Introduction
Adenocarcinoma has gradually become one of the most
common pathological types of lung cancer. In clinical practice,
most lung adenocarcinoma patients eventually suffered relapse
and/or metastasis even when receiving complete excision of the
cancer (1). Metastasis is the main
cause of death in patients with lung adenocarcinoma (2). However, the underlying molecular
mechanisms of cancer metastasis remain poorly understood.
α1-antitrypsin (AAT), also called serine proteinase
inhibitor A1 (Serpin A1), is the most abundant serpin in human
plasma. It is encoded by the protease inhibitor gene
(SERPINA1) locus on the long arm of chromosome 14
(14q31–32.3). A major physiological role of AAT is to protect the
lung from the destructive effects of excess uninhibited neutrophil
elastase. It was shown that the serum levels of AAT were higher in
cancer patients than in healthy controls (3,4).
Additionally, AAT has been found involving in the distant
metastasis of lung adenocarcinoma (5). However, the mechanisms by which the
increase of AAT promotes cancer metastasis remain undefined.
During the course of metastasis, cancer cells
experience detachment, migration, invasion and adhesion. These key
steps are inter-related and are affected by various biochemical
factors. Fibronectin (FN) is one of the most abundant adhesion
proteins and synthesized mainly by hepatocytes. Most FN circulates
in the bloodstream as plasma FN while various cells also secrete
FN, named cellular FN. Some malignant epithelial cells can produce
FN and in some epithelial tumors FN was found upregulated (6,7). FN
plays an important role in cell growth, differentiation, migration
and adhesion (8–10). FN could be recognized by various
cell adhesion receptors, including integrins and dipeptidyl
peptidase IV (DPP IV). Adhesion receptors in vascular endothelial
cells can trigger an intracellular response when activated by
ligands such as FN to facilitate cancer cell extravasating.
Integrins, which are members of a glycoprotein
family, are the most well characterized receptors for FN. They are
composed of α and β subunits with non-covalent bonds connected to
each other. There are at least 24 different integrin heterodimers
that are dimerized by at least 19α and 8β subunits and each
integrin has distinct ligand binding and signaling properties
(11). Endothelial cell surface
express integrins which could recognize and are activated by
ligands in the extracellular environment (12). Integrin α5 is encoded by the ITGA5
gene, which may mediate FN assembly (13). DPP IV is a 110-kDa type II
transmembrane sialoglycoprotein and its expression has been
identified in various epithelial tissues including lung capillary
endothelial cells (14–16). A major function of the DPP IV/FN
adhesion has been reported in the colonization of the lungs by
blood-borne cancer cells (17).
In this study, we investigated the prognostic effect
of AAT expression on lung adenocarcinoma overall survival.
Subsequently, we identified the effects of AAT on lung
adenocarcinoma metastasis in vitro, specifically on cancer
cell migration and the adhesion between cancer cells and vascular
endothelial cells. The effects of FN in these processes were also
explored by modulating its expression levels. Finally, we studied
the influence of AAT and FN on metastatic colonization of lung
adenocarcinoma cells in vivo.
Materials and methods
Clinical samples
Patients who underwent curative surgery and were
diagnosed with lung adenocarcinoma in the department of thoracic
surgery, Nanjing Drum Tower Hospital, the Affiliated Hospital of
Nanjing University Medical School (Nanjing, China) between January
2003 and December 2009 were retrospectively reviewed. Eighty-eight
patients with full medical records and follow-up information were
identified and their formaldehyde fixed and paraffin embedded
(FFPE) lung adenocarcinoma tumor tissues were collected. None of
the 88 patients received radiation therapy or chemotherapy prior to
surgery. The detailed information of the patients is shown in
Table I. This study was approved
by the Institutional Review Board and the Ethics Committee of
Nanjing Drum Tower Hospital and written informed consent was
obtained from each patient.
 | Table IAssociation of AAT expression levels
with clinicopathological characteristics in 88 resected lung
adenocarcinoma patients. |
Table I
Association of AAT expression levels
with clinicopathological characteristics in 88 resected lung
adenocarcinoma patients.
| Patient
characteristics | High AAT expression
no. (%) | Low AAT expression
no. (%) | P-value |
|---|
| Age (years) | | | 0.48 |
| ≤60 | 25 (45.5) | 16 (48.5) | |
| >60 | 30 (54.5) | 17 (51.5) | |
| Sex | | | 0.27 |
| Female | 25 (45.5) | 18 (54.5) | |
| Male | 30 (54.5) | 15 (45.5) | |
| Smoking status | | | 0.22 |
| Never smokers | 36 (65.5) | 25 (75.8) | |
| Smokers | 19 (34.5) | 8 (24.2) | |
| Tumor
differentiation | | | 0.24 |
|
Well+Moderately | 33 (60.0) | 23 (69.7) | |
| Poorly | 22 (40.0) | 10 (30.3) | |
| Regional lymph node
metastasis | | | |
| No | 24 (43.6) | 22 (66.7) | 0.03a |
| Yes | 31 (56.4) | 11 (33.3) | |
| pTNM stage | | | 0.07 |
| I | 15 (27.3) | 17 (51.5) | |
| II | 21 (38.2) | 9 (27.3) | |
| III | 19 (34.5) | 7 (21.2) | |
Immunohistochemistry
Adjacent 3-µm sections of FFPE samples were
made for immunostaining. Each paraffin section was deparaffinized
through dimethylbenzene and rehydrated by graded alcohols. After
rehydration, antigen retrieval of the tisssue was carried by
pressure cooking the slides with citric acid buffer (pH 6.0 for 1
min). Rabbit polyclonal anti-human AAT antibody (1:100, Abcam,
Cambridge, MA, USA) was used. AAT expression status was evaluated
by two independent pathologists who were blinded to patient
clinical characteristics (J. Yang and K. Meng). Five visual fields
of each sample were randomly observed and 100 tumor cells in each
field were counted (×400 magnification). Tumor cells with brown
cytoplasm were considered positive and the staining intensity was
rated as four classes: 3+, strong; 2+, moderate; 1+, weak; and 0,
no staining. Positively stained cells out of 100 tumor cells in
each field were recorded. The average percentage of positive tumor
cells was categorized into the following four classes: 0 for 0%; 1
for 1–33%; 2 for 34–66%; and 3 for 67–100%. The scores of positive
cell percentage and staining intensity were multiplied and
composite scores of 1–3 were defined as low AAT expression, while
scores of 4–9 were considered high AAT expression.
Cell lines
Human lung adenocarcinoma cell lines (A549 and
SPC-A1) and human umbilical vein endothelial cell (HUVEC) were
purchased from the Cell Bank of Chinese Academy of Medical Science
(Shanghai, China) and maintained in our laboratory. A549 and SPC-A1
cells with stable green fluorescent protein (GFP) expression
(A549/GFP and SPC-A1/GFP) were established in our laboratory
through transfecting tumor cells with GFP expressing vector. All
cell lines were cultured and maintained in recommended growth
medium at 37°C in a 5% CO2 humidified atmosphere.
