Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney cancer with approximately 62,700 new RCC cases diagnosed
and 14,240 new mortalities in the United States in 2016, accounting
for approximately 2–3% of all adult malignancies (1). Clear cell renal cell carcinoma
(ccRCC), as the most common histological subtype of RCC, represents
approximately 85% of all primary renal neoplasms (2). Due to lack of early warning signs and
effective treatments for patients with advanced disease,
approximately 20–40% patients were found metastasis at the time of
diagnosis and the 5-year survival rate of RCC is approximately 55%
(1,3,4).
However, the accurate mechanism of the RCC progression remains
unclear. Therefore, there is an urgent need to improve the
understanding of tumor biology in RCC and identify more effective
and highly selective potential therapeutic targets for RCC
treatment.
Annexins, as predominantly cytosolic soluble
proteins, are classified into five families, including vertebrates,
invertebrates, fungi and some groups of unicellular eukaryotes,
plants and protists. Annexins can reversibly bind to negatively
charged phospholipids in a Ca2+ regulated manner
(5). Twelve Annexins common to
vertebrates are known as Annexins A1–A11 and A13 (5,6). The
non-glycosylated phospholipid binding protein, Annexin A5 is
composed of 319 amino acid residues with a molecular mass of ~35.7
kDa (7–9). It is likely that Annexin A5 has a
very short unphosphorylated N-terminus compared with other
Annexins, contributing to a series of functions, such as cell
proliferation and invasion (6,10),
signal transduction (11,12), and anticoagulation (13). Previous studies have reported that
Annexin A5 promoted tumorigenesis and progression in a variety of
cancers, including hepatocarcinoma, colorectal cancer and breast
cancer (14,15). However, the possible relationship
between Annexin A5 and RCC is not clear. Therefore, we analyzed the
expression level of Annexin A5 in RCC tissue samples compared with
normal renal tissue samples and explored its potential biomedical
functions on RCC cell invasion to confirm whether Annexin A5 is a
new molecular biomarker of RCC.
Materials and methods
Patients and RCC samples
All primary RCC and pericarcinous tissues were
obtained from 123 patients with appropriate informed consent at the
Department of Urology of the First Affiliated Hospital of Nanjing
Medical University from February 2008 to August 2011. The follow-up
deadline was January 2016. The specimens were assessed by
immunohistochemistry and the diagnosis was verified by
histopathological examination. The study was approved by the
Institutional Research Ethics Committee of the First Affiliated
Hospital of Nanjing Medical University.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cultured cell lines and
clinical samples using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA) and cDNA was synthesized using Primescript RT Reagent (Takara,
Otsu, Japan) according to the manufacturer's instructions. The
qRT-PCR was performed by using StepOne Plus Real-time PCR system
(Applied Biosystems, Foster City, CA, USA) with SYBR® Premix Ex
Taq™ Reagent (Takara). The following primers were used for qRT-PCR:
Annexin A5, forward: 5′-AGCGGGCTGATGCAGAAAC-3′, reverse:
5′-ACTTCGGGATGTCAACAGAGT-3′; β-actin, forward:
5′-CCTGGCACCCAGCACAAT-3′, reverse: 5′-GCTGATCCACATCTGCTGGAA-3′.
Data analysis was performed with ABI Step One Software version 2.1
and the relative mRNA level was calculated using 2−ΔΔCt
method.
Western blotting
Cells or frozen tissues were lysed in cell lysis
buffer for 30 min on ice and centrifuged at 14,000 × g at 4°C for
15 min. The total protein concentration was calculated by the BCA
Protein Assay kit (Pierce, Rockford, IL, USA). Proteins were
separated by 10% SDS-PAGE gel and transferred onto a polyvinylidene
difluoride membrane (Millipore, Billerica, MA, USA). Western blot
analysis followed a standard procedure. The primary antibody
Annexin A5 was obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). N-cadherin, vimentin, β-catenin, MMP2, MMP9, PI3K,
phospho-PI3K, AKT, phospho-AKT, mTOR, phospho-mTOR were obtained
from Cell Signaling Technology, Danvers, MA, USA. The anti-mouse
and anti-rabbit secondary antibodies were also from Cell Signaling
Technology.
Cell lines and reagents
The human renal cancer cell lines (Caki-1, Caki-2,
ACHN, 769P) and normal epithelial cells of renal tubule (HK2) were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). The cells were cultured in McCoy's 5A or DMEM media
supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA)
and 1% penicillin/streptomycin (Invitrogen) at 5% CO2
and 37°C incubator. LY294002, a phosphatidylinositol 3-kinase
(PI3K) inhibitor, was obtained from Selleck Chemical (Houston, TX,
USA) (no. S1105).
Transfection
Lentivirus packaging cells were transfected with
LV3-pGLV-h1-GFP-puro vector (GenePharma, Shanghai, China)
containing either the Annexin A5 knockdown (shA5-1 and shA5-2) or
Annexin A5 overexpression (A5) and a negative control sequence
(NC), respectively. Lentiviral transduction was performed in Caki-1
and Caki-2 cell lines. Pools of stable transductants were generated
by selection using puromycin (4 μg/ml) for 2 weeks.
Cell proliferation assay
A Cell Counting Kit-8 assay (Dojindo Laboratories,
Kumamoto, Japan) was used to estimate the proliferation potential.
Cells were seeded in 96-well plates with 3000 cells/well. CCK-8
reagents were added into wells after cells grew for 1, 2, 3, and 4
days, respectively, and the absorbance was measured at 450 nm using
a micro-plate reader at 2 h after CCK-8 addition.
Colony formation assay
Cells were seeded into 6-well plates (600
cells/well) and cultured in media containing 10% FBS for 2 weeks.
Then the colonies were fixed with paraform and stained with 0.1%
crystal violet. The colonies were counted and each group were
repeated three times.
Transwell cell migration and invasion
assay
Cell migration or invasion assay were performed
using a 24-well Transwell chamber (Costar, Corning, NY, USA) with
or without Matrigel (Invitrogen). Cells (2×104) were
suspended in serum-free medium and seeded into the upper chambers
which were inserted in the 24-well plate. Medium containing 10% FBS
was added to the lower chamber. Cells were incubated at 37°C for 48
h and then cells on the surface of the upper chambers that did not
migrate through the pores were removed with a cotton swab. Cells
which migrated to the bottom surface of the chamber were stained
with 0.1% crystal violet. Numbers of migratory and invasive cells
were counted in five randomly selected fields and the presented
data represent three individual experiments.
