Introduction
Malignant pleural mesothelioma is an aggressive type
of tumor which is invariably fatal in outcome and is associated
with past asbestos exposure. While mesothelioma is a rare tumor it
continues to have an increased incidence due to the previous
widespread use of asbestos products in industrial nations and
continuing use in some developing countries (1). Understanding the alterations in
molecular signaling that occur in mesotheliomas can help not just
to develop suitable biomarkers but also lead to new targeted
therapies. Many pathways have been identified as potential targets
in mesothelioma by studying molecular changes in patient tumor
samples (2,3). One pathway which has been identified
as a potentially significant pathway in mesothelioma is the Wnt
signaling pathway (4–6).
Aberrant activation of Wnt signaling to drive
proliferation, survival and invasion has been frequently described
in cancer (reviewed in ref. 7).
One mechanism which has been described is the downregulation of
members of a family of regulatory proteins: the secreted frizzled
related proteins (SFRP) (8).
Studies of SFRP family expression and function in mesothelioma
cells and tumors by other laboratories (9–11)
and our own (6) have shown that
one member of this family, SFRP4, is differentially downregulated
in this cancer. There is evidence that downregulation of SFRP4 by
promoter methylation could result in Wnt pathway activation in
mesothelioma (10). Previous
mechanistic studies showing induction of apoptosis and growth
inhibition in response to SFRP4 have used a mesothelioma cell model
which was β-catenin deficient and canonical signaling was not
present (10). Most mesothelioma
tumors and cell lines have been found to express β-catenin
(11–13). The SFRPs consist of two domains, a
Fz-like cysteine rich domain (CRD) and a netrin-like domain (NLD).
Because of its structural similarity to the extracellular domain of
the Fz receptors the SFRP was initially believed to play a role
analogous in binding to Wnt proteins (14), however, more recent studies have
also implicated the NLD (15,16).
Since SFRP4 is the most commonly downregulated SFRP in mesothelioma
(6,9–11) we
undertook to investigate the effect of SFRP4 and its domains in
more relevant β-catenin expressing mesothelioma models which are
capable of canonical Wnt signaling and which express low or
negligible native SFRP4.
In this study we investigate the effects of both
exogenous and endogenous overexpression of SFRP4 upon
proliferation, migration, cell behaviour and downstream signaling.
We report that re-introduction of SFRP4 to mesothelioma cells
inhibits cell proliferation, migration and induces a non-apoptotic
cell death programme. Since SFRP4 expression is widely
downregulated in mesothelioma (9,11)
these findings support the targeting of Wnt signaling in
mesothelioma at the level of upstream regulators as a potential
therapeutic approach. Furthermore, we found that the effects of
SFRP4 were mediated by the NLD providing further insight into the
biology of these important Wnt regulators.
Materials and methods
Cell culture and reagents
The malignant mesothelioma cell lines JU77 and ONE58
were used in this study. These cell lines were originally derived
from pleural effusions of different patients presenting with
malignant pleural mesothelioma (17). All cells were cultured and
maintained in medium R5, which is RPMI-1640 plus 5%
heat-inactivated fetal bovine serum (FBS), 300 mM L-glutamine, 120
µg/ml penicillin and 100 µg/ml streptomycin (all from
Thermo Fisher, Hyclone, VIC, Australia). All cell cultures were
grown at 37°C in a 5% CO2 humidified atmosphere.
Recombinant human proteins Wnt3a and SFRP4 were obtained from
R&D Systems (MN, USA). Cisplatin (Oncotain) was obtained from
Mayne Pharma (VIC, Australia).
MTT assay
Cell viability in response to various treatments was
quantitated by an MTT assay. Cells were seeded into 96-well plates
at a density of 10,000–20,000 cells/well, depending on the
experiment. Following 24-h incubation treatments were added and
cells incubated for a further 24–72 h (depending on experiments)
then the MTT assay was performed as previously described (18) and absorbance was read at 595 nm
with a microplate reader (Enspire, Perkin-Elmer). For each
treatment, the mean absorbance for the replicates was calculated.
The control samples were untreated cells or cells treated with
vehicle alone and control data was set to 100% viable cells and all
other data were normalised to this value based on absorbance value.
The data were expressed as mean ± standard deviation.
Proliferation
Cells were seeded at a density of 5×104
cells/well in 24-well plates and treatments added at 24 h. The
cells were harvested 48 h later by trypsinization and evaluated by
a trypan blue assay using a Countess Automated Cell Counter (Life
Technologies).
Cell migration assay
A scratch wound assay was used to assess cell
migration. Cells were seeded in 6-well plates at a density of
3×105 cells/well and grown to confluency. The monolayers
were scratched using a 10-µl pipette tip to produce 2
crosses per well and washed with PBS to remove any non-adherent
cells and debris. The field of view was kept constant by drawing a
line on the well bottom perpendicular to the centre of T scratch
site and using this as a reference point. The monolayers were
imaged centred on the crosses using a Canon EOS digital camera
mounted on an Olympus CK2 microscope after 0 and 6 h. The open area
of the wound was quantitated by automatic image analysis using
TScratch software (19) with the
default parameter settings.
Assessment of mitochondrial membrane
potential
Mitochondrial outer membrane potential changes were
analysed using the cationic dye JC-1 (5, 5′, 6, 6′-tetrachloro-1,
1′, 3, 3′-tetraethyl-benzimidazolcarbocyanine iodide) essentially
as previously described (20).
Data are presented as the ratio of red to green fluorescence
intensity and mitochondrial depolarization is indicated by a
decrease in this parameter.
Caspase-3 activity assay
Activation of effector caspases was determined using
the Caspase-3 Assay Kit#2 (Molecular Probes, USA) with a modified
protocol as previously described (20).