Lentivirus vectors
Lentivirus vectors with interfering sequences
(5′-AGTCCAACAGCACCAATAT-3′ for AAT (pLenti-shRNA-AATi);
5′-TGGTTGTATCAGGACTTAT-3′ for FN (pLenti-shRNA-FNi);
5′-ACTGTGGATCATCATCCTA-3′ for integrin α5 (pLenti-shRNA-ITGA5i);
5′-AGAAGACAACCTTGACCAT-3′ for DPP IV (pLenti-shRNA-DDP IVi) or with
coding sequence of AAT (pLenti-AAT) were purchased from Lifetech
(Shanghai, China) and Genechem (Shanghai, China) companies. Lung
adenocarcinoma cell lines were infected with a multiplicity of
infection (MOI) as 1:10 in A549 cells and 1:100 in SPC-A1 cells.
Polybrene (Sigma, St. Louis, MO, USA) at the concentration of 8
µg/ml was added to enhance the infection. Blasticidin (0.5
µg/ml) and puromycin (1 µg/ml) were used to screen
the stable infected cells. The modulations of target gene
expression were verified by real-time reverse transcription-PCR
(RT-PCR).
Real-time RT-PCR
Total cellular RNA was extracted from A549 and
SPC-A1 cells after transfecting with targeted gene interfering
sequences (pLenti-shRNA-AATi or pLenti-shRNA-FNi) or coding
sequence of AAT, respectively, using TRIzol (Invitrogen, Waltham,
MA, USA). Subsequently, real-time RT-PCR was performed to determine
AAT and FN expression in the StepOne System (Life Technologies,
Carlsbad, CA, USA). RNA was also extracted from HUVEC after
transfecting with targeted gene interfering sequences
(pLenti-shRNA-ITGA5i or pLenti-shRNA-DDP IVi) and real-time RT-PCR
was performed to determine integrin α5 and DPP IV expression,
respectively. Relative gene expression was determined by the ΔΔCt
method (ΔΔCq) based on glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) levels (18), and results
were expressed as fold change over different conditions.
Western blotting
After A549 and SPC-A1 cells were transfected with
either pLenti-AAT or pLenti-shRNA-AATi, total cell protein were
extracted from cells using RIPA buffer with proteinase inhibitors
(Beyotime, Jiangsu, China) and then resolved by SDS-polyacrylamide
gel electrophoresis and transferred to PVDF membranes. Membranes
were blocked by 5% skim milk for 1 hour and incubated with rabbit
polyclonal anti-human AAT antibody (1:100, Abcam) overnight at 4°C,
followed by incubation with appropriate HRP-conjugated secondary
antibody at optimized concentration. The densitometry of western
blot results was measured using ImageJ software (https://imagej.nih.gov/ij/).
Immunofluorescence studies
In order to demonstrate the influence of AAT on FN
expression and observe the adhesion between adenocarcinoma cells
and HUVECs under various conditions, immunofluorescence was applied
to detect the AAT and FN expression levels in tumor cells and CD31
in HUVECs, with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear
staining. Rabbit anti-human FN antibody and rabbit monoclonal
anti-human CD31 antibody (1:200, Abcam) were used. Rabbit
polyclonal anti-human FN antibody (1:100, Abcam) was applied with a
FITC conjugated rat anti-rabbit secondary antibody and rabbit
monoclonal anti-human CD31 antibody (1:200, Abcam) was applied to
identify the HUVECs with a Cy3 conjugated rat anti-rabbit second
antibody.
Wound healing and Transwell assay
The migration ability of adenocarcinoma cells was
determined by wound healing assay and Transwell assay. AAT
expression levels in lung adenocarcinoma cells were modulated by
pLenti-AAT or pLenti-shRNA-AATi. Human lung adenocarcinoma cells
(A549 and SPC-A1) were cultured in 6-well culture plate
(2.5×105 per well) for 24 h until 90% confluent. Wound
line was created by scratching the plates with a 20 µl
micropipette tip. Migration rate of tumor cells was calculated by
the following formula: healing rate = (the distance before
healing-the distance after healing)/the distance before healing
×100%, in which the distances of gaps were recorded at identical
point.
Transwell chambers (8 µm pore size, Costar,
Fisher Scientific, MA, USA) were also applied. Cells suspended in
100 µl of medium (1×105 cells) were placed into
the top chamber and 600 µl conditioned medium was added to
the bottom well. Cells adherent to the upper surface of the
membrane were removed using a cotton applicator after 24 h
incubation. After fixation in ice cold acetone, cells on the bottom
surface of membrane were stained with 0.4% violet crystal, and
counted by microscopy.
Cell adhesion examination
To analyze the effect of AAT on adhesion of lung
adenocarcinoma cells to vascular endothelial cells, a cancer
cell/endothelial cell co-culture model was established. First,
HUVECs were seeded on sterile cover glasses and cultured in 6-well
plates with endothelial cell growth medium and growth supplement
(Sigma). When HUVECs on cover glasses grew to confluent, all the
cover glasses were transferred into new 6-well plates. At the same
time, single cell solutions of A549/GFP and SPC-A1/GFP cells were
collected and seeded into 6-well plates in which cover glasses with
confluent HUVECs had been placed. After 2 h co-culture, the cover
glasses were collected and washed with PBS, followed by fixation in
cold acetone for immunofluorescence examination.
Expression levels of AAT and FN in tumor cells and
expression levels of integrin α5 and DPP IV in HUVECs were
modulated by lentiviral-mediated deliveries of coding sequence or
interfering sequence.
Animal models
T cell deficient BALB/c nude mice (CByJ.
Cg-Foxn1nu/J) were purchased from Model Animal
Research Center of Nanjing University. Animal use and experiment
protocol were approved by the Institutional Animal Care and Use
Committee of Nanjing Drum Tower Hospital. All surgery was performed
under anesthesia with pentobarbital sodium and ketamine. Animals
were sacrificed by overdose of anesthetics.
Cancer cells (A549 and SPC-A1, respectively) with
AAT upregulation or AAT downregulation were cultured and injected
through lateral tail vein of nude mice (female, 4–6 weeks old) with
a total cell number of 1×106 per mouse. Mice were
divided into following six groups: 1) normal control (NC) in A549;
2) AAT upregulation alone in A549; 3) AAT upregulation followed by
FN downregulation in A549; 4) NC in SPC-A1; 5) AAT upregulation
alone in SPC-A1; 6) AAT upregulation followed by FN downregulation
in SPC-A1. A total of six mice in each group were used. Two months
after injection, total lung tissues of mice were collected and
embedded in paraffin. The whole lung tissues were analyzed by
consecutive sections and hematoxylin and eosin (H&E) staining.
Lung metastasis loci were counted by microscopy.
Statistical analysis
Differences between clinicopathological variables
and AAT expression levels were examined by Chi-square test or
Fisher's exact test if any sample number <5. Overall survival
(OS) was calculated as the time from the date of lung surgery to
death. Patients who were alive at the last contact were censored.
