Introduction
Pancreatic cancer is characterized by a dense
desmoplastic reaction in which extracellular matrix (ECM) proteins
accumulate and surround tumor cells. These ECM proteins not only
provide physical support but also affect the biological properties
of pancreatic cancer cells (1–3).
Recent studies have demonstrated that the integrin family of
heterodimeric transmembrane glycoproteins, each of which are
composed of an α- and a β-subunit, and their related signaling
molecules are associated with the progression of pancreatic cancer
(4–7). Among these signaling molecules, the
Src family kinases (SFKs), a group of non-receptor tyrosine
kinases, are overexpressed in 70% of human pancreatic cancer cases
and their activities are critical for tumor progression (8–11).
Fyn, a key member of the SFKs, mediates 'outside-in'
signaling from the ECM-integrin interaction to affect cellular
processes, including T-cell receptor differentiation, cell
adhesion, migration and apoptosis regulation (12–15).
Our previous study showed that the activation of Fyn promotes cell
proliferation and metastasis in pancreatic cancer (16). However, the role of Fyn in
'inside-out' signaling to regulate pancreatic cancer metastasis is
not completely understood.
Integrins are heterodimers of two type-I membrane
glycoproteins, α and β, which bind to each other non-covalently.
Currently, seventeen α subunits and eight β subunits have been
identified in vertebrates, and different components of integrin
signaling have been shown to be vital for cell survival and cancer
metastasis (17–19). It is well established that
alternatively spliced variants of the α- and β-subunits contribute
to the variety of biological functions of the integrin receptors
(20). In endothelial cells,
alternative splicing of integrin αv have been analyzed by Gauck
et al (21) who found
Cdc2-like kinases as well as DNA topoisomerase I to modulate the
differential isoform expression of Cyr61 and its receptor integrin
αv, the protein expression and secretion in resting as well as in
TNF-α-stimulated human microvascular endothelial cells. Moreover,
these processes affected the endothelial cell proliferation and
pro-angiogenic tube formation by HMEC-1. In pancreatic cancer, the
integrin β1-ECM interaction has been associated with metastasis
(4). Four splice variants (A, B, C
and D), which differ only in the amino acid sequence of the
cytoplasmic domain, have been identified in the integrin β1 family,
and the β1A isoform is widely expressed among all mammalian species
assessed so far. The expression of splice variants B, C and D,
which can inhibit β1A-mediated focal adhesion formation, cell
spreading and motility, has been found to be downregulated or even
lost in various tumor tissues, and may be involved in tumor
progression (22–24). However, the formation of splice
variants of integrin β1 is not yet fully understood.
Splicing of individual precursor messenger
ribonucleic acid (pre-mRNA) is determined by spliceosome proteins
including serine/arginine-rich (SR) proteins and heterogeneous
nuclear ribonucleoproteins (hnRNPs) (25). The SR proteins and hnRNPs function
as trans-acting factors by binding to exonic splicing
enhancers (ESEs) or exonic splicing silencers (ESSs) to regulate
alternative splicing (26–29). Recent reports have shown that hnRNP
E1 binds to the CD44 pre-mRNA, which affects its alternative
splicing (30). However, the
participation of spliceosome proteins in integrin β1 splicing
regulation and the resultant effect on cancer metastasis are
unknown.
As a group of RNA-binding proteins, hnRNPs regulate
the splicing and transportation of mRNA and participate in growth
regulation and carcinogenesis (31–33).
Expression of hnRNP E1 has been detected in several cancer types
(34,35). However, the mechanism by which
hnRNP E1 regulates tumor metastasis is not clear. Our preliminary
study indicated that inhibition of the activity of Fyn
significantly increased hnRNP E1 expression in human pancreatic
cancer cells (36). To determine
whether Fyn modulates pancreatic cancer metastasis via hnRNP E1,
the present study evaluated the association between the metastasis
of pancreatic cancer and the expression of hnRNP E1 and integrin β1
in human pancreatic cancer tissues. Subsequently, the mechanism of
integrin β1 splicing regulation by Fyn/hnRNP E1 signaling in
pancreatic cancer cells was investigated using multiple
experimental approaches.
Materials and methods
Antibodies
Rabbit monoclonal antibodies against SRC
family-phospho Y418 (reactive to Fyn-pY419, ab40660; 1:1,000 for
western blot analysis), integrin β1 (reactive to the extracellular
domain, ab179471, 1:1,000 for western blot analysis) and
p21-activated kinase 1 (ab40852, 1:1,000 for western blot
analysis), rabbit polyclonal antibodies against
serine/arginine-rich splicing factor 1 (ab38017, 1:1,000 for
western blot analysis) and SRp20 (ab73891, 1:1,000 for western blot
analysis) and a mouse monoclonal antibody against hnRNP A1 (ab5832,
1:1000 for western blot analysis) were purchased from Abcam
(Cambridge, UK). Rabbit polyclonal antibodies against hnRNP E1
(sc-28725, 1:500 for western blot analysis) and β-actin (sc-130657,
1:5,000 for western blot analysis) were purchased from Santa Cruz
(Santa Cruz Biotechnology, Dallas, TX, USA). Mouse monoclonal
antibodies against Fyn (P2992, 1:1000 for western blot analysis)
and green fluorescent protein (GFP) (SAB5300167, 1:5000 for western
blot analysis) were purchased from Sigma-Aldrich (St. Louis, MQ,
USA). A rabbit polyclonal antibody against phosphothreonine
(71-8200, 1:500 for western blot analysis) was purchased from
Invitrogen/Thermo Fisher Scientific (Waltham, MA, USA). Goat
anti-rabbit IgG rhodamine-conjugated (31670) and goat anti-rabbit
(31460) or anti-mouse (31430) IgG (1:5,000 for western blot
analysis) peroxidase-conjugated antibodies were purchased from
Pierce Biotechnology (Rockford, IL, USA).
Adenoviral expression of kinase-dead Fyn
(KdFyn) and hnRNP E1-GFP (E1-GFP)
The KdFyn recombinant adenovirus was previously
prepared in our laboratory (16).
The full-length hnRNP E1 coding sequence was obtained from HEK293
RNA using a PrimeScript™ RT-PCR kit (RR014A; Takara Bio, Dalian,
China) according to the manufacturer's protocol. The polymerase
chain reaction (PCR) products were identified by sequencing and
cloned into the multiple cloning site of the pEGFP-N2 plasmid
(6081-1 BD; Biosciences, Bedford, MA, USA) to construct an hnRNP
E1-GFP fusion protein. The hnRNP E1-GFP recombinant adenovirus was
generated using the AdEasy Vector System (240009; Stratagene, La
Jolla, CA, USA) according to the manufacturer's protocol. The GFP
recombinant adenovirus was used as a control vector in the cell
study. Sequences of the hnRNP E1-GFP fusion protein PCR primers are
listed in Table I.
 | Table ISequences of the integrin β1, β1A,
β1C, hnRNP E1-GFP and β-actin PCR primers. |
Table I
Sequences of the integrin β1, β1A,
β1C, hnRNP E1-GFP and β-actin PCR primers.
| Forward primer | Reverse primer | Size (bp) | EFF% |
|---|
| Integrin β1A |
AGAATCCAGAGTGTCCCACTGG |
TTTCCCTCATACTTCGGATTG | 238 | 92.4 |
| Integrin β1C |
TCTGTCGCCCAGCCTGGAGTG |
TTTCCCTCATACTTCGGATTG | 172 | 96.3 |
| integrin β1 |
CCTCATAACAGTCCTGTGCCTAGAAT |
CCAGCCAATGTGGTGAAACCC | 270 | 91.2 |
| hnRNP E1-GFP |
GC(AGATCT)CTCGCCATGGATGCCGGTGT |
CA(GAATTC)GCCCTTCTCAGAGGAAAGCCTGG | 1078 | 93.5 |
| β-actin |
CGGGAAATCGTGCGTGAC |
TGGAAGGTGGACAGCGAGG | 443 | 90.7 |
Cell lines and tissue samples
The human pancreatic cancer cell lines AsPC1, BxPC3,
CFPAC1 and Panc1, and the HEK293 cell line, were cultured in
RPMI-1640 medium (11875093; Thermo Fisher Scientific, Waltham, MA,
USA), Iscove's modified Dulbecco's medium (12440053; Thermo Fisher
Scientific) or Dulbecco's modified Eagle's medium (41965062; Thermo
Fisher Scientific), each supplemented with 10% fetal bovine serum
(FBS, 12657; Thermo Fisher Scientific) and antibiotic-antimycotic
(15240-062; Thermo Fisher Scientific). All cell lines were obtained
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
characterized according to the cell line authentication testing and
used within 6 months after resuscitation.
A total of 152 pancreatic cancer specimens from our
institution were used in the present study, including 76 specimens
that had been cryopreserved in liquid nitrogen, which were used for
RNA analysis, and 76 paraffin-embedded specimens (8330; Thermo
Fisher Scientific), which were used for histological analysis. Each
pancreatic cancer specimen was reviewed by two pathologists.
All human studies were reviewed and approved by the
Ethics Committee of Southwest Hospital and were therefore performed
in accordance with the ethical standards described in the 1975
Declaration of Helsinki, as revised in 1983. Informed consent was
obtained from all individual participants that were included in the
study.
Cell staining for confocal
microscopy
Cells were cultured to 50–70% confluence on
glass-bottom tissue culture dishes (150680; Thermo Fisher
Scientific) and then washed with phosphate-buffered solution (PBS,
10010023; Thermo Fisher Scientific). The cells were fixed with 2%
paraformaldehyde (FB002; Thermo Fisher Scientific), followed by the
addition of glycine buffer (0.1 mM glycine) (G7126; Sigma-Aldrich)
for paraformaldehyde quenching and blocked in 1% bovine serum
albumin (BSA, TS-38839; Thermo Fisher Scientific) in PBS for 1 h.
The hnRNP E1 rabbit polyclonal antibody (sc-28725; Santa Cruz
Biotechnology) was diluted in PBS (1:200) with 1% BSA and incubated
with the cells at 4°C overnight. The cells were then washed with
cold PBS three times prior to incubation with rhodamine-conjugated
goat anti-rabbit IgG (1:500; Pierce Biotechnology) for 45 min at
room temperature. The labeled cells were washed with cold PBS and
imaged using a confocal microscope (Carl Zeiss LSM780; Carl Zeiss
Microscopy GmbH, Jena, Germany). The images were analyzed using ZEN
2012 software (Carl Zeiss Microscopy GmbH).
