Introduction
Cervical cancer is one of the most common
gynecological malignancies in clinical practice, and its incidence
rate is only followed by breast cancer (1–3).
Cervical cancer has a high morbidity and mortality, a poor
prognosis and is a serious threat to women's health (3,4).
However, the pathogenesis and etiology of cervical cancer is not
yet clear.
Brain-derived growth factor (BDNF) is a high
affinity ligand for tropomyosin related kinase B (TrkB) (5,6). The
neurotrophin receptor, TrkB, is one of the members of the
tropomyosin-related kinase (Trk) family (5,6), and
is pivotal to large numbers of biological processes involving the
maturity and development of the nervous system, such as neuronal
differentiation, growth, invasion and survival (7,8).
Previous studies have demonstrated that BDNF/TrkB is involved in
growth, invasion and metastasis in several tumors, including
gastric cancer, lung cancer, hepatocellular carcinoma, and ovarian
and prostate cancer (9–12). The binding of BDNF to TrkB results
in TrkB activation, and the two activated TrkBs combine to form
homodimers, which phosphorylate several tyrosine residues in the
intracellular domain of TrkB. On the one hand, this can enhance the
activity of TrkB itself; on the other hand, the phosphorylation of
tyrosine residues at certain sites forms a site that specifically
binds to PTB and SH2 structures, attracting cell signaling
molecules containing PTB and SH2 structures, which in turn causes
changes in the biological behavior of tumor cells (5,6,13).
It has been confirmed that the BDNF/TrkB signal transduction
pathway is involved in the regulation of tumor cell proliferation,
invasion and metastasis, resistance to chemotherapy and ankylosis
by activating the downstream phosphatidylinositol 3-kinase
(PI3K)/protein kinase B (PKB, also known as Akt) pathway (14). Epithelial-to-mesenchymal transition
(EMT) produces cells with characteristics of stem cells, and is a
hallmark of an increased invasive and migratory potential of cancer
cells (15–17). During the process of EMT, cells
lose cell-cell contacts and intercellular junctions, and undergo
cytoskeletal reorganization (17).
The increased expression of BDNF/TrkB is closely associated with
EMT in several types of tumors, leading to enhanced tumor invasion
and metastasis (18,19). These studies suggested that
BDNF/TrkB plays an important role in several cancers by enhancing
high cell proliferation, invasion and metastasis, the resistance to
apoptosis and poor prognosis.
Recent studies have revealed that extracellular
signal-regulated kinase (ERK) regulates the migration, invasion and
proliferation of cervical cancer cells (20–23).
These results suggest that the enhanced phosphorylation of ERK
promotes cell proliferation, invasion and metastasis (20–23).
There is evidence to indicate that the activation of the PI3K/AKT
pathway contributes to an enhanced invasion and metastasis, and a
poor prognosis in cervical cancers (24,25).
A recent study revealed that the BDNF/TrkB signaling pathway
exerted a marked stimulatory effect on HeLa cells (a cervical
cancer cell line) (26). Another
study reported that TrkB and BDNF expression may play a significant
role in the early events of tumorigenesis in cervical squamous cell
carcinoma (SCC) (27). However,
the underlying mechanisms of the BDNF/TrkB-induced signaling
pathway activation need to be elucidated in cervical cancers.
Hence, although BDNF/TrkB is essential in the tumorignicity of many
tumors (9–12), it remains obscure as to whether the
BDNF/TrkB pathway is associated with metastasis and EMT, and
whether it is linked to the ERK or PI3K/ AKT pathways in the
development of cervical cancer.
In this study, we investigated the role of the
BDNF/TrkB pathway in cervical cancers and cervical cancer cell
lines. Our results revealed that high a BDNF/TrkB expression was
associated with a poor outcome of cervical cancers. Notably, we
also found that a high BDNF/TrkB expression was necessary for cell
proliferation, invasion, metastasis and EMT, processes regulated by
the ERK and PI3K/AKT signaling pathways.
Materials and methods
Patients
This study was approved by our hospital
institutional review board and informed consent was obtained from
all patients. In total, 56 patients with cervical cancer (CC) were
enrolled in this study, aged between 36 to 71 years, with an
average age of 54.6 years. All patients underwent total
hysterectomy or radical mastectomy without pre-operative
radiotherapy and chemotherapy. Paraffin-embedded specimens from
surgical resection, as well as adjacent normal tissues were
collected from The Affiliated Hospital of Southwest Medical
University, Luzhou between January and October, 2016. CC staging
was performed according to the standard of the International Union
of Obstetrics and Gynecology (FIGO) in 1998 (28): 45 cases were characterized as
having disease at the >IIB stage and 11 cases were characterized
as having disease at the ≤IIB. The histological grade of the
samples was as follows: 7 cases of poorly differentiated, 24 cases
of moderately differentiated and 25 cases of well differentiated
tumors. Lymph node metastasis was as follows: 15 cases with lymph
node metastases and 41 cases with no lymph node metastases.
Cell culture
The human cervical cancer cell lines, HeLa, SiHa,
CaSki, C4-1 and C-33 A, and the human papillomavirus immortalized
ectocervical (Ect1/E6E7) cells were purchased from the American
Type Culture Collection (ATCC; Manassas, VA, USA), and grown in
RPMI-1640 medium containing 10% fetal bovine serum (FBS) and
antibiotics at 37°C under a humidified atmosphere with 5%
CO2 and 95% air.
Cell viability assay
Cell viability was determined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenytetrazolium bromide (MTT)
assay kit (Beyotime Institute of Biotechnology, Haimen, China).
Briefly, the cells were seeded in 96-well plates and were
pre-treated with siRNA against TrKB (siTrKB) or negative siRNA for
24 h. Subsequently, a concentration of 0.5 mg/ml of 1 mg/ml
MTT/well was added to the cells followed by incubation for 4 h at
37°C. The optical density at 490 nm was measured using a Bio-Rad
microplate reader (Bio-Rad, Hercules, CA, USA). Cell viability was
calculated as percentage of the average OD value of the
control.
Transwell invasion assay and scratch
migration assay
The invasion assay of the cells was performed using
24-well Transwell plates (Corning Inc., Lowell, MA, USA). Briefly,
1×105 cells in 500 µl of Dulbecco's modified Eagle's
medium (DMEM) were seeded in the upper chamber. The lower chamber
was supplemented with 750 µl of DMEM containing 10% FBS.
Follwowing 24 h of incubation, the upper cells of the membrane were
removed using a cotton-tipped swab. The cells invading the
underside of the membrane were fixed in methanol for 15 min and
stained with 0.5% crystal violet (AppliChem GmbH, Darmstadt,
Germany) for 15 min. The cell numbers from 5 random fields were
calculated and the corresponding images for the selected fields
were captured at ×40 magnification. The assays were performed in
triplicate.
For the scratch assay, the cells were grown in
complete DMEM until 90–100% confluency in cell culture plates. A
wound (5 mm in diameter) was introduced across each plate. Wound
healing growth or the migration of the cells was observed under a
microscope (Olympus CX23; Olympus, Tokyo, Japan) after 24 h.
Animal experiments
BALB/c nude mice (age, 6–8 weeks; sex, male and
female; weight, 20–25g) were purchased from the Medical Laboratory
Animal Center of Guangdong Province (Guangzhou, China) and were
allowed to acclimatize for 2 weeks. Animal experiments in this
study were carried out after obtaining ethics approval from the
Ethics Committee of the Affiliated Hospital of Southwest Medical
University. The mice were maintained in compliance with the
Institutional Animal Care and Use Committee (IACUC) procedures and
guidelines. The mice had free access to food and water and were
kept at a temperature of 20–25℃, and a relative humidity of 60%.
The mice were divided into 3 groups as follows: The control, in
which mice were injected with Hela cells; the siNC group, in which
mice were injected with HeLa cells transfected with negative
control siRNA; and the siTrKB group, in which mice were injected
with HeLa cells transfected with siTrKB; there were 5 mice per
group. HeLa cells (1×105) from all groups were
resuspended in 50 µl phosphate-buffered saline (PBS) before
being injected subcutaneously into the right flanks. Tumors in mice
were allowed to grow for 15 days and the mice were then sacrificed
and tumors removed.
Silencing of TrKB by siRNA
Recombinant adenoviruses encoding siRNA targeting
TrkB and negative TrkB siRNA were designed and constructed by
Sangon Biotech Co., Ltd. (Shanghai, China). The HeLa and CaSki
cells at 2×105 were seeded in 12-well plates for 12 h
prior to transfection. After the cells were grown to 70%
confluence, they were divided into 3 groups and they were
transfected with 30 nM PBS (control group), viruses encoding TrkB
siRNA and negative TrkB siRNA. Following transfection for 48 h, the
cells were collected and used in the experiments.
Immunohistochemical (IHC) staining
The tissue samples were fixed in 10% formalin. All
samples were then routinely processed, paraffin embedded, sectioned
at 5 mm, and then stained with hematoxylin and eosin (H&E). The
sections were deparaffinized in xylene and rehydrated in a graded
series of alcohol solutions according to routine protocol.
Subsequently, the sections were incubated with the appropriate
primary TrKB antibodies (1:100 dilution; Abcam, Cambridge, MA, USA)
and BDNF antibodies (1:100 dilution; Abcam) overnight. After
washing, the slides were incubated for 30 min at room temperature
with goat anti-rat IgG H&L (HRP). Non-specific staining was
blocked with 0.5% casein and 5% normal serum (Invitrogen/ Life
Technologies, Carlsbad, CA, USA). The sections were subsequently
counterstained with hematoxylin (Invitrogen/ Life Technologies).
The percentage of positive cells was determined by counting at
least 200 cells per section in 5 random, non-overlapping fields
under a microscope. Immunostaining was evaluated using a
semi-quantitative scoring system, which was determined by the
staining intensity in conjunction with the percentage of positively
stained cells. Scores of 0, 1+ and 2+ were regarded as weak or
moderate for the expression of TrKB and BDNF protein, whereas
scores of 3+ were considered as positive for the overexpression of
TrKB and BDNF protein.