Kaplan-Meier method and log-rank test were used to calculate the
survival difference. Univariate Cox regression analysis was used to
evaluate the prognostic impact on OS of clinicopathological
variables. Variables were included in multivariate analysis at
P<0.05 in the univariate analysis. Multivariate Cox regression
analysis was used to evaluate the independent prognostic role of
AAT expression. The experimental results are shown as mean ± SEM.
One-way ANOVA, LSD and unpaired t-test were used to analyze the
differences between groups. All analyses were performed with SPSS
software, version 16.0 (SPSS, Inc., Chicago, IL, USA). All tests
were two-sided and performed at a significance level of 0.05.
Results
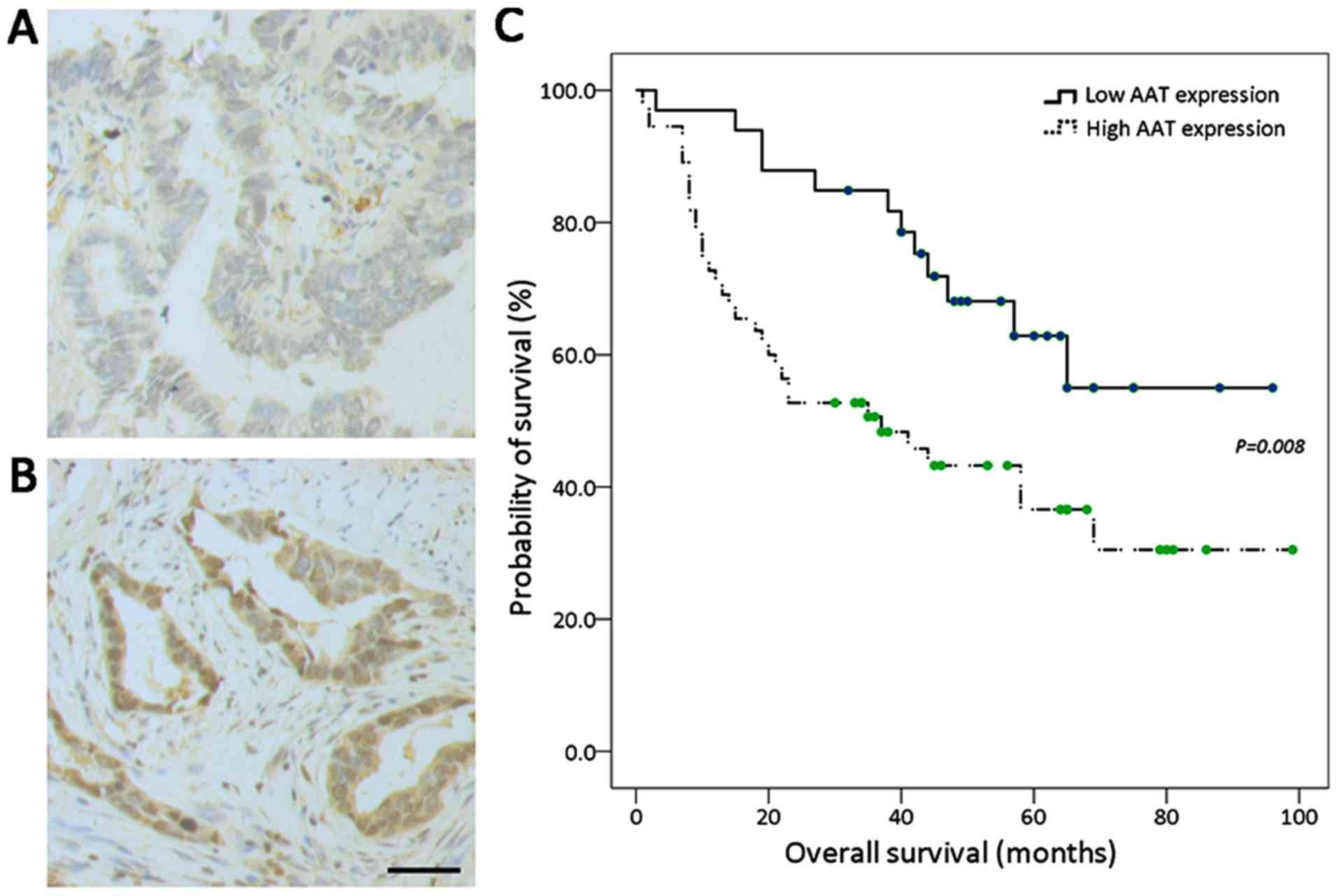
High expression of AAT in tumor tissue is
related to shorter overall survival of lung adenocarcinoma
patients
Positive immunohistochemistry staining for AAT was
mainly localized in the cytoplasm. According to the score of
immunohistochemistry, all lung adenocarcinoma cases were classified
as low AAT expression group (33/88, Fig. 1A) and high expression group (55/88,
Fig. 1B). Clinicopathological
characteristics of included lung adenocarcinoma patients are listed
in Table I by AAT expression
status. There were no significant differences in age, gender,
smoking status, tumor differentiation, or pTNM stage between
patients with high AAT expression and those with low AAT expression
(P≥0.05). The correlation between regional lymph node metastasis
and AAT expression was also examined, with more cases showing
regional lymph node metastasis in the high AAT expression group
(56.4% vs. 33.3%, P=0.03). The median OS of 88 resected lung
adenocarcinoma patients was 58.0 months (95% CI: 37.7–78.3). As
indicated in Fig. 1C, lung
adenocarcinoma patients with high AAT expression in tumor samples
had shorter OS than those with low AAT expression (P=0.008). In
univariate Cox regression analysis, as shown in Table II, tumor differentiation, regional
lymph node metastasis and pTNM stage associated significantly with
OS (P<0.05). There was a significant association of AAT high
expression level with shorter OS (hazard ratio: 2.38; 95% CI: 1.23,
4.63; P=0.01). In multivariate analysis adjusting for variables
significant in the univariate analysis, the significant association
of AAT expression with OS remained (adjusted hazard ratio: 2.05;
95% CI: 1.04–4.06; P=0.04).
 | Table IIPrognostic value of
clinicopathological characteristics and AAT expression in
univariate Cox regression analysis. |
Table II
Prognostic value of
clinicopathological characteristics and AAT expression in
univariate Cox regression analysis.
