Introduction
Glioblastoma multiforme (GBM), a highly malignant
grade 4 glioma, is the most common primary cancer of the brain.
Surgery combined with radiotherapy and chemotherapy is still the
standard treatment for GBM patients (1). However, the high mobility and strong
invasive properties of GBM result in a high inevitable recurrence
rate and a poor median survival of 14.6 months for patients
(2). Thus, there is a pressing
need to reveal the molecular mechanisms of GBM invasion for novel
therapeutic avenue development.
Epithelial-mesenchymal transition (EMT) is a process
in which epithelial cells lose their polarity and adhesion ability
and become mesenchymal stem cells gaining migratory and invasive
properties (3). Accumulating
evidence has showed that EMT also occurs and plays a critical role
in the initiation of metastasis for tumor progression. It is well
known that transforming growth factor-β1 (TGF-β1) signaling pathway
plays a principle role in accelerating epithelial plasticity that
may progress to EMT (4). However,
TGF-β1-induced EMT does not happen in some non-invasive tumor cells
in vitro (5). On top of
that, TGF-β1 has a dual role and it can act as either a tumor
suppressor or promoter depending on the stages and types of the
tumor (3,4). Thus, the detailed mechanism that
TGF-β1 regulates GBM has not been fully explored to date.
MicroRNAs (miRNAs) are small non-coding RNAs that
post-transcriptionally repress target gene expression by inhibiting
translation or promoting mRNA degradation (6). Approximately 35828 miRNAs have been
found to be expressed in 223 species (http://microrna.sanger.ac.uk), which facilitates the
possibility of reciprocal interactions between miRNAs and finely
regulating gene expression by miRNAs. The most compelling evidence
is that the aberrant production of miRNAs has been widely
recognized as a main character of various human diseases, including
developmental abnormalities, autoimmune diseases and cancer
(7–9). Mounting evidence indicated that
miRNAs play pivotal roles in GBM (10,11).
For example, miR-146b-5p is downregulated and triggers the
miR-146b-5p/Hu antigen R (HuR)/lincRNA-p21/β-catenin signaling
pathway in glioma stem cells (GSCs) and miR-146b-5p overexpression
attenuates stemness and radioresistance of GSCs (12).
miR-10b has been reported to be an oncogenic miRNA
which can regulate growth and metastasis of various types of cancer
(13–15). By using pleiotropic mechanisms,
miR-10b widely participated in the regulating of cancer cell
proliferation, migration, invasion and EMT. Although several target
genes of miR-10b have been designated in GBM and other tumors,
their regulation looks cell- and context-specific (13,16,17).
Thus, further studies are still needed to uncover the detailed
mechanisms underlying miR-10b functions in regulating GBM
progression.
In the present study, we investigated the role of
miR-10b in TGF-β1-mediated GBM proliferation, migration and EMT. We
found that miR-10b is apparently upregulated by TGF-β1 in U251 and
U87 cells. Further studies uncovered that TGF-β1 remarkably
promoted GBM cell proliferation, migration and EMT. All these
effects were achieved through regulating miR-10b as miR-10b mimics
promoted, whereas miR-10b inhibitor reversed, the effects of
TGF-β1. In addition, several proliferation-, invasion- and
EMT-associated genes including epithelial cadherin (E-cadherin),
apoptotic protease activating factor 1 (Apaf-1) and phosphatase and
tensin homolog (PTEN) are the targets of miR-10b. When xenograft
models were used to investigate the miR-10b potency as therapeutic
target in vivo, results showed that antagomiR-10b apparently
suppressed tumor progression. In summary, our data collectively
demonstrated that miR-10b can be used as a potential therapeutic
target for the treatment of GBM.
Materials and methods
Clinical specimens
Glioblastoma tissues (n=15) were obtained from the
First Hospital of Jilin University (Changchun, China). All of the
procedures involving specimens obtained from human subjects were
performed under protocols approved by the Jilin University Ethic
Committee. Written informed consent was also obtained from all
subjects before the study. None of the patients received radiation
therapy or chemotherapy before surgical resection. All tissue
samples were snap-frozen in liquid nitrogen and stored at −70°C
until use.
Cell lines and cell culture
Human glioma cell lines U87 and U251 were purchased
from the China Academia Sinica Cell Repository (Shanghai, China).
The cells were cultured in Dulbecco's modified Eagle' medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 50 U/ml
penicillin/streptomycin and 2 mM L-glutamine (Gibco, Carlsbad, CA,
USA). All the cell lines were incubated at 37°C in a CO2
incubator. For cell treatment, 5 ng/ml of TGF-β was added or 100 nM
miR-10b mimics or inhibitor was transfected into the cells with
Lipofectamine 3000 as indicated. All the reagents were purchased
from Life Technologies (Grand Island, NY, USA).
RNA isolation and real-time PCR
Total RNA of GBM cells was isolated with TRIzol, and
cDNA was then generated using the reverse transcription kits
(TaqMan® MicroRNA Reverse Transcription kit for miRNA,
PrimeScript® First Strand cDNA synthesis kit for general
genes) following the protocols of the manufacturer (all from Life
Technologies, Grand Island, NY). Aliquots of the reaction products
were then used for real-time PCR with an ABI PRISM 7500 Fast system
using the following parameters: initial denaturation at 95°C for 10
min, followed by 40 cycles of 95°C for 15 sec, 60°C for 1 min and
72°C for 45 sec. The expression of miR-10b was normalized to U6B
miRNA and E-cadherin, Apaf-1 and PTEN were normalized to GAPDH
mRNA. All PCR experiments were performed in triplicate.
Western blot assay
U251 cells were thoroughly lysed in ice-cold RIPA
buffer (P0013C; Beyotime Institute of Biotechnology, Haimen, China)
for 45 min. Then, ~40 µg protein was subjected to SDS-PAGE
and transferred to polyvinylidene difluoride (PVDF) membranes.
After incubating in blocking buffer [phosphate-buffered saline
(PBS) containing 3% BSA] for 1 h at room temperature, the membranes
were subsequently incubated with E-cadherin (1:3,000; Abcam,
Cambridge, MA, USA), vimentin (1:3,000; Abcam), Apaf-1 (1:3,000; BD
Biosciences, San Diego, CA), PTEN (1:600; Abcam), Tubulin (1:1,000;
CWBio, Beijing, China) or β-actin (1:4,000; CWBio) monoclonal
antibodies and HRP-conjugated secondary antibodies, and the
specific immunoreactive proteins were visualized through enhanced
chemiluminescence.
Vector construction and luciferase
reporter assays
The Dual-luciferase vectors were constructed by
synthesizing the seed sequences in the 3′-UTRs of E-cadherin,
Apaf-1 and PTEN, or the reverse complementary sequence of miR-10b
(rcmiR-10b) and inserting the annealing products into the
psiCHECK-2 vector. The corresponding mutant vectors were also
constructed by introducing 3-bp mutations into the seed sequences.
To verify the specific targeting of these genes by miR-10b, HEK293T
cells were seeded in 24-well plates (1.5×106/well) and
transfected with 0.8 µg of the endotoxin-free recombinant
vectors, either alone or in combination with 50-nM miR-10b
precursors or inhibitors. Luciferase activities were measured 24 h
later using the Dual-luciferase reporter assay system.