Western blot analysis
Cells from all the groups were washed using ice-cold
PBS and lysed for 30 min on ice in lysis buffer (100 mM Tris, 150
mM NaCl, 1% Triton X-100) supplemented with a cocktail of protease
inhibitors (Sigma-Aldrich, St. Louis, MO, USA). Protein
concentrations were examined using the BCA protein assay kit
(Pierce Biotechnology, Rockford, IL, USA) according to the
manufacturer's instructions. Briefly, proteins in the cells were
separated by an SDS polyacrylamide gel (Pharmacia and Biotech,
Piscataway, NJ, USA) electrophoresis, and then electrotransferred
onto polyvinylidene difluoride membranes (Millipore Corp., Bedford,
MA, USA). The blots were then washed with TBST. The membranes were
then blocked with 5% non-fat dry milk. Subsequently, the membranes
were incubated with appropriate antibodies (TrKB, 1:1,000, ab18987;
BDNF, 1:1,000, ab108319; E-Cadherin, 1:100, ab1416; N-cadherin,
1:1,000, ab18203; Vimentin, 1:2,000, ab92547; MMP2, 1:1,000,
ab37150; MMP9, 1:1,000, ab38898; TIMP2, 1:1,000, ab1828; and GAPDH,
1:1,000, ab8245; all from Abcam) at 4°C overnight. The membranes
were washed with PBS and incubated with a 1:2,000 dilution of
peroxidase-conjugated secondary antibodies (A9044; Sigma-Aldrich)
for 1 h. The bands were visualized using the enhanced
chemiluminescence (ECL) western blotting detection system (Amersham
Bioscience, Piscataway, NJ, USA). The intensity of the bands was
determined using ImageJ software (NIH, Bethesda, MD, USA).
RT-qPCR analysis
Total RNA was isolated from the cells in all groups
using the RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to
the manufacturer's instructions. A total of 1 µg total RNA
was converted into cDNA using the TaqMan Reverse Transcription
reagent kit (Applied Biosystems, Foster City, CA, USA).
Quantitative PCR was performed using TaqMan Gene Expression Assays
and the TaqMan Universal PCR Master Mix (Applied Biosystems)
according to the manufacturer's instructions. The amplification and
detection of mRNA were analyzed using a 7500 Real-Time PCR System
(Applied Biosystems). Data were calculated using the
2−∆∆Cq method and normalized to GAPDH levels. The PCR
reactions for each sample were performed in duplicate. The
sequences primers were used as follows: TrkB forward,
5′-TTGCAGCATTTCACTTGGCT-3′ and reverse, 5′-CTGTCATTTAGCCGCGAACA-3′;
BDNF forward, 2, 5′-GAGC CCTGTATCAACCCAGA-3′ and reverse,
5′-TCAAATACCATGCCCCACCT-3′; E-Cadherin forward,
5′-AACAGGATGGCTGAAGGTGA-3′ and reverse, 5′-CCTTCCATGACAGACCCCTT-3′;
N-cadherin forward, 5′-ATATTTCCATCCTGCGCGTG-3′ and reverse,
5′-GTTTGGCCTGGCGTTCTTTA-3′; vimentin forward,
5′-GAGTCCACTGAGTACCGGAG-3′ and reverse, 5′-ACGAGCCATTTCCTCCTTCA-3′;
matrix metalloproteinase (MMP)2 forward, 5′-GGAGTACTGCAAGTTCCCCT-3′
and reverse, 5′-TCAGTGGTGCAGCTGTCATA-3′; MMP9 forward,
5′-TGCCACTTCCCCTTCATCTT-3′ and reverse, 5′-CGTCCT
GGGTGTAGAGTCTC-3′; tissue inhibitor of metallo-proteinases (TIMP)2
forward, 5′-TGATCCCGTGCTACATCTCC-3′ and reverse,
5′-CCTGCTTATGGGTCCTCGAT-3′; and GAPDH forward,
5′-GAGTAAGACCCCTGGACCAC-3′ and reverse,
5′-AACTGGTTGAGCACAGGGTA-3′.
Statistical analysis
The data are expressed as the means ± standard
deviation. An independent t-test and one-way ANOVA was used for the
statistical comparison of two and multiple groups, respectively
followed by Tukey's post hoc test. A value of P<0.05 was
considered to indicate a statistically significant difference. All
statistical analysis were analyzed using SPSS software (SPSS
version 18; SPSS, Inc., Chicago, IL, USA).
Results
Correlations between TrKB and BDNF
expression with clinicopathological parameters
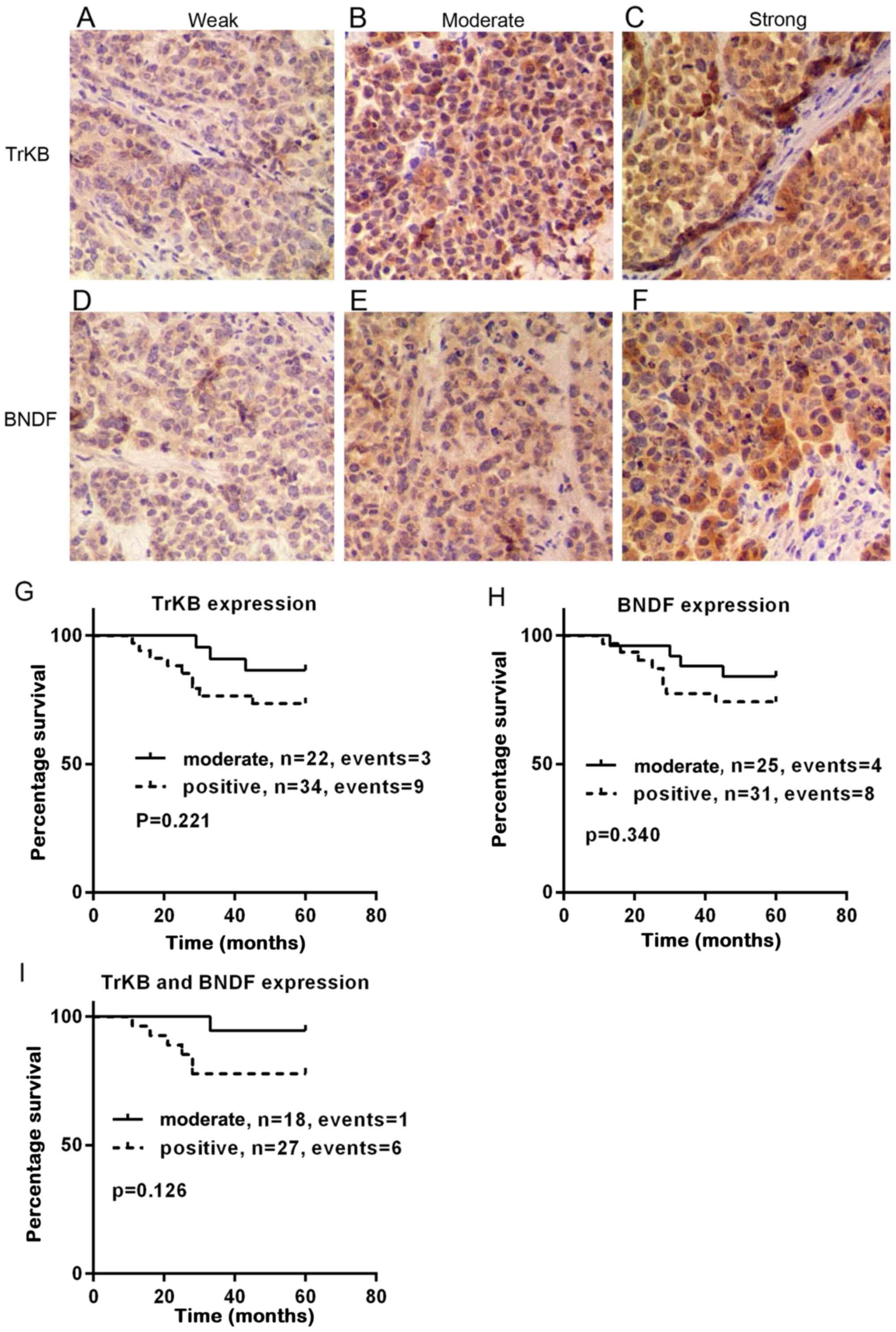
The representative immunohistochemical images for
TrKB and BDNF are shown in Fig.
1A–F. The association between the clinicopathological
parameters and TrKB or BDNF expression are summarized in Table I. Of the 56 patients with cervical
cancer, the age of 39 patients was <50 years. In total, 45 cases
were characterized as having disease at the >IIB stage and 11
cases were characterized as having the disease at the ≤IIB stage.
In addition, 44 patients had squamous cell carcinoma and 12 had
adenocarcinoma. No significant association between TrKB expression
and clinicopathological parameters was observed. However, higher
expression levels were observed in patients with a tumor size of
>4 cm compared to those with a tumor sie of <4 cm (P=0.112).
Patients with poor and moderate differentiation had a higher TrKB
expression compared to those with well differentiation (P=0.061). A
positive TrKB expression was found in 81.8% (n=9) of patients with
the disease at ≥IIB stage, while it was 55.6% in patients with the
disease at <IIB stage. The distribution of TrKB protein was
markedly higher in patients with lymph node metastasis than in
patients with no lymph node metastasis (P=0.074). Similar to TrKB
expression, a high expression of BDNF was found in patients with
moderate and poor differentiation, with the disease at ≥IIB stage
and with lymph node metastasis. In addition, there was a
significant association between BDNF expression and tumor size. The
positive expression of BDNF was 86.7% in patients with a tumor size
of >4 cm (n=13/15), significantly higher than tht of patients
with a tumor size of <4 cm (43.9%; n=18/41) (P=0.004).
 | Table IAssociation of TrKB and BDNF
expression with the clinicopathological characteristics of the
patients with cervical cancer. |
Table I
Association of TrKB and BDNF
expression with the clinicopathological characteristics of the
patients with cervical cancer.
|
Characteristics | No. | TrKB
| BDNF
|
|---|
| Positive n (%) | P-value | Positive n (%) | P-value |
|---|
| Age (years) | | | | | 0.810 |
| <50 | 39 | 24 (61.5) | 0.848 | 22 (56.4) | |
| ≥50 | 17 | 10 (58.8) | | 9 (52.9) | |
| Tumor size
(cm) | | | | | 0.004 |
| <4 | 41 | 22 (53.6) | 0.112 | 18 (43.9) | |
| >4 | 15 | 12 (80) | | 13 (86.7) | |
| Grade of
differentiation | | | | | 0.082 |
| Well | 24 | 11 (45.8) | 0.061 | 10 (41.7) | |
| Moderate | 25 | 18 (72) | | 15 (60) | |
| Poor | 7 | 5 (71.4) | | 6 (85.7) | |
| FIGO stage | | | | | 0.312 |
| <IIB | 45 | 25 (55.6) | 0.171 | 23 (51.1) | |
| ≥IIB | 11 | 9 (81.8) | | 8 (72.7) | |
| Histological
subtype | | | | | 0.123 |
| Squamous cell
carcinoma | 44 | 26 (59.1) | 0.746 | 22 (50) | |
|
Adenocarcinoma | 12 | 8 (66.7) | | 9 (75) | |
| Lymph node | | | | | 0.102 |
| Positive | 15 | 12 (80) | 0.074 | 11 (73.3) | |
| Negative | 41 | 22 (53.7) | | 20 (48.8) | |
Associations between TrKB and BDNF
expression with patient overall survival
To evaluate the association between TrKB or BDNF and
the overall survival of patients with cervical cancer, Kaplan-Meier
analysis was performed. As shown in Fig. 1G–I, the overall survival time was
prolonged in patients with a moderate expression of TrKB and BDNF,
compared to that in patients with a positive expression of TrKB
(P=0.221) and BDNF (P=0.340), respectively. Furthermore, we
compared patients with both TrKB and BDNF positive expression to
patients with both TrKB and BDNF moderate expression. It was
evident that patients with both TrKB and BDNF moderate expression
had a prolonged survival time compared to patients with TrKB and
BDNF positive expression (n=45, P=0.126).
