Introduction
MicroRNAs (miRs), which are 18–25-nucleotide-long
small non-coding RNAs, can cause posttranscriptional repression by
directly binding to the 3′-untranslational region (UTR) of mRNAs
(1). Previous studies have shown
that miRs play important roles in many pivotal biological processes
such as cell growth, proliferation, and death (2,3).
Renal cell carcinoma (RCC) is one of the lethal
urological malignancies in adults, with a high mortality rate of
>40% (4,5). Approximately, 30% patients with
localized RCC develop metastatic recurrence, even after radical
resection of the diseased kidney (6,7).
Despite tremendous development in RCC therapy, patients with
locally advanced and metastatic RCC still have poor prognosis
(8). Therefore, there is an urgent
need to improve the prognosis for patients with RCC and to identify
novel therapeutic targets for controlling the metastatic potential
of RCC and modulating apoptotic pathways in RCC. Since miRs are
important genetic regulators modulating their target genes, miRs
could be good candidates for regulating RCC progression and
development as well as for enhancing cell death. For example,
miR-148b enhances proliferation and apoptosis in human renal cancer
cells by directly targeting MAP3K9 (9). In addition to the tumor-suppressive
effects exerted by the miR, several miRs sensitized renal cancer
cells to anticancer drugs such as sorafenib, imatinib, and 5-FU by
targeting apoptosis-regulating genes (10–12).
In recent years, miR-148a was found to be aberrantly
expressed in various cancers and has been demonstrated to act as an
oncogene or tumor suppressor with crucial roles in the molecular
mechanisms underlying oncogenesis (13–16).
In addition, the ectopic expression of miR-148a attenuated the
paclitaxel resistance of prostate cancer cells by suppressing the
expression of mitogen- and stress-activated kinase 1 (MSK1)
(17). Unfortunately, the
miR-148-involving molecular mechanisms associated with the
regulation of renal cancer cell proliferation and drug sensitivity
are still unknown. Therefore, we investigated the role of miR-148a
in apoptosis and chemosensitivity of human renal cancer cells by
targeting the Ras-related protein 14 (Rab14).
Materials and methods
Cells and materials
Caki cells (human renal cancer cells) were purchased
from the American Type Culture Collection (Rockville, MD, USA), and
maintained in RPMI-1640 medium containing 100 U/ml penicillin, 100
µg/ml streptomycin, and 10% fetal bovine serum.
Anti-caspase-3 antibody was purchased from Enzo Life Sciences, Inc.
(Farmingdale, NY, USA). Anti-PARP antibody was purchased from Cell
Signaling Technology, Inc. (Boston, MA, USA). Rab14 and actin
antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX,
USA). Cisplatin was obtained from Sigma Chemical Co. (St. Louis,
MO, USA). The recombinant human TRAIL was purchased from KOMA
Biotech (Seoul, Korea). The miR-148a mimics and miR-148a inhibitors
were purchased from Ambion (Austin, TX, USA).
Western blotting
Cellular lysates were prepared by suspending
0.5×106 cells in 100 µl of lysis buffer (137 mM
NaCl, 15 mM EGTA, 0.1 mM sodium orthovanadate, 15 mM
MgCl2, 0.1% TritonX-100, 25 mM MOPS, 100 µM
phenyl-methylsulfonyl fluoride, and 20 µM leupeptin,
adjusted to pH 7.2). The cells were disrupted by sonication and
extracted at 4°C for 30 min. The lysate containing proteins was
quantified using bicinchoninic acid (BCA) protein assay kit
(Pierce, Rockford, IL, USA). The proteins were electro-transferred
to Immobilon-P membranes (Millipore Corp., Bedford, MA, USA).
Detection of specific proteins was carried out with an ECL Western
blotting kit (Millipore Corp.), according to the manufacturer's
instructions.
RNA isolation and reverse
transcriptase-polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted from cells using
eazyBlue reagent (Intron Biotechnology, Seongnam-si, Gyeonggi-do,
Korea). cDNA was synthesized from 2 µg of total RNA by using
M-MLV reverse transcriptase (Gibco-BRL, Gaithersburg, MD, USA). The
cDNA for Rab14 and actin were amplified by PCR with specific
primers. For Rab14, the sense and anti-sense primers were
5′-ATGGCAACTGCACCATACAA-3′ and 5′-GCCACAGCAAAGAGGTCACT-3′,
respectively. PCR products were analyzed by agarose gel
electrophoresis and visualized with ethidium bromide.
Flow cytometry-based analysis
Approximately 0.5×106 MDA-MB-231 cell
were suspended in 100 µl of phosphate-buffered saline (PBS),
and 200 µl of 95% ethanol was added while vortexing. The
cells were incubated at 4°C for 1 h, washed with PBS, and
resuspended in 250 µl of 1.12% sodium citrate buffer (pH
8.4), with 12.5 µg of RNase. Incubation was continued at
37°C for 30 min. The cellular DNA was then stained by applying 250
µl of propidium iodide (PI, 50 µg/ml) for 30 min at
room temperature. The stained cells were analyzed by
fluorescence-activated cell sorting (FACS) on a BD FACS Canto II
flow cytometer (BD Biosciences, San Jose, CA, USA) for relative DNA
content based on red fluorescence. Cell undergoing apoptosis will
be a part of the DNA (due to DNA fragmentation during later stages
of apoptosis). These cells may be detected as a 'sub-G1'
population. Cells were further analyzed by flow cytometry using BD
FACS Canto II flow cytometer (BD Biosciences), and a PI/Annexin
staining kit (BD Biosciences).
Luciferase reporter assays
For the basic 3-UTR lucif-erase reporter assay, Caki
cells were transfected with the Rab14 3′-UTR-pmirGLO
Dual-Luciferase reporter plasmid (Promega, Madison, WI), miR-cont,
miR-148a, or anti-miR-148a using Lipofectamine 2000. Luciferase
activity assays were then performed and normalized to
Renilla luciferase activity. The experiments were repeated
three times.
4′,6′-Diamidino-2-phenylindole staining
for nuclear condensation and fragmentation
To examine cellular nuclei, the cells were fixed
with 1% paraformaldehyde on glass slides for 30 min at room
temperature. After fixation, the cells were washed with PBS and 300
nM 4′,6′-diamidino-2-phenylindole solution (Roche, Mannheim,
Germany) was added to the fixed cells for 5 min. After the nuclei
were stained, cells were examined by fluorescence microscopy.
Statistical analysis
Three or more separate experiments were performed.
Statistical analysis was conducted with the paired Student's
t-test. P<0.05 was considered as a significant difference
between the experimental and control groups.