Immunoblot analysis
Cell monolayers were washed once with PBS and lysed
by addition of 1× SDS loading buffer (Bio-Rad) containing 5%
mercaptoethanol (Sigma-Aldrich). The lysate was disrupted by
pipetting and transferred to a microfuge tube on ice. Samples were
sonicated (QSonica, Q125, CT, USA) at amplitude 40, 3× 15 sec at
30-sec intervals then centrifuged at 10,000 × g for 5 min and the
supernatants recovered and stored at −20°C. Proteins were resolved
on 4–12% Mini Protean TGX gels (Bio-Rad) and transferred to
nitrocellulose membrane (Bio-Rad) then probed with the following
antibodies: monoclonal rabbit anti-human β-catenin [Cell Signaling
Technology (CST), MA, USA], polyclonal rabbit anti-Dvl-3 (CST) and
monoclonal mouse anti-β-actin (Sigma-Aldrich). After incubation
with horseradish peroxidase conjugated secondary antibody (Jackson
Immunosearch, PA, USA) chemiluminescent detection was performed
with Clarity ECL substrate (Bio-Rad) and images captured using a
GelDoc XR imaging system (Bio-Rad).
Live cell imaging for nuclear
morphology
At the completion of the experiment the media was
aspirated and cells stained with 1 µg/ml Hoechst 33342
solution (Thermo Scientific) for 5 min. The cells were washed with
PBS and fresh media added before imaging with an Olympus IX-51
inverted fluorescent microscope.
Plasmid constructs and transfection
Expression vectors for the human SFRP4 gene and the
CRD and NLD domains were a kind gift from Professor Robert Friis,
University of Bern, Switzerland. The SFRP4 constructs were prepared
by PCR and cloning into the pEGFP-N1 vector (Clontech, CA, USA) so
that the full length SFRP4, the CRD domain or the NLD C terminal
domain were expressed in frame as amino terminal fusions to the
GFP. The SFRP4, CRD and NLD constructs retained the Kozak and
signal sequences of SFRP4. The parental pEGFP-N1 vector expressing
GFP was used as a control in all transfection experiments although
mock transfected and untreated cultures were routinely included as
assay controls. Plasmid DNA for transfection was prepared using a
HiSpeed Plasmid Midi kit (Qiagen, VIC, Australia). Transient
transfections were performed using FuGENE® HD reagent
and the pEGFP-N1 plasmid vector constructs. Following transfection
with reagent:DNA in a ratio of 3:1 for 24 h, the transfection
reagent was removed from the cells and replaced with standard
complete RPMI medium. Transfection efficiency was assessed by
fluorescence microscopy.
Statistical analysis
Statistical comparison between two groups was
performed using unpaired t-test with Prism v6.07 (GraphPad
software). The difference was determined to be statistically
significant at p<0.05. Determination and statistical comparison
of IC50 was performed by non-linear regression analysis
and F-test using Prism v6.07.
Results
Exogenous SFRP4 downregulates
proliferation and enhances chemosensitivity in mesothelioma
cells
We have previously reported that SFRP4 conditioned
media downregulated mesothelioma cell proliferation (6) and here we conducted experiments to
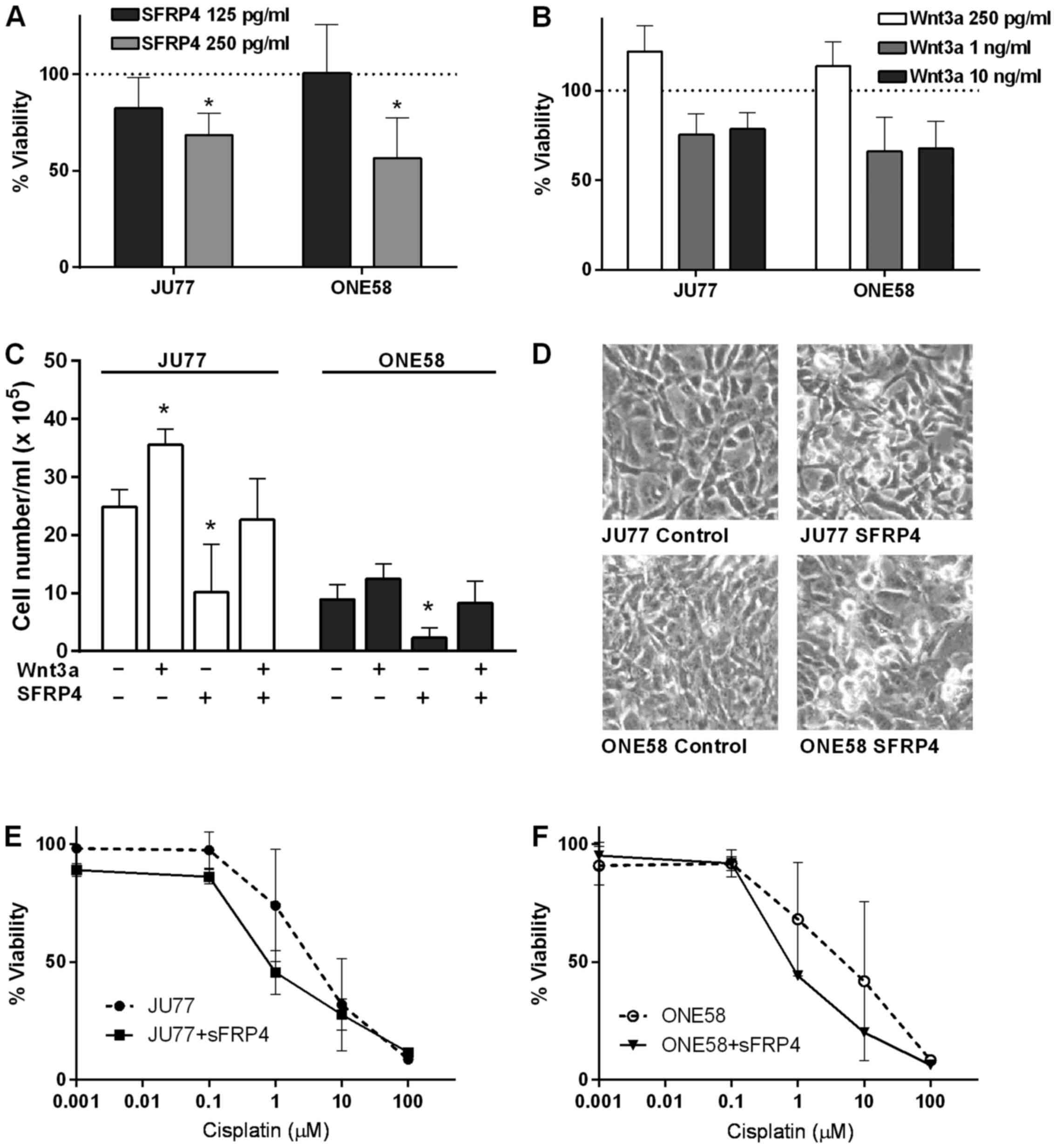
examine the effects of purified recombinant SFRP4. We found that
recombinant SFRP4 caused dose-dependent inhibition of cell
viability/proliferation in both mesothelioma cell lines examined as
determined by MTT assay (Fig. 1A).
