Introduction
Cancer metastasis, the spread of cancer cells from
the primary site to distant organs, causes ~90% of human
cancer-associated mortalities (1).
Cancer cells can metastasize by travelling through blood vessels
and lymphatic vasculature. Certain types of cancer mainly invade
via blood vessels, while others primarily use lymphatic vasculature
(2,3). Therefore, identifying agents that
inhibit lymphatic metastasis are important in cancer therapy.
Previous studies have verified that the lymphatic
vasculature has a passive role in the process of cancer metastasis;
moreover, experimental and clinicopathological data indicate that
lymphatic vessels are induced to undergo dynamic changes that
facilitate cancer cell metastasis (4–6).
These changes include lymphangiogenesis and lymphatic enlargement,
which favor the entry of cancer cells into the lymphatic
vasculature (7). While lymphatic
enlargement involves the proliferation of lymphatic endothelial
cells (LECs) and alterations to lymphatic-associated vascular
smooth muscle cells (LVSMCs), lymphangiogenesis is mediated by the
proliferation and migration of LECs (8–10).
Kallistatin, also known as kallikrein-binding
protein (KBP), is a member of the serine proteinase inhibitor
protein family. Kallistatin rapidly binds to tissue kallikrein and
inhibits its enzymatic activity in vitro (11,12).
Kallistatin has been recognized as an effective agent with a
variety of bioactivities in physiological and pathological
responses, including anti-inflammation, anti-angiogenesis and blood
pressure regulation (13–23). Recently, an increasing number of
studies have demonstrated that kallistatin significantly inhibits
tumor-induced angiogenesis and tumor blood vessel metastasis
(15,24–27).
Other researchers have reported that angiogenesis and
lymphangiogenesis share a similar molecular mechanism (28–37).
Thus, it is important to determine whether the angiogenesis
inhibitor kallistatin also has anti-lymphangiogenic effects.
Materials and methods
Materials and cell culture
Vascular endothelial growth factor receptor-3
(VEGFR-3), phospho-ERK (E4) and ERK 1 (K-23) antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Phospho-Akt (Thr308) and total Akt antibodies were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Lymphatic
vessel endothelial hyaluronan receptor 1 (LYVE-1) antibody was
obtained from Abcam (Cambridge, UK) and β-actin antibody was from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Ceramide C6 was
purchased from Sigma-Aldrich and SC79 from Selleck Chemicals
(Houston, TX, USA). Green fluorescent protein-adenovirus (Ad-GFP),
Ad-kallistatin (Ad-KAL) and kallistatin knock-in transgenic mice
(KAL-TG mice) were provided by Professor Jianxing Ma, Department of
Physiology, The University of Oklahoma Health Sciences Center
(Oklahoma, OK, USA). The transgenic mice were generated through a
contracted service at Transgenic Animal Facility at Stanford
University and confirmed by genotyping with PCR using a forward
primer (5′-AGG GAA GAT TGT GGA TTT GG-3′) and a reverse primer
(5′-ATG AAG ATA CCA GTG ATG CTC-3′) specific for the human
kallistatin cDNA.
Human LECs (hLECs; purchased from ProCell, Wuhan,
China) were cultured in ECM medium (ScienCell, San Diego, CA, USA)
with supplements according to the manufacturer's instructions and
incubated at 37°C in a humidified incubator with 5% CO2.
To maintain uniform conditions, all experiments were performed
using cells between passages 2 and 6.
SGC7901 gastric cancer cells were purchased from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and cultured in 10% FBS-supplemented DMEM medium
(Hyclone; GE Healthcare Life Sciences, Chalfont, UK) and incubated
at 37°C in a humidified incubator at 5% CO2.
Expression and purification of
recombinant kallistatin
The recombinant kallistatin (rKAL) cDNA containing a
sequence encoding the full-length mature peptide was amplified from
the total RNA of rat liver by reverse transcription-PCR as
described previously (54). The
PCR product was cloned into the pET28 vector (Novagen) at the
BamHI and SacI sites in frame with the sequence
encoding the His tag at the 3′-end. The kallistatin/pET28 construct
was introduced into Escherichia coli strain BL-21/DE3
(Novagen). The expression and purification of rKAL protein were
carried out as described previously (54). Briefly, expression of kallistatin
was induced by the addition of iso propylthio-β-galactoside (IPTG)
and carried out for 10 h at 25°C. Periplasmic proteins were
released by breaking down bacteria with ultrasonification and
separated from cells by centrifugation. Kallistatin purification
and LPS deletion were accomplished by dialysis with 1K MWCO
(molecular weight cutoff) dialysis membranes and LPS level was
detected in allowed scope. Identity of recombinant kallistatin was
examined by SDS-PAGE and western blot analysis using antibody
specific to His-tag. Then concentration of recombinant kallistatin
was measured by BCA assay and bacteria were eliminated with a
0.22-µm filter. An average of 20 mg of purified kallistatin
in soluble form was obtained from 1 l of culture.
In vivo experiments
Male BALB/c mice (18–22 g) were obtained from Center
of Experimental Animals, Sun Yat-Sen University (Guangzhou, China).
The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Sun Yat-Sen
University (IACUC SYSU, no. 20061211005). Human gastric cancer
cells SGC-7901 (1×107 cells/0.1 ml) were inoculated
subcutaneously in the middle dorsum of each animal. When tumors
reached a volume of 50 mm3, mice were randomized into
two groups. rKAL-treated group received intraperitoneal injection
of recombinant kallistatin with 48-h intervals, and the total
amount of rKAL was 640 nM/ml blood volume (2.88 mg/kg body mass).
Control group was treated with the same volume of PBS. Tumor growth
was monitored by external measurement in 2 dimensions. Tumor volume
was calculated by the following formula: volume (mm3) =
(length x width2)/2, 30 days after the first injection,
the mice were sacrificed and tumors were dissected, and
weighed.
Cell Counting Kit-8 (CCK8) assay
hLECs were seeded in 96-well plates at a cell
density of 5,000 cells per well and were allowed to attach
overnight. The cells were treated with Ad-KAL/Ad-GFP or rKAL/PBS
for 12–48 h. Cell viability was measured using CCK8 assay (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). The absorbance
value at 450 nm was read using a Sunrise Microplate Reader (Tecan
Group Ltd., Männedorf, Switzerland).
EdU staining
EdU staining was according to the protocol of the
EdU staining kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China), and the images were captured with ZEISS Axio Imager Z1
(Zeiss GmbH, Jena, Germany).
Apoptosis assay
Apoptosis was determined using flow cytometry with a
commercial Annexin V-FITC Apoptosis kit (Vazyme Biotech, Nanjing,
China) according to the manufacturer's protocol. Briefly, following
treatment with different concentrations of rKAL, the cells were
washed in ice-cold PBS and trypsinized gently using a trypsin
solution. After centrifugation to remove the trypsin, the cells
were re-suspended in binding buffer containing Annexin V-FITC and
propidium iodide (PI), then incubated for 15 min at room
temperature in the dark and subsequently analyzed on a Beckman
CytoFLEX flow cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Boyden chamber cell migration assay
Following incubation with rKAL and Ad-GFP/Ad-KAL,
5×104 hLECs were seeded into the upper chamber of the
inserts in 200 µl basal medium, and the bottom chamber was
filled with 500 µl complete ECM as the chemoattractant.
After 12 h, the inserts were washed with PBS, then fixed in 5%
glutaraldehyde for 15 min, and the non-migrated cells in the upper
chamber were removed using cotton swabs. The migrated cells were
stained with crystal violet and imaged using a microscope. Finally,
cell counting was performed using ImageJ software (NIH, Bethesda,
MA, USA).
Wound healing assay
Cells were seeded in 6-well plates and cultured to
100% confluence. The cell monolayer was scratched using a
10-µl pipette tip to produce scratches of a constant width.