Cell invasion assay
Cell invasion assays were performed using 24-well
Transwell inserts with 8 µm pore size (PI8P01250; Merck
Millipore, Billerica, MA, USA) coated with a thin layer of matrigel
(356234; BD Biosciences, Bedford, MA, USA) (1.5
µg/mm2). Cells (1×105 cells in 300
µl serum-free medium) were seeded into the upper chamber,
and the lower compartment was filled with 500 µl of complete
medium. After 24 h at 37°C, non-invading cells were removed by
wiping the upper side of the membrane. Invading cells were fixed,
stained and counted.
Flow cytometry
BxPC3 pancreatic cancer cells were cultured in
RPMI-1640 medium with 10% FBS and antibiotic-anti-mycotic, prior to
harvesteding by trypsinization (25200056; Thermo Fisher
Scientific). Subsequently, the cells were washed with PBS
containing 1% normal goat serum (01-6201; Thermo Fisher Scientific)
and incubated with integrin β1 rabbit monoclonal antibody
(ab179471, 1:500; Abcam) at 4°C for 1 h, followed by
rhodamine-conjugated goat anti-rabbit IgG (31670, 1:500; Pierce
Biotechnology) for 30 min. The stained cells were resuspended in
100 µl PBS and analyzed with a Becton Dickinson FACSort flow
cytometer.
Immunoprecipitation and RNA-protein
immunoprecipitation
Immunoprecipitation was performed by incubating 1
µg hnRNP E1 rabbit polyclonal antibody (sc-28725; Santa Cruz
Biotechnology) or Fyn mouse monoclonal antibody (P2992;
Sigma-Aldrich) with 500 µg extracted proteins overnight at
4°C with constant rotation. The immunocomplexes were captured by
adding protein A agarose (15918014; Invitrogen/Thermo Fisher
Scientific) for 1 h at 4°C with constant rotation. The
immunoprecipitates were analyzed by western blotting.
For RNA-protein immunoprecipitation, 1 mg extracted
proteins were pre-cleared with 20 µl protein A/G plus
agarose (20423; Invitrogen/Thermo Fisher Scientific) and then
incubated with 5 µg of hnRNP E1 rabbit polyclonal antibody
(sc-28725; Santa Cruz Biotechnology), SF2/ASF rabbit polyclonal
antibody (ab38017; Abcam) or hnRNP A1 mouse monoclonal antibody
(ab5832; Abcam) overnight at 4°C with constant rotation. The
co-precipitated RNA was then extracted by phenol (AM9712; Thermo
Fisher Scientific)/chloroform (1.02444; Sigma-Aldrich) and used for
further analysis.
Immunohistochemical staining
Specimens were fixed in formalin (9990916; Thermo
Fisher Scientific), embedded in paraffin and cut into 3-mm
sections. Sections were deparaffinized in xylene (9990501; Thermo
Fisher Scientific), rehydrated in a graded series of ethanol
(E7023; Sigma-Aldrich) solutions and incubated in 3.0% hydrogen
peroxide (TA-060-HP; Thermo Fisher Scientific) in methanol (960055;
Thermo Fisher Scientific) for 30 min to block endogenous peroxidase
activity. Slides were heated at 120°C in an autoclave in 10 mM
sodium citrate (pH 6.0) (71497; Sigma-Aldrich) for 130 sec and then
cooled to room temperature. After blocking with 10% goat serum for
30 min, the sections were incubated overnight at 4°C with the hnRNP
E1 rabbit polyclonal antibody (sc-28725, 1:200; Santa Cruz
Biotechnology). Negative controls were obtained by omitting the
primary antibody. The sections were incubated with
peroxidase-conjugated anti-mouse/rabbit immunoglobulins (K5007;
Dako EnVision™ System; Dako, Copenhagen, Denmark) according to the
manufacturer's protocol for 60 min at 37°C. The peroxidase reaction
was developed with 3,3′-diaminobenzidine as the chromogen, followed
by counterstaining with hematoxylin.
Mass spectrometry
Briefly, 5 mg extracted proteins were pre-cleared
with 50 µl protein A/G Plus agarose and then incubated with
10 µg hnRNP E1 rabbit polyclonal antibody (sc-28725; Santa
Cruz Biotechnology) overnight at 4°C with constant rotation. The
immunocomplexes were captured by adding protein A/G Plus agarose
for 1 h at 4°C. The immunoprecipitates were sent to the BGI
(Shenzhen, China) for mass spectrometry analysis.
Nude mouse xenograft model
Male athymic nu/nu mice, 4–6 weeks of age, were
obtained from the Beijing Animal Facility and provided with food
and water ad libitum. Tumor cells (2×105 tumor
cells in 100 µl of sterile PBS) were transplanted into each
mouse via subcutaneous or intrasplenic injection. Animals were
euthanized after 5–8 weeks, and the subcutaneous tumors and liver
were resected. Primary tumor mass was determined by measuring the
wet weight of the resected tumors and metastasis was determined as
the incidence of tumor nodes present in the liver.
Procedures involving animals and their care were
conducted in conformity with NIH guidelines (NIH Pub. No. 85-23,
revised 1996) and were approved by the Institutional Animal Care
and Use Committee of the Third Military Medical University.
RNA extraction and quantitative
real-time-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from tissues or cultured
cells using RNAiso Plus reagent (9108; Takara) according to the
manufacturer's protocol. The RNA was stored at −80°C, and reverse
transcription of the extracted RNA was performed using the
PrimeScript™ RT reagent kit with genomic DNA Eraser (RR047A;
Takara) according to the manufacturer's protocol. The cDNA was
stored at −20°C. qRT-PCR assays were performed to detect integrin
β1 expression using the One Step SYBR® PrimeScript™
RT-PCR kit (RR086A; Takara) according to the manufacturer's
protocol. The results were normalized to the expression of β-actin.
For quantitative measurement of integrin β1 expression, qRT-PCR was
performed using a CFX96 real-time PCR detection system (Bio-Rad
Laboratories, Hercules, CA, USA) with the following cycling
conditions: 95°C for 30 sec, followed by 40 cycles of 95°C for 5
sec and 60°C for 30 sec. The qRT-PCR results were analyzed and
expressed relative to a pancreatic cancer sample and converted to
fold-change value. The PCR products were separated on a 2% agarose
gel, stained with ethidium bromide and imaged with a video camera
(Vilber Lourmat, Marne-la-Vallée, France). The primers used and the
sizes of all the PCR products for integrin β1 and β-actin are
presented in Table I.
Small interfering RNA (siRNA)
transfection
Target-specific siRNAs for hnRNP E1 (sc-43843),
hnRNP A1 (sc-270345), SF2/ASF (sc-38319) and SRp20 (sc-38338) were
purchased from Santa Cruz Biotechnology and transfected into
pancreatic cancer cells according to the manufacturer's protocol.
In a 6-well tissue culture plate (PIDL06P05; Merck Millipore),
2×105 cells were seeded per well in 2 ml antibiotic-free
normal growth medium supplemented with FBS. The cells were
incubated at 37°C in a CO2 incubator until the cells
were 60–80% confluent. For each transfection, 2–8 µl of
siRNA duplexes were diluted into 100 µl siRNA transfection
medium (31985070; Thermo Fisher Scientific), and 2–8 µl
siRNA transfection reagent (11668019; Thermo Fisher Scientific) was
diluted into 100 µl siRNA transfection medium. The siRNA
duplex was added directly to the diluted transfection reagent using
a pipette and mixed gently by pipetting the solution up and down,
followed by incubation for 45 min at room temperature. The cells
were washed once with 2 ml of siRNA transfection medium, and 0.8 ml
of siRNA transfection medium was added to each tube containing the
siRNA transfection reagent mixture. The solutions were mixed gently
and overlaid onto the washed cells. The cells were incubated for
5–7 h at 37°C in a CO2 incubator. Subsequently, 1 ml of
normal growth medium containing two times the normal serum and
antibiotic concentration (2X normal growth medium) was added
without removing the transfection mixture. The cells were incubated
for an additional 18–24 h. The medium was aspirated and replaced
with fresh 1X normal growth medium. The cells were assayed using
the appropriate protocol at 72 h after the addition of normal
growth medium (2X normal growth medium) in the step above.
Scratch wound assay
Cells were seeded in 6-well plates (PIDL06P05; Merck
Millipore) at a density of >80% confluence. Cells were
transfected with KdFyn, E1-GFP or hnRNP E1 siRNA or the control
vector 24 h after seeding. Wounds were made in confluent monolayers
48 h after transfection by using a pipette tip under vacuum
suction. A straight line was drawn across the bottom of each well,
and then pictures were captured with the line at the bottom of the
viewing field. Wounds were measured from the exact vertical middle
of each image such that the initial measurement and the measurement
24 h later were taken from the same vertical spot in each well.
Representative images are shown for each group, and results are
reported as the percentage of the wound closed.
Western blot analysis
Whole cell protein was extracted with lysis buffer
(25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 5%
glycerol), and 30 µg protein, determined using a BCA protein
assay kit (23227; Pierce Biotechnology), was resolved on a 12%
acrylamide gel and then transferred onto nylon membranes (LC2003;
Thermo Fisher Scientific). The blots were incubated overnight at
4°C with 1% blocking solution in TBS buffer (50 mM Tris-HCl and 150
mM NaCl) and 0.1% Tween-20. The membranes were then incubated with
the primary antibodies. After 2 h at room temperature, the
membranes were washed twice with TBS plus 0.1% Tween-20 and 0.5%
blocking buffer and incubated for 1 h with horseradish
peroxidase-conjugated secondary antibody at room temperature.
Following incubation with the appropriate secondary antibodies, the
membranes were washed and the signals were visualized with Amersham
ECL plus Western blotting detection reagents (28-9829-42 AB; GE
Healthcare Bio-Sciences AB, Stockholm, Sweden). The specific bands
on the autoradiograms were quantitated by densitometry.
Statistical analysis
All analyses were performed using SPSS 19.0
software. The correlation between categorical variables was
evaluated using the χ2 test. Normally distributed data
were analyzed using a Student's t-test; otherwise, the
non-parametric Mann-Whitney test was applied. P<0.05 was
considered to indicate a statistically significant difference.
