Introduction
Angiogenesis is the vital physiological process
involving the growth and remodeling of new blood vessels and is
implicated in a number of diseases including cancer.
Neoangiogenesis is essential for tumor growth as well as crucial
for local and distant metastatization through both blood and
lymphatic vessels (1,2). Therefore, many new targeted therapies
have been developed and they are based on drugs able to bind
vascular endothelial growth factor (VEGF) and its receptor (VEGFR),
which have been shown to be upregulated in tumor and highly
proliferating endothelial cells. Such overexpression has been
associated with progression, metastatization and poor outcome in
particularly aggressive cancers (3,4).
Some of these drugs have been approved for human use and proved to
be effective in many solid tumors (5).
The most widely used in clinical practice is the
anti-VEGF monoclonal antibody (mAb) bevacizumab that binds the free
VEGF. Others, like the tyrosine kinase inhibitors (TKIs) sorafenib
and sunitinib, are able to target the VEGFR2 blocking the signaling
cascade (6). It has been reported
that the majority of patients benefits from targeted therapies, but
a small fraction fails to show even initial benefits. The reasons
may range from the involvement of parallel angiogenic pathways to
the absence of the targets (7).
Therefore, it would be important to predict which patients would
benefit from a specific targeted therapy and several studies
indicated the possibility to image angiogenic markers with the use
of radiopharmaceuticals targeting VEGF or VEGFR (8).
In particular, since VEGFR is expressed also in some
cancer cells, this technique will be useful in both early detection
and cancer treatment monitoring (9–12).
Radiopharmaceuticals to image tumor angiogenesis have been
described in the literature, but many of them showed limitations
that slowed or blocked the shift from preclinical to clinical
trials (13). Among them the most
common were poor or variable binding affinity and exaggerated liver
uptake (14,15). In the present study, we optimized
the radiolabeling of VEGF165 with
99mTechnetium (99mTc) using HYNIC as
bifunctional chelator, obtaining a highly stable
radiopharmaceutical with high in vitro receptor binding
affinity. In vivo we used
99mTc-HYNIC-VEGF165 to image VEGFR expression
in different tumor xenografts and correlated in vivo data
with histological findings.
Materials and methods
Radiolabeling of VEGF-A165 with
99mTc
The human VEGF-A165 analogue with a molecular weight
of 19 kDa was provided by Trophogen Inc. and radiolabeled with
99mTc through an indirect method after conjugation with
the bifunctional chelator 6-hydrazinonicotinamide (HYNIC).
Radiolabeling was optimized by testing several
labeling conditions including different HYNIC:VEGF ratios (1:1, 4:1
and 8:1) and different amounts of tricine (from 0.9 mg/ml to 200
mg/ml PBS) or SnCl2 (from 2 mg/ml to 20 mg/ml 0.1 M
HCl). Briefly, VEGF165 (0.5 mg) was incubated with an
excess of succinimidyl-6-hydrazinonicotinate hydrochloride (SHNH,
SoluLink Inc., San Diego, CA, USA) for 2 h at room temperature in
the dark. At the end of the incubation free SHNH was removed by
size exclusion chromatography using a G-25 Sephadex PD10 column (GE
Healthcare, Little Chalfont, Buckinghamshire, UK) and
nitrogen-purged phosphate buffer saline (pH 7.4) as eluent.
The number of HYNIC groups bound per molecule of
VEGF165 was determined by a molar substitution ratio
(MSR) assay. Briefly, conjugated VEGF165 (2 μl)
was added to a 0.5 mM solution (18 μl) of
2-sulfobenzaldehyde in 0.1 M 2-(N-morpholino) ethanesulfonic
acid (MES) buffer (pH 5.0) and incubated at room temperature for 2
h. Phosphate buffer saline (PBS) alone was used as blank and
duplicates were prepared. After 2 h the absorbance at 345 nm of
each reaction was measured with a spectrophotometer and the number
of HYNIC groups per molecule was calculated as indicated in the
SoluLink data sheet. Radiolabeling was performed incubating 30
μg of VEGF165 (in 100 μl PBS) with 300 MBq
of freshly eluted 99mTcO4− (100
μl), 100 μl of tricine (Sigma-Aldrich Chemicals,
Dorset, UK) and 5 μl SnCl2 (Sigma-Aldrich
Chemicals). Labeling efficiency (LE) and colloids percentage were
measured up to 30 min of incubation. After labeling, an additional
purification by size exclusion chromatography was performed using a
Zeba Spin Column (Thermo Fisher Scientific, Waltham, MA, USA) to
remove any free 99mTcO4−, tricine
and SnCl2.
In vitro quality controls
Quality controls were performed using instant thin
layer chromatography-silica gel (ITLC-SG) strip (Pall Life
Sciences, Port Washington, NY, USA). Results were analyzed by a
radio scanner (Bioscan Inc., Washington, DC, USA) to calculate the
LE of 99mTc-HYNIC-VEGF165. The mobile phase
for LE determination was a 0.9% NaCl solution, whereas the amount
of colloids was determined using a
NH3:H2O:EtOH (1:5:3) solution. Quality
controls were performed before and after the purification with a
Zeba Spin column. Additionally, reverse phase HPLC was carried out
using a C8 Kinetex 4.6×250 mm column and a gradient of
H2O (A) and acetonitrile (B) with 0.1 % TFA. The
following gradient was used: 0–5 min 0–5% B, 5–20 min 5–95% B,
20–25 min 95% B and 25–30 min 95–5% B. Stability assays were
performed adding 100 μl of
99mTc-HYNIC-VEGF165 to a vial containing 900
μl of fresh human blood serum and to another containing 900
μl of 0.9% NaCl solution. Both vials were incubated up to 24
h at 37°C. The radiochemical purity was measured at 1, 3, 6 and 24
h by ITLC analysis. A cysteine challenge assay was performed
incubating the radiolabeled VEGF165 at 37°C for 60 min
with different cysteine concentration, ranging from 1000:1
(cysteine:VEGF165) to 0.1:1 molar ratio. For each time
point, radiochemical purity was evaluated by ITLC as described
above. All known chemical forms of 99mTc-cysteine have
Rf values between 0.5 and 1, when normal saline was used as mobile
phase.
Integrity of the radiolabeled VEGF165
molecule was also checked by sodium dodecyl sulphate-polyacrylamide
gel electrophoresis under non-reducing conditions, according to the
method of Laemmli (16). Proteins
were visualized by staining the gels with Coomassie Brilliant Blue
(Thermo Fisher Scientific). Radioactivity associated with each band
was determined scanning the gel with a radio scanner.
Cell lines
VEGFR+ cell line, human umbilical veins
endothelial cells (HUVEC) were cultured in F-12K medium
supplemented with 10% FCS, 100 IU/ml penicillin, 100 μg/ml
streptomycin, 2 mM L-glutamine and EGM®-2 Bullet kit
(Lonza, Walkersville, MD, USA) (17). The human anaplastic thyroid cancer
cell line (ARO), the human colorectal cancer cell line (HT29) and
the human poorly differentiated thyroid cancer cell line (K1) were
grown in DMEM high glucose (Gibco, Carlsbad, CA, USA) supplemented
with 10% FCS, 100 IU/ml penicillin, 100 μg/ml streptomycin
and 2 mM L-glutamine (18–20).
In vitro binding studies
Measurements of cell uptake and retention of
radiolabeled VEGF-A165, was performed in vitro using the
semi-automatic system LigandTracer™ that allows to follow binding
over time (Ridgeview Instruments AB, Vänge, Sweden) (21). Briefly, 106 HUVEC cells
were seeded in a tilted Petri dish and incubated in a humidified
incubator at 37°C and 5% CO2 for 24 h. The dish was then
placed in the LigandTracer and allowed to rotate continuously for
15 min to induce the release of weakly attached cells. After one
gentle wash, 2 ml of PBS containing radiolabeled VEGF165
(30 nM) were added to the dish and the rotation started, and the
device was stopped when reaching maximal binding. Then the liquid
was removed and replaced with culture medium without radiolabeled
VEGF165 for calculating release of radioactivity from
cells. Association and dissociation curves were obtained analyzing
data by non-linear regression analysis with GraphPad Prism
(GraphPad Software Inc., La Jolla, CA, USA) to calculate the
kon, koff and Kd values.
In vivo studies
Biodistribution and imaging
studies
For animal experiments, approval of the local ethics
committee was obtained and the institutional and national guide for
the care and use of laboratory animals was followed. Imaging
studies were performed with a previously described high-resolution
portable mini-gamma camera (HRC), IP-Guardian (Li-Tech S.r.l.,
Italy) (22). For in vivo
biodistribution studies, 5.5 MBq (190 MBq/nmol, 100 μl) of
radiolabeled VEGF165 were injected in the tail vein of
12 nude CD-1 mice and static planar posterior images were acquired
using the HRC at 1, 3, 6 and 24 h, under light ether anesthesia. At
the end of each imaging point three mice were euthanized and major
organs were collected and counted in a single well
gamma-counter.
