 mediates the
nontranscriptional effect of estrogen on the activation of PI3K/Akt
pathway in endometrial cancer cells. Int J Gynecol Cancer.
23:52–59. 2013. View Article : Google Scholar
|
|
35
|
Wei Y, Zhang Z, Liao H, Wu L, Wu X, Zhou
D, Xi X, Zhu Y and Feng Y: Nuclear estrogen receptor-mediated Notch
signaling and GPR30-mediated PI3K/AKT signaling in the regulation
of endometrial cancer cell proliferation. Oncol Rep. 27:504–510.
2012.
|
|
36
|
Zhang J, Yang Y, Zhang Z, He Y, Liu Z, Yu
Y, Wu S, Cai B and Feng Y: Gankyrin plays an essential role in
estrogen-driven and GPR30-mediated endometrial carcinoma cell
proliferation via the PTEN/PI3K/AKT signaling pathway. Cancer Lett.
339:279–287. 2013. View Article : Google Scholar
|
|
37
|
Wang Y, Zhu Y, Wang Q, Hu H, Li Z, Wang D,
Zhang W, Qi B, Ye J, Wu H, et al: The histone demethylase LSD1 is a
novel oncogene and therapeutic target in oral cancer. Cancer Lett.
374:12–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thambyrajah R, Mazan M, Patel R, Moignard
V, Stefanska M, Marinopoulou E, Li Y, Lancrin C, Clapes T, Möröy T,
et al: GFI1 proteins orchestrate the emergence of haematopoietic
stem cells through recruitment of LSD1. Nat Cell Biol. 18:21–32.
2016. View Article : Google Scholar
|
|
39
|
Lin Y, Kang T and Zhou BP: Doxorubicin
enhances Snail/LSD1-mediated PTEN suppression in a PARP1-dependent
manner. Cell Cycle. 13:1708–1716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hecht JL and Mutter GL: Molecular and
pathologic aspects of endometrial carcinogenesis. J Clin Oncol.
24:4783–4791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ono H, Katagiri H, Funaki M, Anai M,
Inukai K, Fukushima Y, Sakoda H, Ogihara T, Onishi Y, Fujishiro M,
et al: Regulation of phosphoinositide metabolism, Akt
phosphorylation, and glucose transport by PTEN (phosphatase and
tensin homolog deleted on chromosome 10) in 3T3-L1 adipocytes. Mol
Endocrinol. 15:1411–1422. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang CH, Almomen A, Wee YS, Jarboe EA,
Peterson CM and Janát-Amsbury MM: An estrogen-induced endometrial
hyperplasia mouse model recapitulating human disease progression
and genetic aberrations. Cancer Med. 4:1039–1050. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seiler R, Thalmann GN, Rotzer D, Perren A
and Fleischmann A: CCND1/CyclinD1 status in metastasizing bladder
cancer: a prognosticator and predictor of chemotherapeutic
response. Modern Pathol. 27:87–95. 2014. View Article : Google Scholar
|
|
44
|
Dreyer JH, Hauck F, Barros MH and
Niedobitek G: pRb and cyclinD1 complement p16 as
immunohistochemical surrogate markers of HPV infection in head and
neck cancer. Appl Immunohistochem Mol Morphol. Dec 9–2015.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Umekita Y, Ohi Y, Sagara Y and Yoshida H:
Overexpression of cyclinD1 predicts for poor prognosis in estrogen
receptor-negative breast cancer patients. Int J Cancer. 98:415–418.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhong Z, Yeow WS, Zou C, Wassell R, Wang
C, Pestell RG, Quong JN and Quong AA: Cyclin D1/cyclin-dependent
kinase 4 interacts with filamin A and affects the migration and
invasion potential of breast cancer cells. Cancer Res.
70:2105–2114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun X, Tang SC, Xu C, Wang C, Qin S, Du N,
Liu J, Zhang Y, Li X, Luo G, et al: DICER1 regulated let-7
expression levels in p53-induced cancer repression requires cyclin
D1. J Cell Mol Med. 19:1357–1365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Maiques-Diaz A and Somervaille TC: LSD1:
Biologic roles and therapeutic targeting. Epigenomics. 8:1103–1116.
2016. View Article : Google Scholar : PubMed/NCBI
|