Introduction
Epithelial ovarian cancer is the fifth most common
cause of cancer-related death among women in the United States.
Though more than 80% of patients with advanced ovarian cancer
benefit from first-line therapy, 75% of those patients will
experience tumor recurrence due to widespread metastasis within the
abdomen (1,2). The current available treatments for
ovarian cancer include tumor debulking surgery and chemotherapy.
Cisplatin is an important chemotherapeutic drug for the treatment
of ovarian cancer. However, the majority of patients who respond to
cisplatin initially will relapse due to the development of
resistance (3). Thus, there is an
urgent need to search for new agents derived from naturally
occurring secondary metabolites. Since the 1940s, 175 small
molecule cancer drugs have been developed. A total of 131 of those
drugs are considered 'other than synthetic' and 85 drugs are
natural products or their direct derivitives which are mainly
derived from bacteria and plants (4). In recent years, more attention has
been paid to fungi-derived natural products which have promising
anticancer activities. Many fungal metabolites have demonstrated
notable in vitro growth-inhibitory properties against
various human cancer cell lines. Moreover, selected metabolites
have exhibited therapeutic benefits in vivo mouse models
(5). 3-Hydroxyterphenyllin (3-HT;
Fig. 1A), is a metabolite isolated
from Aspergillus candidus. The compound was first discovered
in 1979 (6). It effectively
inhibited the development of sea urchin embryonic development
(7). The inhibitory pattern 3-HT
exhibited was similar to Candidusin B, which is also isolated from
Aspergillus candidus and could suppress DNA and RNA
syntheses in embryos. Other reports suggested that 3-HT possessed
antioxidative properties and showed neither cytotoxic nor genotoxic
traits against human intestine 470 cells (INT 470); though, it
showed protective effects against oxidative damage to INT 407 cells
(8,9). However, the anticancer effects of
3-HT have not been investigated.
In the present study, we investigated the anticancer
effect of 3-HT. Currently, it has been proven that apoptosis is an
important biological pathway of programmed cell death in
multicellular organisms, promoting apoptosis has become a key
strategy for cancer drug discovery (10). Targeting the apoptosis signal
transduction pathway has become pivotal in the implication for
cancer therapy (11). Also,
inducing cell cycle arrest is an effective way to restrict tumor
growth in vitro and in vivo. We have previously
reported that Chaetoglobosin K, a secondary metabolite isolated
from the fungus Diplodia macrospora, could induce apoptosis
and G2 cell cycle arrest in ovarian cancer cells (12). Other reports have also proven that
metabolites isolated from marine-derived fungal metabolites could
induce apoptosis or cell cycle arrest in different human cancer
cell lines (13,14). All these studies provide a
promising prospect for discovering anticancer drugs from fungal
metabolites.
Therefore, considering the lack of published reports
on the anticancer effects of 3-HT in human cancer cells, we aimed
to investigate its anticancer effects and the molecular signaling
pathway using two ovarian cancer cell lines, A2780/CP70 and
OVCAR-3, and a normal human epithelial ovarian cell line, IOSE-364
as in vitro models. Our results demonstrate that 3-HT has
effective anticancer effect and provide foundations for further
studies.
Materials and methods
Materials
3-Hydroxyterphenyllin (3-HT), was obtained from the
Cutler Laboratory (University of Mississippi, Oxford, MS, USA).
3-HT was dissolved in dimethyl sulfoxide (DMSO) to a concentration
of 10 mM and stored at −20°C. Working concentrations of 0, 2, 4, 8,
12 and 16 µM, as for control, DMSO was diluted by cell
culture medium at a final concentration that was equal to the
maximal concentration of the 3-HT solvent. RPMI-1640 medium, bovine
serum albumin (BSA), DMSO, Hoechst 33342 and DCFH-DA were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS),
phosphate-buffered saline (PBS) and propidium iodide (PI) were
purchased from Life Technologies (Grand Island, NY, USA). CellTiter
96® AQueous One Solution Cell Proliferation
assay was purchased from Promega (Madison, WI, USA). Pierce LDH
Cytotoxicity assay kit and Alexa Fluor® 488 Annexin
V/Dead Cell Apoptosis kit were purchased from Thermo Fisher
Scientific (Waltham, MA, USA). Primary antibodies to caspase-3,
caspase-9, p21Waf1/Cip1 (12D1), p38, Bax, Bcl-2, Puma, FADD, cyclin
B1, cyclin A2, cyclin D1, cyclin E1, CDK2, CDK4, cdc2, cdc25c,
cdc25A, p-ATM (Ser1981), ATM, DR5, Fas and γ-H2AX (Ser139) were
purchased from Cell Signaling Inc. (Danvers, MA, USA). Primary
antibodies to p53 (C11), p-p53 (Ser15), PARP-1 (F-2), Bad (C-7),
Bcl-xL (H-5), p-ERK1/2 (Thr202), ERK1 (K-23), chk1 (G4), p-chk2
(Thr68), chk2 (H-300), DR4 (H-130), GAPDH (0411) and the secondary
antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA).
Cell lines and cell culture
The human ovarian carcinoma cell lines, A2780/CP70
and OVCAR-3 were provided by Dr Jiang from the West Virginia
University, the normal ovarian surface epithelial cell line
IOSE-364 was provided by Dr Auersperg from the University of
British Columbia. All cell lines were cultured in RPMI-1640 medium,
supplemented with 10% FBS, and incubated in a humidified incubator
with 5% CO2 at 37°C.
Cell viability assay
The effect of 3-HT on cell viability was measured by
the CellTiter 96® AQueous One Solution Cell
Proliferation assay. A total of 1.0×104 cells/well were
seeded in 96-well plates. After incubation for 24 h, the cells were
treated with different concentrations of 3-HT for 24 h and then 100
µl AQueous One reagent was added to each well and
incubated for another 1 h. Absorbance was measured at 490 nm using
a microplate reader (Synergy™ Multi-Mode; BioTek Instruments, Inc.,
Winooski, VT, USA). Cell viability was expressed as a percentage of
control.
LDH cytotoxicity assay
LDH assay was determined by LDH cytotoxicity assay
kit according to the manufacturer's guidelines. Briefly, cells were
seeded in 96-well plates with the density of 1×104
cells/well. After a 24-h growth period, cells were exposed to 3-HT
at different concentrations for 24 h. After incubation, lysis
buffer and reaction mixture were added to the wells according to
the manufacturer's instruction. The absorbance at 490 and 680 nm
was detected by the microplate reader. LDH cytotoxicity was
calculated using the following formula:
%Cytotoxicity=(Compound-treatedLDHacivity−spontaneous LDH activity)(MaximumLDHacivity−spontaneous LDH activity)×100
Cell cycle analysis
Cell cycle distribution of 3-HT-treated cells was
determined by flow cytometric analysis. Briefly, A2780/CP70 and
OVCAR-3 cell were incubated at a density of ~1×105
cells/well. After exposing with 3-HT at different concentrations
for 24 h, cells were washed twice with PBS and fixed with ice-cold
70% ethanol at 4°C overnight. The fixed cells were washed twice
with PBS followed by incubation with RNase A (180 µg/ml) for
30 min at 37°C. After incubation with PI solution (final
concentration 50 µg/ml) for another 30 min in the dark, cell
cycle analysis was performed by FACSCalibur flow cytometry system
(BD Biosciences, San Jose, CA, USA). A total of 20,000 cells of
each sample were recorded for the analysis. Results were processed
by FCS Software (De Novo Software, Los Angeles, CA, USA).
Hoechst 33342 staining for apoptosis
analysis
Hoechst 33342, a blue fluorescent dye, was used to
analyze the apoptotic effect. Briefly, ~1×104 cells/well
were seeded in 96-well plates. After 24-h incubation, cells were
treated with (0, 2, 4 and 8 µM) 3-HT for 24 h, then washed
with PBS and stained with 10 µg/ml of Hoechst 33342 in PBS
for 15 min at 37°C. After that, cells were assessed by fluorescence
microscopy (Carl Zeiss, Heidelberg, Germany) in a blinded manner to
avoid experimental bias. Apoptotic effect was evaluated through
morphological changes.
Analysis of apoptosis by flow
cytometry
Induction of apoptosis was detected using Alexa
Fluor® 488 Annexin V/Dead Cell Apoptosis kit according
to the manufacturer's protocol. Briefly, after treatment with 3-HT
for 24 h, cells were harvested and washed twice with cold PBS. The
cells were then suspended in 100 µl Annexin-binding buffer
and stained by adding 5 µl Annexin V-fluorescein
isothiocyanate and 1 µl 100 µg/ml PI solution for 15
min in the dark at room temperature. Next 400 µl of
Annexin-binding buffer was added to each sample. Subsequently,
10,000 events of each sample were analyzed using flow cytometry
within 1 h (BD Biosciences).