Higher expression levels of TrKB or BDNF
are observed in cervical cancer tissues than in adjacent normal
tissues
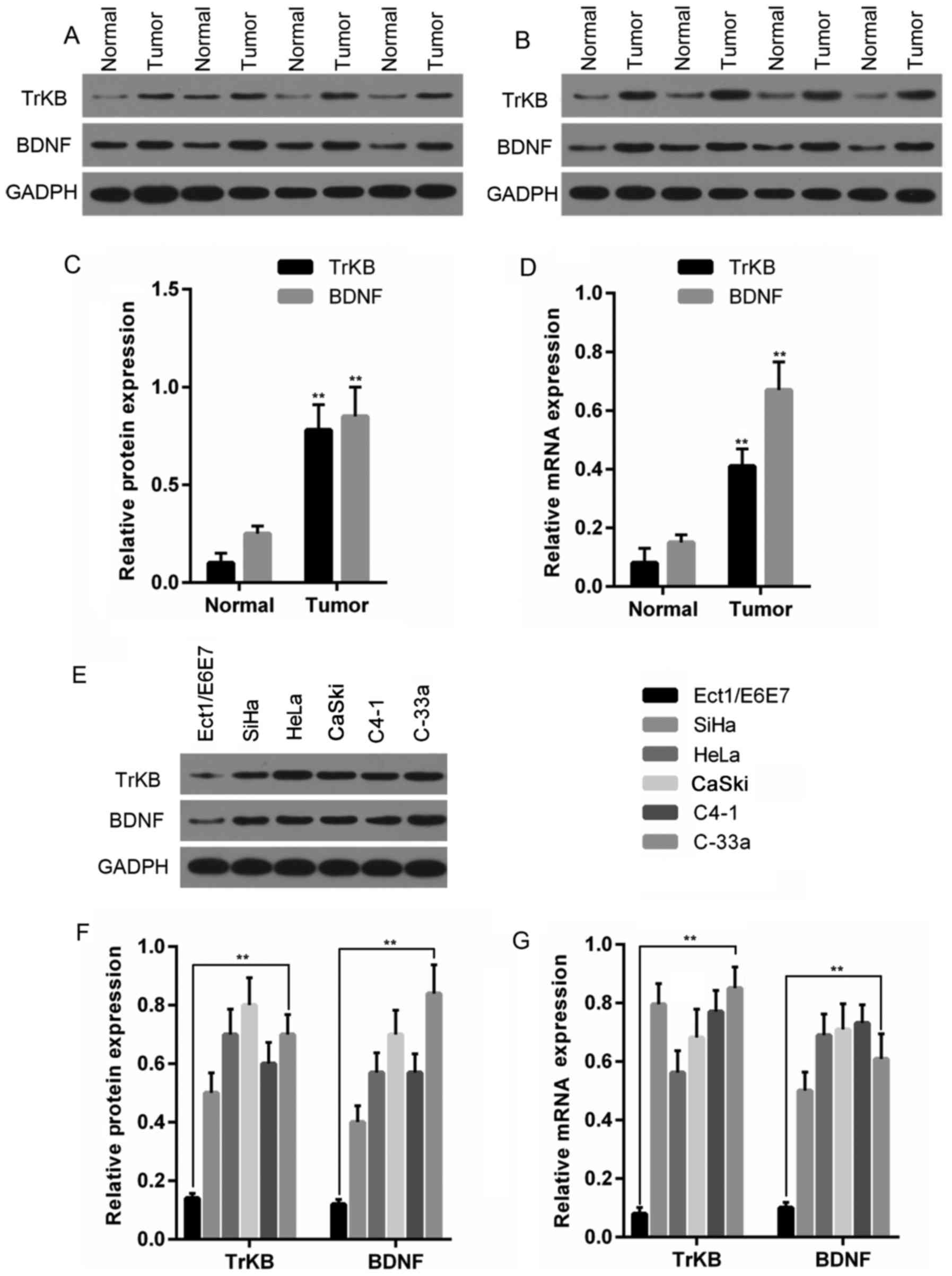
To assess the differences in TrKB and BDNF
expression between cancer tissues and adjacent normal tissues, we
determined the mRNA and the protein expression levels of TrKB and
BDNF in these tissues. In 8 paired cancer tissues and adjacent
normal tissues, the expression at both the protein (Fig. 2A–C) and mRNA (Fig. 2D) level were found to be
overexpressed in cancer tissues compared to those in the adjacent
tissues counterparts.
Expression levels of TrKB and BDNF in
cervical cancer cell lines are higher than those in the human
papillomavirus immortalized ectocervical cells (Ect1/E6E7)
To further explore the role of TrKB and BDNF in
cervical cancer, we examined the expression of TrKB and BDNF in
cervical cancer cell lines and human papillomavirus immortalized
ectocervical cells (Ect1/E6E7). As shown in Fig. 2E–G, compared to the Ect1/E6E7
cells, higher protein and mRNA expression levels of TrKB and BDNF
were observed in the cervical cancer cell lines HeLa, CaSki, CaSki,
C4-1 and C-33 A.
Expression levels of TrKB and BDNF are
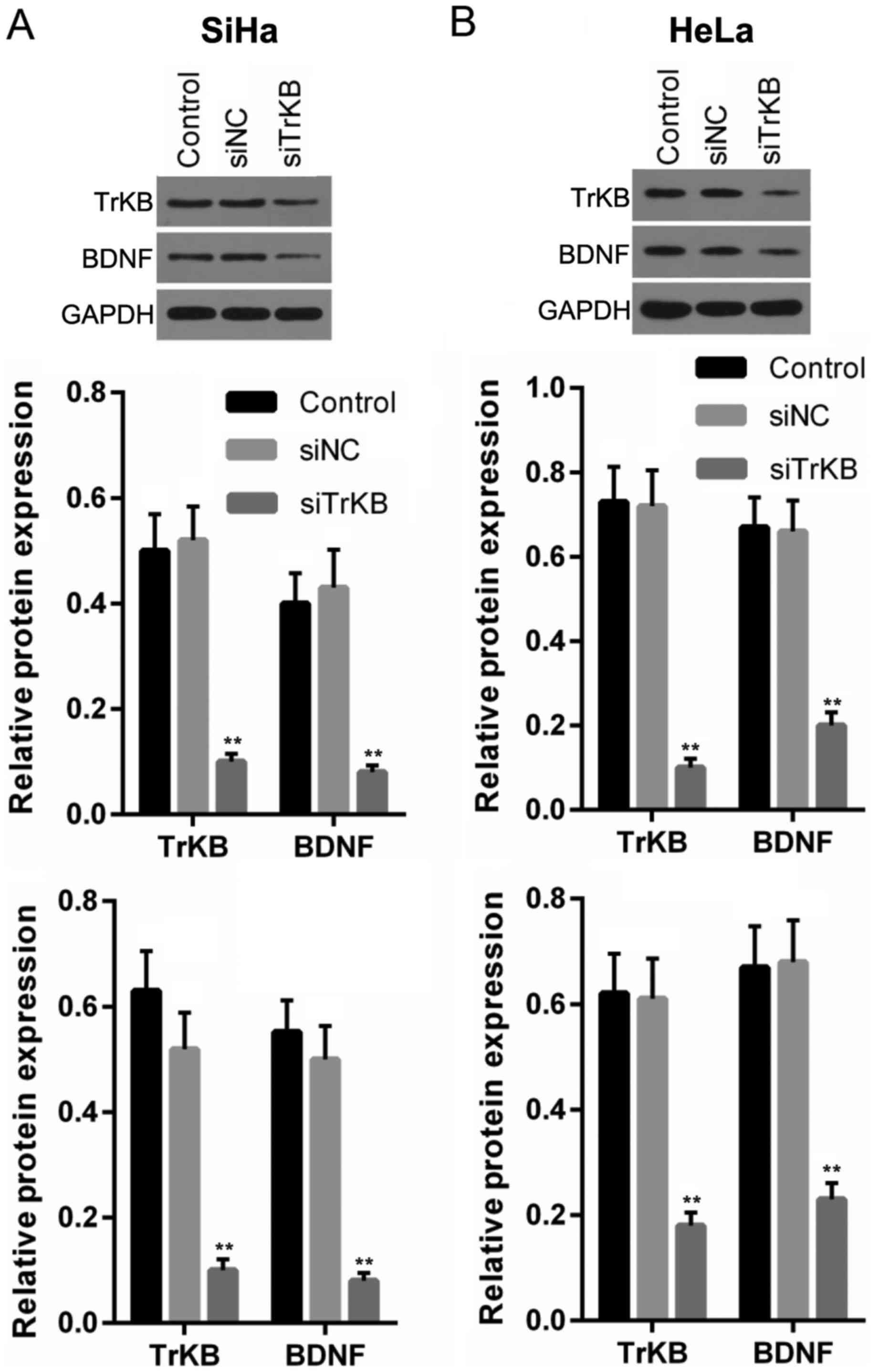
significantly decreased in cells following siRNA transfection
To explore the role of TrKB and BDNF in the cervical
cancer cell lines, HeLa and CaSki, we silenced TrKB with TrKB
siRNA. The results revealed that the protein and mRNA expression
levels of both TrKB and BDNF were clearly downregulated after the
cells were transfected with TrKB siRNA, compared to the levels in
the control and negative siRNA-transfected cells (Fig. 3).