Cells were then incubated with the indicated treatments, and cells
invading the wound line were imaged with a ZEISS Axio Observer Z1.
A video was produced using Axiovison 4.7 software (Zeiss GmbH).
Tube formation assay
A tube formation assay was performed by pipetting
200 µl Matrigel (BD Biosciences, Bedford, MA, USA) into each
well of a 24-well plate, which was then polymerized for 30 min at
37°C. hLECs (2×104) in 200 µl Gibco ECM medium
(Thermo Fisher Scientific Inc., Gaithersburg, MD, USA) with rKAL or
Ad-GFP/KAL were added to each well and incubated at 37°C, 5%
CO2 for 12 h. Images were captured using a bright-field
microscope ZEISS Axio Observer Z1 (Zeiss GmbH).
Immunofluorescence
Histological sections, 5-µm-thick, prepared
from frozen tissue samples, were used for immunofluorescence
analysis. Identification of tissue lymphatic vessels and hLECs was
performed using immunofluorescence with an antibody against mouse
LYVE-1 (Abcam) and VEGFR-3 (Santa Cruz Biotechnology, Inc.).
Immunohistochemistry
Histological sections, 5-µm-thick, prepared
from paraffin-fixed tissue samples, were used for
immunohistochemical analysis. Identification of tissue lymphatic
vessels and hLECs was performed by immunohistochemistry using
antibodies to detect VEGFR-3 (Santa Cruz Biotechnology, Inc.) and
LYVE-1 (Abcam).
Immunoblotting
hLECs transfected with Ad-GFP/Ad-KAL for 48 h were
washed with cold PBS and lysed. The cell lysates were resolved
using SDS-PAGE and transferred onto a nitrocellulose membrane. The
membranes were blocked in 10% skimmed milk for 1 h at room
temperature and probed with antibodies: VEGFR-3, ERK (Santa Cruz
Biotechnology, Inc.), p-ERK, AKT, p-AKT (CST Biotechnology) and
β-actin (Sigma). Membranes were incubated with ECL solution
(Applygen Technologies, Beijing, China) and bound antibodies were
detected using ImageQuant Las 4000mini (GE Healthcare Life
Sciences). Western blot quantification was performed using ImageJ
(NIH).
Statistical analyses
Data are expressed as the mean ± standard deviation.
Multiple comparisons were assessed by one-way analysis of variance
or Student's t-test using SPSS 13.0 software (SPSS Inc., Chicago,
IL, USA) and differences with P<0.05 were considered
statistically significant. All experiments were performed at least
three times.
Results
Kallistatin inhibits proliferation and
promotes apoptosis of hLECs
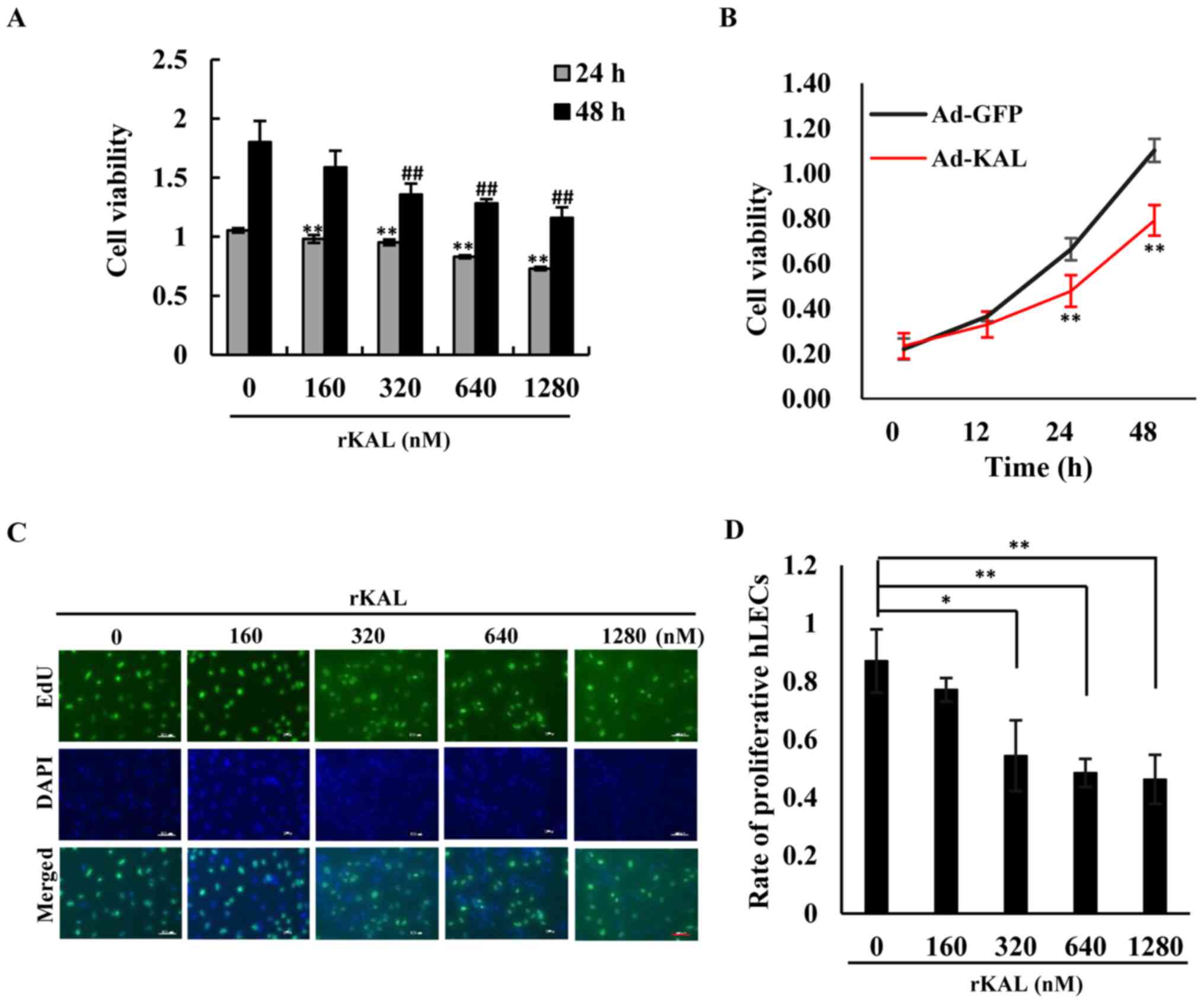
We aimed to determine whether kallistatin had a
direct anti-lymphangiogenic effect on LECs. As the results
demonstrate, kallistatin inhibited the proliferation of hLECs in a
dose- and time-dependent manner. At a dose of 160 nM, kallistatin
had little effect on cell proliferation after a 48-h treatment in
complete medium, whereas it exhibited a significant inhibitory
effect at 320–1,280-nM concentrations (Fig. 1A, C and D). Overexpression of
kallistatin via transfection with Ad-KAL produced a similar effect
on cell proliferation (Fig. 1B).
