Introduction
Distant metastasis, a major prognosis determinant in
patients with colorectal cancer, is present in approximately
one-fifth of all newly diagnosed cases. The 5-year survival of
patients who present with synchronous distant metastases is not
>20% (1,2). Moreover, even if patients are
initially diagnosed with non-metastatic disease, 25–50% of these
patients experience tumour recurrence despite potentially curative
resections (1,3,4). In
order to improve the prognoses of these patients, it is essential
to investigate the aetiology and pathophysiology of metastasis and
find an innovative therapeutic strategy.
It has recently been proposed that cancer stem cells
play a significant role in tumour progression and metastatic
dissemination in various types of cancer (5). A cancer stem cell, defined as ‘a cell
within a tumour that possesses the capacity to self-renew and to
cause the heterogeneous lineages of cancer cells that comprise the
tumour’ (6), is considered to be
an explanation for the resistance of cancer tissues to treatment.
Therefore, tumours with high expression levels of cancer stem cell
markers are expected to exhibit unfavourable prognoses.
However, the impact of cancer stem cell markers on
patient survival remains unclear, particularly in patients with
distant metastasis. Our previous study on liver metastasis using
CD133, the most well known putative stem cell marker in colorectal
cancer, revealed that patients without CD133 expression exhibited
reduced survival (7). Although
this seemed to contradict the cancer stem cell theory, there are
other studies which support these findings (8,9).
Therefore, in this study, we focused on patients
with colon cancer with peritoneal metastasis, as this has not been
investigated to date, at least to the best of our knowledge. The
aim of this study was to determine the impact of cancer stem cell
markers on the prognosis of patients with colon cancer with
peritoneal metastasis. We also examined 2 other putative stem cell
markers, aldehyde dehydrogenase-1 (ALDH1) and leucine-rich
repeating G-protein coupled receptor-5 (Lgr5), in order to examined
whether the findings may be attributed to stemness or whether they
are specific to CD133.
Materials and methods
Patients and tissue specimens
Among 1,990 patients who underwent resection for
primary sporadic colon cancer at the University of Tokyo Hospital
(Tokyo, Japan) between 1997 and 2015, 171 were diagnosed with
synchronous or metachronous peritoneal metastasis. Information on
these patients and their tumour characteristics, treatments and
clinical outcomes were retrieved from patient medical records.
During the former half of the study period, the majority of
surgeries were performed by an open method, while in the latter
half, approximately one third of the surgeries were performed
laparoscopically. A total of 55 patients underwent complete
resections of recognisable metastases, including visible peritoneal
nodules, while the remaining patients had residual tumour tissues.
No patients underwent peritonectomy or hyperthermic intraperitoneal
chemotherapy (HIPEC).
The study protocol was approved by the Research
Ethics Committee at the Graduate School of Medicine, the University
of Tokyo (Tokyo, Japan) (approval no. G3552-3). All participants
provided informed, written informed consent. This study was
conducted in accordance with the 1964 Declaration of Helsinki and
its later amendments.
Peri-operative evaluation
All patients were clinically staged by a physical
examination, colonoscopy and chest-abdomen-pelvis computed
tomography (CT) prior to surgery. Pathological staging was
performed according to the Union for International Cancer Control
TNM Classification of Malignant Tumours, 7th edition (10). Tumours proximal to the splenic
flexure were classified as proximal colon cancer and those distal
to the splenic flexure were classified as distal colon cancer.
Tumours originating in the vermiform appendix and rectum were
excluded. In all cases, regular follow-up examinations were
performed (tumour marker assessments every 3 months,
chest-abdomen-pelvic CT every 6 months and an annual total
colonoscopy). Peritoneal metastasis was diagnosed based on surgical
exploration or imaging studies. No patient underwent systematic
second-look surgery.
Evaluation by immunohistochemistry
Consecutive 3-µm-thick formalin-fixed
paraffin-embedded sections were immunohistochemically stained
manually using the following procedures. Following
deparaffinisation and rehydration, endogenous peroxidase was
blocked with 3% hydrogen peroxidase solution in methanol for 15
min. Heat-induced antigen retrieval was performed in 10 mM sodium
citrate buffer (pH 6.0) using an autoclave. Following non-specific
protein blocking by incubation with 5% bovine serum albumin for 30
min, the slides were incubated overnight with primary antibodies
against CD133 (mouse anti-human polyclonal antibody; AC133; 1:100
dilution; Miltenyi Biotec, Auburn, CA, USA), ALDH1 (clone EP1933Y;
1:200 dilution; Abcam, Cambridge, MA, USA) and Lgr5 (clone
EPR3065Y; 1:100 dilution; LifeSpan Biosciences, Seattle, WA, USA),
in a humidified container at 4°C. They were then incubated using a
Dako Envision kit (Dako, Carpinteria, CA, USA) and also incubated
in 2% 3,3′-diaminobenzidine tetrahydrochloride and 50 mM
tris-buffer containing hydrogen peroxidase as a chromogen following
the manufacturer’s instructions. Meyer’s haematoxylin (Sigma
Chemical Co., St. Louis, MO, USA) was used for counterstaining.