In vivo cell-targeting experiments were
performed in 36 nude CD-1 mice that were divided in three groups.
Each group was injected subcutaneously in the right thigh with
respectively 106 ARO, HT29 and K1 cells mixed with BD
Matrigel® (BD Biosciences, Franklin Lakes, NJ, USA)
(1:1). After tumor growth (approximately 0.6–1 cm3, in
20 days), 5.5 MBq of radiolabeled VEGF165 were
administered i.v. in the tail vein and static planar posterior HRC
images were acquired at 1, 3, 6 and 24 h, under light ether
anesthesia. At each time point 3 mice were euthanized for ex
vivo counting. Major organs and tumors were collected, weighed
and counted for radio activity with a single well gamma-counter
(Gammatom, Italy).
Blocking studies
Blocking studies were performed in four mice
injected with 1 million HT29 cells mixed with Matrigel in the right
thigh. After tumor growth, 5.5 MBq of
99mTc-VEGF165 were injected in the tail vein
and images were acquired with a portable mini-gamma camera at 1 and
3 h post-injection. After 3 days,
99mTc-HYNIC-VEGF165 was pre-incubated for 1 h
with 3.5-fold molar excess of recombinant human VEGFR2-Fc chimera
(BioLegend Inc., San Diego, CA, USA) that act as a soluble decoy
receptor (TRAP) that has been proved to prevent the binding of VEGF
to endothelial cells. After the incubation, a dose of 5.5 MBq was
injected in the tail vein of 3 of the 4 mice previously imaged and
images were acquired with a portable mini-gamma camera at 1 and 3 h
post-injection. After 3 more days, a 100-fold molar excess of
unlabeled VEGF165 (COLD) was injected in the tail vein
of the same 3 mice and after 10 min 5.5 MBq of
99mTc-HYNIC-VEGF165 was injected. Images were
acquired with a portable mini-gamma camera at 1 and 3 h
post-injection. Region of interest were drawn on the tumor and on
the contralateral leg in each image and target-to-background (T/B)
ratios were calculated.
Immunohistochemical analysis
For light microscope immunohistochemical analysis,
small fragments of each excised tumor (ARO, HT29 and K1) were
processed according to ABC/HRP technique (avidin-complexed with
biotinylated peroxidase). These samples were washed in PBS, fixed
in 10% formalin and embedded in paraffin according to a standard
procedure. Serial 3-μm sections were cut using a rotating
microtome, mounted on gelatin-coated slides and processed for
immuno-histochemistry. These sections were de-paraffinized in
xylene and dehydrated. They were immersed in citrate buffer (pH
6.0) and subjected to microwave irradiation twice for 5 min.
Subsequently, all sections were treated for 30 min with 0.3%
hydrogen peroxide in methanol to quench endogenous peroxidase
activity. To block non-specific binding, the slides were incubated
with M.O.M. Mouse Ig Blocking Reagent (Vector Laboratories
Burlingame, Burlingame, CA, USA) for 1 h at room temperature. The
slides were incubated overnight at 4°C with the following
antibodies: i) mouse anti-VEGF monoclonal antibody (Santa Cruz
Biotechnology, Santa Cruz, CA, USA); ii) mouse anti-VEGF receptor 1
(Flt-1/EWC) monoclonal antibody (ab9540; Abcam, Cambridge, UK);
iii) mouse anti-VEGF Receptor 2 (KDR/EIC) monoclonal antibody
(ab9530; Abcam). Optimal antisera dilutions and incubation times
were assessed in a series of preliminary experiments. After
exposure to the primary antibodies, slides were rinsed twice in
phosphate buffer and incubated for 1 h at room temperature with the
appropriate secondary biotinylated goat anti-mouse IgG (Vector
Laboratories Burlingame, BA9200 and BA1000) and with
peroxidase-conjugated avidin (Vectastain Elite ABC kit standard PK
6–100) for 35 min. After a further wash with phosphate buffer,
slides were treated with 0.05% 3,3-diaminobenzidine (DAB) and 0.1%
H2O2 (DAB substrate kit for peroxidase,
Vector Laboratories SK-4100). Finally, sections were counterstained
with Mayer's haematoxylin and observed using a light
microscope.
Negative control experiments were carried out: i) by
omitting the primary antibody; ii) by substituting the primary
antibody with an equivalent amount of non-specific immunoglobulins;
iii) by pre-incubating the primary antibody with the specific
blocking peptide (antigen/antibody = 5 according to supplier's
instructions). The staining assessment was made by two experienced
observers in light microscopy. Immunoreactivity of VEGF, VEGFR1,
and VEGFR2 was assessed in all samples. The intensity of the immune
reaction was assessed micro-densitometrically using an IAS 2000
image analyzer (Delta Sistemi, Rome, Italy). The system was
calibrated taking as zero the background obtained in sections
exposed to non-immune serum. Ten 100 μm2 areas
were delineated in each section using a measuring diaphragm. The
quantitative data regarding the intensity of immune staining were
analyzed statistically using analysis of variance (ANOVA) followed
by Duncan's multiple range test as a post-hoc test.
Results
VEGF165 analogue can be
efficiently radiolabeled with 99mTc
Highest labeling efficiency was obtained when the
analogue was conjugated with a ratio HYNIC:VEGF165 of
8:1. Determination of molar substitution ratio of HYNIC-conjugated
VEGF165 demonstrated that an average of 4.3 molecules of
HYNIC groups were bound per molecule of analogue. Higher ratios
were not selected to avoid over-conjugation of the hormone and
possible structural modification. Optimization of the labeling
procedure of the HYNIC-VEGF165 conjugate (30 μg)
with 99mTc showed that, after 10 min of incubation, the
use of 100 μl of tricine (0.5 mM) and 5 μl of
SnCl2 (50 nM) allowed to obtain the highest LE (65%) and
the lowest amount of colloids (<5%). After purification we were
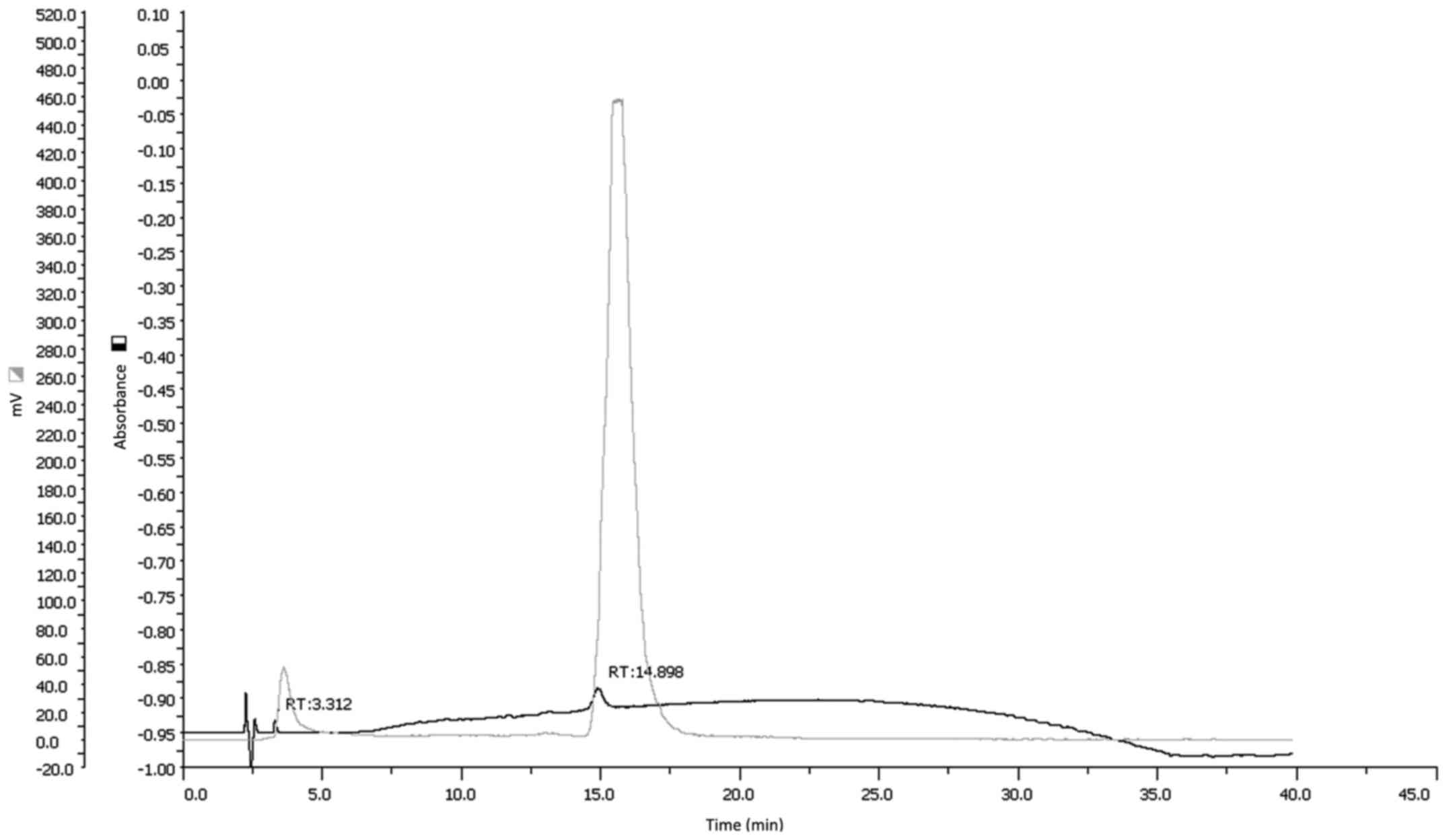
able to obtain a radiochemical purity of >95% as confirmed by
both ITLC and HPLC analysis (Fig.