Results
Activity of Fyn affects alterative
splicing of integrin β1
To determine whether the activity of Fyn affects the
expression of integrin β1 in pancreatic cancer cells, the
expression of active Fyn was analyzed in four pancreatic cancer
cell lines, and BxPC3 pancreatic cancer cells were selected for the
investigation of integrin β1 expression when Fyn activity was
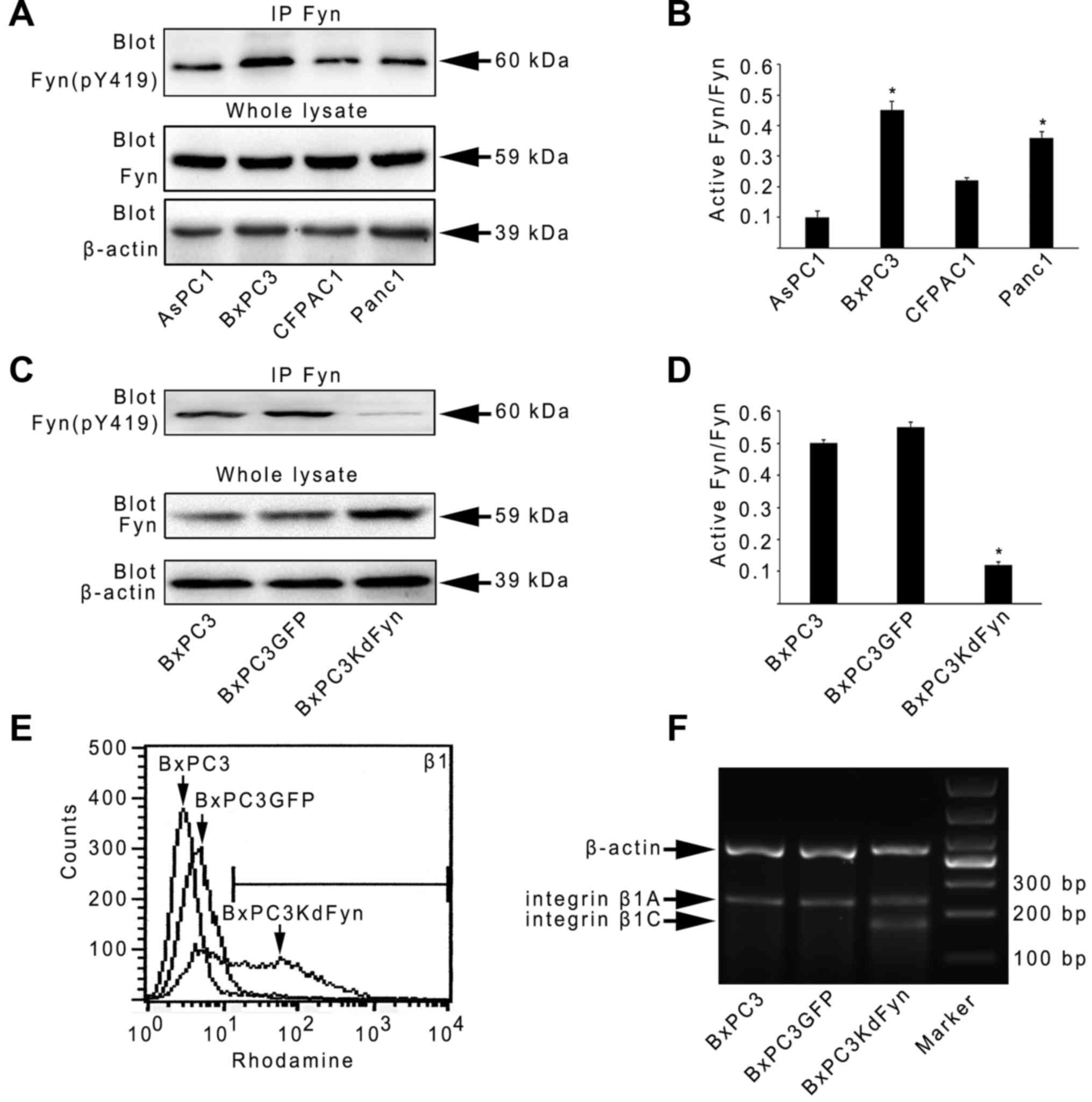
suppressed. As shown in Fig. 1A–E,
BxPC3 cells expressed higher levels of active Fyn than the other
three pancreatic cancer cells. In the KdFyn-transfected BxPC3 cells
(BxPC3KdFyn), the expression of integrin β1 did not decrease;
however, the level of a certain subtype of integrin β1 was
increased, suggesting that suppressing Fyn activity may result in a
change in integrin β1 splicing. Therefore, the splicing of integrin
β1 in BxPC3 cells with different activity levels of Fyn was further
analyzed. Integrin β1B and D were not expressed in these pancreatic
cancer cells, and decreasing the activity of Fyn significantly
promoted the expression of integrin β1C (Fig. 1F). These results suggested that the
activity of Fyn affected the alterative splicing of integrin
β1.
Fyn regulates phosphorylation and nuclear
localization of hnRNP E1 via P21-activated kinase 1 (PAK1), thus
affecting the alterative splicing of integrin β1
To determine whether Fyn regulates the biological
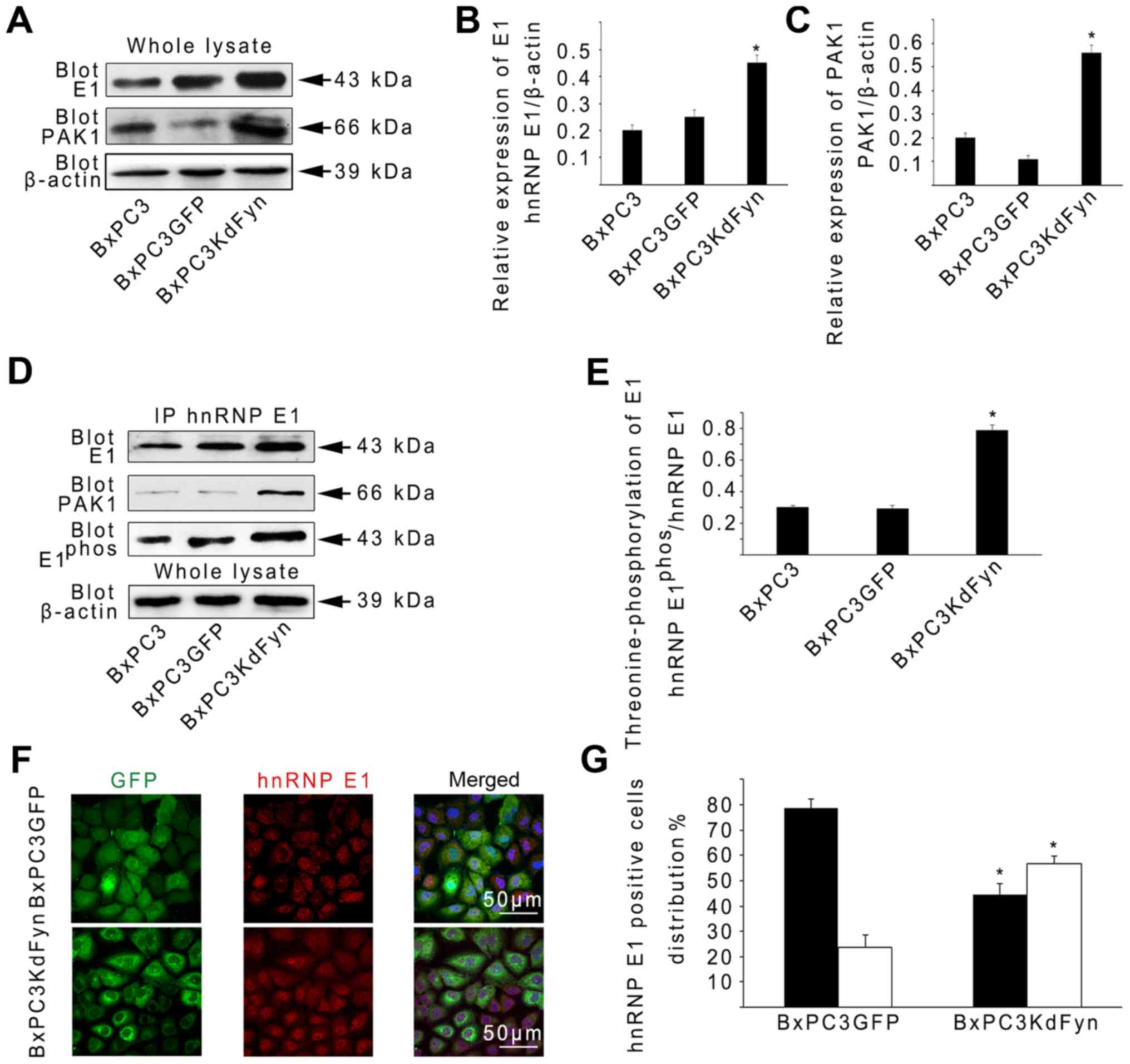
function of hnRNP E1, the protein levels of hnRNP E1 in BxPC3
cancer cells with different Fyn activities were evaluated. The
expression of hnRNP E1 was revealed to be increased ~2-fold in
BxPC3KdFyn cells compared with BxPC3GFP or BxPC3 cells (Fig. 2A and B). It has been shown that
PAK1, one of the evolutionarily conserved families of
serine/threonine protein kinases, contributes to the
threonine-phosphorylation of hnRNP E1 thus activating hnRNP E1 for
RNA processing (37). To further
explore whether the inhibition of Fyn activity affects PAK1
expression, thus promoting hnRNP E1 phosphorylation, PAK1
expression was analyzed in BxPC3 cells with different Fyn activity
levels. PAK1 expression was found to be increased significantly in
BxPC3KdFyn cells compared with the control cells (Fig. 2A and C). Subsequently,
co-immunoprecipitation was used to analyze the
threonine-phosphorylation of hnRNP E1 and the interaction between
hnRNP E1 and PAK1 in BxPC3 cells with different Fyn activity
levels. As shown in Fig. 2D and E,
threonine-phosphorylation of hnRNP E1 was increased ~2.5-fold in
BxPC3KdFyn cells compared with BxPC3 or BxPC3GFP cells. In
addition, inhibition of Fyn activity promoted the interaction of
hnRNP E1 with PAK1 in BxPC3KdFyn cells. Phosphorylated hnRNP E1 is
involved in nuclear localization and mediates a variety of
functions associated with mRNA splicing and stability (37,38).
Therefore, the effect of Fyn activity on hnRNP E1 nuclear
localization was next investigated using confocal
immunofluorescence, revealing that the nuclear distribution of
hnRNP E1 was increased ~2-fold in BxPC3KdFyn cells compared with
BxPC3GFP cells (Fig. 2F and G).