Measurement of mitochondrial membrane
potential (ΔΨm)
The mitochondrial membrane potential was measured by
JC-1 staining (Invitrogen). Cells were treated with (0, 2, 4 and 8
µM) 3-HT for 24 h, then washed twice with PBS followed by
incubation with 10 µg/ml JC-1 for 30 min in an incubator
with 5% CO2 at 37°C. The fluorescence intensity of red
to green was then measured with a fluorescence plate reader at
excitation: emission of 485/595 and excitation: emission of
485/535, respectively.
Western blot analysis
Cells were treated with 3-HT at different
concentrations for 24 h. Total protein was extracted by
M-PER® mammalian protein extraction reagent supplemented
with 1% Halt™ Protease Inhibitor Single-Use Cocktail on ice for 30
min. The protein concentration was measured using BCA protein assay
kit. Equal amounts of protein were separated electrophoretically
with SDS-PAGE gels and transferred to nitrocellulose membranes
using Mini-PROTEAN 3 system (Bio-Rad Laboratories, Hercules, CA,
USA). The membrane was blocked with 5% non-fat milk for 1 h and
followed by incubating with specific primary antibodies at 4°C
overnight. Membranes were washed in TBST three times for 30 min,
then were incubated with secondary antibodies for 2 h at room
temperature. Secondary antibodies were removed through washing the
membranes in TBST three times for 30 min. The membranes were then
exposed in SuperSignal® West Pico Luminol Enhancer
Solution (Thermo Fisher Scientific, Rockford, IL, USA) for 10 min.
The visualization of protein bands was performed by ChemiDoc™ MP
System (Bio-Rad Laboratories). Protein bands were quantified using
NIH ImageJ software and protein levels were normalized by GAPDH as
internal control.
Intracellular ROS measurement
Peroxide-sensitive fluorescent probe DCFH-DA was
used to detect the intracellular ROS production. Cells were treated
with (0, 2, 4 and 8 µM) 3-HT for 24 h and then incubated
with DCFH-DA (10 µM) for 30 min at 37°C. After staining with
DCFH-DA, the fluorescence intensity was measured by microplate
reader with excitation at 485 nm and emission at 528 nm. Total
protein level was detected to normalize the ROS generation; the
results were expressed as percentage of control.
Statistical analysis
All data were presented as mean ± SEM of at least
three independent experiments. Statistical analysis was performed
by Graphpad Prism software and statistical comparison was evaluated
by one-way analysis of variance with Newman-Keuls test. The
significant differences were shown as P<0.05, P<0.01 and
P<0.001.
Results
3-HT suppresses cell growth and induces
cytotoxicity of ovarian cancer cells
We used cell lines A2780/CP70, OVCAR-3 and IOSE-364
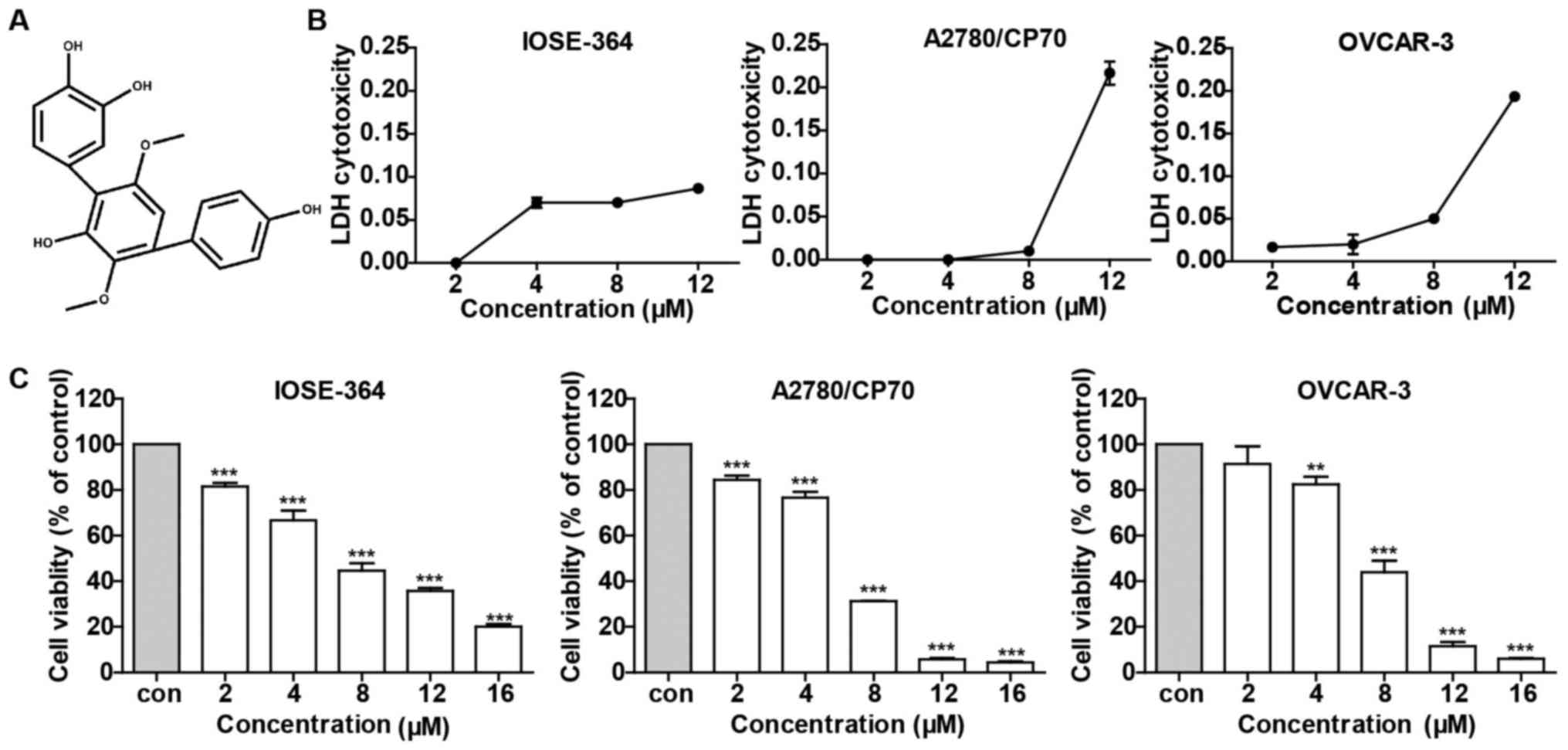
to investigate the effect of 3-HT on ovarian cancer cells. As shown
in Fig. 1C, 3-HT substantially
suppressed ovarian cancer cell growth in a dose-dependent manner,
especially in high doses (12 and 16 µM). In contrast, 3-HT
displayed relatively moderate cytotoxicity toward normal ovarian
surface epithelial cell line IOSE-364 (Fig. 1C). To further explore the
cytotoxicity effect of 3-HT on IOSE-364, A2780/CP70 and OVCAR-3
cells, LDH assay was assessed after treatment with 3-HT at
different concentrations for 24 h. As shown in Fig. 1B, no significant variations were
observed at concentrations ranging from 2 to 8 µM while a
dramatic increase in LDH release was observed at 12 µM in
both A2780/CP70 and OVCAR-3 cells. 3-HT slightly induced LDH
release in IOSE-364 cells, which meant that 3-HT caused less
cytotoxicity in normal ovarian cells than in cancer cells. Taken
together, the results indicated that 3-HT exhibited a growth
inhibitory effect and cytotoxicity on both A2780/CP70 and OVCAR-3
cells in a dose-dependent manner.