The effect of Wnt3a was also determined in a similar experiment and
interestingly it was found that while 250 pg/ml recombinant Wnt3a
did appear to upregulate proliferation, at higher concentrations
there was an inhibitory effect (Fig.
1B) although these effects were not statistically significant.
To further confirm these results we next examined whether SFRP4
could influence the effects of recombinant Wnt3a upon mesothelioma
cell proliferation using a trypan blue assay. These experiments
showed that 250 pg/ml SFRP4 significantly inhibited proliferation
in both JU77 and ONE58 and this effect was blocked by 250 pg/ml
Wnt3a (Fig. 1C). Microscopic
examination of cultures treated with SFRP4 demonstrated significant
morphological effects in both cell lines. Many cells demonstrated
an apparent cytopathic effect with a characteristic loss of
attachment and rounding up appearance after SFRP4 treatment
(Fig. 1D). We next examined
whether SFRP4 influenced the response of mesothelioma cells to
cytotoxic drug insults. Cells were treated with 0.001–100 µM
cisplatin for 48 h in the presence of 250 pg/ml recombinant SFRP4
and assayed for cell proliferation. In both JU77 and ONE58 cells
SFRP4 did appear to sensitize mesothelioma cells to cisplatin
(Fig. 1E and F) with a
statistically significant difference in the IC50 values
as determined by non-linear regression (p=0.0233 and 0.0046
respectively).
SFRP4 inhibits mesothelioma cell
migration
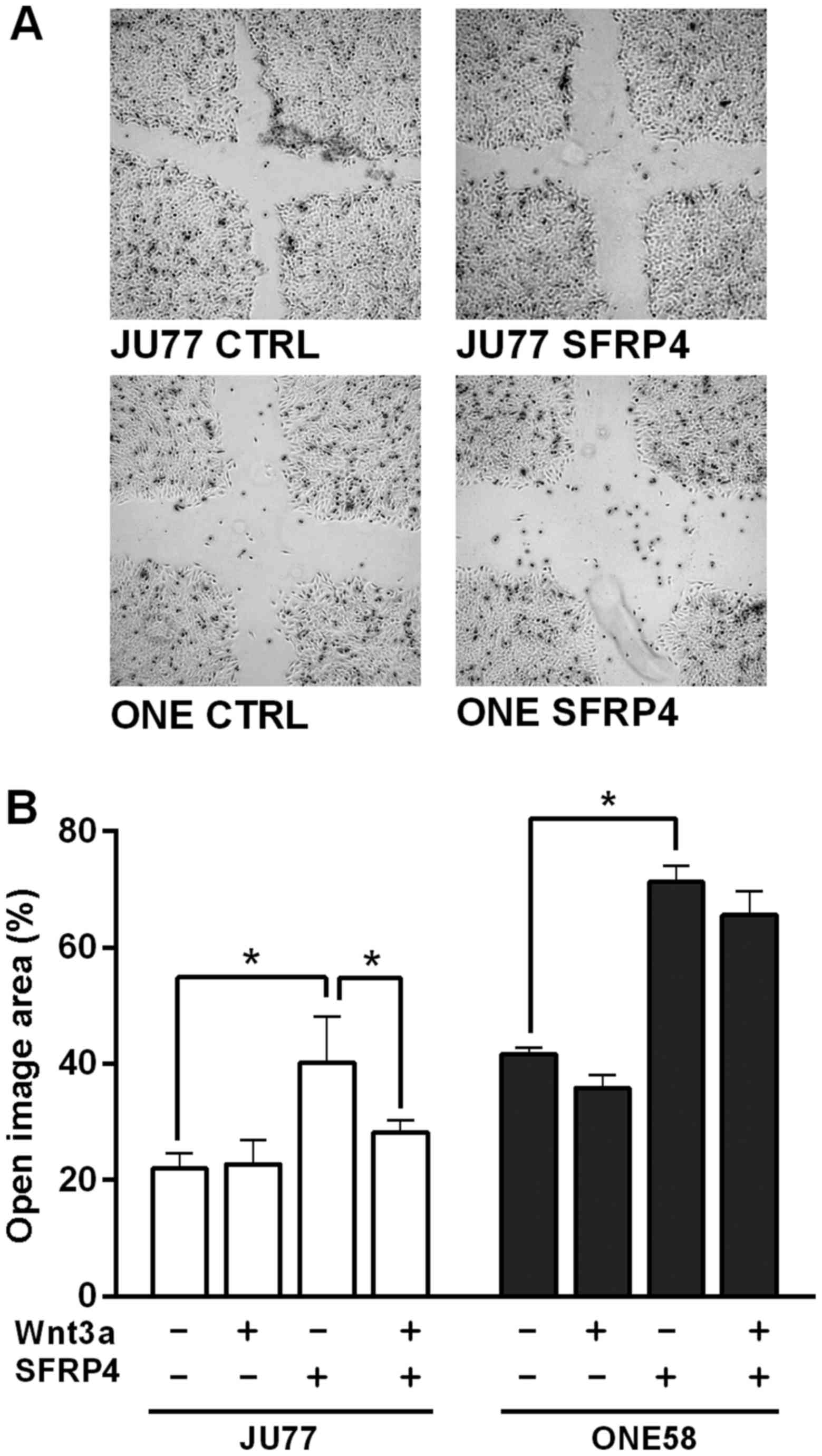
Previous studies have suggested that SFRPs can
affect migration in cancer cells (21). Therefore the effect of SFRP4 and
Wnt3a on the migration of malignant mesothelioma cells was
investigated using a scratch or wound healing assay. In both cell
lines SFRP4 appeared to inhibit wound closure (Fig. 2A) and this result was significant
as confirmed by open wound area image analysis (Fig. 2B). Interestingly, Wnt3a had little
effect upon wound closure in JU77 and ONE58 cells but significantly
ameliorated the effect of SFRP4 in JU77 cells.
