Introduction
Colorectal cancer (CRC) is one of the most commonly
diagnosed types of cancer in both males and females worldwide
(1,2). While the surgical resection of
localized disease may be curative, mortality is primarily caused by
metastatic progression, even with therapeutic regimens and the use
of targeted therapies (3,4). Anoikis is an inherent cellular
mechanism that 'cleans out' the detached epithelial cells in order
to maintain tissue homeostasis and development (5–7).
Cancer cells which survive during detachment from the extracellular
matrix (ECM) and acquire metastatic potential have developed
mechanisms with which resist anoikis (8,9). The
breakdown of anoikis contributes to the development of many types
of cancer, predominantly mammary and colon cancers (10,11).
Therefore, enhancing the response of cancer cells to anoikis is
considered to be an effective strategy for the treatment of
metastatic colorectal cancer.
Marine fungi are an important source of structurally
unique and biologically active natural products (12,13).
In recent years, research has been conducted on the metabolites of
marine fungi and obtained the optimized cultivation parameters with
which to isolate and identify a series of novel and/or bioactive
metabolites (14–17). Gliotoxin and its analogues, the
diketopiperazines of various fungal species, e.g., Aspergrillus
fumigatus, Eurotium chevalieri, Trichoderma virens, Neosartorya
pseudofischeri, and some Prenicillium and
Acremonium species, are known to be immunosuppressive agents
(18) and have also been reported
to have anticancer properties (19–23).
Furthermore, reduced-gliotoxin has previously been shown to possess
the most potent cytotoxic effect compared to other analogues in CRC
cells (14). However, the detailed
effects and molecular mechanisms responsible for the death of CRC
cells induced by reduced-gliotoxin are unclear.
In this study, we examined the potency and of
reduced-gliotoxin against CRC cells and also aimed to elucidate the
underlying mechanisms. We demonstrated that reduced-gliotoxin
triggered rapid cell detachment and induced the apoptosis of CRC
cells. Mechanistically, our data indicated that the anoikis induced
by reduced-gliotoxin was associated with the dysregulation of
multiple signaling pathways and the disruption of
integrin-associated detachment. Furthermore, excessive reactive
oxygen species (ROS) production was induced by reduced-gliotoxin,
resulting in the activation of both endogenous and exogenous
apoptotic pathways, and ultimately culminating in the apoptosis of
CRC cells. The blockade of ROS generation with N-acetylcysteine
(NAC) attenuated the activation of several protein kinase signaling
pathways and cell apoptosis that was induced by reduced-gliotoxin.
Taken together, our findings suggest that reduced-gliotoxin may
prove be a potential candidate for the treatment of CRC.
Materials and methods
Reagents details and use
Reduced-gliotoxin was isolated from the secondary
metabolites of the marine fungus, Neosartorya
pseudofischeri, following the procedure described previously
(14). The purity of
reduced-gliotoxin was >98% as determined by high-performance
liquid chromatography (HPLC). Reduced-gliotoxin was dissolved in
dimethyl sulfoxide (DMSO) to a 50 mM stock solution and stored in a
dark area at −20°C. LY294002, MK-2206 and Z-IETD-FMK (Selleck
Chemicals, Houston, TX, USA) were dissolved in DMSO to a 50 mM, 10
mM, 50 mM solution respectively, and stored at −20°C. The cells
were pre-treated with a concentration gradient of LY294002 (up to
50 µM) MK-2206 (up to 5 µM) or Z-IETD-FMK (up to 50
µM) for 2 h and co-treated with reduced-gliotoxin for 24 h.
NAC (Cat. no. 1009005) and diphenyleneiodonium chloride (DPI Cat.
no. D2926) (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in
DMSO to a 5 mM solution. The cells were pre-treated with a
concentration gradient of NAC (up to 5 mM) or DPI (up to 20 mM) for
2 h and followed by co-treatment with reduced-gliotoxin for 24 h.
Rabbit polyclonal anti-PARP (Cat. no. 9542), mouse monoclonal
anti-caspase-8 (Cat. no. 9746), rabbit monoclonal anti-caspase-9
(Cat. no. 9502), rabbit monoclonal anti-Akt (Cat. no. 4691), rabbit
monoclonal anti-phosphor-Akt (Ser473) (Cat. no. 4060), rabbit
monoclonal anti-integrinβ4 (Cat. no. 14803), rabbit monoclonal
anti-integrinβ1 (Cat. no. 9699), rabbit monoclonal anti-PUMA (Cat.
no. 12450), rabbit monoclonal anti-MCL1 (Cat. no. 5453), rabbit
monoclonal anti caspase-3 (Cat. no. 9665), rabbit monoclonal
anti-phosphor-β-catenin (Thr41/Ser45) (Cat. no. 9565) and rabbit
monoclonal anti-cleaved caspase-3 (Cat. no. 9661) antibodies were
purchased from Cell Signaling Technology (Cell Signaling
Technology, Beverly, MA, USA). Rabbit monoclonal anti-glycogen
synthase kinase (GSK)-3β (Cat. no. ab32391), rabbit monoclonal
anti-phosphor-GSK-3β (Ser9) (Cat. no. ab75814), rabbit monoclonal
anti-β-catenin (Cat. no. ab32572), and rabbit monoclonal
anti-cleaved caspase-9 (Cat. no. ab2324) antibodies were purchased
from Abcam (Abcam, Cambridge, MA, USA). Rabbit polyclonal
anti-GAPDH (Cat. no. 10494-1-AP), and mouse monoclonal anti-ACTB
(Cat. no. 60008-1-Ig) antibodies were purchased from Proteintech
Group (Proteintech Group, Chicago, IL, USA).
Colorectal cancer cell lines and cell
culture
The colorectal cancer cell lines, HCT116 and HT-29,
were purchased from the Culture Collection of Chinese Academy of
Science (Chinese Academy of Science, Shanghai, China). The cells
were cultured in RPMI-1640 medium (#11875500; Gibco/Thermo Fisher
Scientific, Waltham, MA USA) containing 10% fetal bovine serum
(#FBS-22A; Capricorn Scientific, Ebsdorfergrund, Germany) in a
humidified atmosphere of 5% CO2 at 37°C.
Apoptosis assay
The cell apoptotic rate was determined by flow
cytometry using an Annexin V-FITC/PI dual staining kit (Nanjing
KeyGen Biotech, Nanjing, Jiangsu, China), according to the
manufacturer's instructions. Briefly, the CRC cells were seeded in
6-well tissue culture plates, and were then treated with various
concentrations for the indicated periods of time. Cells present in
the supernatant cells were collected by centrifugation at 626 x g
for 10 min. The cells were counted (5×105) and washed
with PBS twice. The cells were then re-suspended in working
solution (100 µl binding buffer with 5 µl Annexin
V-FITC and 5 µl PI staining solution) for 10 min at room
temperature in the dark. Subsequently, 400 µl binding buffer
were added immediately prior to analysis using a flow cytometer (BD
Biosciences, San Jose, CA, USA). The resulting data were analyzed
using BD FACSDiva software, version 6.1.3 (BD Biosciences).