1). Specific activity of resulting
99mTc-HYNIC-VEGF165 was 190 MBq/nmol.
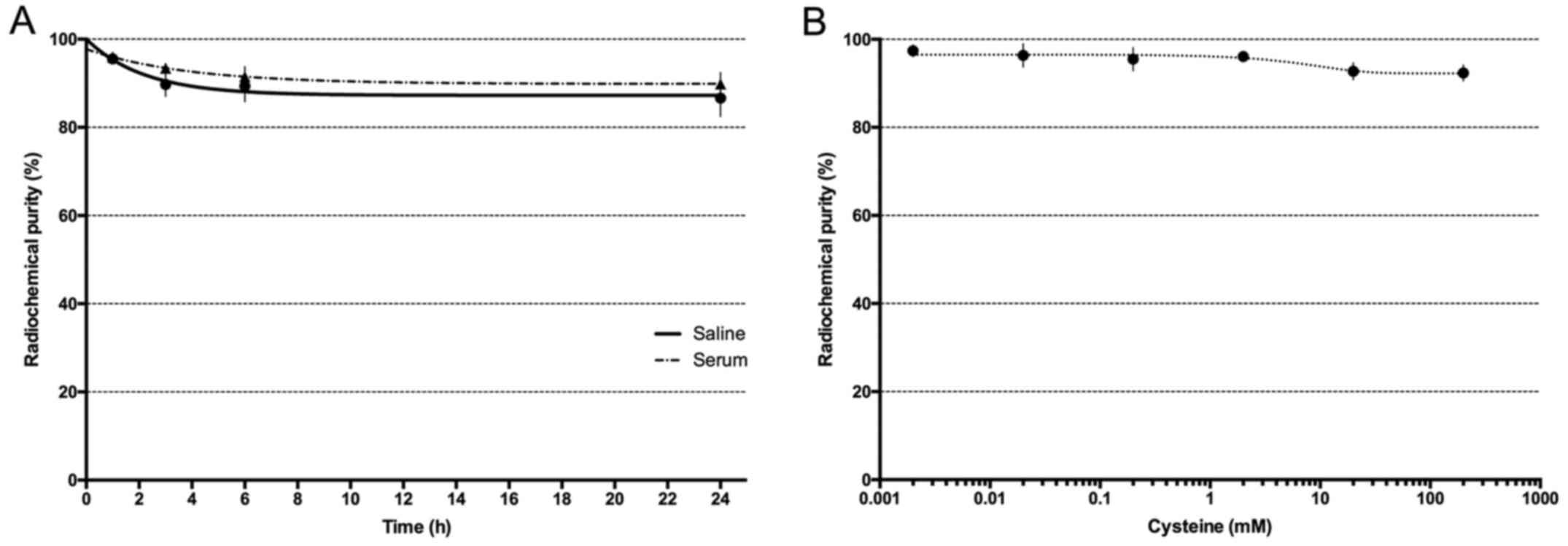
Radiolabeled VEGF165 was stable up to 24 h in both in
human serum and in a 0.9% NaCl solution at 37°C, as well as in
solutions containing increasing cysteine concentrations (Fig. 2A). A slight decrease in the
radiochemical purity was observed only at high cysteine
concentrations (>500:1) (Fig.
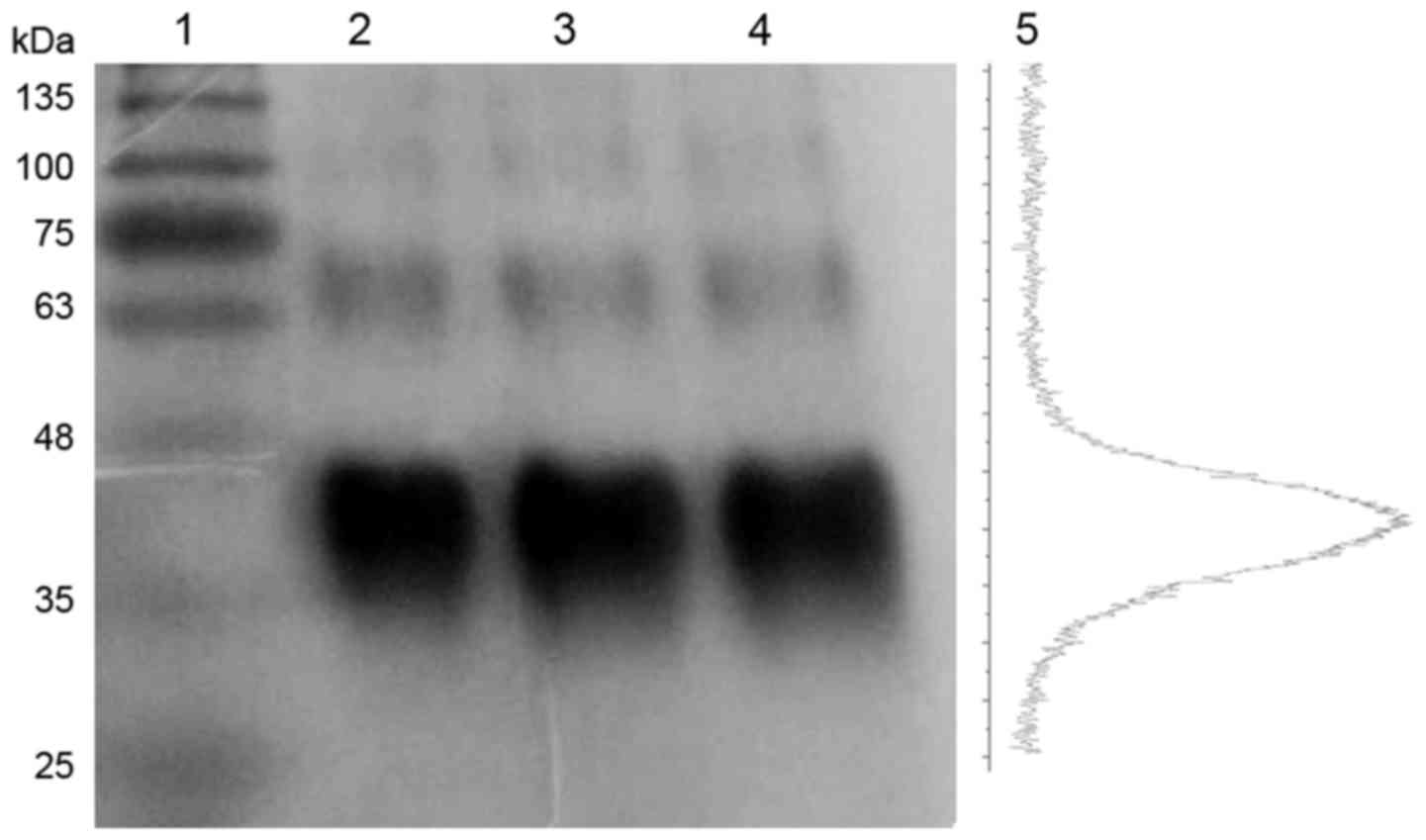
2B). Gel electrophoresis of radiolabeled, conjugated and
unconjugated analogue showed no significant differences and the
absence of significant degradation or aggregation resulting from
conjugation and/or labeling (Fig.
