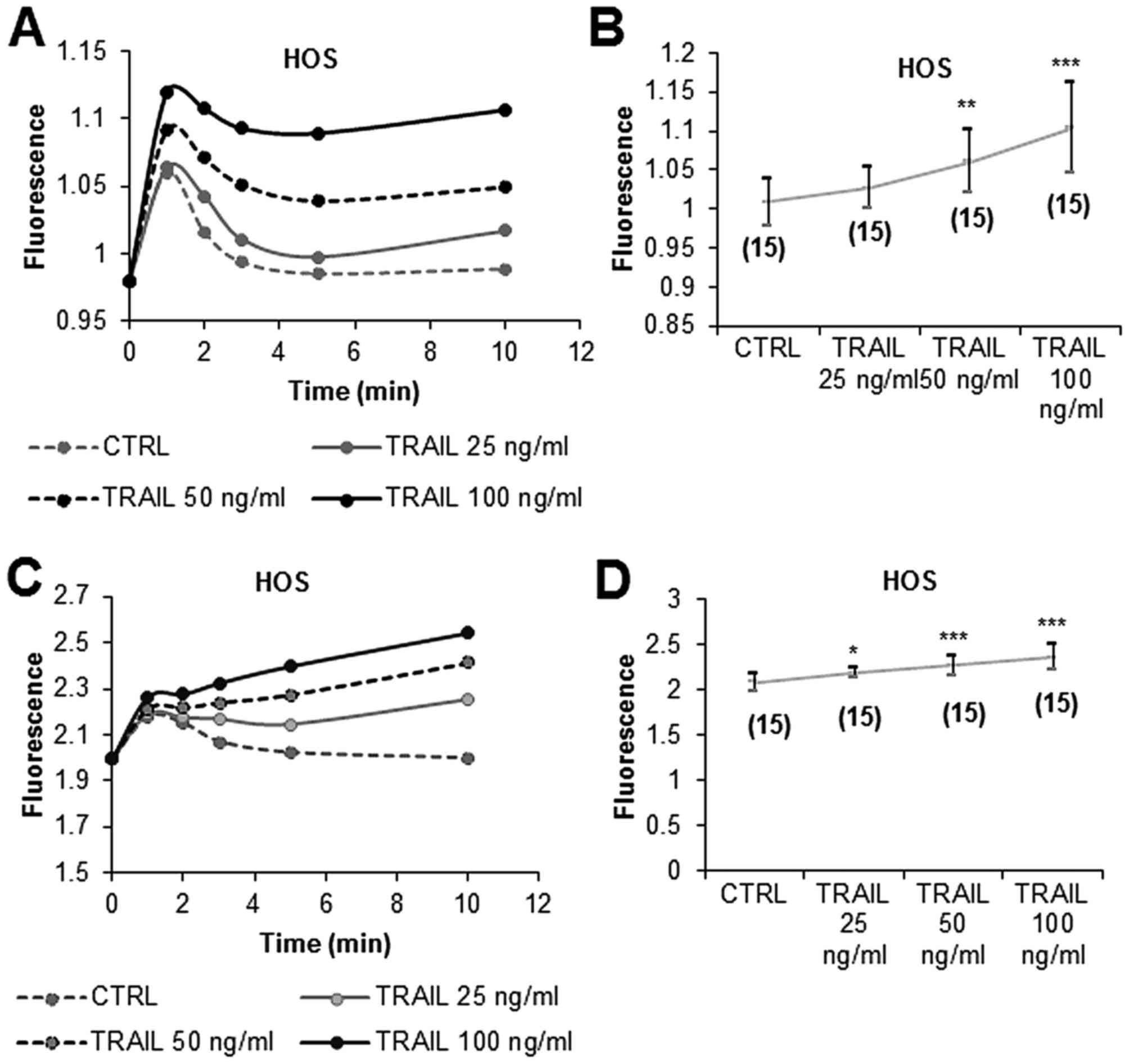
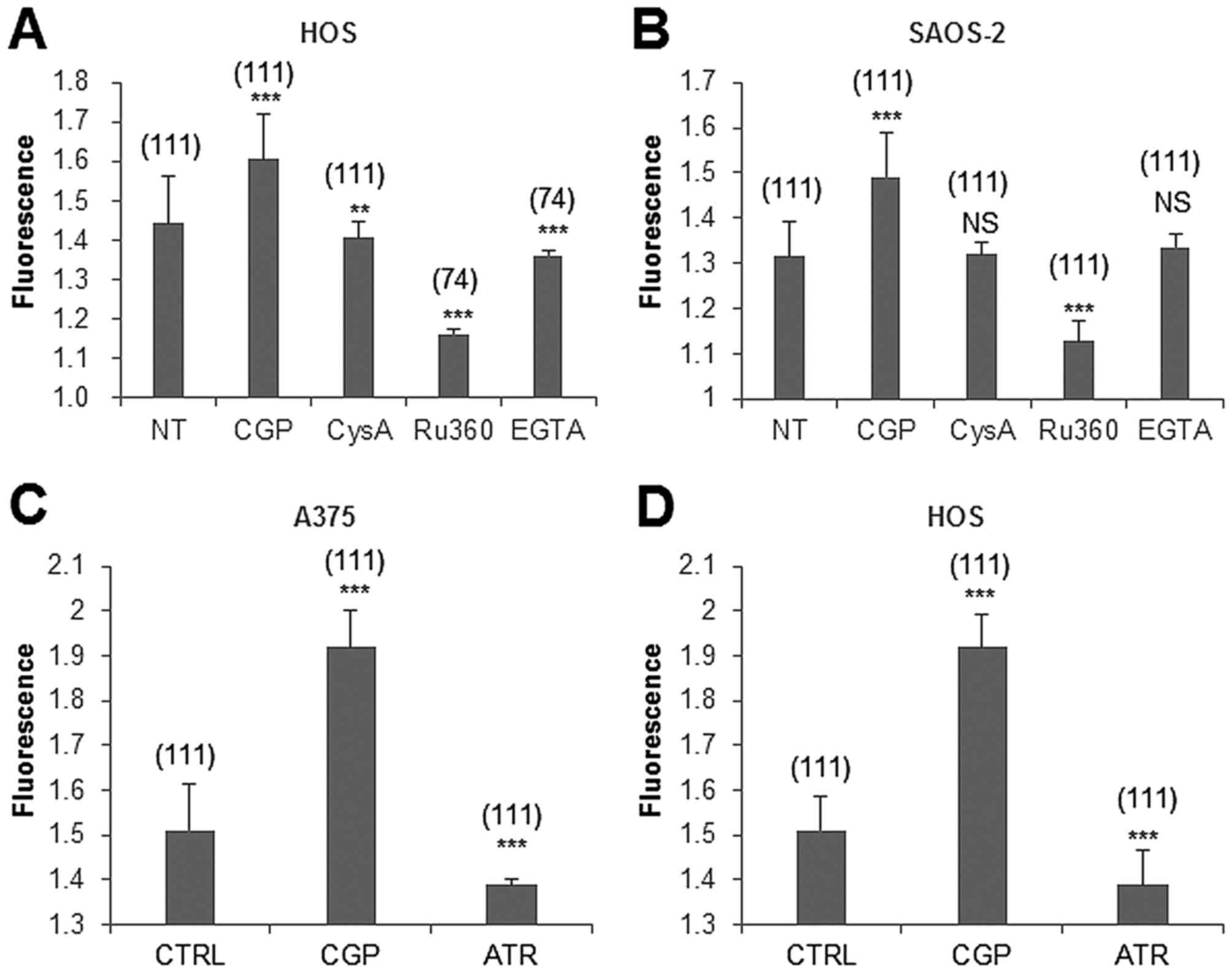
