Introduction
Squamous cell carcinoma of the head and neck (HNSCC)
is one of the most commonly occurring malignancies, and is a major
cause of cancer morbidity and mortality worldwide with an estimated
incidence of 500,000 per year in the USA (1). The overall 5-year survival rate for
pharyngeal and oral squamous cell carcinoma is approximately 60% in
the USA, and has not changed significantly during the past 40 years
(2). Due to the increasing spread
of the human papillomavirus within the oropharyngeal tract, which
represents a new pathogenetic factor, the incidence of HNSCC is
estimated to rise even more (3).
Thus, there is a tremendous need for new treatment options for
HNSCC patients.
Statins are widely used as cholesterol-lowering
drugs, being small-molecule inhibitors of
3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (4). In addition to their common use in the
treatment of lipid disorders, statins have also demonstrated
anticarcinogenic properties in various preclinical in vitro
studies (5,6). This has been attributed mainly to the
inhibition of isoprenoid and cholesterol synthesis, which are both
important processes in the intracellular signaling pathways
(6,7). Several observational human studies
have reported a potential beneficial effect of statin use against
the overall risk of cancer (8–10).
Other studies, however, reported no such protective effects
(11,12). For several specific cancers,
especially for colorectal cancer (13), lung cancer (14,15)
and renal cell carcinoma (16),
protective effects of statin use have been published.
NSAIDs have also been demonstrated to have a
potential chemoprotective effect. This has been explained by an
induction of cell cycle arrest in G1-Phase via inhibition of Akt
(17), inhibition of
Ca2+ ATPase activity (18) or activating p53 and p21 (19). Several studies have reported on a
protective effect of Aspirin on colorectal adenoms as well as
colorectal carcinomas, even in randomized, double-blinded and
placebo-controlled study designs (20–23).
For selective cyclooxygenase (COX)-2 inhibitors such as celecoxib
or rofecoxib, protective effects could also be demonstrated, again
mainly for colorectal carcinoma (24–26).
However, since there have been serious cardiovascular complications
regarding long-term therapy with COX-2-inhibitors (27–29),
the relatively high doses needed for the observed cancer-protective
effect are being questioned. Therefore, a combination with other
drugs with synergistic effects to reduce the dosage of NSAIDs is
warranted.
A combined therapy of statins and NSAIDs has already
been demonstrated to have a synergistic effect on the induction of
apoptosis in prostate and colorectal cancer cells in vitro
(30,31). In vivo, a low-dose
combination of atorvastatin and celecoxib was reported to have
synergistic antitumor effects on colorectal cancer in a xenograft
animal model (32). For colorectal
cancer, population-based studies have also shown synergistic
cancer-protective effects of a combination therapy with statins and
NSAIDs compared to the respective monotherapies (33,34).
Yet for other entities, for example squamous cell carcinoma, few
studies have been conducted to date.
The objective of the present study is to analyze the
possible additive effects of the combined use of simvastatin and
celecoxib on human HNSCC cells in vitro in terms of
viability, cell growth, apoptosis and cell cycle changes.
Additionally, secretion of selected interleukins, namely IL-6 and
IL-8, was analyzed. IL-6 plays a key role in cell proliferation,
apoptosis and differentiation. IL-6 induces activation of Janus
kinase 1/2 (JAK1/2), resulting in phosphorylation of STAT3 at
tyrosine-705 (Y705) (35).
Activation of IL-6/STAT3 signaling has a significant role in
self-renewal and acquisition of malignant features of cancer stem
cells (CSC) (36). Furthermore,
IL-6 levels are highly elevated in metastatic diseases and
increased levels of serum IL-6 are associated with poor disease
outcome and prognosis in human cancers (37,38).
IL-8 is reported to be related to different malignancies due to the
involvement of thrombophilia and angiogenesis (39). Increased secretion of IL-8 has been
shown to increase the metastatic ability of different cancer
entities (40,41). Therefore, analysis of IL-6 and IL-8
was included into the present study.
Materials and methods
Cell culture
The head and neck squamous carcinoma cell lines
PE/CA-PJ 41 and HLaC78 were obtained from ECACC (European
Collection of Cell Cultures, Salisbury, Wiltshire, UK). Cells were
grown in RPMI-expansion medium (RPMI-EM) consisting of RPMI-1640
medium (Biochrom AG, Berlin, Germany) with 10% FCS, 100 U/ml
penicillin, 100 µg/ml streptomycin, 1% sodium pyruvate (100
mM, Biochrom AG), and 1% non-essential amino acids (100-fold
concentration, Biochrom AG). Cells were cultured at 37°C with 5%
CO2 in culture flasks. Medium was replaced every other
day and passaging was performed after reaching 70–80% confluence by
trypsinization, with subsequent washing and seeding in new flasks
or treatment wells. Experiments were performed using cells in the
exponential growth phase.
Exposure to celecoxib and
simvastatin
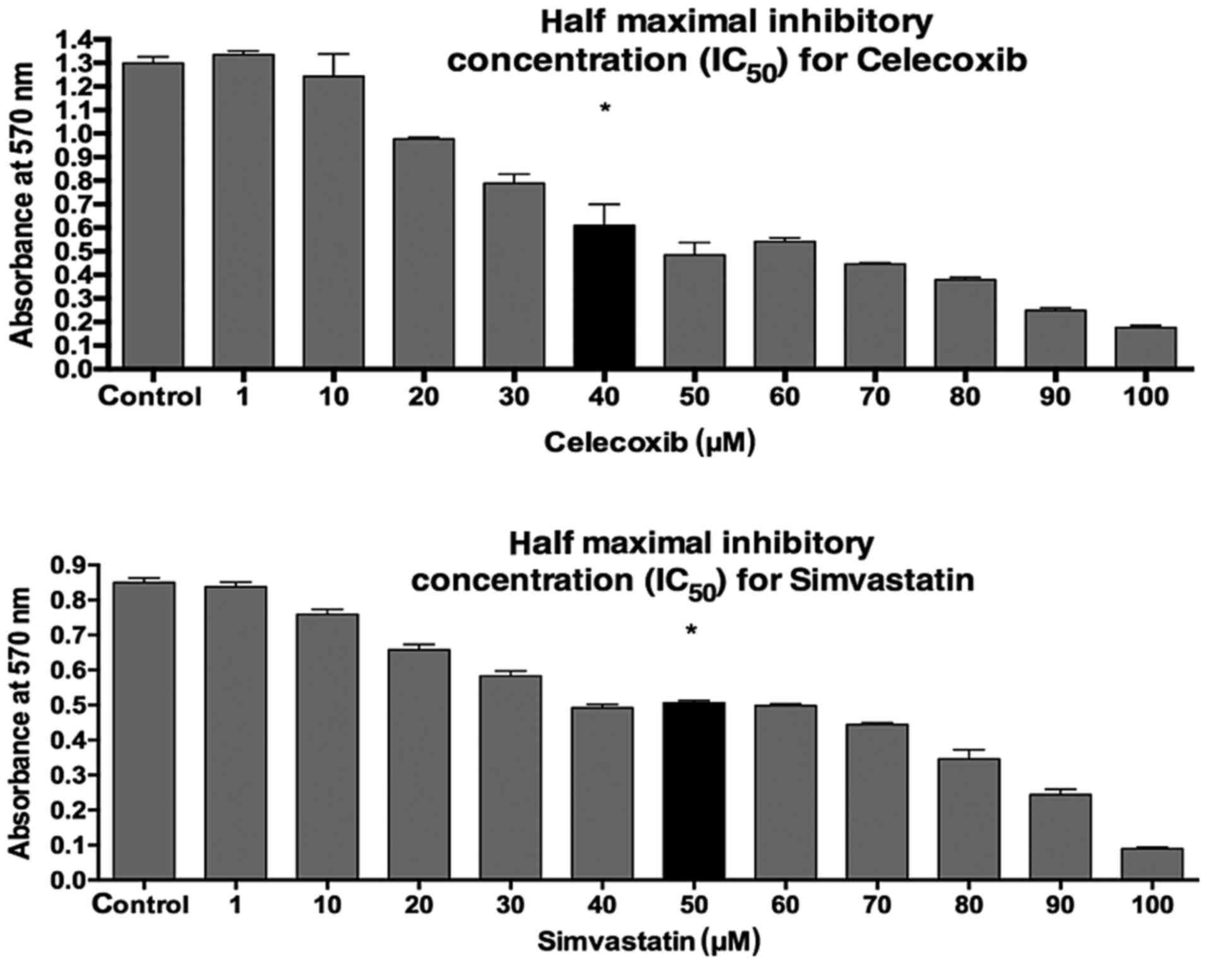
The half-maximal inhibitory concentrations
(IC50) of celecoxib (Pfizer Pharma PFE, Berlin, Germany)
and simvastatin (MIP Pharma, Blieskastel, Germany) on PE/CA-PJ 41
were evaluated with the MTT assay (Fig. 1). To this end, PE/CA-PJ-41 cells
were treated with 40 µM/ml celecoxib, 50 µM/ml
simvastatin or the combination of both. Analytical assays were
performed after 24 h of incubation.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
After 3 days of co-culture, the MTT (Sigma-Aldrich,
Taufkirchen, Germany) colorimetric staining method was performed
according to Mosmann (42) to
determine cell viability. Cells were seeded at 10,000 cells per
well in a 12-well plate. All wells were incubated with 1 ml MTT (1
mg/ml) for 5 h at 37°C and 5% CO2. MTT was then removed
and 1 ml isopropanol was added, followed by another incubation
period of 1 h at 37°C and 5% CO2. Measurement of the
color conversion of the blue formazan dye was performed using a
multi-plate reader (Titertek Multiskan PLUS MK II; Thermo
Labsystems, Thermo Fisher Scientific, Inc., Darmstadt, Germany) at
a wavelength of 570 nm.