3). Poor resolution of the bands is due to the high
glycosylation of the analogues.
Radiolabeled VEGF165 binds
with high affinity to VEGFRs
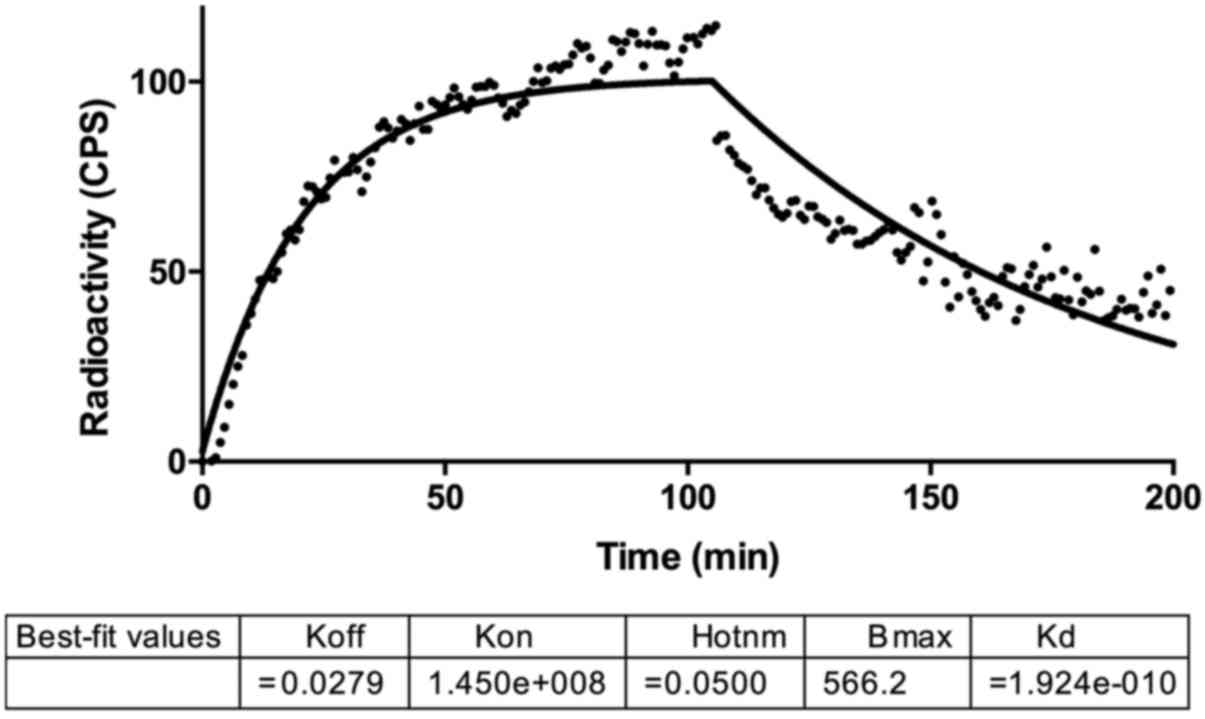
Kinetic biding assay with LigandTracer showed an
increasing uptake of radiolabeled VEGF165 from HUVEC
cells that reached a plateau after 50 min (Fig. 4). Retention studies revealed a slow
dissociation rate from membrane bound receptors in the following 2
h, with a Kd of 192 pM.
99mTc-HYNIC-VEGF165
is able to image tumor xenografts in mice
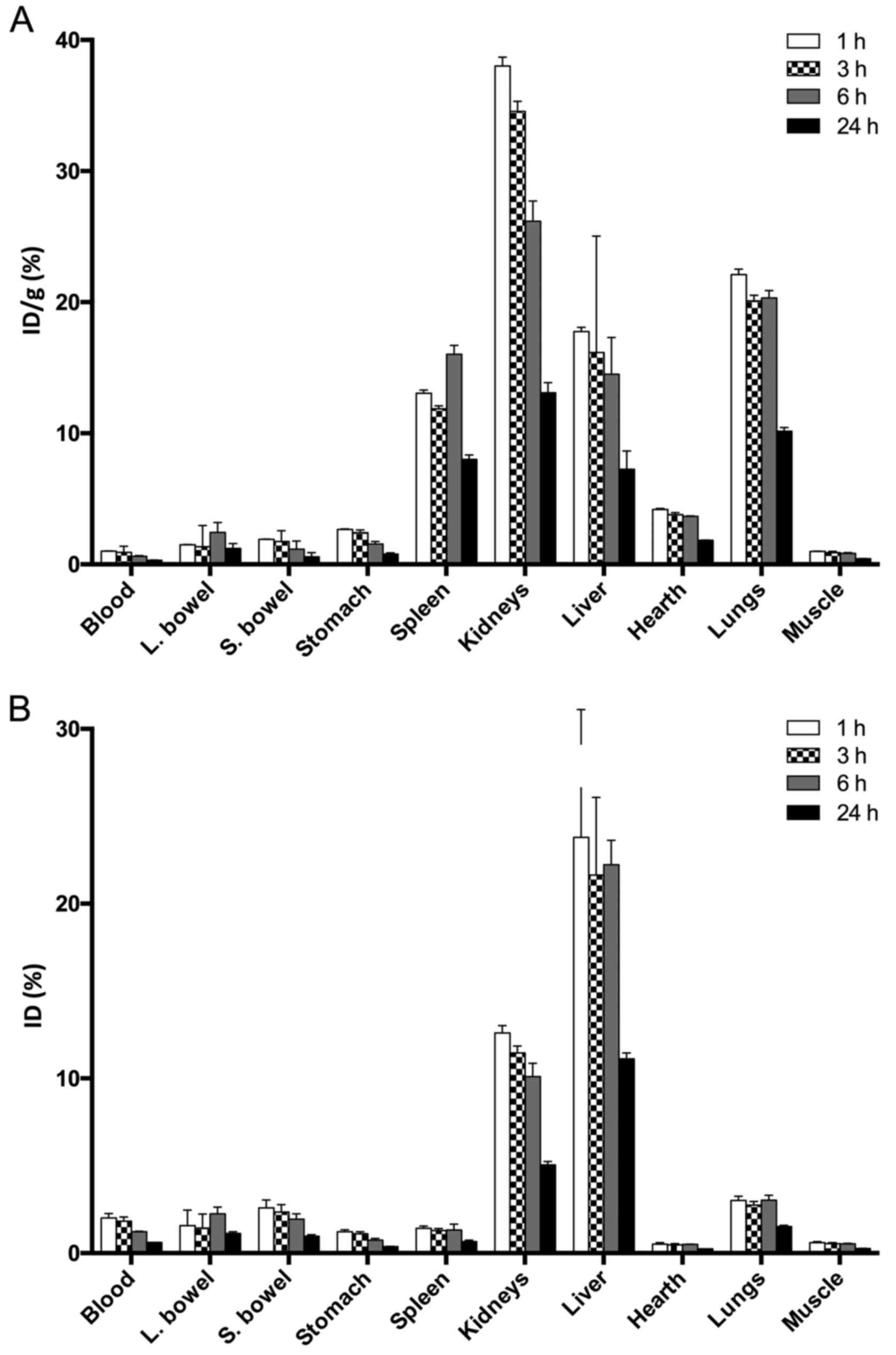
Biodistribution studies with
99mTc-HYNIC-VEGF165 showed a high and
persistent uptake by the liver and a moderate uptake by the kidneys
with almost no signal from other organs and blood pool (Fig. 5). Single organ counting revealed a
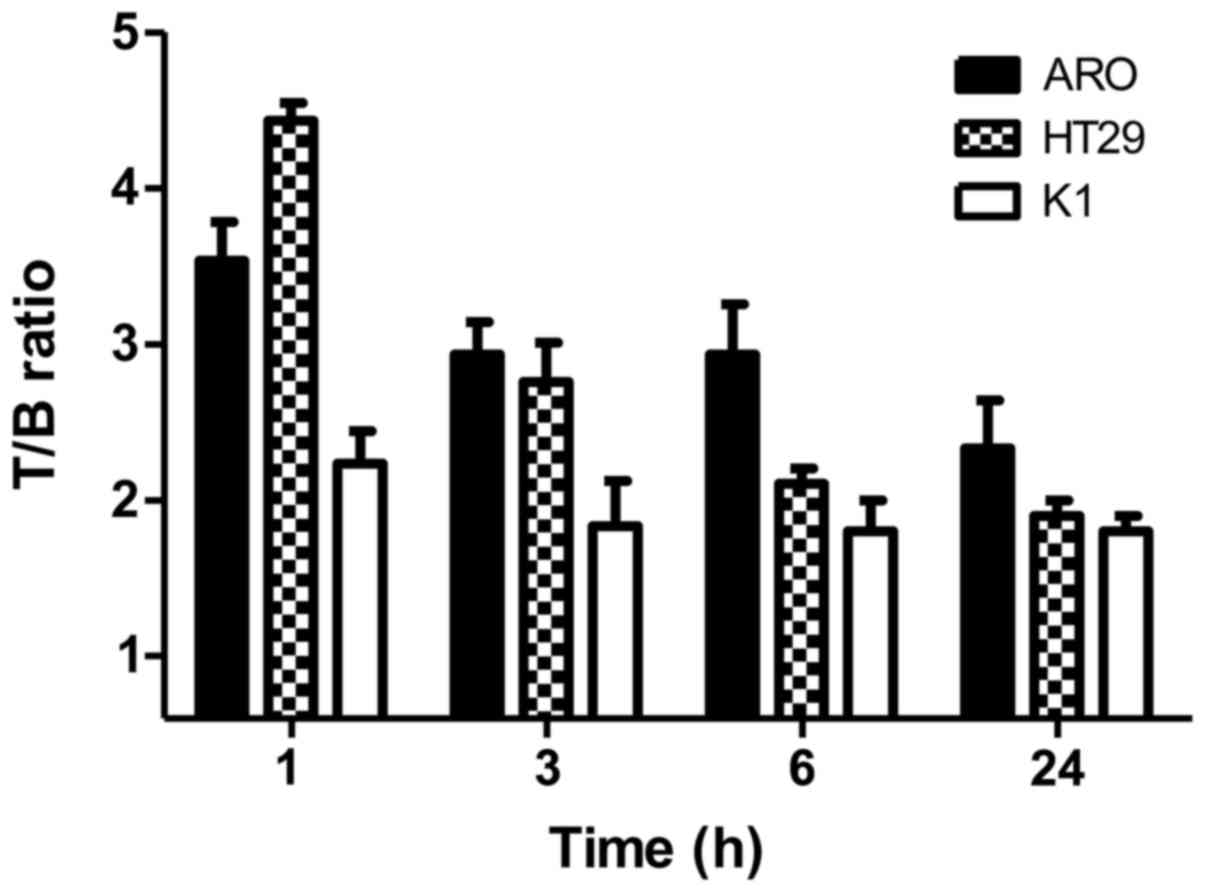
high %ID/g also in the lungs and spleen. In vivo targeting
experiments showed a focal uptake in the right thigh of each group