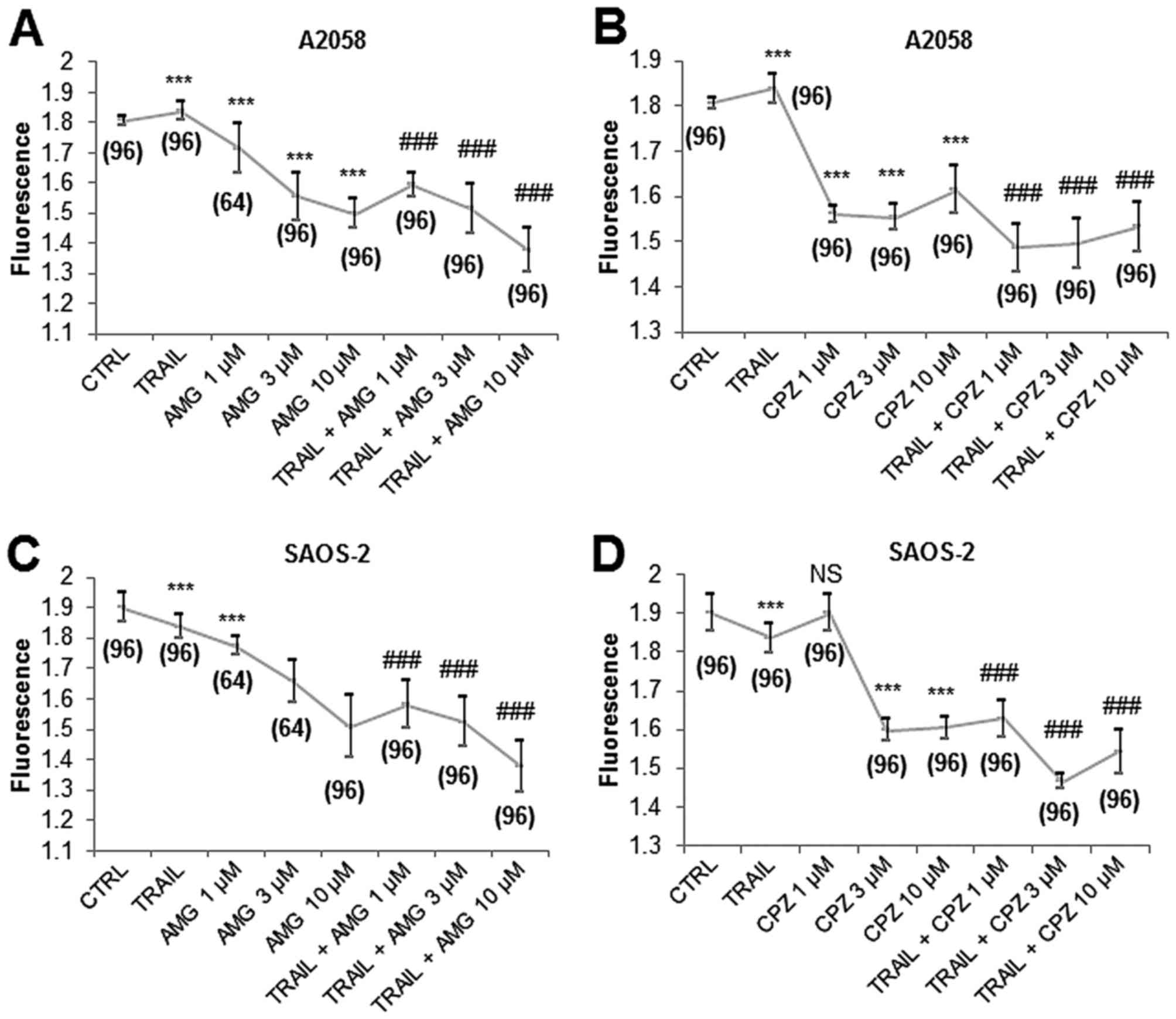