Mechanisms of SFRP4 induces cell death in
mesothelioma cells
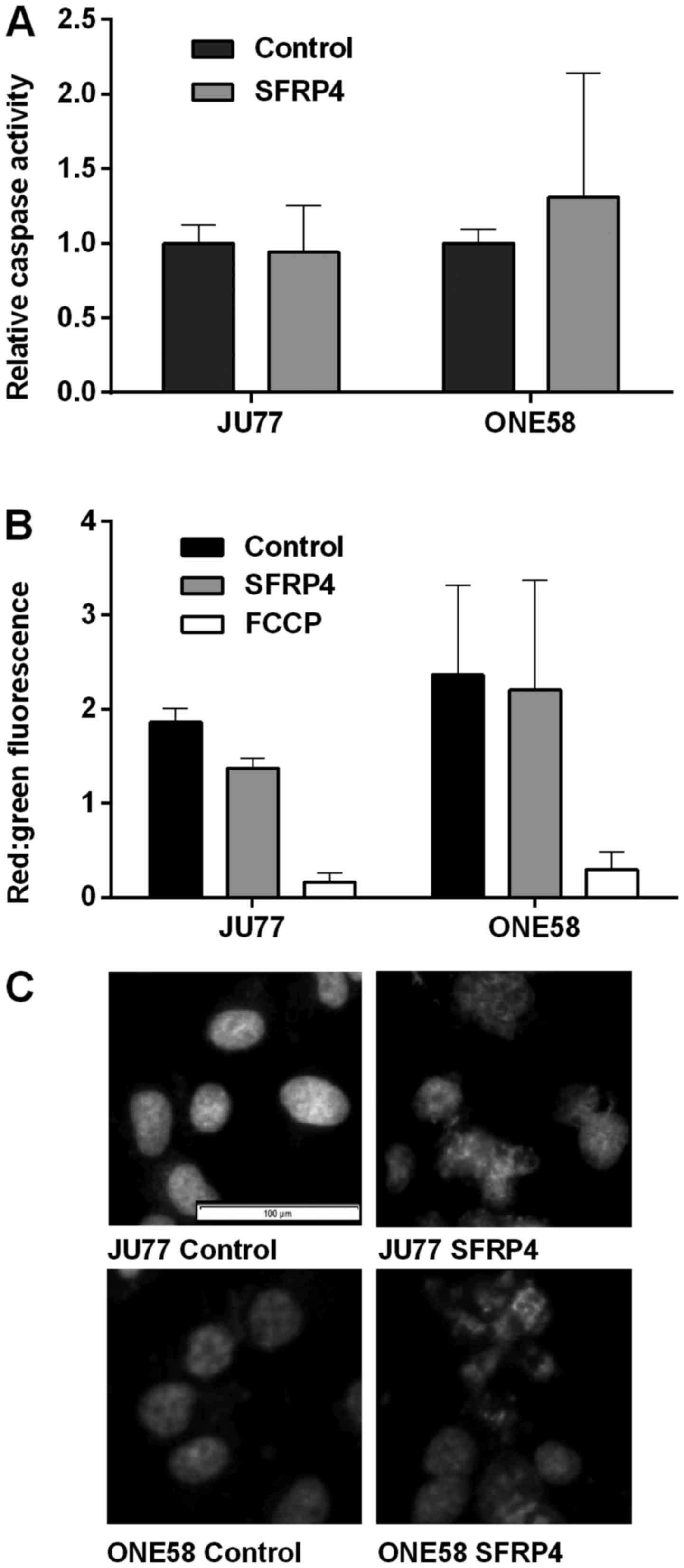
To examine in more detail the cytopathic effect of
SFRP4 observed in cell viability and morphological assays we
endeavored to characterize cell death mechanisms in response to
SFRP4. In JU77 and ONE58 cells SFRP4 did not have a significant
effect upon caspase-3 activation (Fig.
3A). An important event in apoptotic signaling is disruption of
the mitochondrial membrane and loss of the outer membrane potential
(OMP). Only JU77 cells showed a modest decline in OMP with SFRP4
treatment, however, this was not statistically significant and did
not approach the level of the positive control decoupling agent
FCCP (Fig. 3B)
Cells undergoing apoptosis show characteristic
changes in nuclear morphology with chromatin condensation and
nuclear fragmentation. The nuclear morphology of JU77 and ONE58
cells was observed by Hoechst 33342 staining after 48 h of
treatment (Fig. 3C).