Western blot analysis
Western blot analysis was performed as previously
described (24) with minor
modifications. Briefly, total cell lysates were prepared in lysis
buffer containing both protease and phosphatase inhibitors (KeyGEN
Biotech). Protein concentrations were measured using a Bio-Rad
assay kit (Bio-Rad, Hercules, CA, USA). Total proteins were
separated on SDS-PAGE gel and transferred onto PVDF membranes
(Bio-Rad), and the membranes were then blocked with 5% skimmed milk
(BD Biosciences) in Tris-buffered saline with Tween-20 (TBS-T) and
incubated with primary antibodies diluted in primary antibody
solution I (Toyobo, Tokyo, Japan) overnight at 4°C. Anti-PARP,
caspase-8, caspase-9, phosphor-Akt (Ser473), integrinβ4,
integrinβ1, PUMA, MCL1, caspase-3 phosphor-β-catenin (Thr41/Ser45)
cleaved caspase-3, cleaved caspase-9 antibodies were diluted at
1:1,000. Anti-Akt, GSK-3β, phosphor-GSK-3β (Ser9) and β-catenin
antibodies were diluted at 1:2,000. Anti-GAPDH and ACTB antibodies
were diluted at 1:5,000. The following day, the membranes were
washed and incubated with HRP-conjugated goat anti-rabbit IgG (H+L)
(Cat. no. A16096; Invitrogen/Thermo Fisher Scientific) or goat
anti-mouse IgG (H+L) (Cat. no. A16066; Invitrogen/Thermo Fisher
Scientific) secondary antibody diluted at 1:5,000 in blocking
buffer at room temperature for 2 h, followed by ECL (Bio-Rad)
detection using a X-ray film or chemiluminescence equipment
(Bio-Rad). After the detection of protein bands, the membranes were
stripped and re-probed with anti-GAPDH or anti-ACTB antibodies to
confirm equal loading of the samples.
Mitochondrial membrane potential (MMP)
assay
MMP in the cells was detected using the MMP assay
kit with JC-1 (Beyotime, Shanghai, China). According to the
manufacturer's instructions, in brief, the cells were collected
after treatment suspended with 5 µg/ml JC-1 in serum-free
medium. The cells were then incubated at 37°C for 20 min. MMP was
detected by flow cytometry (green fluorescence for monomer form,
Ex/Em: 490/530 nm; red fluorescence for aggregator form, Ex/Em:
525/590 nm).
ROS assay
ROS levels in the cells were monitored using the
Reactive Oxygen Species assay kit (Beyotime). As instructed by the
manual provided by the manufacturer, the cells were treated with
reduced-gliotoxin for the indicated amounts of time, harvested and
suspended with 10 µM of DCFH-DA in a serum-free medium, and
then incubated with dye in 37°C for 20 min. The treated cells were
washed 3 times with serum-free medium and rinsed in PBS. ROS
intensity was measured at an excitation/emission wavelength of
488/525 nm using a flow cytometer (BD Biosciences).
Statistical analysis
All experiments were performed at least 3 times, and
the results are expressed as the means ± SD where applicable. All
data were analyzed by a two-tailed unpaired Student's t-test for
differences between 2 groups and by one-way ANOVA followed with
Fisher's LSD test for multiple comparisons. Statistical analysis
was performed using GraphPad Prism software (GraphPad Software, San
Diego, CA, USA). A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Reduced-gliotoxin triggers rapid cell
detachment and induces the death of CRC cells
It has previously been reported that gliotoxin
triggers rapid cell detachment in mouse embryonic fibroblasts and
BEAS-2B human lung bronchial epithelial cells (25). Moreover, we have previously
reported that gliotoxin induced the apoptosis of CRC cells
(20). Therefore, in this study,
we first examined whether reduced-gliotoxin, an analogue of
gliotoxin, induces the death of CRC cells via the same mechanism.
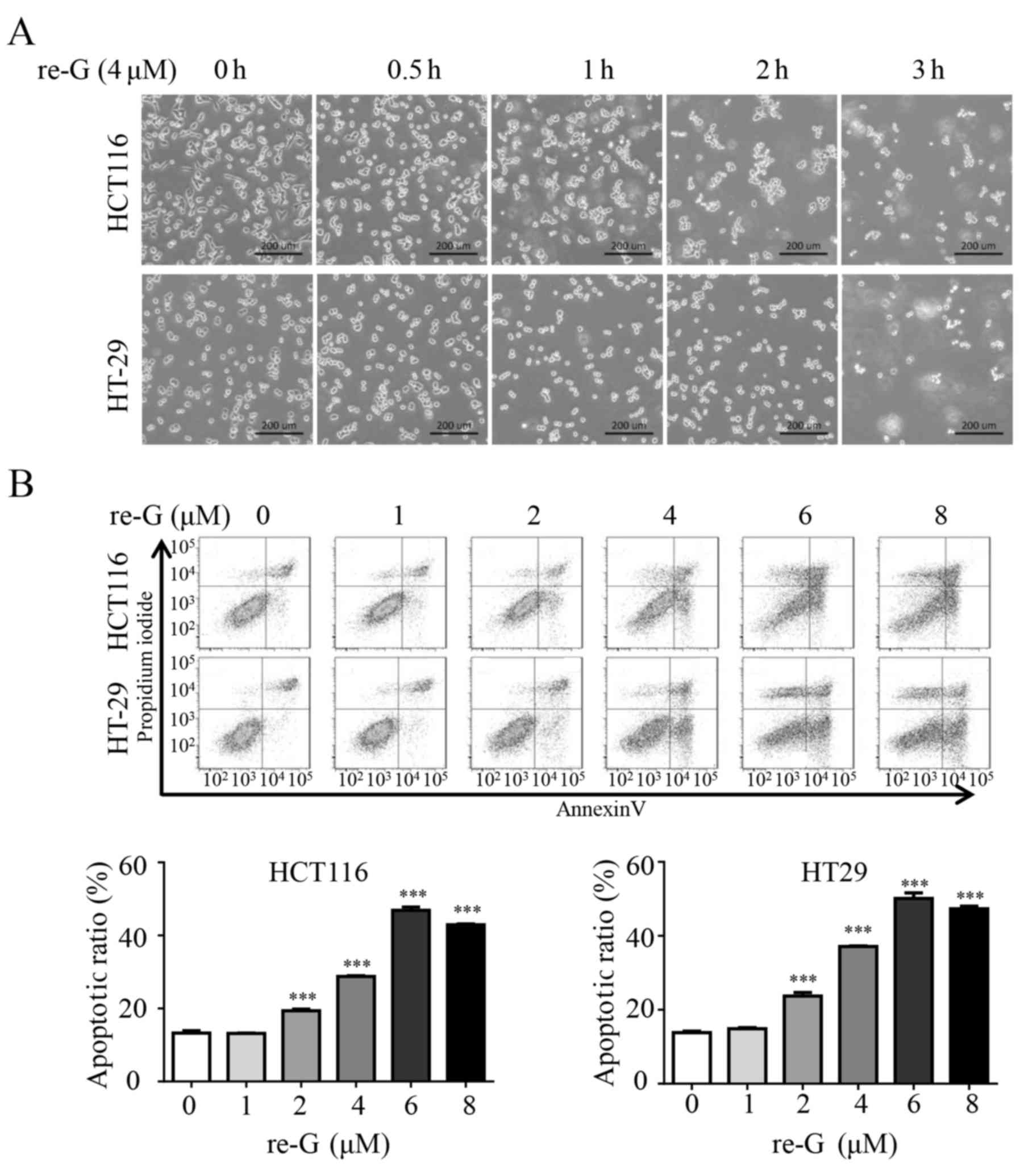
Indeed, we found that reduced-gliotoxin triggered rapid cell
detachment in the HCT116 and HT-29 cells (Fig. 1A). As the detachment of cells can
lead to cell death, we further examined the apoptosis-inducing
effects of reduced-gliotoxin on CRC cells by flow cytometric
analysis. The HCT116 and HT-29 cells were treated with escalating
concentrations of reduced-gliotoxin, followed by Annexin
V-FITC/propidium iodide (PI) staining coupled with flow cytometry.
As shown in Fig. 1B,
reduced-gliotoxin induced cell apoptosis in a dose-dependent
manner.