Xenograft studies
Mouse studies were approved by the Animal Research
Ethics Committee of Nanjing Medical University. The 5-week-old
female nude mice were randomly divided into two groups consisting
of five mice each. The stable cells (7×106) shA5-Caki-1
and the control cells (NC-Caki-1) were suspended in 150 μl
PBS and injected subcutaneously into the flank of each mouse. Tumor
size was calculated (length × width2 ×0.52) once a week.
After 6 weeks, tumors were removed, weighed, fixed, and embedded
for immunohistochemical staining.
Immunohistochemistry
Immunohistochemistry was performed on tissue
microarray to evaluate Annexin A5 protein expression. Tissue
microarray was incubated with a primary antibody against Annexin A5
at 4°C overnight and incubated with HRP conjugated secondary
antibody followed by DAB staining. Immunohistochemistry staining
was assessed by two experienced pathologists. To evaluate the
expression of Annexin A5 in RCC tissues, a semi-quantitative
scoring system (0–3) was used based on the staining intensity of
the tumor tissue: 0, negative; 1, weak positive; 2, moderate
positive; 3, strong positive. Annexin A5 high expresion refers to
scores 2–3 and Annexin A5 low expresion refers to scores 0–1. For
assessing the association of Annexin A5 expression with
clinicopathological characteristics of the RCC patients, following
parameters were included: age (≤60 and >60), gender, tumor size
(≤4 and >4), Histology (clear cell carcinoma and others),
histological stage (grades I, II, III, and IV) and tumor stage (TNM
stages I, II, III, and IV).
Statistical analysis
Statistical analyses were performed with SPSS 22.0
software. All the data were presented as the mean ± SD from three
independent experiments. Student's t-test and the Chi-square test
were used to analyze the differences between groups and survival
curves were drawn by the Kaplan-Meier method. P<0.05 was
considered to indicate a statistical significant difference.
Results
Annexin A5 is highly expressed in RCC
tissues and cell lines
To explore the protein and mRNA expression of
Annexin A5 in RCC cell lines, western blot and qRT-PCR were
performed in four human RCC cell lines (Caki-1, Caki-2, ACHN and
769P) and one normal epithelial cell of renal tubule (HK2). As
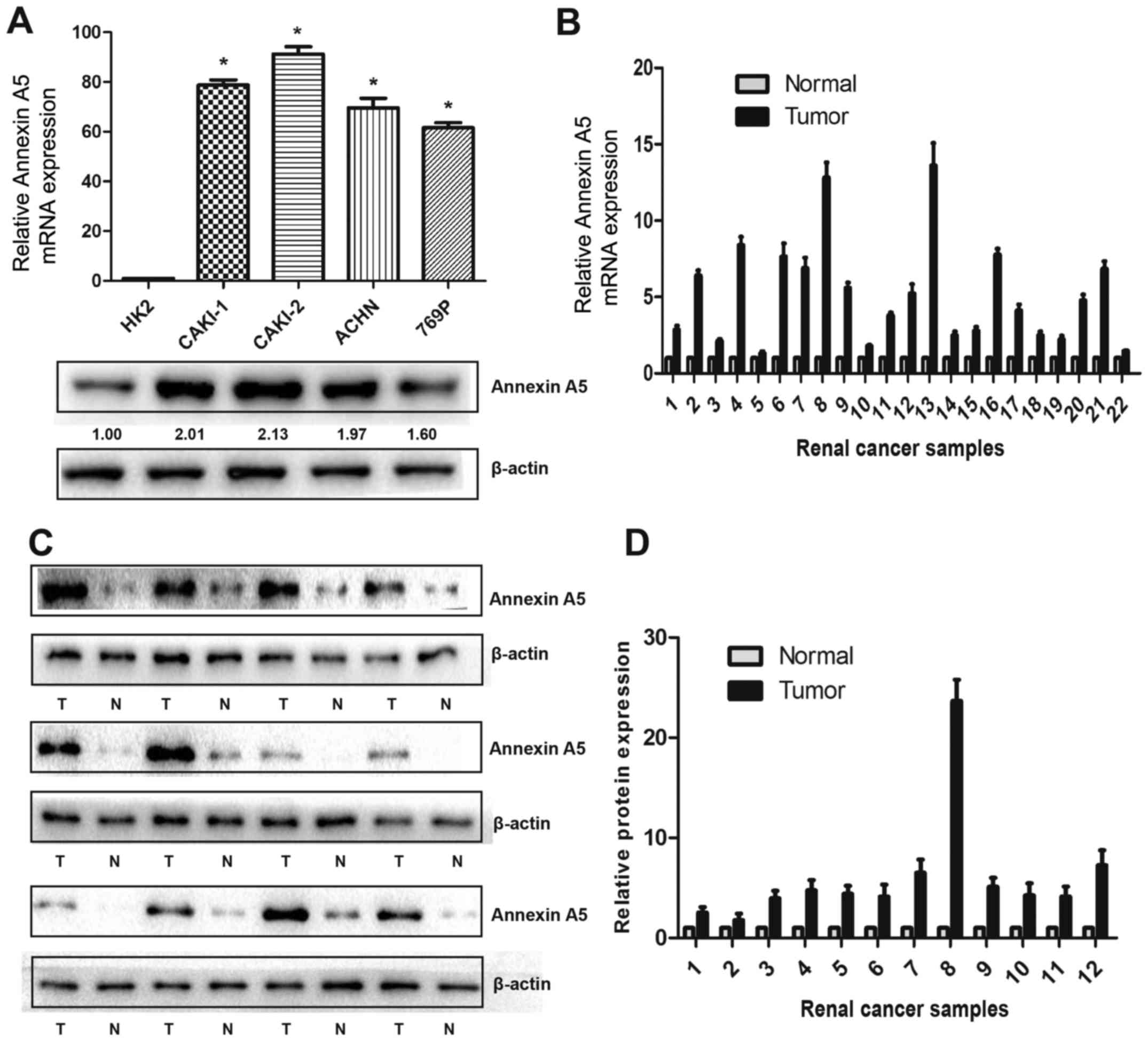
shown in Fig. 1A, Annexin A5 mRNA
was upregulated by 61.2- to 92.4-fold in all RCC cell lines
compared to HK2 and western blot analysis showed similar results.
To assess whether Annexin A5 was also highly expressed in RCC
tissues, 22 pairs of RCC tissues and matched adjacent non-cancerous
tissue were selected for qRT-PCR and western blot, the results
suggested that Annexin A5 expression was markedly upregulated in
both protein and mRNA level compared to the adjacent tissue
(Fig. 1B–D).