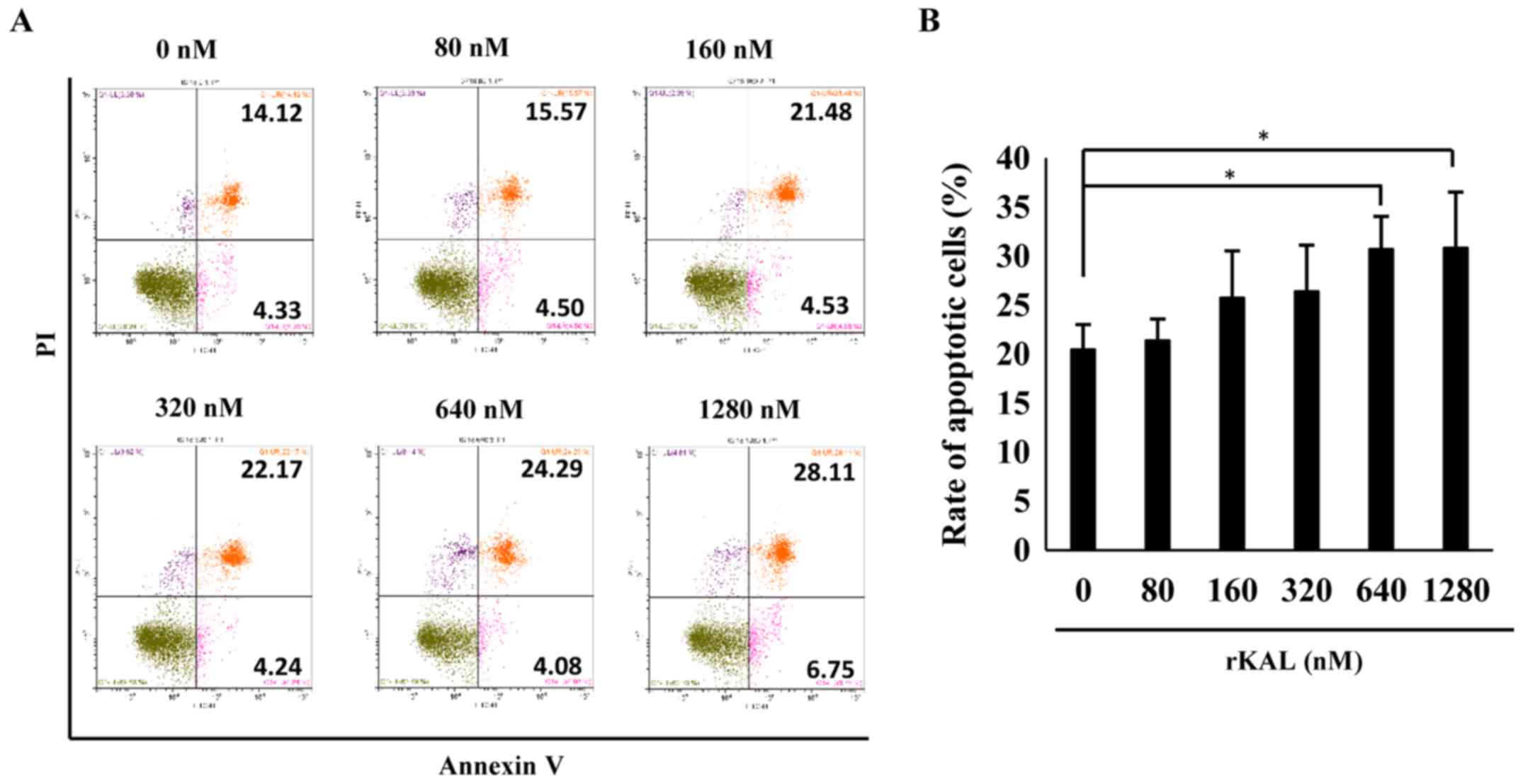
To validate the effect of kallistatin on apoptosis, flow cytometry
was performed to analyze AnnexinV/PI-stained hLECs. Kallistatin
marginally promoted apoptosis of hLECs at doses of 640 and 1,280 nM
(Fig. 2). Taken together, the
results indicate that kallistatin inhibits the survival of hLECs
and directly influences lymphangiogenesis.
Kallistatin inhibits migration of
LECs
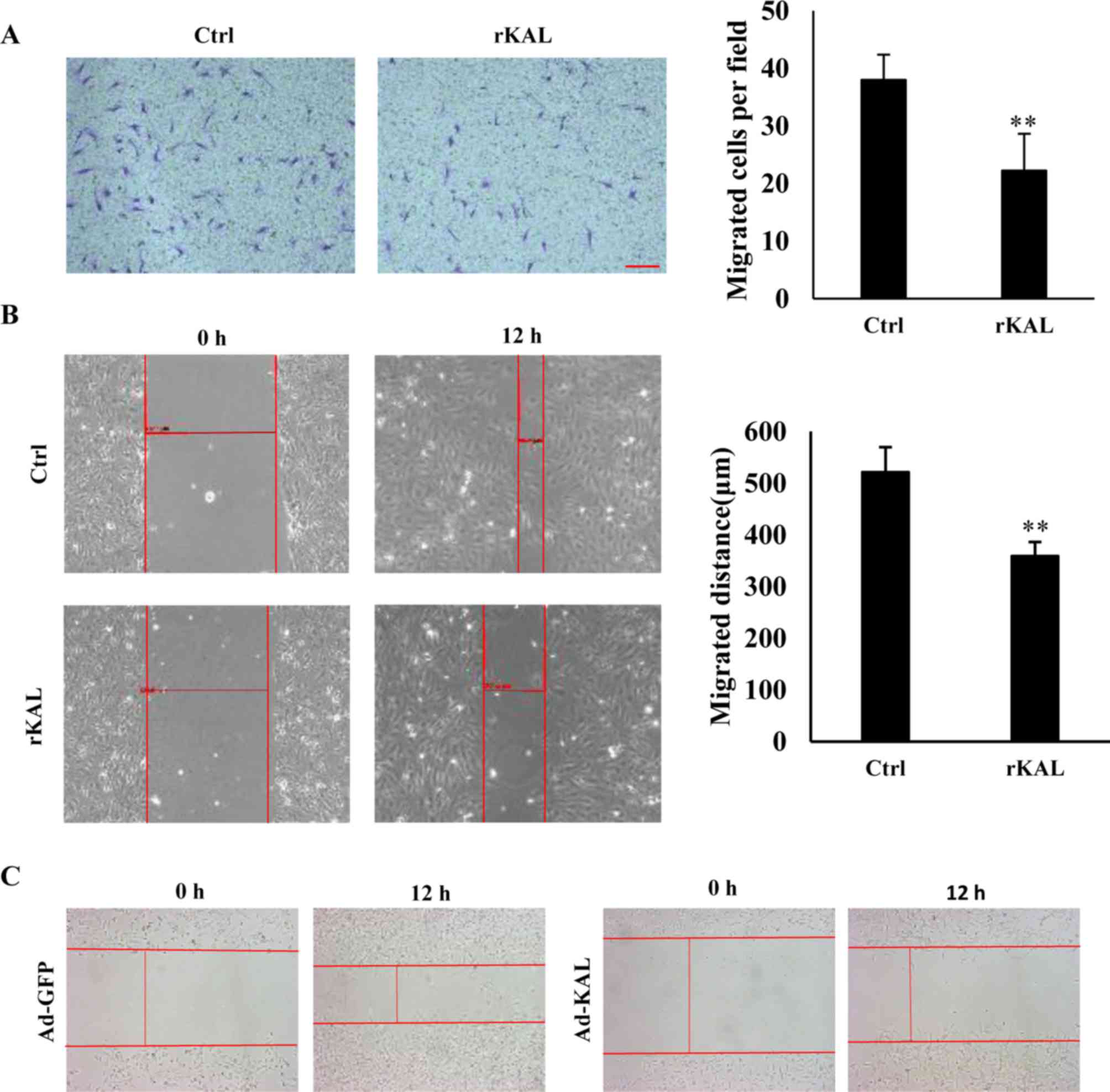
As hLEC migration is involved in the process of
lymphangiogenesis, a Boyden chamber cell migration assay and
wound-healing assay were performed to determine the influence of
kallistatin on the migration of hLECs. Following a 12-h incubation,
kallistatin inhibited the migration of hLECs (Fig. 3A and B). Overexpression of
kallistatin by transfection with Ad-KAL exhibited a similar effect
on cell migration (Fig. 3C). These
results also demonstrate a direct inhibitory effect of kallistatin
on lymphangiogenesis.