3-HT triggers cell cycle arrest at the S
phase
We hypothesized that the reduced cell viability and
increased cytotoxicity after treatment with 3-HT might occur due to
the inhibition of cell progression. To further demonstrate this
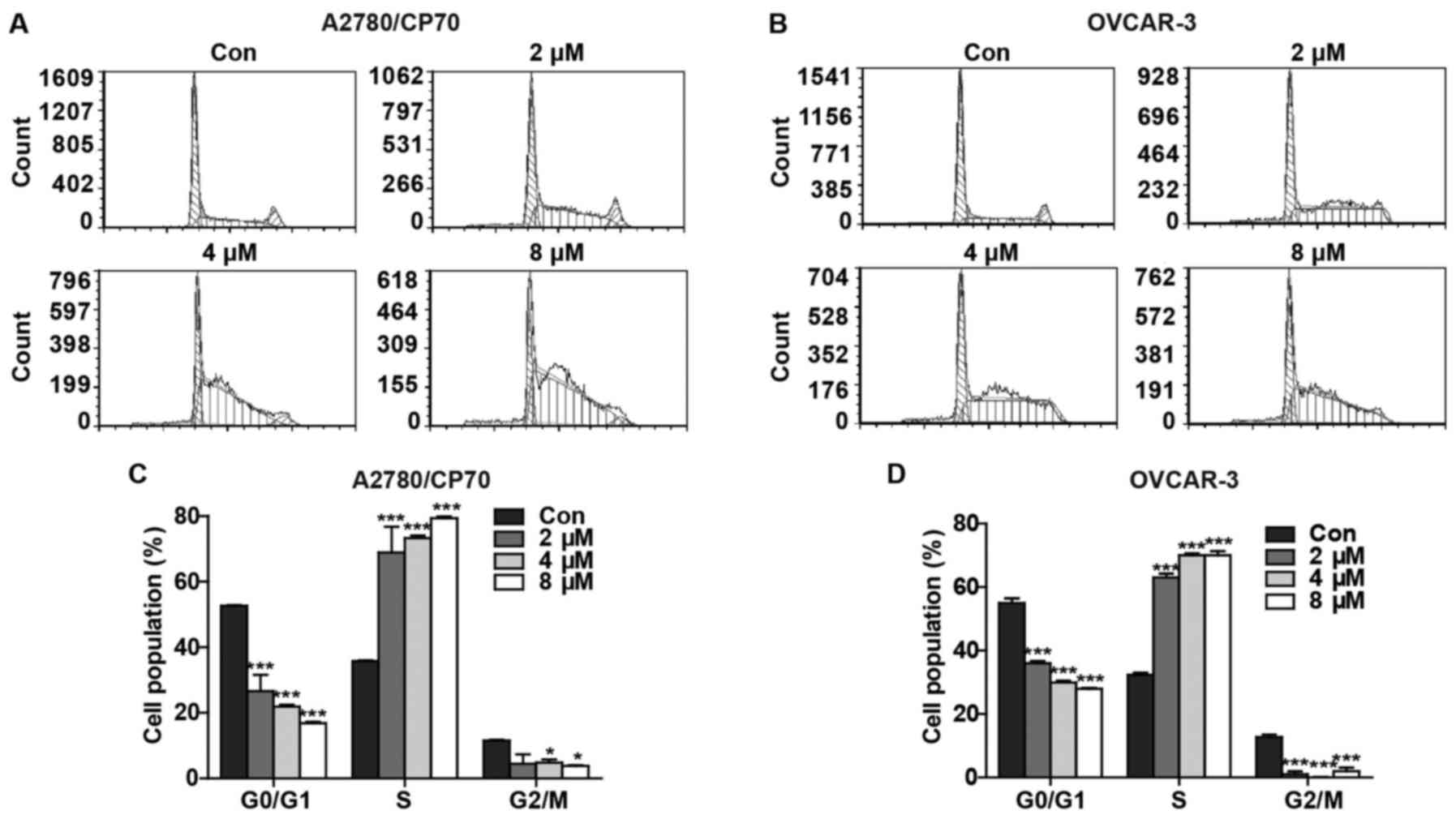
hypothesis, we determined the effect of 3-HT on cell cycle arrest.
After treatment with 3-HT at various concentrations (0, 2, 4 and 8
µM) for 24 h, the percentages of G0/G1, S and G2/M
phase-specific cells were evaluated and plotted. We observed
significant accumulation of cells in S phase in a dose-dependent
manner in both A2780/CP70 (Fig.
2A) and OVCAR-3 cells (Fig.
2B). Compared with the control group, the percentages of
3-HT-treated A2780/CP70 cells at 2, 4 and 8 µM in the S
phase increased from 35.75±0.231 to 68.91±7.885, 73.28±0.749 and
79.37±0.499%, respectively (Fig.
2C). Similarly, the percentages of 3-HT-treated OVCAR-3 cells
at 2, 4 and 8 µM in the S phase increased from 32.28±0.745
to 68.91±1.220, 73.28±0.612 and 79.37±1.258%, respectively
(Fig. 2D). In addition, a decrease
in both G0/G1 and G2/M cell populations occurred concomitant with
the increase in S phase. These results revealed that 3-HT induced S
phase arrest in both ovarian cancer cell lines.
3-HT induces apoptosis of ovarian cancer
cells
Based on the results that 3-HT could induce S phase
arrest in ovarian cancer cells, we next probed whether 3-HT caused
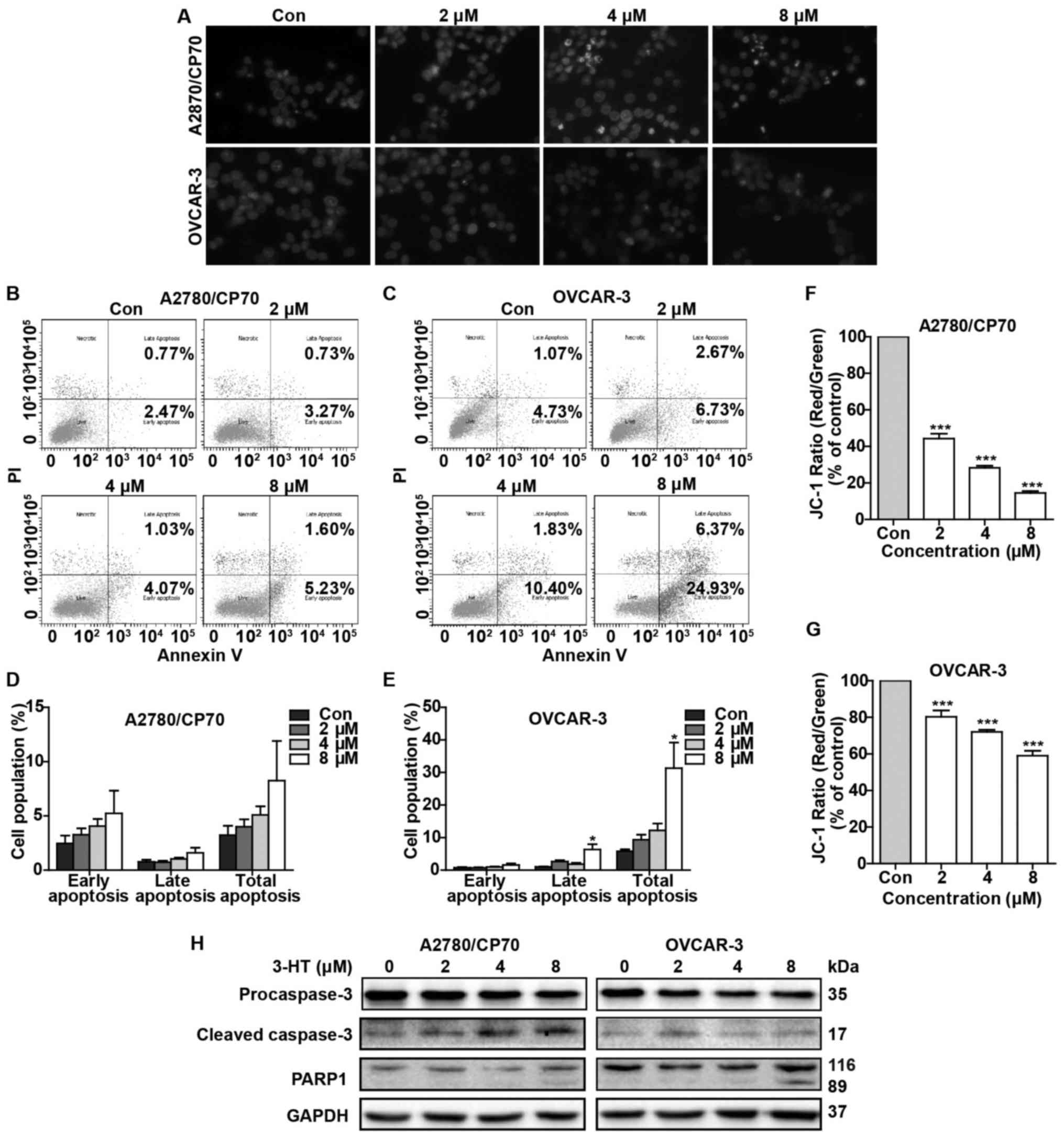
apoptosis. We used Hoechst 33342 staining to assess 3-HT-induced
changes in nuclear morphology. As shown in Fig. 3A, A2780/CP70 and OVCAR-3 cells
treated with 3-HT for 24 h exhibited dramatic nuclear morphology
changes. Typical apoptotic nuclear morphology such as nuclear
shrinkage, fragmentation and condensation were observed (Fig. 3A). We next investigated the
pro-apoptotic effect by using flow cytometric analysis via Annexin
V/PI staining. The results showed that 3-HT-treated OVCAR-3 cells
underwent significant apoptosis in a dose-dependent manner
(Fig. 3C). The apoptotic rate
increased to 31.3% at 8 µM in OVCAR-3 cells vs. 5.8% in the
control group (Fig. 3E). Though
there was no significant difference of apoptotic rate in A2780/CP70
cells (Fig. 3D), we observed a
growth trend of apoptosis rate as the concentration of 3-HT
increased (Fig. 3B).
Loss of mitochondrial membrane potential is
considered a hallmark of apoptosis. To further confirm that 3-HT
induced apoptosis, the mitochondrial membrane potential of
A2780/CP70 and OVCAR-3 cells was measured after treatment with 3-HT
at 0, 2, 4 and 8 µM for 24 h. The JC-1 fluorescence ratio
(red/green) decreased markedly in both ovarian cancer cells
(Fig. 3F and G), which suggested
that depolarization of mitochondrial membrane potential and
apoptosis occurred. Furthermore, apoptosis-related proteins were
also evaluated by western blot analysis. As expected, treatment
with increasing concentrations of 3-HT for 24 h significantly
decreased the procaspase-3 levels in both A2780/CP70 and OVCAR-3
cells (Fig. 3H) and simultaneously
increased the cleaved caspase-3 level in A2780/CP70 cells (Fig. 3H). The 89-kDa cleaved PARP
fragments were detected in both cell types at a high concentration
(8 µM) of 3-HT (Fig. 3H).