| Patient
characteristics | Unadjusted HR (95%
CI) | P-value |
|---|
| Age (years) | | |
| ≤60 | Reference | |
| >60 | 1.07
(0.60–1.93) | 0.81 |
| Gender | | |
| Female | Reference | |
| Male | 1.51 (0.83,
2.75) | 0.17 |
| Smoking status | | |
| Never smokers | Reference | |
| Smokers | 1.17 (0.63,
2.18) | 0.62 |
| Tumor
differentiation | | |
|
Well+Moderately | Reference | |
| Poorly | 2.03 (1.13,
3.64) | 0.02a |
| Regional lymph node
metastasis | | |
| No | Reference | |
| Yes | 2.33 (1.27,
4.27) | 0.006a |
| pTNM stage | | |
| I | Reference | |
| II | 2.40 (1.09,
5.25) | 0.03a |
| III | 3.36 (1.54,
7.31) | 0.002a |
| AAT expression
level | | |
| Low | Reference | |
| High | 2.38 (1.23,
4.63) | 0.01a |
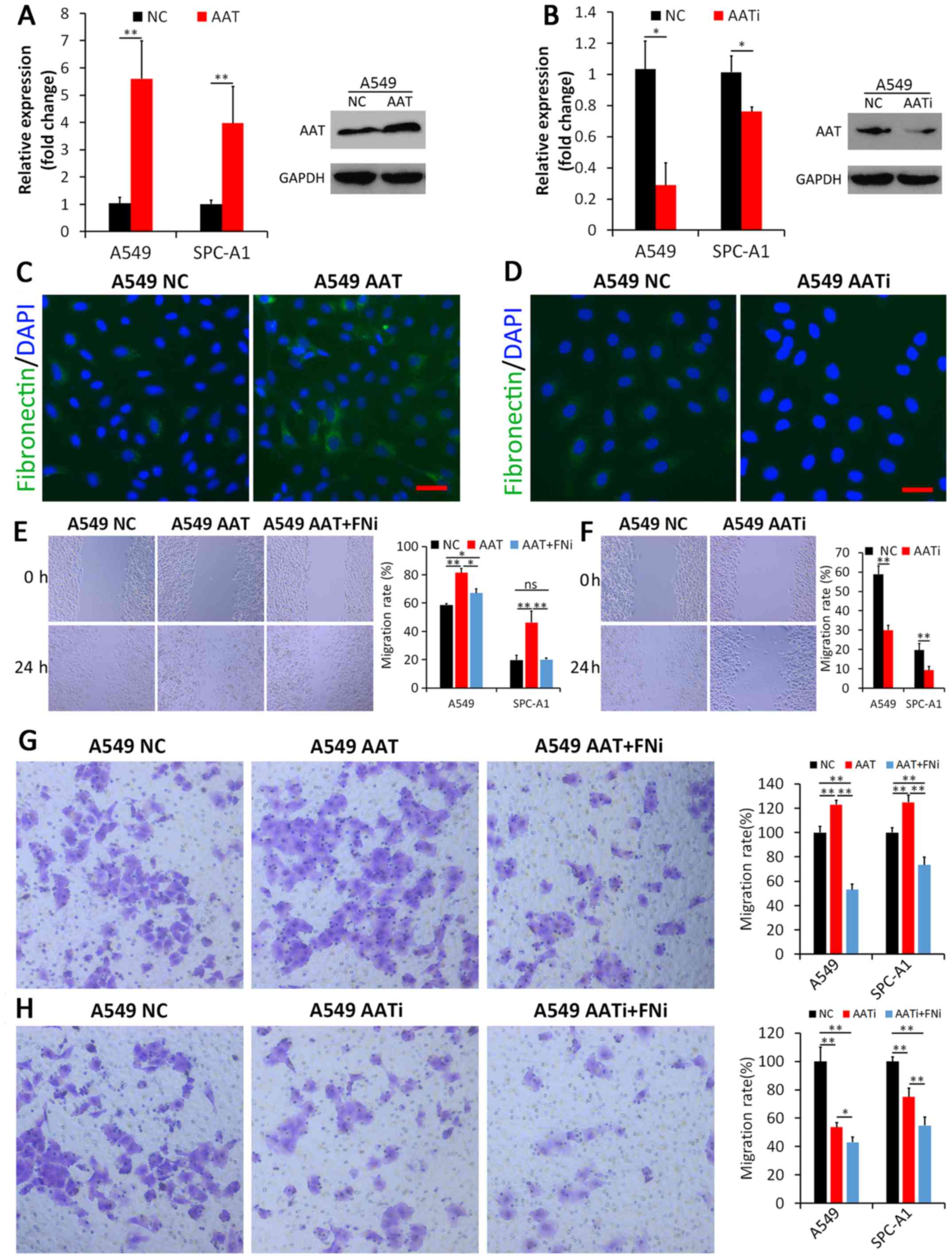
AAT promotes the migration ability of
lung adenocarcinoma cells in vitro by regulating FN expression
AAT expression level was significantly higher after
transfecting with the AAT coding sequence pLenti-AAT; while it was
significantly lower after transfecting with the AAT interfering
sequence pLenti-shRNA-AATi, in both A549 cell line and SPC-A1 cell
line (Fig. 2A and B). The effect
of AAT on FN expression was investigated by immunofluorescence,
which showed that FN expression increased upon upregulation of AAT
expression while decreased upon downregulation of AAT expression in
both A549 cell line (Fig. 2C and
D) and SPC-A1 cell line (data was not shown). These findings
demonstrated that regulating AAT expression could affect FN
expression in lung adenocarcinoma cells.
Wound healing assay and Transwell assay then were
used to examine the effects of AAT and FN on adenocarcinoma cell
migration ability. First, A549 and SPC-A1 cells were transfected
with pLenti-AAT or control vectors respectively. The results of the
wound healing assay showed that the migration rate of
adenocarcinoma cells with AAT upregulation was significantly higher
than that of parental cells transfected with control vectors
(P<0.01, Fig. 2E). Similarly,
AAT downregulation impeded wound healing rate of adenocarcinoma
cells (P<0.01, Fig. 2F).
Second, to examine whether adenocarcinoma cells with higher AAT
expression contribute their increased migration ability to FN, FN
expression was inhibited by transfecting Lenti-shRNA-FNi in lung
adenocarcinoma cells with AAT upregulation. We found that FN
downregulation could reverse the increased migration induced by AAT
upregulation in adenocarcinoma cells (P<0.05, Fig. 2E). Furthermore, Transwell assay was
performed. As shown in Fig. 2G and
Fig. 2H, the transmigration
ability of A549 were significantly increased when transfected with
pLenti-AAT, compared to the control group (P<0.01). Conversely,
the cells showed decreased transmigration upon trasfection of
pLenti-AATi. In addition, when FN expression was inhibited, the
transmigration rate significantly decreased even though AAT was
upregulated, compared to those without FN interference (P<0.01).
If both FN and AAT expression levels were inhibited, A549 cells
showed further decreased transmigration compared to those with only
AAT inhibited (P<0.05). Similar results were also observed in
SPC-A1 cells (P<0.01). These findings indicate that the effect
of AAT on lung adenocarcinoma cell migration might be related to
the expression of FN.