These results suggested that Fyn regulated the phosphorylation and
nuclear localization of hnRNP E1 via PAK1 in pancreatic cancer
cells.
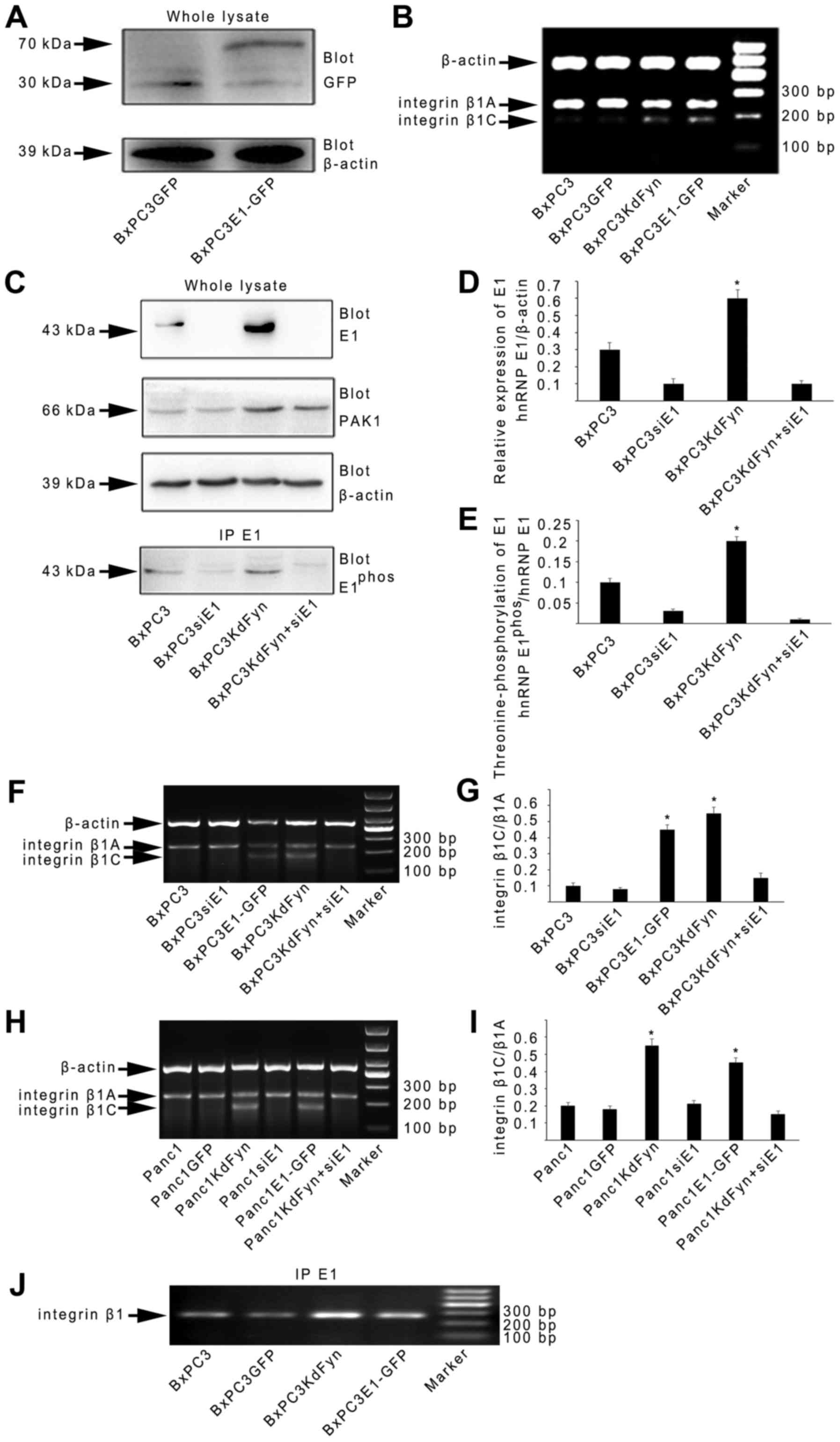
However, to date, the cellular function of hnRNP E1
in integrin β1 splicing has not been reported. Therefore, whether
changes in hnRNP E1 expression could modulate the splicing of
integrin β1 was next investigated. A GFP-hnRNP E1 fusion protein
was expressed from an adenoviral system in BxPC3 cells
(BxPC3E1-GFP) (Fig. 3A). The
expression of integrin β1C was markedly increased in BxPC3E1-GFP
cells (Fig. 3B). Furthermore,
knockdown of the expression of hnRNP E1 by transfection specific
hnRNP E1 siRNA in the BxPC3KdFyn cells (BxPC3KdFyn+siE1) decreased
the expression of integrin β1C (Fig.
3C–G). It was also noted that inhibition of the expression of
hnRNP E1 did not affect PAK1 expression, but significantly
decreased the phosphorylation of hnRNP E1 (Fig. 3E). In addition, inhibition of the
activity of Fyn or overexpression of hnRNP E1 in the Panc1
pancreatic cancer cells which expressed active Fyn (Fig. 1A) also promoted the splicing of
integrin β1C (Fig. 3H and I).
These results suggested that Fyn affected the splicing of integrin
β1 by regulating the phosphorylation and nuclear localization of
hnRNP E1.
hnRNP E1 spliceosome regulates integrin
β1 splicing by using hnRNP A1 and SF2/ASF to recognize the pre-mRNA
of integrin β1
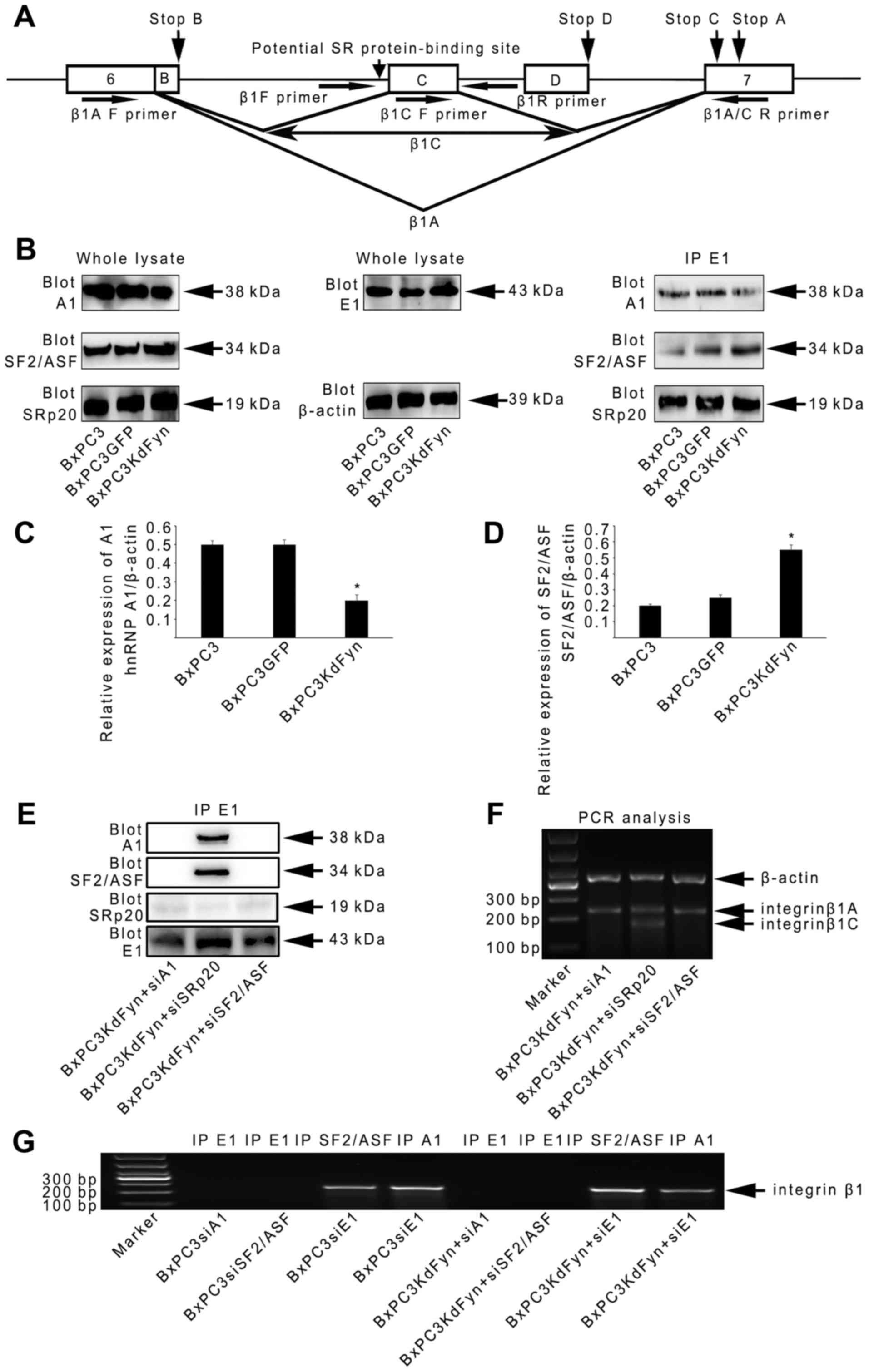
It is established that splicing is performed by the
spliceosome, which is composed of five small ribonucleoproteins
(snRNPs) and hundreds of additional proteins, including the SR
proteins and the hnRNPs. The SR proteins and hnRNPs function as
trans-acting factors by binding to the ESEs or ESSs of
target pre-mRNAs to regulate alternative splicing (39–43).