The anoikis induced by reduced-gliotoxin
is associated with the activation of both endogenous and exogenous
apoptotic pathways
The detachment of epithelial cells from the
extracellular matrix induces cell apoptosis, a phenomenon known as
anoikis (5). Furthermore, anoikis
has been ascribed to the intrinsic and/or the extrinsic apoptotic
pathway (26). Caspase-8 is a
crucial adaptor in the extrinsic apoptotic pathway and caspase-9 is
an important executor in the intrinsic apoptotic pathway (27). Therefore, in this study, we
examined the activation of caspase-8 and caspase-9 in cells treated
with reduced-gliotoxin. The HCT116 and HT-29 cells were treated
with reduced-gliotoxin, followed by the measurement of the levels
of caspase-8, caspase-9 and PARP by western blot analysis. As shown
in Fig. 2A and B,
reduced-gliotoxin decreased the total level of caspase-9, and
increased the levels of cleaved caspase-8 and PARP in a dose- and
time-dependent manner. In addition, reduced-gliotoxin increased the
level of PUMA in a dose-dependent manner, whereas the level of MCL1
was not affected. The status of mitochondria has been shown to be
deeply involved in cell anoikis (11) and the loss of MMP is considered a
hallmark of mitochondrial-associated apoptosis. Therefore, we
performed an MMP assay with JC-1 staining and found that JC-1 was
gradually spread out as a monomer following treatment with
reduced-gliotoxin (Fig. 2C), which
indicated that reduced-gliotoxin may disrupt MMP and trigger
mitochondrial-associated anoikis. The above-mentioned data
suggested that CRC cell apoptosis induced by reduced-gliotoxin
might be associated with both the intrinsic and extrinsic apoptotic
pathways.
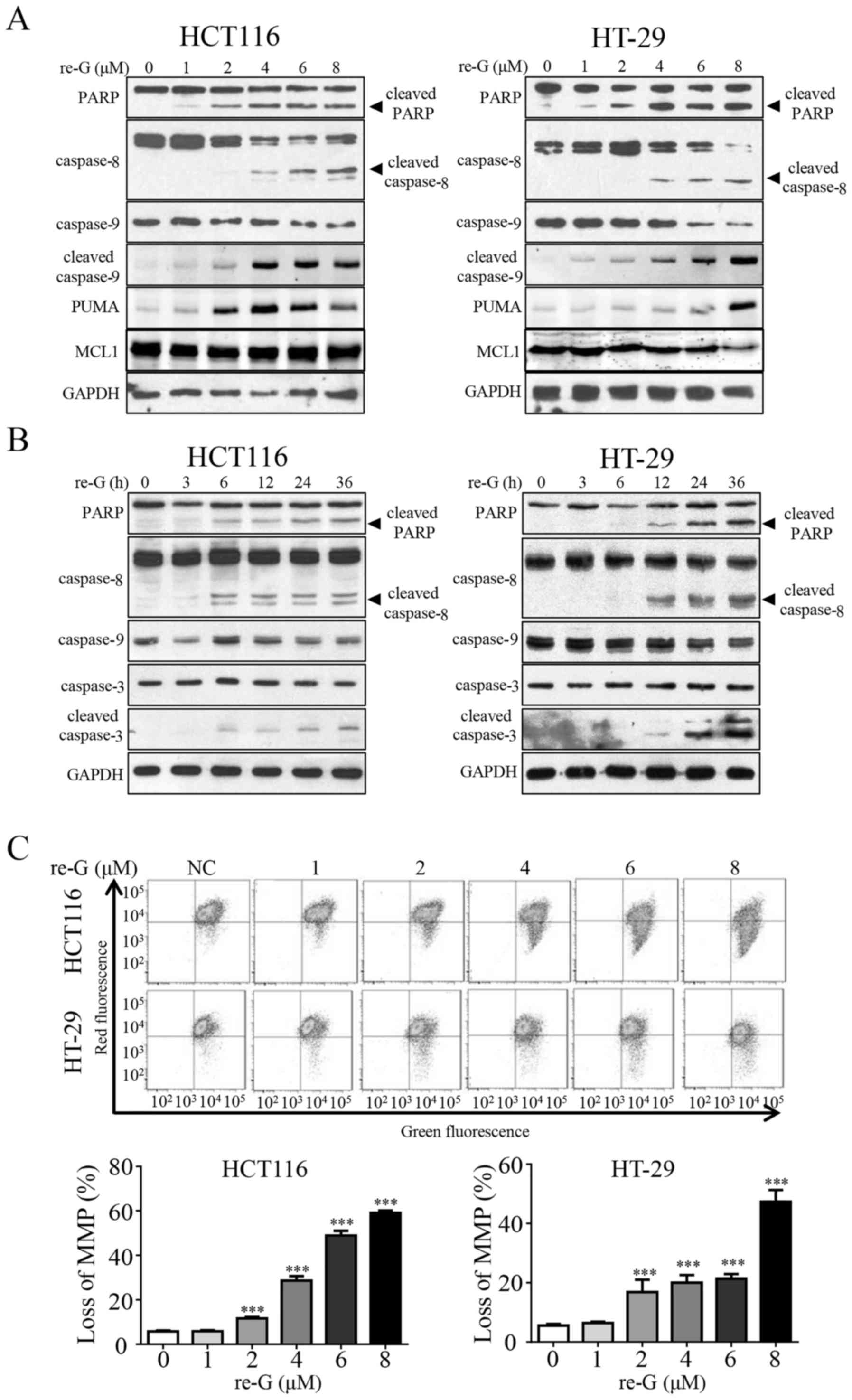 | Figure 2The anoikis induced by
reduced-gliotoxin is associated with the activation of both
endogenous and exogenous apoptotic pathways. (A) Reduced-gliotoxin
induced the cleavage of PARP and the activation of caspase-8 and
caspase-9 in a dosage-dependent manner. The HCT116 and HT-29 cells
were treated with reduced-gliotoxin at increasing concentrations
for 24 h. The expression of PARP, caspase-8, caspase-9, cleaved
caspase-9, PUMA and MCL1 was detected by western blot analysis.