bearing tumor xenografts with a T/B ratio of 4.5 at 1 h p.i in mice
bearing a HT29 xenograft. Animals bearing ARO and K1 cells showed a
T/B of 3.5 and 2.3, respectively, that decreased over time
(Fig. 6).
In vivo binding of
99mTc-HYNIC-VEGF165 can be inhibited by an
excess of unlabeled VEGF or VEGFR2-Fc
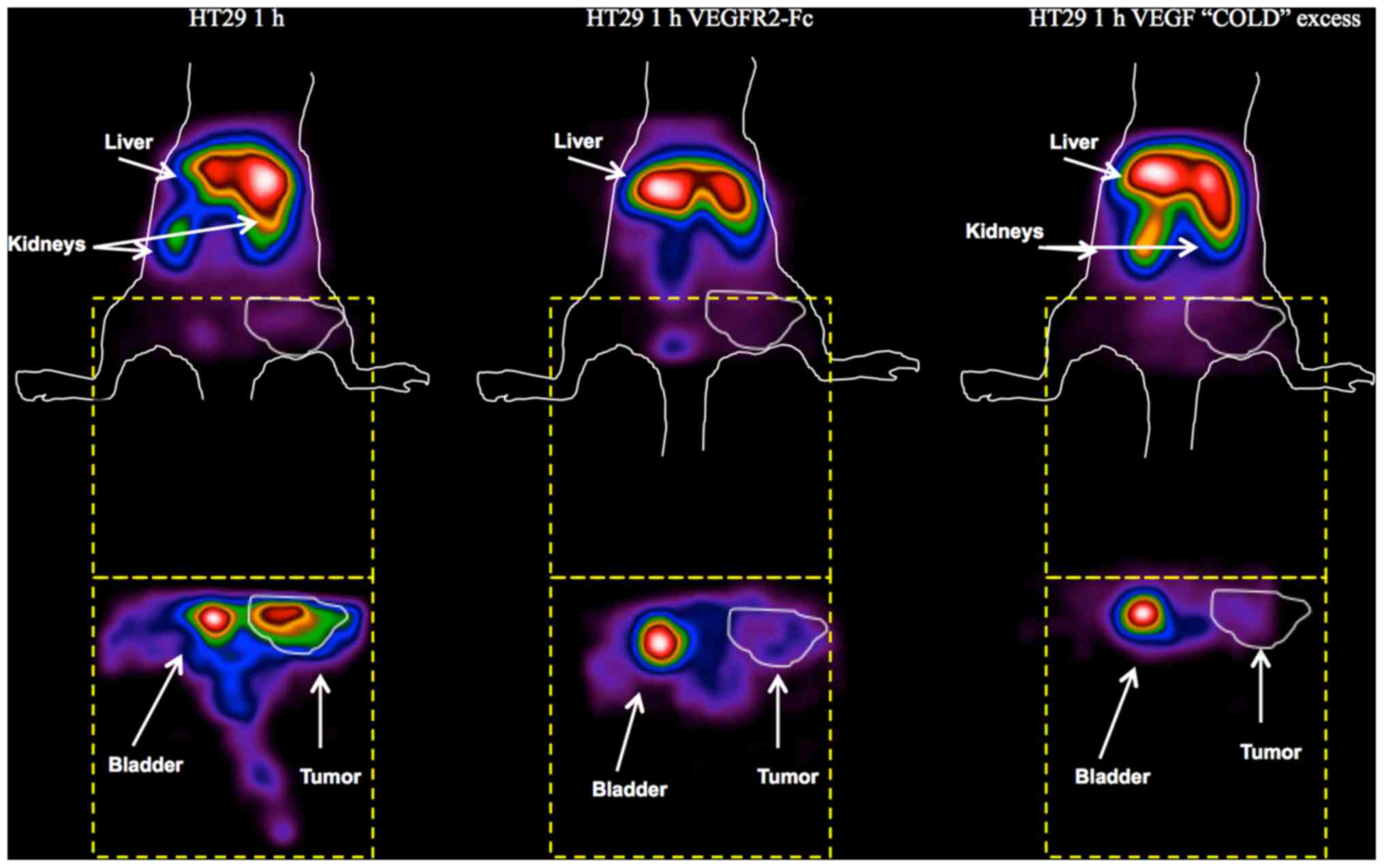
Blocking studies with
99mTc-HYNIC-VEGF165 confirmed the results of
previous targeting experiments. After pre-incubation of the
radiopharmaceutical with recombinant VEGFR2-Fc, a main liver and
spleen uptake, with reduced signal from kidneys, was detected,
resembling the typical biodistribution of a nonspecific
radiolabeled antibody (Fig. 7).
The overall uptake in tissues was lower and the uptake in the tumor
was considerably reduced. Similar findings were obtained after the
pre-injection of a 100-fold molar excess of unlabeled
VEGF165 with the exception of the signal from kidneys,
which was similar to the signal obtained with labeled VEGF only.