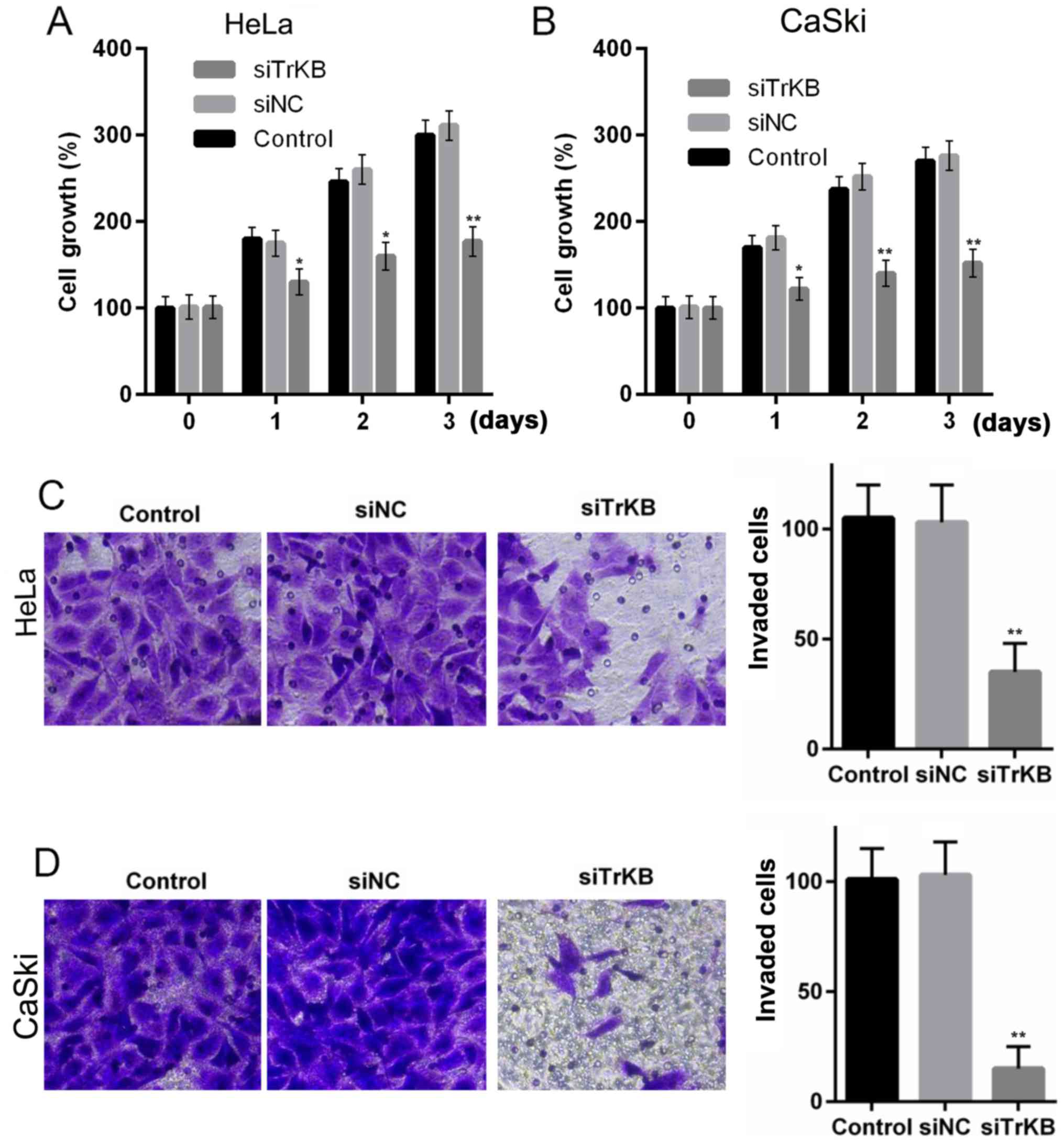
The proliferative, migrative and invasive ability of
the cervical cancer cell lines, HeLa and CaSki, were significantly
suppressed after the cells were transfected with TrKB siRNA. MTT
assay revealed that cell growth was inhibited after the cells were
transfected with TrKB siRNA compared to control and negative
siRNA-transfected cells. For the HeLa cells, the cell viability at
day 3 was ~3-fold of that at day 0 in both the control and negative
siRNA-transfected groups, while in the TrKB siRNA-transfected
group, it was only elevated ~0.7-fold at day 3 compared to day 0
(Fig. 4A). Similarly, for the
CaSki cells, the cell viability was enhanced ~1.5-fold at day 3
compared to that at day 0 in both the control and negative
siRNA-transfected groups, whereas it was only increased ~0.5-fold
at day 3 compared to day 0 (Fig.
4B).
Transwell invasion assay revealed that the invasive
ability was markedly suppressed in the cells in which TrKB was
knocked down compared with the control and negative
siRNA-transfected group. The numbers of invaded cells were ~100 for
both the HeLa and CaSki cells from the control and negative
siRNA-transfected group, whereas the numbers were ~25 and 10 for
the HeLa and CaSki cells from TrKB siRNA-transfected group,
respectively (Fig. 4C and D).
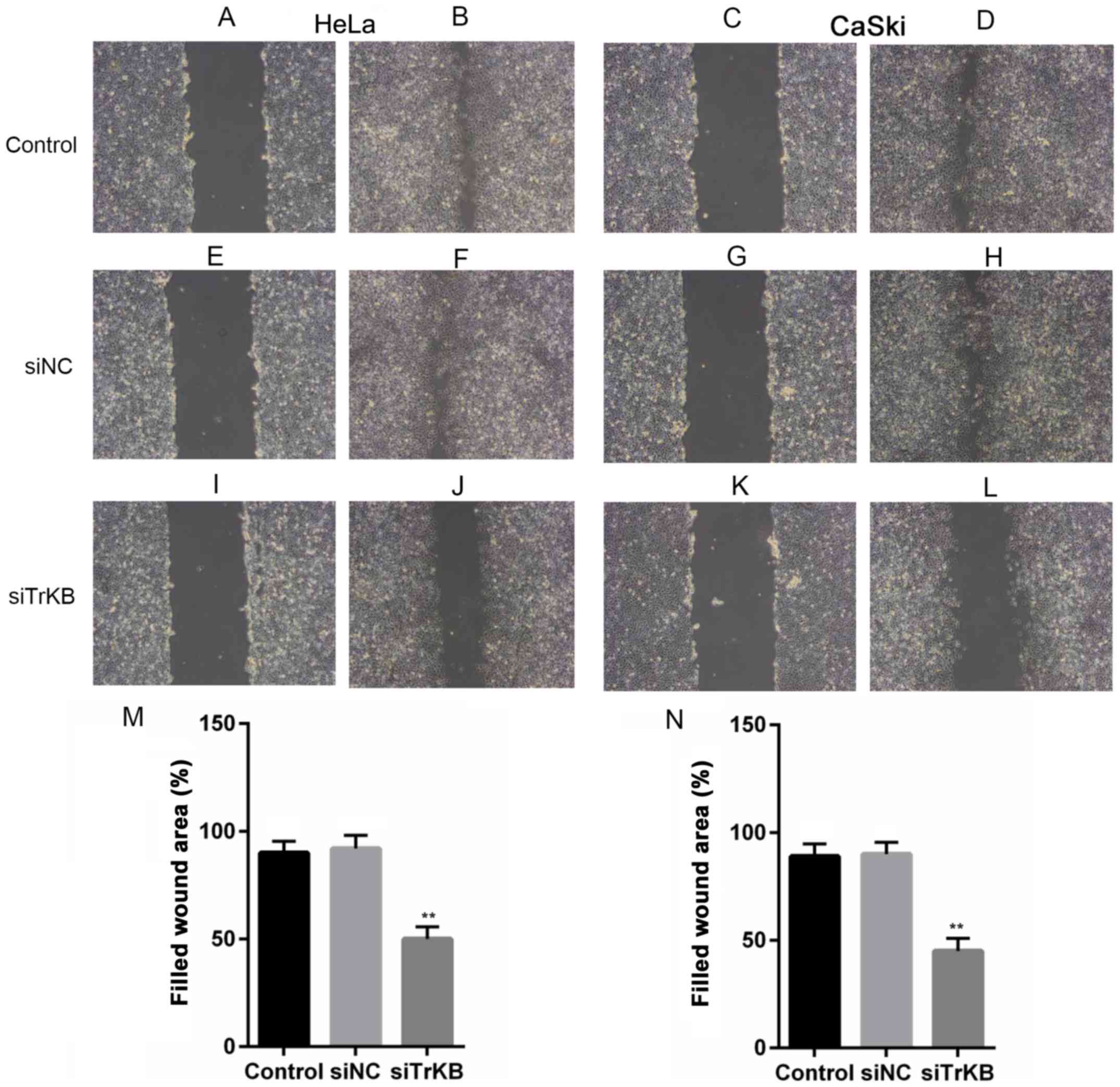
The scratch wound healing assay was performed to
examine the effects of the knockdown of TrKB on the proliferative
and migratory abilities of the cells. It was clearly revealed that
the wound healing ability of the cells from the control and
negative siRNA-transfected groups was significantly higher than
that in the cells transfected with TrKB siRNA. The wound areas were
filled ~90% in both the HeLa and CaSki cells from the control and
negative siRNA-transfected groups, while they were ~50 and 35% in
the HeLa and CaSki from the TrKB siRNA-transfected group,
respectively (Fig. 5).