Interestingly, in cells treated with SFRP4 characteristic apoptotic
changes were not seen although there were distinctive changes in
nuclear morphology. These changes of multinucleated cells with
chromosome vesicles were characteristic of mitotic catastrophe
(22). These changes were
consistently observed in both JU77 and ONE58 cells in response to
SFRP4. Apoptotic nuclei were not observed.
Effect of SFRP4 upon downstream
signaling
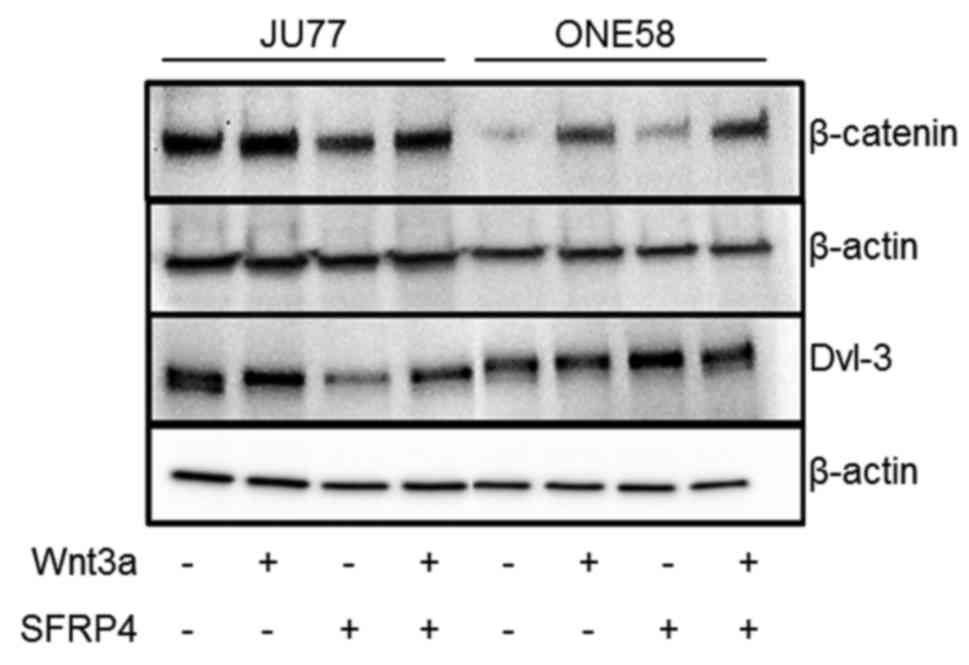
To further understand the effects of SFRP4 upon
mesothelioma cells β-catenin and Dvl-3 were assayed by western
blotting. In both JU77 and ONE58 Wnt3a treatment upregulated
β-catenin levels (Fig. 4), while
in JU77 SFRP4 downregulated β-catenin this was not observed in
ONE58 although basal β-catenin levels were quite low in this cell
line. Similarly, in JU77, Wnt3a induced apparent Dvl-3
phosphorylation while SFRP4 downregulated Dvl-3. Surprisingly,
these effects were not seen in ONE58 despite other assays showing
similar responses to SFRP4 in both cell lines.
Effect of endogenous overexpression of
SFRP4 and domains upon mesothelioma cell proliferation and
morphology
Having established that recombinant SFRP4 had
effects upon mesothelioma cell proliferation, viability and
migration, we next examined the effect of endogenous overexpression
of the full length protein as well as its domains: the NLD and CRD.
Previous studies have reported conflicting evidence regarding the
role of different SFRP domains in Wnt signaling regulation and we
explored this aspect. Following 48 h of transfection the observed
effects were quite small by microscopic examination in comparison
to exogenous treatment with SFRP4 (data not shown). However, at 6
days the effects upon cell morphology of the SFRP4 and NLD
constructs were similar to those seen with recombinant SFRP4
(Fig. 1D). In both cell lines
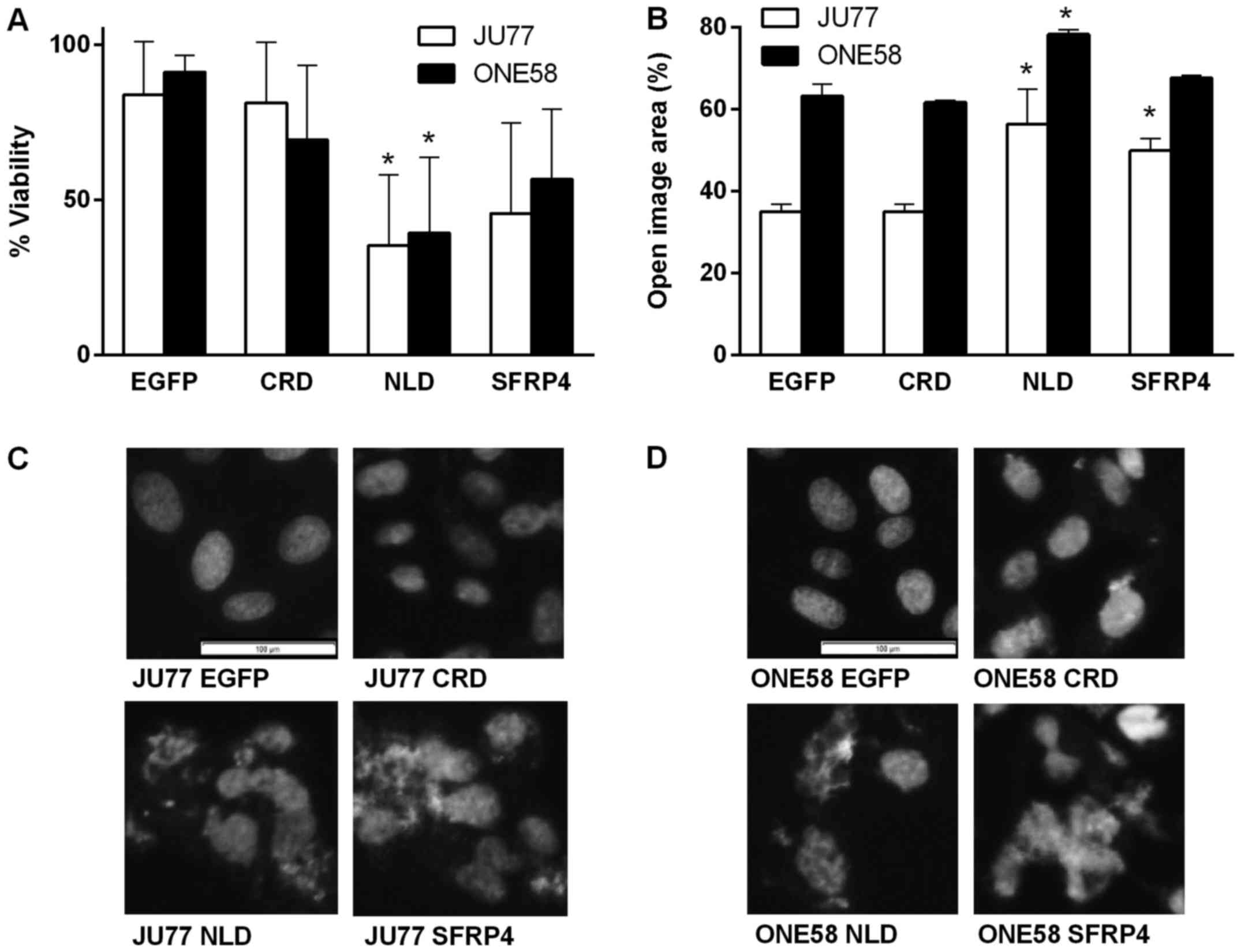
overexpression of SFRP4 and the NLD significantly downregulated
proliferation/viability (Fig. 5A).
The CRD had little effect in either cell line. These results were
consistent with the effect of recombinant SFRP4 and demonstrated
the NLD alone was sufficient for the cell viability effects of
SFRP4.
Effect of endogenous SFRP4 and domain
expression on mesothelioma cell migration
In order to further investigate the effects of
endogenous SFRP4 and domains upon mesothelioma cells transfectants
were investigated using a wound healing assay. The effects of SFRP4
overexpression were not as great as that seen with the endogenous
protein but it was still apparent in both cell lines. Quantitation
of these effects showed that SFRP4 and the NLD significantly
inhibited wound closure while the CRD had no effect (Fig. 5B). These results are consistent
with the effect of recombinant SFRP4 and also demonstrate that as
for proliferation the NLD mediates the major effects of SFRP4 upon
mesothelioma cells.
Overexpression of SFRP4 and its NLD
induce nuclear morphological changes consistent with mitotic
catastrophe
We next examined whether SFRP4 and domain
overexpression elicited similar effects upon nuclear morphology as
those seen with exogenous recombinant protein. We did in fact
observe that in both JU77 and ONE58 cell expression of SFRP4 and
the NLD induced a nuclear morphology characteristic of mitotic
