Introduction
Ovarian cancer, one of the most common malignant
tumors, takes the top spot among all gynecologic cancers with the
high mortality, and is still a serious threat to women's life in
China in the next few decades (1,2). At
present, the combination therapy combining surgery, radiotherapy,
and chemotherapy were mostly adopted for the treatment of ovarian
cancer. Unfortunately, as a result of no early symptoms and
effective biomarker screening, the limitation of diagnosis
technology, >70% of the patients with ovarian cancer have
late-stage, accompanied by distant metastases when they are
diagnosed (3–5). Therefore, the molecular mechanism
research on the development mechanisms of ovarian cancer are
extremely urgent.
MicroRNAs (miRNAs), are a major kind of endogenous
non-coding small RNAs with ~20 nucleotides, participating in
post-transcriptional regulation to affect the biological processes
by targeting the 3′-UTR of target genes (3,6–10).
Many studies have shown that miRNAs, serving as a kind of
regulatory genes, have played important roles in the occurrence and
development of various diseases including the progression and
tumorigenesis of human cancers (4,8,11,12).
Numerous studies have indicated that various miRNAs were relative
to the disease progression of ovarian cancer (3,13–16).
The mechanism and function of miR-28-5p have been studied in
VHL-associated cancer (17),
colorectal cancer (18), and
breast cancer (19). However, the
functional relevance of miR-28-5p in ovarian cancer is unclear.
Epithelial-mesenchymal transition (EMT) is the
biological processe where epithelial cells are transformed into
mesenchymal cells (20–22). Numerous studies have indicated that
the adhesion ability of tumor cells will decrease the migration
capacity of tumor cells. Thus, EMT was relative to metastasis of
tumors. In the process of EMT, the expression level of cell
adhesion molecules (E-cadherin) was decreased; the mesenchymal
markers (vimentin) was increased (23). As is known, F-actin (filamentous)
is essential for important cellular functions, such as the mobility
and contraction of cells and participating in the process of cell
migration (24).
N4BP1 is involved in the normal development by
interacting with the WW and HECT domains of the E3 ubiquitin ligase
Nedd4 and participating in the process to find targets of
ubiquitin-mediated protein degradation (25). It has been indicated that N4BP1 was
related to the related E3 ligase ITCH (26) and was combined with ITCH to
negatively regulate ITCH E3 activity by binding to its substrates
such as p73 and p63, and c-Jun (27). At present, the function of N4BP1 is
unclear, but it has been reported that N4BP1 may have ribonuclease
activity accoording to YacP-like nuclease (NYN) domain, as
characterized nucleases (28).
Research has shown that miR-28-5p negatively regulated the
expression level of N4BP1 dual luciferase reporter gene assay. Our
present study demonstrated the effects of miR-28-5p in ovarian
cancer, the functional mechanism between miR-28-5p and N4BP1, and
the potential downstream genes potentially regulating these
processes, also demonstrating that miR-28-5p could be a potential
therapeutic target for treatment of ovarian cancer.
Materials and methods
Cell lines and transfection
Human epithelial ovarian cancer cell lines ES2 were
obtained from American Type Culture Collection (ATCC, Manassas, VA,
USA) and SKOV3 cells were purchased from College of Life Science,
Hunan Normal University, China. ES2 and SKOV3 cells were cultured
in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad,
CA, USA) including 10% fetal bovine serum (FBS; Invitrogen),
penicillin (100 U/ml), and streptomycin (100 µg/ml) at 37°C
and 5% CO2. For the treatment, 2×105 ES2 or
SKOV3 cells were cultured in 6-well plates and then transfected
with 200 µl mature miR-28-5p mock, mimic, or inhibitor
(GenePharma Co., Ltd., Shanghai, China) for 72 h. All transfections
were completed using Lipofectamine™ 3000 (Invitrogen) according to
the manufacturer's protocols.
Clinical specimens
In this study, the ovarian cancer tissues and
adjacent non-cancerous tissues samples (5 cm from the edge of the
cancer) were collected from the First Affiliated Hospital of Sun
Yat-sen University between 2015 and 2016, and this study obtained
Ethics committee approval of the First Affiliated Hospital of Sun
Yat-sen University. Informed consent was also obtained from each
patient. The ovarian cancer histological diagnosis was assessed on
the basis of the World Health Organization (WHO). All tissue
samples were stored at −80°C.
RNA reverse transcription
Total RNA was extracted from ovarian cancer tissues,
matched adjacent non-cancerous tissues and treated ES2 and SKOV3
cells using the TRIzol reagent (Invitrogen). The RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific) was used to
synthesize cDNAs with random primers according to the
manufacturer's protocols.
Quantitative real-time reverse
transcription PCR (qRT-PCR)
As described previously (29), the mRNA expression levels were
detected using the SYBR-Green PCR Master Mix kit (Takara) in PCR
reaction. The primer sequences for GAPDH are:
5′-TGTTCGTCATGGGTGTGAAC-3′ (forward) and 5′-ATGGCATGGACTGTGGTCAT-3′
(reverse) (internal control). The primer sequences for N4BP1 are:
5′-TATGCAGCCCCTACTCAGTG-3′ (forward) and 5′-GCTCGTTGGTTTCTGCAGAA-3′
(reverse). The primer sequences for hsa-miR-28-5p are:
5′-AAGGAGCUCACAGUCUAUUGAG-3′. The primer sequences for U6 are:
5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-AACGCTTCACGAATTTGCGT-3′
(reverse). All data are reported as the mean ± SD of three
independent experiments.
Western blot analysis
Seventy-two hours after mature miR-28-5p mock,
mimic, or inhibitor transfection, the ES2 and SKOV3 cells were
lysed using lysis buffer containing a protease inhibitor cocktail
(P8340; Sigma-Aldrich, St. Louis, MO, USA). The concentrations of
total proteins were analyzed using BCA Protein Assay kit (Thermo
Fisher Scientific, Rockford, IL, USA). Proteins (30 µg) were
added into each lane on the 8% SDS/PAGE gels based on the molecular
weight of the objective proteins. The 5% skim milk (BD Biosciences)
was used to block the PVDF membranes, and then they were
(Millipore, Billerica, MA, USA) used to incubate the optimal
concentration of primary antibody at 4°C overnight. The next day,
the horseradish peroxidase-conjugated secondary antibodies with a
proper dilution were incubated for 1 h at room temperature. The
experimental results were visualized using the enhanced
chemiluminescence (ECL) substrate kit (Amersham Biosciences, Inc.,
Piscataway, NJ, USA) and the enhanced chemiluminescence detection
system (Amersham Biosciences). The primary antibodies used were the
anti-N4BP1 antibody (rabbit, 1:100, Abcam, Cambridge, MA, USA); the
anti-F-actin (1:200, Cell Signaling Technology, Boston, MA, USA);
the anti-E-cadherin (1:5,000, BD Biosciences, San Jose, CA, USA);
the anti-vimentin (1:1,000, V6630, Sigma-Aldrich); the anti-GAPDH
antibody (1:4,000, Cell Signaling Technology, Beverly, MA, USA) was
used as internal control.
