Introduction
Ovarian cancer has a high mortality rate worldwide,
which is related to the late identification of most ovarian cancer
cases in advanced stages (1). Late
diagnosis indicates that cancer cells are disseminated to
intraperitoneal tissues or metastasized to other organs when the
cancer is first identified. In these cases, chemotherapy is less
likely to be effective, with tumor relapse occurring often,
resulting in poor patient prognosis. Developing therapies that
improve patient outcomes is an important area of focus (1,2).
The experimental procedure that most appropriately
mimic the clinical characteristics of metastasized cancers in
vitro is culturing cancer cells in suspension, also known as
three-dimensional cultures (3–9). The
procedure results in the formation of spheroids, and these
spheroids are often considered to share cancer stem-like
characteristics (3,10–12).
Accordingly, we have recently obtained spheroids by
culturing OVTOKO (ovarian clear cell carcinoma) and SiHa (cervical
squamous cell carcinoma) and demonstrated the common metabolism in
spheroids (10). In the present
study, by performing RNA-seq analysis and using the Kyoto
Encyclopedia of Genes and Genomes (KEGG), we found that the only
common trait between spheroids derived from OVTOKO and SiHa was the
pathway hsp 04510, which is also known as the focal adhesion kinase
(FAK) pathway.
The FAK pathway has been investigated in various
types of solid tumors for the last few decades, and this pathway is
essential in tumor cell-extracellular matrix attachment, migration,
invasion and spheroid formation (13–26).
Inhibiting the FAK pathway is promising as a target in ovarian
serous carcinoma, and currently several types of inhibitors are in
clinical trials (27).
However, to the best of our knowledge, there is no
study reported on the effectiveness of FAK inhibitors in ovarian
clear cell carcinomas. Therefore, we evaluated the effectiveness of
targeting the FAK pathway in ovarian clear cell carcinomas using
the ovarian clear cell carcinoma cell lines OVTOKO, OVISE and
JHOC5. At the same time, we found a potential limitation to
inhibiting FAK pathways from the in vitro study: because the
phosphorylation of FAK was higher in spheroids than adherent cells,
the same doses of FAK inhibitors were not always effective at
inhibiting phosphorylation because inhibition depended on cancer
status and other conditions.
Thus, we investigated other pathways to overcome the
limitation. We have recently demonstrated that the concentration of
glutamine and glutamate were significantly higher in spheroids than
adherent cells (10), and we
investigated the effects of targeting glutamine metabolism in
spheroids, especially focusing on the mTOR pathway, which is a
common downstream pathway between glutamine metabolism and FAK
pathways (28,29). The combination of AOA, a
pan-trans-aminase inhibitor, and PF 573228, an FAK inhibitor,
additively inhibited the mTOR pathway in two of three cell
lines.
In the present study, from an in vitro point
of view, we proposed a rationale for the positive and negative
effects of using FAK inhibitors in ovarian clear cell carcinomas
and suggested that targeting glutamine metabolism could overcome
the limitation of FAK inhibitors by additively inhibiting the mTOR
pathway.
Materials and methods
Cell line and cell culture
The cancer cell lines OVTOKO and OVISE were obtained
from the JCRB Cell Bank (Osaka, Japan), and JHOC5 was obtained from
RIKEN Cell Bank (Tsukuba, Japan). They were cultured in RPMI-1640
medium or DMEM (Wako, Japan), supplemented with 10% fetal bovine
serum (FBS; Invitrogen, USA) and 100 U/ml penicillin/100
µg/ml streptomycin (Wako), and sub-cultured by 0.25%
trypsin/EDTA (Wako) detachment. All cells were grown in a
humidified atmosphere at 37°C with 5% CO2.
Suspension (spheroid-forming)
culture
Dissociated single cells (1×105 cells/ml)
were seeded into ultra-low attachment plates (Corning Inc.,
Corning, NY, USA) and cultured for 48 h unless otherwise described.
To collect spheroids, the medium was centrifuged for 2 min at 100 ×
g, and the supernatants were carefully aspirated.
mRNA-seq data
Adherent-cultured cells and spheroids were collected
after washing and immediately treated with RNAlater (Thermo Fisher
Scientific, Waltham, MA, USA). To construct cDNA libraries with the
TruSeq RNA library kit, 1 µg of total RNA was used. The
protocol consisted of polyA-selected RNA extraction, RNA
fragmentation, random hexamer primed reverse transcription and 100
nucleotide paired-end sequencing by Illumina HiSeq2000 (Illumina,
Inc., San Diego, CA, USA). The libraries were quantified using qPCR
according to the qPCR Quantification Protocol Guide and qualified
using an Agilent Technologies 2100 Bioanalyzer (Agilent
Technologies, Santa Clara, CA, USA). We processed reads from the
sequencer and aligned them to Homo sapiens (hg19) using
Tophat v2.0.13 (30). Tophat
incorporates the Bowtie v2.2.3 algorithm to perform the alignment
(31). Tophat initially removes a
portion of the reads based on the quality information accompanying
each read before mapping reads to the reference genome. The
reference genome sequence of Homo sapiens (hg19) and
annotation data were downloaded from the UCSC table browser
(http://genome.uscs.edu). Gene annotation
information was also used to run Tophat with the '-G' option.
For other parameters in Tophat, the default options
were used. Tophat enables multiple alignments per read (up to 20 by
default) and a maximum of two mismatches when mapping the reads to
the reference. Transcript assembly and abundance estimation were
performed using Cufflinks (32).