Annexin A5 expression is correlated with
clinical stage, histological grade and overall survival in RCC
patients
To validate the association between Annexin A5
expression and clinicopathologic features, 123 cases of RCC were
evaluated by IHC. Patients were divided into a low expression group
(n=56) and a high expression group (n=67) according to the Annexin
A5 expression. As shown in Table
I, although Annexin A5 expression and age (P=0.628), gender
(P=0.576), tumor size (P=0.192) or tumor histology (P=0.637) did
not significantly correlate, Annexin A5 exhibited a signifcantly
positive correlation with histological grade (P=0.007) and TNM
stage (P=0.013). High expression group was correlated with higher
histological grade and TNM stage compared with the low expression
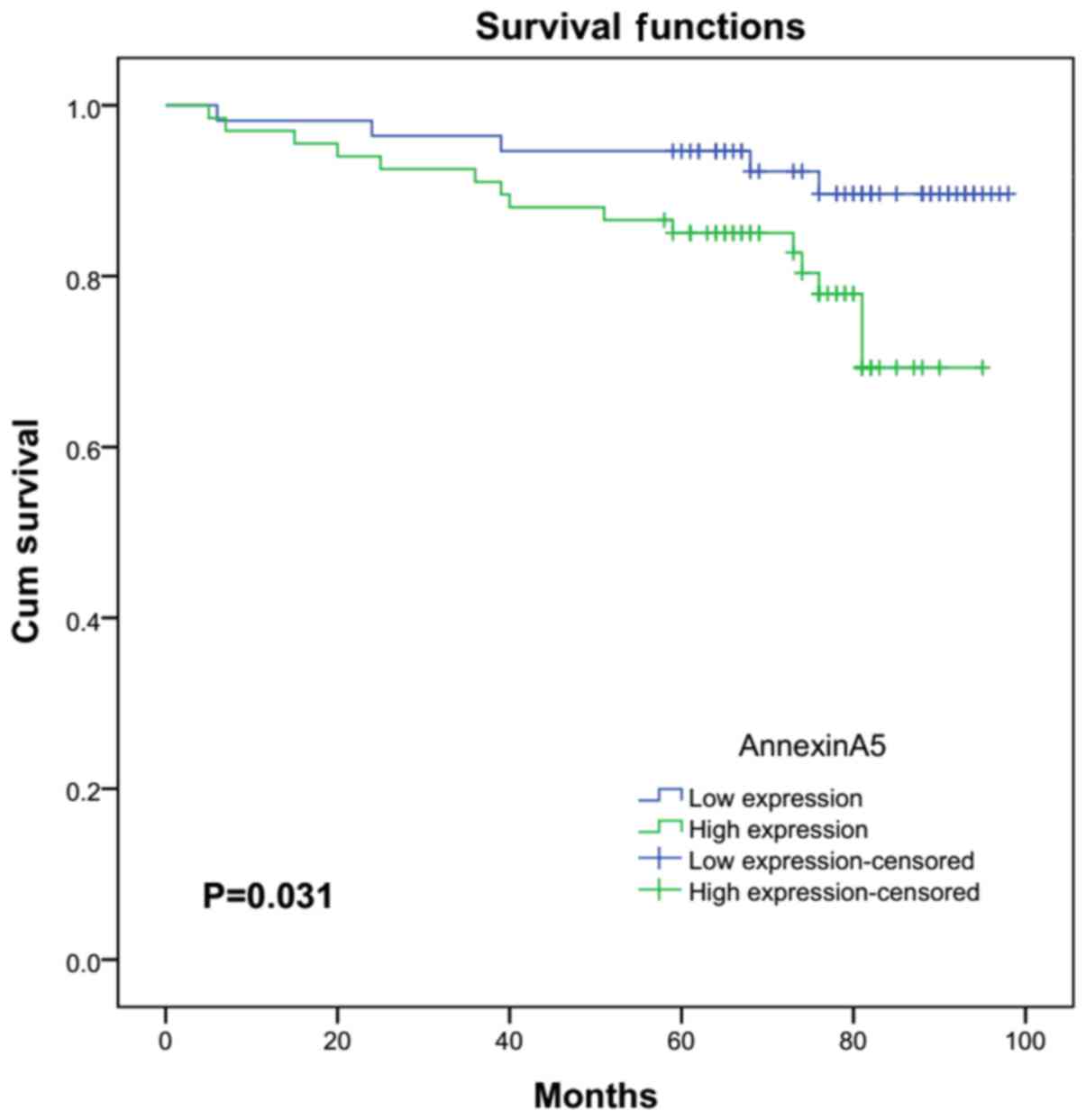
group. As shown in Fig. 2, RCC
patients with high Annexin A5 expression had a significantly
shorter overall survival time than those with low Annexin A5
expression (P=0.031). These data suggested that the upregulated
expression of Annexin A5 might have an important role during RCC
progression.
 | Table IAssociation of Annexin A5 expression
with clinicopathological characteristics of the renal cancer
patients. |
Table I
Association of Annexin A5 expression
with clinicopathological characteristics of the renal cancer
patients.
| Parameters | No. of cases (%) | Annexin A5 expression
| P-value |
|---|
| Low (%) | High (%) |
|---|
| Age (years) | 0.628 |
| ≤60 | 74 (60.2) | 35 (47.3) | 39 (52.7) | |
| >60 | 49 (39.8) | 21 (42.9) | 28 (57.1) | |
| Gender | 0.576 |
| Male | 78 (63.4) | 37 (47.4) | 41 (52.6) | |
| Female | 45 (36.6) | 19 (42.2) | 26 (57.8) | |
| Tumor size
(cm) | 0.192 |
| ≤4 | 58 (47.2) | 30 (51.7) | 28 (48.3) | |
| >4 | 65 (52.8) | 26 (40.0) | 39 (60.0) | |
| Histology | 0.637 |
| Clear cell
carcinoma | 115 (93.5) | 53 (46.1) | 62 (53.9) | |
| Others | 8 (6.5) | 3 (37.5) | 5 (62.5) | |
| Histological
grade | 0.007 |
| I–II | 102 (82.9) | 52 (51.0) | 50 (49.0) | |
| III–IV | 21 (17.1) | 4 (19.0) | 17 (81.0) | |
| TNM stage | 0.013 |
| I | 95 (77.2) | 49 (51.6) | 46 (48.4) | |
| II–IV | 28 (22.8) | 7 (25.0) | 21 (75.0) | |
Annexin A5 promotes cell proliferation in
vitro
To further investigate the functions of Annexin A5
in RCC cell lines, we transfected Caki-1 and Caki-2 cells with
lentivirus to silence or overexpress the expression of Annexin A5.