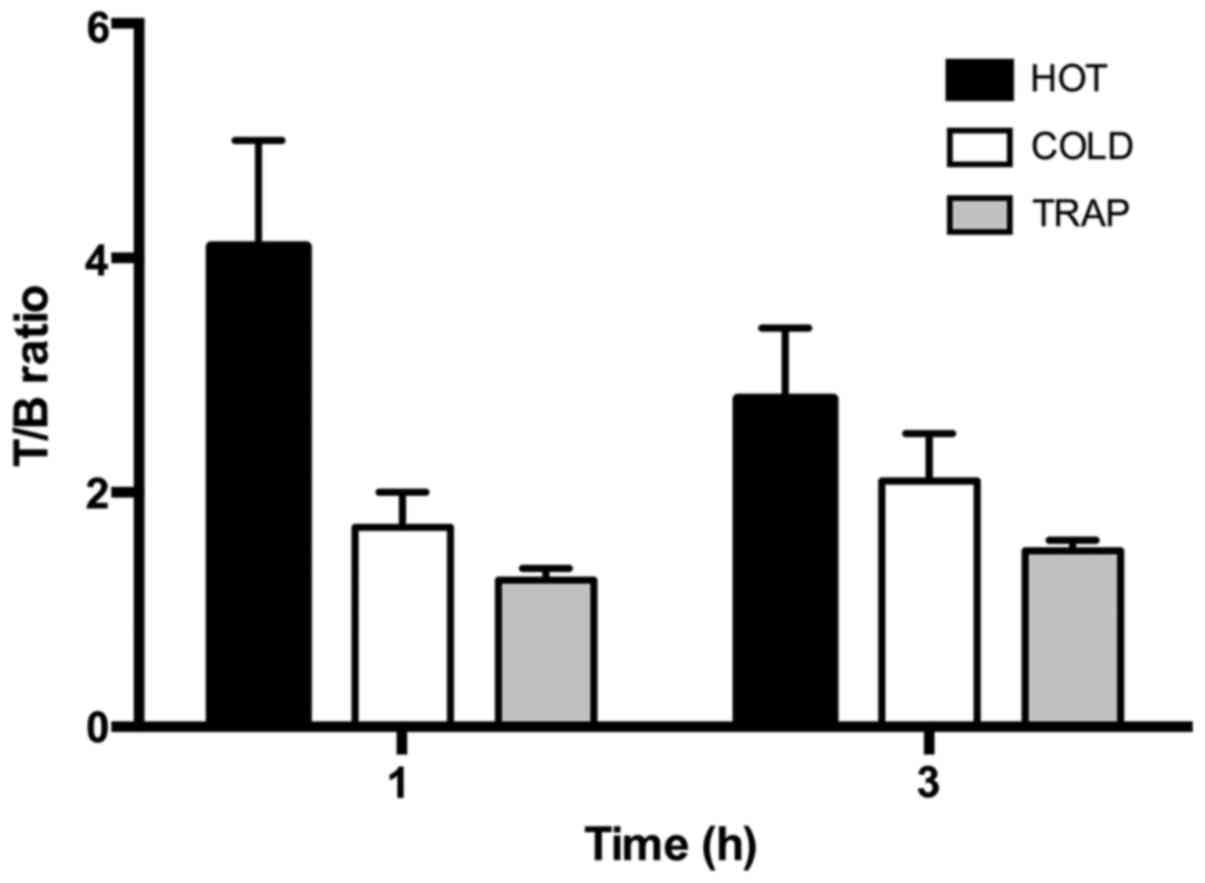
Calculated T/B ratios for the 'HOT' group reflected the data
obtained with the previous experiments with a maximum uptake
reached at 1 h that slowly decreases with time (Fig. 8). The T/B ratio in the TRAP group
was reduced by 70% due to the co-incubation with VEGFR2-Fc at 1 h
and the T/B ratio in the 'COLD' group was reduced by 60% at 1 h.
Minor blocking was evident at 3 h in both TRAP and 'COLD' group
mainly due to the decreased activity in tumors of the control
group.
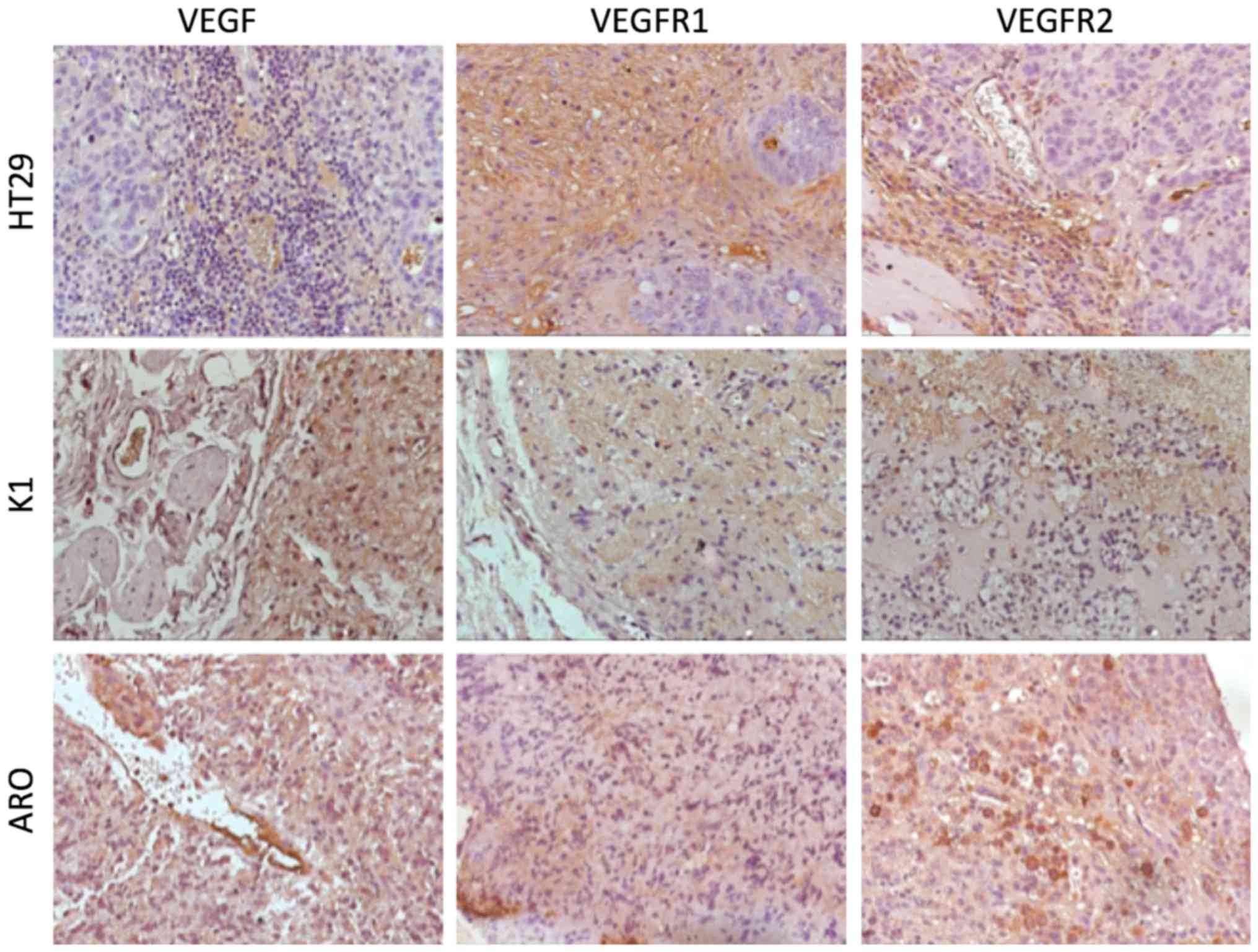
VEGF expression at IHC and T/B ratio of
99mTc-HYNIC-VEGF165 correlates inversely
IHC analysis on excised tumor showed the presence of
VEGF, VEGFR1 and VEGFR2 on both the lesion and the surrounding
vessels to different extent (Fig.
9). After semi-quantitative analysis of expression levels, a
higher amount of free VEGF was present in lesions derived from K1
cell lines (33.2%), followed by HT29 (15.7%) and ARO cells (10.6%).