After aligning reads to the genome, Cufflinks v2.2.1 was used to
assemble aligned reads into transcripts and estimate the abundance
of the reads. To correct sequence expression count bias,
'–max-bundle-frags 50000000' options were used. We also used the
'-G' option to make the best use of known gene annotation
information. For other parameters, default options were used. The
transcript counts in the isoform level were calculated, and the
relative transcript abundances were measured in FPKM (fragments per
kilobase of exon per million fragments mapped) from Cufflinks. Gene
level expression values were also calculated from the transcript
counts. These values were used for later DEG analysis.
mRNA expression
Raw data were calculated as FPKM of each gene for
each sample by Cufflinks software. We excluded genes with zero FPKM
values for more than one total sample. We added a filtered gene
with an FPKM value to facilitate log10 transformation. Filtered
data was transformed by logarithm and normalized by the quantile
normalization method. We finally determined the significant result
by adjusting |fold change| ≥2.
Pathway analysis
Pathway analysis was performed as previously
described (33). The Kyoto
Encyclopedia of Genes and Genomes (KEGG) database was used to
analyze the biological pathways (http://www.genome.jp/kegg/). All data analyses and
visualization of differentially expressed genes was conducted using
R 3.2.2 (www.r-project.org).
Reagent
L-glutamine (2 mM) and HEPES were purchased from
Wako. PF 573228 (1 µM otherwise described), AOA (2 mM) and
dimethyl 2-oxoglutarate (2 mM) were purchased from Sigma-Aldrich
(St. Louis, MO, USA).
Western blotting
The same amount of protein from whole cell lysates
was subjected to SDS-polyacrylamide gel (Bio-Rad, Hercules, CA,
USA) electrophoresis and then electrotransferred onto
polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA).
Membranes were blocked with 5% (w/v) skim milk in TBS/Tween-20 for
1 h at room temperature. After that, the blots were probed with
primary antibodies at 1–500 dilutions overnight at 4°C, followed by
incubation with appropriate secondary antibodies conjugated to
horseradish peroxidase (GE Healthcare, Tokyo, Japan) for 1 h at
room temperature. The secondary antibodies were detected using
Immobilon Western Chemiluminescent HRP Substrate (Millipore)
according to the manufacturer's instructions. Protein bands from
western blotting were relatively quantified with ImageJ (34,35).
Antibody
Anti-GLS antibody, anti-GDH antibody, anti-GOT1
antibody and anti-GPT2 antibody were purchased from abcam (cat#
156876, 89967, 170950 and 101876, respectively).
Anti-PSAT1 antibody was purchased from Proteintech
(cat# 10501-1-AP). Anti-p-FAK (Tyr397) antibody was purchased from
Life Technologies (cat# 44-624G). Anti-FAK antibody was purchased
from BD Biosciences (Bedford, MA, USA) (cat# 610088). Anti-p-Akt
(Ser 473) antibody, anti-Akt antibody, anti-p-S6K (Thr389)
antibody, anti-S6K antibody, anti-p-S6 (Ser 235/236) antibody and
anti-S6 antibody were purchased from Cell signaling technology
(cat# 9271, 9272, 9205, 9202, 4858 and 2217, respectively).
Anti-α-tubulin antibody was purchased from Millipore (cat#
CP06).
Statistical analysis
P-values <0.05 were considered to indicate
statistically significant differences.
Results
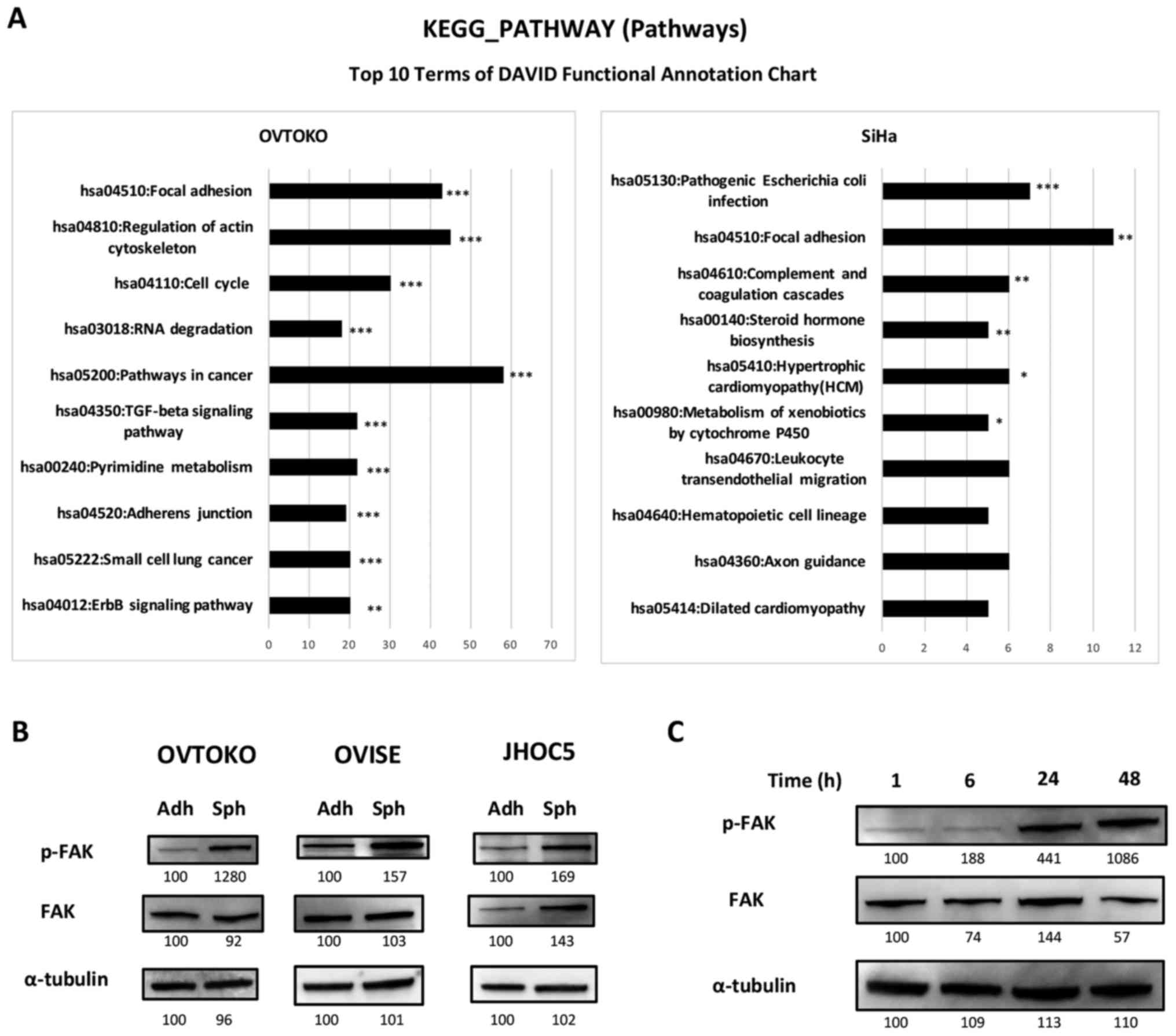
Focal adhesion pathway is the only common
pathway based on KEGG analysis between spheroids from OVTOKO and
SiHa by RNA-seq analysis
We recently obtained spheroids from OVTOKO (ovarian
clear cell carcinoma) and SiHa (cervical squamous cell carcinoma)
(10). Using these spheroids, we
investigated the common pathways, which could be universal and
essential pathways in spheroids. The top 10 significant pathways in
spheroids compared with adherent-cultured cells from each cell line
are shown in Fig. 1. To our
surprise, there was only one pathway that was common between
spheroids from OVTOKO and those from SiHa; it was hsa 04510, the
focal adhesion pathway.