Results
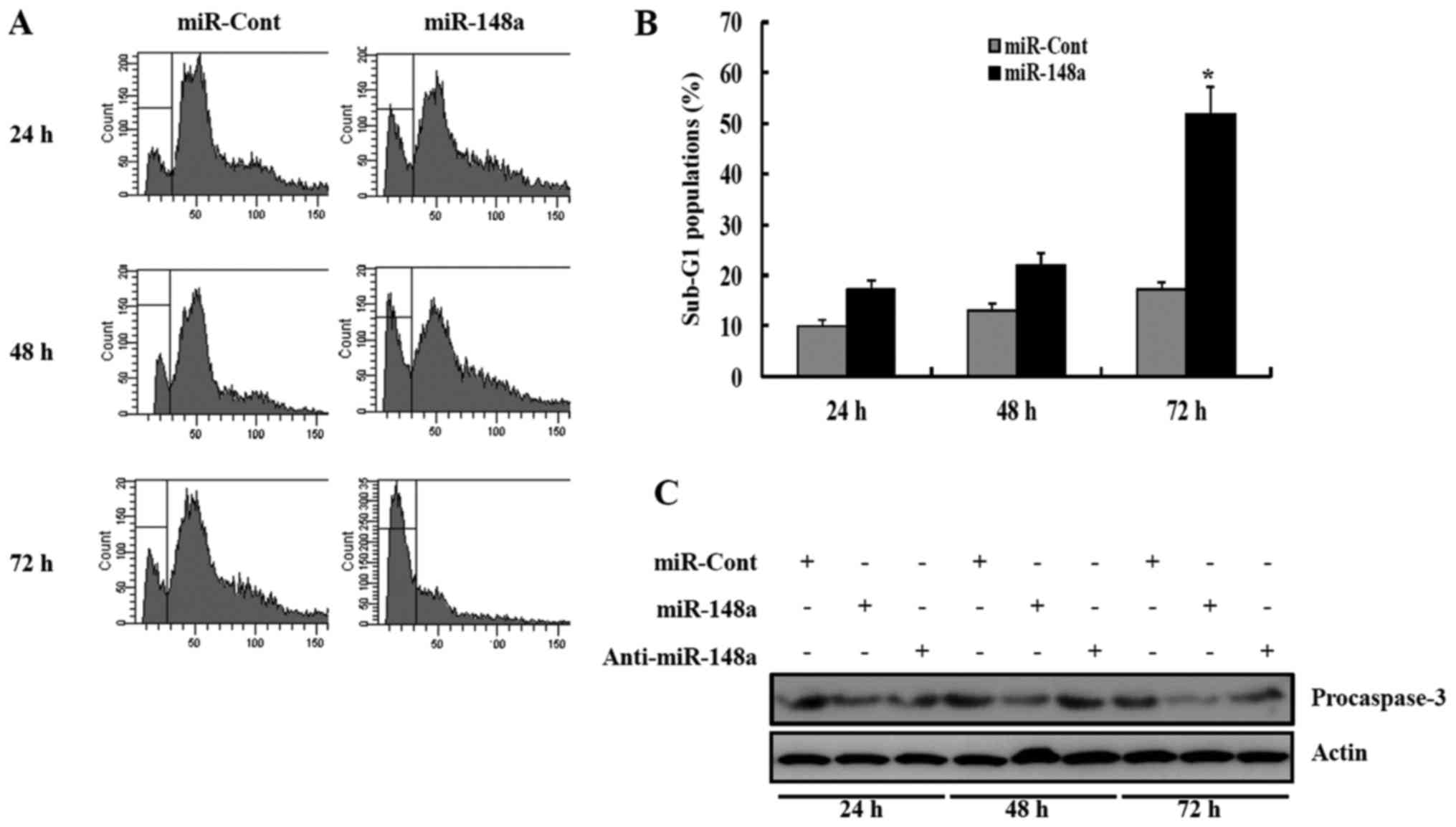
miR-148a inhibits renal cancer cell
proliferation and promotes apoptosis
To examine the functional significance of miR-148a
in RCC, renal cancer cells were transfected with miR-148a. The
percentage of sub-G1 population markedly increased in response to
miR-148a transfection, compared to miRNA-cont transfection of Caki
cells 24 h after transfection (Fig.
1A). We next examined whether transfection with miR-148a
resulted in the activation of caspases in Caki cells. Forced
expression of miR-148a in Caki cells led to a significant decrease
in the protein levels of procaspase-3 precursors at 48 h after
transfection (Fig. 1C). Similarly,
transfection with the miR-148a mimics resulted in the activation of
caspase pathway, compared to miRNA-cont-transfected cells (Fig. 1B).
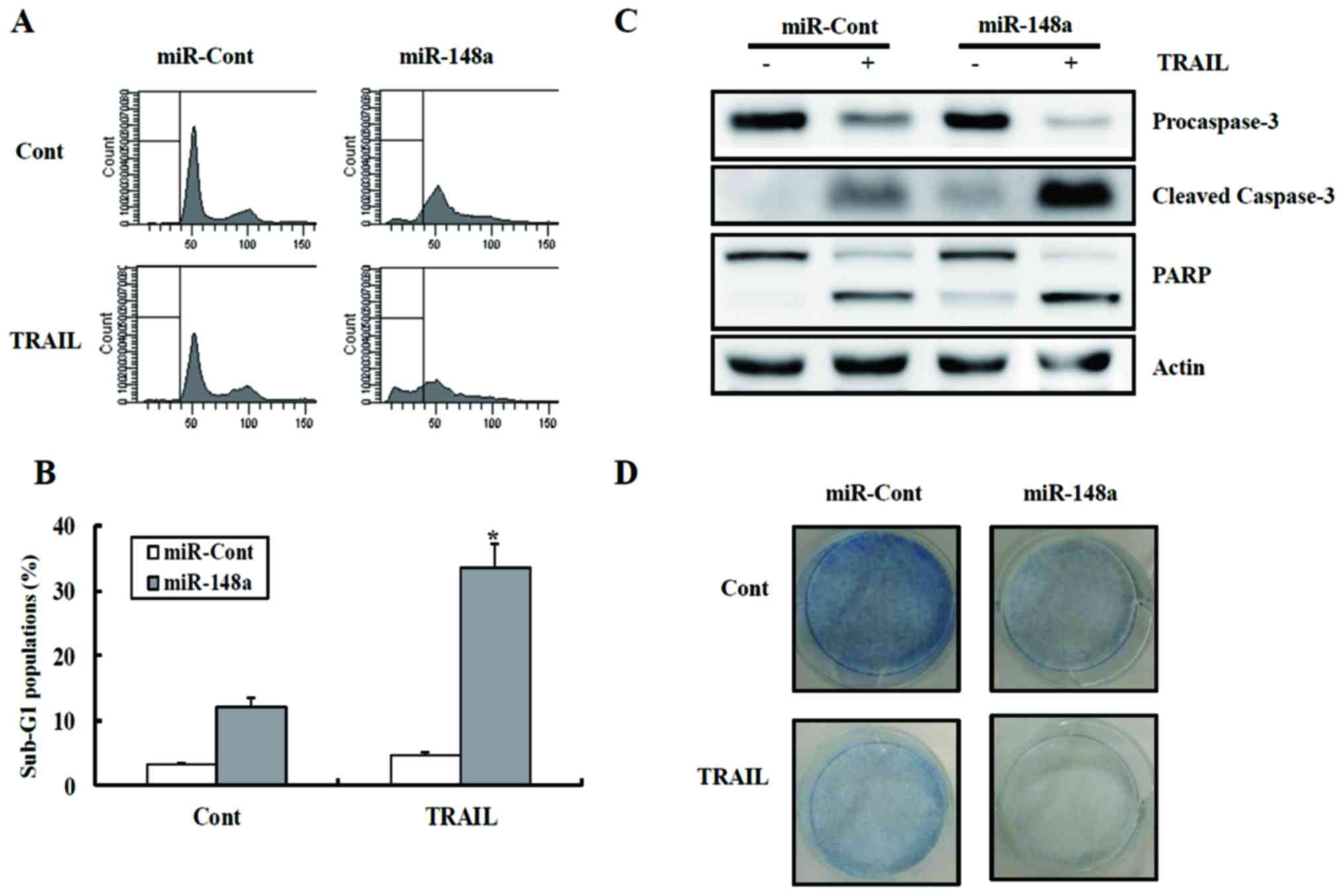
miR-148a sensitizes renal cancer cells to
TRAIL-induced apoptosis
To examine the functional role of miR-148a in
drug-mediated apoptosis in Caki cells, miR-148a-transfected cell
lines were treated with TRAIL and cytotoxicity were examined using
FACS. As shown in Fig. 2A and B,