Immunohistochemistry (IHC) assay
According to the manufacturer's instructions,
immunohistochemistry of N4BP1 was completed on 5-mm formalin fixed;
paraffin-embedded tissue sections which were cut from the FFPE
blocks. The sections were incubated with rabbit anti-N4BP1 antibody
(Abcam) at 4°C overnight. The automated immunostainer (Ventana
Medical Systems, Tucson, AZ, USA) was used to complete the
immunohistochemical stains. The N4BP1 staining results were
classified as 0, negative staining; 1, weak staining; 2, moderate
staining; and 3, intense staining. The negative staining and weak
staining cells were considered as low expressors, and the moderate
staining and intense staining cells were considered to be high
expressors.
Colony forming unit (CFU) assay
The ES2 or SKOV3 cells which were transfected with
miR-28-5p mock, mimics, or inhibitors were seeded into the DMEM
complete medium for 7 days. Then the colons were fixed using
methanol for 15 min, and dyed using Giemsa dye solution for 10 min.
Then the colony forming units were recorded and counted.
MTT assay
The treated ES2 or SKOV3 cells (2,000 cells/well)
were seeded in 96-well plates with complete medium for 24, 48 and
72 h, respectively. The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT,
20 µl/well) solution (5 mg/ml) was added into each well at
the particular point in time. After 4 h, 100 µl dimethyl
sulfoxide solutions were added to dissolve the crystal. The
absorbance was detected using a micro-plate reader (Bio Tek
Instruments, Inc., Winooski, VT, USA) at 490 nm.
Flow cytometric analysis of the cell
cycle
The treated ES2 or SKOV3 cells were resuspended in
PBS containing 70% ethanol, 0.5 mg/ml RNaseA and 0.1 mg/ml
propidium iodide. The images of cell cycle were obtained using the
FACSCalibur (BD Biosciences). The results were analyzed using
FlowJo software (Tree Star Corp., Ashland, OR, USA).
Flow cytometric analysis of the cell
apoptosis
Likewise, the treated ES2 or SKOV3 cell suspension
was stained with FITC-Annexin V and propidium iodide (PI). The flow
cytometry results were analyzed using FlowJo software. Cells were
segmented into four types, such as the viable cells, dead cells,
the early stage apoptotic cells, the late stage apoptotic cells,
and the dead cells.
Wound healing assay
As described previously (30), the treated ES2 or SKOV3 cells were
incubated in 60-mm culture plates, and the small linear wounds were
created. The cell debris was washed off gently. After 24 h, the
results were analyzed by image analysis software (National
Institute of Health, Bethesda, MD, USA). The degree of wound
healing depended on the distance of migration of cells from the
edge of the scratch.
Migration and invasion assays
According to the manufacturer's instructions, the
migration capacity of the treated ES2 or SKOV3 cells were evaluated
in 24-well Transwell cell culture chamber (costar). Treated cells
(200 µl) (2.5×105/100 µl) were incubated
in serum-free media and added into the upper chamber of the
8-µm pore cell culture inserts, and complete medium with 10%
FBS was added to the lower chambers. After 24 h, the migratory
cells were fixed by 4% paraformaldehyde, and stained by 0.1%
crystal violet solution. The number of migratory cells was counted
using a 20× objective microscope. For the invasion assay, 10
µl 1:8 diluted Matrigel (BD Biosciences, San Diego, CA, USA)
was pre-paved into the polycarbonate membrane of Transwell inserts
before the experiment at 37°C for 2 h.
Tumor formation in nude mice
The animal experiments were approved by the
Institutional Committee for Animal Research and performed according
to the Institutional Animal Care and Use Committee. The flanks of
5-week-old BALB/c athymic nude mice were injected subcutaneously
with the ES2 cells treated with negative control or miR-28-5p
agomir (1×107 cells in 100 µl) 5 times for 4
weeks. At the planned time every week, the mice were sacrificed,
the bodies were dissected, the tumor weight was measured and the
tumor volume was calculated based on the formula: V =
πAB2/6, where A means the largest diameter and B means
the perpendicular diameter.
Statistical analysis
The data were analyzed by the Student's t-test and
variance (ANOVA) using SPSS 15.0 software (SPSS, Chicago, IL, USA).
All results are expressed as means ± SE. The statistical
significance was set at P<0.05.
Results
miR-28-5p overexpression in ovarian
cancer tissues
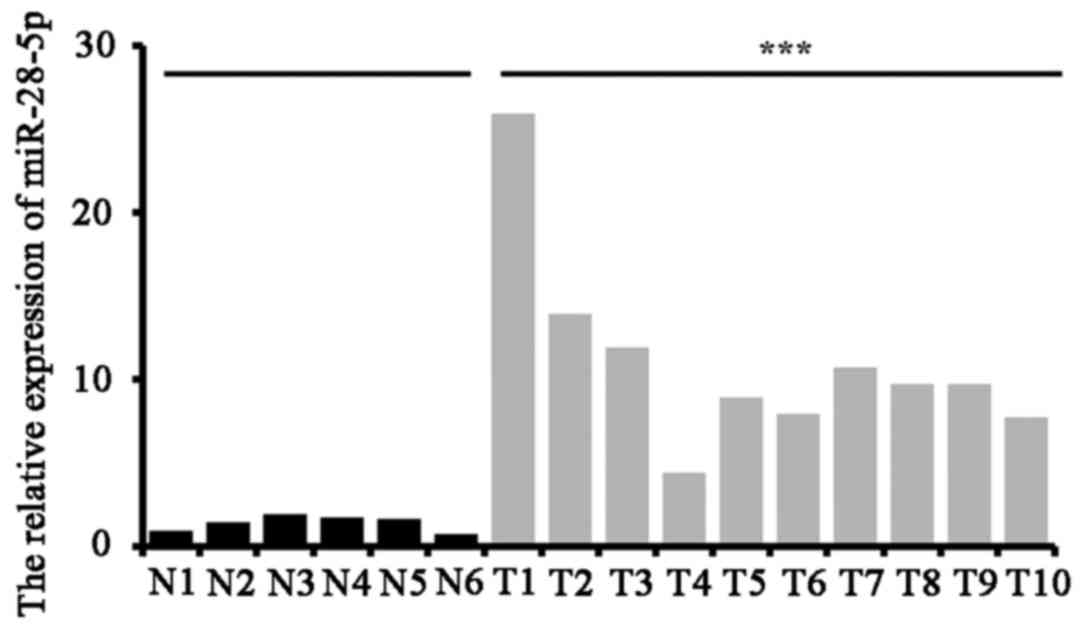
We used the ovarian cancer tissues from 10 patients
and adjacent noncancerous tissues from 6 patients randomly. The
expression level of miR-28-5p was measured by qRT-PCR. The results
indicated that the expression level of miR-28-5p was increased in
ovarian cancer tissues (n=10) compared with adjacent noncancerous
tissues (n=6) (P<0.001) (Fig.