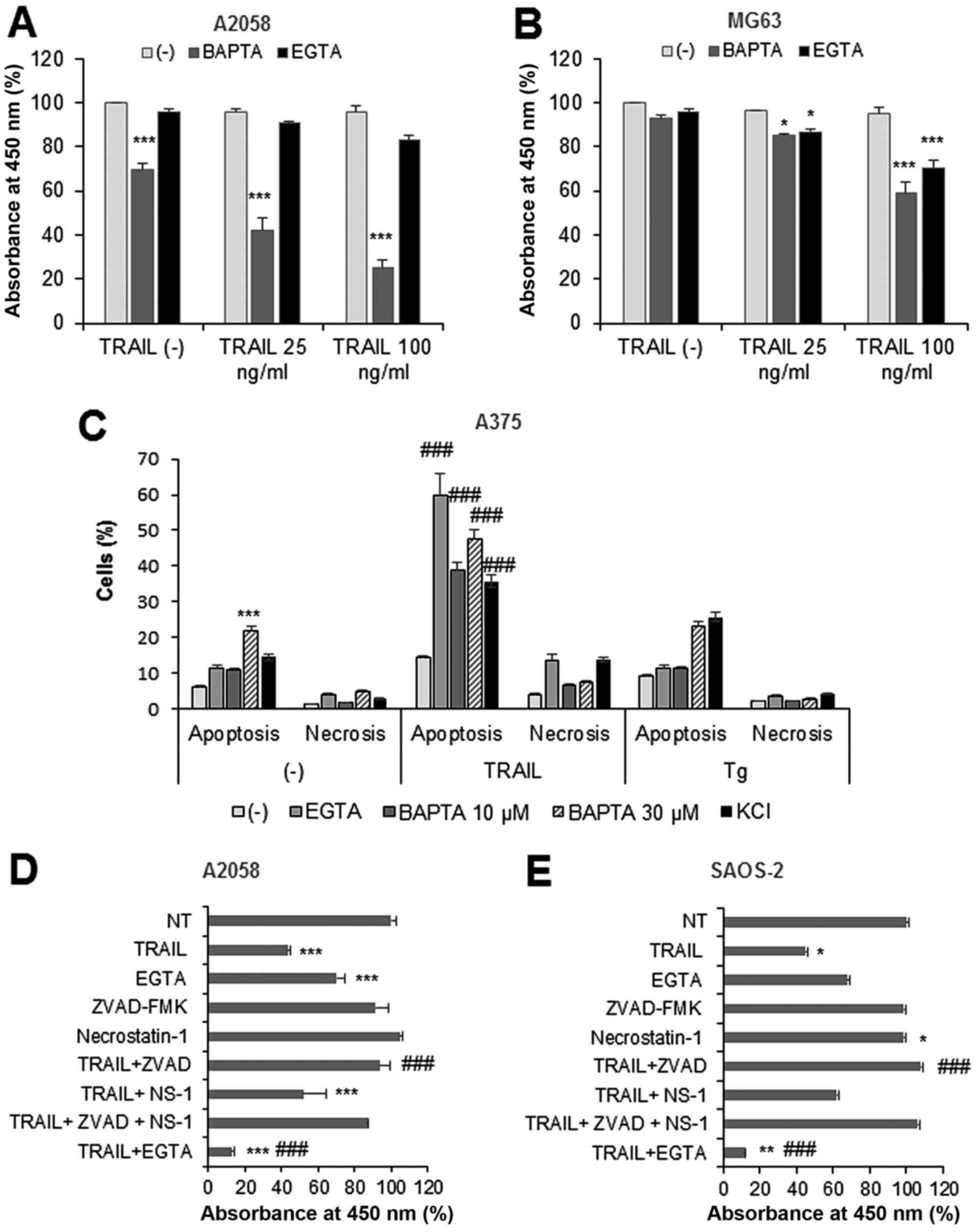
|
1
|
Ivanov VN, Bhoumik A and Ronai Z: Death
receptors and melanoma resistance to apoptosis. Oncogene.