VEGFR1 and -2 were present heterogeneously between tumor cells and
blood vessels, revealing that even cancer may express VEGF
receptors on the plasma membrane. IHC data were compared with the
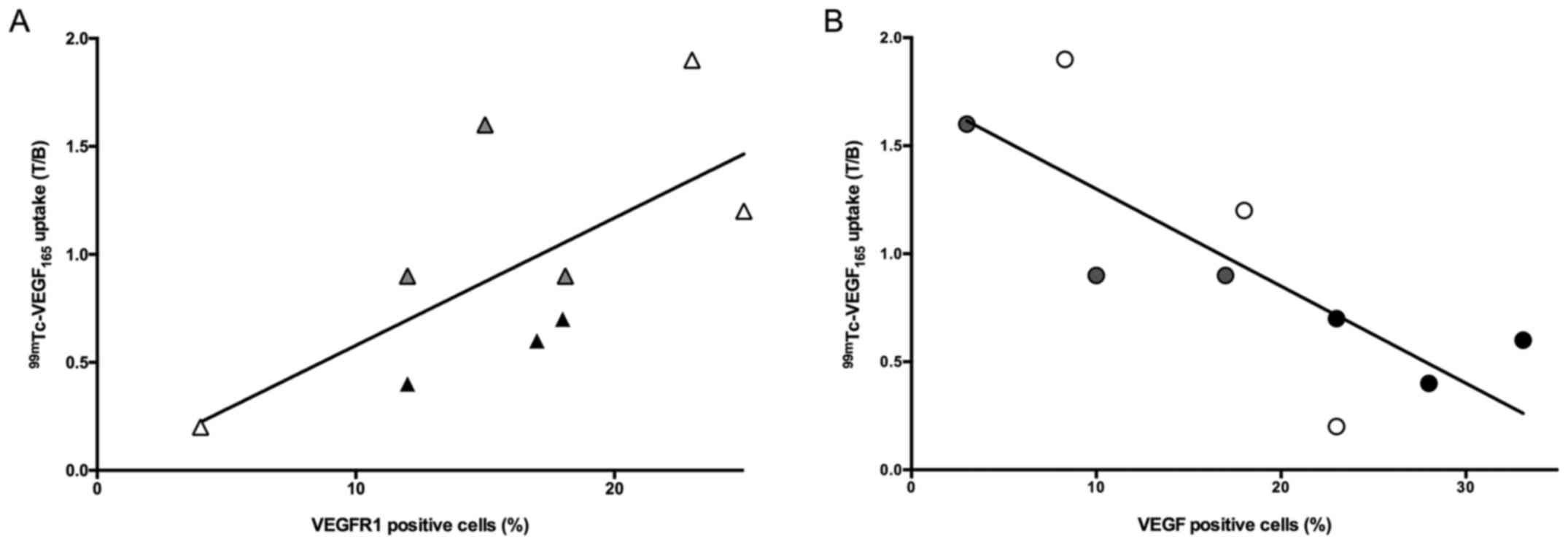
uptake of radioactive VEGF165 and an inverse correlation
was observed between endogenous VEGF and T/B ratio (r2=
0.63; p= 0.03, Fig. 10A). On the
contrary, a positive correlation was observed between radioactive
VEGF165 uptake and VEGFR1 (r2= 0.64; p=0.03,
Fig. 10B). In addition, tumor
weight positively correlates with VEGF production
(r2=0.65; p=0.03) and shows a trend to inversely
correlate with radiolabeled VEGF uptake (r2= 0.31; p=
0.35).
Discussion
Imaging of tumor microenvironment has been described
as a promising approach for non-invasive diagnosis of cancer
metastases and to monitor the efficacy of new drugs (23). Given the role of angiogenesis in
metastatization and tumor growth, VEGF and its receptors are
optimal diagnostic and therapeutic targets (24). Their presence has been reported in
many cancer types and it was correlated with clinical data.
However, given the heterogeneity of VEGFR expression on cancer
cells, their role in tumor dedifferentiation or signaling is still
unclear (13). In undifferentiated
thyroid cancer, the use of TKIs blocking the VEGF/VEGFR pathway
showed its potential as a promising therapeutic approach (25). Unfortunately, severe side effects
have been reported in some patients after long time treatment.
Therefore, a non-invasive diagnostic tool to predict the response
to therapy and evaluate drug efficacy is vitally needed. In the
past many attempts have been made to develop radiopharmaceuticals
to image angiogenesis with promising results. Among them,
111In, 89Zr or 64Cu radiolabeled
bevacizumab was able to efficiently image xenografts from ovarian
cancer, but the high radiation burden to the patient and the low
availability of 89Zr and 64Cu were some of
the drawbacks of its use (26,27).
Other groups tried to use recombinant human VEGF to
overcome the long half-life of mAbs and used radioiodine,
99mTechnetium (99mTc), 64Cu or
68Ga as the isotopes of choice (8,13).
In the present study, we followed the same approach to strengthen
the hypothesis that the use of recombinant human VEGF to target
angiogenesis is a promising methodology to develop non-invasive
diagnostic tools and monitor novel targeted drug development. In
addition we improved the radiolabeling method to produce a high
specific activity and highly stable radiopharmaceutical to bind
VEGFR with high binding affinity and avoid misinterpretation of
in vivo studies. Furthermore, the use of picomolar amounts
of radiolabeled VEGF for a scintigraphic study should not raise any
concern about a potential biologic effect of such
radio-pharmaceuticals and in particular on the pro-angiogenic
effect that VEGF analogues may have on existing blood vessels.
Thus, in vivo results allowed us to image tumor angiogenesis
in xenografts from three different human cell lines with high T/B
ratio between 1 and 3 h post-injection (max T/B at 1 h for HT29 was
4.5). Nevertheless a high liver uptake was observed in all mice
till late time points, confirming previous findings from other
groups (28).
The issue has been raised that VEGF-based probes
uptake in the tumor area is highly heterogeneous, probably because
of the combination of several mechanisms like non-uniform perfusion
of tumor vasculature, differential receptor occupancy by host VEGF
or differential accessibility of VEGF receptors on luminal and
subluminal surfaces of the endothe-lium (29,30).
Moreover, it has been reported by Chen et al that tumor size
negatively influences the uptake of radiolabeled VEGF by the tumor,
probably because of the presence of necrotic areas (31). To address, in particular, the role
of necrosis and endogenous VEGF production, we performed
histological and immunohistochemical analysis of each tumor imaged
with 99mTc-VEGF165. Results confirmed
variability in VEGFR1 and VEGFR2 receptor expression and ligand
occupancy in both host endothelium and cancer cells. The presence
of both receptors has been also confirmed in a recent study by
Meyer et al, but it would be of interest to investigate the
differential contribution of the different VEGFR subtypes (29). Moreover, we confirm that bigger
tumors show lower uptake of radiolabeled VEGF, although we did not
observe the presence of significant necrotic areas in any tumor. On
the other hand, we observed a positive correlation between tumor
size and production of endogenous VEGF (p=0.03), suggesting that
the reduced tumor uptake of the radiopharmaceutical could depend on
saturation of VEGFRs rather than size or necrosis, as previously
suggested (30).
Therefore, this study highlights an important aspect
that has not been considered before: the role of both endogenous
VEGF production and VEGFR expression on imaging strategies. While
for other ligand receptor systems, the endogenous production of the
ligand may not be highly relevant for imaging the receptor ligand
(i.e. IL-2 and IL-2 receptor) (32,33),
herein we show that the high production of endogenous VEGF by tumor
cells hampers the possibility to image its receptors. In light of
our results we can better interpret previously published studies
with radiolabeled VEGF (both VEGF121 and
VEGF165) (34) that
showed very poor tumor uptake, in contrast with studies with
radiolabeled anti-VEGF mAb that showed high tumor uptake (35). Collectively, these data confirm
that the presence of high VEGF levels in tumors, particularly those
advanced with highly hypoxic tumor microenvironment and aggressive
phenotypes, may saturate VEGF receptors, thus limiting the
possibility to image receptors.
Overexpression of soluble VEGFR in some tumors and
significant sequestration of VEGF on cell surface heparin-sulfate
proteoglycans may also contribute to highly variable imaging
results in different tumors. One possible limitation of our study
is the limited number of animals used. However, in mice bearing K1
tumors, or ARO or HT29, we found highly consistent data supporting
the very low variability within the same cell line. Nevertheless,
different tumors showed different levels of VEGF/VEGFR. Another
possible limitation could be that the semi-quantitative evaluation
of VEGF189 that we performed by immunohistochemistry may
not represent the real production of VEGF121 and
VEGF165. However, these forms are splicing variants of
the same molecule and are usually expressed in similar quantities
(36). Overall we believe that the
above considerations may have a limited impact on the final
conclusion that VEGFR imaging in tumors by using radiolabeled VEGF
is extremely variable, influenced by the presence of endogenous
VEGF and unrelated to VEGFR receptor expression. It can be
extrapolated that an accurate in vivo evaluation of tumor
angiogenesis should include both VEGF and VEGFR imaging, unless the
predominant clinical relevance of one over the other is
demonstrated. Finally, the development of a superagonist VEGF
analogue could allow the use of molecule with a greatly increased
affinity for its receptor, overcoming the quenching effect due to
endogenous VEGF (37).
In conclusion, imaging of angiogenesis by targeting
VEGFR with radiolabeled VEGF analogues may be a complementary
approach to evaluate angiogenic status of tumors. This approach may
allow the evaluation of anti-angiogenic drugs at both preclinical
and clinical stages in combination with VEGF imaging. Our results
indicate that VEGFR expression is variable in both tumors and its
imaging is hampered by endogenous VEGF production. Therefore,
additional studies are required to fully understand the VEGF/VEGFR
relationship in different cancers and establish a more accurate and
angiogenic phenotype-determined imaging protocols.
Acknowledgments
This study was funded by grants from the Italian
Association for Cancer Research (AIRC IG-2013 14151 and 13234),
'Sapienza' University research projects and Regione Lazio
(FILAS-RU-2014-1020). We also wish to acknowledge the non-profit
association Nuclear Medicine Discovery for support.