1).
miR-28-5p promotes the progression of
cell cycle and proliferation and inhibits apoptosis in ovarian
cancer cells
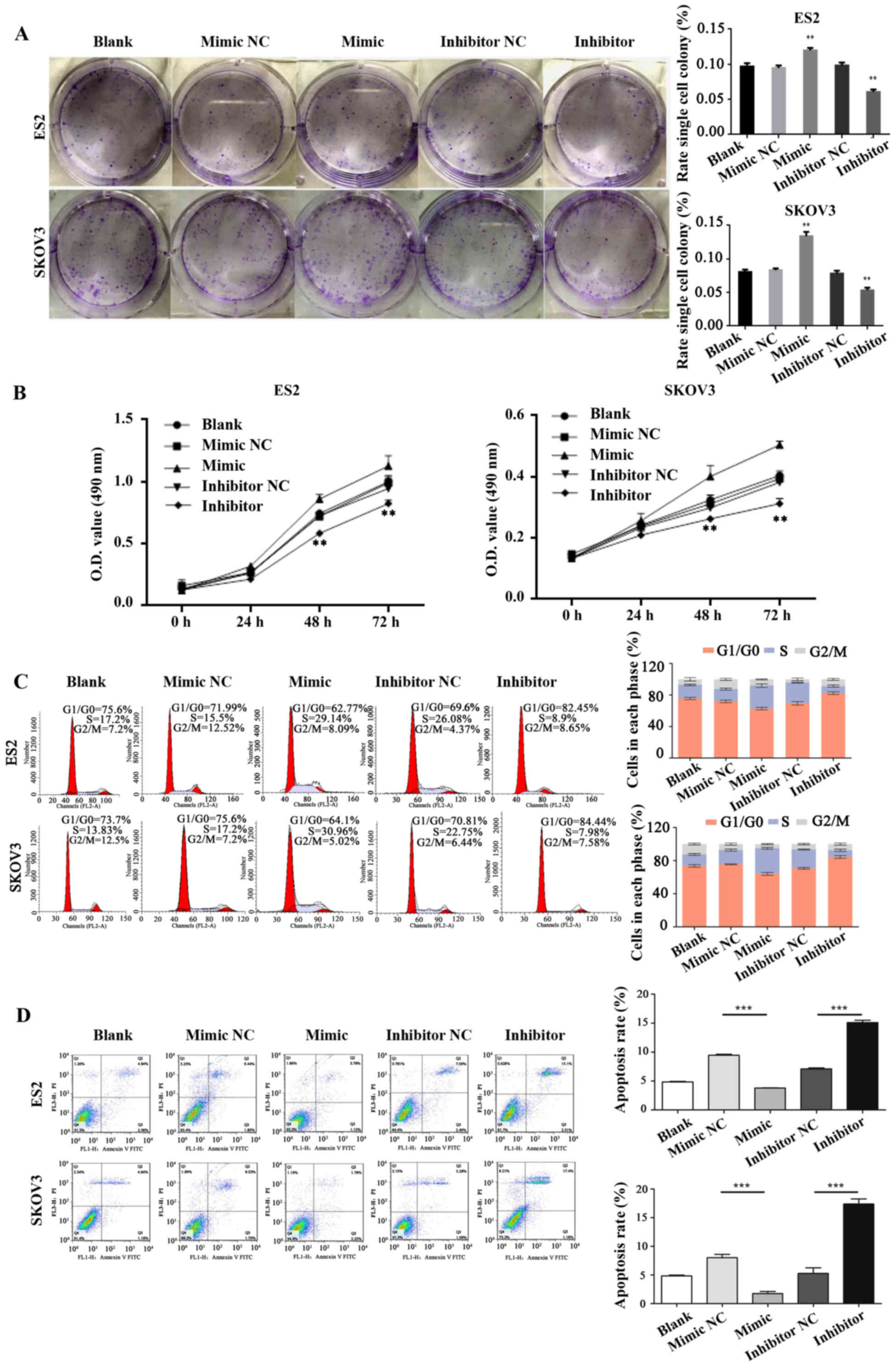
The impacts of miR-28-5p expression levels on the
ability of ovarian cancer cell proliferation, cycle, and apoptosis
was detected in ES2 or SKOV3 cells which were transfected with
mature miR-28-5p mock, mimic, or inhibitor, respectively. The
colony forming unit assay was used to measure the ovarian cell
proliferation ability. Our results found that the proliferation
ability was significantly increased in ES2 or SKOV3 cells
transfected with mature miR-28-5p mimic compared with the control
group (mock) (P<0.001). The proliferation ability was
significantly decreased in ES2 or SKOV3 cells transfected with
mature miR-28-5p inhibitor compared with the control group (mock)
(P<0.001) (W. 2A). Similarly,
the ovarian cell proliferation ability also was detected by MTT
assay, and the results showed that miR-28-5p promoted the
proliferation capacity of ES2 or SKOV3 cells (Fig. 2B). We found that the cell cycle
significantly arrested in G1/G0 in ES2 cells transfected with
mature miR-28-5p inhibitor (Diploid, 100.00%; Dip G1/G0, 82.45%;
Dip S, 8.90%; Dip G2/M, 8.65%) compared with the control group
(Diploid, 100.00%; Dip G1/G0, 69.55%; Dip S, 26.08%; Dip G2/M,
4.37%) by flow cytometry; and the cell cycle significantly arrested
in G1/G0 in SKOV3 cells trans-fected with mature miR-28-5p
inhibitor (Diploid, 100.00%; Dip G1/G0, 84.44%; Dip S, 7.98%; Dip
G2/M, 7.58%) compared with the control group (Diploid, 100.00%; Dip
G1/G0, 70.81%; Dip S, 22.75%; Dip G2/M, 6.44%) (Fig. 2C). The cell apoptosis capacity of
ES2 and SKOV3 cells transfected with mature miR-28-5p inhibitor was
significantly increased compared with the control group by Annexin
V-FITC/PI staining (P<0.001). The cell apoptosis capacity of ES2
and SKOV3 cells transfected with mature miR-28-5p mimic was
significantly decreased compared with the control group
(P<0.001) (Fig. 2D).