Phosphorylation of FAK is increased in
spheroids compared to adherent cells, and the increase is
time-dependent
Inhibition of the FAK pathway is a promising target
in ovarian serous carcinoma (15,25,27,36,37),
and we focused on confirming the effectiveness of targeting the FAK
pathway in ovarian clear cell carcinoma using the ovarian clear
cell carcinoma cell lines OVTOKO, OVISE and JHOC5. We investigated
the expression levels of FAK and the phosphorylation of FAK using
western blots. As shown in Fig.
1B, the phosphorylation of FAK was higher in spheroids than
adherent cells, and it was suggested that the increase was
time-dependent after being in suspension (Fig. 1C).
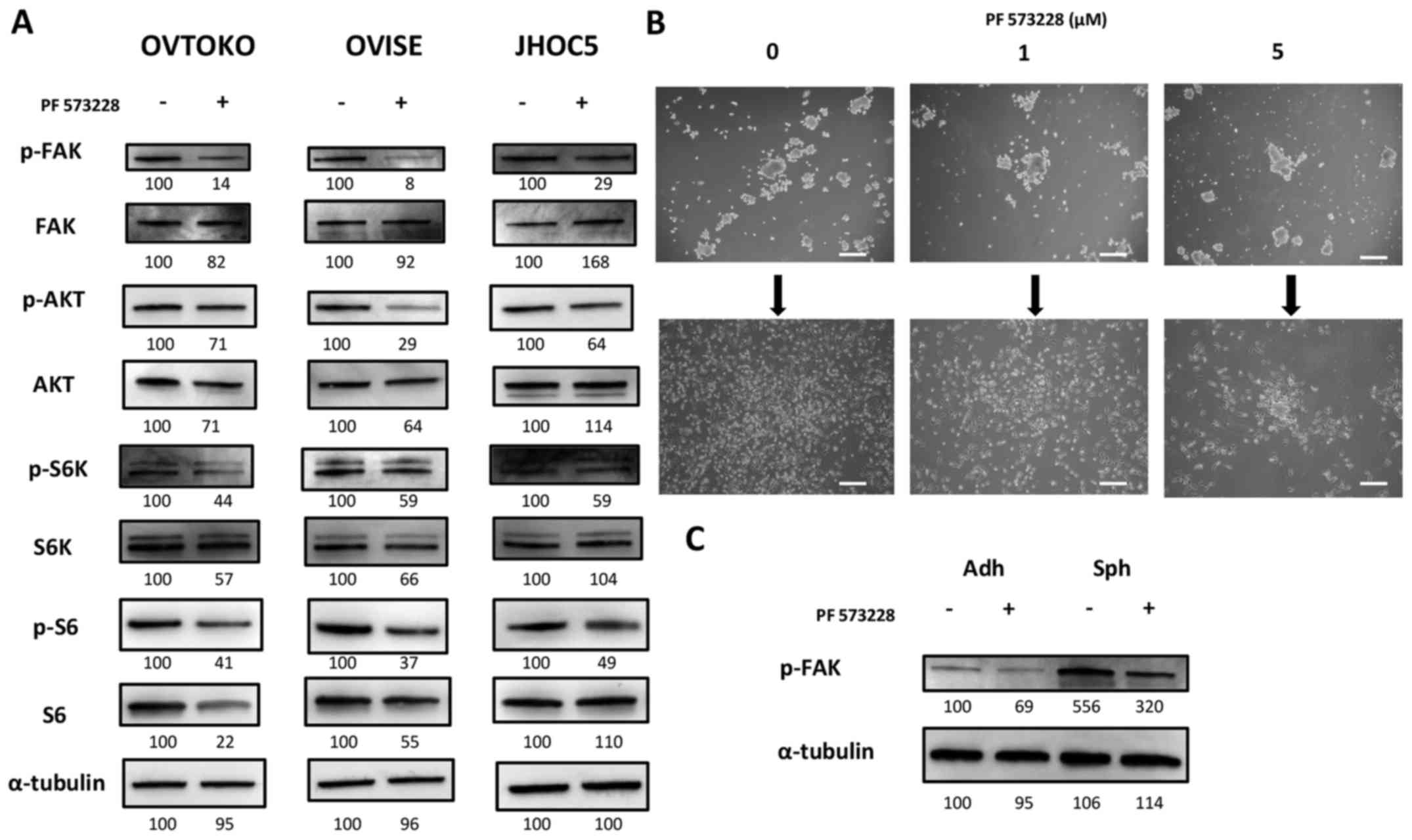
PF 573228, a FAK inhibitor, decreases the
phosphorylation of FAK and inhibits the downstream pathway, the
Akt/mTOR pathway
PF 573228 is a FAK inhibitor, and it was shown to be
effective at inhibiting tumor migration in various types of solid
tumors by inhibiting the phosphorylation of FAK (27). We obtained the expected results,
where PF 573228 decreased the phosphorylation of FAK and inhibited
the downstream pathway, the Akt/mTOR pathway (Fig. 2A). Although it was difficult to
quantify, it appeared that exposing cells in suspension to PF
573228 did not affect spheroid-forming abilities. However, we found
that the attachment and proliferation ability of spheroids cultured
with PF 573228 were deterred when reseeded into adherent plates
(Fig. 2B).