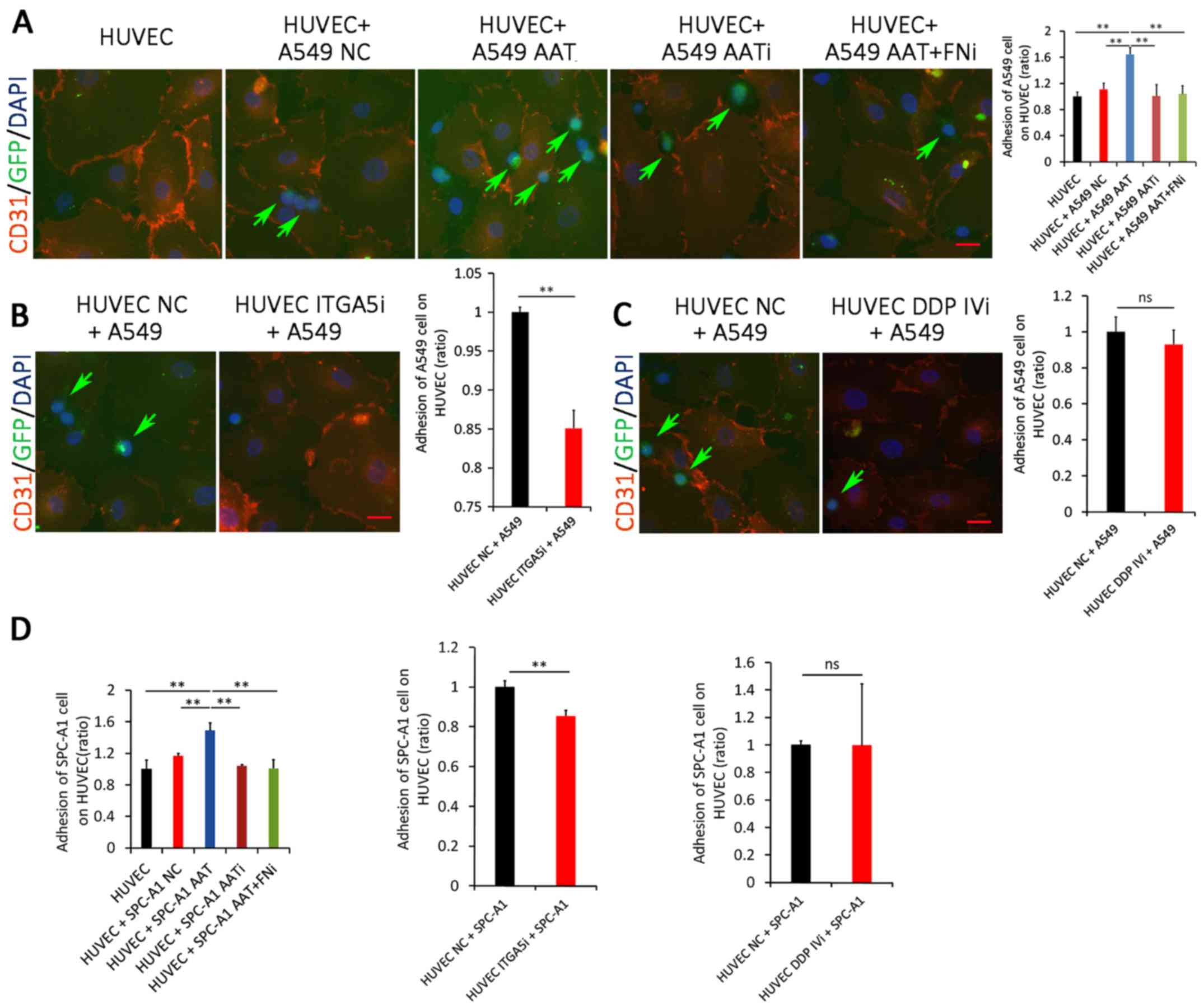
AAT promotes adhesion between lung
adenocarcinoma cells and vascular endothelial cells through
regulating FN expression
To gain further insight into the underlying
molecular mechanism by which AAT promotes lung adenocarcinoma
metastasis, a cancer cell/endothelial cell co-culture model was
established. As indicated in Fig.
3A, more GFP-positive A549 cells adhered to HUVECs when AAT was
upregulated (P<0.01). However, downregulation of AAT did not
further inhibit adhesion between A549 cells and HUVECs compared to
those with the control vector. Additionally, the adhesion of A549
cells to HUVECs significantly decreased if their FN expression was
impaired, compared to those with AAT upregulation alone
(P<0.01). These results suggest that the promotion effect of AAT
on the adhesion ability of lung adenocarcinoma cells to endothelial
cells might be through the effect of FN. Similar results were also
observed in SPC-A1 cells (Fig.
3D).
The functions of FN receptors on vascular
endothelial cells were further examined by RNA-interference-based
studies. Two reported receptors of FN, integrin α5 and DPP IV, were
inhibited in HUVEC, respectively. As indicated in Fig. 3B, downregulation of integrin α5
inhibited adhesion between GFP-positive A549 cells and HUVECs
(P<0.01). However, when DPP IV was knocked down in HUVECs, the
adhesion between A549 cells and HUVECs were not significantly
inhibited (P>0.05, Fig. 3C).
Similar results were also observed in SPC-A1 cells (Fig. 3D). Collectively, these results
suggest that FN may be a functional target of AAT, which is
responsible for AAT-mediated adhesion between lung adenocarcinoma
cells and endothelial cells.
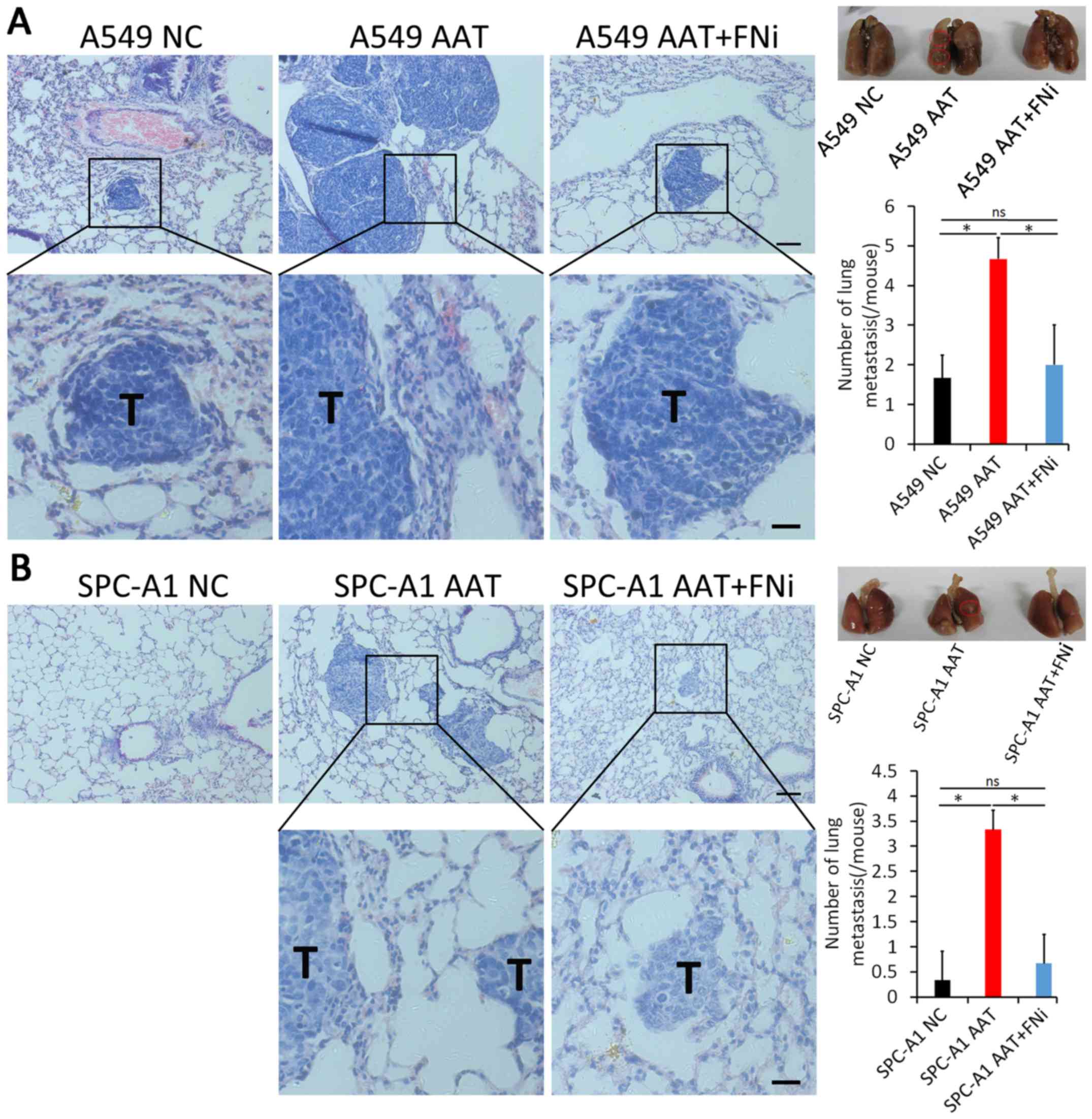
AAT promotes metastasis of lung
adenocarcinoma in vivo
To further confirm whether AAT could promote the
metastatic behavior in vivo, A549 cells with AAT
overexpression were injected into nude mice through the tail vein.