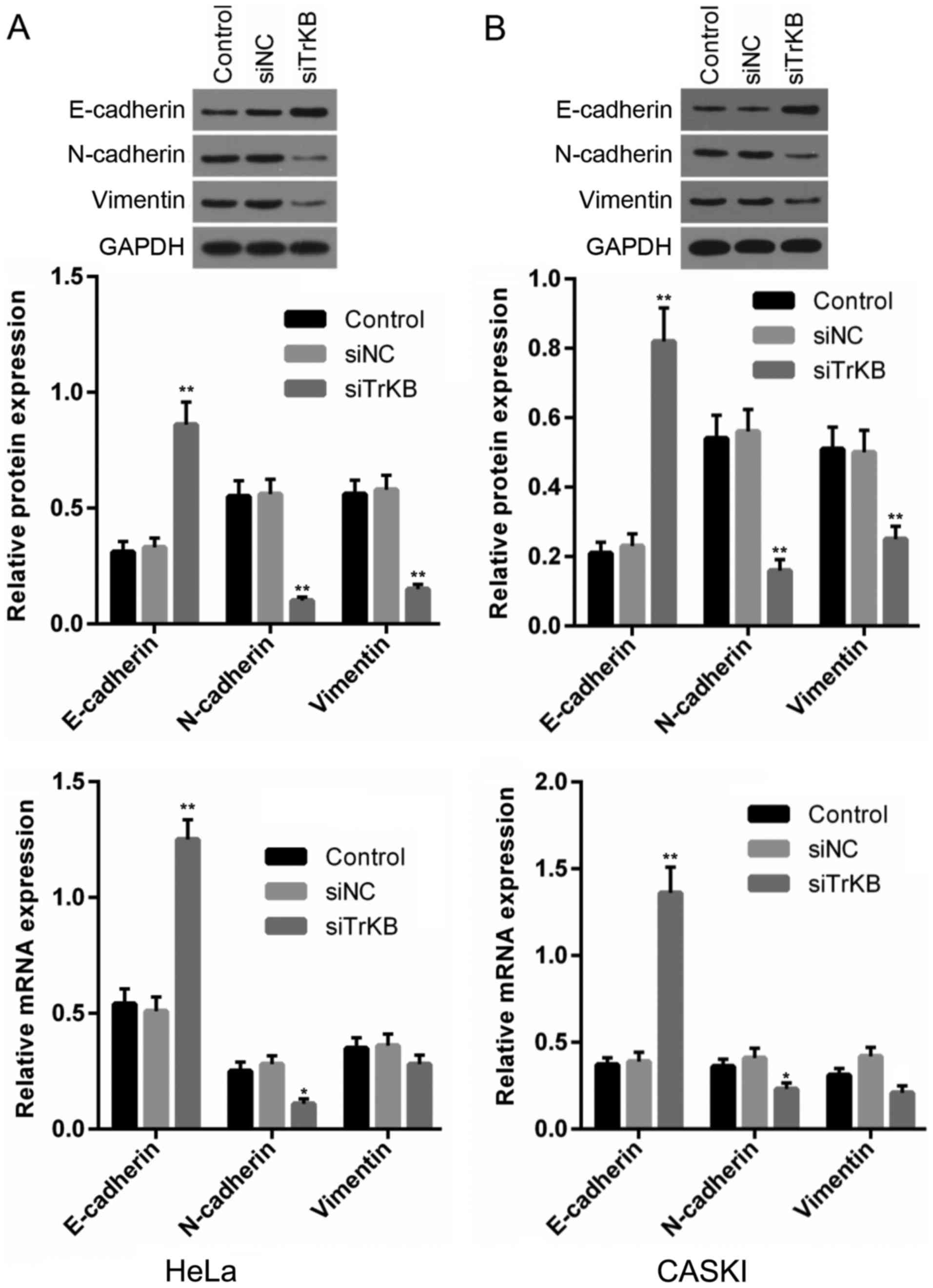
Silencing of TrKB expression inhibits the
expression of cell EMT-related proteins
EMT plays an important role in the invasion and
metastasis of tumor cells. We determined the effects of the
knockdown of TrKB on the expression of EMT-related proteins,
including E-cadherin, N-cadherin and vimentin. It was clearly shown
that in both the HeLa and CaSki cells, E-cadherin expression at
both the protein and mRNA levels was upregulated in the TrKB
siRNA-transfected group compared with that from the control and
negative siRNA-transfected groups (Fig. 6). By contrast, N-cadherin and
vimentin expression levels were downregulated in the TrKB
siRNA-transfected group compared with that from the control and
negative siRNA-transfected groups in both the HeLa and CaSki cells.
These results indicated that the silencing of TrKB inhibited the
migration and invasion of the HeLa and CaSki cells.
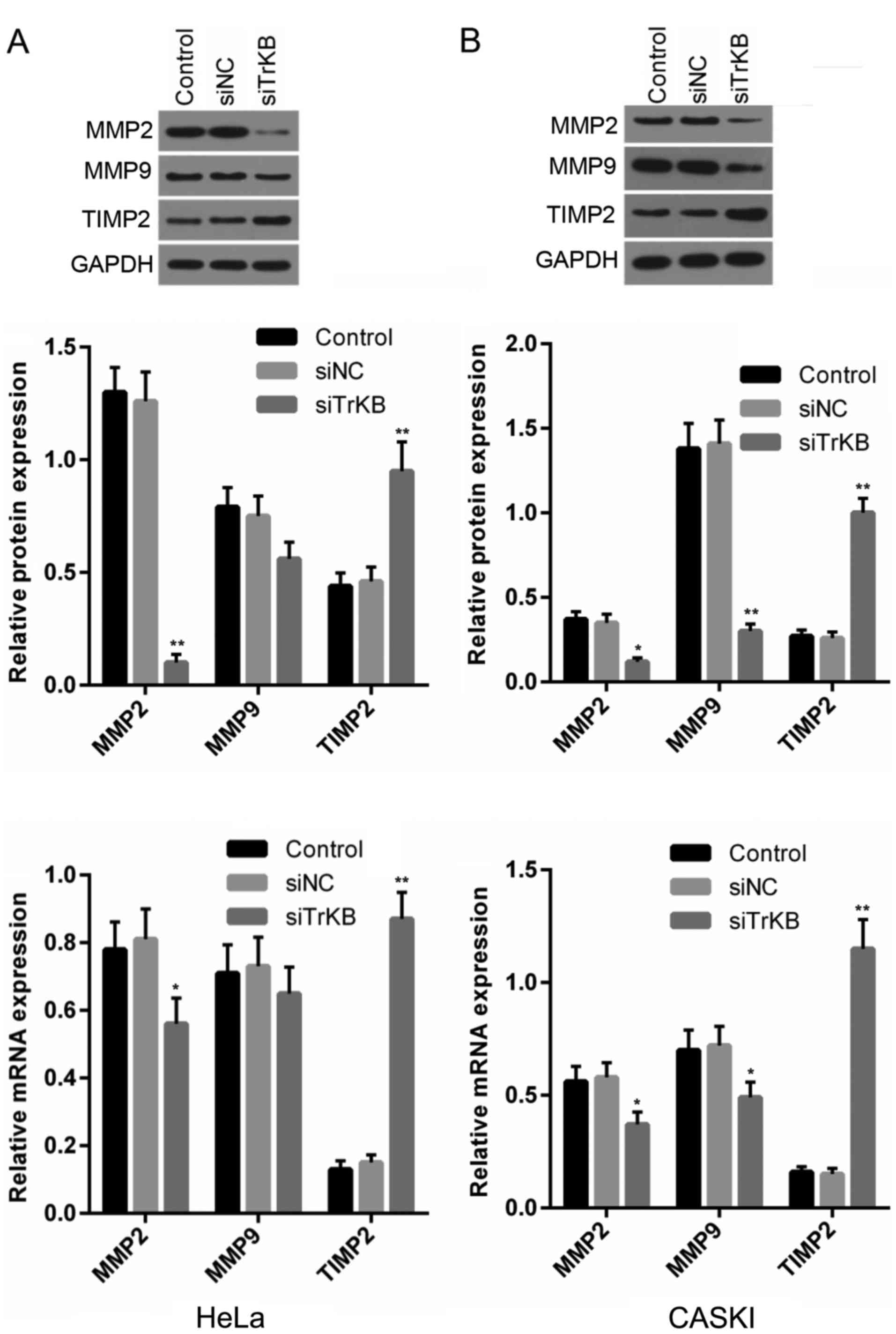
Downregulation of TrKB affects the
expression of proteins associated with invasion
Our above-mentioned experiments suggested that the
cell migratory and invasive abilities were suppressed in both the
HeLa and CaSki cells following the downregulation of TrKB. Hence,
we determined the changes in the expression of proteins associated
with invasion, such as MMP2, MMP9 and TMP2. As shown in Fig. 7, the protein expression of MMP2 was
markedly abrogated in both the HeLa and CaSki cells transfected
with TrKB siRNA compared with that in the control and negative
siRNA-transfected groups, while MMP2 mRNA expression was slightly
decreased in the cells transfected with TrKB siRNA compared with
that in the control and negative siRNA-transfected groups. No
significant changes were observed for MMP9 protein and mRNA
expression in the HeLa cells between all groups, while the MMP9
protein level was markedly downregulated in the cells transfected
with TrKB siRNA compared with the control and negative
siRNA-transfected groups in the CaSki cells. However, the protein
and mRNA expression levels of TMP2 were significantly elevated in
both the HeLa and CaSki cells transfected with TrKB siRNA compared
with that in the control and negative siRNA-transfected groups.
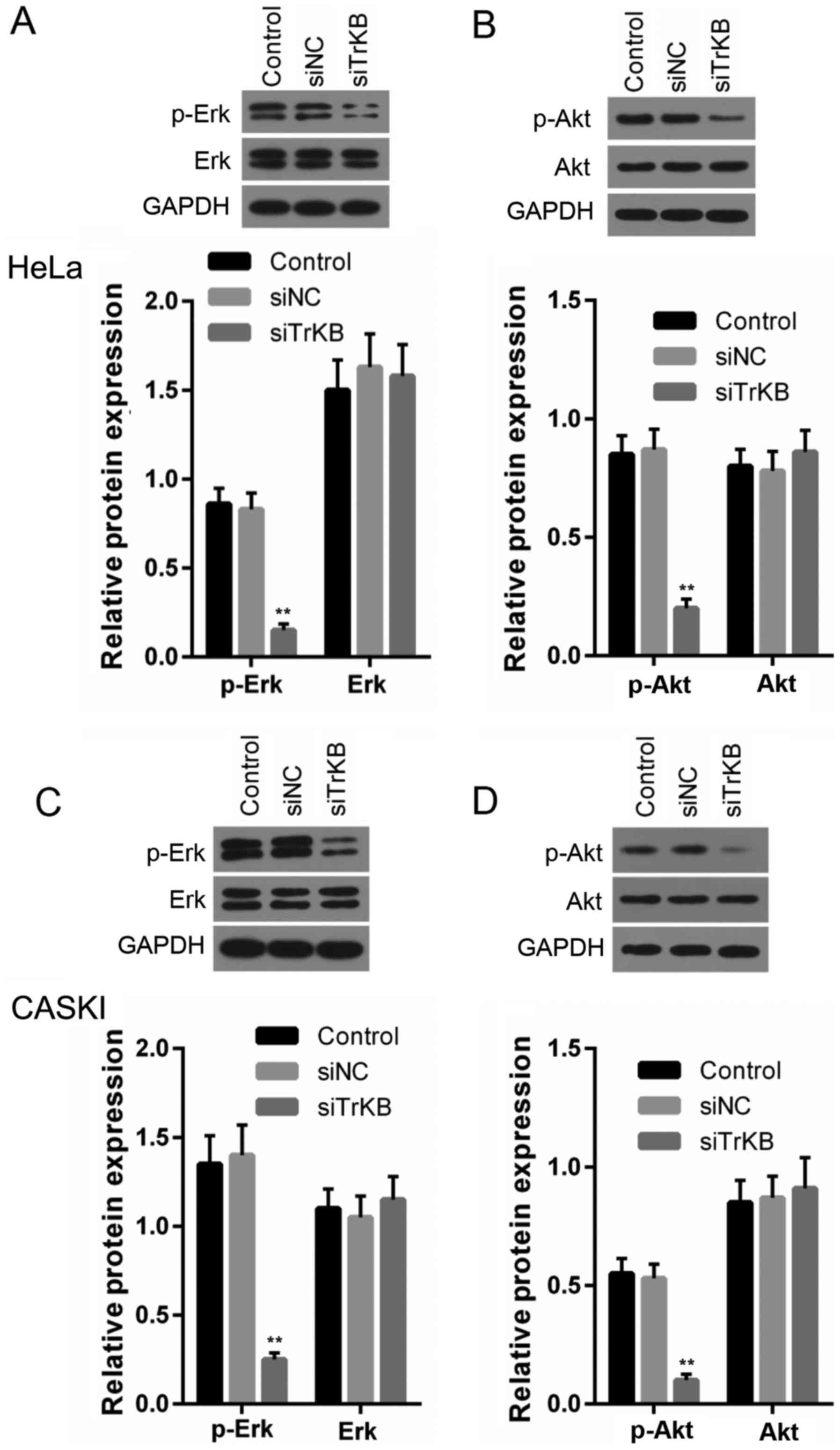
Silencing TrKB expression attenuates the
activation of the ERK and PI3K/AKT pathways
To explore the mechanisms responsible for the
inhibitory effects of the silencing of the expression of TrKB on
the cells, we examined the ERK, PI3K/AKT signaling pathways. As
shown in Fig. 8, the levels of
p-ERK, p-AKT in both the HeLa and CaSki cells were significantly
decreased in the cells transfected with TrKB siRNA compared to
those in the cells from the control and negative siRNA-transfected
groups.