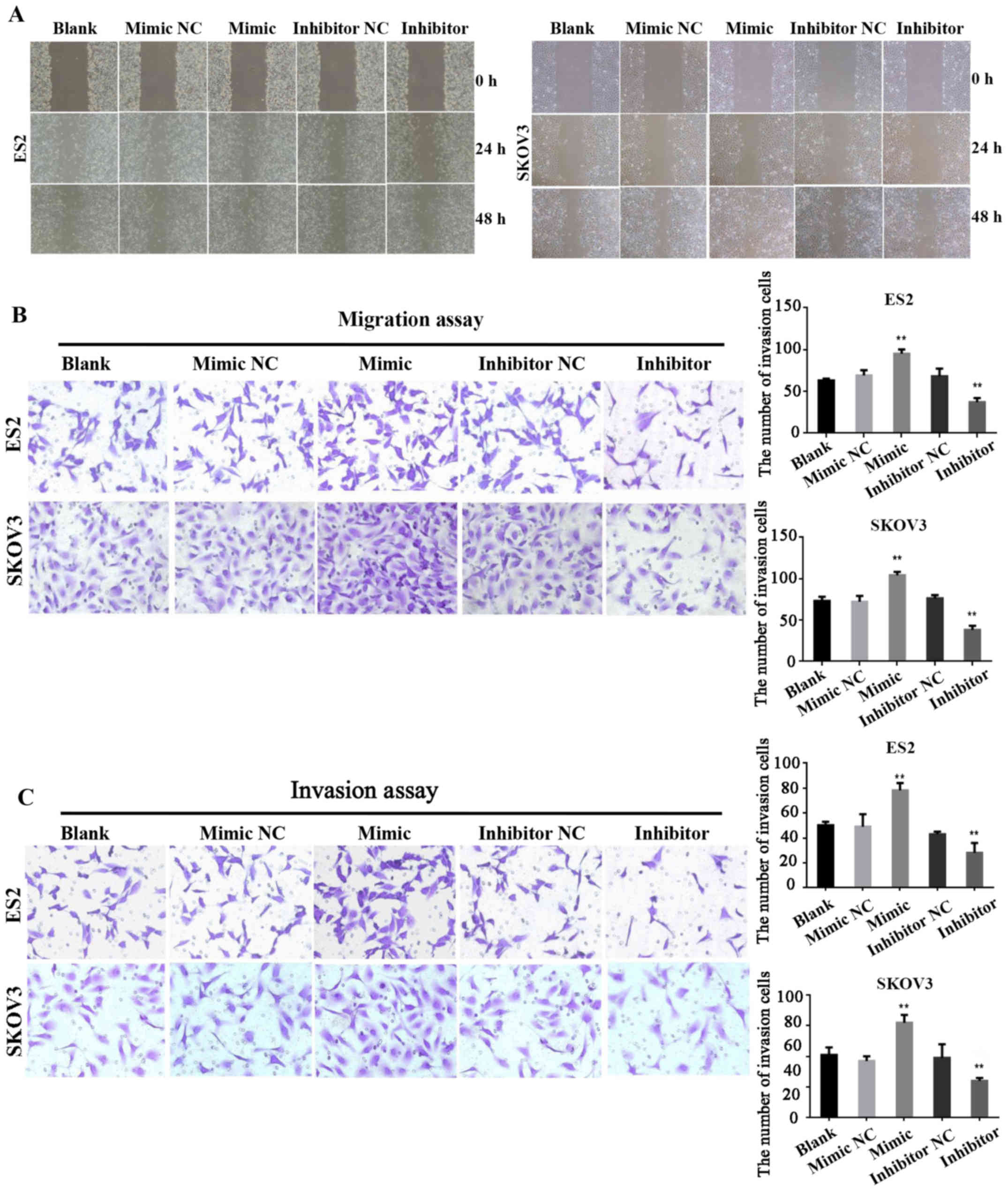
miR-28-5p expressions accelerate
migration and invasion ability of ovarian cancer cells
We further studied the cell migration and invasion
ability which was affected by miR-28-5p in ovarian cancer cells.
Firstly, the migration ability of ES2 and SKOV3 cells transfected
with miR-28-5p mimic, inhibitor or NC were measured by wound
healing assay at 0, 24 and 48 h, respectively. The results
indicated that the cell migration capacity of ES2 and SKOV3 cells
transfected with mature miR-28-5p mimic was significantly increased
compared with the control group (P<0.01); the cell migration
capacity of ES2 and SKOV3 cells transfected with mature miR-28-5p
inhibitor was significantly decreased compared with the control
group (P<0.01) (Fig. 3A).
Secondly, the migration ability was detected using the migration
assay. The results also showed that miR-28-5p expression
accelerated the migration ability of ovarian cancer cells (Fig. 3B). Finally, the results of the
invasion assay also indicated that the cell invasion ability of ES2
and SKOV3 cells transfected with mature miR-28-5p mimic was
significantly increased compared with the control group
(P<0.01); the cell invasion capacity of ES2 cells and SKOV3
transfected with mature miR-28-5p inhibitor was significantly
decreased compared with the control group (P<0.01) (Fig. 3C).