Together, these results demonstrated that 3-HT can induce apoptosis
in ovarian cancer cells.
3-HT induces S phase arrest related with
DNA damage
DNA damage can lead to S phase arrest and result in
DNA damage repair response (15).
To determine whether 3-HT induces DNA damage in ovarian cancer
cells, we evaluated changes of the protein levels of γ-H2AX
(Ser139), p-ATM, ATM, Chk1/2, p53, p-p53 (Ser15), p21 and Cdc25C
after treatment with 3-HT for 24 h. The phosphorylation of H2AX at
Ser139 indicates DNA double-strand breaks. ATM, another sensor of
DNA damage, is phosphorylated after DNA damage (16). Results showed a dramatic increase
of γ-H2AX at Ser-139 in both 3-HT treated ovarian cancer cells
(Fig. 4A–C). Additionally, the
expression of p-ATM significantly increased at the concentration of
8 µM compared with control in A2780/CP70 cells (Fig. 4A and B). The phosphorylation of ATM
can phosphorylate Chk1 and Chk2 which are considered key downstream
checkpoint substrates of ATM, thus, leading to cell cycle arrest.
Treatment with 3-HT resulted in significant increase of the
phosphorylation of Chk2 (Thr68) in a dose-dependent manner in
A2780/CP70 and OVCAR-3 cells (Fig.
4A–C). Chk1 decreased while Chk2 remained unchanged in both
cells (Fig. 4A–C). We concluded
that 3-HT-induced DNA damage involved the activation of ATM-Chk2
pathways. A target of DNA damage-induced phosphorylation is p53
protein. DNA damage results in phosphorylation on Ser15 of p53
(17). Western blotting results
indicated that 3-HT exposure increased phosphorylation of p53
(Ser15) and p53 total protein levels in both ovarian cancer cell
lines (Fig. 4A–C). Whereas, the
downstream protein Cdc25C was downregulated while p21 remained
unchanged (Fig. 4A–C).
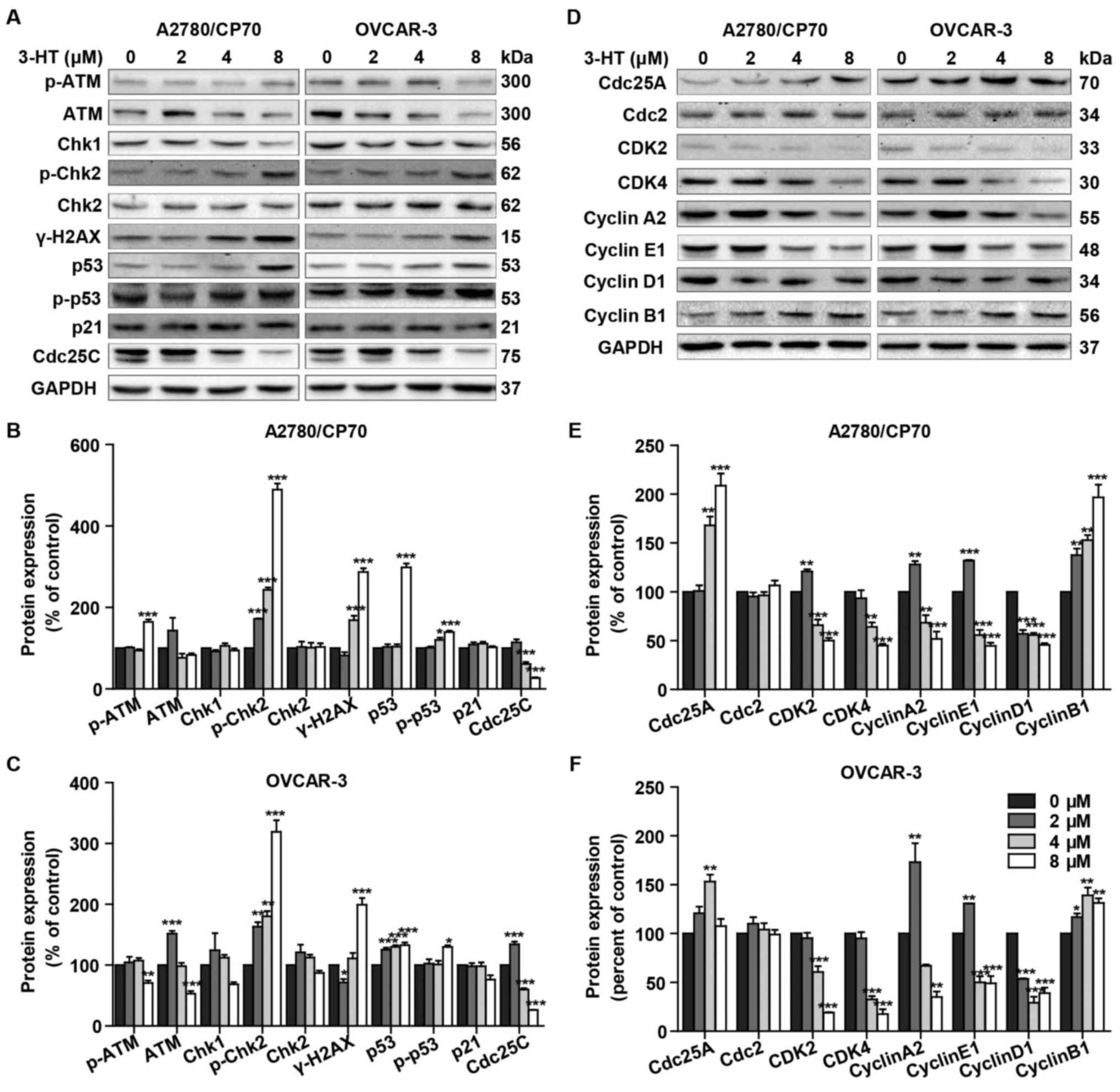 | Figure 4Effect of 3-HT on DNA damage and cell
cycle regulatory proteins in A2780/CP70 and OVCAR-3 cells. (A) The
DNA damage regulatory proteins in A2780/CP70 and OVCAR-3 cells were
detected by western blotting, cells were incubated with 3-HT at 0–8
µM for 24 h, cell lysates were prepared and then subjected
to western blotting, GAPDH was used as internal control. (B and C)
A2780/CP70 and OVCAR-3 protein expression data were expressed as
means ± SEM of three independent experiments.
*P<0.05, **P<0.01,
***P<0.001. (D) The cell cycle regulatory proteins in
A2780/CP70 and OVCAR-3 cells were detected by western blotting,
cells were incubated with 3-HT at 0–8 µM for 24 h, cell
lysates were prepared and then subjected to western blotting, GAPDH
was used as internal control. (E and F) A2780/CP70 and OVCAR-3
protein expression data were expressed as means ± SEM of three
independent experiments. *P<0.05,
**P<0.01, ***P<0.001. |
To further investigate the mechanism of 3-HT-induced
S phase arrest, we then evaluated the expression of cell
cycle-regulatory proteins, such as cyclins, cyclin-dependent
kinases (CDKs), and cell division cycle (Cdc) proteins using
western blotting. Our results showed a dramatic downregulation in
protein levels of CDK2, CDK4, cyclin E1, cyclin A2 and cyclin D1,
while the protein levels of Cdc25A and cyclin B1 were upregulated
(Fig. 4D–F). Cdc2 remained
unchanged in both cancer cell types (Fig. 4D–F). Taken together, these results
indicated that 3-HT induced S phase arrest through regulation of
the expression of the cell cycle proteins.