transfection with miR-148a caused a significant increase in the
fraction of cells in the sub-G1 phase compared to the
miRNA-cont-transfected cells following TRAIL treatment. As shown in
Fig. 2C, treatment of
Caki/miR-148a cells with TRAIL resulted in the cleavage of PARP and
procaspase-3. Treatment with TRAIL decreased the clonogenicity of
Caki/miR-148a cells compared to Caki/miRNA-cont cells (Fig. 2D).
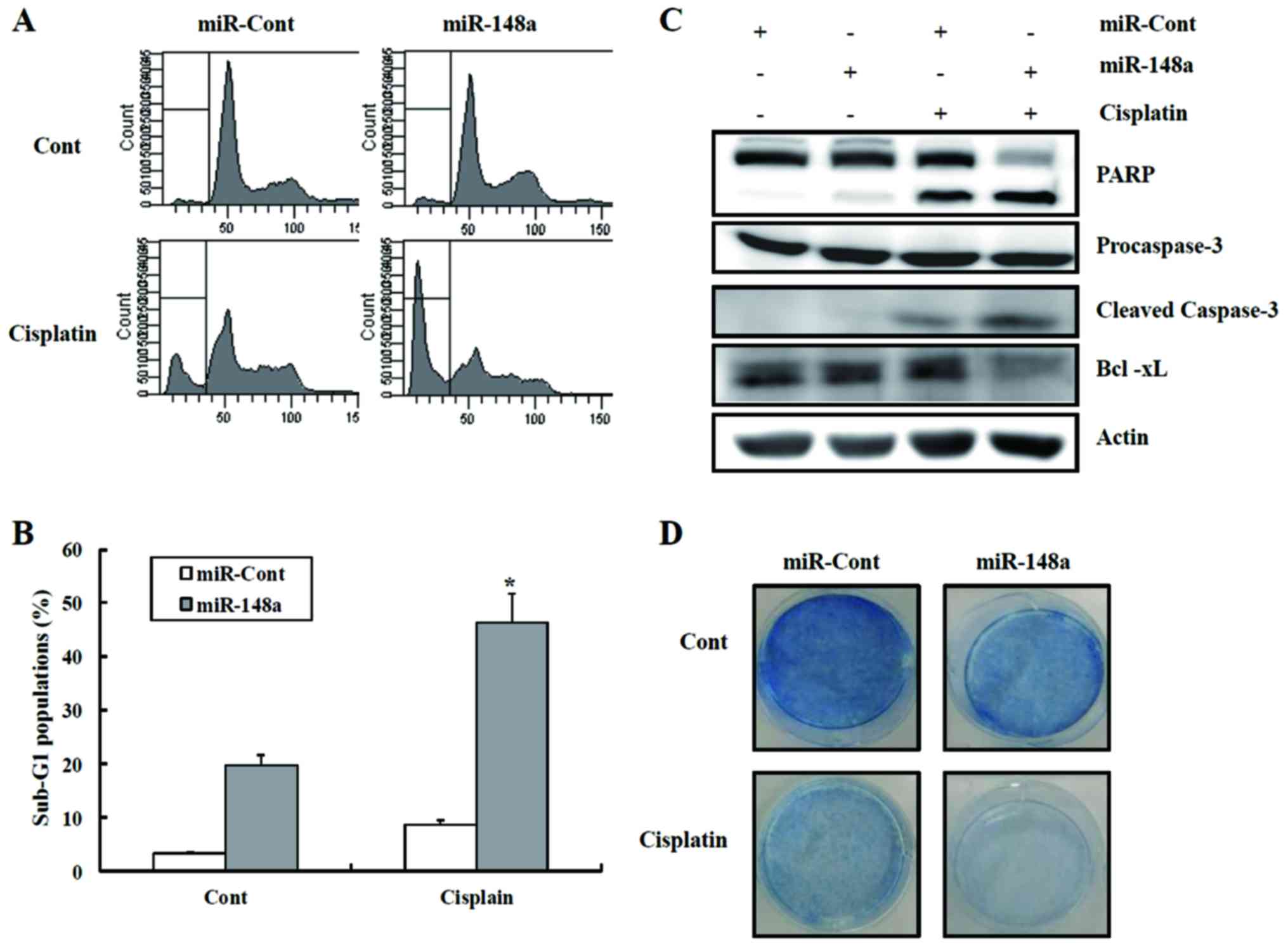
miRNA-148a sensitizes renal cancer cells
to cisplatin-induced apoptosis
We next investigated whether miR-148a could increase
the sensitivity of renal cancer cells to anticancer drugs such as
cisplatin. Cisplatin treatment of miR-148a-transfected cells caused
a marked increase in the fraction of cells in the sub-G1 phase
compared to the cells expressing miRNA-cont, as well as activation
of caspase pathways (Fig. 3A and
B). As shown in Fig. 3C,
cisplatin treatment of miR-148a-transfected cells led to a decrease
in the protein levels of procaspase-3, with the concomitant
cleavage of PARP protein. In addition, treatment with cisplatin
decreased the clonogenicity of Caki/miR-148a cells compared to
Caki/miRNA-cont cells (Fig. 3D).