Kallistatin inhibits tube formation of
LECs
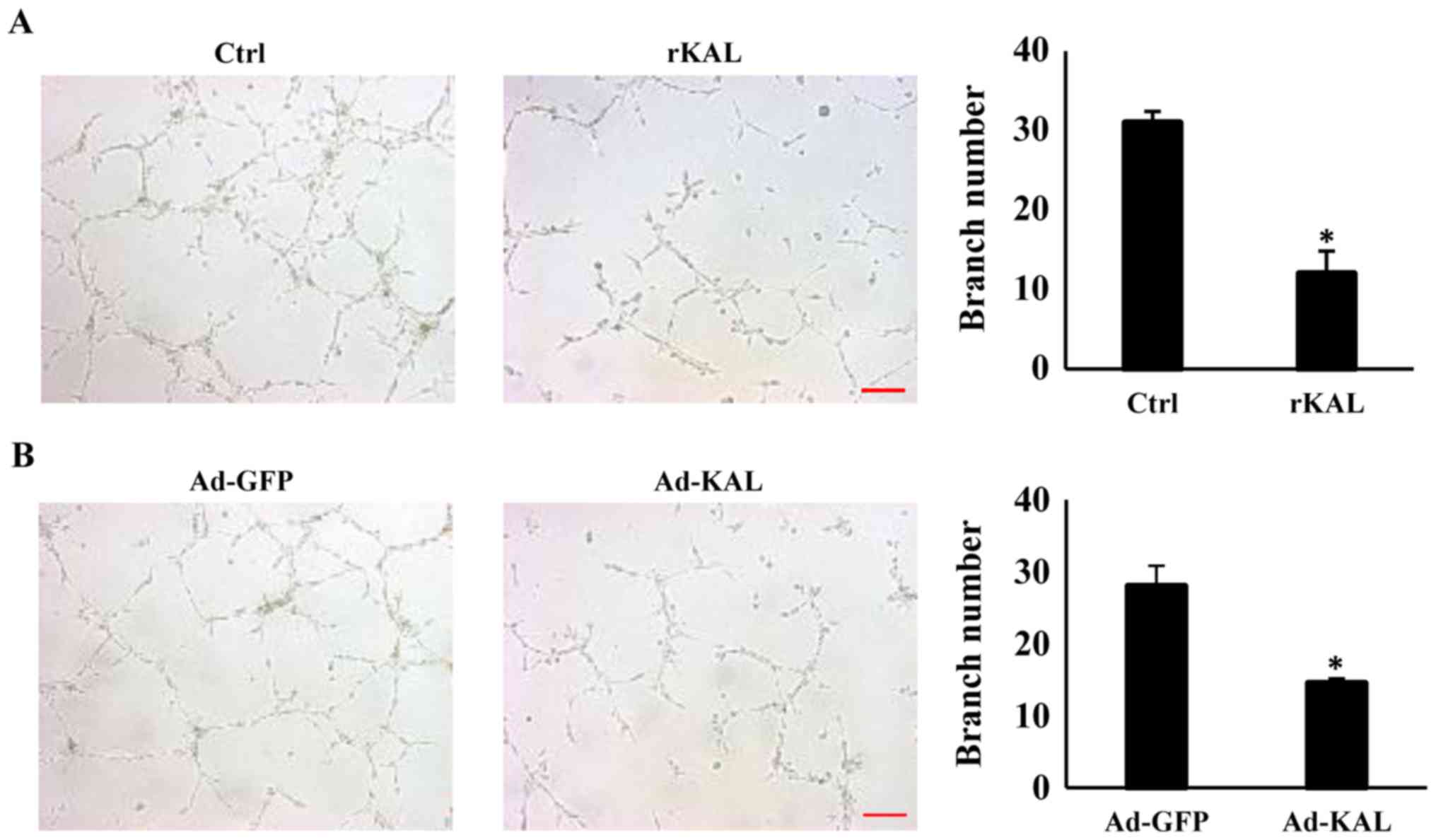
To gain more direct evidence of the inhibitory
effect of kallistatin on lymphangiogenesis, the action of
kallistatin on lymphangiogenic responses was characterized further.
After treatment with 640 nM rKAL or Ad-KAL transfection, the number
of lymphatic tubes formed was significantly reduced, which suggests
that kallistatin is a potent inhibitor of lymphangiogenesis
(Fig. 4). These results showed
that kallistatin exhibited potent inhibitory effect on
lymphangiogenesis ex vivo, we further checked whether this
effect also exists in vivo.
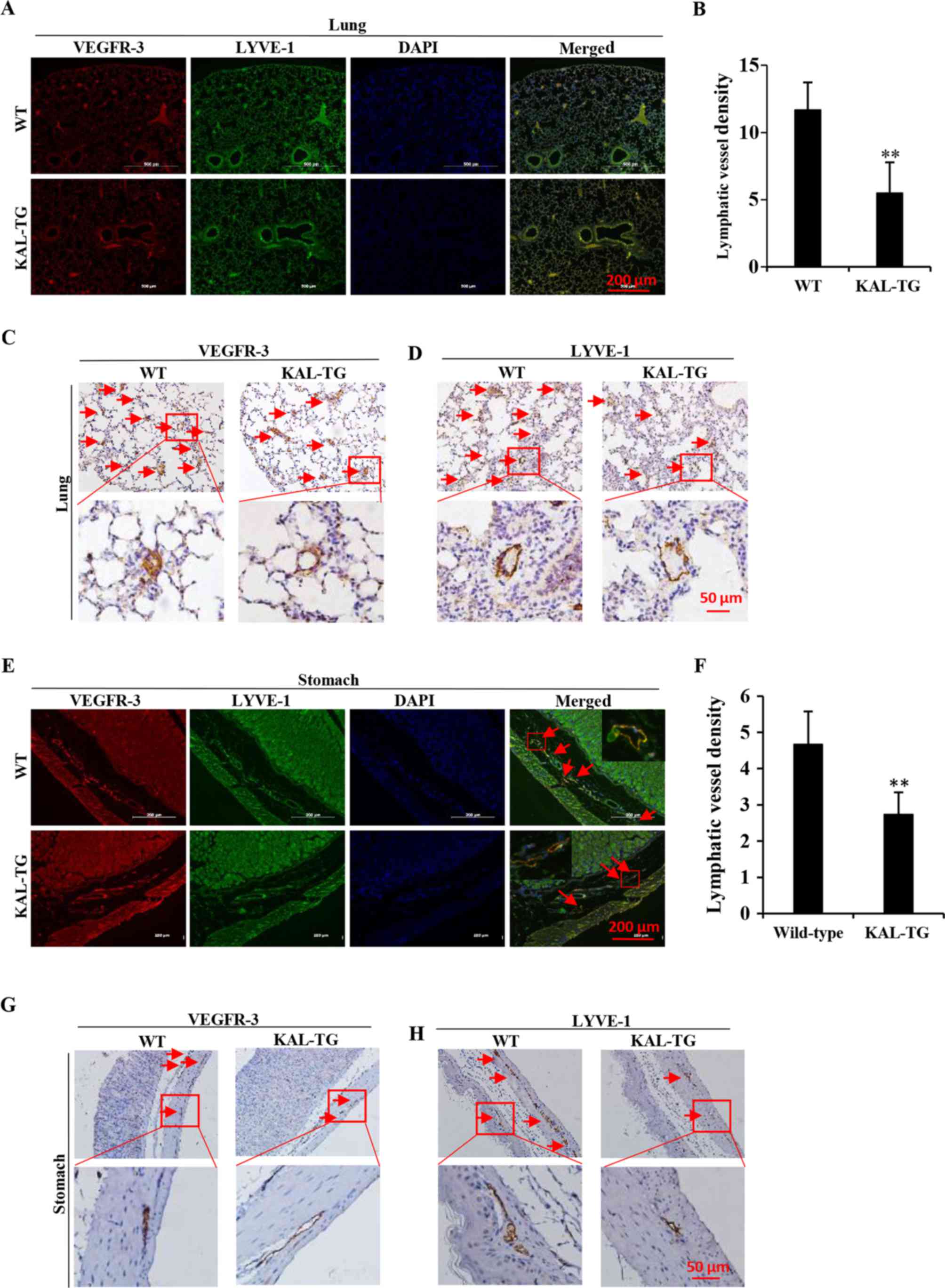
Kallistatin inhibits lymphangiogenesis in
vivo
To investigate the effect of kallistatin on
lymphangiogenesis in vivo, the lymphatic vascular density
(LVD) was assessed in 5 adult wild-type and 5 KAL-TG knock-in
C57BL/6 mice (6–8 weeks). The lymphatic vasculature was stained
with LEC-specific markers, VEGFR-3 and LYVE-1. As the results
demonstrate (Fig. 5), LVD in the
KAL-TG mice was significantly lower than in wild-type mice.