The silenced cells were named as shA5-1 and shA5-2 and the
overexpressed cell lines were called A5, while the matched control
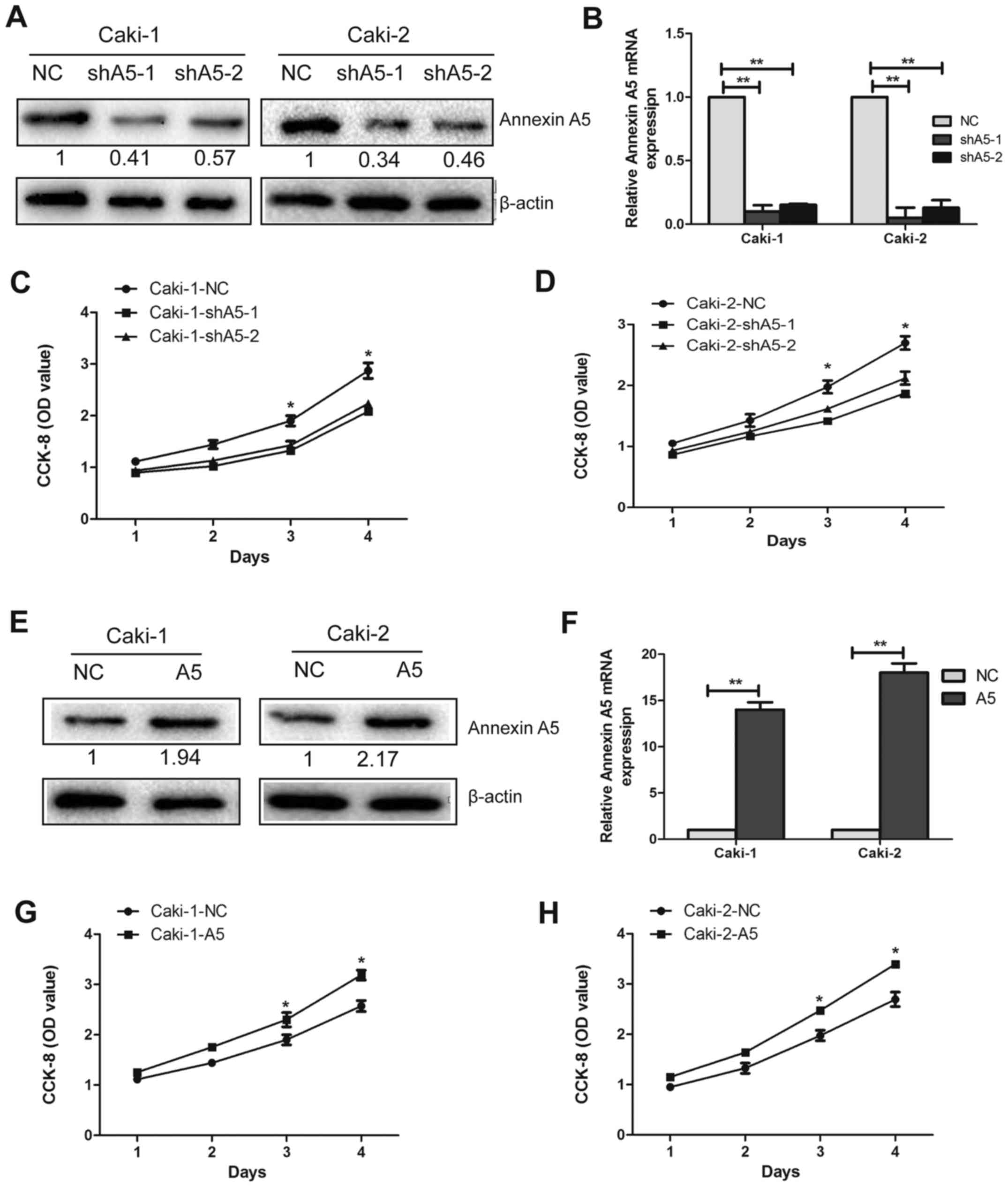
cells were named as NC. The expression levels were confirmed by
western blotting (Fig. 3A and E)
and qRT-PCR (Fig. 3B and F,
P<0.01).
The growth curve analysis demonstrated that the
downregulation of Annexin A5 in Caki-1 and Caki-2 cells
significantly inhibited cell growth compared with the control cells
(Fig. 3C and D, P<0.05).