As the results of the present study identified that hnRNP E1
participated in the regulation of integrin β1 splicing, whether
hnRNP E1 binds to the pre-mRNA of integrin β1 was further analyzed
by RNA immunoprecipitation. As shown in Fig. 3J, the binding of hnRNP E1 to
integrin β1 pre-mRNA in BxPC3 pancreatic cancer cells with
different Fyn activity or hnRNP E1 protein levels suggested that
hnRNP E1 may be a component of the spliceosome complex that
regulates integrin β1 splicing. Therefore, the components of the
hnRNP E1 spliceosome complex extracted from BxPC3KdFyn cells were
next analyzed by mass spectrometry. This revealed two SR proteins
(SRSF1 and SRSF3, also known as SF2/ASF and SRp20) and six other
hnRNPs (hnRNP A1, A3, C1, K, M and U) in the hnRNP E1 spliceosome
complex (Table II). Previous
studies showed a potential SR protein-binding site in the pre-mRNA
of integrin β1 for the splicing of β1C (Fig. 4A (44). Therefore, whether these two SR
proteins and three other hnRNPs (hnRNP A1, C1 and M, which have
been demonstrated to participate in splicing regulation) affect
integrin β1 splicing was examined in this study.
 | Table IIProteins identified by mass
spectrometry in the hnRNP E1 spliceosome of BxPC3KdFyn cells. |
Table II
Proteins identified by mass
spectrometry in the hnRNP E1 spliceosome of BxPC3KdFyn cells.
| Group ID | Protein ID | Protein score | Protein mass | Coverage | No. of unique
peptide | No. of unique
spectrum | Isoelectric
point | Description |
|---|
| 1 | IPI00003865 | 972.09 | 71082.31 | 0.243034056 | 11 | 24 | 5.16 | HSPA8 isoform 1 of
heat shock cognate 71 kDa protein |
| 2 | IPI00216049 | 704.64 | 51229.51 | 0.265658747 | 9 | 17 | 5.18 | HNRNPK,
isoform 1 of heterogeneous nuclear ribonucleoprotein K |
| 3 | IPI00017617 | 526.07 | 69617.93 | 0.140065147 | 7 | 9 | 9.21 | DDX5 probable
ATP-dependent RNA helicase DDX5 |
| 4 | IPI00911039 | 440.23 | 64169.98 | 0.015358362 | 1 | 1 | 5.19 | HSPA1B, HSPA1A cDNA
FLJ54408, highly similar to heat shock 70 kDa protein 1 |
| 5 | IPI00304925 | 440.12 | 70294.14 | 0.014040562 | 1 | 2 | 5.31 | HSPA1B, HSPA1A heat
shock 70 kDa protein 1A/1B |
| 6 | IPI00479217 | 335.99 | 89665.43 | 0.079404467 | 5 | 8 | 5.51 | HNRNPU,
isoform short of heterogeneous nuclear ribonucleoprotein U |
| 7 | IPI00171903 | 323.1 | 77749.42 | 0.115068493 | 6 | 9 | 9.1 | HNRNPM,
isoform 1 of heteronuclear ribonucleoprotein M |
| 8 | IPI00215965 | 316.85 | 38837.09 | 0.112903226 | 3 | 6 | 9.46 | HNRNPA1,
isoform A1-B of heterogeneous nuclear ribonucleoprotein A1 |
| 9 | IPI00420014 | 295.88 | 246006.24 | 0.033707865 | 5 | 5 | 5.95 | SNRNP200 isoform 1
of U5 small nuclear ribonucleoprotein 200 kDa helicase |
| 10 | IPI00216592 | 292.33 | 32374.89 | 0.160409556 | 4 | 10 | 4.69 | HNRNPC,
isoform C1 of heterogeneous nuclear ribonucleoproteins C1/C2 |
| 11 | IPI00419373 | 278.6 | 39798.67 | 0.137566138 | 4 | 5 | 9.31 | HNRNPA3,
isoform 1 of heterogeneous nuclear ribonucleoprotein A3 |
| 12 | IPI00300371 | 236.25 | 136575.15 | 0.045193098 | 4 | 4 | 4.91 | SF3B3 isoform 1 of
splicing factor factor 3B subunit 3 |
| 13 | IPI00552938 | 208.36 | 22271.46 | 0.178571429 | 3 | 6 | 4.95 | RBMX heterogeneous
nuclear ribonucleoprotein G isoform 2 |
| 14 | IPI00215884 | 208.03 | 27841.86 | 0.14516129 | 3 | 5 | 10.77 | SRSF1,
isoform ASF-1 of serine/arginine-rich splicing factor 1 |
| 15 | IPI00641829 | 206.99 | 51103.09 | 0.0248307 | 1 | 1 | 5.67 | SNORD84, DDX39B
isoform 2 of spliceosome RNA helicase DDX39B |
| 16 | IPI00328840 | 178.33 | 27540.9 | 0.189393939 | 3 | 5 | 11.6 | THOC4 THO complex
subunit 4 |
| 17 | IPI00007423 | 173.48 | 28941.37 | 0.079681275 | 2 | 2 | 3.67 | ANP32B isoform 1 of
acidic leucine-rich nuclear phosphoprotein 32 family member B |
| 18 | IPI00017964 | 167.06 | 14021.35 | 0.317460317 | 3 | 4 | 10.98 | SNRPD3 small
nuclear ribonucleoprotein Sm D3 |
| 19 | IPI00010204 | 141.72 | 19545.99 | 0.140243902 | 2 | 4 | 12.15 | SRSF3,
Serine/arginine-rich splicing factor 3 |
| 20 | IPI00031556 | 104.61 | 53809.32 | 0.044210526 | 2 | 2 | 9.49 | U2AF2 isoform 1 of
splicing factor U2AF 65 kDa subunit |
| 21 | IPI00396435 | 91.92 | 91673.47 | 0.031446541 | 2 | 3 | 7.48 | DHX15 putative
pre-mRNA-splicing factor ATP-dependent RNA helicase DHX15 |
| 22 | IPI00009328 | 86.29 | 47126.29 | 0.03406326 | 1 | 2 | 6.69 | EIF4A3 eukaryotic
initiation factor 4A-III |
| 23 | IPI00001757 | 80.34 | 19933.75 | 0.097701149 | 1 | 2 | 5.43 | RBM8A isoform 1 of
RNA-binding protein 8A |
| 24 | IPI00007928 | 79.34 | 274738.05 | 0.003426124 | 1 | 1 | 9.14 | PRPF8
Pre-mRNA-processing- splicing factor 8 |
| 25 | IPI00026089 | 70.91 | 146479.37 | 0.013803681 | 1 | 1 | 7.1 | SF3B1 splicing
factor 3B subunit 1 |
| 26 | IPI00302850 | 64.13 | 13273.36 | 0.092436975 | 1 | 1 | 12.09 | SNRPD1 small
nuclear ribonucleo-protein Sm D1 |
| 27 | IPI00016610 | 60.99 | 37987.14 | 0.04494382 | 1 | 1 | 7.11 | PCBP1,
Poly(rC)-binding protein 1 (hnRNP E1) |
| 28 | IPI00003519 | 60.81 | 110335.65 | 0.012345679 | 1 | 1 | 4.6 | EFTUD2 116 kDa U5
small nuclear ribonucleoprotein component |
| 29 | IPI00027285 | 54.91 | 24764.75 | 0.033333333 | 1 | 1 | 11.75 | SNRPB isoform SM-B'
of small nuclear ribonucleoprotein associated proteins B and
B' |
| 30 | IPI00016572 | 54.82 | 8547.45 | 0.157894737 | 1 | 1 | 9.41 | SNRPG small nuclear
ribonucleo-protein G |
| 31 | IPI00305068 | 43.69 | 107656.09 | 0.015940489 | 1 | 1 | 8.4 | PRPF6
Pre-mRNA-processing factor 6 |
| 32 | IPI00221106 | 41.48 | 100279.02 | 0.013407821 | 1 | 1 | 5.37 | SF3B2 splicing
factor 3B subunit 2 |
| 33 | IPI00329791 | 39.34 | 117802.7 | 0.010669253 | 1 | 1 | 9.87 | DDX46 probable
ATP-dependent RNA helicase DDX46 |
| 34 | IPI00019380 | 38.82 | 92863.93 | 0.013924051 | 1 | 1 | 6.4 | NCBP1 nuclear
cap-binding protein subunit 1 |
| 35 | IPI00012442 | 38.16 | 52189.1 | 0.036480687 | 1 | 1 | 5.21 | G3BP1 Ras
GTPase-activating protein-binding protein 1 |
| 36 | IPI00008943 | 37.53 | 54348.96 | 0.022964509 | 1 | 1 | 6.21 | DDX19B isoform 1 of
ATP-dependent RNA helicase DDX19B |
| 37 | IPI00013180 | 36.66 | 17558.8 | 0.0625 | 1 | 1 | 9 | BUD31 protein BUD31
homolog |
| 38 | IPI00010158 | 34.07 | 14758.48 | 0.061068702 | 1 | 2 | 4.73 | CHRAC1 chromatin
accessibility complex protein 1 |
| 39 | IPI00029266 | 32.15 | 10853.66 | 0.119565217 | 1 | 1 | 9.86 | SNRPE small nuclear
ribonucleo-protein E |
Generally, the alternative splicing of genes is
determined by SR proteins and hnRNPs in a concentration-dependent
manner (45). Therefore, we
analyzed the expression of these SR and hnRNP proteins and their
abundance in the hnRNP E1 complex in pancreatic cancer cells. As
shown in Fig. 4B–D, the expression
of SF2/ASF was significantly increased in BxPC3KdFyn cells compared
with control cells. However, the expression of SRp20 did not
significantly differ among these groups of cells. With regard to
the three hnRNPs, only hnRNP A1 was obviously decreased in
BxPC3KdFyn cells compared with control cells. In addition, the
level of hnRNP A1 was decreased in the hnRNP E1 complex in
BxPC3KdFyn cells when the SF2/ASF level was increased (Fig. 4B). These results suggested that
hnRNP A1 and SF2/ASF may serve important roles in the hnRNP E1
spliceosome complex.
To explore the functions of hnRNP A1 and SF2/ASF in
the spliceosome, target-specific siRNAs were used to knock down
their expression in BxPC3KdFyn cells. As shown in Fig. 4E and F, knockdown of hnRNP A1 or
SF2/ASF in BxPC3KdFyn cells completely eliminated the construction
of the hnRNP E1 complex and ultimately prevented the expression of
integrin β1C. By contrast, knockdown of SRp20 did not affect the
interaction of hnRNP E1 with hnRNP A1 and SF2/ASF, and did not
alter the splicing of integrin β1 in BxPC3KdFyn cells. Therefore,
in the spliceosome complex of BxPC3KdFyn cells, hnRNP A1, hnRNP E1
and SF2/ASF were key proteins responsible for integrin β1
splicing.
To further determine whether hnRNP A1, hnRNP E1 and
SF2/ASF have direct roles in the post-transcriptional modification
of integrin β1 pre-mRNA, endogenous complexes of RNA with hnRNP A1,
E1 and SF2/ASF were extracted from BxPC3KdFyn cells to analyze the
presence of integrin β1 pre-mRNA. hnRNP A1 and SF2/ASF, but not
hnRNP E1, were directly bound to the pre-mRNA of integrin β1
(Fig. 4G). These results suggested
that hnRNP E1 spliceosome regulated integrin β1 splicing by using
hnRNP A1 and SF2/ASF to recognize the pre-mRNA of integrin β1 in
pancreatic cancer cells.