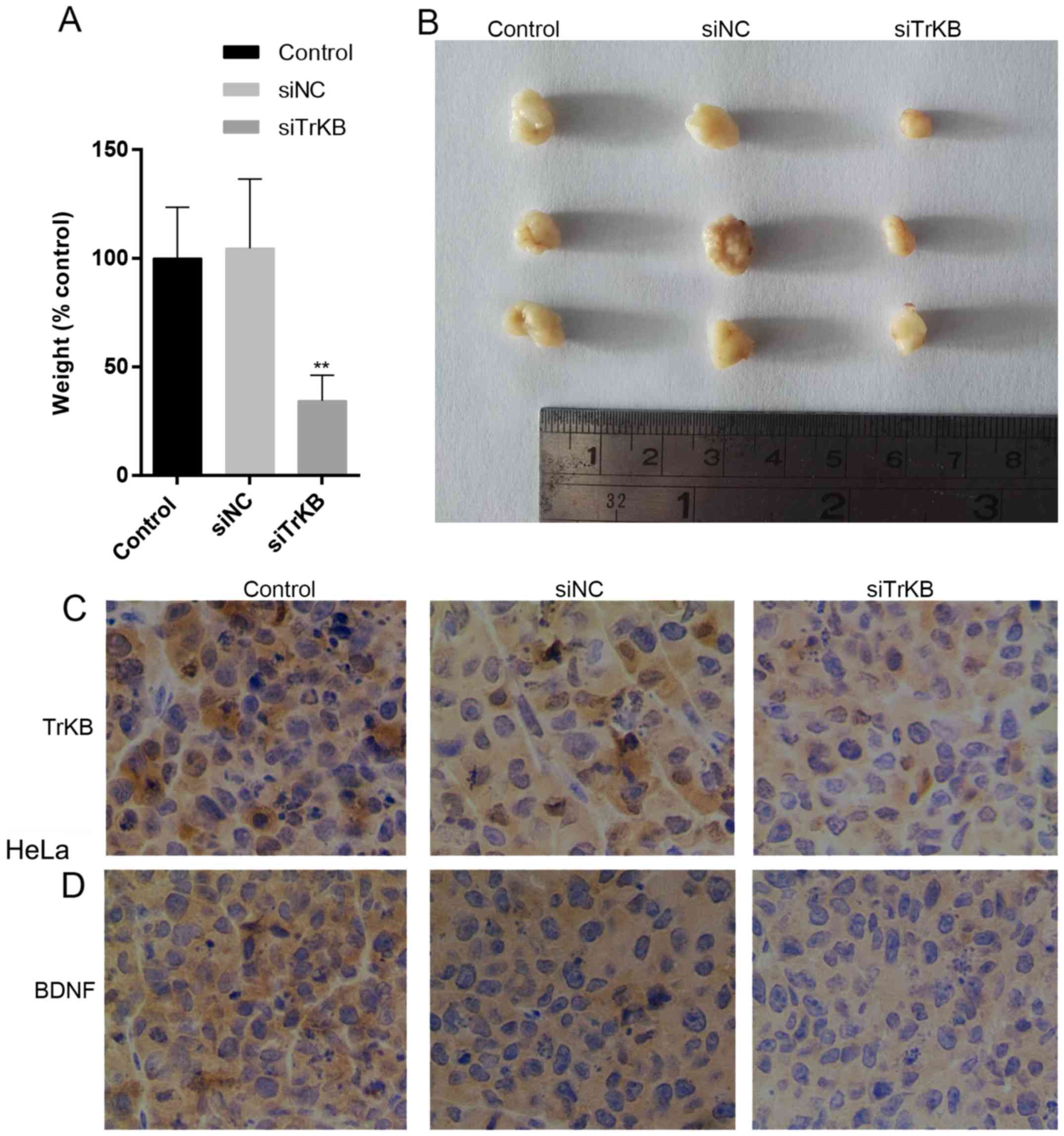
Downregulation of TrKB inhibits tumor
growth
To determine whether the knockdown of TrKB
expression affects the growth of tumors in nude mice, HeLa cells
from the control and negative siRNA-transfected groups were
injected into the nude mice and tumors were allowed to grow for 15
days. As shown in Fig. 9A and B,
the tumors derived from negative siRNA-transfected cells were
similar to those of the controls as regards size and weight, while
tumors derived from siTrKB-transfected cells were smaller and
weighed less. We further examined the expression levels of TrKB and
BDNF in these tumors by immunohistochemical analysis. The results
revealed that both TrKB and BDNF expression levels were
downregulated in tumors derived from siTrKB-transfected cells
compared to the levels in tumors developed by cells from the
control and negative siRNA-transfected groups (Fig. 9C and D). Taken together, these
results indicate that TrKB is essential for tumor growth.
Discussion
Previous studies have shown that a high expression
of BDNF/TrKB is closely associated with EMT and is linked to tumor
growth, invasion and metastasis (18,29).
To the best of our knowledge, the present study is the first to
demonstrate a strong association of a high BDNF/TrKB expression
with tumor size, grade of differentiation, positive lymph node
metastasis and a poor survival of patients with cervical cancer.
However, there was no significant association between TrKB and
clinicopathological parameters. Furthermore, a high BDNF/TrKB
expression enhanced cervical cancer cell growth, migration and
invasion via the promotion of EMT and the upregulation of MMP2 and
MMP9 expression. Our results suggest that BDNF/ TrKB may be
considered as novel biomarkers in cervical cancer. In addition, our
results are supported by those of previous studies in which the
BDNF/TrKB signaling pathway was shown to be essential for EMT, and
for the activation of the PI3K/AKT and Ras/MAPK cascades (30–32).
BDNF was initially known as a neurotrophic factor,
which is essential for maintaining the survival of neurons,
promoting their growth, differentiation and regeneration following
injury in the peripheral and central nervous system (7,33,34).
BDNF expression has been found in many non-union systems, such as
blood vessels (endothelial and smooth muscle cells), bone marrow
and heart, and an increased microvascular density has been observed
in tissues with an overexpression of BDNF (35–37).
In addition, studies have found that BDNF and its receptors are
closely associated with gastric cancer, lung cancer, hepatocellular
carcinoma, ovarian cancer and prostate cancer and other solid
tumors (9–12). Furthermore, BDNF is also known to
be involved in the regulation of endothelial cells and is linked to
angiogenesis (38,39). However, few studies have
investigated the role of BDNF in cervical cancer. In the present
study, a high positive (~60%) expression of BDNF was observed in
cervical cancer tissues. Our results also revealed that the protein
and mRNA expression levels of BDNF were markedly higher in cervical
cancer tissues than in normal tissues. Furthermore, a high
expression of BDNF was also found in several cervical cancer cell
lines.
TrkB is a highly glycosylated molecule consisting of
821 amino acid residues, and the extracellular domain consists of a
signal peptide consisting of 32 amino residues (40). The BDNF-specific receptor is TrkB,
and plays a biological effect mainly dependent on the binding of
the receptor TrkB, promoting of TrkB homodimer formation, and
activating of the receptor tyrosine kinase activity, which
increases the phosphorylation of tyrosine residues (41). Moreover, activated TrkB can
simulate the expression of a variety of proteins and enzymes,
thereby promoting the transcription of immediate and delayed
response genes or by being directly involved in a variety of
physiological responses (40,41).
Additionally, BDNF/ TrkB is not only expressed in multiple myeloma
and neuroblastoma (42), but also
in non-neurogenic tumors, such as lung cancer, liver cancer,
pancreatic cancer, nephroblastoma and even in virus-transformed B
lymphocytes (9–12,43,44).
In particular, the positive expression of BDNF/TrkB may be closely
related to the development and progression of ovarian cancer, which
may promote the development of ovarian epithelial carcinoma
(11,45). In the present study, a high
positive expression of TrKB, as of BDNF, in cervical cancer tissues
and cell lines was observed compared to the normal tissues and
cells, respectively. Furthermore, patients with a positive
expression of both BDNF and TrKB exhibited a poorer prognosis than
those with either BDNF- or TrKB-positive expression alone and with
a negative expression of both. These results can be partially
confirmed by two previous studies (26,27).
It has been found that an autocrine loop exists between TrkB and
BDNF in neuroblastoma and myeloma, in which a high expression of
BDNF induces the increased expression of TrkB (46,47).
Notably, we found that the silencing of TrKB induced the
downregulation of BDNF. Hence, we hypothesized that there other
mechanisms involved and these warrant further investigation.