Colony assay
PE/CA-PJ 41 were seeded into 6-well plates at a
concentration of 2.5×103 cells/well in triplicate.
Celecoxib (40 µM), 50 µM simvastatin or the
combination of both were added to defined well plates. PE/CA-PJ 41
cultivated in RPMI-EM served as the control. Cells were incubated
for 14 days. After 2 weeks the well plates were stained with
crystal violet, and colonies were counted manually.
Annexin V-propidium iodide test
The BD Pharmingen Annexin V-APC kit (BD Biosciences,
Heidelberg, Germany) was used to evaluate apoptosis on HLaC78 and
PE/CA-PJ 41. After 3 days of co-culture, cells in suspension and
adherent cells were harvested, then washed twice with PBS and
resuspended in 1:10 binding buffer [0.1 M HEPES (Sigma-Aldrich) (pH
7.4), 1.4 M NaCl, 25 mM CaCl2] at a concentration of
1×106 cells/ml. Aliquots of this cell suspension (100
µl; 1×105 cells) were then transferred to a 5 ml
culture tube. Propidium iodide (5 µl) and Annexin V-APC (5
µl) were added to each aliquot. After 15 min of incubation
at room temperature in the dark, the cells were resuspended with
400 µl 1:10 binding buffer. A FACSCanto flow cytometer was
used to analyze the samples with BD FACSDiva version 5.0.3 software
(BD Biosciences). Only cells with damaged membranes were stained by
propidium iodide.
Cell cycle analysis
To analyze the effect of celecoxib and salinomycin
on the cell cycle of PE/CA-PJ-41 and HLaC78, 1×105 cells
were cultivated in 12-well plates in triplicate. Following a 48 h
period, PE/CA-PJ-41 cells were trypsinized and washed twice with
cold PBS. Cells were then fixed in 1ml of 70% cold ethanol in test
tubes and incubated for 2 h at 4°C in the dark. After incubation,
cells were centrifuged at 500 × g for 5 min at 4°C and resuspended
in 500 µl propidium iodide (BD Bioscience). After another
incubation at 4°C in the dark for 15 min, cells were analyzed with
flow cytometry within 1 h. PE/CA-PJ-41 cultivated in RPMI-EM served
as the control.
IL-6/IL-8 ELISA
For measurement of the secretion of IL-6 and IL-8,
the super natants were collected (centrifugation, 150 × g for 5 min
at 37°C) after 3 days of co-culture and stored at −20°C in sterile
tubes until further use. RPMI-EM served as the control. Human IL-6
and IL-8 kits (catalog nos. 950.030.192 and 950.050.192,
respectively; Diaclone SAS, Besançon, France) were used and the
experiments were performed in duplicate. The ELISA plate was read
at 450 nm (Titertek Multiskan PLUS MK II). The concentrations of
IL-6 and IL-8 were determined by constructing a standard curve
using recombinant IL-6 and IL-8.
Statistical analysis
The data collected was transferred to standard
spreadsheets and statistically analyzed using GraphPad Prism
software (version 4.0; GraphPad Software, Inc., San Diego, CA,
USA). Data are presented as the mean ± standard deviation of three
experiments, unless otherwise stated. The Gaussian distribution was
tested via first column analysis. One-way analysis of variance
followed by Tukey's multiple comparison test was used.
Additionally, multiplicity adjusted p-values were determined.
p<0.05 was used to indicate a statistically significant
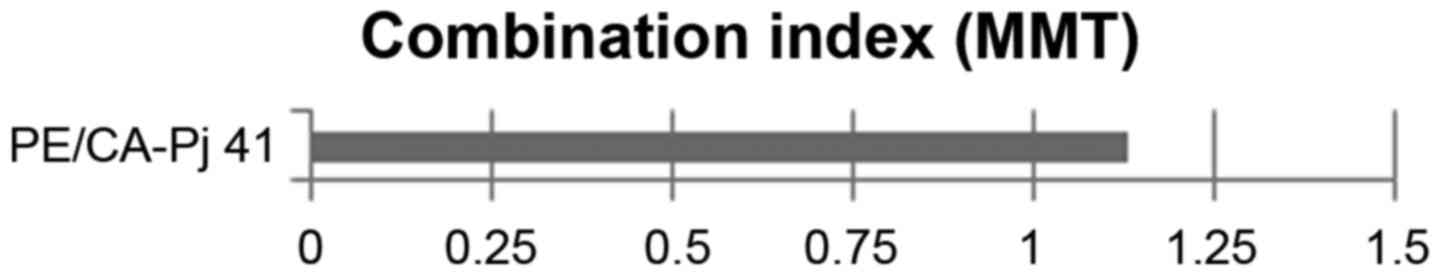
difference. The combination index (CI) was applied to evaluate the
interaction between celecoxib and simvastatin for PE/CA-PJ 41. CI
analysis provides qualitative information on the nature of drug
interaction, and the CI index, a numerical value calculated as
described below, also provides a quantitative measure of the extent
of drug interaction (43).
CI=CA,X:ICX,A+CB,X:ICX,B
CA,X and CB,X are the concentrations of drug
A and drug B used in combination to achieve x% drug effect
(IC75, IC50). ICX,A and
ICX,B are the concentrations required for single agents
to achieve the same effect. A CI of <0.85 was deemed to indicate
synergy, a CI of >1.15 was deemed to indicate antagonism.
Additive effects were assumed at an CI between 0.85 and 1.15.