Sensitivity and impact of FAK pathway
inhibition depends on the cancer cell status
The results above were expected based on previous
studies and the presence of FAK inhibitors in clinical trials
(27,37–40).
We also determined that the effectiveness of inhibiting FAK
pathways might depend on cancer cell status. The phosphorylation of
FAK in spheroids was higher than adherent cells, and exposing them
to 1 µM PF 573228 still resulted in a higher phosphorylation
level of FAK compared with that of the non-exposed adherent cells
(Fig. 2C).
Glutamine metabolism is essential to
maintain the mTOR pathway of cells in suspension
We recently demonstrated that the concentration of
glutamine and glutamate were significantly increased in spheroids
compared to adherent cells (10),
and we investigated the effects of targeting glutamine metabolism
on spheroids, especially focusing on the mTOR pathway. This was our
focus because it is a common downstream pathway between glutamine
metabolism and FAK pathways and because inhibiting the mTOR pathway
is a promising target for the treatment for ovarian clear cell
carcinoma (28,29,36,41,42).
To confirm the significance of glutamine metabolism in cells in
suspension, we cultured cells in suspension with or without
glutamine. We found that the mTOR pathway, which is represented by
the phosphorylation levels of S6K and S6, was inhibited when cells
were cultured without glutamine. We also found that the effect was
salvaged by supplementation of cell-permeable type α-KG, which are
the intermediates of both glutaminolysis and the tricarboxylic acid
(TCA) cycle (Fig. 3A) (43–45).
This result suggested the involvement of glutaminolysis in
maintaining the mTOR pathway. We then investigated major enzymes
related to glutamine metabolism, including glutaminase (GLS),
glutamate dehydrogenase (GDH), aspartate aminotransferase (GOT1),
phosphohydroxythreonine aminotransferase (PSAT1) and alanine
aminotransferase (GPT2) (46,47).
We could not detect GPT2 (data not shown). We found that the
expression of GOT1 was commonly increased in spheroids compared to
adherent cells among the cell lines (Fig. 3B). Amino-oxyacetate (AOA), a
pan-transaminase inhibitor (48–50),