22:3152–3161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guiho R, Biteau K, Heymann D and Redini F:
TRAIL-based therapy in pediatric bone tumors: How to overcome
resistance. Future Oncol. 11:535–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ashkenazi A: Targeting the extrinsic
apoptosis pathway in cancer. Cytokine Growth Factor Rev.
19:325–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amarante-Mendes GP and Griffith TS:
Therapeutic applications of TRAIL receptor agonists in cancer and
beyond. Pharmacol Ther. 155:117–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Miguel D, Lemke J, Anel A, Walczak H
and Martinez-Lostao L: Onto better TRAILs for cancer treatment.
Cell Death Differ. 23:733–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang S: The promise of cancer therapeutics
targeting the TNF-related apoptosis-inducing ligand and TRAIL
receptor pathway. Oncogene. 27:6207–6215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sayers TJ: Targeting the extrinsic
apoptosis signaling pathway for cancer therapy. Cancer Immunol
Immunother. 60:1173–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herrero-Martín G, Høyer-Hansen M,
García-García C, Fumarola C, Farkas T, López-Rivas A and Jäättelä
M: TAK1 activates AMPK-dependent cytoprotective autophagy in
TRAIL-treated epithelial cells. EMBO J. 28:677–685. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He W, Wang Q, Xu J, Xu X, Padilla MT, Ren
G, Gou X and Lin Y: Attenuation of TNFSF10/TRAIL-induced apoptosis
by an autophagic survival pathway involving TRAF2- and
RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy. 8:1811–1821.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jouan-Lanhouet S, Arshad MI,
Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van
Herreweghe F, Takahashi N, Sergent O, Lagadic-Gossmann D,
Vandenabeele P, et al: TRAIL induces necroptosis involving
RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ.
19:2003–2014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sosna J, Philipp S, Fuchslocher Chico J,
Saggau C, Fritsch J, Föll A, Plenge J, Arenz C, Pinkert T, Kalthoff
H, et al: Differences and similarities in TRAIL- and tumor necrosis
factor-mediated necroptotic signaling in cancer cells. Mol Cell
Biol. 36:2626–2644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dimberg LY, Anderson CK, Camidge R,
Behbakht K, Thorburn A and Ford HL: On the TRAIL to successful
cancer therapy? Predicting and counteracting resistance against
TRAIL-based therapeutics. Oncogene. 32:1341–1350. 2013. View Article : Google Scholar
|
|
13
|
Hempel N and Trebak M: Crosstalk between
calcium and reactive oxygen species signaling in cancer. Cell
Calcium. Jan 18–2017.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Villalobos C, Sobradillo D,
Hernández-Morales M and Núñez L: Calcium remodeling in colorectal
cancer. Biochim Biophys Acta. 1864:843–849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Danese A, Patergnani S, Bonora M,
Wieckowski MR, Previati M, Giorgi C and Pinton P: Calcium regulates
cell death in cancer: Roles of the mitochondria and
mitochondria-associated membranes (MAMs). Biochim Biophys Acta. Jan
10–2017.Epub ahead of print. View Article : Google Scholar
|
|
16
|
Nilius B and Szallasi A: Transient
receptor potential channels as drug targets: From the science of
basic research to the art of medicine. Pharmacol Rev. 66:676–814.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orrenius S, Gogvadze V and Zhivotovsky B:
Calcium and mitochondria in the regulation of cell death. Biochem
Biophys Res Commun. 460:72–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Storr SJ, Carragher NO, Frame MC, Parr T
and Martin SG: The calpain system and cancer. Nat Rev Cancer.