As shown in Fig. 4A, the number of
lung metastatic loci in the AAT overexpression group was remarkably
higher compared to the NC group (P<0.05). We further examined
the effect of FN on lung colonization of A549 cells by
downregulating FN expression in addition to upregulating AAT
expression in immunocompromised nude mice. Results showed that
downregulation of FN could induce decreased lung metastasis even
though AAT was upregulated (Fig.
4A, P<0.05). Similar results were observed in SPC-A1 cells
(Fig. 4B, P<0.05). The results
indicate that AAT could significantly promote the metastasis of
lung adenocarcinoma cells in the nude mouse xenograft model, which
may be through regulating FN expression.
Discussion
In this study, we investigated the effect of
α1-antitrypsin (AAT) on lung cancer metastasis. We found that AAT
expression was associated with overall survival in resected lung
adenocarcinoma. Furthermore, we identified that AAT was able to
promote migration and adhesion of lung adenocarcinoma cells through
enhancing the expression of FN. In the experiment with nude mouse
xenograft model, AAT upregulation could significantly promote
metastasis of lung adenocarcinoma cells, while FN downregulation
could reverse the promotion effect. These results suggested that
AAT may be a therapeutic target for lung adenocarcinoma
metastasis.
Metastasis is the most critical complication of
malignancies and remains a big challenge to the effective treatment
for lung adenocarcinoma. Our understanding of the molecular
mechanisms regarding lung adenocarcinoma metastasis is still
incomplete. AAT is a glycoprotein synthesized primarily by
hepatocytes, with smaller amounts synthesized by intestinal
epithelial cells, neutrophils, pulmonary alveolar cells,
macrophages, and cancer cells (19,20).
AAT has long been recognized as an important anti-protease which
protects the lung from the destructive effects of major proteases
such as neutrophil elastase. In recent years, AAT has gradually
been found to have an impact on lung cancer metastasis. Increased
serum AAT concentration was a poor prognostic marker for non-small
cell lung cancer (21). In this
study, we demonstrated that AAT expression in tumor tissue was a
prognostic marker for lung adenocarcinoma patients, with higher
expression associated with shorter overall survival. Our
immunohistochemistry results showed a positive correlation between
AAT expression and the frequency of regional lymph node
involvement, suggesting that AAT overexpression could be important
for the remodeling process during lung adenocarcinoma development
and metastasis. However, the molecular mechanism of AAT influencing
cancer metastasis has still not been completely defined.
The migratory ability of cancer cells is one
critical parameter of the metastatic cascade. Our findings
demonstrated that AAT overexpression could promote migration of
lung adenocarcinoma cells, but this promotion effect could be
reversed by FN downregulation. FN plays key roles in promoting
oncogenic transformation and has been associated with cell
migration and invasion in various malignancies, including lung
cancer (22,23). Digiacomo et al found that FN
stimulated the migration of murine or human macrophages and the
activation of SFK/FAK complex, while the macrophage migration
depended on FAK activity (24).
This phenomenon may be extended to explain the effect of FN on
tumor cell migration. FN could interact with the integrins and then
lead to the activation of many signaling pathways, including
c-Met/FAK/Src and FAK-PI3K/Akt pathways, which regulate cancer cell
adhesion and migration (25,26).
Mitra et al suggested that FN may bind integrin α5β3 on
cancer cells and subsequently activate the FAK/Src-dependent
signaling pathway (27). FN could
stimulate the secretion of MMP-9 through the MEK1/ERK and the
PI-3K/Akt-dependent pathways in breast cancer cells, thereby
triggering invasion of tumor cells (28). Thus, FN may be a significant factor
in the process of AAT promoting migration of lung adenocarcinoma
cells.
We have examined the mRNA levels of FN in lung
adenocarcinoma cells (A549 and SPC-A1) after upregulation or
downregulation of the AAT expression. There was no significant
correlation between mRNA levels of FN and AAT in the two cell
lines. As a result, AAT might regulate the expression of FN through
an indirect way. As indicated in Fig.
2, FN protein was upregulated in both cytoplasm and surface of
lung adenocarcinoma cells after upregulation of AAT. We
hypothesized that AAT could prevent degradation of FN intra- and
extra-adenocarcinoma cells. It has been widely accepted that the
physiological role of AAT is to inhibit the destructive effects of
excess uninhibited neutrophil elastase (19), which means that AAT could prevent
degradation of FN through inhibiting proteases.
Once tumor cells are circulating, they are in a
suspended state and require additional cellular activities to
enable their colonization in distant organs, which is mostly
initiated by adhesive interactions with the endothelium. In the
present study we showed that the adhesion between lung
adenocarcinoma cells and vascular endothelium was regulated by
expression levels of AAT and FN. FN could adhere to integrin α5
expressed on the endothelium. The specificity of integrin α5/FN
adhesion was confirmed by reduced adhering ability when lung
adenocarcinoma cells were treated with integrin α5 interfering
sequence. Interactions between FN and integrins play important
roles in cancer metastasis (29).
Through specifically binding to integrins, such as α5β1 or α5β3, FN
may activate multiple signal pathways, and then regulate malignant
cellular metastasis (30,31).
In our study, however, DDP IV failed to show
significant effect on adhesion between adenocarinoma cells and
endothelial cells. Some studies disclosed that the integrin-binding
domains located in FN were different from the DPP IV-binding sites,
indicating that they may operate in a different manner (32,33).