cancelled the salvation effect of α-KG (Fig. 3C) on the mTOR pathway when
inhibited by glutamine depletion.
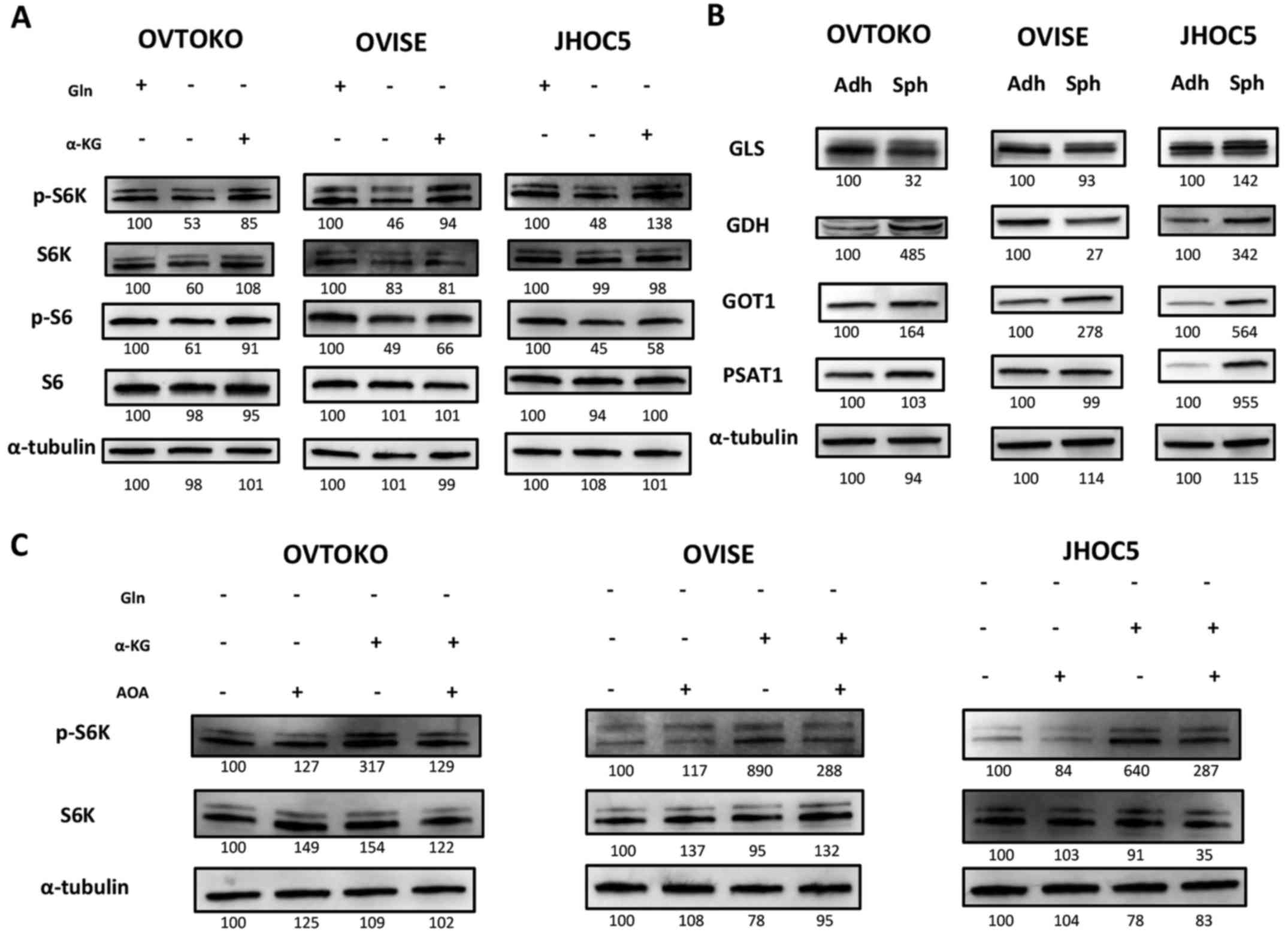 | Figure 3Significance of glutamine metabolism
in cells in suspension. (A) Glutamine metabolism impact on the mTOR
pathway in cells in suspension. Cells were cultured for 6 h with or
without 2 mM glutamine and 2 mM dimethyl 2-oxoglutarate. Glutamine
depletion inhibited the mTOR pathway and dimethyl 2-oxoglutarate
salvaged it. This suggested that glutamine metabolism had some
impacts on the mTOR pathway of cells in suspension. (B) Expression
of enzymes related to glutamine metabolism between adherent cells
and spheroids. Expression of enzymes related during glutamine
metabolism (GLS, GDH, GOT1, PSAT1 and GPT2) were investigated by
western blotting. Only GOT1 was commonly increased in spheroids
compared to adherent cells among all cell lines. The expression of
GPT2 could not be confirmed. (C) The effect of AOA, a
pan-transaminase inhibitor, on the mTOR pathway related to
glutamine metabolism for cells in suspension. Cells in suspension
were cultured for 6 h with or without 2 mM dimethyl 2-oxoglutarate
and 2 mM AOA. The salvation effect of dimethyl 2-oxoglutarate on
the mTOR pathway inhibited by glutamine depletion was cancelled by
AOA. α-KG, dimethyl 2-oxoglutarate; GLS, glutaminase; GDH,
glutamate dehydrogenase; GOT, aspartate aminotransferase; PSAT1,
phosphohydroxythreonine aminotransferase; GPT2, alanine
amino-transferase; Adh, adherent-cultured cells; and Sph,
spheroids. |
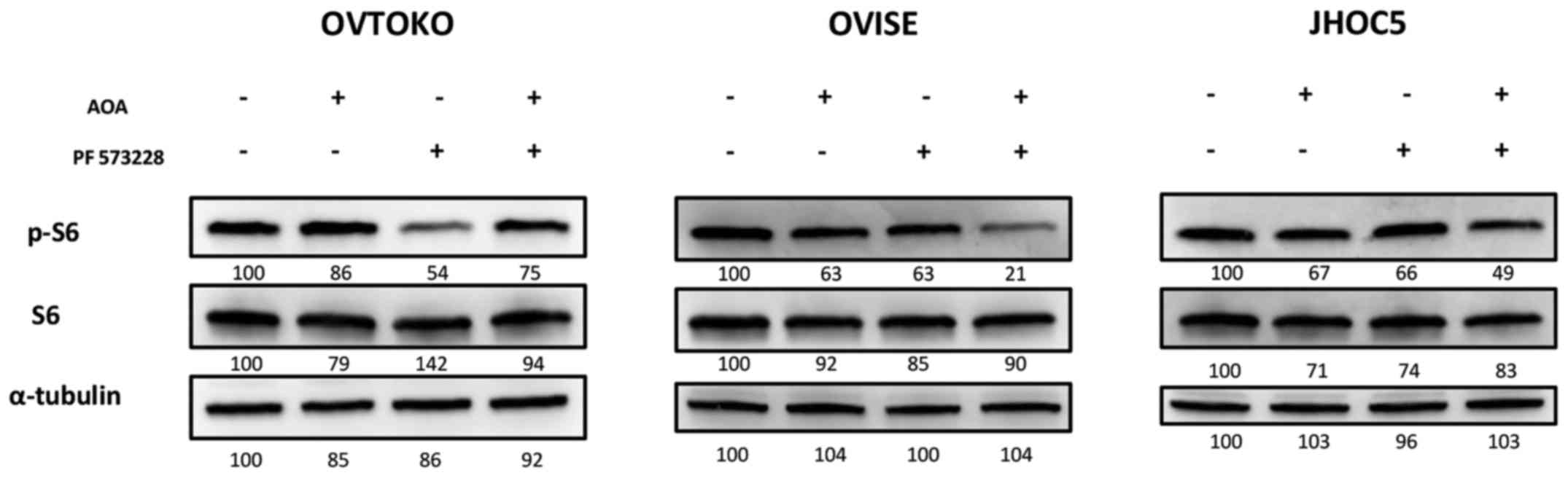
Targeting glutamine metabolism additively
inhibited the mTOR pathway of spheroids when combined with a FAK
inhibitor
We then investigated the synergistic effect of AOA
combined with a FAK inhibitor on the mTOR pathway in ovarian clear
cell carcinoma. We found that exposing cells in suspension to AOA
and PF 573228 additively inhibited the mTOR pathway in OVISE and
JHOC5 (Fig. 4). The combined
effect could not be observed in spheroids from OVTOKO. Rather, to
our surprise, it appeared that adding AOA cancelled the inhibitory
effect of PF 573228 on the mTOR pathway (Fig. 4). We were unsure what caused the
adverse effect of AOA in spheroids from OVTOKO, which is discussed
later.