Results
MTT assay
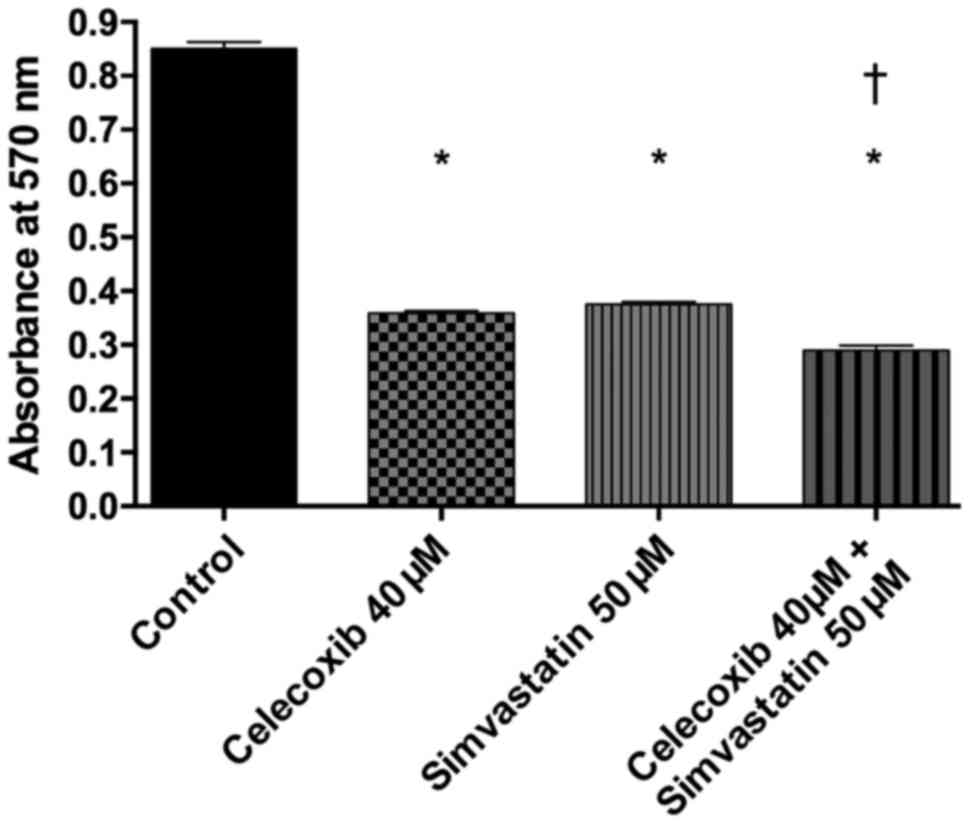
Viability of PE/CA-PJ-41 cells was analyzed using
the MTT assay (Fig. 2). It
revealed a significantly lower viability after addition of
celecoxib, simvastatin and the combination of both compared to the
control group (p<0.05 for all three). The combination of
treatment with celecoxib and simvastatin also proved to decrease
tumor cell viability significantly more compared to celecoxib and
simvastatin alone (p<0.05 for both). Between treatment with
celecoxib and simvastatin, no significant difference was found
(p>0.05). The CI was calculated as 1.13, indicating a moderate
additive effect (Fig. 3).
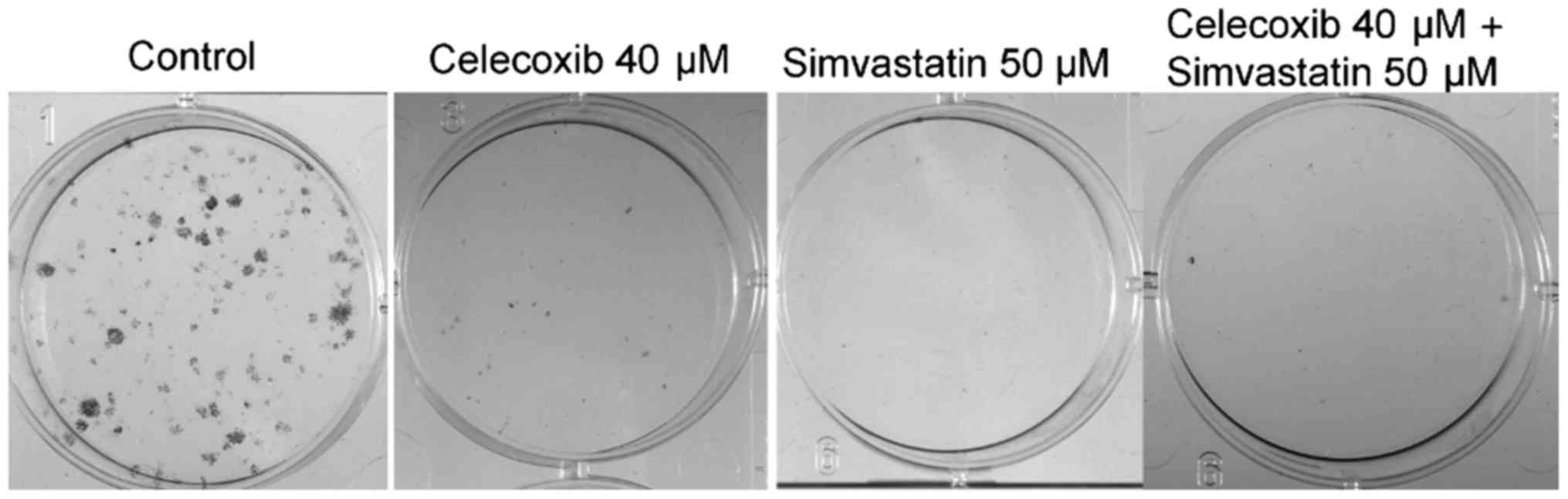
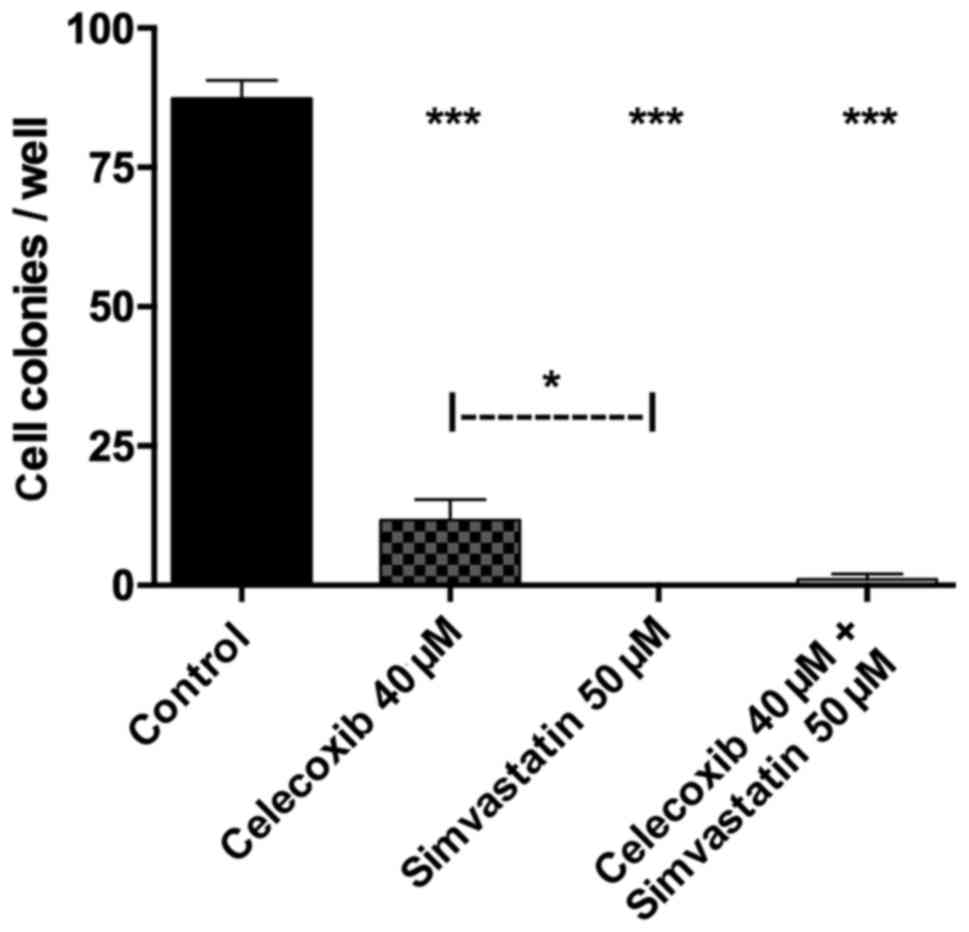
Colony assay
Tumor cell proliferation was analyzed using a colony
assay (Figs. 4 and 5). Treatment with celecoxib, simvastatin
and the combination of both showed significantly reduced cell
colonies compared to the control group (p<0.05 for all three).