Effect of Fyn and hnRNP E1 on the
invasion and metastasis of pancreatic cancer cells in vitro and in
vivo
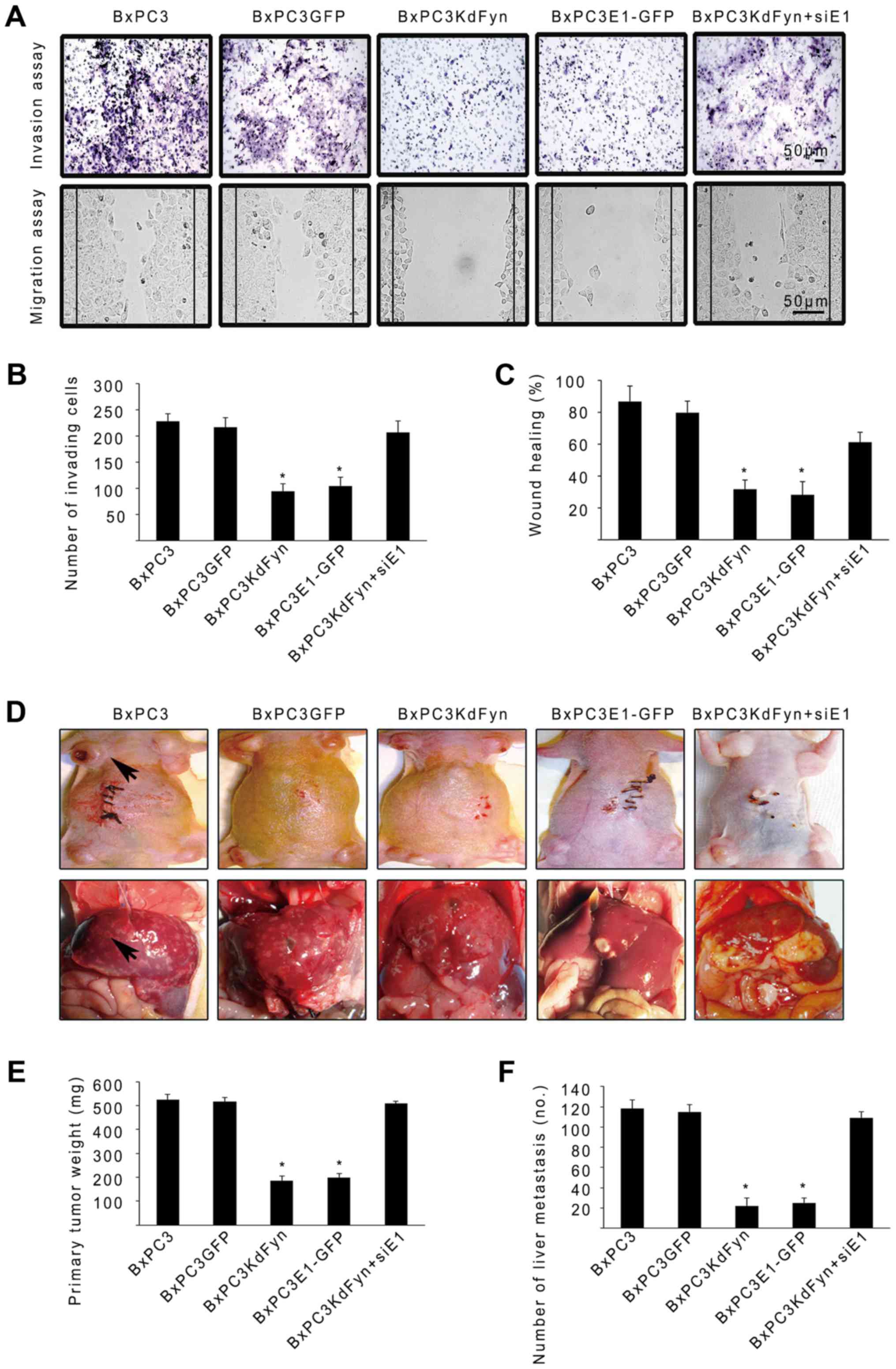
Transwell and wound-healing assays were employed to
determine the effect of Fyn and hnRNP E1 on the invasion and
migration of pancreatic cancer cells in vitro. As shown in
Fig. 5A–C, inhibition of the
activity of Fyn or overexpression of hnRNP E1 significantly
decreased the number of invading cells and the migration of BxPC3
pancreatic cancer cells. By contrast, knockdown of the expression
of hnRNP E1 in the BxPC3KdFyn cells promoted the invasion and
migration of tumor cells. Subsequently, the effects of Fyn and
hnRNP E1 on pancreatic cancer cell tumorigenesis and metastasis
were investigated in vivo by subcutaneous or intrasplenic
injection of 2×105 tumor cells into nude mice. After 5–8
weeks, the mice were sacrificed to detect subcutaneous tumors and
liver metastases, the findings revealed that inhibition of Fyn
activity or overexpression of exogenous hnRNP E1 within BxPC3
pancreatic cancer cells significantly decreased the primary tumor
mass and liver metastases. Furthermore, knockdown of hnRNP E1 in
the BxPC3KdFyn cells promoted the growth of the primary tumor mass
and liver metastases (Fig. 5D–F).
These results suggested that Fyn/hnRNP E1 signaling regulated the
invasion and metastases of pancreatic cancer cells in vitro
and in vivo.
Expression of hnRNP E1 and integrin β1
are associated with metastasis of pancreatic cancer
Our data showed that Fyn, hnRNP E1 and integrin β1
were involved in the metastases of pancreatic cancer cells.
Therefore, we next analyzed the expression of these proteins in
pancreatic cancer using the Human Protein Atlas database and
pancreatic cancer tissues.
In the Human Protein Atlas database, the expression
of hnRNP E1 was positive in pancreatic cancer and the malignant
cells exhibited moderate to strong cytoplasmic positivity
(http://www.proteinatlas.org/ENSG00000169564-PCBP1/cancer/tissue/pancreatic+cancer).
In pancreatic cancer tissues obtained from our institution, the
mRNA expression of integrin β1A was correlated with lymph node
metastasis (P=0.001), hepatic metastasis (P=0.005) and the tumor
stage (P=0.002) of pancreatic cancer. By contrast, only cytoplasmic
and not nuclear, expression of hnRNP E1 was correlated with lymph
node metastasis (P=0.004), hepatic metastasis (P=0.011) and the
tumor stage of pancreatic cancer (P=0.001) (Table III). In addition, the cytoplasmic
expression of hnRNP E1 was positively correlated with the mRNA
expression of integrin β1A (Table
IV). Therefore, the expression of hnRNP E1 and integrin β1A
were associated with metastasis of pancreatic cancer.
 | Table IIIAssociations between the expression
of integrin β1A, hnRNP E1 and the categorical clinicopathological
parameters of pancreatic carcinoma. |
Table III
Associations between the expression
of integrin β1A, hnRNP E1 and the categorical clinicopathological
parameters of pancreatic carcinoma.
| Parameters | Integrin β1A mRNA
expression
| Cytoplasmic hnRNP
E1 protein expression
|
|---|
| Median expression
(range) | P-value | Low | High | P-value |
|---|
| Age (years) | | 0.183 | | | 0.805 |
| <65 | 44.343
(24.355–82.093) | | 27 | 29 | |
| ≥65 | 28.273
(17.489–58.197) | | 9 | 11 | |
| Sex | | 0.847 | | | 0.092 |
| Male | 37.853
(18.741–74.376) | | 5 | 12 | |
| Female | 28.671
(23.728–79.674) | | 31 | 28 | |
| Tumor size
(cm) | | 0.517 | | | 0.961 |
| ≤2 | 55.170
(23.557–80.806) | | 16 | 18 | |
| >2 | 29.847
(18.257–74.357) | | 20 | 22 | |
| Tumor location | | 0.601 | | | 0.108 |
| Head | 35.482
(21.230–74.376) | | 34 | 33 | |
| Body and tail | 64.364
(27.496–79.674) | | 2 | 7 | |
| Lymph node
metastasis | | 0.001 | | | 0.004 |
| Negative | 28.051
(10.770–57.022) | | 27 | 17 | |
| Positive | 70.630
(34.876–96.331) | | 9 | 23 | |
| Vascular
invasion | | 0.150 | | | 0.258 |
| Negative | 36.222
(25.467–77.838) | | 30 | 29 | |
| Positive | 29.816
(5.563–64.032) | | 6 | 11 | |
| Neural
invasion | | 0.147 | | | 0.426 |
| Negative | 29.361
(9.563–56.324) | | 11 | 9 | |
| Positive | 40.934
(25.618–80.162) | | 25 | 31 | |
| Duodenal
invasion | | 0.328 | | | 0.417 |
| Negative | 27.934
(9.758–63.527) | | 8 | 6 | |
| Positive | 37.038
(24.087–78.756) | | 28 | 34 | |
| Hepatic
metastases | | 0.005 | | | 0.011 |
| Negative | 29.847
(16.785–69.192) | | 35 | 31 | |
| Positive | 72.487
(64.115–106.964) | | 1 | 9 | |
|
Differentiation | | 0.56 | | | 0.404 |
| Well | 49.798
(25.015–69.084) | | 1 | 4 | |
| Moderate | 35.482
(18.741–80.892) | | 26 | 25 | |
| Poor | 28.189
(25.769–30.836) | | 9 | 11 | |
| T-stage (UICC) | | 0.419 | | | 0.077 |
| T1, 2 | 35.482
(25.467–77.188) | | 26 | 21 | |
| T3, 4 | 39.998
(12.114–76.003) | | 10 | 19 | |
| Tumor stage
(UICC) | | 0.002 | | | 0.001 |
| I | 28.632
(13.919–44.015) | | 21 | 8 | |
| II | 46.717
(18.839–82.530) | | 14 | 22 | |
| III, IV | 76.003
(54.517–117.941) | | 1 | 10 | |
 | Table IVCorrelation between the expressions
of β1A (mRNA) and hnRNP E1 (protein). |
Table IV
Correlation between the expressions
of β1A (mRNA) and hnRNP E1 (protein).
| n | Spearman rank
correlation analysis
|
|---|
| Correlation
coefficient | P-value |
|---|
| hnRNP E1 protein
expression β1A mRNA expression | 76 | 0.556 | <0.01 |
Discussion
The present study revealed a novel mechanism by
which Fyn/hnRNP E1 signaling regulates pancreatic cancer metastasis
by affecting the alternative splicing of integrin β1. The results
demonstrated that inhibition of Fyn activity upregulated the
expression of PAK1 and promoted the phosphorylation and nuclear
localization of hnRNP E1, which affected the alterative splicing of
integrin β1. Subsequently, an hnRNP E1 spliceosome complex
including SR proteins and hnRNPs was shown to regulate the
alternative splicing of integrin β1. In this spliceosome complex,
hnRNP A1, hnRNP E1 and SF2/ASF were key proteins responsible for
integrin β1 splicing. Importantly, hnRNP A1 and SF2/ASF directly
bound to the pre-mRNA of integrin β1 to achieve
post-transcriptional regulation. In vivo and in vitro
studies demonstrated that suppression of Fyn activity and/or
overexpression of hnRNP E1 significantly decreased the invasion and
metastasis of pancreatic cancer cells. Finally, the expression of
hnRNP E1 and integrin β1A were associated with the metastasis of
pancreatic cancer.