EdU proliferation assay
EdU (5-ethynyl-2′-deoxyuridine) proliferation assay
was performed to measure cell proliferation. In brief, cells
treated as indicated were seeded in 48-well plates
(2×104 cells/well) and cultured for 24 h. Subsequently,
the cells were incubated for 3 h in serum-free DMEM supplemented
with 30 µM EdU (Guangzhou RiboBio, Co., Ltd., Guangzhou,
China) after being washed in PBS thrice. Afterwards the cells were
fixed with 4% polyformaldehyde in PBS at room temperature for 30
min. Finally, cells were incubated with Apollo staining solution
and Hoechst 33342 for 30 min each. Proliferation index was
presented as the percentage of EdU-positive cells relative to the
total cell numbers. Images were acquired using a fluorescent
microscope (Olympus IX73) and cells selected from five random
fields were counted.
Wound closure assay
U251 and U87 cells were treated as indicated and a
wound closure assay was performed to evaluate the cell migration
ability. The cells were plated at 1.5×106 cells/ml in
12-well dishes and stayed in the incubator at 37°C until a
confluent cell layer was established. A scratch in the cells was
then made with a sterile pipette tip, and both the numbers and the
average distance that cells moved from the edge of the scratch
towards the center were measured 24 h later.
Nude mouse xenograft model
Animal experiments were careful performed following
the guidelines of Jilin University Institutional Animal Care and
Use Committee (IACUC) and were approved by the Institutional Animal
Ethics Committee of Jilin University. U251 cells (1×106)
transfected with antag-miR-10b, agomiR-10b or miR-scramble were
resuspended in HBSS and injected subcutaneously into the flank
region of female athymic (nu/nu) mice aged at 4–6 weeks (Beijing,
China). The tumors were allowed to grow to average volume of 200
mm3 prior to initiation of treatment. PBS and the
miR-scramble were used as negative controls. The tumor volume (V)
was measured every other day with a slide caliper and calculated by
the formula: V = 4/3 × π [length/2 × (width/2)2]. All
mice were sacrificed after 18 days of treatment. Finally, the mice
were sacrificed, and the tumors were isolated and snap-frozen in
liquid nitrogen for following experiments.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism software. All data from at least three independent
experiments were analyzed with the Student's t-test; P<0.05 were
considered statistically significant.
Results
miR-10b is upregulated in
TGF-β1-stimulated GBM cells
Considering that TGF-β1 plays critical and
paradoxical roles in GBM but the detailed mechanism is still far
from elucidated, we wondered whether miRNAs play critical roles in
TGF-β1-treated GBM cells. U251 and U87 cells were treated with 5
ng/ml TGF-β1 for 24 h, and total RNAs were isolated from the cells
and the miRNA expression profile was determined by miRNA
microarray. Approximately 20 miRNAs were differentially expressed
between the TGF-β1-treated and control cells, among which miR-10b
was significantly upregulated by TGF-β1 in U251 and U87 cells.
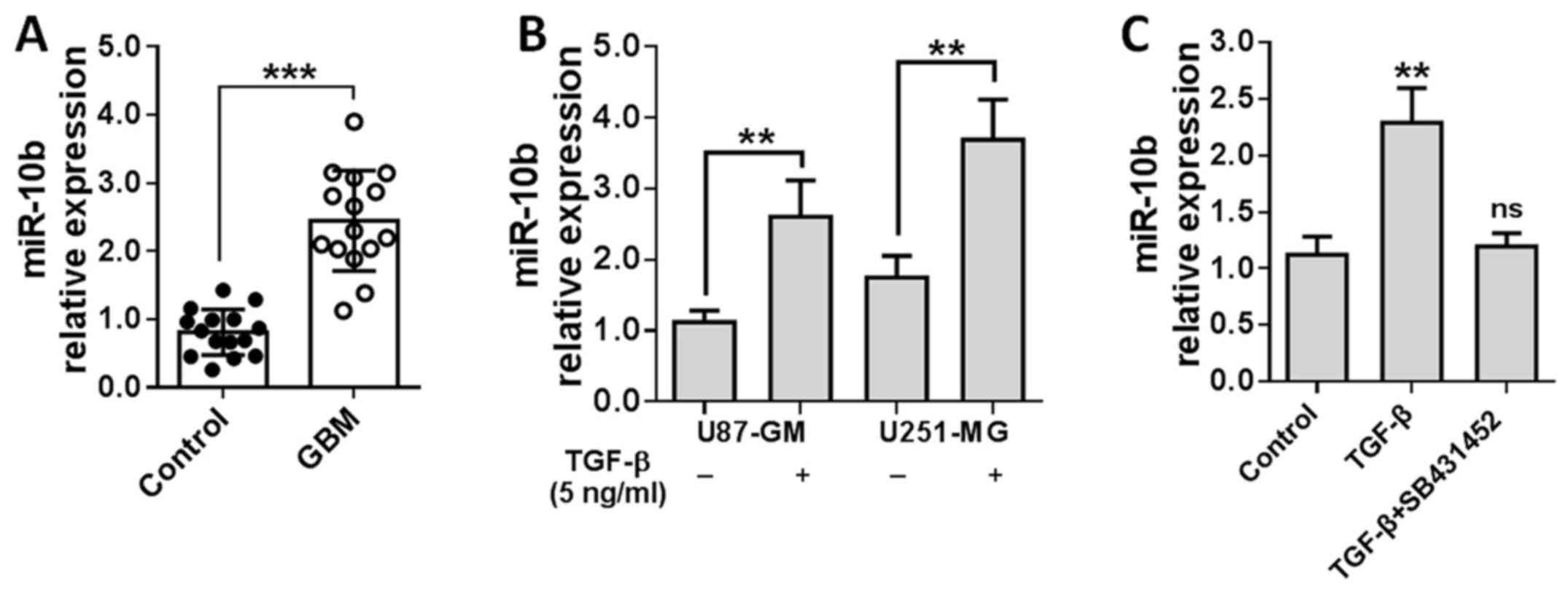
To confirm this finding, miR-10b level was detected
in the specimens collected from 15 GBM patients with real-time PCR.
As shown in Fig. 1A, miR-10b was
significantly overexpressed in GBM tissues relative to adjacent
non-tumor tissues. Then, U251 and U87 cells were stimulated by
TGF-β1 for 24 h and miR-10b levels were measured. Results showed
that miR-10b expression was elevated ~2–3-fold in TGF-β1-treated
GBM cells (Fig. 1B). To further
determine whether miR-10b upregulation is TGF-β1 specific, we
treated U251 cells with TGF-β1 in the presence of TGF-β receptor
inhibitor (SB431452). As shown in Fig.
1C, blockade of TGF-β1 signaling notably reversed the induction
of miR-10b. All these data suggested that miR-10b is induced in GBM
in a TGF-β1-dependent manner.
miR-10b mediates TGF-β1-induced GBM cell
proliferation
Although several studies found that miR-10b is
predominantly expressed in GBM but absent in normal brain tissues
(18–20), whether miR-10b participate in
TGF-β1-mediated GBM cell proliferation, migration and EMT are not
previously reported.