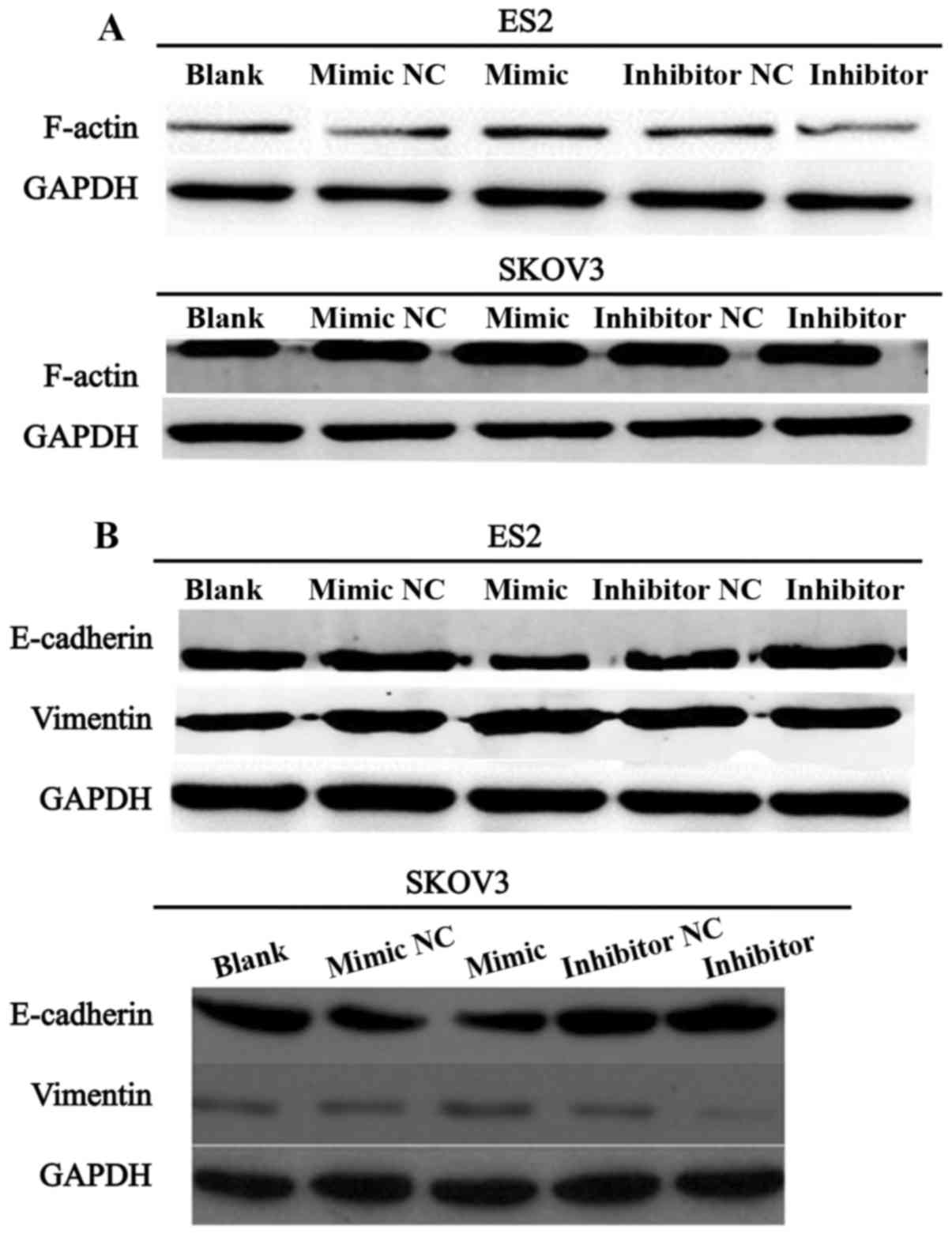
miR-28-5p regulates F-actin, E-cadherin,
and vimentin expression
We further investigated whether miR-28-5p expression
influenced the protein expression level of F-actin. Western blot
assay was performed to detect the protein expression level of
F-actin in ES2 and SKOV3 cells transfected with miR-28-5p mimic,
inhibitor or NC. As showed in Fig.
4A, the expression level of F-actin was significantly
upregulated in ES2 and SKOV3 cells transfected with mature
miR-28-5p mimic compared with the control group. Conversely,
F-actin expression was significantly downregulated in ES2 and SKOV3
cells transfected with mature miR-28-5p inhibitor in comparison
with the control group. Furthermore, we measured the expression
levels of proteins associated with epithelial-mesenchymal
transition (EMT) in ES2 and SKOV3 cells transfected with miR-28-5p
mimic, inhibitor or NC. The results indicated that miR-28-5p
downregulated the protein expression level of E-cadhetin and
upregulated the protein expression level of vimentin in ES2 and
SKOV3 cells (Fig. 4B). Therefore,
our investigation demonstrated that miR-28-5p promotes the progress
of EMT in ovarian carcinoma cells.