3-HT induces ROS accumulation and
activates the MAPK signaling pathway
Since many anticancer compounds could induce ROS
generation and activate MAPK signaling pathway ultimately causing
apoptosis, we then examined the effects of 3-HT on ROS generation
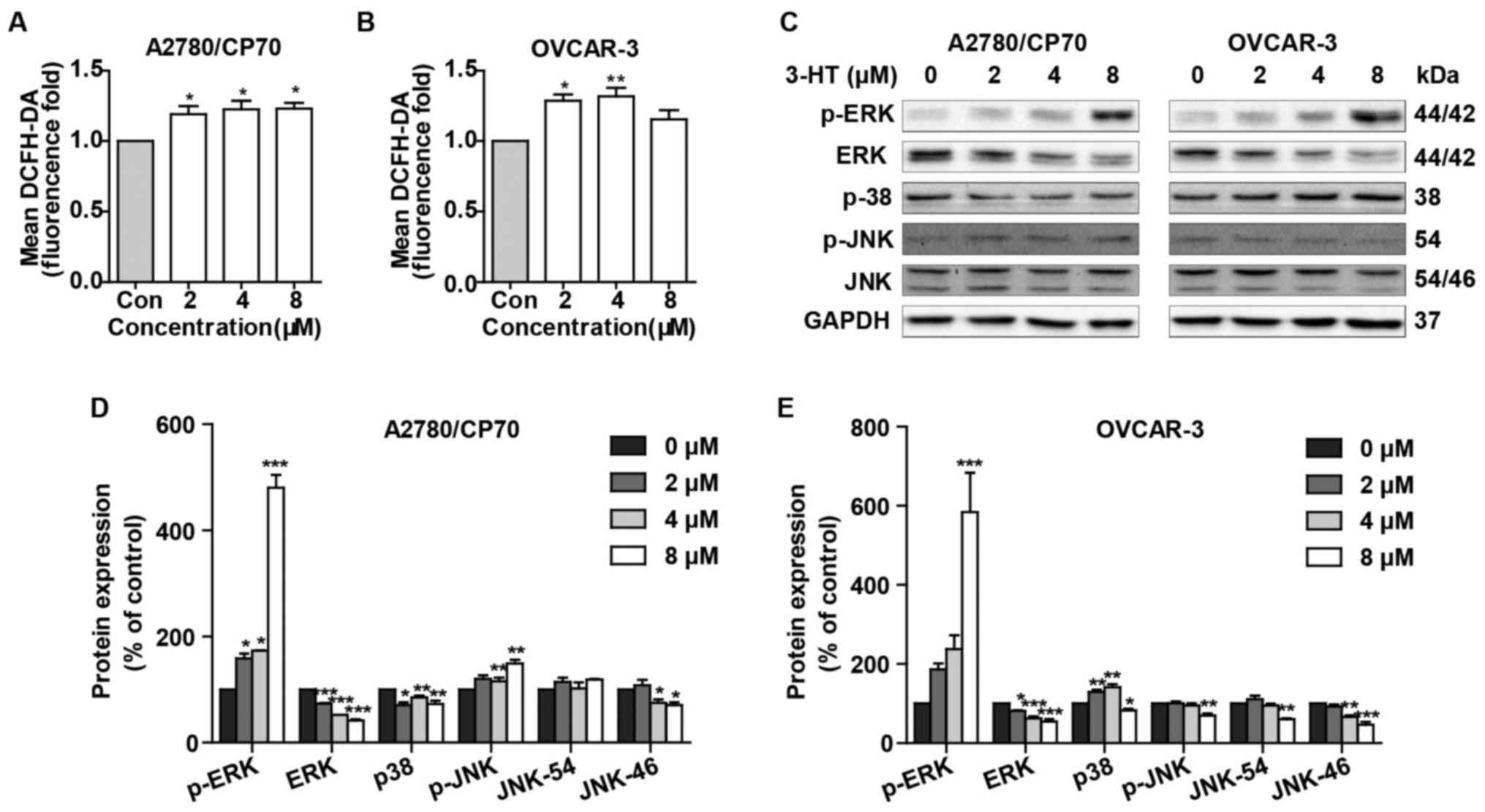
and the MAPK signaling pathway. As shown in Fig. 5A and B, treatment with 3-HT at 2, 4
and 8 µM for 24 h demonstrated higher ROS levels compared
with the control group in both cell lines. ROS levels increased by
1.19- (2 µM), 1.23- (4 µM), and 1.23- (8
µM)-fold by 3-HT over that in control groups in A2780/CP70
cells (Fig. 5A). Similar results
were obtained in OVCAR-3 cells, the ROS levels increased by 1.28-
(2 µM), 1.32- (4 µM), and 1.15- (8 µM)-fold
compared with control groups (Fig.
5B). These results suggested that 3-HT elevated ROS levels
dose-dependently in ovarian cancer cells.
We then determined the effects of 3-HT on p38, c-Jun
N-terminal kinase (JNK), and extracellular regular protein kinase
(ERK), the three main proteins of MAKPs family. Results showed that
3-HT significantly induced activation of ERK1/2 (Fig. 5C–E). The protein level of p-38
decreased in A2780/CP70 cells, while it increased in OVCAR-3 cells
(Fig. 5C–E). Also, p-JNK protein
levels were increased in A2780/CP70 cells and decreased in OVCAR-3
cells (Fig. 5C–E). The protein
level of total JNK was inhibited in both cell types (Fig. 5C–E).
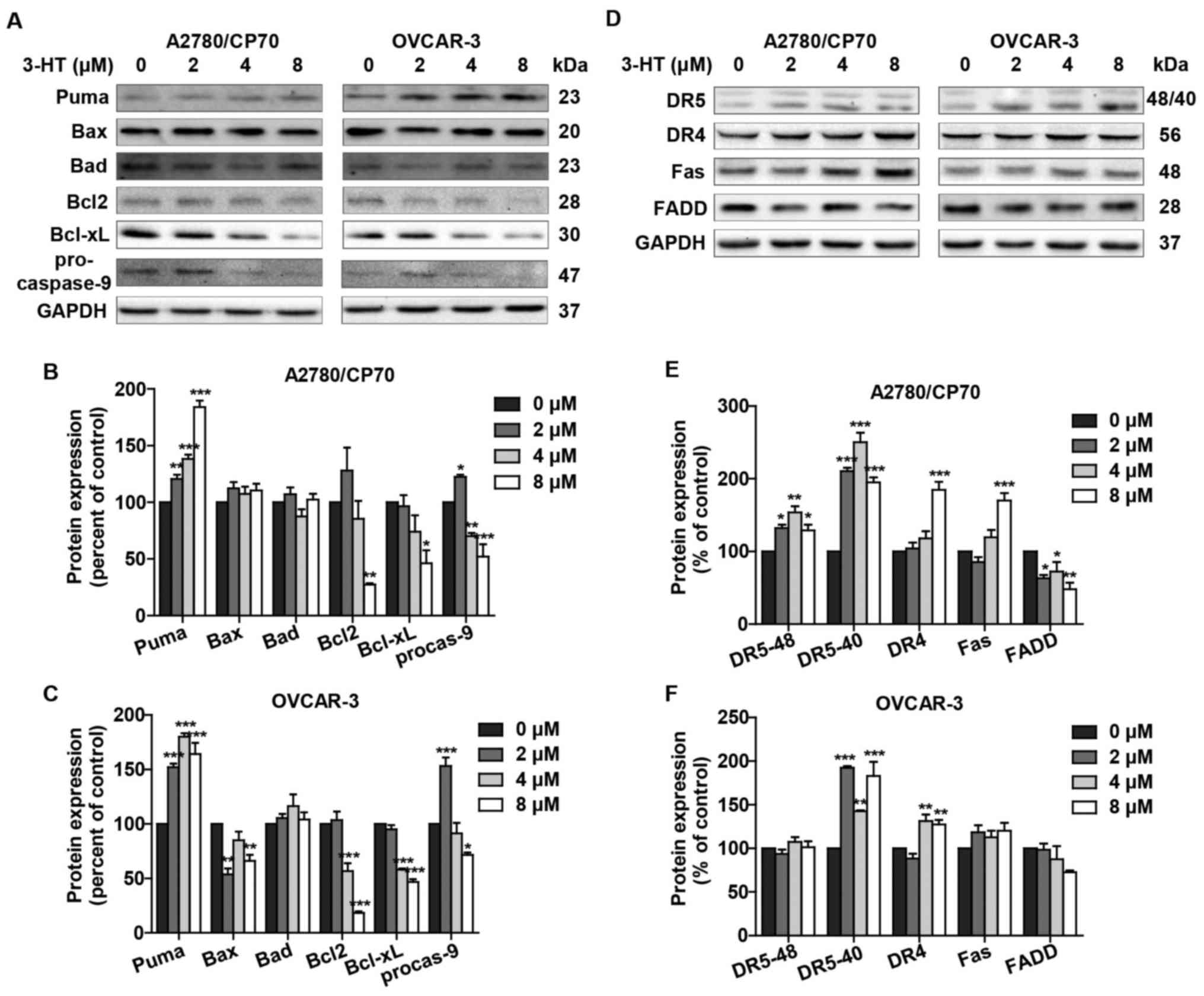
3-HT induces apoptosis via intrinsic and
extrinsic apoptotic pathways
The two best-understood apoptotic activation
mechanisms are the intrinsic and the extrinsic pathways.
Considering the fact that 3-HT induced apoptosis in both A2780/CP70
and OVCAR-3 cells, we next examined whether the intrinsic and/or
extrinsic apoptotic pathways were/was involved in the apoptotic
effect by western blotting. We first detected the intrinsic
apoptotic pathway related proteins such as Puma, Bax, Bad, Bcl2,
Bcl-xL and procaspase-9. Puma protein expression was significantly
upregulated in A2780/CP70 and OVCAR-3 cells (Fig. 6A–C). The level of pro-apoptotic
protein Bax remained unaffected in A2780/CP70 cells (Fig. 6A and B); however, it slightly
decreased in OVCAR-3 cells (Fig. 6A
and C). Another pro-apoptotic protein Bad showed no significant
changes in either cell type (Fig.