Colony forming was higher when incubated with celecoxib alone
versus simvastatin alone (p<0.05). The combination of both drugs
showed no significant difference compared to celecoxib (p>0.05)
or simvastatin (p>0.05) alone.
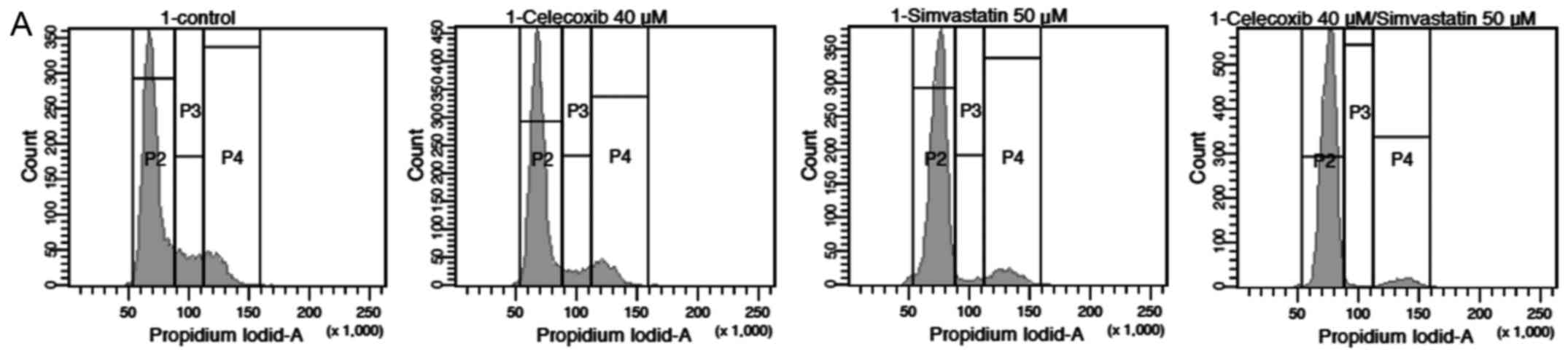
Annexin V-propidium iodide test
Annexin V-propidium iodide analysis of PE/CA-PJ 41
(Fig. 6A) and HLaC78 (Fig. 6B) revealed enhanced apoptosis and
necrosis after simvastatin treatment alone and after the
combination of both compared to the control group (p<0.05 for
both). The combination of both drugs induced higher rates of
apoptosis and necrosis compared to simvastatin and celecoxib alone
(p<0.05). Addition of celecoxib alone had no significant
difference on apoptosis or necrosis compared to the control
(p>0.05).
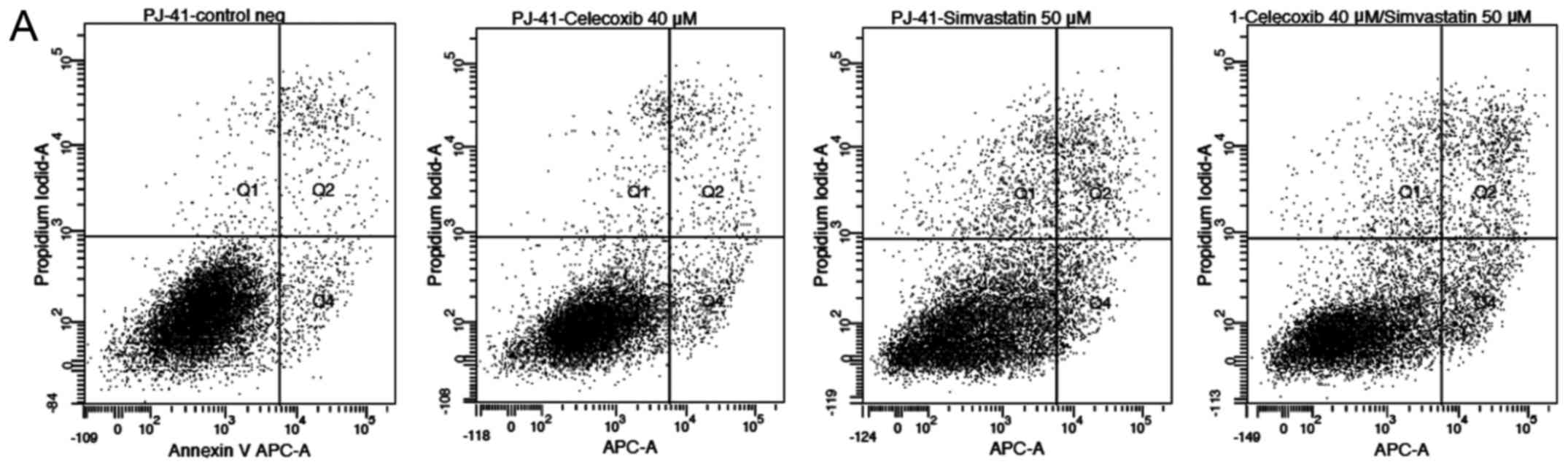 | Figure 6(A) Annexin V-propidium iodide
analysis of PE/CA-PJ 41. Apoptosis and necrosis is increased for
simvastatin as well as the combination of both compared to the
control group (p<0.05 for both). The combination of both drugs
also showed higher rates of apoptosis and necrosis compared to
simvastatin and celecoxib alone (p<0.05). Celecoxib alone showed
no significant difference in apoptosis or necrosis compared to the
control (p>0.05; data not shown). Q1, % of damaged cells; Q2, %
of necrotic cells; Q3, % of viable cells; Q4, % of apoptotic cells.
APC-A, allophycocyanin-A. (B) Annexin V-propidium iodide analysis
of HLaC78. Apoptosis and necrosis is increased for simvastatin as
well as the combination of both compared to the control group
(p<0.05 for both). The combination of both drugs also showed
higher rates of apoptosis and necrosis compared to simvastatin and
celecoxib alone (p<0.05). Celecoxib alone showed no significant
difference in apoptosis or necrosis compared to the control
(p>0.05; data not shown). Q1, % of damaged cells; Q2, % of
necrotic cells; Q3, % of viable cells; Q4, % of apoptotic cells.
APC-A, allophycocyanin-A. |
Cell cycle analysis
For both cell lines used, cell cycle analysis showed
a significant increase in cells in G0/G1-phase when treated with
celecoxib and simvastatin in combination compared to the control
group (p<0.05), while celecoxib (p>0.05) and simvastatin
(p>0.05) alone had no significant effect. The effect of the
combination therapy was also significant compared to celecoxib
(p<0.05) and simvastatin (p<0.05) alone (Fig. 7A and B).