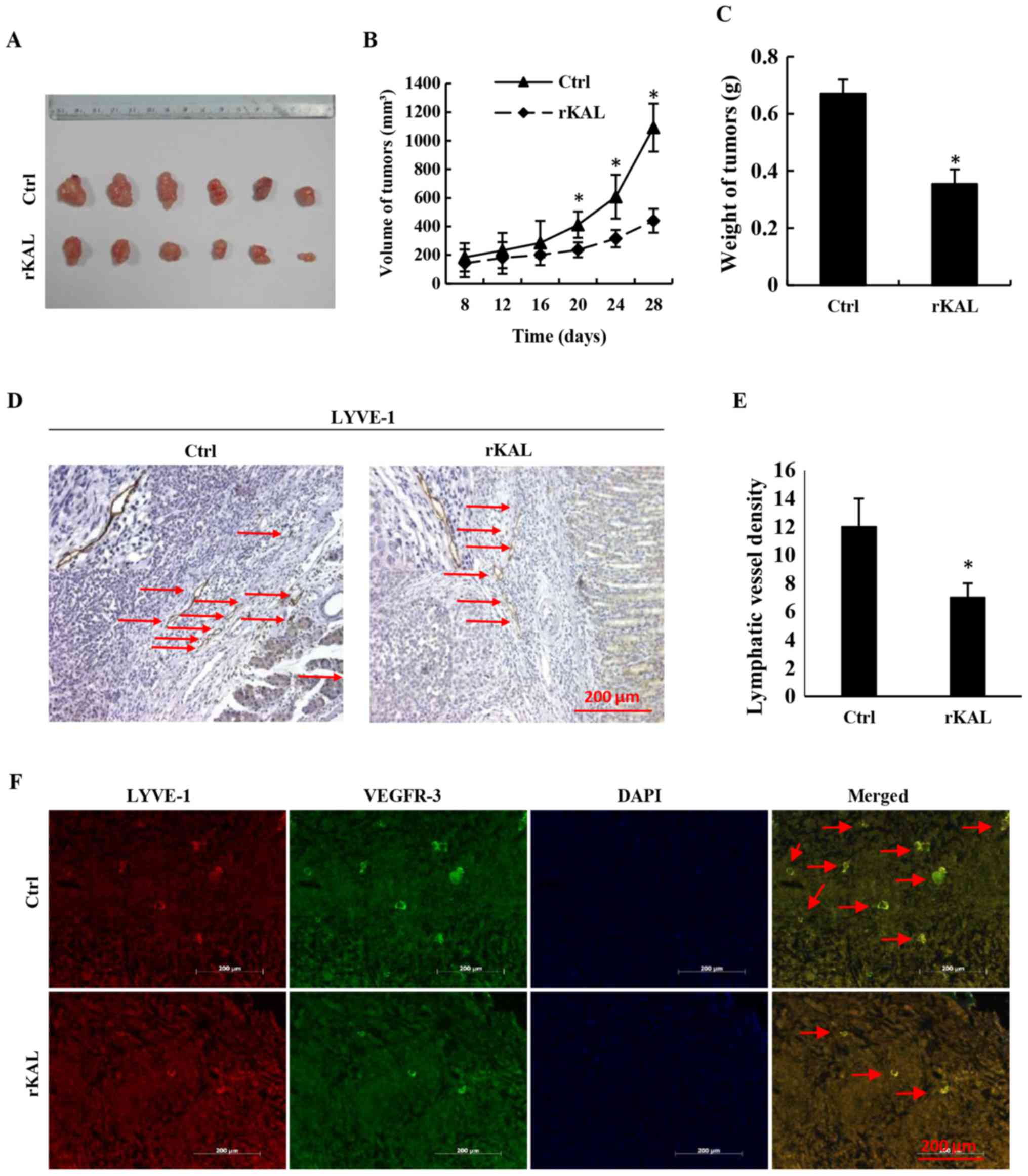
Additionally, recombinant kallistatin was used to treat nude mice
with gastric cancer xenografts, and the LVD in the primary tumors
was subsequently analyzed by staining for lymphatics. Similarly, we
observed that kallistatin reduced the LVD in the gastric tumors
(Fig. 6). Taken together, these
results indicate that kallistatin also exerts anti-lymphan-giogenic
effects in vivo.
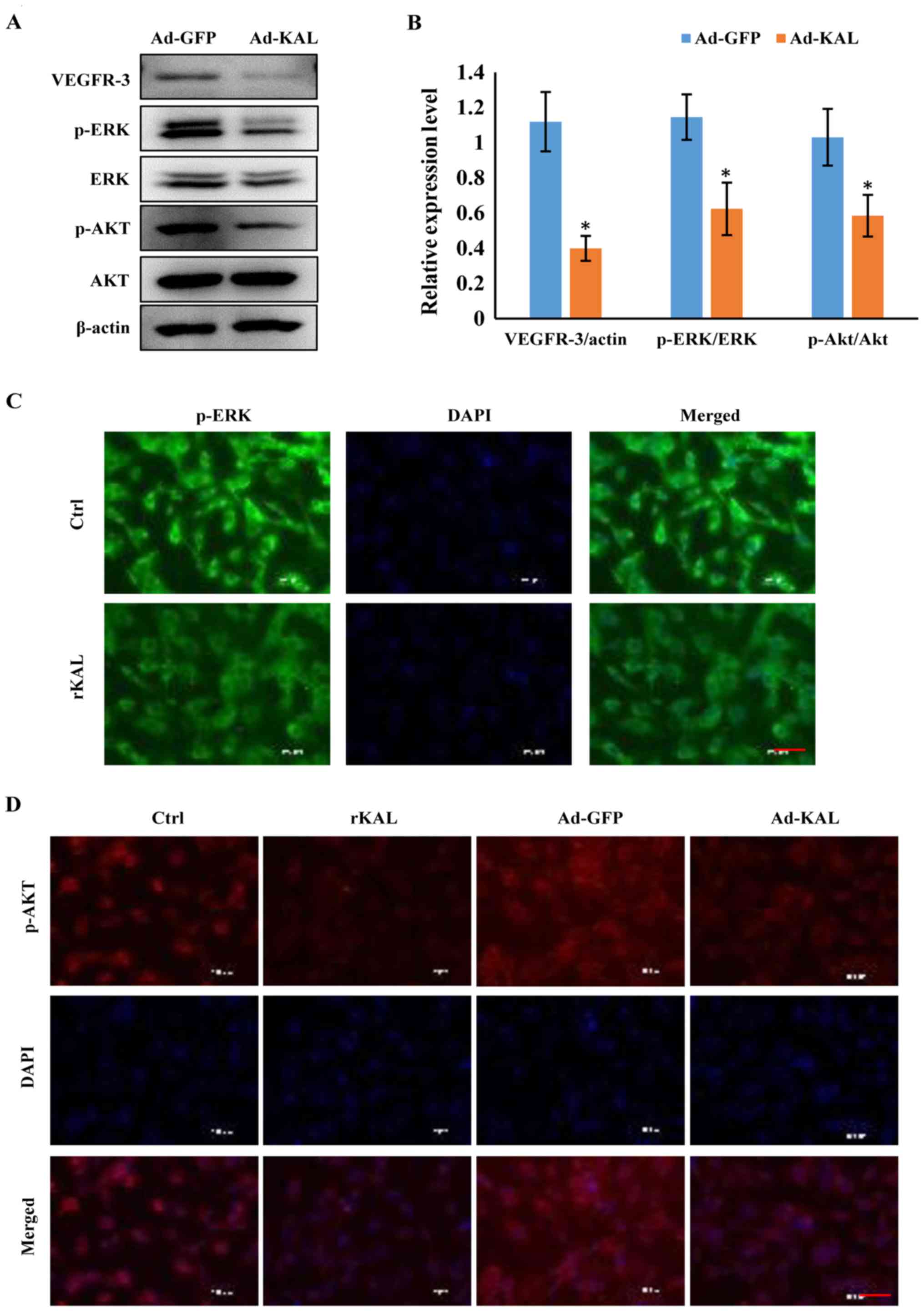
Kallistatin inhibits expression of
VEGFR-3 and downstream signaling pathways in LECs
As a VEGF-C-specific receptor, VEGFR-3 has critical
roles in lymphangiogenesis. Therefore, we investigated the actions
of kallistatin on VEGFR-3 expression. After 48 h of Ad-KAL
transfection, expression of VEGFR-3 in hLECs was reduced (Fig. 7A). Additionally, the
phosphorylation of the downstream signaling proteins ERK and Akt
was decreased by Ad-KAL transfection, whereas there was no
observable effect on total ERK and Akt expression (Fig. 7B and C). To understand the effect
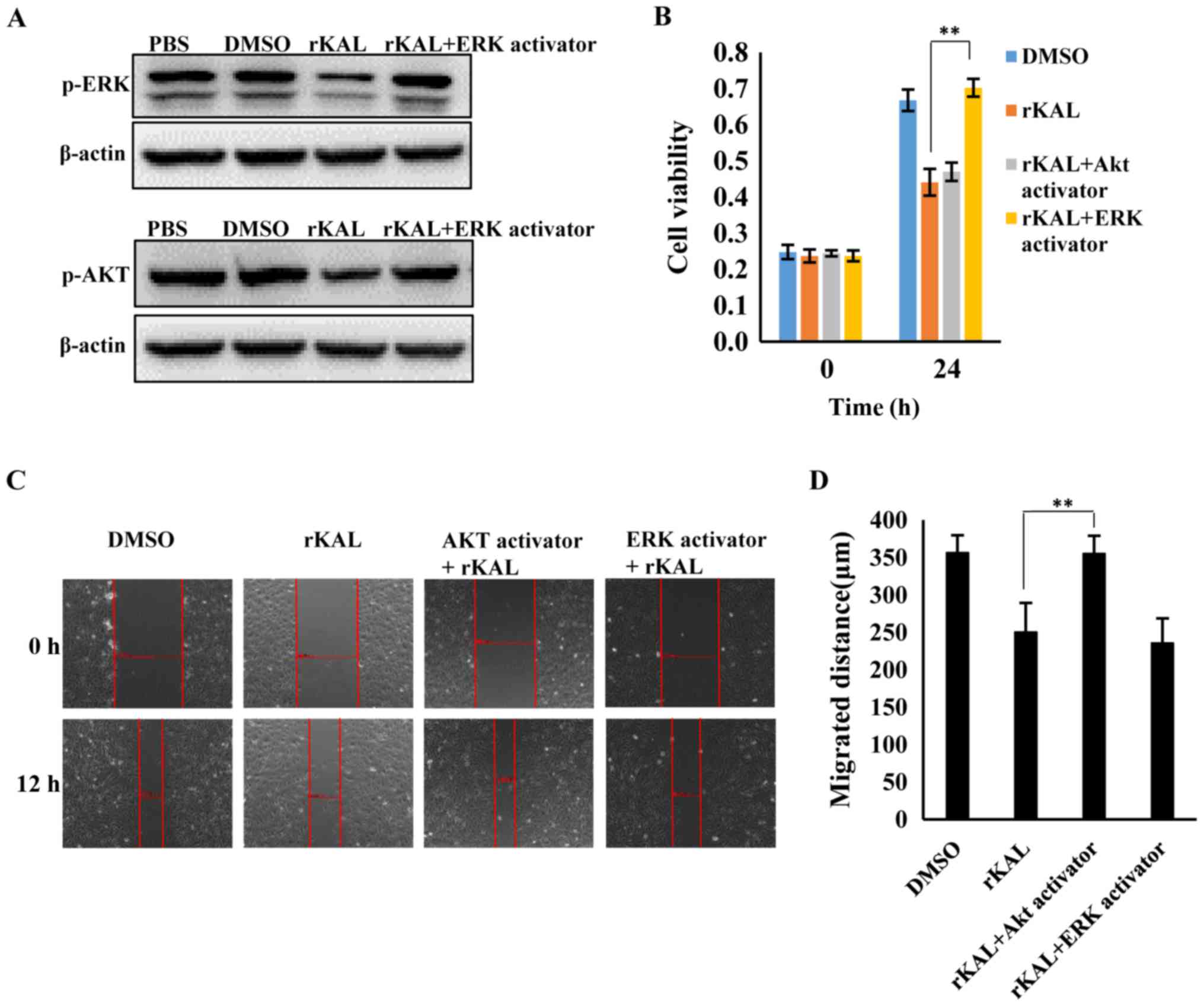
of kallistatin further, hLECs were treated with rKAL, and
simultaneously, ERK and Akt signaling were activated using ceramide
C6 or SC79, respectively. The proliferation and migration of hLECs
was subsequently analyzed. The inhibition of proliferation induced
by rKAL was rescued by ERK activation using ceramide C6, and
inhibition of migration induced by rKAL was counteracted by Akt
activation using SC79 (Fig. 8).
These outcomes suggest that kallistatin inhibits proliferation and
migration of hLECs by reducing the activation of ERK and Akt
signaling, respectively.
Discussion