As shown in Fig. 3E, miRNA-148
plus cisplatin treatment enhanced the number of TUNEL-positive
cells. These results indicate that the miRNA-148 plus
cisplatin-induced apoptosis were involved in the activation of
caspase-dependent apoptotic pathways.
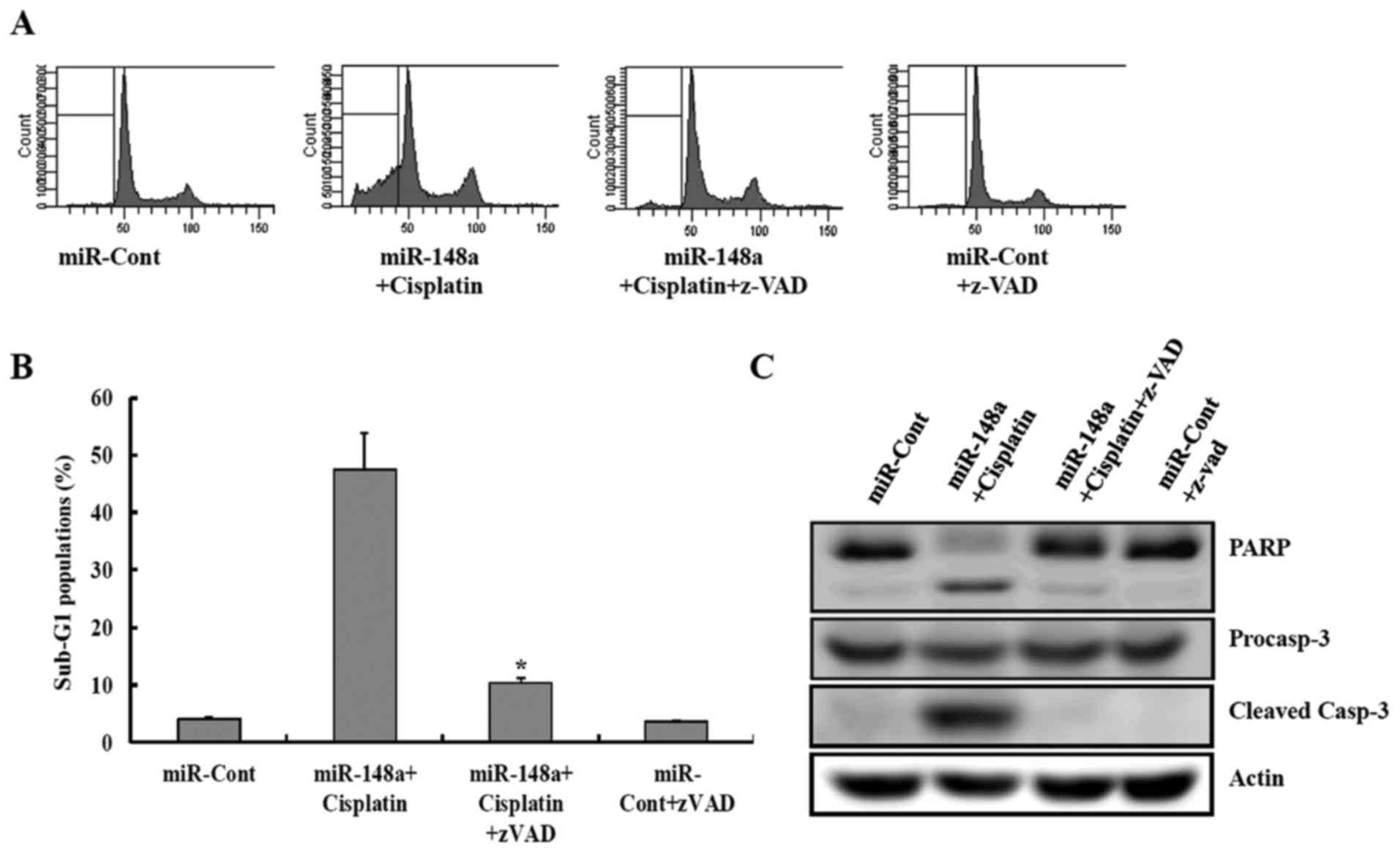
miR-148a plus cisplatin-induced apoptosis
was involved in the activation of caspase-dependent apoptotic
pathways
This study next examined whether the activation of
caspase pathway plays a critical role in miRNA-148 plus
cisplatin-induced apoptosis. miR-148a plus cisplatin-induced
apoptosis was completely prevented by pretreatment with the general
and potent inhibitor of caspases, the z-VAD-fmk, as determined by
FACS analysis (Fig. 4A and B). In
addition, z-VAD-fmk treatment completely prevented these
caspase-related events such as cleavage of procaspase-3 and PARP
(Fig. 4C).
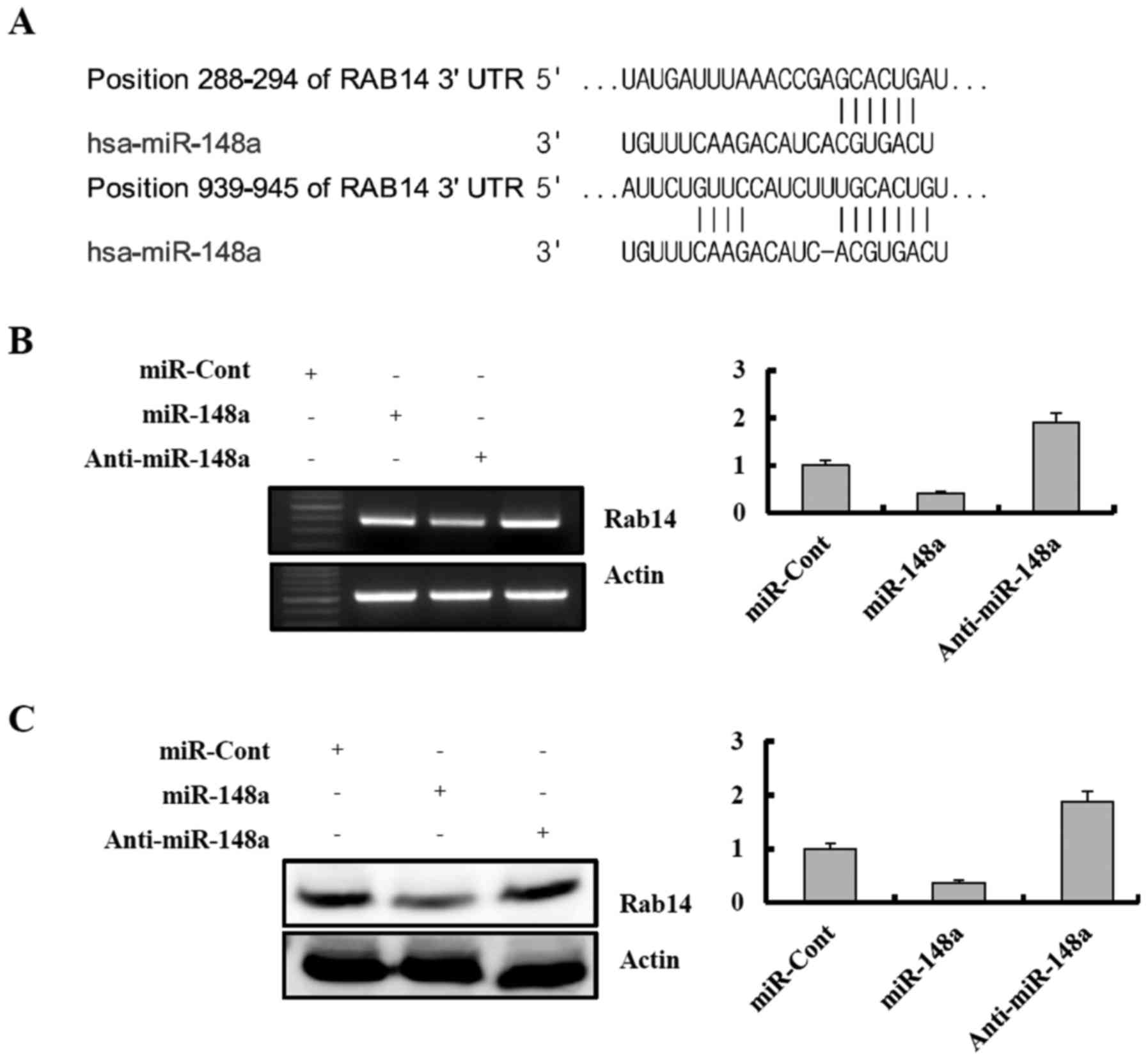
miR-148a post-transcriptionally reduces
Rab14 expression by directly targeting its 3′-UTR
A bioinformatic analysis program, TargetScan, was
used to identify putative protein-coding gene targets of miR-148a.