Whereas, upregulated Annexin A5 expression enhanced cell growth
markedly in Caki-1 and Caki-2 cells (Fig. 3G and H, P<0.05). In addition, a
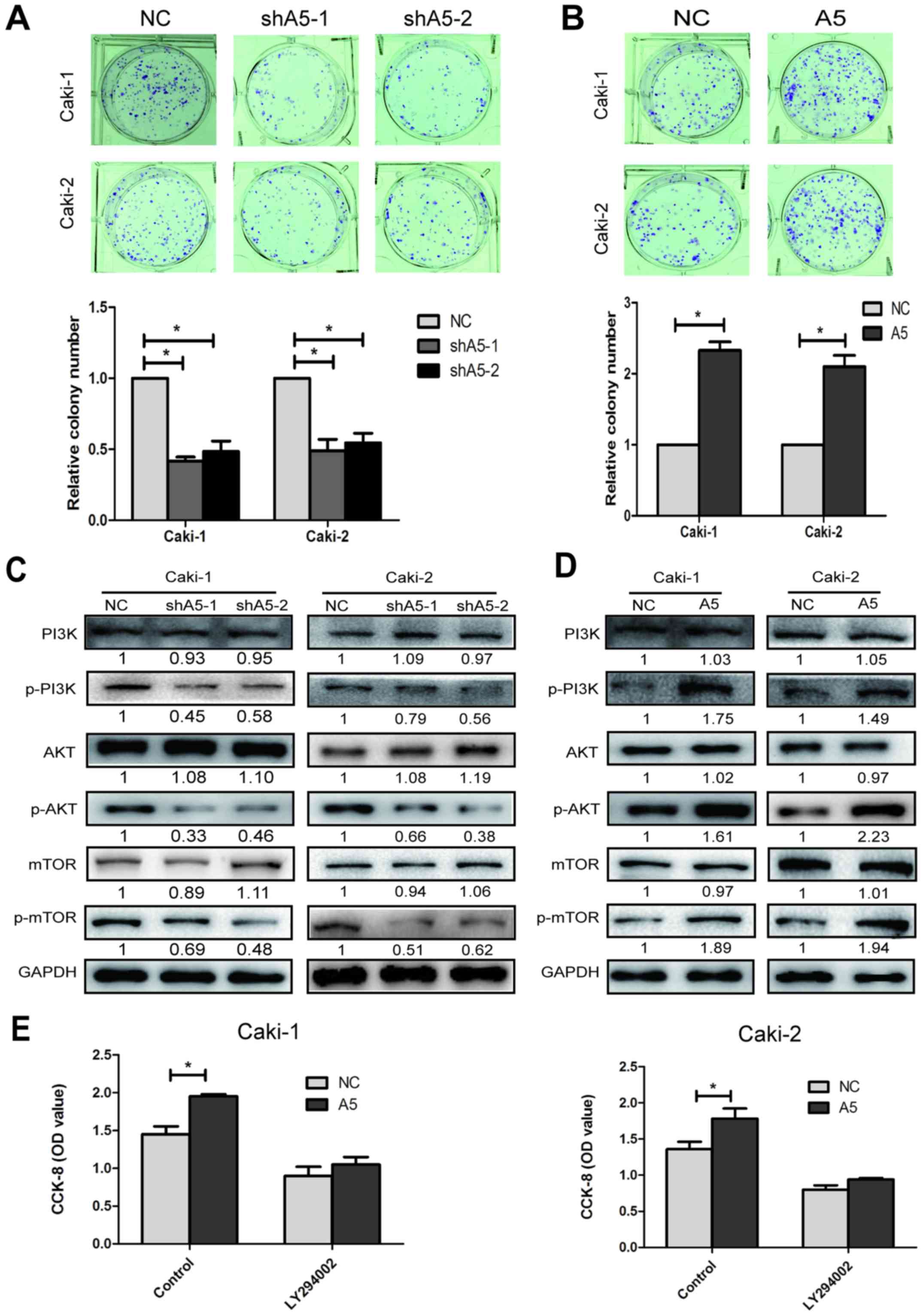
colony formation assay was also performed to further investigate
the effect of Annexin A5 on the proliferation. The results
indicated that Annexin A5 knockdown contributed significantly to
decrease cell colony formation efficiency, whereas inducing Annexin
A5 expression markedly enhanced the ability of Caki-1 and Caki-2
cells to form colonies (Fig. 4A and
B, P<0.05).
To further study the mechanism by which Annexin A5
overexpression or knockdown affected proliferation, we investigated
the effects of Annexin A5 overexpression or knockdown on the
PI3K/AKT/mTOR pathway. Western blot analysis suggested that the
levels of phospho-PI3K, phospho-AKT and phospho-mTOR decreased in
the Annexin A5 knockdown cells, while increased in the Annexin A5
over-expressed cells. However, the total levels of PI3K, AKT and
mTOR had no obvious change in Annexin A5 overexpression or
knockdown cells (Fig. 4C and D,
P<0.05).
To further confirm our hypothesis, we used PI3K
inhibitor (LY294002) at a dose of 20 μM to observe the role
of PI3K/AKT/mTOR pathway in Annexin A5-regulated cell
proliferation. The dose of LY294002 selected was the dose of
IC50 to Caki-1 cells. After treated with LY294002 for 48
h, Caki-1 and Caki-2 with Annexin A5 overexpression (A5) and
control cells (NC) were used for CCK8 analysis. As shown in
Fig. 4E, Annexin A5 overexpression
increased the cell proliferation while LY294002 reversed the cell
growth promoting trend by Annexin A5. Overall, these results
suggested that Annexin A5 positively regulated cell proliferation
in RCC and might be mediated by the phosphorylation of
PI3K/AKT/mTOR pathway.
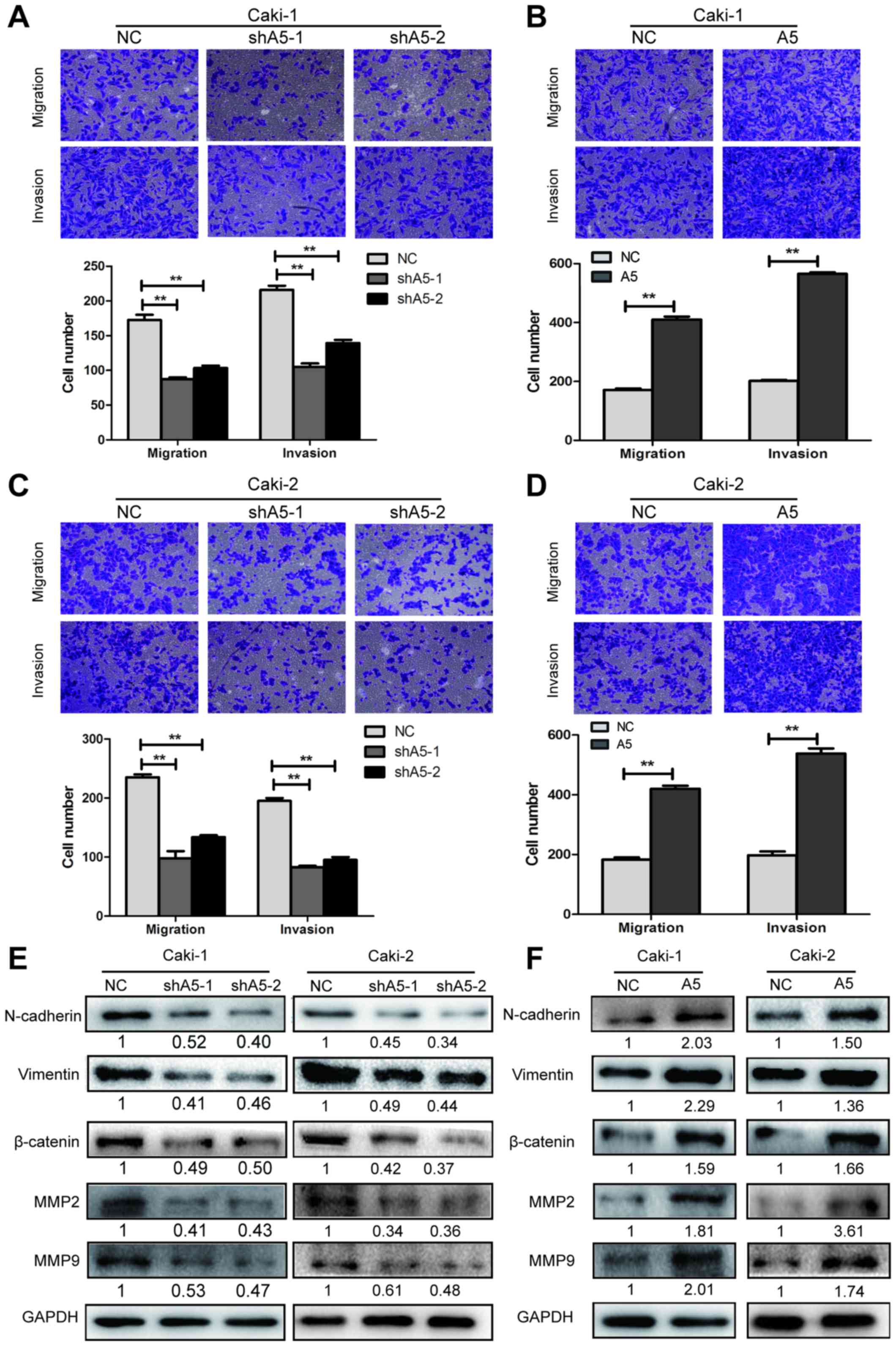
Annexin A5 promotes cell migratory and
invasive potential
To assess the potential role of Annexin A5 in
regulating the migration and invasion ability of RCC cells,
Transwell migration and invasion assays were performed in Caki-1
and Caki-2 cells with Annexin A5 overexpression or knockdown.