DPP IV might not be an indispensable receptor for FN in mediating
adhesion between lung adenocarcinoma cells and endothelial
cells.
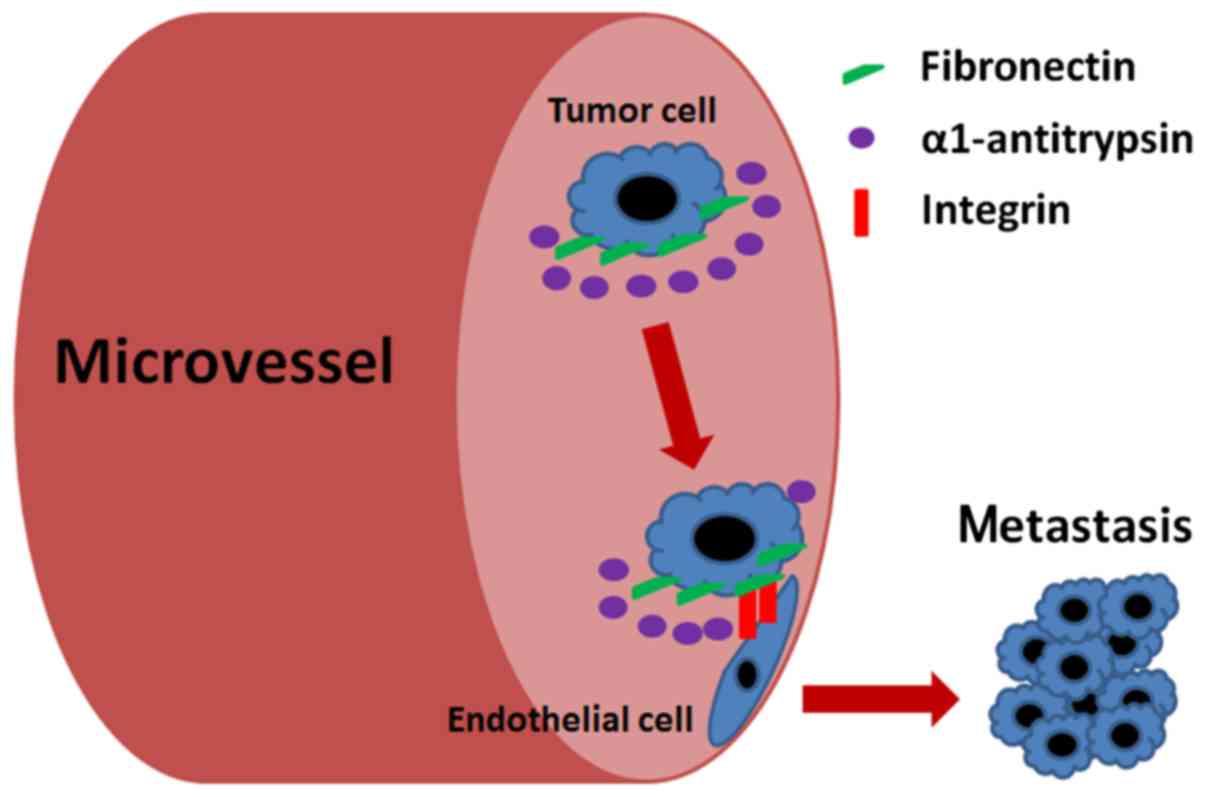
As stated above, our results offered a scheme by
which AAT facilitates lung adenocarcinoma metastasis. As shown in
Fig. 5, lung adenocarcinoma cells
may express high levels of AAT. As AAT is the most abundant
proteinase inhibitor within the lung, it may inhibit the
proteolytic activity of proteinases targeting at FN, and recruit
more FN overexpressed on the surface of cancer cells. This
situation may further increase the adhesion of adenocarcinoma cells
to endothelial cells through FN interacting with integrins. Binding
of FN to integrins may not only serve as a way for cell adhesion,
but also generate the traction needed for cell migration.
Noteworthy, it has been shown that AAT could have an
anti-apoptotic role in alveolar cells (34). Under certain conditions, inhibition
of apoptosis in lung tissue by AAT may become a pathological
mechanism that leads to lung cancer development and metastasis. On
the other hand, AAT could be produced by various tumor cells.
Therefore, one can speculate a link between tumor cell propensity
to produce and secrete AAT and tumor progression or metastasis.
Additionally, AAT may mediate immune tolerance (35), and might help cancer cells escape
immune surveillance to metastasis. FN upregulation has been found
in several types of malignant tumors and its high expression
positively correlates with metastasis (36,37).
AAT may modulate the expression of FN through signaling pathways.
This may represent crosstalk that is of prognostic relevance in
lung cancer. The association between FN and AAT needs further
research.
In conclusion, our study explored the mechanisms by
which AAT promotes lung adenocarcinoma metastasis and identified
the effect of FN during the processes. Our findings offer useful
information for an understanding of the mechanisms of lung
adenocarcinoma metastasis.
Acknowledgments
We would like to thank Dr Kui Meng (Department of
Pathology, Nanjing Drum Tower Hospital, The Affiliated Hospital of
Nanjing University Medical School, Nanjing, China) for his
technical support in the evaluation of the pathological samples.
This work was supported by the grants from the Natural Science
Foundation of Jiangsu Province (no. BK20130089), the National
Natural Science Foundation of China (no. 81501972), and the Nanjing
Municipal Health and Family Planning Commission (no. YKK15063).
References
|
1
|
Travis WD: Pathology of lung cancer. Clin
Chest Med. 23:65–81. viii2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Comunale MA, Rodemich-Betesh L, Hafner J,
Wang M, Norton P, Di Bisceglie AM, Block T and Mehta A: Linkage
specific fucosylation of alpha-1-antitrypsin in liver cirrhosis and
cancer patients: Implications for a biomarker of hepatocellular
carcinoma. PLoS One. 5:e124192010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Akawi ZJ, Abu-Awad AM, Sharara AM and
Khader Y: The importance of alpha-1 antitrypsin (alpha1-AT) and
neopterin serum levels in the evaluation of non-small cell lung and
prostate cancer patients. Neuro Endocrinol Lett. 31:113–116.
2010.PubMed/NCBI
|
|
5
|
Zelvyte I, Wallmark A, Piitulainen E,
Westin U and Janciauskiene S: Increased plasma levels of serine
proteinase inhibitors in lung cancer patients. Anticancer Res.