catastrophe which was consistent with that shown in Fig. 3 (Fig.
5C and D). Cells expressing EGFP alone or the CRD did not
display this effect (Fig. 5C and
D).
Discussion
The experiments described in this study were
initiated based upon findings by other laboratories (9–11)
and our own study (6) showing
differential downregulation of SFRP4 in mesothelioma cells and
tissues. Evidence from the literature has demonstrated that most
mesotheliomas are β-catenin-positive (4,12,13).
Therefore it was of great interest to us to investigate the
biological effects and downstream signaling of SFRP4 in β-catenin
expressing mesothelioma cell models. We established that both
exogenous recombinant SFRP4 and endogenous SFRP4 overexpression had
similar effects resulting in downregulation of proliferation and
migration. Furthermore, a novel finding of our study was that SFRP4
induced a cytopathic effect that was apparently distinct from
apoptosis and consistent with mitotic catastrophe. Notably, we were
also able to provide evidence enhancing our understanding of the
biology of SFRPs since we found that the effects of SFRP4 upon
mesothelioma cells were largely mediated by the netrin-like
domain.
Overall the findings of our study are consistent
with downregulation of SFRP4 playing a role in the dysregulation of
Wnt signaling which promotes mesothelioma pathogenesis. These
findings broadly agree with those reported in other cancers where
SFRP4 has been reported to act as a tumor suppressor (23–25)
although there are conflicting reports in some cancers (26–28).
We found that SFRP4 both downregulated mesothelioma proliferation
and antagonized the effects of Wnt3a alone. Interestingly, the dose
response study of Wnt3a revealed a biphasic response in
proliferation. These effects may be due to feedback regulation of
the pathway at high Wnt concentrations leading to downregulation of
β-catenin levels (29). A key
finding of this study was that the cytopathic effect of SFRP4 upon
mesothelioma cells occurred via alternative pathways characteristic
of mitotic catastrophe (22) and
this contrasts with literature reports of SFRP4 inducing apoptosis
in other cell types (10,30,31).
This observation was confirmed by SFRP4 overexpression experiments
and while it has been reported that ultimately mitotic catastrophe
can trigger apoptosis we did not observe it here (22).
Wnt signaling is known to target genes involved in
cell migration (7), however, most
studies which have investigated the role of SFRP4 in cancer have
focused upon proliferation or apoptosis although SFRP4 has been
shown to regulate migration of endothelial and ovarian cancer cells
(24,32). We found that migration of both cell
lines was greatly inhibited by SFRP4. We know from a previous study
that these mesothelioma cells express Wnt2b, Wnt3 and Wnt4 and
Wnt5a although Wnt2b and Wnt4 are downregulated (6). Hence, endogenously expressed Wnt3 and
Wnt5a are possible SFRP4 interacting partners which may mediate
this effect. The fact that Wnt3a alone had little effect upon
migration but did reduce the effect of SFRP4 in JU77 cells suggests
that the target of SFRP4 interaction to influence migration may be
subject to competition by Wnt3a in these cells but not in ONE58. It
has recently been reported that SFRP4 does not bind with Wnt3a or
inhibit Wnt3a signaling (33).