Transwell migration assays revealed that Annexin A5 knockdown
reduced the migration capability of Caki-1 and Caki-2 cells and
overexpressing Annexin A5 signifcantly increased the ability of
migration. The Transwell invasion assays showed similar results
(Fig. 5A–D, P<0.01).
To further explore the mechanism by which Annexin A5
affects cell migration and invasion, western blotting was performed
to determine the expression levels of N-cadherin, vimentin,
β-catenin, MMP2 and MMP9, which are related to regulating the cell
adhesion and metastasis. The results demonstrated that the
expression of these proteins was significantly downregulated in
Annexin A5 knockdown cells while they were upregulated in Annexin
A5 overexpressing cells (Fig. 5E and
F). These data suggested that Annexin A5 could promote
migration and invasion potential in RCC cells and the underlying
mechanism might be the upregulation of adhesion and
metastasis-related molecules.
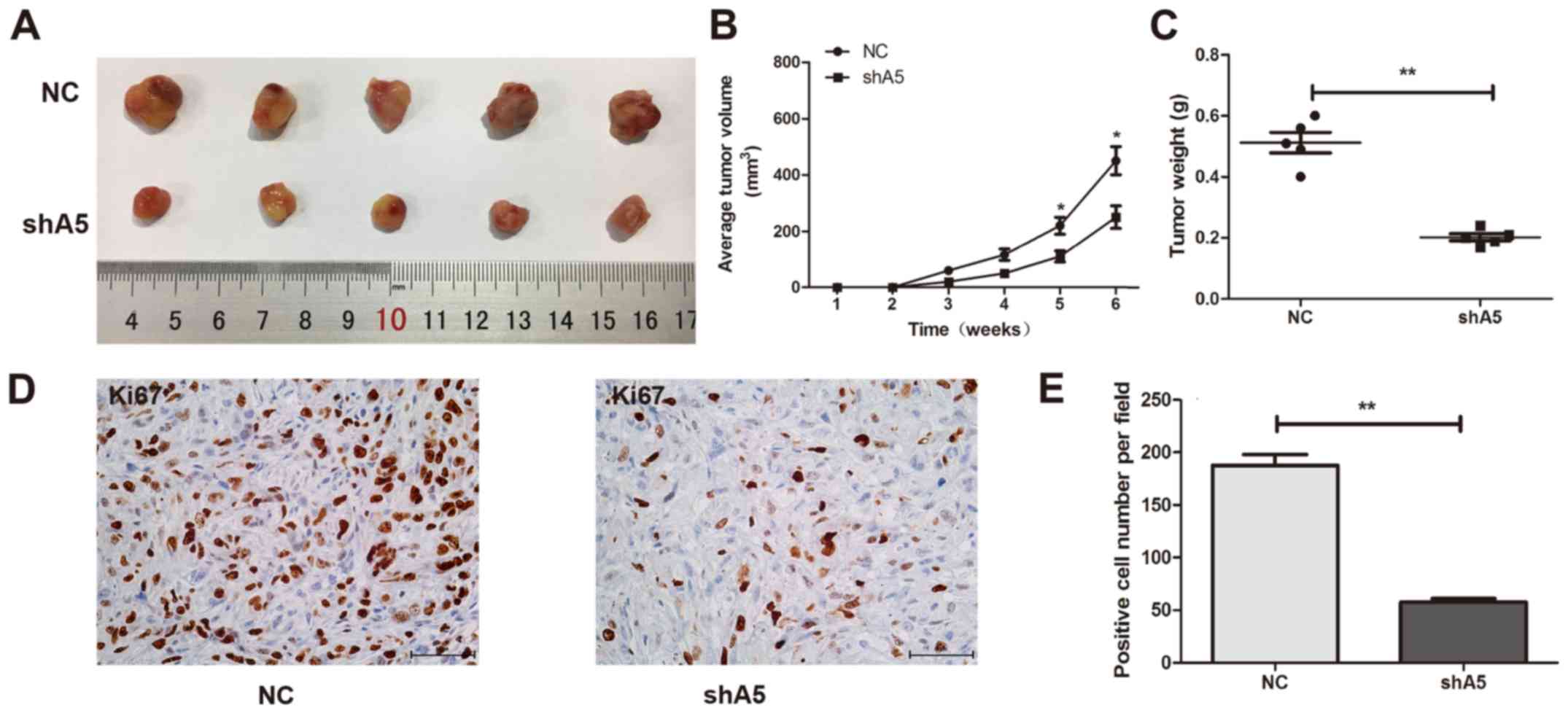
Knockdown of Annexin A5 impedes
tumorigenesis in vivo
To study the effect of Annexin A5 expression on
tumor growth in vivo, we constructed subcutaneous xenograft
tumor model using Caki-1 cells with Annexin A5 knockdown and
control cells in the female nude mice. As shown in Fig. 6A and B, tumors from the Annexin A5
knockdown group (shA5) grew markedly slower than those from control
group (NC). The mean tumor weight of shA5 group was lower compared
to the NC group (Fig. 6C) and
Ki-67, a proliferation marker of tumor, was markedly decreased in
tumors from shA5 group (Fig. 6D and
E). Collectively, these results showed that Annexin A5
knockdown could significantly impede tumor growth in
vivo.