GAPDH was used as a loading control. (B) Reduced-gliotoxin induced
the cleavage of PARP, and caspase-8, caspase-9 and caspase3
activation in a time-dependent manner. The HCT116 and HT-29 cells
were treated with 4 µM reduced-gliotoxin for the indicated
amounts of time, and the levels of PARP, caspase-8, caspase-9,
caspase-3 and cleaved caspase-3 were detected by western blot
analysis. GAPDH was used as a loading control. (C)
Reduced-gliotoxin induced the loss of mitochondrial membrane
potential (MMP) loss in the HCT116 and HT-29 cells. The cells were
treated with increasing concentrations of reduced-gliotoxin for 24
h, and the loss of MMP was detected by JC-1 staining coupled with
flow cytometry. All the values were expressed as the means ± SD
(n=3). ***P<0.0001 vs. untreated cells, as shown by
one-way ANOVA with Fisher's LSD test. re-G, reduced-gliotoxin. |
The anoikis induced by reduced-gliotoxin
is associated with integrinβ1, integrinβ4 and β-catenin
degradation
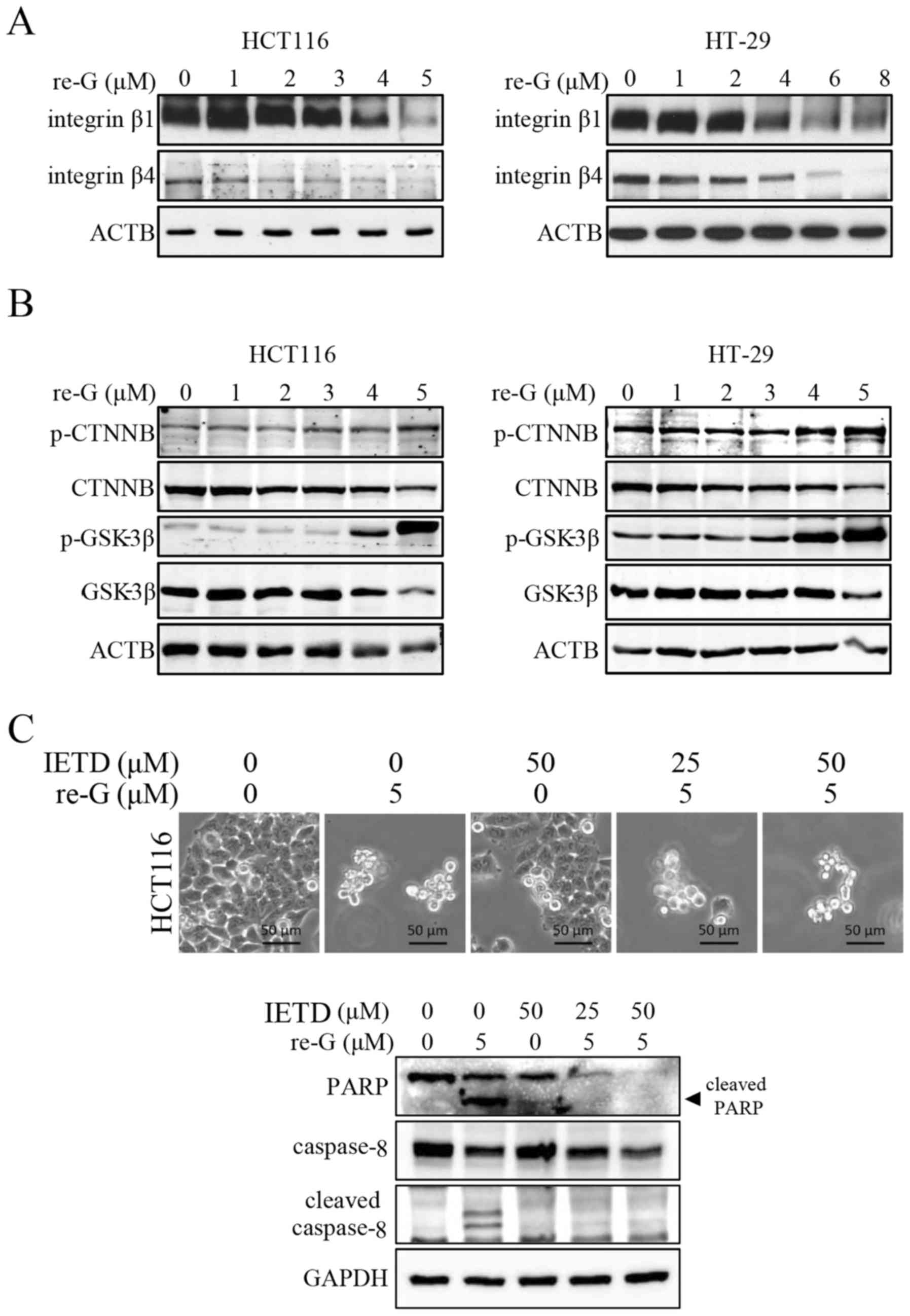
Given that integrin signaling supports cancer cell
metastasis via resisting anoikis (28,29),
western blot analysis was performed to evaluate the protein
expression of integrinβ1 and integrinβ4. As shown in Fig. 3A, treatment of the HCT116 and HT-29
cells with reduced-gliotoxin resulted in a decrease in the protein
levels of integrinβ1 and integrinβ4. As the loss of β-catenin has
been implicated in triggering anoikis in colon carcinoma (30), we also examined whether
reduced-gliotoxin decreases the protein level of β-catenin by
western blot analysis. As shown in Fig. 3B, reduced-gliotoxin decreased
β-catenin expression in a dose-dependent manner. The disengagement
of integrin from the ECM leads to the activation of both the
extrinsic and intrinsic apoptotic pathways (31). Thus, in order to verify the
predominant pathway involved in the execution of the apoptosis of
these cells, we used a caspase-8 inhibitor (Z-IETD-FMK) to block
the extrinsic apoptotic pathway. As shown in Fig. 3C, the inhibition of caspase-8
activity did not reverse cell detachment and death induced by
reduced-gliotoxin.
The anoikis induced by reduced-gliotoxin
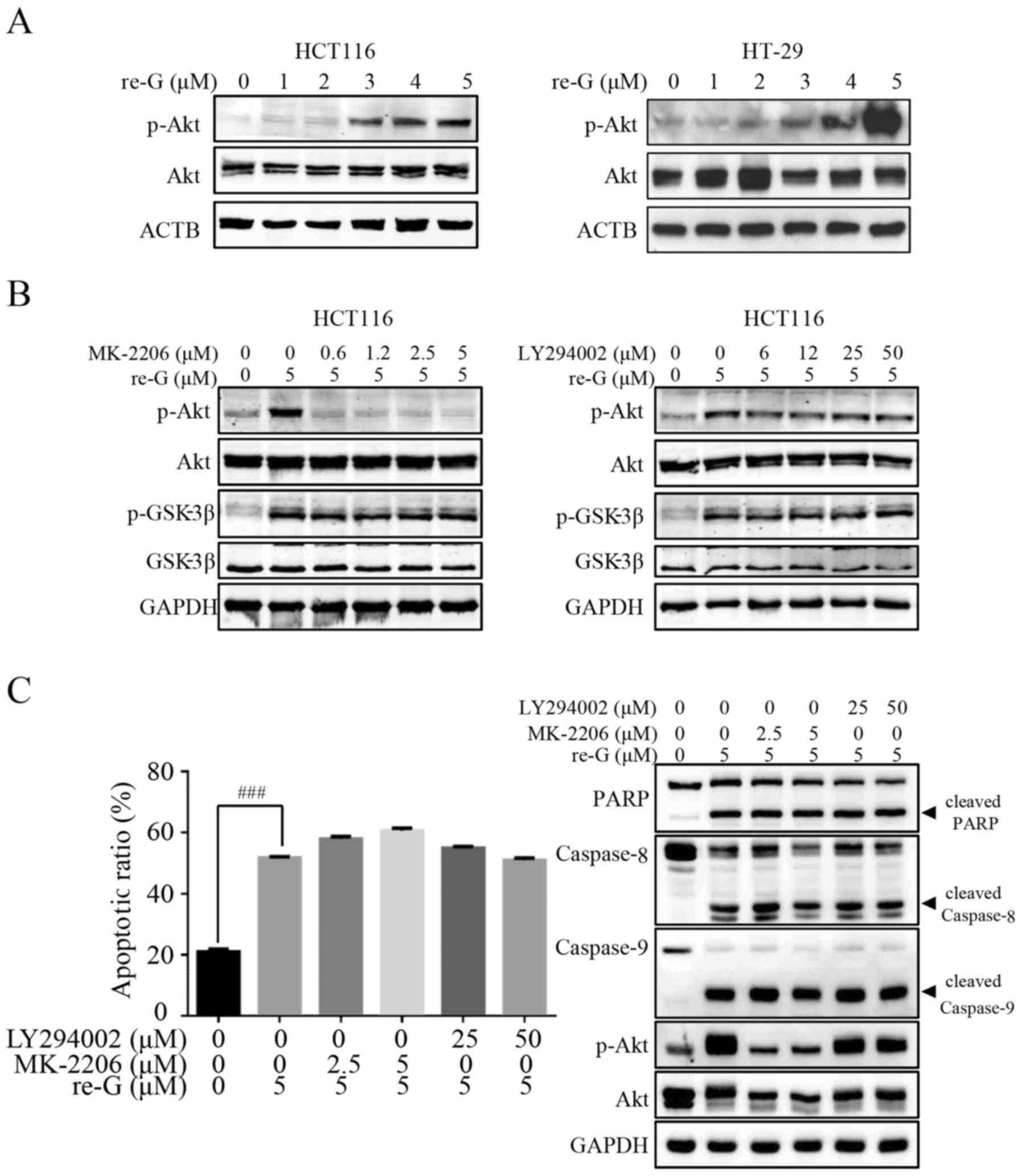
is independent of Akt activation or GSK-3β inactivation
As the phosphorylation of β-catenin by GSK-3β
results in β-catenin degradation (32), we then examined whether the
promotion of β-catenin degradation induced by reduced-gliotoxin
required GSK-3β activity. Of note, reduced-gliotoxin treatment did
not modify the expression of pan GSK-3β, but induced its
phosphorylation on its Ser9 residue, which was shown to trigger its
inactivation (Fig. 3B). These
results indicate that the degradation of β-catenin by
reduced-gliotoxin occurs independently of GSK-3β activity. During
tumorigenesis, PI3K/Akt signaling has been shown to inhibit GSK-3β
through phosphorylation at Ser9 (33). Thus, we then examined the
phosphorylation of Akt at Ser473, which is strongly dependent upon
PI3K activity. As shown in Fig.