It is well known that the TGF-β1 exerts both tumor
promoting and tumor suppressive functions during cancer
progression, in a variety of cancers, depending on the stages and
types of the tumors. To investigate the effects of TGF-β1 on GBM
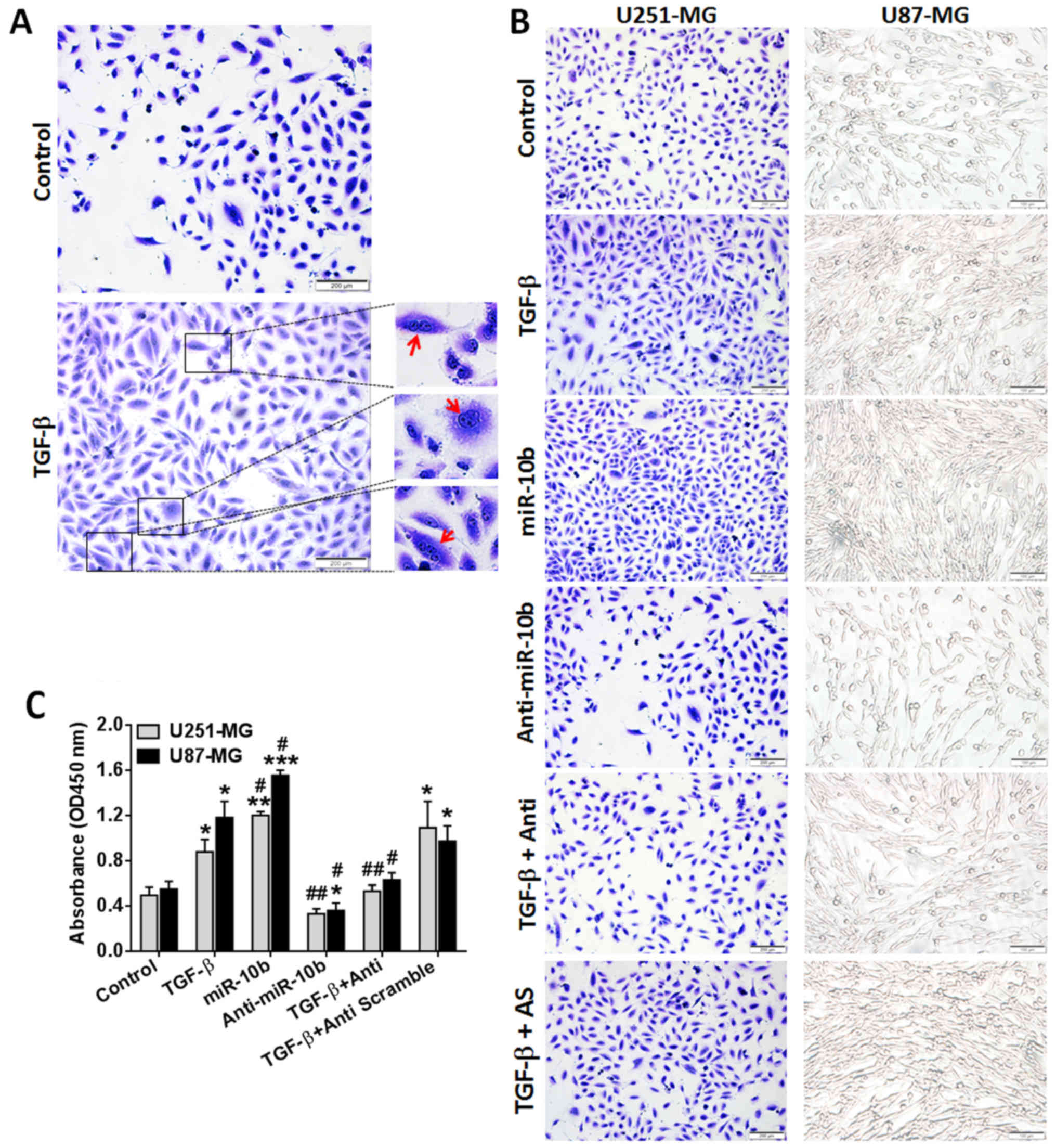
cells, U87 cells were treated with TGF-β1 for 72 h and cells were
stained with crystal violet. As shown in Fig. 2A, more plurinuclear cells were
found in TGF-β1-treated cells than untreated ones, which indicated
that TGF-β1-treated sample contains a reasonable number of
proliferating cells under mitosis. Then, both U87 and U251 cells
were treated with TGF-β1 and cells were counted 72 h later. Results
showed that TGF-β1 exerted potent proliferation-stimulation effect
on GBM cells (Fig. 2B and C, panel
2). As miR-10b was upregulated by TGF-β1, we speculated that
miR-10b mediated the effects of TGF-β1 on U251 and U87 cells. To
verify this hypothesis, U251 and U87 cells were treated with
miR-10b mimics (miR-10b), miR-10b inhibitor (anti-miR-10b), either
alone or in the combination with TGF-β1. Results showed that
miR-10b mimics (Fig. 2B and C,
panel 3) promoted, whereas miR-10b inhibitor suppressed (Fig. 2B and C, panel 4) GBM cell
proliferation. When treated in combination with TGF-β1 and
anti-miR-10b, TGF-β1-mediated cell proliferation was remarkably
reversed (Fig. 2B and C, panel 5).
This is a unique feature of the miR-10b inhibitor as the scramble
inhibitor (Fig. 2B and C, panel 6)
has no effect on TGF-β1. All the data suggested that TGF-β1
promotes GBM cell proliferation at least partially through
regulating miR-10b.
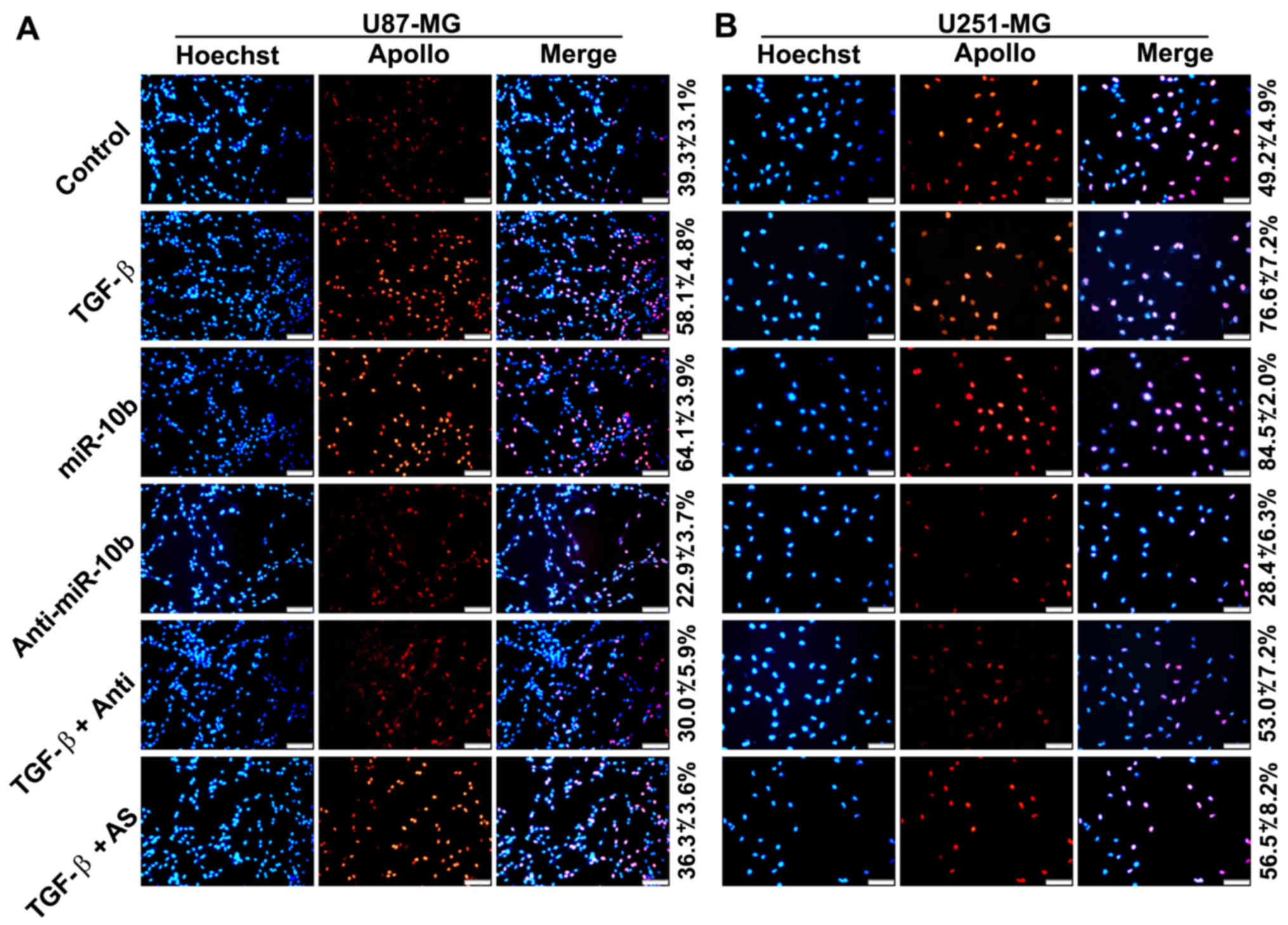
To further confirm that TGF-β1 directly stimulated
GBM cell proliferation, we treated U251 and U87 cells with TGF-β1
and determined the cell growth by EdU proliferation assays. Results
showed that growth of the cells was apparently accelerated in the
presence of TGF-β1 (Fig. 3A and
B). In addition, the effect of TGF-β1 was remarkably shielded
in the presence of miR-10a inhibitors. All these data collectively
indicated that miR-10b mediated TGF-β1-induced GBM cell
proliferation.