E-cadherin is linked to the morphogenesis of
epithelial cells, and to the maintenance of the integrity of
epithelial tissues (48,49). A decreased E-cadherin expression is
associated with the invasion and metastasis of tumors, including
gastric, breast and pancreatic endocrine cancer (48–52).
Recent experiments have indicated that the activation of BDNF/TrkB
can lead to the occurrence of EMT (30). In addition, it has been reported
that a high expression of BDNF/TrkB leads to EMT in head and neck
squamous carcinoma cell lines (31), and is associated with EMT in
colorectal cancer cells, leading to enhanced tumor metastasis
(32). In agreement with these
findings, our results suggested that the downregulated expression
of E-cadherin was an important stimulus of EMT, which was mediated
by the activation of the BDNF/TrkB pathway in cervical cancer cell
lines. We found that a high expression of N-cadherin and vimentin
was closely associated with the activation of the BDNF/TrkB
pathway. N-cadherin and vimentin have been implicated in the
process EMT, resulting in phenotypic changes in many cancer cells
(53,54). In the present study, the silencing
of TrKB induced a decrease in both N-cadherin and vimentin, which
supported the stimulatory effect of BDNF/TrkB on EMT observed in
other studies (30,31,53,54).
We further examined the effects of the knockdown of TrKB by siRNA
on the proliferation, migration and invasion of cervical cancer
cell lines. Our results revealed that the proliferation, migration
and invasion of the cervical cancer cell lines, HeLa and CaSki,
were suppressed after the cells were transfected with TrKB siRNA.
MMP2 and MMP9 are members of the MMP family and have been
implicated in the invasion and metastasis of cancer cells (55). In this study, the decreased
expression of MMP2 and MMP9 supported the suppressed proliferation,
migration and invasion of the cervical cancer cell lines (MMP9 was
not significantly affected in the HeLa cells and CaSki cells)
transfected with TrKB siRNA. TIMP2, as previously demonstrated
(56), was related to inhibition
of cell migration and invasion, and its expression was increased in
cells in which TrKB was knocked down. These results suggest that
BDNF/TrkB exerts a potent simulatory effect on cell growth,
migration and invasion via the regulation of E-cadherin,
N-cadherin, vimentin, MMP2, MMP9 and TIMP2.
Recent studies have indicated that when TrkB binds
to its ligand, BDNF, multiple signaling cascades can be stimulated,
including the ERK and PI3K/AKT pathways (57–59).
In addition, the TrkB/BDNF signaling pathway induces EMT and
enhances cell invasion and metastasis by regulating downstream
genes, such as E-cadherin, N-cadherin and vimentin (18,19).
Therefore, in this study we examined whether the TrkB/ BDNF
signaling pathway is linked to the ERK and PI3K/AKT pathways,
thereby inducing cell proliferation, migration and invasion. Our
results revealed that high levels of phosphorylated ERK were
observed in the HeLa and CaSki cells. We found that the
phosphorylation of ERK was evidently decreased after the cells were
transfected with TrKB siRNA. Moreover, the phosphorylation of
PI3K/AKT was also observed to be markedly decreased after the
silencing of TrKB in the HeLa and CaSki cells. Furthermore, the
decrease in TrkB expression affected the expression of EMT-related
transcription factors, such as E-cadherin, N-cadherin and vimentin.
Additionally, the expression levels of genes associated with cell
invasion, such as MMP2, MMP9 and TIMP2 were also affected by the
silencing of TrKB in the cells. These results suggest that the
downregulation of TrkB/BDNF signaling suppresses cell metastasis,
proliferation and invasion by reducing downstream gene expression
via the inhibition of the ERK and PI3K/AKT pathways.
In conclusion, we identified two new molecular
biomarkers, TrkB/BDNF, present in cervical cancer that regulate
cell proliferation, invasion and metastasis. Furthermore, we
demonstrate that the TrkB/BDNF pathway may be a potential
therapeutic candidate for the targeted therapy of cervical
cancer.
References
|
1
|
Singhal P, Hussain S, Thakur N, Batra S,
Salhan S, Bhambani S and Bharadwaj M: Association of MDM2 and p53
polymorphisms with the advancement of cervical carcinoma. DNA Cell
Biol. 32:19–27. 2013. View Article : Google Scholar
|
|
2
|
Fukushima K, Ogawa S, Tsukimori K,
Kobayashi H and Wake N: Can we diagnose invasive cervical cancer
during pregnancy as precise as in nonpregnant women? Maternal and
perinatal outcome in pregnancies complicated with cervical cancers.
Int J Gynecol Cancer. 19:1439–1445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qu J, Lu W, Li B, Lu C and Wan X: WWOX
induces apoptosis and inhibits proliferation in cervical cancer and
cell lines. Int J Mol Med. 31:1139–1147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao Y, Shen L, Chen X, Qian Y, Zhou Q,
Wang Y, Li K, Liu M, Zhang S and Huang X: High expression of PKM2
as a poor prognosis indicator is associated with radiation
resistance in cervical cancer. Histol Histopathol. 30:1313–1320.
2015.PubMed/NCBI
|
|
5
|
Okugawa Y, Tanaka K, Inoue Y, Kawamura M,
Kawamoto A, Hiro J, Saigusa S, Toiyama Y, Ohi M, Uchida K, et al:
Brain-derived neurotrophic factor/tropomyosin-related kinase B
pathway in gastric cancer. Br J Cancer. 108:121–130. 2013.
View Article : Google Scholar :
|
|
6
|
Tanaka K, Okugawa Y, Toiyama Y, Inoue Y,
Saigusa S, Kawamura M, Araki T, Uchida K, Mohri Y and Kusunoki M:
Brain-derived neurotrophic factor (BDNF)-induced
tropomyosin-related kinase B (Trk B) signaling is a potential
therapeutic target for peritoneal carcinomatosis arising from
colorectal cancer. PLoS One 9: e96410. 2014
|
|
7
|
Kelly-Spratt KS, Klesse LJ and Parada LF:
BDNF activated TrkB/IRR receptor chimera promotes survival of
sympathetic neurons through Ras and PI-3 kinase signaling. J
Neurosci Res. 69:151–159. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kimura A, Namekata K, Guo X, Harada C and
Harada T: Neuroprotection, growth factors and BDNF-TrkB signalling
in retinal degeneration. Int J Mol Sci. 17:15842016. View Article : Google Scholar :
|
|
9
|
Okamura K, Harada T, Wang S, Ijichi K,
Furuyama K, Koga T, Okamoto T, Takayama K, Yano T and Nakanishi Y:
Expression of TrkB and BDNF is associated with poor prognosis in
non-small cell lung cancer. Lung Cancer. 78:100–106. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi B, Lee EJ, Shin MK, Park YS, Ryu MH,
Kim SM, Kim EY, Lee HK and Chang EJ: Upregulation of brain-derived
neurotrophic factor in advanced gastric cancer contributes to bone
metastatic osteolysis by inducing long pentraxin 3. Oncotarget.
7:55506–55517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Au CW, Siu MK, Liao X, Wong ES, Ngan HY,
Tam KF, Chan DC, Chan QK and Cheung AN: Tyrosine kinase B receptor
and BDNF expression in ovarian cancers - Effect on cell migration,
angiogenesis and clinical outcome. Cancer Lett. 281:151–161. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bronzetti E, Artico M, Forte F,
Pagliarella G, Felici LM, D'Ambrosio A, Vespasiani G and Bronzetti
B: A possible role of BDNF in prostate cancer detection. Oncol Rep.
19:969–974. 2008.PubMed/NCBI
|
|
13
|
Huang YT, Lai PC, Wu CC, Hsu SH, Cheng CC,
Lan YF and Chiu TH: BDNF mediated TrkB activation is a survival
signal for transitional cell carcinoma cells. Int J Oncol.
36:1469–1476. 2010.PubMed/NCBI
|
|
14
|
Xiang J, Pan J, Chen F, Zheng L, Chen Y,
Zhang S and Feng W: L-3-n-butylphthalide improves cognitive
impairment of APP/ PS1 mice by BDNF/TrkB/PI3K/AKT pathway. Int J
Clin Exp Med. 7:1706–1713. 2014.
|
|
15
|
Taube JH, Herschkowitz JI, Komurov K, Zhou
AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et
al: Core epithelial-to-mesenchymal transition interactome
gene-expression signature is associated with claudin-low and
metaplastic breast cancer subtypes. Proc Natl Acad Sci USA.
107:15449–15454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang CC, Zhu LF, Xu XH, Ning TY, Ye JH and
Liu LK: Membrane type 1 matrix metalloproteinase induces an
epithelial to mesenchymal transition and cancer stem cell-like
properties in SCC9 cells. BMC Cancer. 13:1712013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cufí S, Vazquez-Martin A,
Oliveras-Ferraros C, Martin-Castillo B, Joven J and Menendez JA:
Metformin against TGFβ-induced epithelial-to-mesenchymal transition
(EMT): From cancer stem cells to aging-associated fibrosis. Cell
Cycle. 9:4461–4468. 2010. View Article : Google Scholar
|
|
18
|
Bao W, Qiu H, Yang T, Luo X, Zhang H and
Wan X: Upregulation of TrkB promotes epithelial-mesenchymal
transition and anoikis resistance in endometrial carcinoma. PLoS
One. 8:e706162013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ricci A, De Vitis C, Noto A, Fattore L,
Mariotta S, Cherubini E, Roscilli G, Liguori G, Scognamiglio G,
Rocco G, et al: TrkB is responsible for EMT transition in malignant
pleural effusions derived cultures from adenocarcinoma of the lung.