4A, treatment of the CRC cells with reduced-gliotoxin increased
the level of phosphorylated Akt at Ser473 in a
concentration-dependent manner. Furthermore, we used the
PI3K-specific inhibitor, LY294002, and the Akt inhibitor, MK-2206,
to examine the role of PI3K/Akt/GSK-3β in the anoikis induced by
reduced-gliotoxin. As shown in Fig.
4B, Akt activity was inhibited by treatment with escalating
concentrations of MK-2206, whereas the inhibition of GSK-3β induced
by reduced-gliotoxin was not affected. Likewise, the activation of
Akt induced by reduced-gliotoxin was also not affected by
pre-treatment with the PI3K specific inhibitor, LY294002 (Fig. 4B). These findings thus suggest that
reduced-gliotoxin triggers the phosphorylation of Akt and that this
occurs independently of PI3K activity, while reduced-gliotoxin
triggers the phosphorylation of GSK-3β and that this occurs
independently of the PI3K/Akt pathway. We also examined the
apoptosis-promoting effects of reduced-gliotoxin by flow cytometric
analysis and western blot analysis in the presence or absence of
PI3K/Akt inhibitors. As shown in Fig.
4C, PI3K/Akt inhibition did not affect the cell detachment and
death induced by reduced-gliotoxin.
Excessive ROS production is the upstream
regulator of the anoikis induced by reduced-gliotoxin
Gliotoxin has been found to increase ROS generation
and induce oxidative stress in mouse fibroblasts (34), human hepatic stellate cells
(35) and cultured macrophages
(36). ROS generation is
associated with the mitochondria, and as shown above,
reduced-gliotoxin triggered the loss of MMP (Fig. 2B). Therefore, we examined the
intracellular ROS levels by flow cytometry in the cells with or
without reduced-gliotoxin treatment. As shown in Fig. 5A, reduced-gliotoxin increased ROS
production in a dose-dependent manner. The two main sources of ROS
are the mitochondria and NADPH oxidase (NOX). In order to verify
the main source of ROS generation induced by treatment with
reduced-gliotoxin, we used DPI to block NOX activity. As it shown
in Fig. 5B, DPI reduced most of
the ROS generation. To identify the role of ROS in the anoikis
induced by reduced-gliotoxin, NAC, as a ROS scavenger, was used. Of
note, it was found that NAC almost completely abolished cell
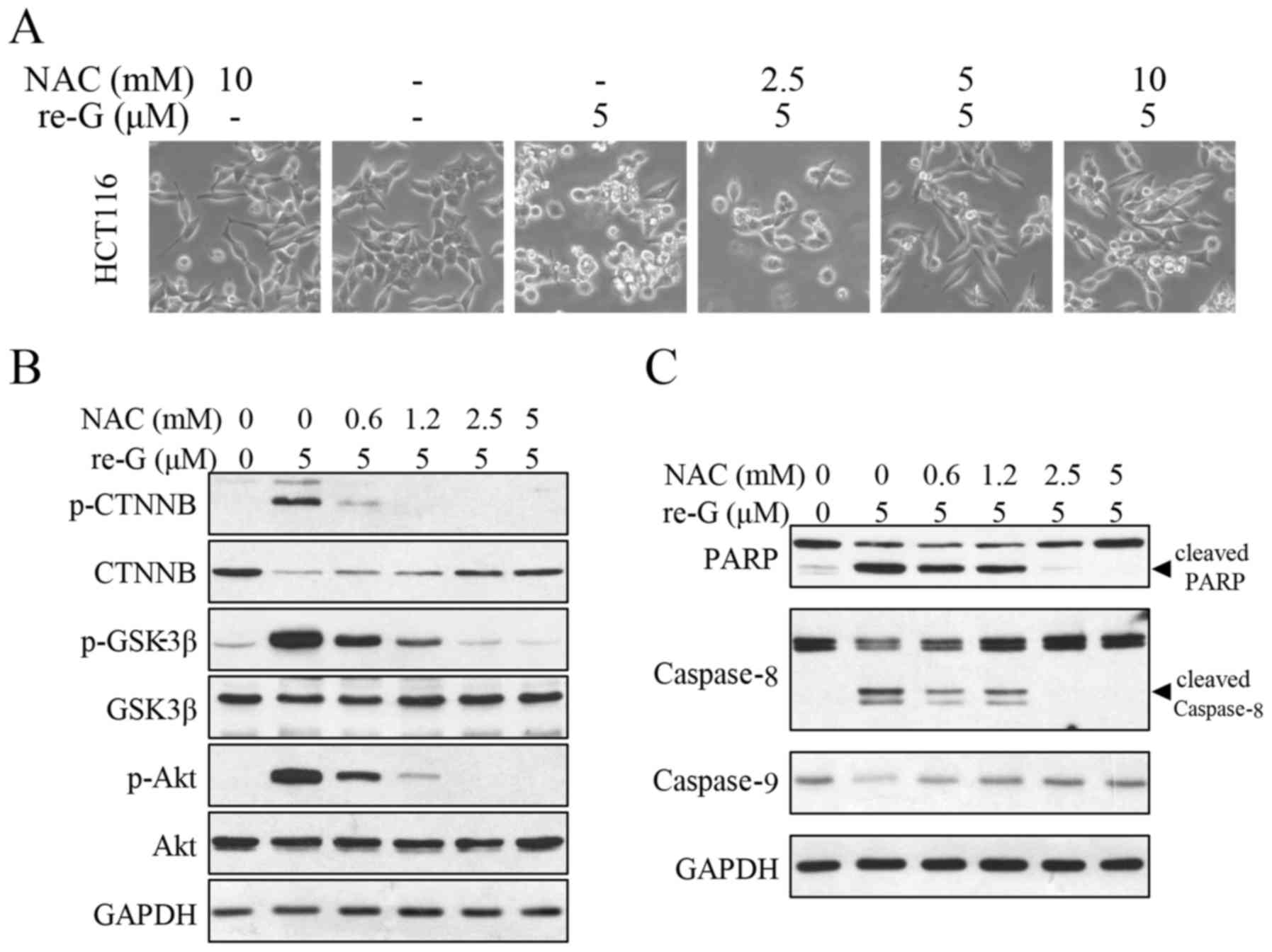
anoikis induced by reduced-gliotoxin (Fig. 5C). We further examined the effects
of NAC on cell detachment, and multi-signaling pathway and caspase
cascade activation induced by reduced-gliotoxin. As shown in
Fig. 6A, the cell detachment
induced by reduced-gliotoxin was attenuated by pre-treatment with
NAC. In addition, β-catenin degradation, Akt activation and GSK-3β
inhibition induced by reduced-gliotoxin-induced were all reversed
(Fig. 6B). Additionally, the
decrease in the levels of PARP, caspase-8 cleavage and
pro-caspase-9 induced by reduced-gliotoxin were all reversed by NAC
pre-treatment (Fig. 6C). These
results suggest that ROS induction mediates the apoptotic pathways
activated by reduced-gliotoxin and that ROS are a critical upstream
regulator of the anoikis induced by reduced-gliotoxin.