Discussion
We examined the positive and negative effects of
targeting the FAK pathway in ovarian clear cell carcinoma and
suggested that targeting glutamine metabolism might overcome the
limitation of inhibiting the FAK pathway by additively inhibiting
the mTOR pathway of cancer stem-like properties.
Ovarian cancer is one of the leading cause of death
in the world. Fatality results because it seldom induces symptoms
and is often identified during advanced stages (1). In these cases, chemotherapy is less
likely to be effective, and it often results in tumor relapse. The
experimental procedures that reflect the clinical characteristics
of metastasized cancers in vitro are culturing cancer cells
in suspension, which results in the formation of spheroids
(3,4).
Accordingly, we performed RNA-seq analysis in
spheroids obtained from OVTOKO (ovarian clear cell carcinoma) and
SiHa (cervical squamous cell carcinoma) to find targets that were
universal and essential pathways in spheroids. We found that the
only common pathway based on KEGG was hsp 04510, the focal adhesion
pathway. The focal adhesion pathway has been investigated in
various types of solid tumors for the last few decades, and
inhibition of FAK is a promising target in ovarian serous
adenocarcinoma (15,25,27,36,37).
Thus, we evaluated the effectiveness of targeting the FAK pathway
in ovarian clear cell carcinoma using the ovarian clear cell
carcinoma cell lines OVTOKO, OVISE and JHOC5.
In the first half of this study, we demonstrated
that the phosphorylation of FAK was increased in spheroids compared
to adherent cells, and inhibiting FAK phosphorylation resulted in
deterring spheroid attachment onto adherent plates. These findings
suggested that targeting the FAK pathway can be effective in
targeting cancer stem cells because spheroids are known to share
cancer stem-like features (positive effect) (3,10–12).
We also demonstrated the limitation of targeting the FAK pathway
from an in vitro study (negative effect). The
phosphorylation levels of FAK in spheroids were high, and even as
much as 1 µM PF 573228 could not completely inhibit the
phosphorylation of FAK. These results intrigued us to determine a
way to overcome it. In the latter half of this study, we approached
FAK and the downstream mTOR pathway from a glutamine metabolism
perspective.
We recently demonstrated that the concentrations of
glutamine and glutamate were significantly increased in spheroids
compared to adherent cells (10).
Glutamine metabolism is known to be essential in the mTOR pathway
(28). Taken together, we
speculated that targeting glutamine metabolism could be a
breakthrough for the potential limitation of targeting FAK in
ovarian clear cell carcinoma. Among enzymes related to glutamine
metabolism, only the expression of GOT1 was commonly increased in
spheroids compared to adherent cells among all cell lines. The
combination of AOA, a pan-transaminase inhibitor, and PF 573228, an
FAK inhibitor, additively inhibited the mTOR pathway in two of
three cell lines.
The combined effect was not observed in spheroids
from OVTOKO. Alternatively, it seemed that adding AOA cancelled the
inhibitory effect of PF 573228 on the mTOR pathway. We are not sure
what caused the adverse effect of AOA in spheroids from OVTOKO.
Previous studies demonstrated that the KRAS mutation caused a
metabolic switch and changed the significance of GOT1 in
glutaminolysis in pancreatic cancer (50). However, it seemed that the KRAS
mutation was not the one that caused our results, based on our
previous study that investigated the mutation of each oncogene in
ovarian clear cell carcinoma cell lines (51). Finding a biomarker and features to
predict the combinational effect of targeting glutamine metabolism
and FAK will be our future direction.
In conclusion, from the in vitro perspective,
we proposed that targeting glutamine metabolism with properly
chosen targets could enhance the inhibitory effect of FAK
inhibitors on the mTOR pathway in cancer stem cell-like
properties.
Acknowledgments
This work was supported by JSPS KAKENHI grant number
26293357 and 15H06172, and by the Practical Research for Innovative
Cancer Control from Japan Agency for Medical Research and
Development, AMED.
References
|
1
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tancioni I, Uryu S, Sulzmaier FJ, Shah NR,
Lawson C, Miller NL, Jean C, Chen XL, Ward KK and Schlaepfer DD:
FAK Inhibition disrupts a β5 integrin signaling axis controlling
anchorage-independent ovarian carcinoma growth. Mol Cancer Ther.