6A–C). Anti-apoptotic proteins Bcl-2 and Bcl-xL were inhibited
after treatment with 3-HT (Fig.
6A–C). The procaspase-9 protein level was also inhibited in
both cell lines (Fig. 6A–C). These
results suggested that the intrinsic apoptotic pathway was involved
in 3-HT-induced apoptosis.
We further checked the expression levels of
extrinsic apoptotic pathway related proteins. The levels of DR4 and
Fas receptor increased in A2780/CP70 cells; however, no significant
changes were observed in OVCAR-3 cells (Fig. 6D and F). FADD protein expression
levels were downregulated. We also observed that protein levels of
DR5 were upregulated significantly in A2780/CP70 and OVCAR-3 cells
(Fig. 6D–F). The results above
indicated that the extrinsic apoptotic pathway was also involved in
3-HT-induced apoptosis in ovarian cancer cells.
Discussion
The major problem facing current cancer research is
the resistance of cancer to chemotherapy and molecularly targeted
therapies (18). Resistance to
platinum-based drugs continues to be a major factor leading to
therapeutic failure for ovarian cancer (19). In the present study, we first
investigated whether 3-HT, the metabolite isolated from
Aspergillus candidus, could exhibit anticancer effects in
vitro. Our results clearly demonstrate that 3-HT exhibited
significant cell viability inhibition effect against ovarian cancer
cells due to the induction of S phase arrest and apoptosis at low
concentrations. The IC50 values of 3-HT for the growth
of A2780/CP70 and OVCAR-3 cells were 5.77 and 6.97 µM,
respectively. These results were consistent with previous reports
that many metabolites of fungi inhibit cell proliferation in
various cancer cell types (13,20,21).
However, 3-HT also resulted in the loss of cell viability in
IOSE-364. In LDH assay, significant alterations of LDH leakage
levels were observed in both ovarian cancer cell lines while 3-HT
caused slightly less LDH release in IOSE-364 cells. These results
clearly suggested that 3-HT caused cytotoxic effects in both
ovarian cancer cells. However, 3-HT was less cytotoxic to normal
ovarian epithelial surface cells, IOSE-364. The MTS and LDH assays
both suggested 3-HT demonstrated different effect on ovarian cancer
cells and normal cells. Ideal anticancer drugs are expected to be
cytotoxic to cancer cells while being selective towards normal
cells with minimal cytotoxicity (22). The present study demonstrated the
3-HT selectivity towards ISOE-364 cells by increasing LDH release
in A2780/CP70 and OVCAR-3 cells indicating targeted cytotoxicity.
Cell cycle regulation plays an important role in tumorigenesis and
tumor progression; thus, the molecules involved in cell cycle
regulation become potential targets for therapeutic interventions
(23). The eukaryotic cell cycle
includes four sequential phases, G1, S, G2 and M. S and M phases
are arguably the most pivotal phases pertaining to DNA replication
and the creation of two new daughter cells (24). Flow cytometric analysis provided
evidence that A2780/CP70 and OVCAR-3 cells were arrested at S phase
after 3-HT treatment. Previous studies have shown that natural
products and their derivatives are considered leads to the cell
cycle pathway in cancer chemotherapy treatments (25). Some chemotherapy drugs like
5-fluorouracil and 6-mercaptopurine are commonly used to treat
lukemias, ovarian and breast cancers, and other types of cancers by
damaging cancer cells during the S phase (26). In addition, several other natural
compounds, which have exhibited S phase arrest, have also been
shown to induce apoptosis (27–29).
In the present study, 3-HT reduced the cell viability of ovarian
cancer cells partly through arresting cell cycle at S phase, thus,
can become a candidate for further research to treat ovarian cancer
in the future. Given the importance of the induction of apoptosis
in cytotoxicity, we also evaluated the apoptotic effect of 3-HT on
ovarian cancer cells using several methods. Nuclear chromatin
condensation and nuclear DNA fragmentation are typical
morphological hallmarks of apoptosis (30). These changes were clearly observed
in both ovarian cancer cell lines after treatment with 3-HT by
Hoechst 33342 staining. Annexin V/PI staining further confirmed the
number of apoptotic cells increased with increased concentrations
of 3-HT. The loss of mitochondrial membrane potential is considered
as another hallmark of early apoptosis. Our results showed a
dose-dependent reduction of mitochondrial membrane potential in
both cancer cell lines; thus, indicating that 3-HT induced
apoptosis is related to mitochondrial damage. Protein cleavage is
another key hallmark of apoptosis (31). The central role in the initiation
of apoptosis is caspase-3 activation and the induction of cleavage
of PARP by caspase-3 (32). In
this study, the induction of caspase-3 and PARP cleavage indicated
that 3-HT induced apoptosis was caspase-dependent. Collectively,
all these results indicated that the anti-proliferation effect of
3-HT on ovarian cancer cells was also mediated by induction of
apoptosis. Therefore, our results indicated that the
anti-proliferative effects of 3-HT against ovarian cancer cells are
correlated strongly with S phase arrest and apoptosis. To further
elucidate the possible mechanisms that 3-HT induced cell cycle
arrest at S phase, the expression of cell cycle regulatory proteins
was determined by western blot analysis. Cyclin-dependent kinases
(CDKs) are a family of protein kinases that regulate the cell cycle
progression. 3-HT significantly inhibited the expression of cyclin
E1, cyclin A2 and CDK2; thus, preventing the formation of cyclin
E-CDK2 and cyclin A2-CDK2 complexes, which play pivotal role in the
initiation and progression of the S phase (33), ultimately leading to S phase
arrest. The results were in accordance with previous studies that
natural compounds induced S phase arrest by inhibiting the
expression of cyclin E, cyclin A2 and CDK in different types of
human cancer cells (27,28). A previous study reported that
h-PNAS-4 induced S phase arrest in ovarian cancer cells via
activation of the Cdc25A-Cdk2-cyclin E/cyclin A pathway, the
expression of cyclin E and cyclin A were upregulated while Cdc25A
was inhibited (34). However, in
this study, we found that Cdc25A was increased while cyclin E and
cyclin A were inhibited. The inhibition of cyclin E and cyclin A
prevented the formation of cyclin E/CDK2 and cyclin A/CDK2
complexes and leading to the S phase arrest. 3-HT downregulated the
expression of CDK4 and cyclin D1, as cyclin D1 is only suppressed
in S phase and its inhibition is an index for S phase arrest
(34). The downregulation of
cyclin D1/Cdk4 complex was also observed in a previous report in
resveratrol-induced cell arrest in colon cancer cells (35). We thus, concluded that the
downregulation of CDK4 and cyclin D1 contributed to the S phase
arrest in A2780/CP70 and OVCAR-3 cells. Moreover, the upregulation
of cyclin B1 induced by 3-HT was also observed in A2780/CP70 cells.
Several reports also found an increase of cyclin B1 that was
correspondent with the S phase arrest induced by different
compounds in various cancer lines (36–38).
These results indicated that 3-HT induced S phase arrest stemmed
from the inactivation of cyclin E/Cdk2, cyclin A/Cdk2 and cyclin
D1/Cdk4 complexes. The upregulation of cyclin B1 also contributed
to the S phase arrest. Cell cycle arrest might be associated with
the induction of DNA damage via activation of ATM/p53-mediated DNA
damage response in MCF-7 cells (39). ATM is a DNA damage sensor that
participates in the detection of DNA double-stranded breaks.
Studies have indicated that ATM is activated when double-stranded
breaks occur, and activated ATM results in the phosphorylation of
p53 at Ser15 in response to DNA damage (40,41).
ATM could also directly phosphorylate H2AX at Ser139, which is
considered an early event in response to DNA damage (42). Chk1 and Chk2 are involved in
channeling DNA damage signals from ATR and ATM in mammalian cells,
respectively. Other research has shown that Chk2 at Thr68 is
phosphorylated by ATM in response to DNA damage (43,44).
Indeed, in the present study, 3-HT treatment led to the
upregulation of p-ATM in A2780/CP70 cells. The DNA double strand
breaks that occurred in A2780/CP70 and OVCAR-3 cells were indicated
by the significant upregulation of γ-H2AX. Total p53 and
phosphory-lation of p53 at Ser15 were dramatically increased in
both ovarian cancer cell lines; furthermore, a significant
induction of p-Chk2 was observed in a dose-dependent manner in both
A2780/CP70 and OVCAR-3 cells. We also observed significant
inhibition of Cdc25C in both cancer cell types. A previous study
has reported that the activation of the ATM/ATR-Chk1/2-Cdc25C
pathway is a central mechanism in S phase arrest in OVCAR-3 cells
induced by resveratrol (38). Our
results strongly suggest this pathway was involved in the 3-HT
induced S phase arrest in A2780/CP70 and OVCAR-3 cells.
The intrinsic and the extrinsic apopotic pathways
are well documented. The intrinsic pathway is mediated by molecules
released from mitochondria (45).