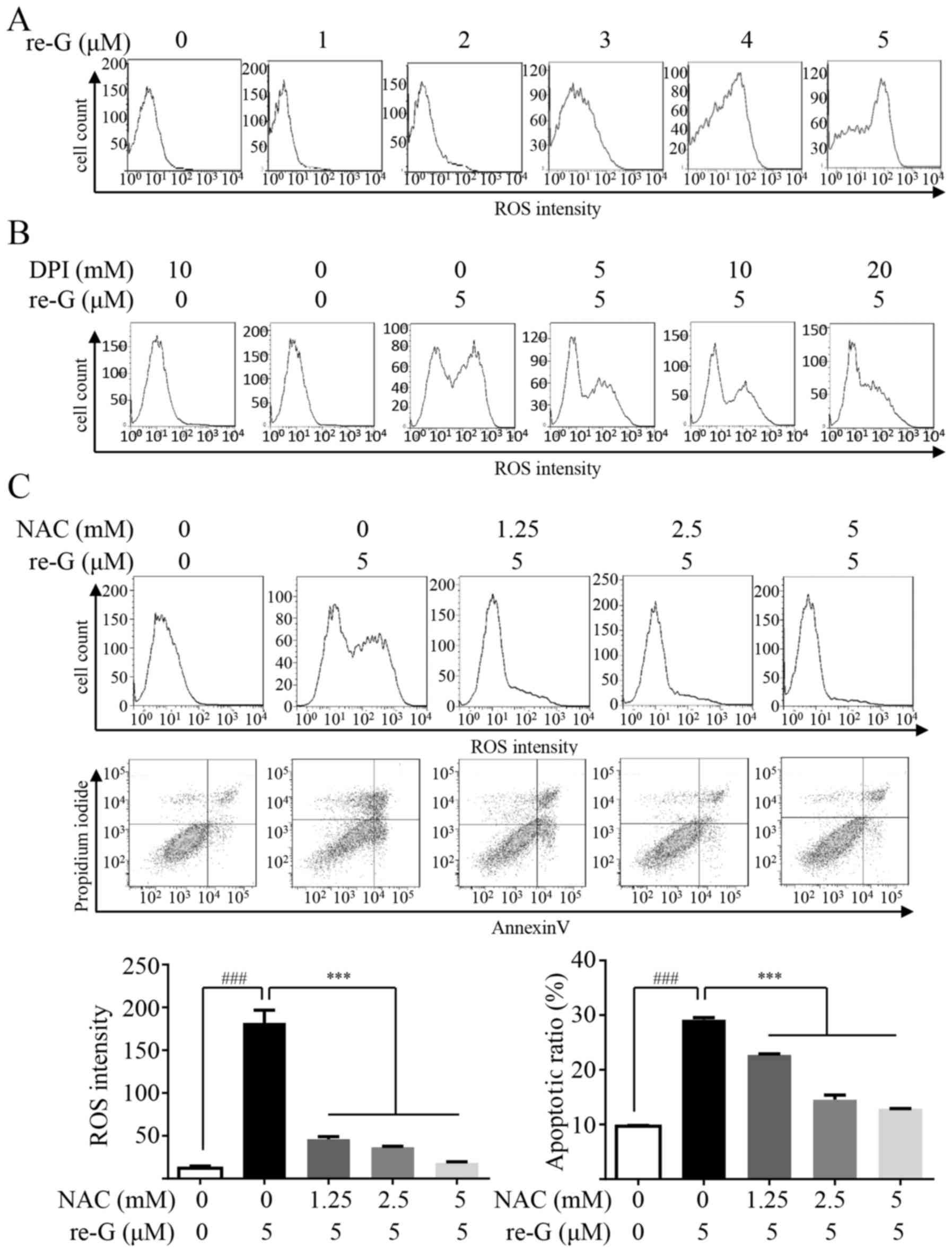 | Figure 5The anoikis induced by
reduced-gliotoxin in CRC cells is dependent on reactive oxygen
species (ROS) generation. (A) Reduced-gliotoxin induced ROS
production in a dose-dependent manner. The cells were treated with
the indicated concentrations of reduced-gliotoxin for 24 h, and ROS
generation was assessed by 2,7-dichlorodi-hydrofluorescein
diacetate (DCFH-DA) staining with flow cytometry. (B) The cells
were treated with reduced-gliotoxin (5 µM) in the absence
and presence of 5, 10 and 20 mM of the NOX inhibitor, DPI, for 24
h, and ROS production were then analyzed by flow cytometry (C) The
cells were treated with reduced-gliotoxin (5 µM) in the
absence and presence of 1.25 and 2.5 mM of the ROS scavenger, NAC,
for 24 h, and ROS production and apoptotic cell pollutions were
then analyzed by flow cytometry. ROS density and the apoptotic rate
were shown in the bar graphs; all the values are expressed as the
means ± SD. A two-tailed unpaired Student's t-test was used to
determine significant differences between the cells treated with or
without reduced-gliotoxin, ###P<0.001. One-way ANOVA
with Fisher's LSD test was used to determine significant
differences between the cells treated with NAC and/or
reduced-gliotoxin, ***P<0.001. re-G,
reduced-gliotoxin. |
Discussion
There is evidence to suggest that gliotoxin is a
promising anticancer reagent that induces cytotoxic effects on
cancer cells (21–23). Previously, we observed that
reduced- exerted the most potent anticancer effects compared to
other gliotoxin analogues (14).