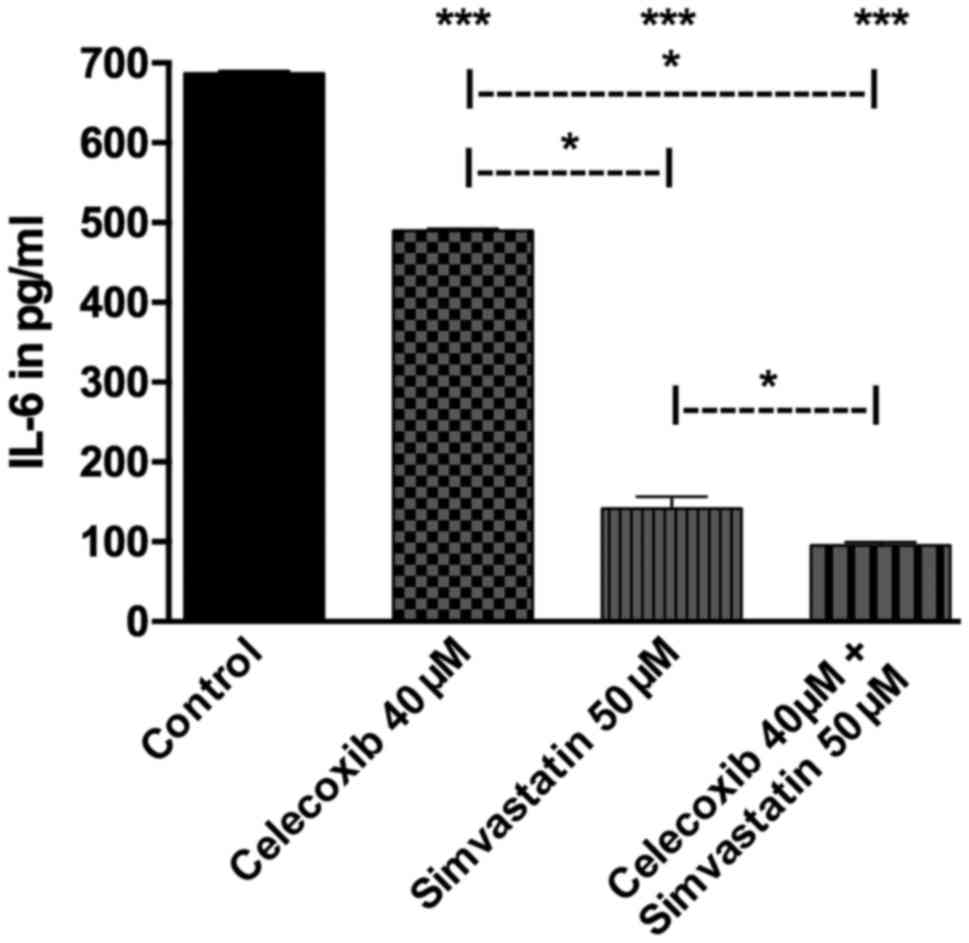
Quantitative analysis of IL-6
Treatment with celecoxib, simvastatin and the
combination of both all showed a lower secretion of IL-6 compared
to the control group (p<0.05 for all three). Addition of
simvastatin proved to decrease IL-6 secretion significantly more
than celecoxib (p<0.05). The combined treatment of both drugs in
turn showed less IL-6 than celecoxib (p<0.05) or simvastatin
alone (p<0.05; Fig. 8).
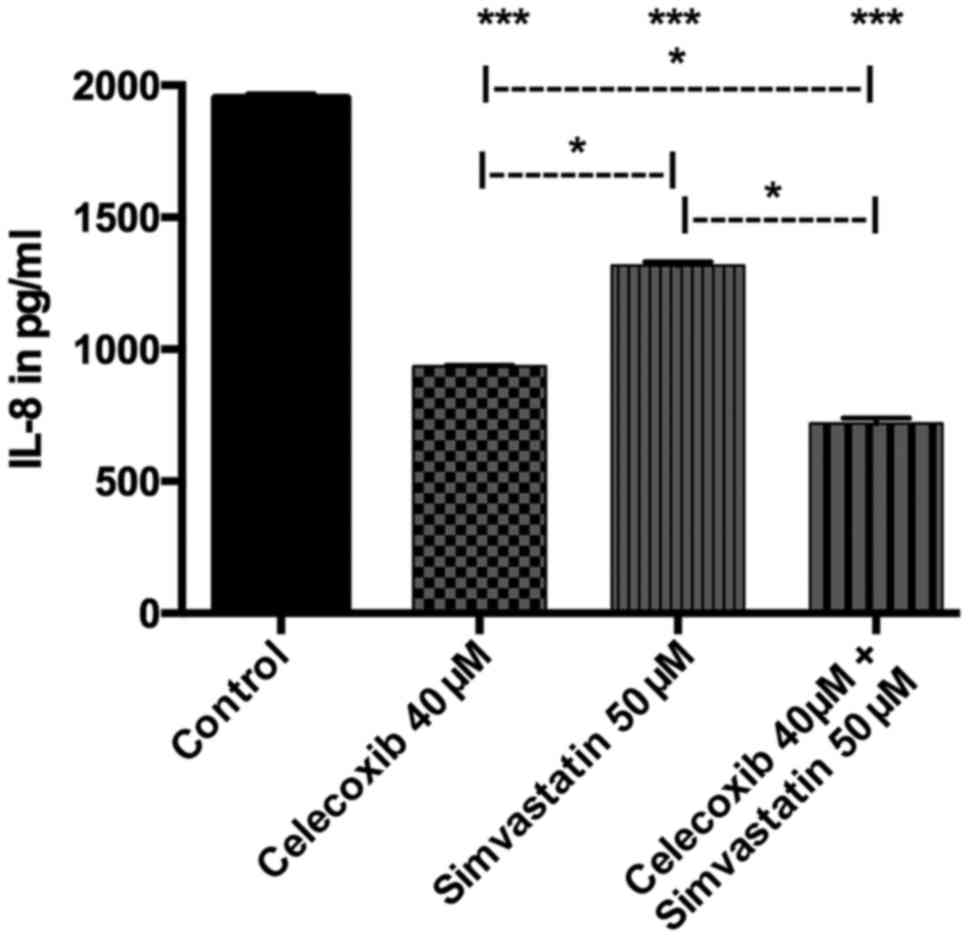
Quantitative analysis of IL-8
Treatment with celecoxib, simvastatin and the
combination of both all revealed a lower secretion of IL-8 compared
to the control group (p<0.05 for all three). Incubation with
celecoxib showed significantly decreased IL-8 secretion than with
simvastatin (p<0.05). The combined treatment of both drugs
proved to reduce IL-8-production significantly more than celecoxib
(p<0.05) or simvastatin alone (p<0.05; Fig. 9).
Discussion
Despite many advances in the therapy of HNSCC,
survival rates remain low (44).
Anticancer drug treatment for HNSCC today is mostly reserved for
palliative chemotherapy regimens, which include cytostatic agents
such as cisplatin, 5-FU or docetaxel as well as monoclonal
antibodies such as cetuximab. However, these drugs offer small
benefit with respect to progression-free survival, while in turn
inducing severe side effects further limiting the use in cancer
patients (45). Therefore,
research for identifying new treatment options with reduced
toxicities is warranted.
Simvastatin is an inhibitor of HNG-CoA reductase, an
enzyme of the mevalonate synthesis pathway, which in turn inhibits
formation of downstream lipid isoprenoids such as farnesyl
pyrophosphate (FFP) and geranylgeranyl pyrophosphate (GGPP)
(46). This in turn results in the
side effect of decreasing cell proliferation via inhibition of Ras
oncogenes (47). Statins have also
been shown to induce apoptosis, reduce serum-stimulated Ras
activity and increase messenger RNA (mRNA) and protein expression
of the proapoptotic proteins Bax and Bad in esophageal carcinoma
cell lines (48).
NSAIDs in general (21–23),
as well as selective COX-2-inhibitors (24–26),
have already proven to be potent tumor-protective substances in
vitro and in vivo. However, relatively high doses of
NSAIDs or selective COX-2-inhibitors are needed to achieve the
desired effects, which causes problems for long-term therapy due to
the cardiovascular risks of these drugs (27–29).
Thus, a combination of NSAIDs with other possibly synergistic
drugs, for example statins, could be a solution for reducing the
required doses for each.
The combination of statins and NSAIDs has already
been demonstrated to have synergistic effects on colorectal cancer
cells in vitro (30,31)
and in an animal model in vivo (32). Yet, as of now few studies have
evaluated the effects of this combination therapy on HNSCC cells.