Discussion
RCC remains a major clinical challenge due to its
poor long-term prognosis. Various oncogenes and suppressor genes
are involved in the tumorigenesis of RCC (15). Therefore, it is urgent to find
appropriate RCC biomarkers which are essential to tumor
progression. Recent studies have suggested that Annexin A5, a
calcium-dependent phospholipid-binding protein, could promote
tumorigenesis and progression in certain types of cancers (14,15,17).
This study focused on the biological functions of Annexin A5 and
its potential clinical value in RCC.
Among the RCC cell lines analyzed, Annexin A5 was
found to be highly expressed in RCC cells compared to HK2.
Consistent with this, Annexin A5 was frequently upregulated in RCC
tissues and acted as a predictor of clinical stage, histological
grade and overall survival in RCC. Therefore, Annexin A5 might be
used as a clinical marker for RCC, although more pathological data
are required to confirm this conclusion. Moreover, two RCC cell
lines Caki-1 and Caki-2 were used as cell models to demonstrate the
potential role of Annexin A5 in RCC cells. In both cell lines,
knockdown of Annexin A5 markedly reduced cell growth rate and
colony formation efficiency, while overexpression of Annexin A5
signifcantly accelerated cell proliferation. Similarly, knockdown
of Annexin A5 increased, whereas overexpression of Annexin A5
decreased the migratory and invasive potential in RCC cells. In
addition, xenograft studies showed Annexin A5 downregulated cells
formed smaller tumors compared to the control cells. These data
suggested that Annexin A5 played as a tumor promoter in RCC. To the
best of our knowledge, this is the first study to explore the
relationship between Annexin A5 and RCC tumorigenesis and
progression.
It has been reported that Annexin A5 was upregulated
in various cancers including hepatocarcinoma (16), breast cancer (17), cervical cancer (18), colorectal adenocarcinoma (19) and glioma (20). In this study, Annexin A5 was also
highly expressed in RCC which was in accord with the previous
studies. However, Annexin A5 was negatively related with
tumorigenesis in thyroid cancer and diffuse large B-cell lymphoma
(21,22). Therefore, Annexin A5 might play a
dual role in cancer depending on tissue specificity.
Uncontrolled proliferation and inappropriate cell
survival are one of the characteristics in cancer and these
processes are commonly regulated by PI3K/Akt/mTOR pathway (23). The PI3K/Akt/mTOR signaling pathway
is activated by several tyrosine kinase receptors and plays an
important role in the regulation of many aspects of cell function
including metabolism, proliferation, protein synthesis and survival
(24). It has been reported that
the abnormal expression of Annexin A5 may lead to aberrant
activation of PKC and cellular signal transduction, which may
contribute to tumorigenesis (14).
In glioblastoma multiforme (GBM), Annexin A5 promoted GBM
progression and chemoresistance to temozolomide through a
PI3K-dependent mechanism (25).
Our study demonstrated that overexpression of Annexin A5 activated
the PI3K/Akt/mTOR signaling pathway which might be involved in RCC
cell proliferation. Moreover, PI3K inhibitor (LY294002) could
reverse the cell growth promoting trend by Annexin A5.
Migration and invasion are the major events in the
metastasis of cancer (26). The
EMT refers to the conversion of epithelial cells to mesenchymal
cells, which is crucial in the progress of tumor metastasis
(27). EMT is characterized by a
loss of E-cadherin and an increase in non-epithelial cadherins
including N-cadherin and vimentin (28). MMPs belong to a family of
zinc-dependent endopeptidases which can degrade the extracellular
matrix (29). Especially, MMPs
have remarkable effects on tumor invasion and metastasis (30). To explore the underlying mechanism
by which Annexin A5 contributes to cell migration and invasion of
RCC, we investigated the expression of EMT-related proteins and
MMPs. Among the tested cell lines, N-cadherin, vimentin and
β-catenin were significantly upregulated in Annexin A5
overexpression group and Annexin A5 markedly increased MMP2 and
MMP9 expression. Therefore, we propose that Annexin A5 may promote
migration and invasion of RCC by regulating EMT and extracellular
matrix degradation. However, the role of these metastasis-related
makers in Annexin A5-induced progression of RCC should be further
studied in vivo in subsequent studies.
In addition, Annexin A5 also enhanced
chemoresistance in gastric cancer and nasopharyngeal carcinoma
which might be associated with the multidrug resistance protein
(MRP) (14). A study showed that
the protein expression of MRP and Annexin A5 were concurrently
elevated in drug-resistance of gastric cancer cells SGC-7901/DDP
compared to SGC-7901. Downregulation of Annexin A5 in SGC-7901/DDP
cells decreased the expression of MRP and increase the sensitivity
to cisplatin, paclitaxel and 5-Fu (31). Future studies are required to
explore the role of Annexin A5 in the chemosensitivity of RCC.
Moreover, Annexin A1, A2, A4, and A5 played a vital role in breast
cancer, pancreatic cancer, and laryngeal carcinoma, alone and/or
synergistically (32). It would be
of interest to investigate whether other Annexin family members
play a role, alone or coupled with Annexin A5 in RCC.
In conclusion, our study suggested that Annexin A5
was frequently highly expressed in RCC and might be used as a novel
prognostic indicator for RCC. In vitro and in vivo
experiments validated the promotion function of Annexin A5 in RCC
proliferation and metastasis via activating the PI3K/Akt/mTOR
signaling pathway and regulating EMT process and MMPs expression.
Annexin A5 may become a novel therapeutic target and prognosis
factor for the future treatment of RCC.
Acknowledgments
This work was supported by the National Natural
Science Funding of China (nos. 81600514, 81370781, 81670608).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu W, Wang Y, Jiang Y, Zhang W and Li Y:
Genetic analysis and clinicopathological features of ALK-rearranged
renal cell carcinoma in a large series of resected Chinese renal
cell carcinoma patients and literature review. Histopathology. Feb
15–2017.Epub ahead of print. View Article : Google Scholar
|
|
3
|
Golovastova MO, Korolev DO, Tsoy LV,
Varshavsky VA, Xu WH, Vinarov AZ, Zernii EY, Philippov PP and
Zamyatnin AA Jr: Biomarkers of renal tumors: The current state and
clinical perspectives. Curr Urol Rep. 18:32017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choueiri TK and Motzer RJ: Systemic
therapy for metastatic renal-cell carcinoma. N Engl J Med.