miR-28-5p downregulates N4BP1 expression
in human ovarian cancer
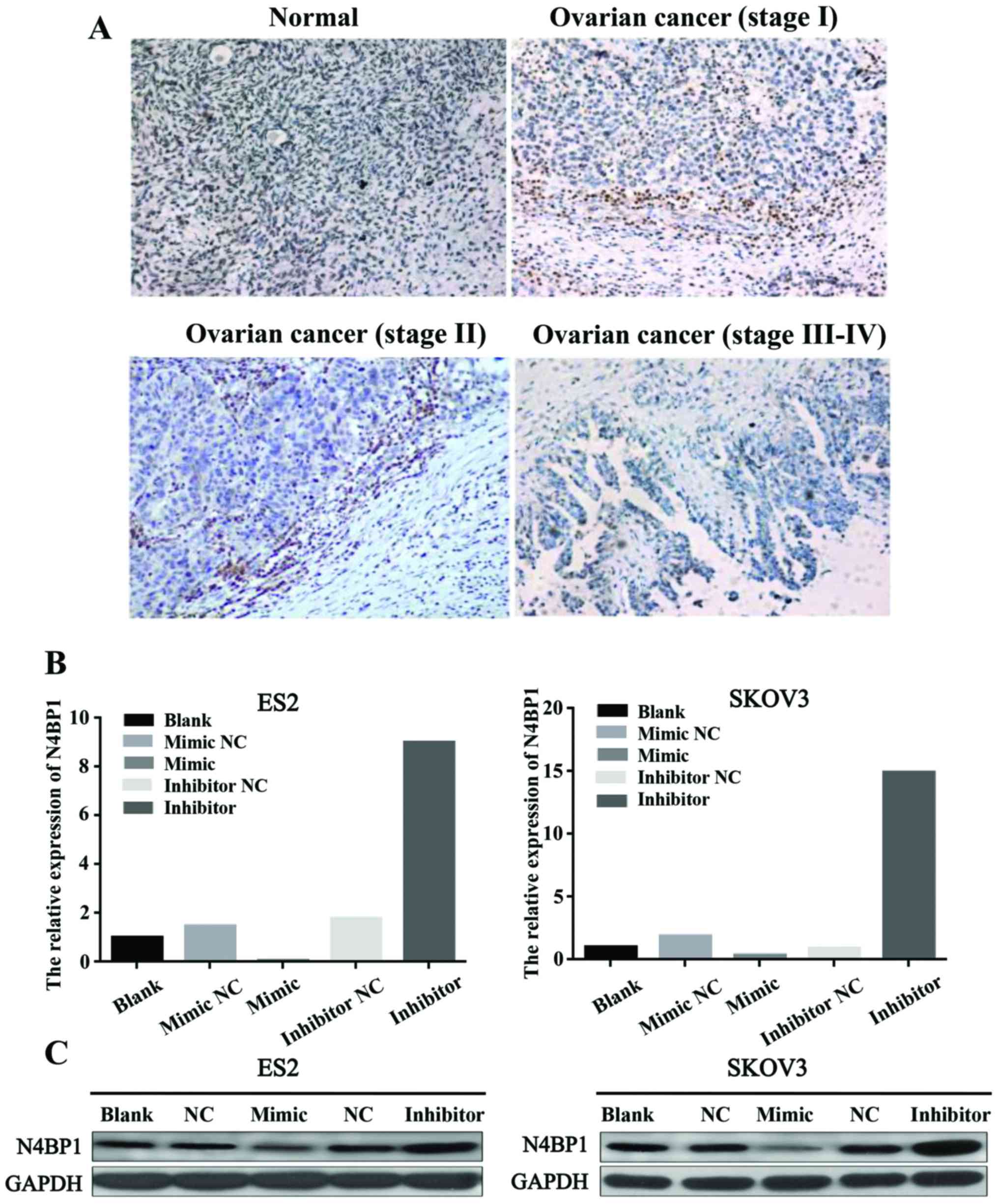
To explore the protein expression level of N4BP1 in
human ovarian cancer tissues, we performed IHC analysis of the
human ovarian cancer tissues. The results indicated that the
expression of N4BP1 was lower in ovarian cancer tissues compared
with normal ovarian tissues. Therefore, we concluded that N4BP1 has
low expression in human ovarian cancer (Fig. 5A). To further obtain the mechanism
of miR-28-5p in ovarian cancer, we studied the relationship between
miR-28-5p and N4BP1. The qRT-PCR results revealed that miR-28-5p
inhibited the mRNA expression level of N4BP1 in ES2 and SKOV3 cells
(Fig. 5B). We also detected the
protein expression level of N4BP1 using western blot analysis. We
found that miR-28-5p inhibited the protein expression level of
N4BP1 in ES2 and SKOV3 cells (Fig.
5C). Therefore, we proved that miR-28-5p downregulated N4BP1
expression in human ovarian cancer.
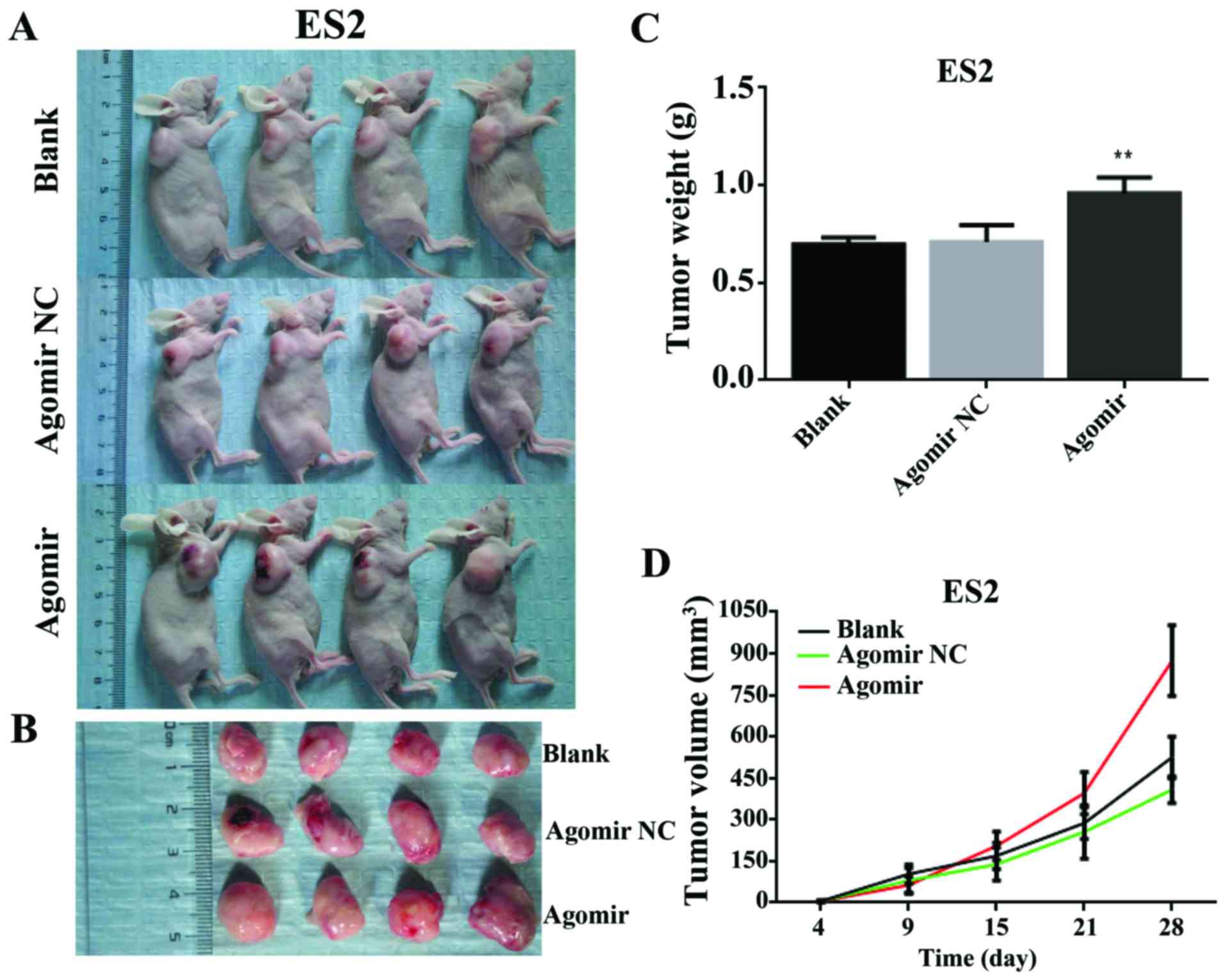
miR-28-5p promotes the growth of ovarian
tumor in vivo
To assess the effect of miR-28-5p on tumorigenesis
in vivo, ES2-blank, ES2-NC, and ES2-agomir cells were
implanted subcutaneously into nude mice. The mice were sacrificed
at 4, 9, 15, 21 and 28 days respectively (Fig. 6A), the bodies were dissected, and
the tumor removed (Fig. 6B). Tumor
weight was measured and tumor volume was calculated. The results
showed that mice injected with ES2-agomir cells developed larger
tumors than those injected with ES2-blank, or ES2-NC cells (P<0.