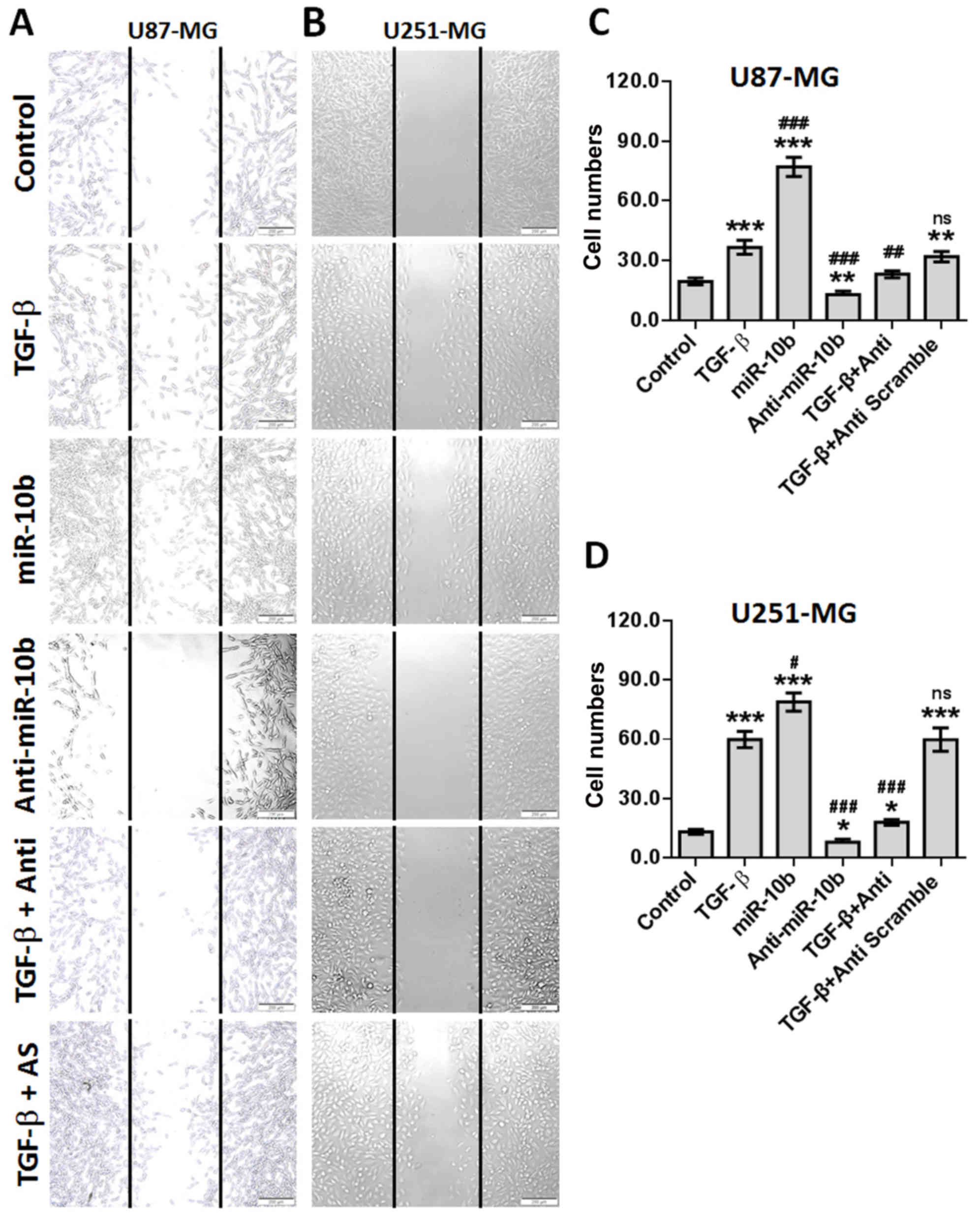
miR-10b enhances GBM cell migration
The migration, invasion and infiltration of tumor
cells are one of the significant contributors to mortality in GBM
patients. Although GBM cells can acquire enhanced invasive features
following stimulation with some secretory cytokines like TGF-β1 and
which contributes to the heterogeneity of GBM (21,22),
the detailed mechanism are still unclear. Several genes including
ZEB1, Crk-like (CrkL) and HOXA13 have been reported to be
associated with TGF-β1-induced migration of GBM cells (22–24).
To measure the roles that miR-10b may play in TGF-β1-regulated GBM
cell migration, the wound closure assay was performed and as
expected, treatment with TGF-β1 or miR-10b mimics significantly
promoted the sound healing ability of both U87 (Fig. 4A and C) and U251 (Fig. 4B and D) cells, whereas the miR-10b
inhibitor effectively weakened the effects of TGF-β1. These data
reveal that miR-10b is involved in the regulation of GBM cell
migration.