Thus, the present study focused on the synergistic effects of
celecoxib and simvastatin on HNSCC cells in vitro.
The analyses showed significant reduction in
PE/CA-PJ-41 tumor cell proliferation and viability after addition
of celecoxib or simvastatin alone, with the effect increasing even
more using a combination of both substances. This confirms results
of colorectal cancer cells and prostate cancer cells treated with
celecoxib and simvastatin in combination (30–32,46).
The underlying mechanisms of these anticarcinogenic effects are not
completely understood, however.
In the present study, these antitumor effects were
mainly caused by apoptosis and, to a much lesser extent, by
necrosis. By inhibition of HMG-CoA reductase, statins inhibit the
synthesis of isoprenoids essential for membrane localization and
subsequent activation of signaling proteins such as Ras, Rho and
Rac, leading to increased apoptosis (4). Moreover, the reduction of cholesterol
synthesis via statins and their inhibition of the Akt pathway has
been shown to promote apoptosis in cancer cells (7). NSAIDs, on the other hand, also have a
variety of possible mechanisms that determine their
anticarcinogenic properties. Besides inhibition of Ca2+
ATPase activity (18), increase in
ceramid levels (49), and
inhibition of transcription activity of NFκB (50), NSAIDs have also shown the potential
for inhibition of the Akt pathway, as does simvastatin (17). Even in concentrations that do not
induce direct inhibition of Akt by celecoxib itself, it could be
demonstrated that celecoxib significantly synergized atorvastatin
to inhibit Akt-phosphorylation, indicating a pivotal synergistic
effect of both substances regarding Akt-pathway-induced apoptosis
(46).
In addition, significant cell cycle arrest in
G0/G1-phase could be demonstrated for the combination therapy in
the present study. Celecoxib has already been shown to induce cell
cycle arrest at G1-phase via increased expression of
cyclin-dependent kinase (CDK) inhibitors for various tumor cell
types (51,52). The combination of atorvastatin and
celecoxib has also been demonstrated to cause cell cycle arrest at
G0/G1-phase at a significantly higher level than both substances
alone (46). Thus, the induction
of cell cycle arrest at G0/G1-phase could also be a potential
synergistic effect. Since G0/G1-arrest inhibits the proliferation
of tumor cells, it is a vital target for anticancer therapeutics.
However, whether this G0/G1-arrest is irreversible, as has been
described for Terfenadine (53),
or perhaps even reversible, remains unclear since the present study
only measured one time point after treatment. Preliminary analysis
hint at an increase of cyclin-dependent kinase inhibitors
p21Cip1/Waf1 and p27Kip1 as a possible
mechanism behind the G0/G1-arrest. However, in the present study no
complete analysis of cell cycle protein expression has been
conducted, limiting the information in this regard. It will be part
of a future investigation at our institution.
The present study also revealed a significantly
lower secretion of IL-6 and IL-8 by the tumor cells after addition
of simvastatin and celecoxib combined rather than alone. IL-6 is a
cytokine which, among other functions, induces STAT3
phosphorylation via IL-6 receptors and Janus family kinases (JAK),
and is thereby involved in cell proliferation, angiogenesis and
apoptosis (54,55). For hepatocellular carcinoma,
celecoxib has already been demonstrated to inhibit
IL-6/IL-6-receptor-induced JAK2/STAT3 phosphorylation (56). In a study focusing on arthritis,
celecoxib significantly reduced secretion of IL-6 and IL-8 in
synovial fluid (57). Similarly,
simvastatin was also shown to inhibit IL-6 and IL-8 production in
rheumatoid arthritis (58).
However, the effect of a combination of celecoxib and simvastatin
on IL-6-secretion has not been investigated thus far, and in
particular not for HNSCC.
Interleukin-8 (IL-8), one of the ELR+ CXC
family of chemokines, is a potent proangiogenic factor and its
expression is associated with angiogenesis, tumor progression and
survival in patients with cancer (59,60).
NSAIDs have been shown to have inhibitory effects on angiogenesis
for pancreatic tumors in a mouse model, even by COX-independent
mechanisms (61), and have proven
to inhibit other proangiogenetic factors such as
matrixmetalloprotease (MMP)-2 and -9, as well as early growth
response factor EGR-1 (62–64).
Simvastatin could be demonstrated to inhibit the production of IL-6
and IL-8 as well as cell proliferation in patients with rheumatoid
arthritis (58). To our knowledge,
the present study is the first to evaluate the effect of a
combination of celecoxib and simvastatin on IL-8 production, and
therefore the angiogenesis of tumor cells, which may be of great
value in the treatment of metastatic cancer considering the
critical role of angiogenesis.
Still, there is much controversy about the
concentrations of simvastatin and celecoxib used in in vitro
experiments, regarding the expected in vivo dose necessary
to achieve similar effects in humans (65). Most in vitro studies
regarding cancer therapy have come to use concentrations of 40–60
µM of simvastatin (66).
Although it is true that the in vitro concentrations
generally used in oncologic studies are higher than the expected
dose in vivo, there is also the problem of accumulation in
the target organ, the liver, which at least partially makes up for
the difference in concentrations (67). The same is valid for celecoxib,
where the concentrations generally used in vitro are also
slightly higher than the expected in vivo doses achievable
in humans (68). Therefore, to
further reduce possible side effects when examining the
co-medication in vivo, lower concentrations should also be
tested for possible further synergistic effects in the future.
Since especially simvastatin intervenes in the
synthesis of Cholesterol, a key in cellular integrity, there could
be possible damage to regular human cells as well. Gauthaman et
al, on the other hand, already showed that simvastatin
decreased viability and proliferation of cancer cells and cancer
stem cells, but had no effect on normal human stem cells (69). This, coupled with the years of
clinical practice and experience with possible side effects of both
substances, may still make it worthwhile to further examine their
possible use as anticancer drugs.
In conclusion, it could be demonstrated that a
combination of celecoxib and simvastatin has significant
synergistic effects on reducing tumor cell proliferation and
viability in HNSCC cells in vitro. These antitumor effects
are based on apoptosis and cell cycle arrest in G0/G1-phase.
Furthermore, a reduction in the secretion of IL-6 and IL-8 could be
shown, indicating additional ways this synergism works to inhibit
tumor growth, such as via antiangiogenesis. At present, medical
tumor therapy for HNSCC is still limited. However, since both
substances have a good risk-benefit ratio based on their long-term
clinical use as lipid-lowering and anti-inflammational drugs, at
least in concentrations used in the present study, their combined
use for cancer therapy clearly warrants further investigation.
Future studies will need to elucidate the intracellular mechanisms
behind these effects. Especially analysis of mitogenic and other
signaling pathways are relevant targets for further
investigations.
Acknowledgments
This study was supported by the Rudolf Bartling
Foundation.
References
|
1
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herrero R, Castellsagué X, Pawlita M,
Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B,
Pintos J, et al IARC Multicenter Oral Cancer Study Group: Human
papillomavirus and oral cancer: The International Agency for
Research on Cancer multicenter study. J Natl Cancer Inst.
95:1772–1783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao H and Yang CS: Combination regimen
with statins and NSAIDs: A promising strategy for cancer
chemoprevention. Int J Cancer. 123:983–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prasanna P, Thibault A, Liu L and Samid D:
Lipid metabolism as a target for brain cancer therapy: Synergistic
activity of lovastatin and sodium phenylacetate against human
glioma cells. J Neurochem. 66:710–716. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia Z, Tan MM, Wong WW, Dimitroulakos J,
Minden MD and Penn LZ: Blocking protein geranylgeranylation is
essential for lovastatin-induced apoptosis of human acute myeloid
leukemia cells. Leukemia. 15:1398–1407. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhuang L, Kim J, Adam RM, Solomon KR and
Freeman MR: Cholesterol targeting alters lipid raft composition and
cell survival in prostate cancer cells and xenografts. J Clin
Invest. 115:959–968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blais L, Desgagné A and LeLorier J:
3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the
risk of cancer: A nested case-control study. Arch Intern Med.