The central finding of this study was that
kallistatin inhibits lymphangiogenesis in vivo and in
vitro. Lymphangiogenesis involves various processes in LECs,
including proliferation, migration and tube formation. Our data
indicate that kallistatin inhibits lymphangiogenesis by promoting
apoptosis and suppressing proliferation, migration and tube
formation of LECs.
Tumor-induced lymphangiogenesis and lymphatic
remodeling play an important role in lymphatic metastasis, similar
to the role of angiogenesis in metastasis via blood vessels
(3,38,39).
While angiogenesis has received much attention over the past few
decades, and many anti-angiogenic agents, such as sorafenib, have
entered clinical use, there is a lack of drugs designed to inhibit
lymphangiogenesis. The present study demonstrates that kallistatin
is an active anti-lymphangiogenic agent that potently inhibits
lymphangiogenesis in vitro and in vivo.
Lymphangiogenesis requires careful coordination of complex cellular
events, including proliferation, migration and tube formation
(3). The proliferation, survival
and migration of hLECs are central to the process of
lymphangiogenesis. As a potent angiogenesis inhibitor, kallistatin
exhibits significant effects on proliferation, apoptosis, migration
and tube formation of vascular endothelial cells (26,40).
Interestingly, we found that kallistatin has similar effects on
LECs, with significantly reduced proliferation, migration and tube
formation of LECs in vitro following treatment with
kallistatin. Moreover, kallistatin also promoted the apoptosis of
LECs. Taken together, these results suggest that kallistatin is an
effective inhibitor of lymphangiogenesis.