The TargetScan miRNA target predictions showed that Rab14 3-UTR
contained two potential binding sites for miR-148a at the
nucleotides 288 and 939 (Fig 5A,
http://www.targetscan.org/cgi-bin/targetscan/vert_61/view_gene.cgi-taxid=9606&rs=NM_016322&members=miR-148ab-3p/152&showcnc=0&shownc=0).
To determine whether exogenous miR-148a could repress Rab14
expression, Caki cells were transiently transfected with premature
miR-148a or a control miRNA (miRNA-cont) for 24 h. Rab14 expression
was analyzed by RT-PCR and western blotting. As shown in Fig. 5B and C, ectopic expression of
miR-148a inhibited Rab14 mRNA and protein expression in a
dose-dependent manner. In contrast, transfection with anti-miR-148a
resulted in an increase in Rab14 expression in Caki cells (Fig. 5B and C).
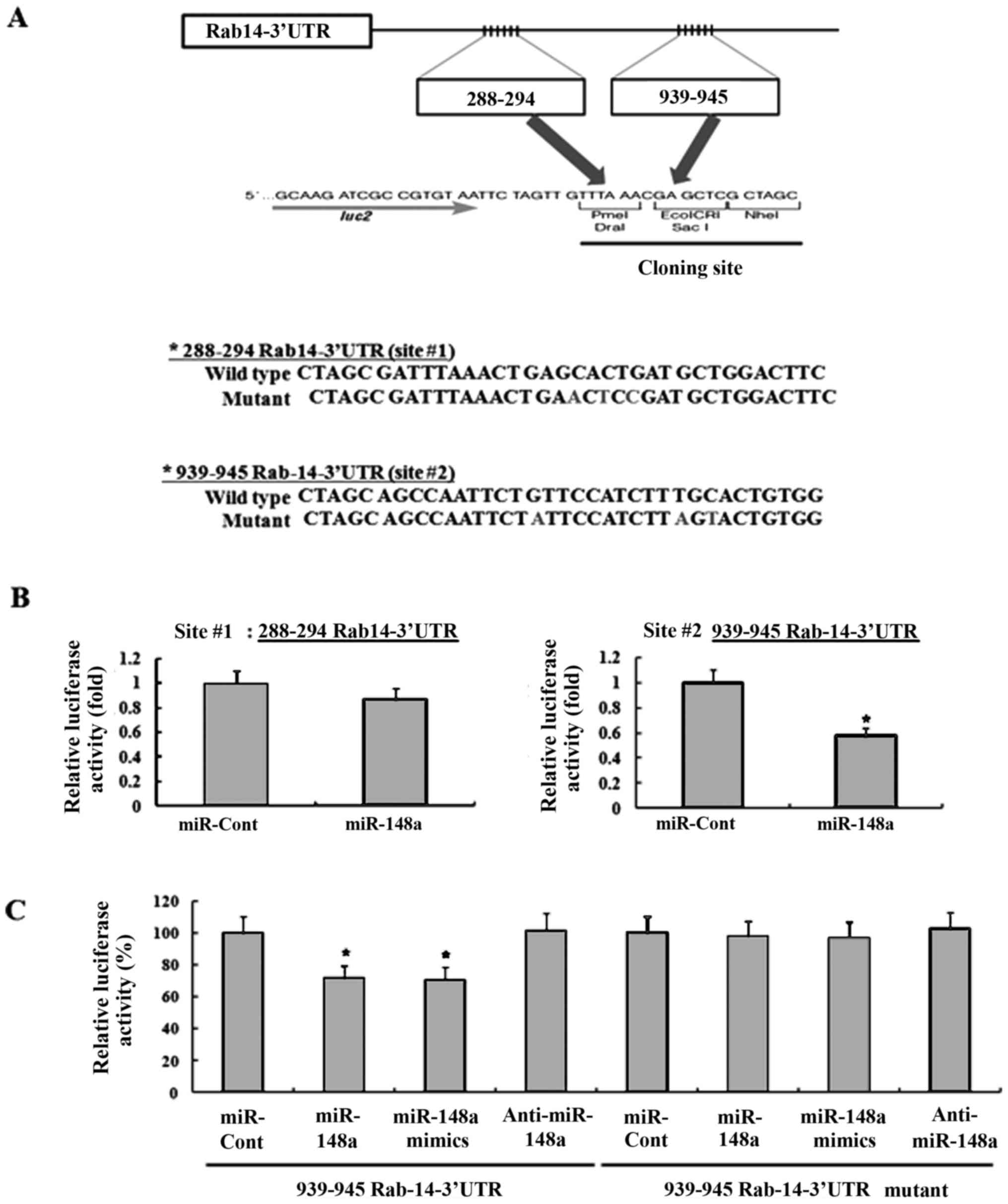
Next, it was investigated whether the 3′-UTR of
Rab14 was a functional target of miR-148a in RCC. As miR-148a could
bind to two different regions of the 3-UTR of Rab14 mRNA (Fig. 5A), we investigated which of the two
regions was involved in miR-148a binding. The predicted
miRNA-binding sequences of Rab14 (sites 1 and 2) were cloned into
the downstream region of a luciferase reporter construct
(pmirGLO-Rab14 #1 and pmirGLO-Rab14 #2, Fig. 6A). Caki cells were transiently
transfected with these constructs in the presence of either
pre-miR-148a or miRNA-cont. As shown in Fig. 6B, miR-148a markedly reduced the
luciferase activity of pmirGLO-Rab14#2 compared to miRNA-cont, but
miR-148a slightly decreased the luciferase activity of
pmirGLO-Rab14 #1. These data suggested that miR-148a specifically
bound to the 3-UTR of RAb14 at nucleotide 939 and impaired Rab14
expression. In addition, miR-148a mimics significantly reduced the
luciferase activity, compared to miRNA-cont. In contrast, the
luciferase activity of the reporter vector containing a mutated
3′-UTR in Rab14 was unaffected by miR-148a (Fig. 6C).