160:2363–2368. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Graaf MR, Beiderbeck AB, Egberts AC,
Richel DJ and Guchelaar HJ: The risk of cancer in users of statins.
J Clin Oncol. 22:2388–2394. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Friis S, Poulsen AH, Johnsen SP,
McLaughlin JK, Fryzek JP, Dalton SO, Sørensen HT and Olsen JH:
Cancer risk among statin users: A population-based cohort study.
Int J Cancer. 114:643–647. 2005. View Article : Google Scholar
|
|
11
|
Olsen JH, Johansen C, Sørensen HT,
McLaughlin JK, Mellemkjaer L, Steffensen FH and Fraumeni JF Jr:
Lipid-lowering medication and risk of cancer. J Clin Epidemiol.
52:167–169. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaye JA and Jick H: Statin use and cancer
risk in the General Practice research Database. Br J Cancer.
90:635–637. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poynter JN, Gruber SB, Higgins PD, Almog
R, Bonner JD, Rennert HS, Low M, Greenson JK and Rennert G: Statins
and the risk of colorectal cancer. N Engl J Med. 352:2184–2192.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khurana V, Bejjanki HR, Caldito G and
Owens MW: Statins reduce the risk of lung cancer in humans: A large
case-control study of US veterans. Chest. 131:1282–1288. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Farwell WR, Scranton RE, Lawler EV, Lew
RA, Brophy MT, Fiore LD and Gaziano JM: The association between
statins and cancer incidence in a veterans population. J Natl
Cancer Inst. 100:134–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khurana V, Caldito G and Ankem M: Statins
might reduce risk of renal cell carcinoma in humans: Case-control
study of 500,000 veterans. Urology. 71:118–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kulp SK, Yang YT, Hung CC, Chen KF, Lai
JP, Tseng PH, Fowble JW, Ward PJ and Chen CS:
3-phosphoinositide-dependent protein kinase-1/Akt signaling
represents a major cyclooxygenase-2-independent target for
celecoxib in prostate cancer cells. Cancer Res. 64:1444–1451. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson AJ, Hsu AL, Lin HP, Song X and
Chen CS: The cyclooxygenase-2 inhibitor celecoxib perturbs
intracellular calcium by inhibiting endoplasmic reticulum
Ca2+-ATPases: A plausible link with its anti-tumour
effect and cardiovascular risks. Biochem J. 366:831–837. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luciani MG, Campregher C and Gasche C:
Aspirin blocks proliferation in colon cells by inducing a G1 arrest
and apoptosis through activation of the checkpoint kinase ATM.
Carcinogenesis. 28:2207–2217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thun MJ, Henley SJ and Patrono C:
Nonsteroidal anti-inflammatory drugs as anticancer agents:
Mechanistic, pharmacologic, and clinical issues. J Natl Cancer
Inst. 94:252–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giardiello FM, Hamilton SR, Krush AJ,
Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR and
Offerhaus GJ: Treatment of colonic and rectal adenomas with
sulindac in familial adenomatous polyposis. N Engl J Med.
328:1313–1316. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Labayle D, Fischer D, Vielh P, Drouhin F,
Pariente A, Bories C, Duhamel O, Trousset M and Attali P: Sulindac
causes regression of rectal polyps in familial adenomatous
polyposis. Gastroenterology. 101:635–639. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nugent KP, Farmer KC, Spigelman AD,
Williams CB and Phillips RK: Randomized controlled trial of the
effect of sulindac on duodenal and rectal polyposis and cell
proliferation in patients with familial adenomatous polyposis. Br J
Surg. 80:1618–1619. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steinbach G, Lynch PM, Phillips RK,
Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y,
Fujimura T, et al: The effect of celecoxib, a cyclooxygenase-2
inhibitor, in familial adenomatous polyposis. N Engl J Med.
342:1946–1952. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bertagnolli MM, Eagle CJ, Zauber AG,
Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J,
Corle D, et al APC Study Investigators: Celecoxib for the
prevention of sporadic colorectal adenomas. N Engl J Med.
355:873–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arber N, Eagle CJ, Spicak J, Rácz I, Dite
P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, et al PreSAP
Trial Investigators: Celecoxib for the prevention of colorectal
adenomatous polyps. N Engl J Med. 355:885–895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bresalier RS, Sandler RS, Quan H,
Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D,
Lanas A, et al Adenomatous Polyp Prevention on Vioxx (APPROVe)
Trial Investigators: Cardiovascular events associated with
rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J
Med. 352:1092–1102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kerr DJ, Dunn JA, Langman MJ, Smith JL,
Midgley RS, Stanley A, Stokes JC, Julier P, Iveson C, Duvvuri R, et
al VICTOR Trial Group: Rofecoxib and cardiovascular adverse events
in adjuvant treatment of colorectal cancer. N Engl J Med.
357:360–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Solomon SD, McMurray JJ, Pfeffer MA,
Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E and
Bertagnolli M; Adenoma Prevention with Celecoxib (APC) Study
Investigators: Cardiovascular risk associated with celecoxib in a
clinical trial for colorectal adenoma prevention. N Engl J Med.
352:1071–1080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Swamy MV, Cooma I, Reddy BS and Rao CV:
Lamin B, caspase-3 activity, and apoptosis induction by a
combination of HMG-CoA reductase inhibitor and COX-2 inhibitors: A
novel approach in developing effective chemopreventive regimens.
Int J Oncol. 20:753–759. 2002.PubMed/NCBI
|
|
31
|
Agarwal B, Rao CV, Bhendwal S, Ramey WR,
Shirin H, Reddy BS and Holt PR: Lovastatin augments
sulindac-induced apoptosis in colon cancer cells and potentiates
chemopreventive effects of sulindac. Gastroenterology. 117:838–847.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng X, Cui XX, Avila GE, Huang MT, Liu
Y, Patel J, Kong AN, Paulino R, Shih WJ, Lin Y, et al: Atorvastatin
and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice.
Clin Cancer Res. 13:5480–5487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoffmeister M, Chang-Claude J and Brenner
H: Individual and joint use of statins and low-dose aspirin and
risk of colorectal cancer: A population-based case-control study.
Int J Cancer. 121:1325–1330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Flick ED, Habel LA, Chan KA, Van Den Eeden
SK, Quinn VP, Haque R, Orav EJ, Seeger JD, Sadler MC, Quesenberry
CP Jr, et al: Statin use and risk of prostate cancer in the
California Men's Health Study cohort. Cancer Epidemiol Biomarkers
Prev. 16:2218–2225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Taga T, Hibi M, Hirata Y, Yamasaki K,
Yasukawa K, Matsuda T, Hirano T and Kishimoto T: Interleukin-6
triggers the association of its receptor with a possible signal
transducer, gp130. Cell. 58:573–581. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He G, Dhar D, Nakagawa H, Font-Burgada J,
Ogata H, Jiang Y, Shalapour S, Seki E, Yost SE, Jepsen K, et al:
Identification of liver cancer progenitors whose malignant
progression depends on autocrine IL-6 signaling. Cell. 155:384–396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Trikha M, Corringham R, Klein B and Rossi
JF: Targeted anti-interleukin-6 monoclonal antibody therapy for
cancer: a review of the rationale and clinical evidence. Clin
Cancer Res. 9:4653–4665. 2003.PubMed/NCBI
|
|
38
|
Zhang GJ and Adachi I: Serum interleukin-6
levels correlate to tumor progression and prognosis in metastatic
breast carcinoma. Anticancer res. 19:1427–1432. 1999.PubMed/NCBI
|
|
39
|
Zabaleta J, Su LJ, Lin HY, Sierra RA, Hall
MC, Sartor AO, Clark PE, Hu JJ and Ochoa AC: Cytokine genetic
polymorphisms and prostate cancer aggressiveness. Carcinogenesis.