In this study, we examined the potency of reduced-gliotoxin against
CRC cells and the underlying mechanisms. We demonstrated that
reduced-gliotoxin triggered rapid cell detachment and induced the
apoptosis of CRC cells. Mechanistically, our data indicated that
the anoikis induced by reduced-gliotoxin induced is associated with
the disruption of integrin-related cell detachment and multiple
signaling pathways. Furthermore, reduced-gliotoxin induced ROS
production, resulting in the activation of both endogenous and
exogenous apoptotic pathways and eventually resulting in the
apoptosis of CRC cells. The blockade of ROS generation by NAC
completely reversed several reduced-gliotoxin-induced protein
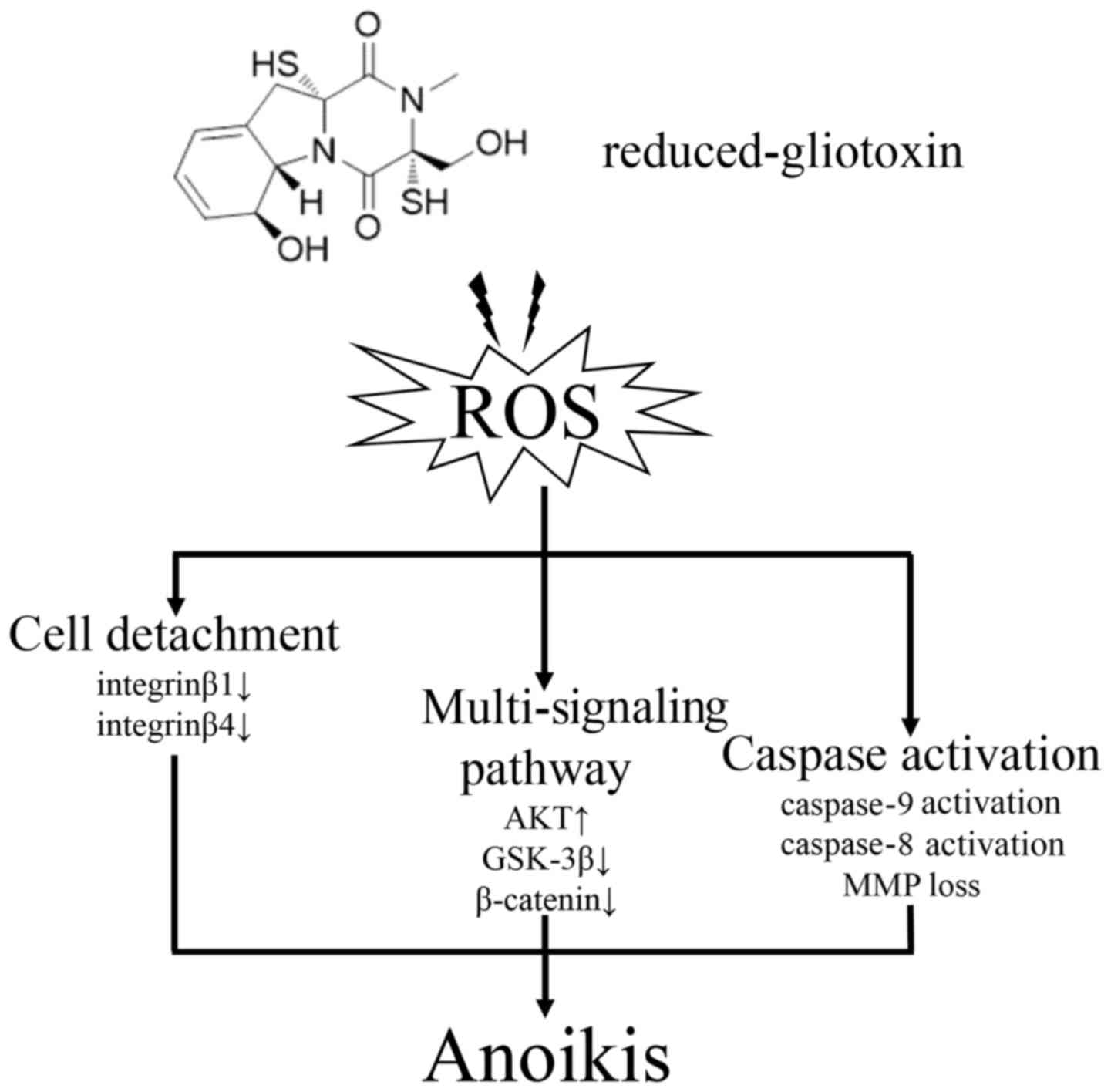
kinase signaling pathways and cell anoikis (Fig. 7). Taken together, these studies
suggest that reduced-gliotoxin may be a potential candidate for the
treatment of CRC.
Anoikis is defined as apoptosis that is induced by
inadequate or inappropriate cell matrix interaction (11). Anoikis plays an important role in
tissue homeostasis, disease development and cancer metastasis
(5). Resistance to anoikis
characterizes the ability of cancer cells to circumvent barriers to
migration and invasion, thereby promoting metastatic progression
(31). Therefore, a novel method
that can enhance or induce anoikis to disrupt the settling of
metastatic cancer cells at a distal site is an attractive strategy.
In this study, we demonstrated that reduced-gliotoxin induced cell
death by inducing anoikis (Fig.
1). Anoikis either disrupts mitochondrial homeostasis or
triggers cell surface death receptors; hence, it is involved in
both the intrinsic and extrinsic apoptotic pathways (11). In this study, the anoikis induced
by reduced-gliotoxin was characterized by the activation of
caspase-8, caspase-9 and PARP cleavage, suggesting that the
intrinsic apoptotic pathway mainly mediates this event.
The induction of anoikis is caused by an absence of
adhesive interaction between epithelial cells and their surrounding
ECM. Integrins involved in the cell-ECM and cell-cell communication
play an important role in regulating anoikis and metastasis
(37). The overexpression of
integrinβ1 and integrinβ4, which promote cell invasion and
metastasis, are associated with a clinically aggressive phenotype
of different types of cancer, including CRC (38–40).
The inhibition of cell surface integrins is able to influence the
first step of anoikis. It has been suggested this may prove to be
an attractive target for CRC therapy (41). In this context, in this study, we
demonstrated that the anoikis induced by reduced-gliotoxin might be
associated with integrinβ1 and integrinβ4 degradation (Fig. 3). Previously, we reported that
gliotoxin blocked the Wnt/β-catenin signaling pathway by inducing
the degradation of β-catenin in CRC cells. A similar result was
observed: Reduced-gliotoxin induced β-catenin phosphorylation and
decreased the protein expression levels. β-catenin is a major
oncoprotein that is often activated in CRC and promotes tumor
progression. Our data indicated that reduced-gliotoxin induced CRC
cell anoikis and this was not only associated with the degradation
of proteins interacting with ECM, but also with adherens junction
proteins. Furthermore, we demonstrated that the degradation of
β-catenin was accompanied by Akt activation and GSK-3β
inactivation.
Reduced-gliotoxin can be oxidized to gliotoxin, thus
releasing ROS, and it can also form mixed disulfides by thiol
groups with proteins or antioxidants, such as glutathione (42). Therefore, it is not surprising to
observe that ROS was produced by CRC cells treated with
reduced-gliotoxin, whereas pre-treatment with NAC blocked ROS
production and cell anoikis. In tumorigenesis, GSK-3β is the most
important kinase for the abnormal phosphorylation of β-catenin, and
the inactivation of GSK-3β promotes the accumulation of β-catenin.
In this study, reduced-gliotoxin triggered the activation of Akt
and the inactivation of GSK-3β. Moreover, the activation of Akt and
the inactivation of GSK-3β were abrogated by pre-treatment with
NAC. NAC inhibited the phosphorylation of Akt; it subsequently
inactivated GSK-3β by phosphorylating at Ser9 as usual. However,
inhibiting the activation of Akt with LY294002 or MK-2206 did not
attenuate the inactivation of GSK-3β. Thus, ROS may act
independently of Akt activation to mediate reduced-gliotoxin, which
inactivates GSK-3β activity. Based on these findings, it is likely
that ROS are crucial factors in the induction of anoikis and act as
upstream signaling molecules in initiating cell anoikis.
Glossary
Abbreviations
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
NAC
|
N-acetylcysteine
|
|
MMP
|
mitochondrial membrane potential
|
|
ECM
|
extracellular matrix
|
Acknowledgments
The authors would like to thank Rose-Ann Thomas for
English proofreading the manuscript.
Notes
[1]
Funding
This study was supported by National Natural Science
Foundation of China (81672413 and 81402418); Doctoral Funds of
Ministry of Education of China (2016M592583); Guangdong Provincial
Department of Science and Technology (2014B020212016); Guangdong
Natural Science Funds (2015A030310096); Guangzhou Science
Technology and Innovation Commission (2016201604030007;
2016201604030003); Overseas Excellent Professor Project, Ministry
of Education, China; Japan Ministry of Education, Culture, Sports,
Science and Technology (MEXT) for Program of Japan Initiative for
Global Research Network on Infectious Diseases (J-GRID).
[2] Availability
of data and materials
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
JC, QL, LH, CW, ML, ZZ, FW and LH performed the cell
and molecular biology experiments; WL contributed to the marine
natural productions; JC and QL wrote the manuscript; AI revised the
manuscript and provided professional advice during the whole
research process. HL and XY designed the experiments and analyzed
the results. All authors read and approved the final
manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
März L and Piso P: Treatment of peritoneal
metastases from colorectal cancer. Gastroenterol Rep (Oxf).
3:298–302. 2015.
|
|
5
|
Gilmore AP: Anoikis. Cell Death Differ.