11:364–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moretti D, Del Bello B, Allavena G and
Maellaro E: Calpains and cancer: Friends or enemies? Arch Biochem
Biophys. 564:26–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akita M, Suzuki-Karasaki M, Fujiwara K,
Nakagawa C, Soma M, Yoshida Y, Ochiai T, Tokuhashi Y and
Suzuki-Karasaki Y: Mitochondrial division inhibitor-1 induces
mitochondrial hyperfusion and sensitizes human cancer cells to
TRAIL-induced apoptosis. Int J Oncol. 45:1901–1912. 2014.PubMed/NCBI
|
|
21
|
Suzuki Y, Inoue T, Murai M,
Suzuki-Karasaki M, Ochiai T and Ra C: Depolarization potentiates
TRAIL-induced apoptosis in human melanoma cells: Role for
ATP-sensitive K+ channels and endoplasmic reticulum
stress. Int J Oncol. 41:465–475. 2012.PubMed/NCBI
|
|
22
|
Suzuki Y, Yoshimaru T, Inoue T and Ra C:
Mitochondrial Ca2+ flux is a critical determinant of the
Ca2+ dependence of mast cell degranulation. J Leukoc
Biol. 79:508–518. 2006. View Article : Google Scholar
|
|
23
|
Marchi S and Pinton P: The mitochondrial
calcium uniporter complex: Molecular components, structure and
physiopathological implications. J Physiol. 592:829–839. 2014.
View Article : Google Scholar :
|
|
24
|
Tosatto A, Sommaggio R, Kummerow C,
Bentham RB, Blacker TS, Berecz T, Duchen MR, Rosato A, Bogeski I,
Szabadkai G, et al: The mitochondrial calcium uniporter regulates
breast cancer progression via HIF-1α. EMBO Mol Med. 8:569–585.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang X, Wang B, Zeng H, Cai C, Hu Q, Cai
S, Xu L, Meng X and Zou F: Role of the mitochondrial
Ca2+ uniporter in
Pb2+-induced oxidative stress in human
neuroblastoma cells. Brain Res. 1575:12–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tochigi M, Inoue T, Suzuki-Karasaki M,
Ochiai T, Ra C and Suzuki-Karasaki Y: Hydrogen peroxide induces
cell death in human TRAIL-resistant melanoma through intracellular
superoxide generation. Int J Oncol. 42:863–872. 2013.PubMed/NCBI
|
|
27
|
Curry MC, Peters AA, Kenny PA,
Roberts-Thomson SJ and Monteith GR: Mitochondrial calcium uniporter
silencing potentiates caspase-independent cell death in MDA-MB-231
breast cancer cells. Biochem Biophys Res Commun. 434:695–700. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Stefani D, Patron M and Rizzuto R:
Structure and function of the mitochondrial calcium uniporter
complex. Biochim Biophys Acta. 1853:2006–2011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brand MD: Electroneutral efflux of
Ca2+ from liver mitochondria. Biochem J. 225:413–419.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Altschuld RA, Hohl CM, Castillo LC, Garleb
AA, Starling RC and Brierley GP: Cyclosporin inhibits mitochondrial
calcium efflux in isolated adult rat ventricular cardiomyocytes. Am
J Physiol. 262:H1699–H1704. 1992.PubMed/NCBI
|
|
31
|
Bernardi P and von Stockum S: The
permeability transition pore as a Ca2+ release channel:
New answers to an old question. Cell Calcium. 52:22–27. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Lisa F, Carpi A, Giorgio V and Bernardi
P: The mitochondrial permeability transition pore and cyclophilin D
in cardioprotection. Biochim Biophys Acta. 1813:1316–1322. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gutiérrez-Aguilar M and Baines CP:
Structural mechanisms of cyclophilin D-dependent control of the
mitochondrial permeability transition pore. Biochim Biophys Acta.
1850:2041–2047. 2015. View Article : Google Scholar :
|
|
34
|
Klingenberg M: The ADP and ATP transport
in mitochondria and its carrier. Biochim Biophys Acta.