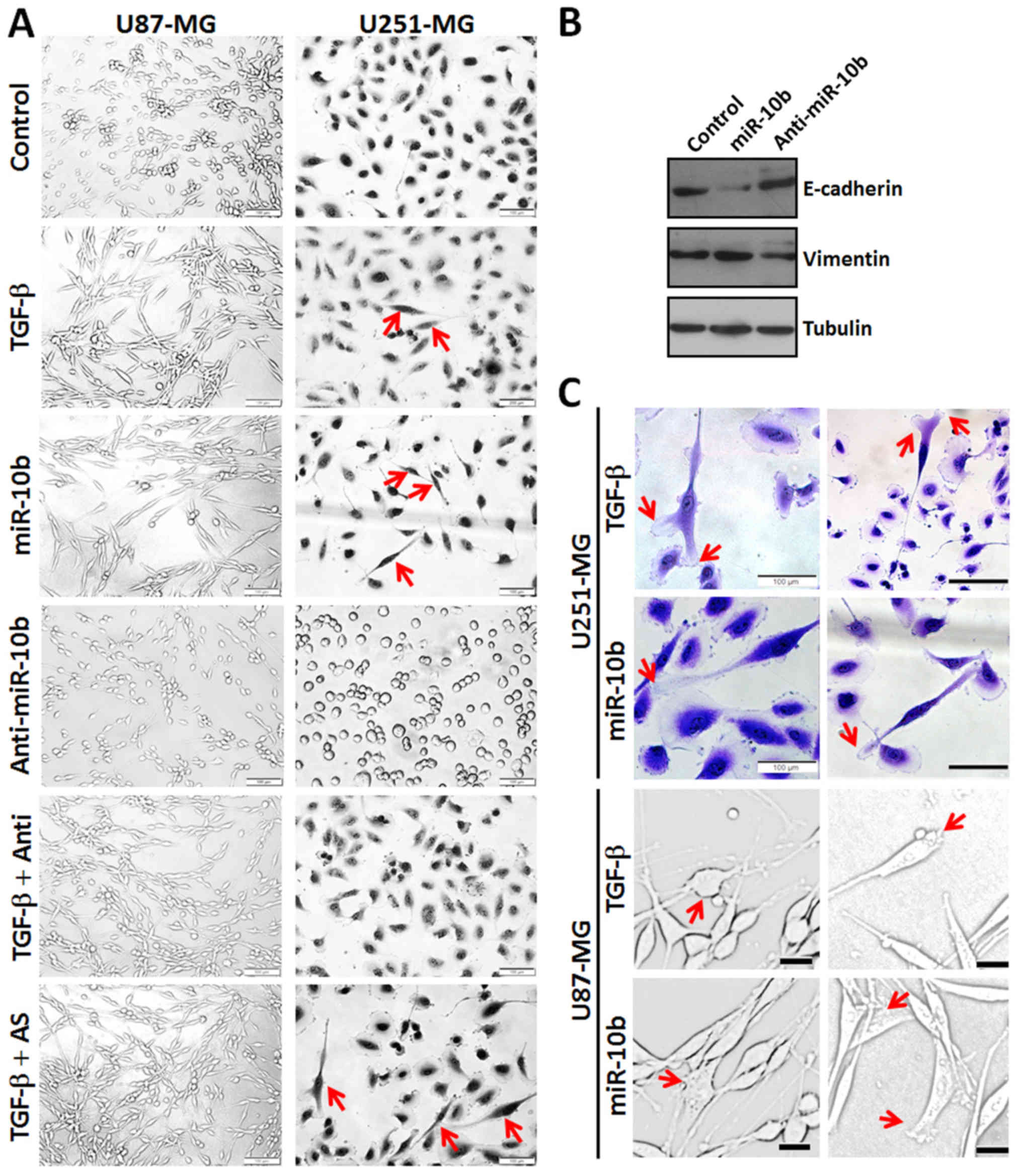
miR-10b promotes EMT and lamellipodia
formation in GBM cell lines
Considering EMT is a common feature of various
tumors which may be closely associated with tumor invasion and
metastasis and TGF-β1 is one of the most potent inducers of EMT
(25,26). In addition, TGF-β1 is also an
important cytokine in the GBM microenvironment (27,28).
We investigated whether miR-10b participates in the EMT of GBM
cells. As shown in Fig. 5A,
treatment with TGF-β1 (panel 2) or miR-10b mimics (panel 3) induced
a remarkable morphological change of U87 and U251 cells from pebble
shape to the long fusiform shape, reminiscent of EMT. However,
miR-10b inhibitor makes the cells round and of
cobblestone-appearance (panel 4). To further confirm whether
miR-10b induce EMT in GBM cells, we measured the expression level
of EMT-associated markers in U251 cells by western blot analysis.
We found that the expression of E-cadherin, a classical epithelial
marker, was apparently suppressed after miR-10b treatment, whereas
vimentin, a mesenchymal marker, was increased significantly
(Fig. 5B). Consistently, miR-10b
inhibitor-transfected cells showed reverse properties. These data
indicated that miR-10b is involved in TGF-β1-induced EMT in GBM
cells.
It is well known that both morphologic changes in
EMT and migration and invasion of GBM cells require dynamic
reorganization of the actin cytoskeleton including lamellipodial
extensions, focal adhesions and stress fiber formation at the
leading edge of GBM. We assessed whether miR-10b participates in
the reorganization of the actin cytoskeleton. As shown in Fig. 5C, TGF-β1 and miR-10b mimics induced
significant lamellipodia formation in U87 and U251 cells. This is
consistent with the aforementioned data that miR-10b possesses the
property to mediate TGF-β1-induced GBM cell migration and EMT.
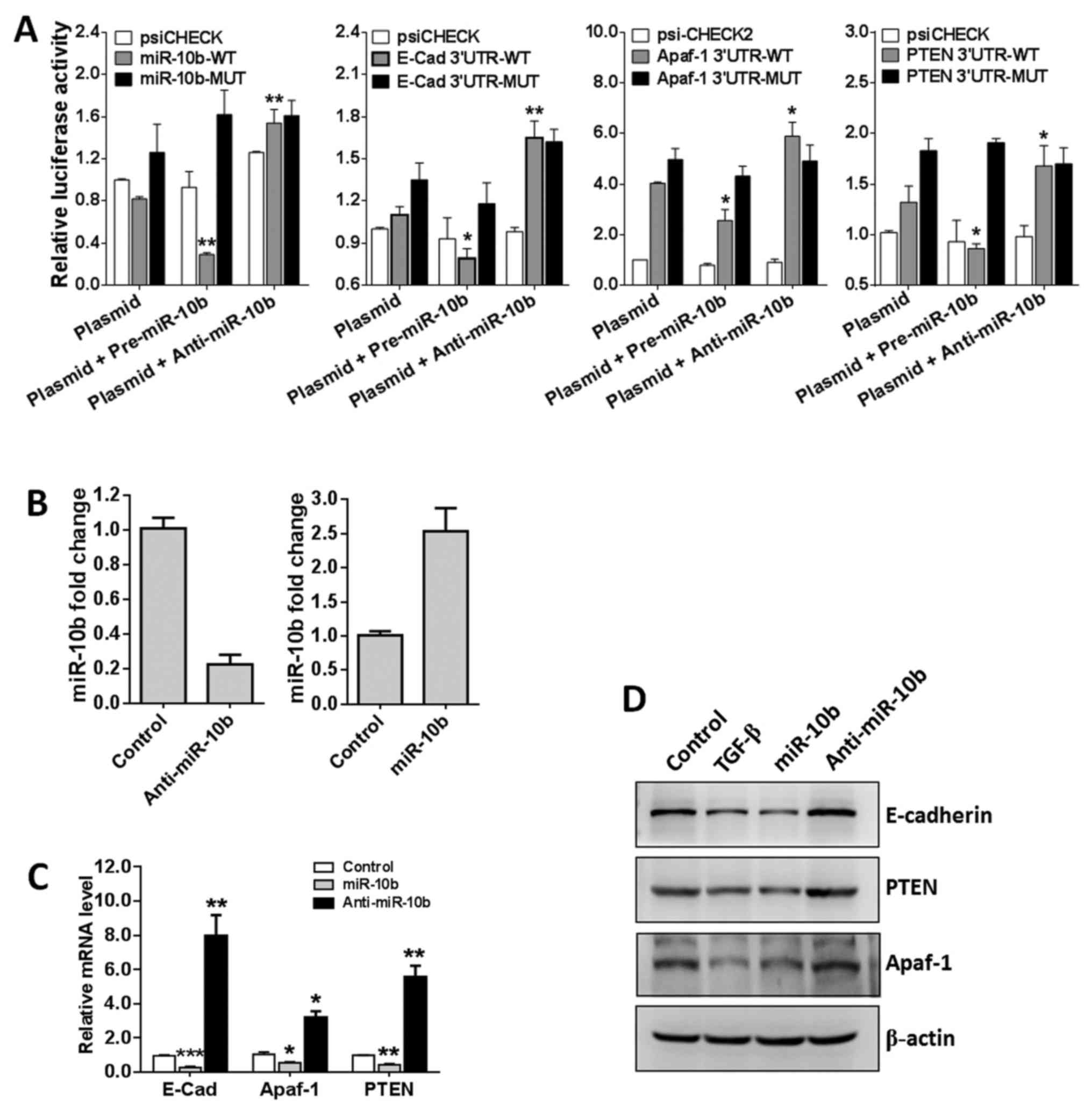
miR-10b targets E-cadherin, caspase-9,
Apaf-1 and PTEN
To investigate the pathological role of miR-10b in
GBM, miR-10b target genes were predicted using microRNA online
prediction software (http://www.microRNA.org). We focused on
proliferation-, invasion- and EMT-related genes and E-cadherin,
Apaf-1 and PTEN are screened as miR-10b candidate target genes for
further validation. E-cadherin is encoded by the CDH1 gene which
mediates cell-cell contact at the basolateral membrane and is a
hallmark of epithelial cells. Loss of E-cadherin is a marker of EMT
(29). Apaf-1 is a cofactor of
caspase-9 and can regulate mitochondria-mediated apoptosis
(30). PTEN is a specific tumor
suppressor gene which can regulate GBM cell growth, apoptosis,
adhesion, invasion and metastasis and has been used for GBM
prognosis evaluation (31). All
these genes are closely related with the proliferation, invasion
and EMT of various tumor cells.