This is inconsistent with our results which suggest that there is
some interaction between SFRP4 and Wnt3a and that SFRP4 can
antagonize the effects of Wnt3a. These discrepancies emphasize the
context dependency of Wnt signaling since SFRPs may also act by
interactions with Fz receptors and may not need to directly bind
Wnts (34). Furthermore, this
supports some mechanistic differences between the two cell lines
despite the phenotypic effects of SFRP4 being broadly similar. This
was most frequently seen in experiments using ONE58 where Wnt3a had
a less pronounced phenotypic effect (Figs. 1C and 2B) although it clearly induced β-catenin
accumulation which was more easily observed in ONE58 (Fig. 4). These results suggest that basal
β-catenin levels are higher in JU77 so that it was more difficult
to discern the effect of Wnt3a. These phenotypic differences in
response to Wnt3a may reflect the different repertoire of Fz
receptors expressed in these two cell lines (6).
Overall it was found that endogenous SFRP4
expression exerted similar effects on proliferation, migration and
nuclear morphology to those found using exogenous protein. The key
finding here was that the effects of SFRP4 were also seen when
cells were transfected with NLD expression constructs but not in
cells overexpressing the CRD domain. This is significant in the
context of our understanding of SFRPs since it is consistent with
recent studies that show that the NLD of other SFRPs bind with Wnts
at high affinity and can antagonize Wnt signaling (15,16,34)
while the CRD most likely acts through interactions with Fz
receptors (34).
In conclusion, SFRP4 which is downregulated in
mesothelioma is able to inhibit proliferation, migration and induce
nuclear changes characteristic of mitotic catastrophe when
reintroduced to mesothelioma cells using recombinant protein or
overexpression. A key finding of our study is that these effects
are mainly caused by the functions of the SFRP4 netrin-like domain
and the CRD has limited effect in these models. SFRP4 is also able
to inhibit signaling by Wnt3a in mesothelioma cells. Overall the
data provide supporting evidence for the targeting of the Wnt
pathway in mesothelioma and new evidence regarding the biology of
SFRP4 which is likely to have relevance to other members of this
family and their role in regulation of Wnt signaling and tumor
biology.
Acknowledgments
This study was supported by a grant from the Cancer
Council of Western Australia. V.P. was a recipient of a Curtin
International Postgraduate Research Scholarship. A.D. was supported
by strategic research funds from the School of Biomedical Sciences
(Curtin University), Commercialisation Advisory Board of Curtin
University, and Actinogen Ltd., Perth, Australia.
References
|
1
|
van Meerbeeck JP, Scherpereel A, Surmont
VF and Baas P: Malignant pleural mesothelioma: The standard of care
and challenges for future management. Crit Rev Oncol Hematol.
78:92–111. 2011. View Article : Google Scholar
|
|
2
|
Zucali PA, Ceresoli GL, De Vincenzo F,
Simonelli M, Lorenzi E, Gianoncelli L and Santoro A: Advances in
the biology of malignant pleural mesothelioma. Cancer Treat Rev.
37:543–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rascoe PA, Jupiter D, Cao X, Littlejohn JE
and Smythe WR: Molecular pathogenesis of malignant mesothelioma.
Expert Rev Mol Med. 14:e122012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uematsu K, Kanazawa S, You L, He B, Xu Z,
Li K, Peterlin BM, McCormick F and Jablons DM: Wnt pathway
activation in mesothelioma: Evidence of Dishevelled overexpression
and transcriptional activity of beta-catenin. Cancer Res.
63:4547–4551. 2003.PubMed/NCBI
|
|
5
|
Uematsu K, Seki N, Seto T, Isoe C,
Tsukamoto H, Mikami I, You L, He B, Xu Z, Jablons DM, et al:
Targeting the Wnt signaling pathway with dishevelled and cisplatin
synergistically suppresses mesothelioma cell growth. Anticancer
Res. 27B:4239–4242. 2007.
|
|
6
|
Fox SA, Richards AK, Kusumah I, Perumal V,
Bolitho EM, Mutsaers SE and Dharmarajan AM: Expression profile and
function of Wnt signaling mechanisms in malignant mesothelioma
cells. Biochem Biophys Res Commun. 440:82–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar
|
|
8
|
Surana R, Sikka S, Cai W, Shin EM, Warrier
SR, Tan HJ, Arfuso F, Fox SA, Dharmarajan AM and Kumar AP: Secreted
frizzled related proteins: Implications in cancers. Biochim Biophys
Acta. 1845:53–65. 2014.
|
|
9
|
Lee AY, He B, You L, Dadfarmay S, Xu Z,
Mazieres J, Mikami I, McCormick F and Jablons DM: Expression of the
secreted frizzled-related protein gene family is downregulated in
human mesothelioma. Oncogene. 23:6672–6676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He B, Lee AY, Dadfarmay S, You L, Xu Z,
Reguart N, Mazieres J, Mikami I, McCormick F and Jablons DM:
Secreted frizzled-related protein 4 is silenced by hypermethylation
and induces apoptosis in beta-catenin-deficient human mesothelioma
cells. Cancer Res. 65:743–748. 2005.PubMed/NCBI
|
|
11
|
Kohno H, Amatya VJ, Takeshima Y, Kushitani
K, Hattori N, Kohno N and Inai K: Aberrant promoter methylation of
WIF-1 and SFRP1, 2, 4 genes in mesothelioma. Oncol Rep. 24:423–431.