376:354–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mirsaeidi M, Gidfar S, Vu A and
Schraufnagel D: Annexins family: Insights into their functions and
potential role in pathogenesis of sarcoidosis. J Transl Med.
14:892016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laohavisit A and Davies JM: Annexins. New
Phytol. 189:40–53. 2011. View Article : Google Scholar
|
|
7
|
Bouter A, Carmeille R, Gounou C, Bouvet F,
Degrelle SA, Evain-Brion D and Brisson AR: Review: Annexin-A5 and
cell membrane repair. Placenta. 36(Suppl 1): S43–S49. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lauritzen SP, Boye TL and Nylandsted J:
Annexins are instrumental for efficient plasma membrane repair in
cancer cells. Semin Cell Dev Biol. 45:32–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fatimathas L and Moss SE: Annexins as
disease modifiers. Histol Histopathol. 25:527–532. 2010.PubMed/NCBI
|
|
10
|
Mussunoor S and Murray GI: The role of
annexins in tumour development and progression. J Pathol.
216:131–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerke V, Creutz CE and Moss SE: Annexins:
Linking Ca2+ signalling to membrane dynamics. Nat Rev
Mol Cell Biol. 6:449–461. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rescher U and Gerke V: Annexins - unique
membrane binding proteins with diverse functions. J Cell Sci.
117:2631–2639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hayes MJ and Moss SE: Annexins and
disease. Biochem Biophys Res Commun. 322:1166–1170. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng B, Guo C, Guan H, Liu S and Sun MZ:
Annexin A5 as a potential marker in tumors. Clin Chim Acta.
427:42–48. 2014. View Article : Google Scholar
|
|
15
|
Guo W, Xue J, Shi J, Li N, Shao Y, Yu X,
Shen F, Wu M, Liu S and Cheng S: Proteomics analysis of distinct
portal vein tumor thrombi in hepatocellular carcinoma patients. J
Proteome Res. 9:4170–4175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cojocaru E, Lozneanu L, Giuşcă SE, Căruntu
ID and Danciu M: Renal carcinogenesis - insights into signaling
pathways. Rom J Morphol Embryol. 56:15–19. 2015.
|
|
17
|
Hong M, Park N and Chun YJ: Role of
annexin a5 on mitochondria-dependent apoptosis induced by
tetramethoxystilbene in human breast cancer cells. Biomol Ther
(Seoul). 22:519–524. 2014. View Article : Google Scholar
|
|
18
|
Bae SM, Lee CH, Cho YL, Nam KH, Kim YW,
Kim CK, Han BD, Lee YJ, Chun HJ and Ahn WS: Two-dimensional gel
analysis of protein expression profile in squamous cervical cancer
patients. Gynecol Oncol. 99:26–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue G, Hao LQ, Ding FX, Mei Q, Huang JJ,
Fu CG, Yan HL and Sun SH: Expression of Annexin A5 is associated
with higher tumor stage and poor prognosis in colorectal
adenocarcinomas. J Clin Gastroenterol. 43:831–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rajcevic U, Petersen K, Knol JC, Loos M,
Bougnaud S, Klychnikov O, Li KW, Pham TV, Wang J, Miletic H, et al:
iTRAQ-based proteomics profiling reveals increased metabolic
activity and cellular cross-talk in angiogenic compared with
invasive glioblastoma phenotype. Mol Cell Proteomics. 8:2595–2612.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sofiadis A, Becker S, Hellman U,
Hultin-Rosenberg L, Dinets A, Hulchiy M, Zedenius J, Wallin G,
Foukakis T, Hoog A, et al: Proteomic profiling of follicular and
papillary thyroid tumors. Eur J Endocrinol. 166:657–667. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Zhang Y, Liu X, Ma J, Liu P, Hu C
and Zhang G: Annexin A5 inhibits diffuse large B-cell lymphoma cell
invasion and chemoresistance through phosphatidylinositol 3-kinase
signaling. Oncol Rep. 32:2557–2563. 2014.PubMed/NCBI
|
|
23
|
Yu JS and Cui W: Proliferation, survival
and metabolism: The role of PI3K/AKT/mTOR signalling in
pluripotency and cell fate determination. Development.
143:3050–3060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Wu C, Chen N, Gu H, Yen A, Cao L,
Wang E and Wang L: PI3K/Akt/mTOR signaling pathway and targeted
therapy for glioblastoma. Oncotarget. 7:33440–33450.
2016.PubMed/NCBI
|
|
25
|
Wu L, Yang L, Xiong Y, Guo H, Shen X,
Cheng Z, Zhang Y, Gao Z and Zhu X: Annexin A5 promotes invasion and
chemoresistance to temozolomide in glioblastoma multiforme cells.
Tumour Biol. 35:12327–12337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin K, Li T, van Dam H, Zhou F and Zhang
L: Molecular insights into tumour metastasis: Tracing the dominant
events. J Pathol. Dec 30;2016Epub ahead of print. View Article : Google Scholar
|
|
27
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Itoigawa Y, Harada N, Harada S, Katsura Y,
Makino F, Ito J, Nurwidya F, Kato M, Takahashi F, Atsuta R, et al:
TWEAK enhances TGF-β-induced epithelial-mesenchymal transition in
human bronchial epithelial cells. Respir Res. 16:482015. View Article : Google Scholar
|
|
29
|
Kapral M, Wawszczyk J, Jurzak M, Dymitruk
D and Weglarz L: Evaluation of the expression of metalloproteinases
2 and 9 and their tissue inhibitors in colon cancer cells treated
with phytic acid. Acta Pol Pharm. 67:625–629. 2010.
|
|
30
|
Wang Q, Yu W, Huang T, Zhu Y and Huang C:
RUNX2 promotes hepatocellular carcinoma cell migration and invasion
by upregulating MMP9 expression. Oncol Rep. 36:2777–2784.
2016.PubMed/NCBI
|
|
31
|
Wu X, Tang Y, Huang W and Wu Y:
Identification of proteins interacting with multidrug resistance
protein in gastric cancer. World J Gastroenterol. 19:3568–3673.
2011.
|
|
32
|
Deng S, Wang J, Hou L, Li J, Chen G, Jing
B, Zhang X and Yang Z: Annexin A1, A2, A4 and A5 play important
roles in breast cancer, pancreatic cancer and laryngeal carcinoma,
alone and/or synergistically. Oncol Lett. 5:107–112. 2013.
|