Cell Cycle. 12:1696–1703. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Zhang Y, Liu S, Zhang Q, Wang Y,
Tong L, Chen X, Ji Y, Shang Q, Xu B, et al: Metadherin confers
chemoresistance of cervical cancer cells by inducing autophagy and
activating ERK/NF-κB pathway. Tumour Biol. 34:2433–2440. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ming P, Cai T, Li J, Ning Y, Xie S, Tao T
and Tang F: A novel arylbenzofuran induces cervical cancer cell
apoptosis and G1/S arrest through ERK-mediated Cdk2/cyclin-A
signaling pathway. Oncotarget. 7:41843–41856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Q, Liang Y, Zhang T, Wang K and Yang
X: ER-α36 mediates estrogen-stimulated MAPK/ERK activation and
regulates migration, invasion, proliferation in cervical cancer
cells. Biochem Biophys Res Commun. 487:625–632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Im SR and Jang YJ: Aspirin enhances
TRAIL-induced apoptosis via regulation of ERK1/2 activation in
human cervical cancer cells. Biochem Biophys Res Commun. 424:65–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Yang Z, Song W, Zhou L, Li Q, Tao K,
Zhou J, Wang X, Zheng Z, You N, et al: Overexpression of Bmi-1
contributes to the invasion and metastasis of hepatocellular
carcinoma by increasing the expression of matrix metalloproteinase
(MMP)-2, MMP-9 and vascular endothelial growth factor via the
PTEN/I3K/Akt pathway. Int J Oncol. 43:793–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li B, Tsao SW, Li YY, Wang X, Ling MT,
Wong YC, He QY and Cheung ALM: Id-1 promotes tumorigenicity and
metastasis of human esophageal cancer cells through activation of
PI3K/AKT signaling pathway. Int J Cancer. 125:2576–2585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cornelio DB, DE Farias CB, Prusch DS,
Heinen TE, Dos Santos RP, Abujamra AL, Schwartsmann G and Roesler
R: Influence of GRPR and BDNF/TrkB signaling on the viability of
breast and gynecologic cancer cells. Mol Clin Oncol. 1:148–152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moon A, Won KY, Lee JY, Kang I, Lee SK and
Lee J: Expression of BDNF, TrkB, and p53 in early-stage squamous
cell carcinoma of the uterine cervix. Pathology. 43:453–458. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Benedet JL, Bender H, Jones H III and Ngan
HY: FIGO staging classifications and clinical practice guidelines
in the management of gynecologic cancers. Int J Gynaecol Obstet.
70:209–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alonso-Alconada L, Eritja N, Muinelo-Romay
L, Barbazan J, Lopez-Lopez R, Matias-Guiu X, Gil-Moreno A, Dolcet X
and Abal M: ETV5 transcription program links BDNF and promotion of
EMT at invasive front of endometrial carcinomas. Carcinogenesis.
35:2679–2686. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia S, Wang W, Hu Z, Shan C, Wang L, Wu B,
Yang Z, Yang X and Lei D: BDNF mediated TrkB activation contributes
to the EMT progression and the poor prognosis in human salivary
adenoid cystic carcinoma. Oral Oncol. 51:64–70. 2015. View Article : Google Scholar
|
|
31
|
Lee J, Jiffar T and Kupferman ME: A novel
role for BDNF-TrkB in the regulation of chemotherapy resistance in
head and neck squamous cell carcinoma. PLoS One. 7:e302462012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujikawa H, Tanaka K, Toiyama Y, Saigusa
S, Inoue Y, Uchida K and Kusunoki M: High TrkB expression levels
are associated with poor prognosis and EMT induction in colorectal
cancer cells. J Gastroenterol. 47:775–784. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wong J: Neurotrophin Signaling and
Alzheimer's Disease Neurodegeneration - Focus on BDNF/TrkB
Signaling, Trends in Cell Signaling Pathways in Neuronal Fate
Decision. Wislet-Gendebien Dr Sabine: InTech. 2013, View Article : Google Scholar : Available from:
https://www.intechopen.com/books/trends-in-cell-signaling-pathways-in-neuronal-fate-decision/neurotrophin-signaling-and-alzheimer-s-disease-neurodegeneration-focus-on-bdnf-trkb-signaling.
|
|
34
|
Eberhardt KA, Irintchev A, Al-Majed AA,
Simova O, Brushart TM, Gordon T and Schachner M: BDNF/TrkB
signaling regulates HNK-1 carbohydrate expression in regenerating
motor nerves and promotes functional recovery after peripheral
nerve repair. Exp Neurol. 198:500–510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Berner A, Reichert JC, Müller MB, Zellner
J, Pfeifer C, Dienstknecht T, Nerlich M, Sommerville S, Dickinson
IC, Schütz MA, et al: Treatment of long bone defects and
non-unions: From research to clinical practice. Cell Tissue Res.
347:501–519. 2012. View Article : Google Scholar
|
|
36
|
Chen J, Li Y and Chopp M: Intracerebral
transplantation of bone marrow with BDNF after MCAo in rat.
Neuropharmacology. 39:711–716. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fulgenzi G, Tomassoni-Ardori F, Babini L,
Becker J, Barrick C, Puverel S and Tessarollo L: BDNF modulates
heart contraction force and long-term homeostasis through truncated
TrkBT1 receptor activation. J Cell Biol. 210:1003–1012. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pouneh Kermani BH: BDNF: A newly described
mediator of angiogenesis. Trends Cardiovasc Med. 17:1402007.
View Article : Google Scholar
|
|
39
|
Hong JH, Park HM, Byun KH, Lee BH, Kang WC
and Jeong GB: BDNF expression of macrophages and angiogenesis after
myocardial infarction. Int J Cardiol. 176:1405–1408. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jerónimo-Santos A, Vaz SH, Parreira S,
Rapaz-Lérias S, Caetano AP, Buée-Scherrer V, Castrén E, Valente CA,
Blum D, Sebastião AM, et al: Dysregulation of TrkB receptors and
BDNF function by amyloid-β peptide is mediated by calpain. Cereb
Cortex. 25:3107–3121. 2015. View Article : Google Scholar
|
|
41
|
Feng N, Huke S, Zhu G, Tocchetti CG, Shi
S, Aiba T, Kaludercic N, Hoover DB, Beck SE, Mankowski JL, et al:
Constitutive BDNF/ TrkB signaling is required for normal cardiac
contraction and relaxation. Proc Natl Acad Sci USA. 112:1880–1885.
2015. View Article : Google Scholar
|
|
42
|
Brodeur GM, Nakagawara A, Yamashiro DJ,
Ikegaki N, Liu XG, Azar CG, Lee CP and Evans AE: Expression of
TrkA, TrkB and TrkC in human neuroblastomas. J Neurooncol.
31:49–55. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eggert A, Grotzer MA, Zhao H, Brodeur GM
and Evans AE: Expression of the neurotrophin-receptor TrkB predicts
outcome in nephroblastomas: Results of a pilot-study. Klin Padiat .
213:191–196. 2001.In German.
|
|
44
|
Wang HY, Crupi D, Liu J, Stucky A,
Cruciata G, Di Rocco A, Friedman E, Quartarone A and Ghilardi MF:
Repetitive transcranial magnetic stimulation enhances BDNF-TrkB
signaling in both brain and lymphocyte. J Neurosci. 31:11044–11054.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Siu MK, Wong OG and Cheung AN: TrkB as a
therapeutic target for ovarian cancer. Expert Opin Ther Targets.
13:1169–1178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang ZF, Ho DW, Lau CK, Tam KH, Lam CT,
Poon RTP and Fan ST: Platelet activation during tumor development,
the potential role of BDNF-TrkB autocrine loop. Biochem Biophys Res
Commun. 346:981–985. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Polakowski N, Terol M, Nash I, Hoang K,
Gazon H, Wurm T, Césaire R, Péloponèse JM, Mesnard JM and Lemasson
I: A BDNF/TrKB autocrine loop stimulated by HBZ is involved in
HTLV-1-infected cell-survival. Retrovirology. 11 (Suppl 1):
O572014. View Article : Google Scholar :
|
|
48
|
Bondow BJ, Faber ML, Wojta KJ, Walker EM
and Battle MA: E-cadherin is required for intestinal morphogenesis
in the mouse. Dev Biol. 371:1–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fournier MV, Fata JE, Martin KJ, Yaswen P
and Bissell MJ: Interaction of E-cadherin and PTEN regulates
morphogenesis and growth arrest in human mammary epithelial cells.
Cancer Res. 69:4545–4552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tamura G, Yin J, Wang S, Fleisher AS, Zou
T, Abraham JM, Kong D, Smolinski KN, Wilson KT, James SP, et al:
E-Cadherin gene promoter hypermethylation in primary human gastric
carcinomas. J Natl Cancer Inst. 92:569–573. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Eger A, Aigner K, Sonderegger S, Dampier
B, Oehler S, Schreiber M, Berx G, Cano A, Beug H and Foisner R:
DeltaEF1 is a transcriptional repressor of E-cadherin and regulates
epithelial plasticity in breast cancer cells. Oncogene.
24:2375–2385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chetty RI, Serra S and Asa SL: Loss of
membrane localization and aberrant nuclear E-cadherin expression
correlates with invasion in pancreatic endocrine tumors. Am J Surg
Pathol. 32:413–419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang X, Liu G, Kang Y, Dong Z, Qian Q and
Ma X: N-cadherin expression is associated with acquisition of EMT
phenotype and with enhanced invasion in erlotinib-resistant lung
cancer cell lines. PLoS One. 8:e576922013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nakajima S, Doi R, Toyoda E, Tsuji S, Wada
M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, et al: N-cadherin
expression and epithelial-mesenchymal transition in pancreatic
carcinoma. Clin Cancer Res. 10:4125–4133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kumar B, Koul S, Petersen J, Khandrika L,
Hwa JS, Meacham RB, Wilson S and Koul HK: 38mitogen-activated
protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer
by modulation of MMP-2 and MMP-9 activity. Cancer Res. 70:832–841.
2010. View Article : Google Scholar
|
|
56
|
Lu KV, Jong KA, Rajasekaran AK, Cloughesy
TF and Mischel PS: Upregulation of tissue inhibitor of
metalloproteinases (TIMP)-2 promotes matrix metalloproteinase
(MMP)-2 activation and cell invasion in a human glioblastoma cell
line. Lab Invest. 84:8–20. 2004. View Article : Google Scholar
|
|
57
|
Kumamaru E, Numakawa T, Adachi N and
Kunugi H: Glucocorticoid suppresses BDNF-stimulated MAPK/ERK
pathway via inhibiting interaction of Shp2 with TrkB. FEBS Lett.
585:3224–3228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Revest JM, Le Roux A, Roullot-Lacarrière
V, Kaouane N, Vallée M, Kasanetz F, Rougé-Pont F, Tronche F,
Desmedt A and Piazza PV: BDNF-TrkB signaling through Erk1/2 MAPK
phosphorylation mediates the enhancement of fear memory induced by
glucocorticoids. Mol Psychiatry. 19:1001–1009. 2014. View Article : Google Scholar
|
|
59
|
Yao RQ, Qi DS, Yu HL, Liu J, Yang LH and
Wu XX: Quercetin attenuates cell apoptosis in focal cerebral
ischemia rat brain via activation of BDNF-TrkB-I3K/Akt signaling
pathway. Neurochem Res. 37:2777–2786. 2012. View Article : Google Scholar : PubMed/NCBI
|