24:241–247. 2004.PubMed/NCBI
|
|
6
|
Ioachim E, Charchanti A, Briasoulis E,
Karavasilis V, Tsanou H, Arvanitis DL, Agnantis NJ and Pavlidis N:
Immunohistochemical expression of extracellular matrix components
tenascin, fibronectin, collagen type IV and laminin in breast
cancer: Their prognostic value and role in tumour invasion and
progression. Eur J Cancer. 38:2362–2370. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
David L, Nesland JM, Holm R and
Sobrinho-Simões M: Expression of laminin, collagen IV, fibronectin,
and type IV collagenase in gastric carcinoma. An
immunohistochemical study of 87 patients. Cancer. 73:518–527. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams CM, Engler AJ, Slone RD, Galante
LL and Schwarzbauer JE: Fibronectin expression modulates mammary
epithelial cell proliferation during acinar differentiation. Cancer
Res. 68:3185–3192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han S, Khuri FR and Roman J: Fibronectin
stimulates non-small cell lung carcinoma cell growth through
activation of Akt/mammalian target of rapamycin/S6 kinase and
inactivation of LKB1/AMP-activated protein kinase signal pathways.
Cancer Res. 66:315–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alizadeh AM, Shiri S and Farsinejad S:
Metastasis review: From bench to bedside. Tumour Biol.
35:8483–8523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burgett ME, Lathia JD, Roth P, Nowacki AS,
Galileo DS, Pugacheva E, Huang P, Vasanji A, Li M, Byzova T, et al:
Direct contact with perivascular tumor cells enhances integrin αvβ3
signaling and migration of endothelial cells. Oncotarget.
7:43852–43867. 2016.PubMed/NCBI
|
|
13
|
Schwarzbauer JE and DeSimone DW:
Fibronectins, their fibrillogenesis, and in vivo functions. Cold
Spring Harb Perspect Biol. 3:a0050412011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hartel-Schenk S, Gossrau R and Reutter W:
Comparative immunohistochemistry and histochemistry of dipeptidyl
peptidase IV in rat organs during development. Histochem J.
22:567–578. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson RC, Zhu D, Augustin-Voss HG and
Pauli BU: Lung endothelial dipeptidyl peptidase IV is an adhesion
molecule for lung-metastatic rat breast and prostate carcinoma
cells. J Cell Biol. 121:1423–1432. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piazza GA, Callanan HM, Mowery J and
Hixson DC: Evidence for a role of dipeptidyl peptidase IV in
fibronectin-mediated interactions of hepatocytes with extracellular
matrix. Biochem J. 262:327–334. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng HC, Abdel-Ghany M, Elble RC and
Pauli BU: Lung endothelial dipeptidyl peptidase IV promotes
adhesion and metastasis of rat breast cancer cells via tumor cell
surface-associated fibronectin. J Biol Chem. 273:24207–24215. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Sun Z and Yang P: Role of imbalance
between neutrophil elastase and alpha 1-antitrypsin in cancer
development and progression. Lancet Oncol. 5:182–190. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen XL, Zhou L, Yang J, Shen FK, Zhao SP
and Wang YL: Hepatocellular carcinoma-associated protein markers
investigated by MALDI-TOF MS. Mol Med Rep. 3:589–596. 2010.
View Article : Google Scholar
|
|
21
|
Li Y, Krowka MJ, Qi Y, Katzmann JA, Song
Y, Li Y, Mandrekar SJ and Yang P: Alpha1-antitrypsin deficiency
carriers, serum alpha1-antitrypsin concentration, and non-small
cell lung cancer survival. J Thorac Oncol. 6:291–295. 2011.
View Article : Google Scholar
|
|
22
|
Jia D, Yan M, Wang X, Hao X, Liang L, Liu
L, Kong H, He X, Li J and Yao M: Development of a highly metastatic
model that reveals a crucial role of fibronectin in lung cancer
cell migration and invasion. BMC Cancer. 10:3642010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao Y, Liu X, Lu W, Chen Y, Wu X, Li M,
Wang XA, Zhang F, Jiang L, Zhang Y, et al: Fibronectin promotes
cell proliferation and invasion through mTOR signaling pathway
activation in gallbladder cancer. Cancer Lett. 360:141–150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Digiacomo G, Tusa I, Bacci M, Cipolleschi
MG, Dello Sbarba P and Rovida E: Fibronectin induces macrophage
migration through a SFK-FAK/CSF-1R pathway. Cell Adh Migr. 2:1–11.
2016. View Article : Google Scholar
|
|
25
|
Vakonakis I and Campbell ID: Extracellular
matrix: From atomic resolution to ultrastructure. Curr Opin Cell
Biol. 19:578–583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yousif NG: Fibronectin promotes migration
and invasion of ovarian cancer cells through up-regulation of
FAK-PI3K/Akt pathway. Cell Biol Int. 38:85–91. 2014. View Article : Google Scholar
|
|
27
|
Mitra AK, Sawada K, Tiwari P, Mui K, Gwin
K and Lengyel E: Ligand-independent activation of c-Met by
fibronectin and α(5) β(1)-integrin regulates ovarian cancer
invasion and metastasis. Oncogene. 30:1566–1576. 2011. View Article : Google Scholar
|
|
28
|
Maity G, Choudhury PR, Sen T, Ganguly KK,
Sil H and Chatterjee A: Culture of human breast cancer cell line
(MDA-MB-231) on fibronectin-coated surface induces pro-matrix
metalloproteinase-9 expression and activity. Tumour Biol.
32:129–138. 2011. View Article : Google Scholar
|
|
29
|
Subbaram S and Dipersio CM: Integrin α3β1
as a breast cancer target. Expert Opin Ther Targets. 15:1197–1210.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han S, Sidell N and Roman J: Fibronectin
stimulates human lung carcinoma cell proliferation by suppressing
p21 gene expression via signals involving Erk and Rho kinase.
Cancer Lett. 219:71–81. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Knowles LM, Gurski LA, Engel C, Gnarra JR,
Maranchie JK and Pilch J: Integrin αvβ3 and fibronectin upregulate
Slug in cancer cells to promote clot invasion and metastasis.
Cancer Res. 73:6175–6184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mohri H: Interaction of fibronectin with
integrin receptors: Evidence by use of synthetic peptides.
Peptides. 18:899–907. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng HC, Abdel-Ghany M and Pauli BU: A
novel consensus motif in fibronectin mediates dipeptidyl peptidase
IV adhesion and metastasis. J Biol Chem. 278:24600–24607. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petrache I, Fijalkowska I, Zhen L, Medler
TR, Brown E, Cruz P, Choe KH, Taraseviciene-Stewart L, Scerbavicius
R, Shapiro L, et al: A novel antiapoptotic role for
alpha1-antitrypsin in the prevention of pulmonary emphysema. Am J
Respir Crit Care Med. 173:1222–1228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ozeri E, Mizrahi M, Shahaf G and Lewis EC:
α-1 antitrypsin promotes semimature, IL-10-producing and readily
migrating tolerogenic dendritic cells. J Immunol. 189:146–153.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Malik G, Knowles LM, Dhir R, Xu S, Yang S,
Ruoslahti E and Pilch J: Plasma fibronectin promotes lung
metastasis by contributions to fibrin clots and tumor cell
invasion. Cancer Res. 70:4327–4334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lal A, Lash AE, Altschul SF, Velculescu V,
Zhang L, McLendon RE, Marra MA, Prange C, Morin PJ, Polyak K, et
al: A public database for gene expression in human cancers. Cancer
Res. 59:5403–5407. 1999.PubMed/NCBI
|