To determine whether these genes are real target
genes of miR-10b in GBM cells, we constructed luciferase reporter
vectors containing the theoretical seed sequences in the 3′-UTR of
E-cadherin, Apaf-1 and PTEN as well as the corresponding mutant
vectors. All these endo-free vectors were transfected into HEK293T
cells, either alone or in combination with miR-10b precursor or
inhibitor. The luciferase activities were then analyzed 24 h later.
As shown in Fig. 6A, miR-10b
precursor remarkably suppressed the activity of RLuc containing the
seed sequences of E-cadherin, Apaf-1 and PTEN compared with the
empty vector group. In contrast, miR-10b inhibitor greatly
upregulates RLuc activity. As expected, when the mutant vectors
were transfected, neither miR-10b precursor nor inhibitor has
apparent effects on the RLuc activity. All these data demonstrated
that miR-10b specifically targets E-cadherin, Apaf-1 and PTEN.
To further confirm that miR-10b targets E-cadherin,
Apaf-1 and PTEN, U251 cells were transfected with miR-10b precursor
of inhibitor (Fig. 6B) and target
gene mRNA and proteins levels were detected. Results showed that
miR-10b precursor enforced expression of miR-10a markedly
repressed, whereas miR-10b inhibitor significantly promoted
E-cadherin, Apaf-1 and PTEN transcription (Fig. 6C). Finally, western blotting was
used to detect the protein levels of these genes and the data were
consistent with the mRNA levels (Fig.
6D). Collectively, these findings indicated that E-cadherin,
Apaf-1 and PTEN are specifically regulated by miR-10b in GBM cells
and TGF-β1 regulates these molecules at least partly via repressing
miR-10b.
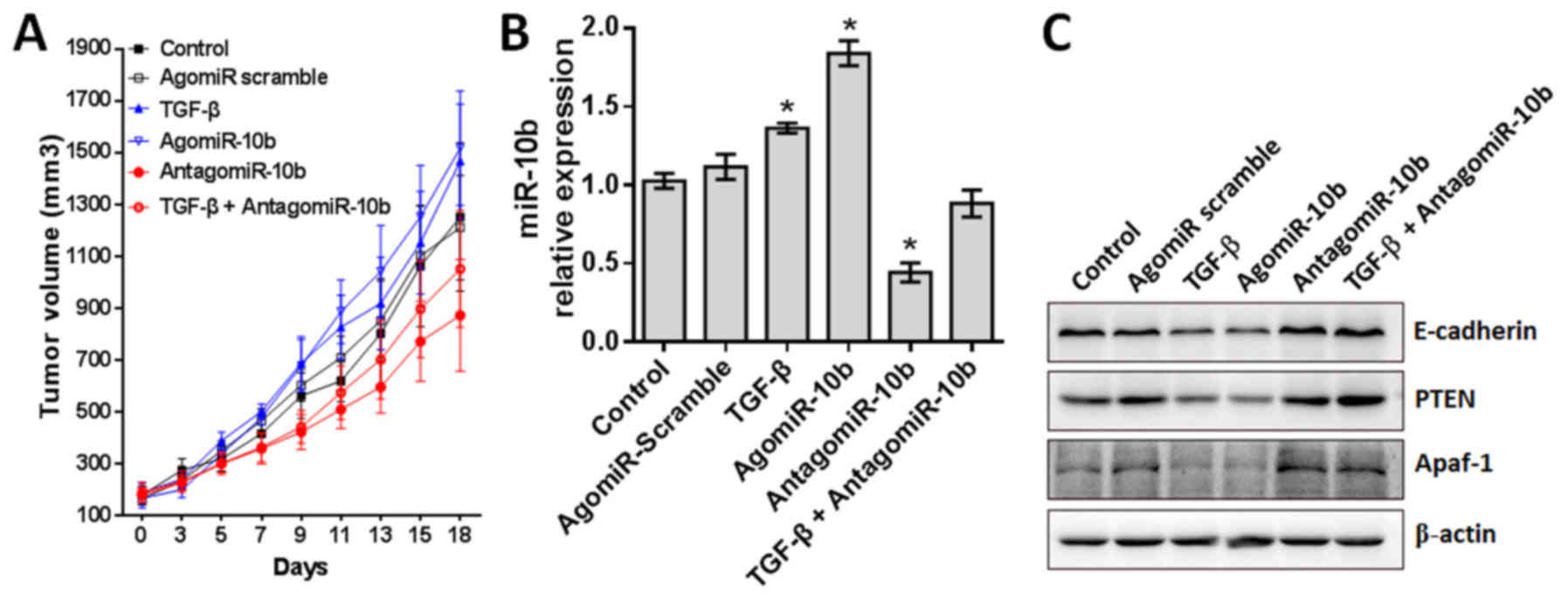
AntagomiR-10b enhances antitumor efficacy
in glioblastoma xenografts
Our in vitro studies proved that TGF-β1
regulates GBM cell proliferation, migration and EMT at least
partially through promoting miR-10b expression. Thus, miR-10b can
be used as a therapeutic target in GBM treatment. To evaluate these
effects in vivo, U87 cells were stably transfected with 400
nM agomiR-10b (mimics), antagomiR-10b (inhibitor) or agomir-control
(negative control) and subcutaneous xenografts were established by
subcutaneously injecting 2×106 wild-type or stably
transfected U87 cells (n=10 mice/group). Tumor growth was under
surveillance and tumor volumes were calculated with equation. As
indicated in Fig. 7A, treatment
with TGF-β1 or miR-10b agomir significantly promoted GBM tumor
growth, whereas the miR-10b antagomir remarkably inhibited tumor
growth, even in the presence of TGF-β1. Consistent with in
vitro results, the expression of miR-10b is increased in TGF-β1
or miR-10b agomir treated tumor tissues, and decreased in miR-10b
antagomir treated tumor tissues regardless of TGF-β1 treatment. As
expected, the expression of downstream target of miR-10b is also
consistent with the expression of miR-10b (Fig. 7B and C). Collectively, these data
positively support our in vitro data that miR-10b acts as a
key factor downstream of TGF-β1, contributing to GBM tumor
growth.