13:2050–2061. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedrich J, Ebner R and Kunz-Schughart
LA: Experimental anti-tumor therapy in 3-D: Spheroids - old hat or
new challenge? Int J Radiat Biol. 83:849–871. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friedrich J, Seidel C, Ebner R and
Kunz-Schughart LA: Spheroid-based drug screen: Considerations and
practical approach. Nat Protoc. 4:309–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beck HC, Gosau M, Kristensen LP and
Morsczeck C: A site-specific phosphorylation of the focal adhesion
kinase controls the formation of spheroid cell clusters. Neurochem
Res. 39:1199–1205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heyman L, Kellouche S, Fernandes J, Dutoit
S, Poulain L and Carreiras F: Vitronectin and its receptors partly
mediate adhesion of ovarian cancer cells to peritoneal mesothelium
in vitro. Tumour Biol. 29:231–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanjoni I, Walsh C, Uryu S, Tomar A, Nam
JO, Mielgo A, Lim ST, Liang C, Koenig M, Sun C, et al: PND-1186 FAK
inhibitor selectively promotes tumor cell apoptosis in
three-dimensional environments. Cancer Biol Ther. 9:764–777. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ward KK, Tancioni I, Lawson C, Miller NL,
Jean C, Chen XL, Uryu S, Kim J, Tarin D, Stupack DG, et al:
Inhibition of focal adhesion kinase (FAK) activity prevents
anchorage-independent ovarian carcinoma cell growth and tumor
progression. Clin Exp Metastasis. 30:579–594. 2012. View Article : Google Scholar
|
|
9
|
Tancioni I, Miller NL, Uryu S, Lawson C,
Jean C, Chen XL, Kleinschmidt EG and Schlaepfer DD: FAK activity
protects nucleostemin in facilitating breast cancer spheroid and
tumor growth. Breast Cancer Res. 17:472015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sato M, Kawana K, Adachi K, Fujimoto A,
Yoshida M, Nakamura H, Nishida H, Inoue T, Taguchi A, Takahashi J,
et al: Spheroid cancer stem cells display reprogrammed metabolism
and obtain energy by actively running the tricarboxylic acid (TCA)
cycle. Oncotarget. 7:33297–33305. 2016.PubMed/NCBI
|
|
11
|
Shield K, Ackland ML, Ahmed N and Rice GE:
Multicellular spheroids in ovarian cancer metastases: Biology and
pathology. Gynecol Oncol. 113:143–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alanko J, Mai A, Jacquemet G, Schauer K,
Kaukonen R, Saari M, Goud B and Ivaska J: Integrin endosomal
signalling suppresses anoikis. Nat Cell Biol. 17:1412–1421. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lark AL, Livasy CA, Calvo B, Caskey L,
Moore DT, Yang X and Cance WG: Overexpression of focal adhesion
kinase in primary colorectal carcinomas and colorectal liver
metastases: immunohistochemistry and real-time PCR analyses. Clin
Cancer Res. 9:215–222. 2003.PubMed/NCBI
|
|
15
|
Sood AK, Coffin JE, Schneider GB, Fletcher
MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD and Hendrix
MJ: Biological significance of focal adhesion kinase in ovarian
cancer: Role in migration and invasion. Am J Pathol. 165:1087–1095.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beierle EA, Massoll NA, Hartwich J,
Kurenova EV, Golubovskaya VM, Cance WG, McGrady P and London WB:
Focal adhesion kinase expression in human neuroblastoma:
Immunohistochemical and real-time PCR analyses. Clin Cancer Res.
14:3299–3305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Casanova I, Parreño M, Farré L, Guerrero
S, Céspedes MV, Pavon MA, Sancho FJ, Marcuello E, Trias M and
Mangues R: Celecoxib induces anoikis in human colon carcinoma cells
associated with the deregulation of focal adhesions and nuclear
translocation of p130Cas. Int J Cancer. 118:2381–2389. 2006.
View Article : Google Scholar
|
|
18
|
Chen CH, Shyu MK, Wang SW, Chou CH, Huang
MJ, Lin TC, Chen ST, Lin HH and Huang MC: MUC20 promotes aggressive
phenotypes of epithelial ovarian cancer cells via activation of the
integrin β1 pathway. Gynecol Oncol. 140:131–137. 2016. View Article : Google Scholar
|
|
19
|
Chen YY, Wang ZX, Chang PA, Li JJ, Pan F,
Yang L, Bian ZH, Zou L, He JM and Liang HJ: Knockdown of focal
adhesion kinase reverses colon carcinoma multicellular resistance.
Cancer Sci. 100:1708–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong D, Chen F and Sima NI: Inhibition of
focal adhesion kinase induces apoptosis in bladder cancer cells via
Src and the phosphatidylinositol 3-kinase/Akt pathway. Exp Ther
Med. 10:1725–1731. 2015.PubMed/NCBI
|
|
21
|
Lark AL, Livasy CA, Dressler L, Moore DT,
Millikan RC, Geradts J, Iacocca M, Cowan D, Little D, Craven RJ, et
al: High focal adhesion kinase expression in invasive breast
carcinomas is associated with an aggressive phenotype. Mod Pathol.
18:1289–1294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu W, Bloom DA, Cance WG, Kurenova EV,
Golubovskay VM and Hochwald SN: FAK and IGF-IR interact to provide
survival signals in human pancreatic adenocarcinoma cells.
Carcinogenesis. 29:1096–1107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Madan R, Smolkin MB, Cocker R, Fayyad R
and Oktay MH: Focal adhesion proteins as markers of malignant
transformation and prognostic indicators in breast carcinoma. Hum
Pathol. 37:9–15. 2006. View Article : Google Scholar
|
|
24
|
Rentala S, Chintala R, Guda M, Chintala M,
Komarraju AL and Mangamoori LN: Atorvastatin inhibited
Rho-associated kinase 1 (ROCK1) and focal adhesion kinase (FAK)
mediated adhesion and differentiation of
CD133+CD44+ prostate cancer stem cells.
Biochem Biophys Res Commun. 441:586–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stone RL, Baggerly KA, Armaiz-Pena GN,
Kang Y, Sanguino AM, Thanapprapasr D, Dalton HJ, Bottsford-Miller
J, Zand B, Akbani R, et al: Focal adhesion kinase: An alternative
focus for anti-angiogenesis therapy in ovarian cancer. Cancer Biol
Ther. 15:919–929. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sulzmaier FJ, Jean C and Schlaepfer DD:
FAK in cancer: Mechanistic findings and clinical applications. Nat
Rev Cancer. 14:598–610. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee BY, Timpson P, Horvath LG and Daly RJ:
FAK signaling in human cancer as a target for therapeutics.
Pharmacol Ther. 146:132–149. 2015. View Article : Google Scholar
|
|
28
|
Durán RV, Oppliger W, Robitaille AM,
Heiserich L, Skendaj R, Gottlieb E and Hall MN: Glutaminolysis
activates Rag-mTORC1 signaling. Mol Cell. 47:349–358. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kassem L and Abdel-Rahman O: Targeting
mTOR pathway in gynecological malignancies: Biological rationale
and systematic review of published data. Crit Rev Oncol Hematol.
108:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trapnell C, Pachter L and Salzberg SL:
TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics.
25:1105–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Langmead B, Trapnell C, Pop M and Salzberg
SL: Ultrafast and memory-efficient alignment of short DNA sequences
to the human genome. Genome Biol. 10:R252009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trapnell C, Williams BA, Pertea G,
Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ and Pachter
L: Transcript assembly and quantification by RNA-Seq reveals
unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:511–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, He RQ, Dang YW, Zhang XL, Wang X,
Huang SN, Huang WT, Jiang MT, Gan XN, Xie Y, et al: Comprehensive
analysis of the long noncoding RNA HOXA11-AS gene interaction
regulatory network in NSCLC cells. Cancer Cell Int. 16:892016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fujimoto A, Kawana K, Taguchi A, Adachi K,
Sato M, Nakamura H, Ogishima J, Yoshida M, Inoue T, Nishida H, et
al: Inhibition of endoplasmic reticulum (ER) stress sensors
sensitizes cancer stem-like cells to ER stress-mediated apoptosis.
Oncotarget. 7:51854–51864. 2016.PubMed/NCBI
|
|
35
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kang Y, Hu W, Ivan C, Dalton HJ, Miyake T,
Pecot CV, Zand B, Liu T, Huang J, Jennings NB, et al: Role of focal
adhesion kinase in regulating YB-1-mediated paclitaxel resistance
in ovarian cancer. J Natl Cancer Inst. 105:1485–1495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McGrail DJ, Khambhati NN, Qi MX, Patel KS,
Ravikumar N, Brandenburg CP and Dawson MR: Alterations in ovarian
cancer cell adhesion drive taxol resistance by increasing
microtubule dynamics in a FAK-dependent manner. Sci Rep.
5:95292015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Infante JR, Camidge DR, Mileshkin LR, Chen
EX, Hicks RJ, Rischin D, Fingert H, Pierce KJ, Xu H, Roberts WG, et
al: Safety, pharmacokinetic, and pharmacodynamic phase I
dose-escalation trial of PF-00562271, an inhibitor of focal
adhesion kinase, in advanced solid tumors. J Clin Oncol.
30:1527–1533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schultze A and Fiedler W: Therapeutic
potential and limitations of new FAK inhibitors in the treatment of
cancer. Expert Opin Investig Drugs. 19:777–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Walsh C, Tanjoni I, Uryu S, Tomar A, Nam
J-O, Luo H, Phillips A, Patel N, Kwok C, McMahon G, et al: Oral
delivery of PND-1186 FAK inhibitor decreases tumor growth and
spontaneous breast to lung metastasis in pre-clinical models.
Cancer Biol Ther. 9:778–790. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mabuchi S, Kuroda H, Takahashi R and
Sasano T: The PI3K/AKT/mTOR pathway as a therapeutic target in
ovarian cancer. Gynecol Oncol. 137:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yamamoto D, Sonoda Y, Hasegawa M,
Funakoshi-Tago M, Aizu-Yokota E and Kasahara T: FAK overexpression
upregulates cyclin D3 and enhances cell proliferation via the PKC
and PI3-kinase-Akt pathways. Cell Signal. 15:575–583. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hou P, Kuo CY, Cheng CT, Liou JP, Ann DK
and Chen Q: Intermediary metabolite precursor
dimethyl-2-ketoglutarate stabilizes hypoxia-inducible factor-1α by
inhibiting prolyl-4-hydroxylase PHD2. PLoS One. 9:e1138652014.
View Article : Google Scholar
|
|
44
|
Zhao J, Peng L, Luo Z, Cui R and Yan M:
Inhibitory effects of dimethyl α-ketoglutarate in hepatic stellate
cell activation. Int J Clin Exp Pathol. 8:5471–5477.
2015.PubMed/NCBI
|
|
45
|
Mariño G, Pietrocola F, Kong Y, Eisenberg
T, Hill JA, Madeo F and Kroemer G: Dimethyl α-ketoglutarate
inhibits maladaptive autophagy in pressure overload-induced
cardiomyopathy. Autophagy. 10:930–932. 2014. View Article : Google Scholar
|
|
46
|
Jin L, Alesi GN and Kang S: Glutaminolysis
as a target for cancer therapy. Oncogene. 35:3619–3625. 2016.
View Article : Google Scholar
|
|
47
|
Yang L, Moss T, Mangala LS, Marini J, Zhao
H, Wahlig S, Armaiz-Pena G, Jiang D, Achreja A, Win J, et al:
Metabolic shifts toward glutamine regulate tumor growth, invasion
and bioenergetics in ovarian cancer. Mol Syst Biol. 10:7282014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Feld FM, Nagel PD, Weissinger SE, Welke C,
Stenzinger A, Möller P and Lennerz JK: GOT1/AST1 expression status
as a prognostic biomarker in pancreatic ductal adenocarcinoma.
Oncotarget. 6:4516–4526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thornburg JM, Nelson KK, Clem BF, Lane AN,
Arumugam S, Simmons A, Eaton JW, Telang S and Chesney J: Targeting
aspartate aminotransferase in breast cancer. Breast Cancer Res.
10:R842008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Son J, Lyssiotis CA, Ying H, Wang X, Hua
S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et
al: Glutamine supports pancreatic cancer growth through a
KRAS-regulated metabolic pathway. Nature. 496:101–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kashiyama T, Oda K, Ikeda Y, Shiose Y,
Hirota Y, Inaba K, Makii C, Kurikawa R, Miyasaka A, Koso T, et al:
Antitumor activity and induction of TP53-dependent apoptosis toward
ovarian clear cell adenocarcinoma by the dual PI3K/mTOR inhibitor
DS-7423. PLoS One. 9:e872202014. View Article : Google Scholar : PubMed/NCBI
|