Alternative splicing of the mRNA encoding certain
integrin subunits increases diversity within the integrin family,
and alters the function of the specific integrin (22). Previous studies have shown that
splicing of integrin αv affected the proliferation and
pro-angiogenic properties of human endothelial cells (21). Four splice variants (A, B, C and D)
have been identified in the integrin β1 family, and the β1A isoform
is known to be widely expressed among all mammalian species tested
to date. Splice variants B, C and D which inhibit β1A-mediated
focal adhesion formation, focal adhesion kinase phosphorylation,
fibronectin matrix assembly, cell spreading and motility, have been
shown to be downregulated or even absent in many types of tumor
tissues and may be involved in tumor progression (22,46–49).
However, the formation of splice variants of integrin β1 has not
been fully described. In the present study, inhibition of Fyn
activity and/or overexpression of hnRNP E1 was found to
significantly promote the splicing of the integrin β1C variant in
pancreatic cancer cells and decrease the invasion and migration of
tumor cells in vitro, thus eventually suppressing
tumorigenesis and metastasis of pancreatic cancer cells in
vivo. In human pancreatic cancer tissues obtained from our
institution, the expression of hnRNP E1 and integrin β1A were
associated with the metastasis of pancreatic cancer. These results
suggest that Fyn and hnRNP E1 affect pancreatic cancer metastasis
by participating in the regulation of integrin β1 splicing.
As a member of the SFKs, Fyn has been shown to
affect many cellular processes, including mRNA splicing (50,51).
However, the mechanism by which Fyn regulates integrin β1
alternative splicing has not been reported. In the present study,
the activity of Fyn was demonstrated to regulate the alternative
splicing of integrin β1 via hnRNP E1. Inhibition of Fyn activity
significantly promoted the phosphorylation and nuclear localization
of hnRNP E1, which affected the alterative splicing of integrin β1
in pancreatic cancer cells. In addition, overexpression of hnRNP E1
significantly promoted the splicing of integrin β1C. By contrast,
knockdown of hnRNP E1 expression eliminated integrin β1C splicing
induced by KdFyn. These results suggest that Fyn acts though hnRNP
E1 to regulate the alternative splicing of integrin β1.
Additionally, inhibition of Fyn activity upregulated PAK1, which
phosphorylated hnRNP E1 through direct interaction. Therefore, Fyn
functions through PAK1 to induce phosphorylation and nuclear
localization of hnRNP E1, which regulates integrin β1 alternative
splicing.
The different components of the spliceosome
determine the splicing of individual pre-mRNAs and assist in the
accurate recognition of splice sites (52,53).
The hnRNPs are involved in processing heterogeneous nuclear RNAs
into mature mRNAs, as well as functioning as trans-acting
factors in the regulation of gene expression under the
phosphorylation of serine and threonine residues or methylation of
arginine residues (54). The SR
proteins, which are phosphorylated by the SR-specfic protein kinase
family, including cdc2-like kinase, are key determinants of exon
identity, and function as molecular adaptors to link the pre-mRNA
to the splicing machinery (55–58).
In the present study, hnRNP E1 was found to form a complex with SR
proteins and hnRNPs, which coordinately regulated the alternative
splicing of integrin β1 in pancreatic cancer cells. In this
spliceosome complex, hnRNP E1, hnRNP A1 and SF2/ASF served key
roles in the regulation of integrin β1 splicing. Firstly, the
amounts of hnRNP A1 and SF2/ASF in the hnRNP E1 spliceosome complex
were demonstrated to be inversely correlated with one another among
BxPC3 cells with different Fyn activity levels, suggesting that
hnRNP A1 and SF2/ASF may have opposing roles in integrin β1
splicing. This phenomenon is consistent with a previous report,
which showed that these two proteins have competing roles in
regulation of alternative splicing of the Ron gene (59). Additionally, in the present study,
knockdown of either hnRNP A1 or SF2/ASF completely eliminated the
formation of the hnRNP E1 spliceosome complex and ultimately
prohibited the alternative splicing of integrin β1 in pancreatic
cancer cells, suggesting that hnRNP A1 and SF2/ASF were the key
proteins responsible for integrin β1 splicing. Furthermore, RNA
immunoprecipitation revealed that hnRNP A1 and SF2/ASF, but not
hnRNP E1, directly bound to the pre-mRNA of integrin β1, suggesting
that the hnRNP E1 spliceosome complex acts via binding of hnRNP A1
and SF2/ASF to the pre-mRNA of integrin β1. Therefore, the hnRNP E1
spliceosome regulates integrin β1 splicing via hnRNP A1- and
SF2/ASF-mediated recognition of the pre-mRNA of integrin β1.
In conclusion, the results of the present study
provide a potential explanation for how the Fyn/hnRNP E1
spliceosome signaling regulates pancreatic cancer metastasis.
Inhibition of Fyn activity promoted the phosphorylation and nuclear
localization of hnRNP E1, leading to construction of the hnRNP E1
spliceosome complex, thus resulting in alterative splicing of
integrin β1. In the hnRNP E1 spliceosome complex, hnRNP A1 and
SF2/ASF are responsible for binding to the pre-mRNA of integrin β1
to achieve posttranscriptional modification. Suppression of Fyn
and/or overexpression of hnRNP E1 significantly decreased the
invasion and metastasis of pancreatic cancer cells. Thus, by
altering integrin β1 splicing, the Fyn/hnRNP E1 spliceosome signal
modulates the metastasis of pancreatic cancer.
Acknowledgments
The authors would like to thank Professors Lei Cai
and Xiaobin Feng for their assistance with the writing assistance
and proofreading of the present study. This work was supported by
the National Natural Science Foundation of China under the grant
no. 81430063; and the Natural Science Foundation of Southwest
Hospital under the grant no. SWH2016JCYB-46.
References
|
1
|
Binker MG, Binker-Cosen MJ, Binker-Cosen
AA and Cosen-Binker LI: Microenvironmental factors and
extracellular matrix degradation in pancreatic cancer. JOP.
15:280–285. 2014.PubMed/NCBI
|
|
2
|
Erkan M: Understanding the stroma of
pancreatic cancer: Co-evolution of the microenvironment with
epithelial carcinogenesis. J Pathol. 231:4–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lunardi S, Muschel RJ and Brunner TB: The
stromal compartments in pancreatic cancer: Are there any
therapeutic targets? Cancer Lett. 343:147–155. 2014. View Article : Google Scholar
|
|
4
|
Grzesiak JJ, Ho JC, Moossa AR and Bouvet
M: The integrin-extracellular matrix axis in pancreatic cancer.
Pancreas. 35:293–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grzesiak JJ, Tran Cao HS, Burton DW,
Kaushal S, Vargas F, Clopton P, Snyder CS, Deftos LJ, Hoffman RM
and Bouvet M: Knockdown of the beta(1) integrin subunit reduces
primary tumor growth and inhibits pancreatic cancer metastasis. Int
J Cancer. 129:2905–2915. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walsh N, Clynes M, Crown J and O'Donovan
N: Alterations in integrin expression modulates invasion of
pancreatic cancer cells. J Exp Clin Cancer Res. 28:1402009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao H, Zeng ZZ, Fay KS, Veine DM,
Staszewski ED, Morgan M, Wilder-Romans K, Williams TM, Spalding AC,
Ben-Josef E, et al: Role of α5β1 integrin up-regulation in
radiation-induced invasion by human pancreatic cancer cells. Transl
Oncol. 4:282–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hilbig A: Src kinase and pancreatic
cancer. Recent Results Cancer Res. 177:179–185. 2008. View Article : Google Scholar
|
|
9
|
Je DW, O YM, Ji YG, Cho Y and Lee DH: The
inhibition of SRC family kinase suppresses pancreatic cancer cell
proliferation, migration, and invasion. Pancreas. 43:768–776. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma YC, Shi C, Zhang YN, Wang LG, Liu H,
Jia HT, Zhang YX, Sarkar FH and Wang ZS: The tyrosine kinase c-Src
directly mediates growth factor-induced Notch-1 and Furin
interaction and Notch-1 activation in pancreatic cancer cells. PLoS
One. 7:e334142012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagathihalli NS and Merchant NB:
Src-mediated regulation of E-cadherin and EMT in pancreatic cancer.
Front Biosci (Landmark Ed). 17:2059–2069. 2012. View Article : Google Scholar
|
|
12
|
Chaimowitz NS, Falanga YT, Ryan JJ and
Conrad DH: Fyn kinase is required for optimal humoral responses.
PLoS One. 8:e606402013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chapman NM, Yoder AN and Houtman JC:
Non-catalytic functions of Pyk2 and Fyn regulate late stage
adhesion in human T cells. PLoS One. 7:e530112012. View Article : Google Scholar
|
|
14
|
Du CP, Tan R and Hou XY: Fyn kinases play
a critical role in neuronal apoptosis induced by oxygen and glucose
deprivation or amyloid-β peptide treatment. CNS Neurosci Ther.