Discussion
GBM is the most common and aggressive cancer within
the brain and it represents ~15% of brain tumors. Although surgery,
chemotherapy and radiation have been used to treat GBM patients,
the high mobility and strong invasive properties of GBM result in a
high inevitable recurrence rate and a poor median survival for
patients (2,32). The most common survival is 12–15
months with only <3–5% of patients surviving more than 5 years
(32). Thus, there is a pressing
need to reveal the molecular mechanisms of GBM proliferation and
invasion for novel therapeutic avenue development.
TGF-β1 is a multifaceted cytokine that can regulate
proliferation, differentiation and other functions of various cell
types including T cell (33), B
cell (34,35), myeloid cell and tumor cells via
both Smad-dependent and Smad-independent signaling pathways
(36,37). TGF-β1 plays vital roles in
controlling tumor progression. On one hand, TGF-β1 can indirectly
promote tumor cell proliferation and metastasis through promoting
regulatory T cell (Treg) differentiation. The
CD4+Foxp3+Treg cells can hinder effective
host immune responses against cancer cells and abundant Treg cell
infiltration into tumors is usually associated with poor clinical
outcomes (38,39). On the other hand, TGF-β1 can
directly regulate tumor progression with different activities at
different developmental stages (40,41).
However, the broad spectrum expression and functional diversity of
TGF-β1, and sometimes even coexisting with complicated tumor
circumstances or other cytokines, make it difficult to clarify the
roles that TGF-β1 played in tumor development. As a result, the
data on TGF-β1 in various tumors are controversial and it has a
dual role as either a tumor suppressor or promoter depending on the
stages and types of the tumor (3,4).
Thus, further studies are still needed to elucidate the detailed
mechanisms and to clarify the universal rule and essence of TGF-β1
in GBM and other tumors.
As an oncogenic miRNA, miR-10b can regulate growth
and metastasis of various types of cancer (13–15).
miR-10b widely participated in the regulating of breast cancer
proliferation and metastasis (42,43).
However, miR-10b may play diverse roles in different tumors.
Accumulating evidence showed that miR-10b is increased in breast
cancers (43), but only reduced
expression of miR-10b was found in cervical cancer (44). The expression level of miR-10b in
colorectal cancer is paradoxical as reported by different groups
(45,46). All these data collectively
indicated that the activity of miR-10b is accurately tuned in
different tumors or the same tumor at different stages. Although
several target genes of miR-10b have been designated in GBM and
other tumors, their regulation appears cell- and context-specific
(13,16,17).
Thus, further studies are still needed to uncover the detailed
mechanisms underlying miR-10b functions in regulating GBM
progression.
Here, we investigated the role of miR-10b in
TGF-β1-mediated GBM proliferation, migration and EMT. We found that
miR-10b is apparently upregulated by TGF-β1 in U251 and U87 cells.
Further studies uncovered that TGF-β1 significantly promoted GBM
cell proliferation, migration and EMT. All these effects were
achieved through regulating miR-10b as miR-10b mimics promoted,
whereas miR-10b inhibitor reversed, the effects of TGF-β1 on U251
and U87 cell proliferation, migration and EMT. In addition, several
proliferation-, migration- and EMT-associated genes including
epithelial cadherin (E-cadherin), apoptotic protease activating
factor 1 (Apaf-1) and phosphatase and tensin homolog (PTEN) are the
targets of miR-10b. When xenograft models were used to investigate
the miR-10b potency as therapeutic target in vivo, results
showed that antagomiR directed against miR-10b remarkably
suppressed tumor progression. In summary, our data collectively
demonstrated that TGF-β1 functions in GBM at least partially trough
regulating miR-10b expression and our findings provide a rationale
for targeting TGF-β1 or miR-10b for the treatment of GBM.
Taken together, our data demonstrated the mechanism
of TGF-β1 and miR-10b in regulating GBM cell proliferation,
migration and EMT. TGF-β1 promotes miR-10b expression and the
latter molecule then regulates GBM cell proliferation, migration
and EMT through suppressing its downstream target genes E-cadherin,
Apaf-1 and PTEN. Modulation of TGF-β1 and miR-10b may effectively
regulate these tumor-associated genes and both TGF-β1 and miR-10b
can be candidate targets for GBM treatment.
Acknowledgments
The present study was supported by grants from the
Natural Science Fund of China (nos. 81301884, 81401883 and
81302173), the Science and Technology Department of Jilin Province
(nos. 20140520036JH, 20160414052GH and 20160101053JC), the Bethune
Project Plan B of Jilin University (no. 450060521279) and the First
Prize of China Postdoctoral Science Foundation (no.
2016M590264).
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Otsuki N, Konno T, Kurahashi T, Suzuki S,
Lee J, Okada F, Iuchi Y, Homma T and Fujii J: The SOD1 transgene
expressed in erythroid cells alleviates fatal phenotype in congenic
NZB/NZW-F1 mice. Free Radic Res. 50:793–800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie LP, Chen QX, Huang H, Liu XD, Chen HT
and Zhang RQ: Inhibitory effects of cupferron on the monophenolase
and diphenolase activity of mushroom tyrosinase. Int J Biochem Cell
Biol. 35:1658–1666. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown KA, Aakre ME, Gorska AE, Price JO,
Eltom SE, Pietenpol JA and Moses HL: Induction by transforming
growth factor-beta1 of epithelial to mesenchymal transition is a
rare event in vitro. Breast Cancer Res. 6:R215–R231. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei F, Liu Y, Guo Y, Xiang A, Wang G, Xue
X and Lu Z: miR-99b-targeted mTOR induction contributes to
irradiation resistance in pancreatic cancer. Mol Cancer. 12:812013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mu N, Gu J, Huang T, Zhang C, Shu Z, Li M,
Hao Q, Li W, Zhang W, Zhao J, et al: A novel NF-κB/YY1/microRNA-10a
regulatory circuit in fibroblast-like synoviocytes regulates
inflammation in rheumatoid arthritis. Sci Rep. 6:200592016.
View Article : Google Scholar
|
|
9
|
Wu W, He C, Liu C, Cao AT, Xue X,
Evans-Marin HL, Sun M, Fang L, Yao S, Pinchuk IV, et al: miR-10a
inhibits dendritic cell activation and Th1/Th17 cell immune
responses in IBD. Gut. 64:1755–1764. 2015. View Article : Google Scholar
|
|
10
|
Nikaki A, Piperi C and Papavassiliou AG:
Role of microRNAs in gliomagenesis: Targeting miRNAs in
glioblastoma multiforme therapy. Expert Opin Investig Drugs.
21:1475–1488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Costa PM, Cardoso AL, Mano M and de Lima
MC: MicroRNAs in glioblastoma: Role in pathogenesis and
opportunities for targeted therapies. CNS Neurol Disord Drug
Targets. 14:222–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang W, Yu H, Shen Y, Liu Y, Yang Z and
Sun T: MiR-146b-5p overexpression attenuates stemness and
radioresistance of glioma stem cells by targeting
HuR/lincRNA-p21/β-catenin pathway. Oncotarget. 7:41505–41526.
2016.PubMed/NCBI
|
|
13
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakata K, Ohuchida K, Mizumoto K,
Kayashima T, Ikenaga N, Sakai H, Lin C, Fujita H, Otsuka T, Aishima
S, et al: MicroRNA-10b is overexpressed in pancreatic cancer,
promotes its invasiveness, and correlates with a poor prognosis.