01) (Fig. 6C and D).
Discussion
Ovarian cancer is one of the most universal cancers
in women and ranks the sixth in female cancers in the world
resulting in ~125,000 deaths each year (31). Due to technical limitations, the
patients have advanced ovarian carcinoma when they are diagnosed.
There are only 30% of advanced-stage ovarian cancer patients who
can survive >5 years after diagnosis (32). Therefore, it is urgent to study the
pathological mechanism in ovarian cancer for exploiting new
diagnosis and new treating method against this malignancy.
MicroRNAs (miRNAs), a class of small non-coding
RNAs, regulate mRNAs. Many studies showed that miRNAs have
important functions, such as cell proliferation, metastasis,
inflammation, and angiogenesis of tumor by targeting mRNAs in
various cancers (33,34). Many studies have indicated that
miRNAs are closely related to the development and progression of
diverse diseases (8,11,12).
There are reports that various miRNAs such as miR-141, miR-200a,
miR-199a, miR-223, miR-100, and miR-9 are associated with the
development process of ovarian cancer (15,35–37).
The mechanism and function of miR-28-5p have been proven in
colorectal cancer (18) and
hepatocellular carcinoma (38,39).
However, the functional relevance of miR-28-5p in ovarian cancer is
still not known. In our study, we found that miR-28-5p had higher
expression in ovarian cancer tissues in comparison with adjacent
ovarian tissues. miR-28-5p promoted the progression of ovarian
cancer cell cycle, proliferation, migration and invasion, and
inhibited cell apoptosis in vitro. Moreover, miR-28-5p
promoted the growth of ovarian tumor in vivo.
Epithelial-mesenchymal transition (EMT) is the core
of normal embryonic development, and a biological process that the
epithelial cells are translated into the mesenchymal phenotype
cells (40). It has played an
important role in the development of embryonic, chronic
inflammation, tissue reconstruction, cancer metastasis, and many
fibrotic diseases (41). In the
process of EMT, a main characteristic is that the expression level
of cell adhesion molecules (E-cadherin) was decreased; and the the
expression level of mesenchymal markers (such as vimentin) was
increased (23). Some studies
indicated that EMT has very important effect in tumor invasion and
metastasis (42). Therefore, the
process of EMT is related to invasion, metastasis and drug
resistance of tumors. In this study, we indicated that miR-28-5p
downregulated the protein expression level of E-cadherin and
upregulated the protein expression level of vimentin in ES2 and
SKOV3 cells. Therefore, our studies demonstrated that miR-28-5p
promotes the progress of EMT in ovarian carcinoma cells. Previous
studies have shown that F-actin is interrelated with the mobility,
contraction, and migration of cells (24,43).
Our results indicated that miR-28-5p increased the protein
expression level of F-actin. This demonstrated that miR-28-5p may
promote the progress of migration in ovarian cancer cells.
N4BP1 is closely related to the normal development
by acting on the related E3 ligase ITCH (26). It was supposed that N4BP1 may have
ribonuclease activity because the YacP-like nuclease (NYN) domain
possesses nucleases (28).
Therefore, N4BP1 plays a major role in gene regulation. However,
the function and mechanism research of N4BP1 have not been reported
in ovarian cancer. Therefore, in this study, we indicated that the
expression level of N4BP1 was lower in ovarian cancer tissues
compared with normal ovarian tissues. miR-28-5p inhibited the mRNA
expression level of N4BP1 in ES2 and SKOV3 cells. Therefore, we
came to a conclusion that miR-28-5p downregulated N4BP1 expression
in human ovarian cancer. This further revealed the important roles
of N4BP1, which miR-28-5p promoted in the development and
progression of ovarian cancer through inhibition of N4BP1.
In conclusion, this study indicated that miR-28-5p
and N4BP1 had high expression in ovarian cancer tissues. miR-28-5p
promoted the progression of ovarian cancer cell cycle,
proliferation, migration and invasion, and inhibited apoptosis
in vitro and promoted the growth of ovarian tumor in
vivo. miR-28-5p promoted the progress of EMT in ovarian
carcinoma cells. In addition, we indicated that miR-28-5p
downregulated N4BP1 expression in human ovarian cancer. This
further revealed that miR-28-5p promoted the development and
progression of ovarian cancer through inhibition of N4BP1. This
study may provide the potential theoretical foundation for the
diagnosis and treatment of ovarian cancer.
Acknowledgments
This study was supported by Natural Science
Foundation of Guangdong Province (nos. S2012010006150 and
S2012040006148) and Science and Technology Planning Project of
Guangdong Province, China (no. 2014A020212710).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zaman MS, Maher DM, Khan S, Jaggi M and
Chauhan SC: Current status and implications of microRNAs in ovarian
cancer diagnosis and therapy. J Ovarian Res. 5:442012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heintz AP, Odicino F, Maisonneuve P, Quinn
MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S and Beller U:
Carcinoma of the ovary. FIGO 26th Annual Report on the Results of
Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 95(Suppl
1): S161–S192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. MicroRNAs en route to the clinic: Progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baer C, Claus R and Plass C: Genome-wide
epigenetic regulation of miRNAs in cancer. Cancer Res. 73:473–477.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Leva G and Croce CM: The role of
microRNAs in the tumori-genesis of ovarian cancer. Front Oncol.