Compared with angiogenesis, the molecular mechanisms
regulating lymphangiogenesis are less well established.
Understanding the functions and regulatory pathways of this system
will undoubtedly lead to novel therapeutic targets and
corresponding drugs.
By binding to its cellular receptor, VEGFR-3, VEGF-C
induce VEGFR-3 phosphorylation and activates downstream signaling
pathways (41). Many inhibitors of
lymphangiogenesis or angiogenesis, such as sorafenib and
regorafenib, are VEGF receptor tyrosine kinase inhibitors, which
inhibit the phosphorylation of VEGFR-3 (42–46),
while other drugs act by downregulating the expression of VEGFR-3
(47). Our current study shows
that kallistatin modulates VEGFR-3 signaling by inhibiting its
expression. Since VEGFR-3 plays an important role in tumor-induced
lymphangiogenesis, the data suggest that kallistatin treatment may
also inhibit lymphatic metastasis. Huang et al reported that
kallistatin may reduce the phosphorylation of VEGFR-2 in human
umbilical vein endo-thelial cells, by which it can inhibit
angiogenesis (26). As the
majority of the lymphatic endothelium is derived from venous
endothelial cells, it is not surprising that they share similar
signaling systems.
As kallistatin reduces VEGFR-3 expression, and VEGFR
tyrosine kinase inhibitors inhibit phosphorylation of
VEGFR-2/VEGFR-3, their combination may synergistically inhibit
tumor angiogenesis and lymphangiogenesis. Many VEGFR tyrosine
kinase inhibitors, such as regorafenib and sorafenib, produce
severe adverse effects during treatment, limiting their clinical
applications (43–46). This synergistic interaction could
be utilized to develop new strategies to increase the efficacy
and/or reduce the toxicity of agents that interfere with VEGFRs. By
disrupting both VEGFR-2 and VEGFR-3, kallistatin is a potential
dual-effect agent that could be used to target cancers that spread
via blood vessels and the lymphatic vasculature.
Consistent with the inhibition of VEGFR-3,
kallistatin also reduced activation of the downstream signaling
pathways of VEGFR-3, such as phosphorylation of ERK and Akt. ERK
has been previously shown to regulate proliferation of lymphatic
endothelium, and Akt has been shown to have a critical role in cell
migration (48–53). Therefore, the inhibitory effects of
kallistatin on these signaling proteins are consistent with its
suppression of cell proliferation and migration of LECs.
Taken together, this study demonstrated that
kallistatin inhibited lymphangiogenesis in vitro and in
vivo through inhibition of VEGFR-3/ERK and VEGFR-3/Akt
signaling. As lymphangiogenesis plays an important role in tumor
metastasis, our study suggests that kallistatin may be a useful
inhibitor of lymphatic metastasis.
Acknowledgments
This study was supported by National Nature Science
Foundation of China, grant nos. 81272338, 81272515, 81370945,
81471033, 81572342, 81570871, 81570764 and 81600641; National Key
Sci-Tech Special Project of China, grant nos. 2013ZX09102053 and
2015GKS355. Program for Doctoral Station in University, grant no.
20130171110053; Key Project of Nature Science Foundation of
Guangdong Province, China, grant nos. 015A030311043 and
2016A030311035. Guandong Natural Science Fund, grant nos.
2014A030313073, 2015A030313103 and 2015A030313029. Guandong Science
and Technology Project (2014A020212023 and 2015B090903063); Key
Sci-tech Research Project of Guangzhou Municipality, China, grant
nos. 2014J4100162 and 201508020033; Changjiang Scholars and
Innovative Research Team in University, no. 985 project PCSIRT
0947; Fundamental Research Funds for the Central Universities of
China (Youth Program 13ykpy06, 31610046 and 16ykpy24). National
Nature Science Foundation of China, no. 81502507. Sci-tech Research
Project of Guangzhou, no. 201607010200.
References
|
1
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stacker SA, Williams SP, Karnezis T,
Shayan R, Fox SB and Achen MG: Lymphangiogenesis and lymphatic
vessel remodelling in cancer. Nat Rev Cancer. 14:159–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshimatsu Y, Miyazaki H and Watabe T:
Roles of signaling and transcriptional networks in pathological
lymphangiogenesis. Adv Drug Deliv Rev. 99B:161–171. 2016.
View Article : Google Scholar
|
|
5
|
Liu L, Lin C, Liang W, Wu S, Liu A, Wu J,
Zhang X, Ren P, Li M and Song L: TBL1XR1 promotes lymphangiogenesis
and lymphatic metastasis in esophageal squamous cell carcinoma.
Gut. 64:26–36. 2015. View Article : Google Scholar
|
|
6
|
Hirakawa S: From tumor lymphangiogenesis
to lymphvascular niche. Cancer Sci. 100:983–989. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dadras SS, Paul T, Bertoncini J, Brown LF,
Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC and Detmar
M: Tumor lymphangiogenesis: A novel prognostic indicator for
cutaneous melanoma metastasis and survival. Am J Pathol.
162:1951–1960. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He Y, Rajantie I, Pajusola K, Jeltsch M,
Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T and
Alitalo K: Vascular endothelial cell growth factor receptor
3-mediated activation of lymphatic endothelium is crucial for tumor
cell entry and spread via lymphatic vessels. Cancer Res.
65:4739–4746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karpanen T, Egeblad M, Karkkainen MJ, Kubo
H, Ylä-Herttuala S, Jäättelä M and Alitalo K: Vascular endothelial
growth factor C promotes tumor lymphangiogenesis and
intra-lymphatic tumor growth. Cancer Res. 61:1786–1790.
2001.PubMed/NCBI
|
|
10
|
Karnezis T, Shayan R, Caesar C, Roufail S,
Harris NC, Ardipradja K, Zhang YF, Williams SP, Farnsworth RH, Chai
MG, et al: VEGF-D promotes tumor metastasis by regulating
prostaglandins produced by the collecting lymphatic endothelium.
Cancer Cell. 21:181–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang MY, Day J, Chao L and Chao J: Human
kallistatin, a new tissue kallikrein-binding protein: Purification
and characterization. Adv Exp Med Biol. 247B:1–8. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chao J and Chao L: Biochemistry,
regulation and potential function of kallistatin. Biol Chem Hoppe
Seyler. 376:705–713. 1995.PubMed/NCBI
|
|
13
|
Miao RQ, Murakami H, Song Q, Chao L and
Chao J: Kallistatin stimulates vascular smooth muscle cell
proliferation and migration in vitro and neointima formation in
balloon-injured rat artery. Circ Res. 86:418–424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chao J, Miao RQ, Chen V, Chen LM and Chao
L: Novel roles of kallistatin, a specific tissue kallikrein
inhibitor, in vascular remodeling. Biol Chem. 382:15–21. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miao RQ, Agata J, Chao L and Chao J:
Kallistatin is a new inhibitor of angiogenesis and tumor growth.
Blood. 100:3245–3252. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stadnicki A, Mazurek U, Plewka D and
Wilczok T: Intestinal tissue kallikrein-kallistatin profile in
inflammatory bowel disease. Int Immunopharmacol. 3:939–944. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chao J, Yin H, Yao YY, Shen B, Smith RS Jr
and Chao L: Novel role of kallistatin in protection against
myocardial ischemia-reperfusion injury by preventing apoptosis and
inflammation. Hum Gene Ther. 17:1201–1213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen B, Hagiwara M, Yao YY, Chao L and
Chao J: Salutary effect of kallistatin in salt-induced renal
injury, inflammation, and fibrosis via antioxidative stress.