Surgery. 150:916–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mussnich P, D'Angelo D, Leone V, Croce CM
and Fusco A: The High Mobility Group A proteins contribute to
thyroid cell transformation by regulating miR-603 and miR-10b
expression. Mol Oncol. 7:531–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gabriely G, Yi M, Narayan RS, Niers JM,
Wurdinger T, Imitola J, Ligon KL, Kesari S, Esau C, Stephens RM, et
al: Human glioma growth is controlled by microRNA-10b. Cancer Res.
71:3563–3572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma L, Reinhardt F, Pan E, Soutschek J,
Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW and Weinberg RA:
Therapeutic silencing of miR-10b inhibits metastasis in a mouse
mammary tumor model. Nat Biotechnol. 28:341–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teplyuk NM, Uhlmann EJ, Wong AH, Karmali
P, Basu M, Gabriely G, Jain A, Wang Y, Chiocca EA, Stephens R, et
al: MicroRNA-10b inhibition reduces E2F1-mediated transcription and
miR-15/16 activity in glioblastoma. Oncotarget. 6:3770–3783. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teplyuk NM, Uhlmann EJ, Gabriely G,
Volfovsky N, Wang Y, Teng J, Karmali P, Marcusson E, Peter M, Mohan
A, et al: Therapeutic potential of targeting microRNA-10b in
established intracranial glioblastoma: First steps toward the
clinic. EMBO Mol Med. 8:268–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gabriely G, Teplyuk NM and Krichevsky AM:
Context effect: microRNA-10b in cancer cell proliferation, spread
and death. Autophagy. 7:1384–1386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang M, Kleber S, Röhrich M, Timke C, Han
N, Tuettenberg J, Martin-Villalba A, Debus J, Peschke P, Wirkner U,
et al: Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor
LY2109761 enhances radiation response and prolongs survival in
glioblastoma. Cancer Res. 71:7155–7167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Joseph JV, Conroy S, Tomar T,
Eggens-Meijer E, Bhat K, Copray S, Walenkamp AM, Boddeke E,
Balasubramanyian V, Wagemakers M, et al: TGF-β is an inducer of
ZEB1-dependent mesenchymal transdifferentiation in glioblastoma
that is associated with tumor invasion. Cell Death Dis.
5:e14432014. View Article : Google Scholar
|
|
23
|
Duan R, Han L, Wang Q, Wei J, Chen L,
Zhang J, Kang C and Wang L: HOXA13 is a potential GBM diagnostic
marker and promotes glioma invasion by activating the Wnt and TGF-β
pathways. Oncotarget. 6:27778–27793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lv S, Qin J, Yi R, Coreman M, Shi R, Kang
H and Yao C: CrkL efficiently mediates cell proliferation,
migration, and invasion induced by TGF-β pathway in glioblastoma. J
Mol Neurosci. 51:1046–1051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiong M, Jiang L, Zhou Y, Qiu W, Fang L,
Tan R, Wen P and Yang J: The miR-200 family regulates
TGF-β1-induced renal tubular epithelial to mesenchymal transition
through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J
Physiol Renal Physiol. 302:F369–F379. 2012. View Article : Google Scholar
|
|
27
|
Hardee ME, Marciscano AE, Medina-Ramirez
CM, Zagzag D, Narayana A, Lonning SM and Barcellos-Hoff MH:
Resistance of glioblastoma-initiating cells to radiation mediated
by the tumor microenvironment can be abolished by inhibiting
transforming growth factor-β. Cancer Res. 72:4119–4129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joseph JV, Balasubramaniyan V, Walenkamp A
and Kruyt FAE: TGF-β as a therapeutic target in high grade gliomas
- promises and challenges. Biochem Pharmacol. 85:478–485. 2013.
View Article : Google Scholar
|
|
29
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soengas MS, Alarcón RM, Yoshida H, Giaccia
AJ, Hakem R, Mak TW and Lowe SW: Apaf-1 and caspase-9 in
p53-dependent apoptosis and tumor inhibition. Science. 284:156–159.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fang M, Zhong XY, Du B, Lin CL, Luo F,
Tang LJ and Chen J: Role of DJ-1-induced PTEN down-regulation in
migration and invasion of human glioma cells. Chin J Cancer.
29:988–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gallego O: Nonsurgical treatment of
recurrent glioblastoma. Curr Oncol. 22:e273–e281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wahl SM, Hunt DA, Wong HL, Dougherty S,
McCartney-Francis N, Wahl LM, Ellingsworth L, Schmidt JA, Hall G,
Roberts AB, et al: Transforming growth factor-beta is a potent
immunosuppressive agent that inhibits IL-1-dependent lymphocyte
proliferation. J Immunol. 140:3026–3032. 1988.PubMed/NCBI
|
|
34
|
Letterio JJ and Roberts AB: Regulation of
immune responses by TGF-beta. Annu Rev Immunol. 16:137–161. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kehrl JH, Thevenin C, Rieckmann P and
Fauci AS: Transforming growth factor-beta suppresses human B
lymphocyte Ig production by inhibiting synthesis and the switch
from the membrane form to the secreted form of Ig mRNA. J Immunol.
146:4016–4023. 1991.PubMed/NCBI
|
|
36
|
Li MO, Wan YY, Sanjabi S, Robertson AK and
Flavell RA: Transforming growth factor-beta regulation of immune
responses. Annu Rev Immunol. 24:99–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qin H, Wang L, Feng T, Elson CO, Niyongere
SA, Lee SJ, Reynolds SL, Weaver CT, Roarty K, Serra R, et al:
TGF-beta promotes Th17 cell development through inhibition of
SOCS3. J Immunol. 183:97–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
deLeeuw RJ, Kost SE, Kakal JA and Nelson
BH: The prognostic value of FoxP3+ tumor-infiltrating
lymphocytes in cancer: A critical review of the literature. Clin
Cancer Res. 18:3022–3029. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang Y, Woosley AN, Sivalingam N,
Natarajan S and Howe PH: Cathepsin-B-mediated cleavage of
disabled-2 regulates TGF-β-induced autophagy. Nat Cell Biol.
18:851–863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Akhurst RJ and Derynck R: TGF-beta
signaling in cancer - a double-edged sword. Trends Cell Biol.
11:S44–S51. 2001.PubMed/NCBI
|
|
42
|
Knirsh R, Ben-Dror I, Modai S, Shomron N
and Vardimon L: MicroRNA 10b promotes abnormal expression of the
proto-oncogene c-Jun in metastatic breast cancer cells. Oncotarget.
7:59932–59944. 2016.PubMed/NCBI
|
|
43
|
Bahena-Ocampo I, Espinosa M,
Ceballos-Cancino G, Lizarraga F, Campos-Arroyo D, Schwarz A,
Garcia-Lopez P, Maldonado V and Melendez-Zajgla J: miR-10b
expression in breast cancer stem cells supports self-renewal
through negative PTEN regulation and sustained AKT activation. EMBO
Rep. 17:10812016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zou D, Zhou Q, Wang D, Guan L, Yuan L and
Li S: The Downregulation of MicroRNA-10b and its role in cervical
cancer. Oncol Res. 24:99–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vychytilova-Faltejskova P, Pesta M, Radova
L, Liska V, Daum O, Kala Z, Svoboda M, Kiss I and Slaby O:
Genome-wide microRNA expression profiling in primary tumors and
matched liver metastasis of patients with colorectal cancer. Cancer
Genomics Proteomics. 13:311–316. 2016.PubMed/NCBI
|
|
46
|
Wang Y, Li Z, Zhao X, Zuo X and Peng Z:
miR-10b promotes invasion by targeting HOXD10 in colorectal cancer.
Oncol Lett. 12:488–494. 2016.PubMed/NCBI
|