1778:1978–2021. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zamorano S, Rojas-Rivera D, Lisbona F,
Parra V, Court FA, Villegas R, Cheng EH, Korsmeyer SJ, Lavandero S
and Hetz C: A BAX/BAK and cyclophilin D-independent intrinsic
apoptosis pathway. PLoS One. 7:e377822012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Briston T, Lewis S, Koglin M, Mistry K,
Shen Y, Hartopp N, Katsumata R, Fukumoto H, Duchen MR, Szabadkai G,
et al: Identification of ER-000444793, a Cyclophilin D-independent
inhibitor of mitochondrial permeability transition, using a
high-throughput screen in cryopreserved mitochondria. Sci Rep.
6:377982016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bevan S, Hothi S, Hughes G, James IF, Rang
HP, Shah K, Walpole CS and Yeats JC: Capsazepine: A competitive
antagonist of the sensory neurone excitant capsaicin. Br J
Pharmacol. 107:544–552. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang
TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, et al: AMG 9810
[(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4]
dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1)
antagonist with antihyperalgesic properties. J Pharmacol Exp Ther.
313:474–484. 2005. View Article : Google Scholar
|
|
39
|
Zhao R and Tsang SY: Versatile roles of
intracellularly located TRPV1 channel. J Cell Physiol.
232:1957–1965. 2017. View Article : Google Scholar
|
|
40
|
Thomas KC, Roberts JK, Deering-Rice CE,
Romero EG, Dull RO, Lee J, Yost GS and Reilly CA: Contributions of
TRPV1, endovanilloids, and endoplasmic reticulum stress in lung
cell death in vitro and lung injury. Am J Physiol Lung Cell Mol
Physiol. 302:L111–L119. 2012. View Article : Google Scholar :
|
|
41
|
Stock K, Kumar J, Synowitz M, Petrosino S,
Imperatore R, Smith ES, Wend P, Purfürst B, Nuber UA, Gurok U, et
al: Neural precursor cells induce cell death of high-grade
astrocytomas through stimulation of TRPV1. Nat Med. 18:1232–1238.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mellier G and Pervaiz S: The three Rs
along the TRAIL: Resistance, re-sensitization and reactive oxygen
species (ROS). Free Radic Res. 46:996–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Suzuki-Karasaki M, Ochiai T and
Suzuki-Karasaki Y: Crosstalk between mitochondrial ROS and
depolarization in the potentiation of TRAIL-induced apoptosis in
human tumor cells. Int J Oncol. 44:616–628. 2014.
|
|
44
|
Voltan R, Secchiero P, Casciano F, Milani
D, Zauli G and Tisato V: Redox signaling and oxidative stress:
Cross talk with TNF-related apoptosis inducing ligand activity. Int
J Biochem Cell Biol. 81:364–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kozai D, Ogawa N and Mori Y: Redox
regulation of transient receptor potential channels. Antioxid Redox
Signal. 21:971–986. 2014. View Article : Google Scholar
|
|
46
|
Mergler S, Derckx R, Reinach PS, Garreis
F, Böhm A, Schmelzer L, Skosyrski S, Ramesh N, Abdelmessih S, Polat
OK, et al: Calcium regulation by temperature-sensitive transient
receptor potential channels in human uveal melanoma cells. Cell
Signal. 26:56–69. 2014. View Article : Google Scholar
|
|
47
|
Chien CS, Ma KH, Lee HS, Liu PS, Li YH,
Huang YS and Chueh SH: Dual effect of capsaicin on cell death in
human osteosarcoma G292 cells. Eur J Pharmacol. 718:350–360. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Marchi S, Lupini L, Patergnani S, Rimessi
A, Missiroli S, Bonora M, Bononi A, Corrà F, Giorgi C, De Marchi E,
et al: Downregulation of the mitochondrial calcium uniporter by
cancer-related miR-25. Curr Biol. 23:58–63. 2013. View Article : Google Scholar :
|