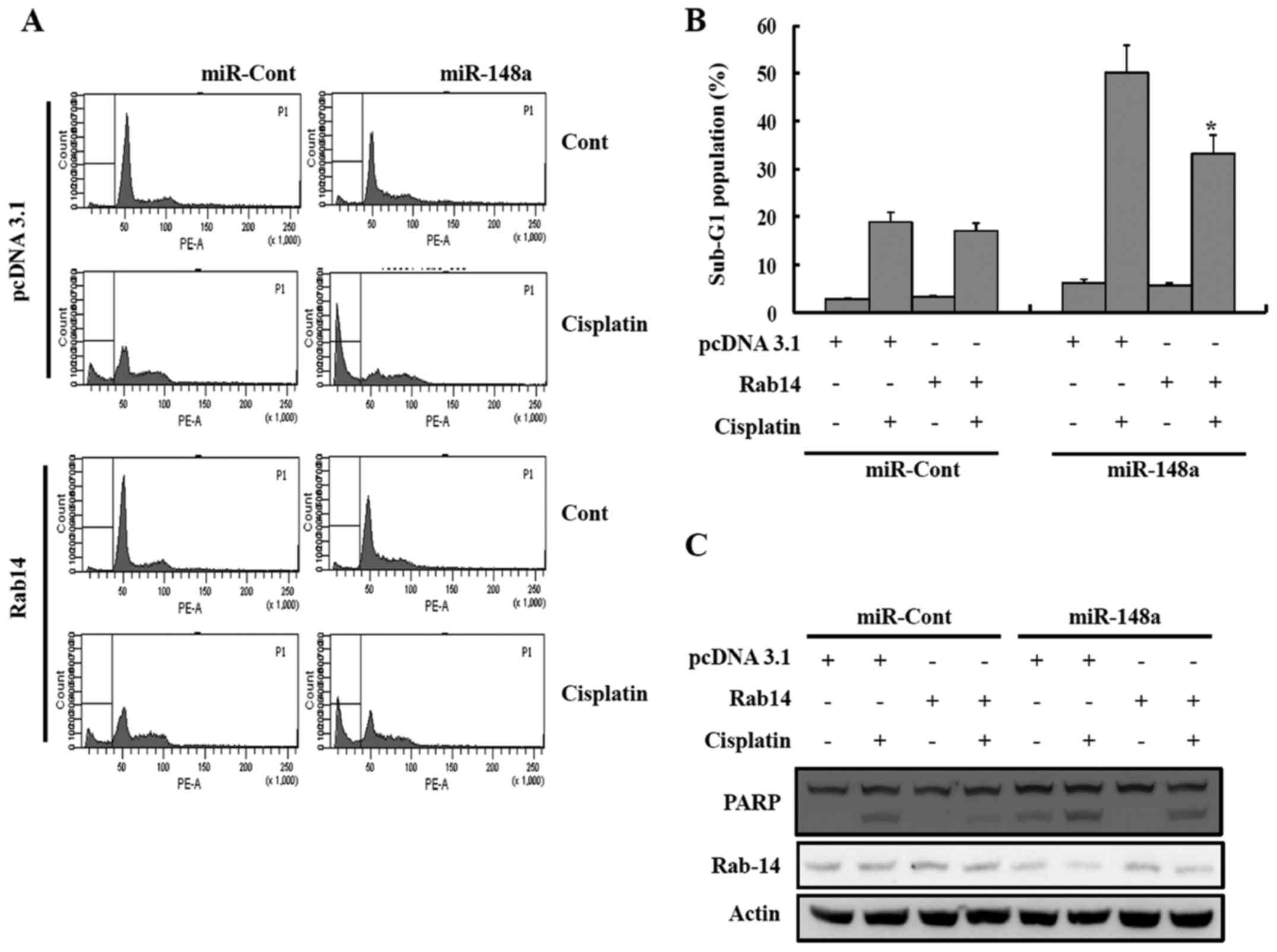
Overexpression of Rab14 decreases the
sensitivity to cisplatin
As miR-148a can inhibit Rab14 expression by directly
inhibiting the Rab14 transcript, it was investigated whether an
increase of Rab14 expression could reduce the sensitivity to
cisplatin. Therefore, miR-148a was ectopically expressed in Caki
cells, together with a construct containing the Rab14-coding
sequence but lacking the 3′-UTR of the Rab14 mRNA or an empty
vector. After treatment with cisplatin, the accumulation of the
sub-G1 population was lower in the Caki/Rab14 cells compared to the
Caki/vector cells, indicating that the restoration of Rab14
counteracted the effects of miR-148a on the sensitivity to
cisplatin in renal cancer cells (Fig.
7).