Abbreviations:
|
FCS
|
fetal calf serum
|
|
HRC
|
high-resolution portable mini-gamma
camera
|
|
HYNIC
|
6-hydrazinonicotinamide
|
|
ITLC
|
instant thin layer chromatography
|
|
LE
|
labeling efficiency
|
|
mAb
|
monoclonal antibody
|
|
MES
|
2-(N-morpholino) ethanesulfonic
acid
|
|
PBS
|
phosphate buffered saline
|
|
99mTc
|
99mTechnetium
|
|
TKI
|
tyrosine kinase inhibitors
|
|
VEGF
|
vascular endothelial growth
factor
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
References
|
1
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Araujo-Filho VJ, Alves VA, de Castro
IV, Lourenço SV, Cernea CR, Brandão LG and Ferraz AR: Vascular
endothelial growth factor expression in invasive papillary thyroid
carcinoma. Thyroid. 19:1233–1237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
5
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gruber JJ and Colevas AD: Differentiated
thyroid cancer: Focus on emerging treatments for radioactive
iodine-refractory patients. Oncologist. 20:113–126. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gotink KJ and Verheul HM: Anti-angiogenic
tyrosine kinase inhibitors: What is their mechanism of action?
Angiogenesis. 13:1–14. 2010. View Article : Google Scholar :
|
|
8
|
Blankenberg FG, Levashova Z, Sarkar SK,
Pizzonia J, Backer MV and Backer JM: Noninvasive assessment of
tumor VEGF receptors in response to treatment with pazopanib: A
molecular imaging study. Transl Oncol. 3:56–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toi M, Inada K, Suzuki H and Tominaga T:
Tumor angiogenesis in breast cancer: Its importance as a prognostic
indicator and the association with vascular endothelial growth
factor expression. Breast Cancer Res Treat. 36:193–204. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Folkman J: Angiogenesis: An organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iagaru A, Chen X and Gambhir SS: Molecular
imaging can accelerate anti-angiogenic drug development and
testing. Nat Clin Pract Oncol. 4:556–557. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taurone S, Galli F, Signore A, Agostinelli
E, Dierckx RA, Minni A, Pucci M and Artico M: VEGF in nuclear
medicine: Clinical application in cancer and future perspectives
(Review). Int J Oncol. 49:437–447. 2016.PubMed/NCBI
|
|
13
|
Goel HL and Mercurio AM: VEGF targets the
tumour cell. Nat Rev Cancer. 13:871–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dijkgraaf I and Boerman OC: Molecular
imaging of angiogenesis with SPECT. Eur J Nucl Med Mol Imaging.
37(Suppl 1): S104–S113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai W and Chen X: Multimodality molecular
imaging of tumor angiogenesis. J Nucl Med. 49(Suppl 2): 113–128.
2008. View Article : Google Scholar
|
|
16
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zenner HL, Collinson LM, Michaux G and
Cutler DF: High-pressure freezing provides insights into
Weibel-Palade body biogenesis. J Cell Sci. 120:2117–2125. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ke CC, Liu RS, Yang AH, Liu CS, Chi CW,
Tseng LM, Tsai YF, Ho JH, Lee CH and Lee OK: CD133-expressing
thyroid cancer cells are undifferentiated, radioresistant and
survive radioiodide therapy. Eur J Nucl Med Mol Imaging. 40:61–71.
2013. View Article : Google Scholar
|
|
19
|
Paudyal B, Paudyal P, Shah D, Tominaga H,
Tsushima Y and Endo K: Detection of vascular endothelial growth
factor in colon cancer xenografts using bevacizumab based near
infrared fluorophore conjugate. J Biomed Sci. 21:352014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Challeton C, Branea F, Schlumberger M,
Gaillard N, de Vathaire F, Badie C, Antonini P and Parmentier C:
Characterization and radiosensitivity at high or low dose rate of
four cell lines derived from human thyroid tumors. Int J Radiat
Oncol Biol Phys. 37:163–169. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Björke H and Andersson K: Automated,
high-resolution cellular retention and uptake studies in vitro.
Appl Radiat Isot. 64:901–905. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Soluri A, Massari R, Trotta C, Montani L,
Iurlaro G, Mangano AM, Scopinaro F and Scafè R: New imaging probe
with crystals integrated in the collimator's square holes. Nucl
Instrum Methods Phys Res A. 554:331–339. 2005. View Article : Google Scholar
|
|
23
|
Galli F, Iodice V, Lauri C and Signore A:
New approaches to image thyroid cancer cells and microenvironment.
Q J Nucl Med Mol Imaging. 59:184–196. 2015.PubMed/NCBI
|
|
24
|
Stacy MR, Maxfield MW and Sinusas AJ:
Targeted molecular imaging of angiogenesis in PET and SPECT: A
review. Yale J Biol Med. 85:75–86. 2012.PubMed/NCBI
|
|
25
|
Schneider TC, Abdulrahman RM, Corssmit EP,
Morreau H, Smit JW and Kapiteijn E: Long-term analysis of the
efficacy and tolerability of sorafenib in advanced radio-iodine
refractory differentiated thyroid carcinoma: Final results of a
phase II trial. Eur J Endocrinol. 167:643–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stollman TH, Scheer MG, Franssen GM,
Verrijp KN, Oyen WJ, Ruers TJ, Leenders WP and Boerman OC: Tumor
accumulation of radiolabeled bevacizumab due to targeting of cell-
and matrix-associated VEGF-A isoforms. Cancer Biother Radiopharm.
24:195–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Van Dongen GA, Huisman MC, Boellaard R,
Harry Hendrikse N, Windhorst AD, Visser GW, Molthoff CF and Vugts
DJ: 89Zr-immuno-PET for imaging of long circulating drugs and
disease targets: Why, how and when to be applied? Q J Nucl Med Mol
Imaging. 59:18–38. 2015.
|
|
28
|
Blankenberg FG, Backer MV, Levashova Z,
Patel V and Backer JM: In vivo tumor angiogenesis imaging with
site-specific labeled (99m)Tc-HYNIC-VEGF. Eur J Nucl Med Mol
Imaging. 33:841–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meyer JP, Edwards KJ, Kozlowski P, Backer
MV, Backer JM and Lewis JS: Selective imaging of VEGFR-1 and
VEGFR-2 receptors using 89Zr-labeled single-chain VEGF mutants. J
Nucl Med. 57:1811–1816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Backer MV, Levashova Z, Patel V, Jehning
BT, Claffey K, Blankenberg FG and Backer JM: Molecular imaging of
VEGF receptors in angiogenic vasculature with single-chain
VEGF-based probes. Nat Med. 13:504–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen K, Cai W, Li ZB, Wang H and Chen X:
Quantitative PET imaging of VEGF receptor expression. Mol Imaging
Biol. 11:15–22. 2009. View Article : Google Scholar
|
|
32
|
Signore A, Chianelli M, Annovazzi A,
Bonanno E, Spagnoli LG, Pozzilli P, Pallone F and Biancone L:
123I-interleukin-2 scin-tigraphy for in vivo assessment
of intestinal mononuclear cell infiltration in Crohn's disease. J
Nucl Med. 41:242–249. 2000.PubMed/NCBI
|
|
33
|
Signore A, Capriotti G, Chianelli M,
Bonanno E, Galli F, Catalano C, Quintero AM, De Toma G, Manfrini S
and Pozzilli P; Action LADA Group: Detection of insulitis by
pancreatic scin-tigraphy with 99mTc-labeled IL-2 and MRI
in patients with LADA (Action LADA 10). Diabetes Care. 38:652–658.
2015.PubMed/NCBI
|
|
34
|
Kang CM, Koo HJ, Choe YS, Choi JY, Lee KH
and Kim BT: 68Ga-NODAGA-VEGF121 for in vivo
imaging of VEGF receptor expression. Nucl Med Biol. 41:51–57. 2014.
View Article : Google Scholar
|
|
35
|
Gaykema SB, Brouwers AH, Lub-de Hooge MN,
Pleijhuis RG, Timmer-Bosscha H, Pot L, van Dam GM, van der Meulen
SB, de Jong JR, Bart J, et al: 89Zr-bevacizumab PET imaging in
primary breast cancer. J Nucl Med. 54:1014–1018. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cai C, Böttcher MC, Werner JA and Mandic
R: Differential expression of VEGF121, VEGF165 and VEGF189 in
angiomas and squamous cell carcinoma cell lines of the head and
neck. Anticancer Res. 30:805–810. 2010.PubMed/NCBI
|
|
37
|
Galli F, Manni I, Piaggio G, Balogh L,
Weintraub BD, Szkudlinski MW, Fremont V, Dierckx RA and Signore A:
(99m)Tc-labeled-rhTSH analogue (TR1401) for imaging poorly
differentiated metastatic thyroid cancer. Thyroid. 24:1297–1308.
2014. View Article : Google Scholar : PubMed/NCBI
|