Hypertension. 51:1358–1365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen B, Gao L, Hsu YT, Bledsoe G, Hagiwara
M, Chao L and Chao J: Kallistatin attenuates endothelial apoptosis
through inhibition of oxidative stress and activation of Akt-eNOS
signaling. Am J Physiol Heart Circ Physiol. 299:H1419–H1427. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu SL, Tsai CY, Luo YH, Kuo CF, Lin WC,
Chang YT, Wu JJ, Chuang WJ, Liu CC, Chao L, et al: Kallistatin
modulates immune cells and confers anti-inflammatory response to
protect mice from group A streptococcal infection. Antimicrob
Agents Chemother. 57:5366–5372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li P, Bledsoe G, Yang ZR, Fan H, Chao L
and Chao J: Human kallistatin administration reduces organ injury
and improves survival in a mouse model of polymicrobial sepsis.
Immunology. 142:216–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li P, Guo Y, Bledsoe G, Yang Z, Chao L and
Chao J: Kallistatin induces breast cancer cell apoptosis and
autophagy by modulating Wnt signaling and microRNA synthesis. Exp
Cell Res. 340:305–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yiu WH, Wong DW, Wu HJ, Li RX, Yam I, Chan
LY, Leung JC, Lan HY, Lai KN and Tang SC: Kallistatin protects
against diabetic nephropathy in db/db mice by suppressing
AGE-RAGE-induced oxidative stress. Kidney Int. 89:386–398. 2016.
View Article : Google Scholar
|
|
24
|
Diao Y, Ma J, Xiao WD, Luo J, Li XY, Chu
KW, Fung P, Habib N, Farzaneh F and Xu RA: Inhibition of
angiogenesis and HCT-116 xenograft tumor growth in mice by
kallistatin. World J Gastroenterol. 13:4615–4619. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang KF, Huang XP, Xiao GQ, Yang HY, Lin
JS and Diao Y: Kallistatin, a novel anti-angiogenesis agent,
inhibits angiogenesis via inhibition of the NF-κB signaling
pathway. Biomed Pharmacother. 68:455–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang KF, Yang HY, Xing YM, Lin JS and
Diao Y: Recombinant human kallistatin inhibits angiogenesis by
blocking VEGF signaling pathway. J Cell Biochem. 115:575–584. 2014.
View Article : Google Scholar
|
|
27
|
Sun HM, Mi YS, Yu FD, Han Y, Liu XS, Lu S,
Zhang Y, Zhao SL, Ye L, Liu TT, et al: SERPINA4 is a novel
independent prognostic indicator and a potential therapeutic target
for colorectal cancer. Am J Cancer Res. 6:1636–1649.
2016.PubMed/NCBI
|
|
28
|
Scavelli C, Weber E, Aglianò M, Cirulli T,
Nico B, Vacca A and Ribatti D: Lymphatics at the crossroads of
angiogenesis and lymphangiogenesis. J Anat. 204:433–449. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scavelli C, Vacca A, Di Pietro G, Dammacco
F and Ribatti D: Crosstalk between angiogenesis and
lymphangiogenesis in tumor progression. Leukemia. 18:1054–1058.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van den Eynden GG, Van der Auwera I, Van
Laere SJ, Trinh XB, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB
and Van Marck EA: Comparison of molecular determinants of
angiogenesis and lymphangiogenesis in lymph node metastases and in
primary tumours of patients with breast cancer. J Pathol.
213:56–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adams RH and Alitalo K: Molecular
regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell
Biol. 8:464–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garmy-Susini B and Varner JA: Roles of
integrins in tumor angiogenesis and lymphangiogenesis. Lymphat Res
Biol. 6:155–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gomes FG, Nedel F, Alves AM, Nör JE and
Tarquinio SB: Tumor angiogenesis and lymphangiogenesis:
Tumor/endothelial crosstalk and cellular/microenvironmental
signaling mechanisms. Life Sci. 92:101–107. 2013. View Article : Google Scholar :
|
|
34
|
Sasahira T, Ueda N, Yamamoto K, Kurihara
M, Matsushima S, Bhawal UK, Kirita T and Kuniyasu H: Prox1 and
FOXC2 act as regulators of lymphangiogenesis and angiogenesis in
oral squamous cell carcinoma. PLoS One. 9:e925342014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Riabov V, Gudima A, Wang N, Mickley A,
Orekhov A and Kzhyshkowska J: Role of tumor associated macrophages
in tumor angiogenesis and lymphangiogenesis. Front Physiol.
5:752014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Escobedo N and Oliver G:
Lymphangiogenesis: Origin, specification, and cell fate
determination. Annu Rev Cell Dev Biol. 32:677–691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Corliss BA, Azimi MS, Munson JM, Peirce SM
and Murfee WL: Macrophages: An inflammatory link between
angiogenesis and lymphangiogenesis. Microcirculation. 23:95–121.
2016. View Article : Google Scholar :
|
|
38
|
Paduch R: The role of lymphangiogenesis
and angiogenesis in tumor metastasis. Cell Oncol. 39:397–410. 2016.
View Article : Google Scholar
|
|
39
|
Orellana C: Is lymphangiogenesis as
important as angiogenesis? Lancet Oncol. 6:2652005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu B, Lu L, Cai W, Yang X, Li C, Yang Z,
Zhan W, Ma JX and Gao G: Kallikrein-binding protein inhibits growth
of gastric carcinoma by reducing vascular endothelial growth factor
production and angiogenesis. Mol Cancer Ther. 6:3297–3306. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tammela T and Alitalo K:
Lymphangiogenesis: Molecular mechanisms and future promise. Cell.
140:460–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Morabito A, De Maio E, Di Maio M, Normanno
N and Perrone F: Tyrosine kinase inhibitors of vascular endothelial
growth factor receptors in clinical trials: Current status and
future directions. Oncologist. 11:753–764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al TARGET Study Group: Sorafenib in advanced clear-cell
renal-cell carcinoma. N Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu S, Chen JJ, Kudelka A, Lu J and Zhu X:
Incidence and risk of hypertension with sorafenib in patients with
cancer: A systematic review and meta-analysis. Lancet Oncol.
9:117–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Demetri GD, Reichardt P, Kang YK, Blay JY,
Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M,
Joensuu H, et al GRID study investigators: Efficacy and safety of
regorafenib for advanced gastrointestinal stromal tumours after
failure of imatinib and sunitinib (GRID): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:295–302. 2013. View Article : Google Scholar
|
|
46
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al CORRECT Study Group: Regorafenib monotherapy for previously
treated metastatic colorectal cancer (CORRECT): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:303–312. 2013. View Article : Google Scholar
|
|
47
|
Wang W, Sukamotoh E, Xiao H and Zhang G:
Curcumin inhibits lymphangiogenesis in vitro and in vivo. Mol Nutr
Food Res. 59:2345–2354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cheng GZ, Park S, Shu S, He L, Kong W,
Zhang W, Yuan Z, Wang LH and Cheng JQ: Advances of AKT pathway in
human oncogenesis and as a target for anti-cancer drug discovery.
Curr Cancer Drug Targets. 8:2–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tan L, Song X, Sun X, Wang N, Qu Y and Sun
Z: ART3 regulates triple-negative breast cancer cell function via
activation of Akt and ERK pathways. Oncotarget. 7:46589–46602.
2016.PubMed/NCBI
|
|
50
|
Kaliszczak M, Trousil S, Ali T and Aboagye
EO: AKT activation controls cell survival in response to HDAC6
inhibition. Cell Death Dis. 7:e22862016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16(Suppl 2):
S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar
|
|
53
|
Gao L, Li P, Zhang J, Hagiwara M, Shen B,
Bledsoe G, Chang E, Chao L and Chao J: Novel role of kallistatin in
vascular repair by promoting mobility, viability, and function of
endothelial progenitor cells. J Am Heart Assoc. 3:e0011942014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gao G, Shao C, Zhang SX, Dudley A, Fant J
and Ma JX: Kallikrein-binding protein inhibits retinal
neovascularization and decreases vascular leakage. Diabetologia.
46:689–698. 2003. View Article : Google Scholar : PubMed/NCBI
|