12(Suppl 2): 1473–1477. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiarugi P and Giannoni E: Anoikis: A
necessary death program for anchorage-dependent cells. Biochem
Pharmacol. 76:1352–1364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grossmann J: Molecular mechanisms of
'detachment-induced apoptosis - Anoikis'. Apoptosis. 7:247–260.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simpson CD, Anyiwe K and Schimmer AD:
Anoikis resistance and tumor metastasis. Cancer Lett. 272:177–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagaprashantha LD, Vatsyayan R, Lelsani
PC, Awasthi S and Singhal SS: The sensors and regulators of
cell-matrix surveillance in anoikis resistance of tumors. Int J
Cancer. 128:743–752. 2011. View Article : Google Scholar
|
|
10
|
Shanmugathasan M and Jothy S: Apoptosis,
anoikis and their relevance to the pathobiology of colon cancer.
Pathol Int. 50:273–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frisch SM and Screaton RA: Anoikis
mechanisms. Curr Opin Cell Biol. 13:555–562. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cragg GM and Newman DJ: Natural products:
A continuing source of novel drug leads. Biochim Biophys Acta.
1830:3670–3695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saleem M, Ali MS, Hussain S, Jabbar A,
Ashraf M and Lee YS: Marine natural products of fungal origin. Nat
Prod Rep. 24:1142–1152. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang WL, Le X, Li HJ, Yang XL, Chen JX,
Xu J, Liu HL, Wang LY, Wang KT, Hu KC, et al: Exploring the
chemodiversity and biological activities of the secondary
metabolites from the marine fungus Neosartorya pseudofischeri. Mar
Drugs. 12:5657–5676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bugni TS and Ireland CM: Marine-derived
fungi: A chemically and biologically diverse group of
microorganisms. Nat Prod Rep. 21:143–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lan WJ, Fu SJ, Xu MY, Liang WL, Lam CK,
Zhong GH, Xu J, Yang DP and Li HJ: Five new cytotoxic metabolites
from the marine fungus Neosartorya pseudofischeri. Mar Drugs.
14:182016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan DF, Lan WJ, Wang KT, Huang L, Jiang CW
and Li HJ: Two chlorinated benzofuran derivatives from the marine
fungus Pseudallescheria boydii. Nat Prod Commun. 10:621–622.
2015.PubMed/NCBI
|
|
18
|
Yamada A, Kataoka T and Nagai K: The
fungal metabolite gliotoxin: Immunosuppressive activity on
CTL-mediated cytotoxicity. Immunol Lett. 71:27–32. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Takada K, Takemoto Y, Yoshida M,
Nogi Y, Okada S and Matsunaga S: Gliotoxin analogues from a
marine-derived fungus, Penicillium sp., and their cytotoxic and
histone methyltransferase inhibitory activities. J Nat Prod.
75:111–114. 2012. View Article : Google Scholar
|
|
20
|
Chen J, Wang C, Lan W, Huang C, Lin M,
Wang Z, Liang W, Iwamoto A, Yang X and Liu H: Gliotoxin inhibits
proliferation and induces apoptosis in colorectal cancer cells. Mar
Drugs. 13:6259–6273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nguyen VT, Lee JS, Qian ZJ, Li YX, Kim KN,
Heo SJ, Jeon YJ, Park WS, Choi IW, Je JY, et al: Gliotoxin isolated
from marine fungus Aspergillus sp induces apoptosis of human
cervical cancer and chondrosarcoma cells. Mar Drugs. 12:69–87.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hubmann R, Hilgarth M, Schnabl S, Ponath
E, Reiter M, Demirtas D, Sieghart W, Valent P, Zielinski C, Jäger
U, et al: Gliotoxin is a potent NOTCH2 transactivation inhibitor
and efficiently induces apoptosis in chronic lymphocytic leukaemia
(CLL) cells. Br J Haematol. 160:618–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan XQ and Harday J: Electromicroscopic
observations on gliotoxin-induced apoptosis of cancer cells in
culture and human cancer xenografts in transplanted SCID mice. In
Vivo. 21:259–265. 2007.PubMed/NCBI
|
|
24
|
Burnette WN: 'Western blotting':
Electrophoretic transfer of proteins from sodium dodecyl sulfate -
polyacrylamide gels to unmodified nitrocellulose and radiographic
detection with antibody and radioiodinated protein A. Anal Biochem.
112:195–203. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geissler A, Haun F, Frank DO, Wieland K,
Simon MM, Idzko M, Davis RJ, Maurer U and Borner C: Apoptosis
induced by the fungal pathogen gliotoxin requires a triple
phosphorylation of Bim by JNK. Cell Death Differ. 20:1317–1329.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maamer-Azzabi A, Ndozangue-Touriguine O
and Bréard J: Metastatic SW620 colon cancer cells are primed for
death when detached and can be sensitized to anoikis by the
BH3-mimetic ABT-737. Cell Death Dis. 4:e8012013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ata R and Antonescu CN: Integrins and cell
metabolism: An intimate relationship impacting cancer. Int J Mol
Sci. 18:182017. View Article : Google Scholar
|
|
29
|
Alanko J, Mai A, Jacquemet G, Schauer K,
Kaukonen R, Saari M, Goud B and Ivaska J: Integrin endosomal
signalling suppresses anoikis. Nat Cell Biol. 17:1412–1421. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoo BH, Masson O, Li Y, Khan IA, Gowda PS
and Rosen KV: Anoikis of colon carcinoma cells triggered by
β-catenin loss can be enhanced by tumor necrosis factor receptor 1
antagonists. Oncogene. 34:4939–4951. 2015. View Article : Google Scholar
|
|
31
|
Paoli P, Giannoni E and Chiarugi P:
Anoikis molecular pathways and its role in cancer progression.
Biochim Biophys Acta. 1833:3481–3498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of beta-catenin
phosphorylation/degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo J: Glycogen synthase kinase 3beta
(GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett.
273:194–200. 2009. View Article : Google Scholar
|
|
34
|
Pardo J, Urban C, Galvez EM, Ekert PG,
Müller U, Kwon-Chung J, Lobigs M, Müllbacher A, Wallich R, Borner
C, et al: The mitochondrial protein Bak is pivotal for
gliotoxin-induced apoptosis and a critical host factor of
Aspergillus fumigatus virulence in mice. J Cell Biol. 174:509–519.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kweon YO, Paik YH, Schnabl B, Qian T,
Lemasters JJ and Brenner DA: Gliotoxin-mediated apoptosis of
activated human hepatic stellate cells. J Hepatol. 39:38–46. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suen YK, Fung KP, Lee CY and Kong SK:
Gliotoxin induces apoptosis in cultured macrophages via production
of reactive oxygen species and cytochrome c release without
mitochondrial depolarization. Free Radic Res. 35:1–10. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ruoslahti E and Reed JC: Anchorage
dependence, integrins, and apoptosis. Cell. 77:477–478. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Beaulieu JF: Integrin α6β4 in colorectal
cancer. World J Gastrointest Pathophysiol. 1:3–11. 2010. View Article : Google Scholar
|
|
39
|
Scartozzi M, Giampieri R, Loretelli C,
Mandolesi A, del Prete M, Biagetti S, Alfonsi S, Faloppi L,
Bianconi M, Bittoni A, et al: Role of β4 integrin in
HER-3-negative, K-RAS wild-type metastatic colorectal tumors
receiving cetuximab. Future Oncol. 9:1207–1214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fujita S, Watanabe M, Kubota T, Teramoto T
and Kitajima M: Alteration of expression in integrin beta 1-subunit
correlates with invasion and metastasis in colorectal cancer.
Cancer Lett. 91:145–149. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pelillo C, Mollica H, Eble JA, Grosche J,
Herzog L, Codan B, Sava G and Bergamo A: Inhibition of adhesion,
migration and of α5β1 integrin in the HCT-116 colorectal cancer
cells treated with the ruthenium drug NAMI-A. J Inorg Biochem.
160:225–235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scharf DH, Brakhage AA and Mukherjee PK:
Gliotoxin - bane or boon? Environ Microbiol. 18:1096–1109. 2016.
View Article : Google Scholar
|