18:754–761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yadav V and Denning MF: Fyn is induced by
Ras/PI3K/Akt signaling and is required for enhanced
invasion/migration. Mol Carcinog. 50:346–352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen ZY, Cai L, Bie P, Wang SG, Jiang Y,
Dong JH and Li XW: Roles of Fyn in pancreatic cancer metastasis. J
Gastroenterol Hepatol. 25:293–301. 2010. View Article : Google Scholar
|
|
17
|
Kapp TG, Rechenmacher F, Sobahi TR and
Kessler H: Integrin modulators: a patent review. Expert Opin Ther
Pat. 23:1273–1295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Landowski TH, Gard J, Pond E, Pond GD,
Nagle RB, Geffre CP and Cress AE: Targeting integrin α6 stimulates
curative-type bone metastasis lesions in a xenograft model. Mol
Cancer Ther. 13:1558–1566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun C, Zargham R, Shao Q, Gui X, Marcus V,
Lazaris A, Salman A, Metrakos P, Qu X and Gao Z: Association of
CD98, integrin β1, integrin β3 and Fak with the progression and
liver metastases of colorectal cancer. Pathol Res Pract.
210:668–674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moro L, Perlino E, Marra E, Languino LR
and Greco M: Regulation of beta1C and beta1A integrin expression in
prostate carcinoma cells. J Biol Chem. 279:1692–1702. 2004.
View Article : Google Scholar
|
|
21
|
Gauck S, Schultheiss HP, Rauch U and
Eisenreich A: Modulation of the isoform expression of Cyr61 and
Integrin-αv in human microvascular endothelial cells. Cardiovasc
Syst. 1:82013. View Article : Google Scholar
|
|
22
|
de Melker AA and Sonnenberg A: Integrins:
Alternative splicing as a mechanism to regulate ligand binding and
integrin signaling events. BioEssays. 21:499–509. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fornaro M, Steger CA, Bennett AM, Wu JJ
and Languino LR: Differential role of β1C and
β1A integrin cytoplasmic variants in modulating focal
adhesion kinase, protein kinase B/AKT, and Ras/Mitogen-activated
protein kinase pathways. Mol Biol Cell. 11:2235–2249. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moro L, Greco M, Maiorano E, Selvaggi L,
Marra E and Perlino E: Transcriptional regulation of beta1 integrin
expression in the physio/pathological states of human endometrial
tissues. Int J Oncol. 26:457–465. 2005.PubMed/NCBI
|
|
25
|
Eisenreich A: Regulation of vascular
function on posttranscriptional level. Thrombosis. 2013:9487652013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barash Y and Garcia JV: Predicting
alternative splicing. Methods Mol Biol. 1126:411–423. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kotlajich MV, Crabb TL and Hertel KJ:
Spliceosome assembly pathways for different types of alternative
splicing converge during commitment to splice site pairing in the A
complex. Mol Cell Biol. 29:1072–1082. 2009. View Article : Google Scholar :
|
|
28
|
Schor IE, Gómez Acuña LI and Kornblihtt
AR: Coupling between transcription and alternative splicing. Cancer
Treat Res. 158:1–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Verbeeren J, Niemelä EH, Turunen JJ, Will
CL, Ravantti JJ, Lührmann R and Frilander MJ: An ancient mechanism
for splicing control: U11 snRNP as an activator of alternative
splicing. Mol Cell. 37:821–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang T, Huang XH, Dong L, Hu D, Ge C,
Zhan YQ, Xu WX, Yu M, Li W, Wang X, et al: PCBP-1 regulates
alternative splicing of the CD44 gene and inhibits invasion in
human hepatoma cell line HepG2 cells. Mol Cancer. 9:722010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barboro P, Ferrari N and Balbi C: Emerging
roles of heterogeneous nuclear ribonucleoprotein K (hnRNP K) in
cancer progression. Cancer Lett. 352:152–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dery KJ, Gaur S, Gencheva M, Yen Y,
Shively JE and Gaur RK: Mechanistic control of carcinoembryonic
antigen-related cell adhesion molecule-1 (CEACAM1) splice isoforms
by the heterogeneous nuclear ribonuclear proteins hnRNP L, hnRNP
A1, and hnRNP M. J Biol Chem. 286:16039–16051. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garayoa M, Man YG, Martínez A, Cuttitta F
and Mulshine JL: Downregulation of hnRNP A2/B1 expression in tumor
cells under prolonged hypoxia. Am J Respir Cell Mol Biol. 28:80–85.
2003. View Article : Google Scholar
|
|
34
|
Chaudhury A, Hussey GS, Ray PS, Jin G, Fox
PL and Howe PH: TGF-beta-mediated phosphorylation of hnRNP E1
induces EMT via transcript-selective translational induction of
Dab2 and ILEI. Nat Cell Biol. 12:286–293. 2010.PubMed/NCBI
|
|
35
|
Zhang HY and Dou KF: PCBP1 is an important
mediator of TGF-β-induced epithelial to mesenchymal transition in
gall bladder cancer cell line GBC-SD. Mol Biol Rep. 41:5519–5524.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen ZY, Cai L, Zhu J, Chen M, Chen J, Li
ZH, Liu XD, Wang SG, Bie P, Jiang P, et al: Fyn requires HnRNPA2B1
and Sam68 to synergistically regulate apoptosis in pancreatic
cancer. Carcinogenesis. 32:1419–1426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Meng Q, Rayala SK, Gururaj AE, Talukder
AH, O'Malley BW and Kumar R: Signaling-dependent and coordinated
regulation of transcription, splicing, and translation resides in a
single coregulator, PCBP1. Proc Natl Acad Sci USA. 104:5866–5871.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lian WX, Yin RH, Kong XZ, Zhang T, Huang
XH, Zheng WW, Yang Y, Zhan YQ, Xu WX, Yu M, et al: THAP11, a novel
binding protein of PCBP1, negatively regulates CD44 alternative
splicing and cell invasion in a human hepatoma cell line. FEBS
Lett. 586:1431–1438. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hodson MJ, Hudson AJ, Cherny D and Eperon
IC: The transition in spliceosome assembly from complex E to
complex A purges surplus U1 snRNPs from alternative splice sites.
Nucleic Acids Res. 40:6850–6862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ritchie DB, Schellenberg MJ and MacMillan
AM: Spliceosome structure: Piece by piece. Biochim Biophys Acta.
1789:624–633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shcherbakova I, Hoskins AA, Friedman LJ,
Serebrov V, Corrêa IR Jr, Xu MQ, Gelles J and Moore MJ: Alternative
spliceosome assembly pathways revealed by single-molecule
fluorescence microscopy. Cell Rep. 5:151–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van der Feltz C, Anthony K, Brilot A and
Pomeranz Krummel DA: Architecture of the spliceosome. Biochemistry.
51:3321–3333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Will CL and Lührmann R: Spliceosome
structure and function. Cold Spring Harb Perspect Biol. 3:32011.
View Article : Google Scholar
|
|
44
|
Svineng G, Fässler R and Johansson S:
Identification of beta1C-2 a novel variant of the integrin beta1
subunit generated by utilization of an alternative splice acceptor
site in exon C. Biochem J. 330:1255–1263. 1998. View Article : Google Scholar
|
|
45
|
Busch A and Hertel KJ: Evolution of SR
protein and hnRNP splicing regulatory factors. Wiley Interdiscip
Rev RNA. 3:1–12. 2012. View Article : Google Scholar
|
|
46
|
Manzotti M, Dell'Orto P, Maisonneuve P,
Fornaro M, Languino LR and Viale G: Down-regulation of
β1C integrin in breast carcinomas correlates with high
proliferative fraction, high histological grade, and larger size.
Am J Pathol. 156:169–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Meredith JE Jr, Kiosses WB, Takada Y and
Schwartz MA: Mutational analysis of cell cycle inhibition by
integrin beta1C. J Biol Chem. 274:8111–8116. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Moro L, Greco M, Ditonno P, Battaglia M,
Marra E and Perlino E: Transcriptional regulation of the beta1C
integrin splice variant in human prostate adenocarcinoma. Int J
Oncol. 23:1601–1606. 2003.PubMed/NCBI
|
|
49
|
Perlino E, Lovecchio M, Vacca RA, Fornaro
M, Moro L, Ditonno P, Battaglia M, Selvaggi FP, Mastropasqua MG,
Bufo P, et al: Regulation of mRNA and protein levels of beta1
integrin variants in human prostate carcinoma. Am J Pathol.
157:1727–1734. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Saito YD, Jensen AR, Salgia R and Posadas
EM: Fyn: A novel molecular target in cancer. Cancer. 116:1629–1637.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Paronetto MP, Achsel T, Massiello A,
Chalfant CE and Sette C: The RNA-binding protein Sam68 modulates
the alternative splicing of Bcl-x. J Cell Biol. 176:929–939. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mattioli C, Pianigiani G and Pagani F:
Cross talk between spliceosome and microprocessor defines the fate
of pre-mRNA. Wiley Interdiscip Rev RNA. 5:647–658. 2014.PubMed/NCBI
|
|
53
|
Wahl MC, Will CL and Lührmann R: The
spliceosome: Design principles of a dynamic RNP machine. Cell.
136:701–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Han SP, Tang YH and Smith R: Functional
diversity of the hnRNPs: Past, present and perspectives. Biochem J.
430:379–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Eisenreich A, Boltzen U, Poller W,
Schultheiss HP and Rauch U: Effects of the Cdc2-like kinase-family
and DNA topoisomerase I on the alternative splicing of eNOS in
TNF-alpha-stimulated human endothelial cells. Biol Chem.
389:1333–1338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Eisenreich A, Bogdanov VY, Zakrzewicz A,
Pries A, Antoniak S, Poller W, Schultheiss HP and Rauch U:
Cdc2-like kinases and DNA topoisomerase I regulate alternative
splicing of tissue factor in human endothelial cells. Circ Res.
104:589–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Eisenreich A, Zakrzewicz A, Huber K,
Thierbach H, Pepke W, Goldin-Lang P, Schultheiss HP, Pries A and
Rauch U: Regulation of pro-angiogenic tissue factor expression in
hypoxia-induced human lung cancer cells. Oncol Rep. 30:462–470.
2013.PubMed/NCBI
|
|
58
|
Howard JM and Sanford JR: The RNAissance
family: SR proteins as multifaceted regulators of gene expression.
Wiley Interdiscip Rev RNA. 6:93–110. 2015. View Article : Google Scholar
|
|
59
|
Bonomi S, di Matteo A, Buratti E, Cabianca
DS, Baralle FE, Ghigna C and Biamonti G: HnRNP A1 controls a
splicing regulatory circuit promoting mesenchymal-to-epithelial
transition. Nucleic Acids Res. 41:8665–8679. 2013. View Article : Google Scholar : PubMed/NCBI
|