Cytochrome c and AIF are released from the mitochondria to
the cytosol, and caspase-9 is activated during the prosess
(46). Caspase-9 plays a key role
in the intrinsic pathway through activating caspase-3 and caspase-7
(47). In this study, procaspase-9
was decreased and cleaved caspase-3 was upregulated in both ovarian
cancer cells indicating that 3-HT triggered the intrinsic apoptotic
pathway. Bcl-2 family proteins are considered key regulators of the
intrinsic pathway. The mitochondrial membrane permeabilization is
governed by either pro-apoptotic (Puma, Bax, Bad and Bak) or
anti-apoptotic (Bcl-2, Bcl-xL,Bcl-B and Bcl-W) proteins (48). Puma is a pro-apoptotic factor which
served as a direct mediator of p53-associated apoptosis. The
expression of Puma can induce apoptosis in human cancer cells
(49). Puma can transduce death
signals to mitochondria where it induces mitochondrial dysfunction
and caspase activation by binding and inhibiting multidomain Bcl-2
family members (50). A previous
report found that Puma initiates apoptosis partly through
dissociating Bax and Bcl-xL (51).
In this study, 3-HT treatment significantly upregulated the protein
level of Puma and downregulated Bcl2 and Bcl-xL in both ovarian
cancer cell lines. Together with the downregulation of
pro-caspase-9 and activation of caspase-3, our results strongly
suggested that the intrinsic apoptotic pathway was involved in
3-HT-mediated apoptosis. The extrinsic apoptotic pathway is
triggered by binding death ligands of the tumor necrosis factor
(TNF) family to death receptors (DRs) (52). Here, the protein level of Fas was
upregulated in 3-HT-treated ovarian cancer cells; furthermore, 3-HT
markedly upregulated the proteins levels of DR4 and DR5. Similar
results were found in paclitaxel triggered apoptosis in prostate
cancer cells through upregulation of DR4 and DR5 protein levels
(53). Our results showed that the
protein expression of FADD was downregulated in both ovarian cancer
cell types. A previous study also observed upregulation of DR4 and
downregulation of FADD in TRAIL-mediated apoptosis in prostate
carcinoma LNCap cells (51). These
results indicated that the extrinsic apoptotic pathway was also
involved in 3-HT-induced apoptosis. It has been reported that
damaging anticancer agents can upregulate p53 proteins levels,
which subsequently upregulate DR4 and DR5 expression (54,55).
Our results found that 3-HT induced DNA damage and resulted in the
upregulation of p53 as well as DR4 and DR5 in both ovarian cancer
cell lines. The specific role of p53 in 3-HT induced apoptosis
worth further investigation.
The accumulation of ROS is an early event connected
with cancer cell apoptosis induced by DNA damage (56,57).
Excessive ROS can induce apoptosis. Previous studies have indicated
that ROS induced apoptosis is mediated by p38 MAPK, JNK and ERK
activation (58,59). ERK is a signaling molecule that
plays a key role in cell survival and differentiation and can be
activated by extracellular stimuli such as DNA-damaging agents
(23). In this study, we observed
that 3-HT significantly increased ROS production. Exposure to 3-HT
induced ERK1/2 phosphorylation in both ovarian cancer cell lines
and resulted in the upregulation of p-JNK in A2780/CP70 cells.
Similar results were reported in HEMA and TEGDMA induced apoptosis
by the formation of ROS and activation of MAP-kinases ERK, JNK and
p38 (58). ERK activation can
result in S phase arrest and apoptosis in human pancreatic cancer
cells (60). Previous reports have
also shown that activation of ERK is likely playing a role in
2,3-DCPE-mediated S phase arrest in human colon cancer cells
(23). In the present study, we
did not elucidate the specific mechanism of ROS generation and ERK
activation in 3-HT-induced apoptosis and S phase in ovarian cancer
cells, but the results provide fundamental evidence for further
underlying the role of ROS generation and ERK activation in
apoptosis.
In summary, the present study indicated for the
first time that 3-HT, the metabolite of Aspergillus
candidus, significantly inhibits proliferation of A2780/CP70
and OVCAR-3 cells. 3-HT treatment caused DNA damage and cell cycle
arrest in the S phase. The results also indicated that 3-HT induced
cell apoptosis by activating both the intrinsic pathway and the
extrinsic death receptor pathway. The generation of ROS and
activation of ERK also play an important role in 3-HT induced
anti-proliferation effect on ovarian cancer cells. Thus, this study
demonstrated that 3-HT should be considered as an important
anti-proliferative and pro-apoptotic agent for ovarian cancer and
needs further investigation.
Acknowledgments
We thank Dr Kathy Brundage from the Flow Cytometry
Core at the West Virginia University for providing technical help
on apoptosis and cell cycle analysis. This research was supported
by the NIH grants P20RR016477 from the National Center for Research
Resources and P20GM103434 from the National Institute for General
Medical Sciences (NIGMS) awarded to the West Virginia IDeA Network
of Biomedical Research Excellence. The present study was also
supported by the grant number P20GM104932 from NIGMS, a component
of the National Institutes of Health (NIH) and its contents are
solely the responsibility of the authors and do not necessarily
represent the official view of NIGMS or NIH. This study was also
supported by the COBRE grant GM102488/RR032138, the ARIA S10 grant
RR020866, the FORTESSA S10 grant OD016165.
References
|
1
|
Hennessy BT, Coleman RL and Markman M:
Ovarian cancer. Lancet. 374:1371–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J and Wu GS: Role of autophagy in
cisplatin resistance in ovarian cancer cells. J Biol Chem.
289:17163–17173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evidente A, Kornienko A, Cimmino A,
Andolfi A, Lefranc F, Mathieu V and Kiss R: Fungal metabolites with
anticancer activity. Nat Prod Rep. 31:617–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Velkov T, Roberts KD, Nation RL, Thompson
PE and Li J: Pharmacology of polymyxins: New insights into an 'old'
class of antibiotics. Future Microbiol. 8:711–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi A, Takemoto A, Koshimizu K and
Kawazu K: p-Terphenyls with cytotoxic activity toward sea urchin
embryos. Agric Biol Chem. 49:867–868. 1985. View Article : Google Scholar
|
|
8
|
Yen GC, Chang YC, Sheu F and Chiang HC:
Isolation and characterization of antioxidant compounds from
Aspergillus candidus broth filtrate. J Agric Food Chem.
49:1426–1431. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yen GC, Chiang HC, Wu CH and Yeh CT: The
protective effects of Aspergillus candidus metabolites against
hydrogen peroxide-induced oxidative damage to Int 407 cells. Food
Chem Toxicol. 41:1561–1567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fulda S: Targeting apoptosis for
anticancer therapy. Semin Cancer Biol. 31:84–88. 2015. View Article : Google Scholar
|
|
12
|
Li B, Gao Y, Rankin GO, Rojanasakul Y,
Cutler SJ, Tu Y and Chen YC: Chaetoglobosin K induces apoptosis and
G2 cell cycle arrest through 53-dependent pathway in
cisplatin-resistant ovarian cancer cells. Cancer Lett. 356(2 Pt B):
418–433. 2015. View Article : Google Scholar
|
|
13
|
Chen YF, Wang SY, Shen H, Yao XF, Zhang FL
and Lai D: The marine-derived fungal metabolite, terrein, inhibits
cell proliferation and induces cell cycle arrest in human ovarian
cancer cells. Int J Mol Med. 34:1591–1598. 2014.PubMed/NCBI
|
|
14
|
Li YX, Himaya SW, Dewapriya P, Zhang C and
Kim SK: Fumigaclavine C from a marine-derived fungus Aspergillus
fumigatus induces apoptosis in MCF-7 breast cancer cells. Mar
Drugs. 11:5063–5086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou B-BS and Elledge SJ: The DNA damage
response: Putting checkpoints in perspective. Nature. 408:433–439.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bakkenist CJ and Kastan MB: DNA damage
activates ATM through intermolecular autophosphorylation and dimer
dissociation. Nature. 421:499–506. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siliciano JD, Canman CE, Taya Y, Sakaguchi
K, Appella E and Kastan MB: DNA damage induces phosphorylation of
the amino terminus of 53. Genes Dev. 11:3471–3481. 1997. View Article : Google Scholar
|
|
18
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar
|
|
20
|
Elaasser M, Abdel-Aziz M and El-Kassas R:
Antioxidant, antimicrobial, antiviral and antitumor activities of
pyranone derivative obtained from Aspergillus candidus. J Microbiol
Biotechnol Res. 1:5–17. 2011.
|
|
21
|
Koul M, Meena S, Kumar A, Sharma PR,
Singamaneni V, Riyaz-Ul-Hassan S, Hamid A, Chaubey A, Prabhakar A,
Gupta P, et al: Secondary metabolites from endophytic fungus
penicillium pinophilum induce ROS-mediated apoptosis through
mitochondrial pathway in pancreatic cancer cells. Planta Med.
82:344–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blagosklonny MV: Overcoming limitations of
natural anticancer drugs by combining with artificial agents.