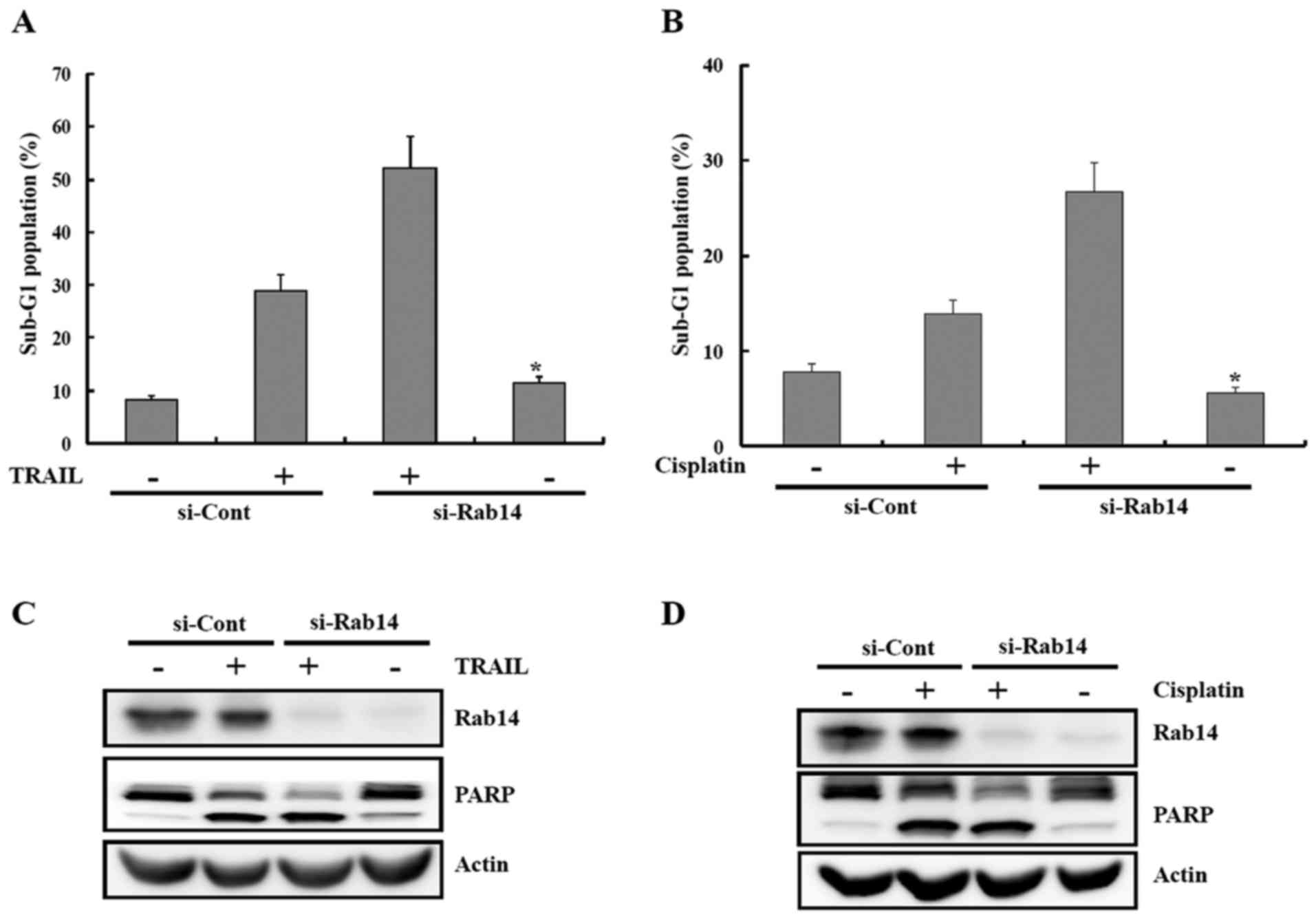
siRab14-mediated downregulation of Rab14
enhances the sensitivity to various apoptotic stimuli
To determine whether the anticancer effects of
miR-148a in renal cancer cell lines were due to Rab14 inhibition or
interaction with another gene, Caki cells were transiently
transfected with a small interfering RNA (siRNA) specific to Rab14
(siRab14) or a scrambled siRNA negative control (siCont). The
si-Rab14 was able to knock down the expression of Rab14 (Fig. 8C and D). Depletion of Rab14 by
siRNA significantly increased the sensitivity of the cells to
apoptosis-inducing drugs, including TRAIL and cisplatin (Fig. 8).
Discussion
The present study showed that miR-148a resulted in
apoptosis of human renal cancer cells via activating the caspase
pathway. Moreover, ectopic expression of miR-148a enhanced the
anticancer drug sensitivity of renal cancer cells. Rab14 was
identified as a direct and functional target of miR-148a, and Rab14
expression was negatively regulated at the posttranscriptional
level by miR-148a in renal cancer cells. Finally, we found that the
anticancer effect of miR-148a on renal cancer is, at least partly,
via the suppression of Rab14 expression.
Downregulation of miR-148a has been identified in
various types of human cancer, including gastric cancer, breast
cancer, hepatocellular carcinoma, and pancreatic ductal
adenocarcinoma, and is therefore considered a tumor-suppressive
miRNA (18–21). Moreover, miR-148a overexpression
sensitized the cancer cells to anticancer drugs. For example,
ectopic expression of miR-148a sensitized the cells to TRAIL via
the down-modulation of matrix metalloproteinase 15 (MMP15) and
Rho-associated kinase 1 (ROCK1) in non-small cell lung cancer
(22). In addition, enforced
expression of miR-148a promotes paclitaxel-induced apoptosis of
ovarian cancer cells by targeting PDIA3 (23). miR-148a was found to induce
apoptosis and activate the caspase-dependent pathway, indicating
that it might function as a tumor suppressor in renal cancer cells.
Next, we investigated the effects of miR-148a on the sensitivity to
apoptotic stimuli such as TRAIL and cisplatin. Introduction of
miR-148a increased the sensitivity of Caki cells to apoptotic
stimuli, indicating that miR-148a can promote the sensitivity of
renal cancer cells to cisplatin or TRAIL.
Previous studies have shown that the direct targets
of miR-148a include MSK1, TGIF2, DNMT3, and PXR (17,24–26).
The present study showed that Rab14 is a direct target of miR-148a
in renal cancer Caki cells and that some of the tumor-suppressive
effects of miR-148a might be mediated through the downregulation of
Rab14 expression. Rab14 is a member of the RAS oncogene family of
small GTPases involved in human oncogenesis (27,28).
These studies suggest that Rab14 dysfunction might be involved in
human cancers and other diseases. Rab14 has been reported to play a
vital role in human non-small cell lung cancer (29). Therefore, it is necessary to
identify the upstream regulators of Rab14 in order to suppress
tumor growth and increase drug susceptibility.
Previous studies have shown that ectopic expression
of miRNAs such as miRNA-451 and miR-338-3p induces growth
inhibition and enhances apoptosis by inhibiting Rab14 expression in
lung cancer (29,30). Our data also showed that miR-148a
directly targets Rab14 by interacting with the second binding site
in the 3′-UTR, which is involved in miR-148a-induced apoptosis, and
enhancing the sensitivity to TRAIL or cisplatin in renal cancer
cells. The inhibition of Rab14 by siRab14 was also found to be
associated with an increase in the drug susceptibility of Caki
cells. Rab14 overexpression could partially block the effects
induced by miR-148a in Caki cells. These results indicated that
Rab14 might work as an oncogenic factor in renal cancer cells.
In conclusion, the present study showed that Rab14
was a direct target of miR-148a, and miR-148a/Rab14 interaction
played an important role in the regulation of apoptosis as well as
enhancement of drug sensitivity in renal cancer cells. Thus,
miR-148a could be considered as a potential target for renal cancer
therapy.
Acknowledgments
This study was supported by a grant of Yeungnam
University Medical Center (2013).