2010.PubMed/NCBI
|
|
12
|
Abutaily AS, Collins JE and Roche WR:
Cadherins, catenins and APC in pleural malignant mesothelioma. J
Pathol. 201:355–362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Orecchia S, Schillaci F, Salvio M, Libener
R and Betta P-G: Aberrant E-cadherin and gamma-catenin expression
in malignant mesothelioma and its diagnostic and biological
relevance. Lung Cancer. 45(Suppl 1): S37–S43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin K, Wang S, Julius MA, Kitajewski J,
Moos M Jr and Luyten FP: The cysteine-rich frizzled domain of
Frzb-1 is required and sufficient for modulation of Wnt signaling.
Proc Natl Acad Sci USA. 94:11196–11200. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhat RA, Stauffer B, Komm BS and Bodine
PVN: Structure-function analysis of secreted frizzled-related
protein-1 for its Wnt antagonist function. J Cell Biochem.
102:1519–1528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopez-Rios J, Esteve P, Ruiz JM and
Bovolenta P: The Netrin-related domain of Sfrp1 interacts with Wnt
ligands and antagonizes their activity in the anterior neural
plate. Neural Dev. 3:192008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manning LS, Whitaker D, Murch AR, Garlepp
MJ, Davis MR, Musk AW and Robinson BW: Establishment and
characterization of five human malignant mesothelioma cell lines
derived from pleural effusions. Int J Cancer. 47:285–290. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Whittell LR, Batty KT, Wong RPM, Bolitho
EM, Fox SA, Davis TME and Murray PE: Synthesis and antimalarial
evaluation of novel isocryptolepine derivatives. Bioorg Med Chem.
19:7519–7525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gebäck T, Schulz MMP, Koumoutsakos P and
Detmar M: TScratch: A novel and simple software tool for automated
analysis of monolayer wound healing assays. Biotechniques.
46:265–274. 2009.PubMed/NCBI
|
|
20
|
Cregan IL, Dharmarajan AM and Fox SA:
Mechanisms of cisplatin-induced cell death in malignant
mesothelioma cells: Role of inhibitor of apoptosis proteins (IAPs)
and caspases. Int J Oncol. 42:444–452. 2013.
|
|
21
|
Roth W, Wild-Bode C, Platten M, Grimmel C,
Melkonyan HS, Dichgans J and Weller M: Secreted Frizzled-related
proteins inhibit motility and promote growth of human malignant
glioma cells. Oncogene. 19:4210–4220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vakifahmetoglu H, Olsson M and Zhivotovsky
B: Death through a tragedy: Mitotic catastrophe. Cell Death Differ.
15:1153–1162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Horvath LG, Lelliott JE, Kench JG, Lee CS,
Williams ED, Saunders DN, Grygiel JJ, Sutherland RL and Henshall
SM: Secreted frizzled-related protein 4 inhibits proliferation and
metastatic potential in prostate cancer. Prostate. 67:1081–1090.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ford CE, Jary E, Ma SSQ, Nixdorf S,
Heinzelmann-Schwarz VA and Ward RL: The Wnt gatekeeper SFRP4
modulates EMT, cell migration and downstream Wnt signalling in
serous ovarian cancer cells. PLoS One. 8:e543622013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Warrier S, Balu SK, Kumar AP, Millward M
and Dharmarajan A: Wnt antagonist, secreted frizzled-related
protein 4 (sFRP4), increases chemotherapeutic response of glioma
stem-like cells. Oncol Res. 21:93–102. 2013. View Article : Google Scholar
|
|
26
|
Turashvili G, Bouchal J, Burkadze G and
Kolar Z: Wnt signaling pathway in mammary gland development and
carcinogenesis. Pathobiology. 73:213–223. 2006. View Article : Google Scholar
|
|
27
|
Huang D, Yu B, Deng Y, Sheng W, Peng Z,
Qin W and Du X: SFRP4 was overexpressed in colorectal carcinoma. J
Cancer Res Clin Oncol. 136:395–401. 2010. View Article : Google Scholar
|
|
28
|
Mortensen MM, Høyer S, Lynnerup A-S,
Ørntoft TF, Sørensen KD, Borre M and Dyrskjøt L: Expression
profiling of prostate cancer tissue delineates genes associated
with recurrence after prostatectomy. Sci Rep. 5:160182015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lustig B, Jerchow B, Sachs M, Weiler S,
Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM,
Birchmeier W and Behrens J: Negative feedback loop of Wnt signaling
through upregulation of conductin/axin2 in colorectal and liver
tumors. Mol Cell Biol. 22:1184–1193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Drake JM, Friis RR and Dharmarajan AM: The
role of sFRP4, a secreted frizzled-related protein, in ovulation.
Apoptosis. 8:389–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maganga R, Giles N, Adcroft K, Unni A,
Keeney D, Wood F, Fear M and Dharmarajan A: Secreted Frizzled
related protein-4 (sFRP4) promotes epidermal differentiation and
apoptosis. Biochem Biophys Res Commun. 377:606–611. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muley A, Majumder S, Kolluru GK, Parkinson
S, Viola H, Hool L, Arfuso F, Ganss R, Dharmarajan A and Chatterjee
S: Secreted frizzled-related protein 4: An angiogenesis inhibitor.
Am J Pathol. 176:1505–1516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carmon KS and Loose DS: Development of a
bioassay for detection of Wnt-binding affinities for individual
frizzled receptors. Anal Biochem. 401:288–294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodriguez J, Esteve P, Weinl C, Ruiz JM,
Fermin Y, Trousse F, Dwivedy A, Holt C and Bovolenta P: SFRP1
regulates the growth of retinal ganglion cell axons through the Fz2
receptor. Nat Neurosci. 8:1301–1309. 2005. View Article : Google Scholar : PubMed/NCBI
|