30:1358–1362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li A, Varney ML and Singh RK: Expression
of interleukin 8 and its receptors in human colon carcinoma cells
with different metastatic potentials. Clin Cancer res. 7:3298–3304.
2001.PubMed/NCBI
|
|
41
|
Ren Y, Poon RT, Tsui HT, Chen WH, Li Z,
Lau C, Yu WC and Fan ST: Interleukin-8 serum levels in patients
with hepatocellular carcinoma: correlations with
clinicopathological features and prognosis. Clin Cancer res.
9:5996–6001. 2003.PubMed/NCBI
|
|
42
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chan GG, Tai BC, Liang S, Lim DT and Soo
KC: Squamous cell carcinoma of the head and neck (HNSCC) -
multi-modality treatment and impact on survival. Asian J Surg.
25:35–40. 2002.
|
|
45
|
Patil V, Joshi A, Noronha V, Deodhar J,
Bhattacharjee A, Dhumal S, Chandrakanth MV, Karpe A, Talreja V,
ChandRasekharan A, et al: Expectations and preferences for
palliative chemotherapy in head and neck cancers patients. Oral
Oncol. 63:10–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xiao H, Zhang Q, Lin Y, Reddy BS and Yang
CS: Combination of atorvastatin and celecoxib synergistically
induces cell cycle arrest and apoptosis in colon cancer cells. Int
J Cancer. 122:2115–2124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ogunwobi OO and Beales IL: Statins inhibit
proliferation and induce apoptosis in Barrett's esophageal
adenocarcinoma cells. Am J Gastroenterol. 103:825–837. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kundu N, Smyth MJ, Samsel L and Fulton AM:
Cyclooxygenase inhibitors block cell growth, increase ceramide and
inhibit cell cycle. Breast Cancer Res Treat. 76:57–64. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim SH, Song SH, Kim SG, Chun KS, Lim SY,
Na HK, Kim JW, Surh YJ, Bang YJ and Song YS: Celecoxib induces
apoptosis in cervical cancer cells independent of cyclooxygenase
using NF-kappab as a possible target. J Cancer res Clin Oncol.
130:551–560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Grösch S, Tegeder I, Niederberger E,
Bräutigam L and Geisslinger G: COX-2 independent induction of cell
cycle arrest and apoptosis in colon cancer cells by the selective
COX-2 inhibitor celecoxib. FASEB J. 15:2742–2744. 2001.PubMed/NCBI
|
|
52
|
Kardosh A, Blumenthal M, Wang WJ, Chen TC
and Schönthal AH: Differential effects of selective COX-2
inhibitors on cell cycle regulation and proliferation of
glioblastoma cell lines. Cancer Biol Ther. 3:55–62. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu JD, Wang YJ, Chen CH, Yu CF, Chen LC,
Lin JK, Liang YC, Lin SY and Ho YS: Molecular mechanisms of G0/G1
cell-cycle arrest and apoptosis induced by terfenadine in human
cancer cells. Mol Carcinog. 37:39–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Berishaj M, Gao SP, Ahmed S, Leslie K,
Al-Ahmadie H, Gerald WL, Bornmann W and Bromberg JF: Stat3 is
tyrosine-phosphorylated through the interleukin-6/glycoprotein
130/Janus kinase pathway in breast cancer. Breast Cancer Res.
9:R322007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bollrath J, Phesse TJ, von Burstin VA,
Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T,
Canli O, Schwitalla S, et al: gp130-mediated Stat3 activation in
enterocytes regulates cell survival and cell-cycle progression
during colitis-associated tumorigenesis. Cancer Cell. 15:91–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu Y, Liu A, Li H, Li C and Lin J:
Celecoxib inhibits interleukin-6/interleukin-6 receptor-induced
JAK2/STAT3 phosphorylation in human hepatocellular carcinoma cells.
Cancer Prev Res (Phila). 4:1296–1305. 2011. View Article : Google Scholar
|
|
57
|
Bianchi M, Broggini M, Balzarini P,
Franchi S and Sacerdote P: Effects of nimesulide on pain and on
synovial fluid concentrations of substance P, interleukin-6 and
interleukin-8 in patients with knee osteoarthritis: Comparison with
celecoxib. Int J Clin Pract. 61:1270–1277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yokota K, Miyazaki T, Hirano M, Akiyama Y
and Mimura T: Simvastatin inhibits production of interleukin 6
(IL-6) and IL-8 and cell proliferation induced by tumor necrosis
factor-alpha in fibroblast-like synoviocytes from patients with
rheumatoid arthritis. J Rheumatol. 33:463–471. 2006.PubMed/NCBI
|
|
59
|
Yuan A, Yang PC, Yu CJ, Chen WJ, Lin FY,
Kuo SH and Luh KT: Interleukin-8 messenger ribonucleic acid
expression correlates with tumor progression, tumor angiogenesis,
patient survival, and timing of relapse in non-small-cell lung
cancer. Am J respir Crit Care Med. 162:1957–1963. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Masuya D, Huang C, Liu D, Kameyama K,
Hayashi E, Yamauchi A, Kobayashi S, Haba R and Yokomise H: The
intratumoral expression of vascular endothelial growth factor and
interleukin-8 associated with angiogenesis in nonsmall cell lung
carcinoma patients. Cancer. 92:2628–2638. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wei D, Wang L, He Y, Xiong HQ, Abbruzzese
JL and Xie K: Celecoxib inhibits vascular endothelial growth factor
expression in and reduces angiogenesis and metastasis of human
pancreatic cancer via suppression of Sp1 transcription factor
activity. Cancer res. 64:2030–2038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lee HC, Park IC, Park MJ, An S, Woo SH,
Jin HO, Chung HY, Lee SJ, Gwak HS, Hong YJ, et al: Sulindac and its
metabolites inhibit invasion of glioblastoma cells via
down-regulation of Akt/PKB and MMP-2. J Cell Biochem. 94:597–610.
2005. View Article : Google Scholar
|
|
63
|
Ostrowski J, Wocial T, Skurzak H and
Bartnik W: Do altering in ornithine decarboxylase activity and gene
expression contribute to antiproliferative properties of COX
inhibitors? Br J Cancer. 88:1143–1151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Peluffo GD, Stillitani I, Rodríguez VA,
Diament MJ and Klein SM: Reduction of tumor progression and
paraneoplastic syndrome development in murine lung adenocarcinoma
by nonsteroidal antiinflammatory drugs. Int J Cancer. 110:825–830.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Björkhem-Bergman L, Lindh JD and Bergman
P: What is a relevant statin concentration in cell experiments
claiming pleiotropic effects? Br J Clin Pharmacol. 72:164–165.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gauthaman K, Fong CY and Bongso A:
Statins, stem cells, and cancer. J Cell Biochem. 106:975–983. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Thelen KM, Rentsch KM, Gutteck U, Heverin
M, Olin M, Andersson U, von Eckardstein A, Björkhem I and Lütjohann
D: Brain cholesterol synthesis in mice is affected by high dose of
simvastatin but not of pravastatin. J Pharmacol Exp Ther.
316:1146–1152. 2006. View Article : Google Scholar
|
|
68
|
Lai GH, Zhang Z and Sirica AE: Celecoxib
acts in a cyclooxygenase-2-independent manner and in synergy with
emodin to suppress rat cholangiocarcinoma growth in vitro through a
mechanism involving enhanced Akt inactivation and increased
activation of caspases-9 and -3. Mol Cancer Ther. 2:265–271.
2003.PubMed/NCBI
|
|
69
|
Gauthaman K, Manasi N and Bongso A:
Statins inhibit the growth of variant human embryonic stem cells
and cancer cells in vitro but not normal human embryonic stem
cells. Br J Pharmacol. 157:962–973. 2009. View Article : Google Scholar : PubMed/NCBI
|