Trends Pharmacol Sci. 26:77–81. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu H, Zhang L, Wu S, Teraishi F, Davis
JJ, Jacob D and Fang B: Induction of S-phase arrest and 21
overexpression by a small molecule
2[[3-(2,3-dichlorophenoxy)propyl] amino]ethanol in correlation with
activation of ERK. Oncogene. 23:4984–4992. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Williams GH and Stoeber K: The cell cycle
and cancer. J Pathol. 226:352–364. 2012. View Article : Google Scholar
|
|
25
|
Newman DJ, Cragg GM, Holbeck S and
Sausville EA: Natural products and derivatives as leads to cell
cycle pathway targets in cancer chemotherapy. Curr Cancer Drug
Targets. 2:279–308. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheung-Ong K, Giaever G and Nislow C:
DNA-damaging agents in cancer chemotherapy: Serendipity and
chemical biology. Chem Biol. 20:648–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao Y, Ling J, Zhang G, Liu F, Tao S, Han
Z, Chen S, Chen Z and Le H: Cordycepin induces cell cycle arrest
and apoptosis by inducing DNA damage and up-regulation of 53 in
leukemia cells. Cell Cycle. 14:761–771. 2015. View Article : Google Scholar :
|
|
28
|
Lee YS, Choi KM, Kim W, Jeon YS, Lee YM,
Hong JT, Yun YP and Yoo HS: Hinokitiol inhibits cell growth through
induction of S-phase arrest and apoptosis in human colon cancer
cells and suppresses tumor growth in a mouse xenograft experiment.
J Nat Prod. 76:2195–2202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Joe AK, Liu H, Suzui M, Vural ME, Xiao D
and Weinstein IB: Resveratrol induces growth inhibition, S-phase
arrest, apoptosis, and changes in biomarker expression in several
human cancer cell lines. Clin Cancer Res. 8:893–903.
2002.PubMed/NCBI
|
|
30
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bratton SB and Cohen GM: Apoptotic death
sensor: An organelle's alter ego? Trends Pharmacol Sci. 22:306–315.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eastman A: Cell cycle checkpoints and
their impact on anticancer therapeutic strategies. J Cell Biochem.
91:223–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yeo EJ, Ryu JH, Chun YS, Cho YS, Jang IJ,
Cho H, Kim J, Kim MS and Park JW: YC-1 induces S cell cycle arrest
and apoptosis by activating checkpoint kinases. Cancer Res.
66:6345–6352. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wolter F, Akoglu B, Clausnitzer A and
Stein J: Downregulation of the cyclin D1/Cdk4 complex occurs during
resveratrol-induced cell cycle arrest in colon cancer cell lines. J
Nutr. 131:2197–2203. 2001.PubMed/NCBI
|
|
36
|
Zaffaroni N, Silvestrini R, Orlandi L,
Bearzatto A, Gornati D and Villa R: Induction of apoptosis by taxol
and cisplatin and effect on cell cycle-related proteins in
cisplatin-sensitive and -resistant human ovarian cells. Br J
Cancer. 77:1378–1385. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang CZ, Calway TD, Wen XD, Smith J, Yu C,
Wang Y, Mehendale SR and Yuan CS: Hydrophobic flavonoids from
Scutellaria baicalensis induce colorectal cancer cell apoptosis
through a mitochondrial-mediated pathway. Int J Oncol.
42:1018–1026. 2013.PubMed/NCBI
|
|
38
|
Tyagi A, Singh RP, Agarwal C, Siriwardana
S, Sclafani RA and Agarwal R: Resveratrol causes Cdc2-tyr15
phosphorylation via ATM/ATR-Chk1/2-Cdc25C pathway as a central
mechanism for S phase arrest in human ovarian carcinoma Ovcar-3
cells. Carcinogenesis. 26:1978–1987. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lam M, Carmichael AR and Griffiths HR: An
aqueous extract of Fagonia cretica induces DNA damage, cell cycle
arrest and apoptosis in breast cancer cells via FOXO3a and 53
expression. PLoS One. 7:e401522012. View Article : Google Scholar
|
|
40
|
Abe K and Matsuki N: Measurement of
cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) reduction activity and lactate dehydrogenase release
using MTT. Neurosci Res. 38:325–329. 2000. View Article : Google Scholar
|
|
41
|
Henkels KM and Turchi JJ:
Cisplatin-induced apoptosis proceeds by caspase-3-dependent and
-independent pathways in cisplatin-resistant and -sensitive human
ovarian cancer cell lines. Cancer Res. 59:3077–3083.
1999.PubMed/NCBI
|
|
42
|
Burma S, Chen BP, Murphy M, Kurimasa A and
Chen DJ: ATM phosphorylates histone H2AX in response to DNA
double-strand breaks. J Biol Chem. 276:42462–42467. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahn JY, Schwarz JK, Piwnica-Worms H and
Canman CE: Threonine 68 phosphorylation by ataxia telangiectasia
mutated is required for efficient activation of Chk2 in response to
ionizing radiation. Cancer Res. 60:5934–5936. 2000.PubMed/NCBI
|
|
44
|
Yuan Z, Guo W, Yang J, Li L, Wang M, Lei
Y, Wan Y, Zhao X, Luo N, Cheng P, et al: PNAS-4, an early DNA
damage response gene, induces S phase arrest and apoptosis by
activating checkpoint kinases in lung cancer cells. J Biol Chem.
290:14927–14944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Green DR: Apoptotic pathways: Paper wraps
stone blunts scissors. Cell. 102:1–4. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Akasaka Y, Ito K, Fujita K, Komiyama K,
Ono I, Ishikawa Y, Akishima Y, Sato H and Ishii T: Activated
caspase expression and apoptosis increase in keloids: Cytochrome c
release and caspase-9 activation during the apoptosis of keloid
fibroblast lines. Wound Repair Regen. 13:373–382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ding H, Han C, Zhu J, Chen CS and
D'Ambrosio SM: Celecoxib derivatives induce apoptosis via the
disruption of mitochondrial membrane potential and activation of
caspase 9. Int J Cancer. 113:803–810. 2005. View Article : Google Scholar
|
|
48
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu J, Zhang L, Hwang PM, Kinzler KW and
Vogelstein B: PUMA induces the rapid apoptosis of colorectal cancer
cells. Mol Cell. 7:673–682. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yu J and Zhang L: PUMA, a potent killer
with or without p53. Oncogene. 27(Suppl 1): S71–S83. 2008.
View Article : Google Scholar
|
|
51
|
Siddiqui IA, Malik A, Adhami VM, Asim M,
Hafeez BB, Sarfaraz S and Mukhtar H: Green tea polyphenol EGCG
sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated
apoptosis and synergistically inhibits biomarkers associated with
angiogenesis and metastasis. Oncogene. 27:2055–2063. 2008.
View Article : Google Scholar
|
|
52
|
Sayers TJ: Targeting the extrinsic
apoptosis signaling pathway for cancer therapy. Cancer Immunol
Immunother. 60:1173–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nimmanapalli R, Perkins CL, Orlando M,
O'Bryan E, Nguyen D and Bhalla KN: Pretreatment with paclitaxel
enhances apo-2 ligand/tumor necrosis factor-related
apoptosis-inducing ligand-induced apoptosis of prostate cancer
cells by inducing death receptors 4 and 5 protein levels. Cancer
Res. 61:759–763. 2001.PubMed/NCBI
|
|
54
|
Wu GS, Burns TF, McDonald ER III, Jiang W,
Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, et al:
KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor
gene. Nat Genet. 17:141–143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Guan B, Yue P, Clayman GL and Sun SY:
Evidence that the death receptor DR4 is a DNA damage-inducible,
p53-regulated gene. J Cell Physiol. 188:98–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rogalska A, Marczak A, Gajek A, Szwed M,
Śliwińska A, Drzewoski J and Jóźwiak Z: Induction of apoptosis in
human ovarian cancer cells by new anticancer compounds, epothilone
A and B. Toxicol In Vitro. 27:239–249. 2013. View Article : Google Scholar
|
|
57
|
Zhang Y, Zheng S, Zheng JS, Wong KH, Huang
Z, Ngai SM, Zheng W, Wong YS and Chen T: Synergistic induction of
apoptosis by methylseleninic acid and cisplatin, the role of
ROS-ERK/AKT-p53 pathway. Mol Pharm. 11:1282–1293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Samuelsen JT, Dahl JE, Karlsson S,
Morisbak E and Becher R: Apoptosis induced by the monomers HEMA and
TEGDMA involves formation of ROS and differential activation of the
MAP-kinases p38 JNK and ERK. Dent Mater. 23:34–39. 2007. View Article : Google Scholar
|
|
59
|
Zhang X, Wang X, Wu T, Li B, Liu T, Wang
R, Liu Q, Liu Z, Gong Y and Shao C: Isoliensinine induces apoptosis
in triple-negative human breast cancer cells through ROS generation
and p38 MAPK/JNK activation. Sci Rep. 5:125792015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yadav V, Varshney P, Sultana S, Yadav J
and Saini N: Moxifloxacin and ciprofloxacin induces S-phase arrest
and augments apoptotic effects of cisplatin in human pancreatic
cancer cells via ERK activation. BMC Cancer. 15:5812015. View Article : Google Scholar : PubMed/NCBI
|