References
|
1
|
Huang X, Liang M, Dittmar R and Wang L:
Extracellular microRNAs in urologic malignancies: Chances and
challenges. Int J Mol Sci. 14:14785–14799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carrington JC and Ambros V: Role of
microRNAs in plant and animal development. Science. 301:336–338.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Spronsen DJ, de Weijer KJ, Mulders PF
and De Mulder PH: Novel treatment strategies in clear-cell
metastatic renal cell carcinoma. Anticancer Drugs. 16:709–717.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hadoux J, Vignot S and De La Motte Rouge
T: Renal cell carcinoma: Focus on safety and efficacy of
temsirolimus. Clin Med Insights Oncol. 4:143–154. 2010. View Article : Google Scholar
|
|
6
|
Zisman A, Pantuck AJ, Wieder J, Chao DH,
Dorey F, Said JW, deKernion JB, Figlin RA and Belldegrun AS: Risk
group assessment and clinical outcome algorithm to predict the
natural history of patients with surgically resected renal cell
carcinoma. J Clin Oncol. 20:4559–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dutcher JP: Recent developments in the
treatment of renal cell carcinoma. Ther Adv Urol. 5:338–353. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen B, Duan L, Yin G, Tan J and Jiang X:
miR-381, a novel intrinsic WEE1 inhibitor, sensitizes renal cancer
cells to 5-FU by up-regulation of Cdc2 activities in 786-O. J
Chemother. 25:229–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nie F, Liu T, Zhong L, Yang X, Liu Y, Xia
H, Liu X, Wang X, Liu Z, Zhou L, et al: MicroRNA-148b enhances
proliferation and apoptosis in human renal cancer cells via
directly targeting MAP3K9. Mol Med Rep. 13:83–90. 2016.
|
|
11
|
Gao C, Peng FH and Peng LK: MiR-200c
sensitizes clear-cell renal cell carcinoma cells to sorafenib and
imatinib by targeting heme oxygenase-1. Neoplasma. 61:680–689.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mu W, Hu C, Zhang H, Qu Z, Cen J, Qiu Z,
Li C, Ren H, Li Y, He X, et al: miR-27b synergizes with anticancer
drugs via p53 activation and CYP1B1 suppression. Cell Res.
25:477–495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murata T, Takayama K, Katayama S, Urano T,
Horie-Inoue K, Ikeda K, Takahashi S, Kawazu C, Hasegawa A, Ouchi Y,
et al: miR-148a is an androgen-responsive microRNA that promotes
LNCaP prostate cell growth by repressing its target CAND1
expression. Prostate Cancer Prostatic Dis. 13:356–361. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi M, Cuatrecasas M, Balaguer F,
Hur K, Toiyama Y, Castells A, Boland CR and Goel A: The clinical
significance of MiR-148a as a predictive biomarker in patients with
advanced colorectal cancer. PLoS One. 7:e466842012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Song Y, Wang Y, Luo J and Yu W:
MicroRNA-148a suppresses epithelial-to-mesenchymal transition by
targeting ROCK1 in non-small cell lung cancer cells. Mol Cell
Biochem. 380:277–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia J, Guo X, Yan J and Deng K: The role
of miR-148a in gastric cancer. J Cancer Res Clin Oncol.
140:1451–1456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujita Y, Kojima K, Ohhashi R, Hamada N,
Nozawa Y, Kitamoto A, Sato A, Kondo S, Kojima T, Deguchi T, et al:
MiR-148a attenuates paclitaxel resistance of hormone-refractory,
drug-resistant prostate cancer PC3 cells by regulating MSK1
expression. J Biol Chem. 285:19076–19084. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liffers ST, Munding JB, Vogt M, Kuhlmann
JD, Verdoodt B, Nambiar S, Maghnouj A, Mirmohammadsadegh A, Hahn SA
and Tannapfel A: MicroRNA-148a is down-regulated in human
pancreatic ductal adenocarcinomas and regulates cell survival by
targeting CDC25B. Lab Invest. 91:1472–1479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang SH, Li X, Zhou LS, Cao ZW, Shi C,
Zhou CZ, Wen YG, Shen Y and Li JK: microRNA-148a suppresses human
gastric cancer cell metastasis by reversing
epithelial-to-mesenchymal transition. Tumour Biol. 34:3705–3712.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Q, Jiang Y, Yin Y, Li Q, He J, Jing Y,
Qi YT, Xu Q, Li W, Lu B, et al: A regulatory circuit of
miR-148a/152 and DNMT1 in modulating cell transformation and tumor
angiogenesis through IGF-IR and IRS1. J Mol Cell Biol. 5:3–13.
2013. View Article : Google Scholar :
|
|
21
|
Zhang SL and Liu L: microRNA-148a inhibits
hepatocellular carcinoma cell invasion by targeting
sphingosine-1-phosphate receptor 1. Exp Ther Med. 9:579–584.
2015.PubMed/NCBI
|
|
22
|
Joshi P, Jeon YJ, Laganà A, Middleton J,
Secchiero P, Garofalo M and Croce CM: MicroRNA-148a reduces
tumorigenesis and increases TRAIL-induced apoptosis in NSCLC. Proc
Natl Acad Sci USA. 112:8650–8655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao S, Wen Z, Liu S, Liu Y, Li X, Ge Y
and Li S: MicroRNA-148a inhibits the proliferation and promotes the
paclitaxel-induced apoptosis of ovarian cancer cells by targeting
PDIA3. Mol Med Rep. 12:3923–3929. 2015.PubMed/NCBI
|
|
24
|
Takagi S, Nakajima M, Mohri T and Yokoi T:
Post-transcriptional regulation of human pregnane X receptor by
micro-RNA affects the expression of cytochrome P450 3A4. J Biol
Chem. 283:9674–9680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zuo J, Xia J, Ju F, Yan J, Zhu A, Jin S,
Shan T and Zhou H: MicroRNA-148a can regulate runt-related
transcription factor 3 gene expression via modulation of DNA
methyltransferase 1 in gastric cancer. Mol Cells. 35:313–319. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian Y, Wei W, Li L and Yang R:
Down-Regulation of miR-148a promotes metastasis by DNA methylation
and is associated with prognosis of skin cancer by targeting TGIF2.
Med Sci Monit. 21:3798–3805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takai Y, Sasaki T and Matozaki T: Small
GTP-binding proteins. Physiol Rev. 81:153–208. 2001.PubMed/NCBI
|
|
28
|
Agarwal R, Jurisica I, Mills GB and Cheng
KW: The emerging role of the RAB25 small GTPase in cancer. Traffic.
10:1561–1568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang R, Wang ZX, Yang JS, Pan X, De W and
Chen LB: MicroRNA-451 functions as a tumor suppressor in human
non-small cell lung cancer by targeting ras-related protein 14
(RAB14). Oncogene. 30:2644–2658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun J, Feng X, Gao S and Xiao Z:
microRNA-338-3p functions as a tumor suppressor in human
non-small-cell lung carcinoma and targets Ras-related protein 14.
Mol Med Rep. 11:1400–1406. 2015.
|