3:1532013. View Article : Google Scholar
|
|
13
|
Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim
JH, Kim JW and Kim S: MicroRNA expression profiles in serous
ovarian carcinoma. Clin Cancer Res. 14:2690–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dahiya N and Morin PJ: MicroRNAs in
ovarian carcinomas. Endocr Relat Cancer. 17:F77–F89. 2010.
View Article : Google Scholar :
|
|
15
|
Mateescu B, Batista L, Cardon M, Gruosso
T, de Feraudy Y, Mariani O, Nicolas A, Meyniel JP, Cottu P,
Sastre-Garau X, et al: miR-141 and miR-200a act on ovarian
tumorigenesis by controlling oxidative stress response. Nat Med.
17:1627–1635. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dahiya N, Sherman-Baust CA, Wang TL,
Davidson B, Shih IeM, Zhang Y, Wood W III, Becker KG and Morin PJ:
MicroRNA expression and identification of putative miRNA targets in
ovarian cancer. PLoS One. 3:e24362008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hell MP, Thoma CR, Fankhauser N,
Christinat Y, Weber TC and Krek W: miR-28–5p promotes chromosomal
instability in VHL-associated cancers by inhibiting Mad2
translation. Cancer Res. 74:2432–2443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Almeida MI, Nicoloso MS, Zeng L, Ivan C,
Spizzo R, Gafà R, Xiao L, Zhang X, Vannini I, Fanini F, et al:
Strand-specific miR-28-5p and miR-28-3p have distinct effects in
colorectal cancer cells. Gastroenterology. 142:886–896.e9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Q, Zhu F and Chen P: miR-7 and miR-218
epigenetically control tumor suppressor genes RASSF1A and Claudin-6
by targeting HoxB3 in breast cancer. Biochem Biophys Res Commun.
424:28–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Creighton CJ, Gibbons DL and Kurie JM: The
role of epithelial-mesenchymal transition programming in invasion
and metastasis: A clinical perspective. Cancer Manag Res.
5:187–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sheppard D: Epithelial-mesenchymal
interactions in fibrosis and repair. Transforming growth factor-β
activation by epithelial cells and fibroblasts. Ann Am Thorac Soc.
12(Suppl 1): S21–S23. 2015. View Article : Google Scholar
|
|
23
|
Fraga CH, True LD and Kirk D: Enhanced
expression of the mesenchymal marker, vimentin, in hyperplastic
versus normal human prostatic epithelium. J Urol. 159:270–274.
1998. View Article : Google Scholar
|
|
24
|
McGough A, Pope B, Chiu W and Weeds A:
Cofilin changes the twist of F-actin: Implications for actin
filament dynamics and cellular function. J Cell Biol. 138:771–781.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murillas R, Simms KS, Hatakeyama S,
Weissman AM and Kuehn MR: Identification of developmentally
expressed proteins that functionally interact with Nedd4 ubiquitin
ligase. J Biol Chem. 277:2897–2907. 2002. View Article : Google Scholar
|
|
26
|
Oberst A, Malatesta M, Aqeilan RI, Rossi
M, Salomoni P, Murillas R, Sharma P, Kuehn MR, Oren M, Croce CM, et
al: The Nedd4-binding partner 1 (N4BP1) protein is an inhibitor of
the E3 ligase Itch. Proc Natl Acad Sci USA. 104:11280–11285. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma P, Murillas R, Zhang H and Kuehn
MR: N4BP1 is a newly identified nucleolar protein that undergoes
SUMO-regulated polyubiquitylation and proteasomal turnover at
promyelocytic leukemia nuclear bodies. J Cell Sci. 123:1227–1234.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anantharaman V and Aravind L: The NYN
domains: Novel predicted RNAses with a PIN domain-like fold. RNA
Biol. 3:18–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang L, Lai YK, Zhang J, Wang H, Lin MC,
He ML and Kung HF: Targeting S100P inhibits colon cancer growth and
metastasis by Lentivirus-mediated RNA interference and proteomic
analysis. Mol Med. 17:709–716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Madhyastha HK, Radha KS, Nakajima Y, Omura
S and Maruyama M: uPA dependent and independent mechanisms of wound
healing by C-phycocyanin. J Cell Mol Med. 12B:2691–2703. 2008.
View Article : Google Scholar
|
|
31
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Paris PL, Chen J, Ngo V, Yao H,
Frazier ML, Killary AM, Liu CG, Liang H, Mathy C, et al: Next
generation sequencing of pancreatic cyst fluid microRNAs from low
grade-benign and high grade-invasive lesions. Cancer Lett.
356B:404–409. 2015. View Article : Google Scholar
|
|
34
|
Stahlhut C and Slack FJ: MicroRNAs and the
cancer phenotype: Profiling, signatures and clinical implications.
Genome Med. 5:1112013. View
Article : Google Scholar
|
|
35
|
Cheng W, Liu T, Wan X, Gao Y and Wang H:
MicroRNA-199a targets CD44 to suppress the tumorigenicity and
multidrug resistance of ovarian cancer-initiating cells. FEBS J.
279:2047–2059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nagaraja AK, Creighton CJ, Yu Z, Zhu H,
Gunaratne PH, Reid JG, Olokpa E, Itamochi H, Ueno NT, Hawkins SM,
et al: A link between mir-100 and FRAP1/mTOR in clear cell ovarian
cancer. Mol Endocrinol. 24:447–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu X, Tian J, Zhang L, Chen Y and Hao Q:
Involvement of microRNA-93, a new regulator of PTEN/Akt signaling
pathway, in regulation of chemotherapeutic drug cisplatin
chemosensitivity in ovarian cancer cells. FEBS Lett. 586:1279–1286.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou SL, Hu ZQ, Zhou ZJ, Dai Z, Wang Z,
Cao Y, Fan J, Huang XW and Zhou J: miR-28–5p-IL-34-macrophage
feedback loop modulates hepatocellular carcinoma metastasis.
Hepatology. 63:1560–1575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi X and Teng F: Down-regulated miR-28-5p
in human hepatocellular carcinoma correlated with tumor
proliferation and migration by targeting insulin-like growth
factor-1 (IGF-1). Mol Cell Biochem. 408:283–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesen-chymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
De Wever O, Demetter P, Mareel M and
Bracke M: Stromal myofibroblasts are drivers of invasive cancer
growth. Int J Cancer. 123:2229–2238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lauffenburger DA and Horwitz AF: Cell
migration: A physically integrated molecular process. Cell.
84:359–369. 1996. View Article : Google Scholar : PubMed/NCBI
|