Expression was defined as positive when CD133, ALDH1
and Lgr5 staining was found in >5, 20 and 50% of the tumour
samples, respectively (7,11,12),
by examining 1,000 tumour cells in 10 fields (100 cells/field) with
high-power (×200) microscopy (11). The evaluation was performed
independently by 2 clinicians (H.N. and J.K.) who were blinded to
the clinical findings. Discrepancies between their findings were
resolved by discussion.
Statistical analysis
Categorical variables were described using
frequencies and percentages. Correlation was evaluated using
Fisher’s exact test or a Chi-squared test. The distributions of
continuous variables were described using medians and the
interquartile ranges. The Mann-Whitney test was used for
comparisons. Overall survival was defined as the duration between
the date of primary tumour resection and the date of death from any
cause. Disease-free survival was defined as the duration between
the date of complete resection for metastasis and the date of
recurrence or death from any cause. These outcomes were calculated
using the Kaplan-Meier method and compared using the log-rank test.
Univariate and multivariate Cox regression analyses were performed
to investigate patient and tumour characteristics associated with
the prognosis of metachronous peritoneal metastasis. The variables
for multivariate analysis were selected using the model selection
approach by Collett (13). Through
the time-to-event analysis, hazard ratios (HRs) and 95% confidence
intervals (CIs) were generated. Associations were considered
significant for P-values <0.05 in general; however, a level of
significance of 0.15 was used for Collett’s univariable screening
(13). Data were statistically
analysed using the statistical program R version 3.3.1 (http://www.R-project.org/).
Results
Immunohistochemical expression in primary
tumours and clinicopathological variables
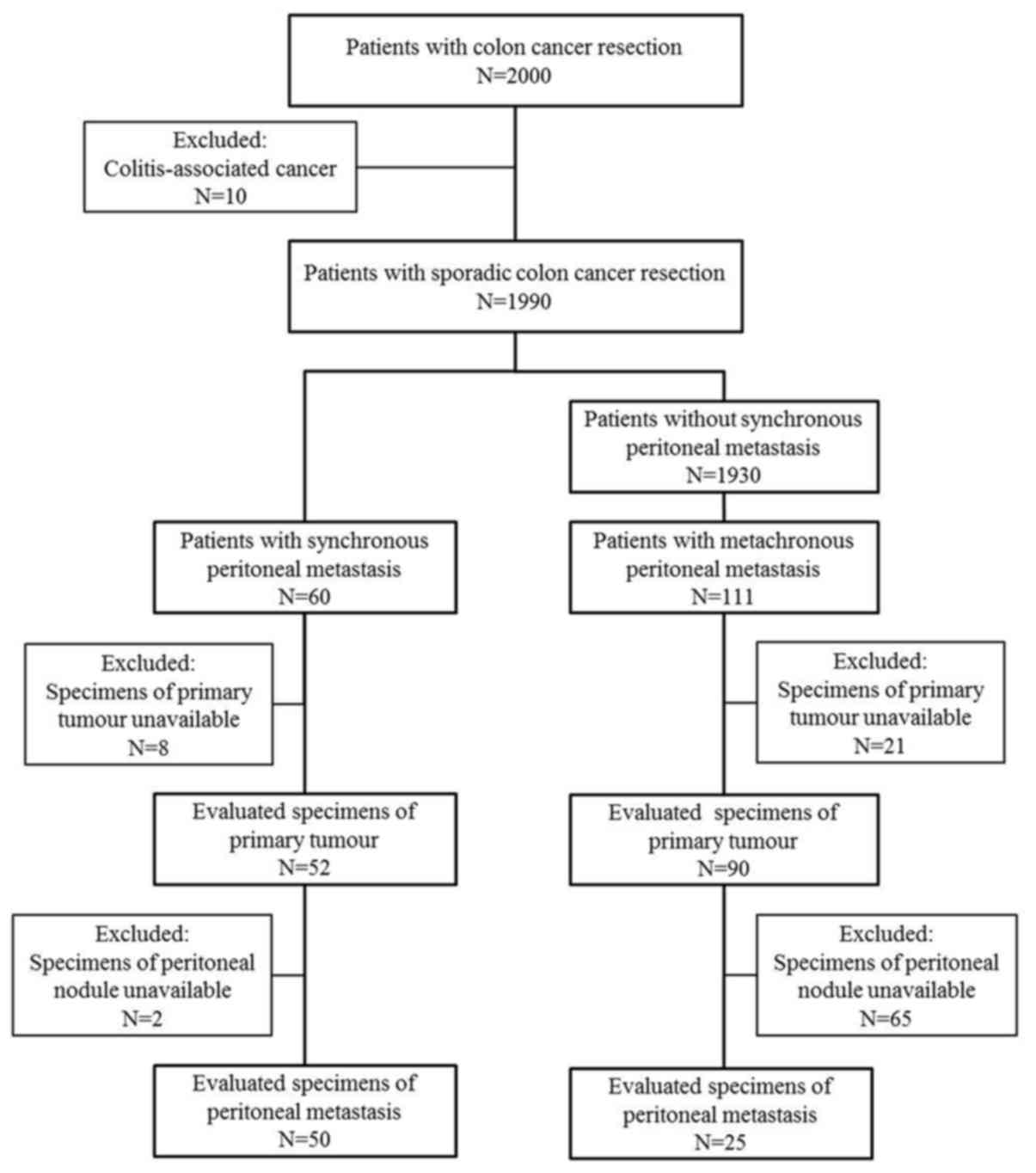
Specimens of 142 cases were available for the
immunohistological evaluation of primary tumours, and those of 75
cases were available for the evaluation of metastatic peritoneal
nodules, as shown in Fig. 1. The
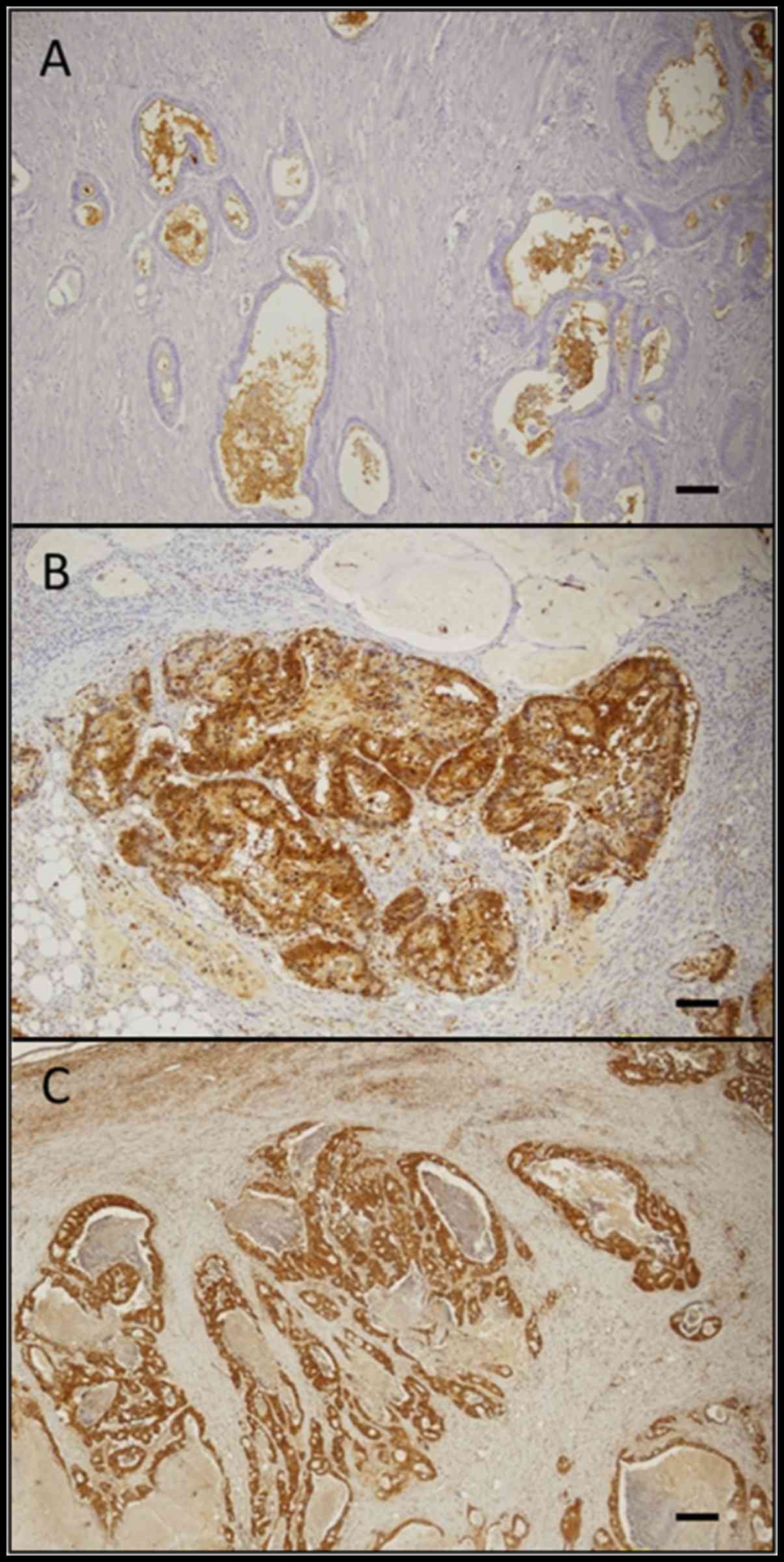
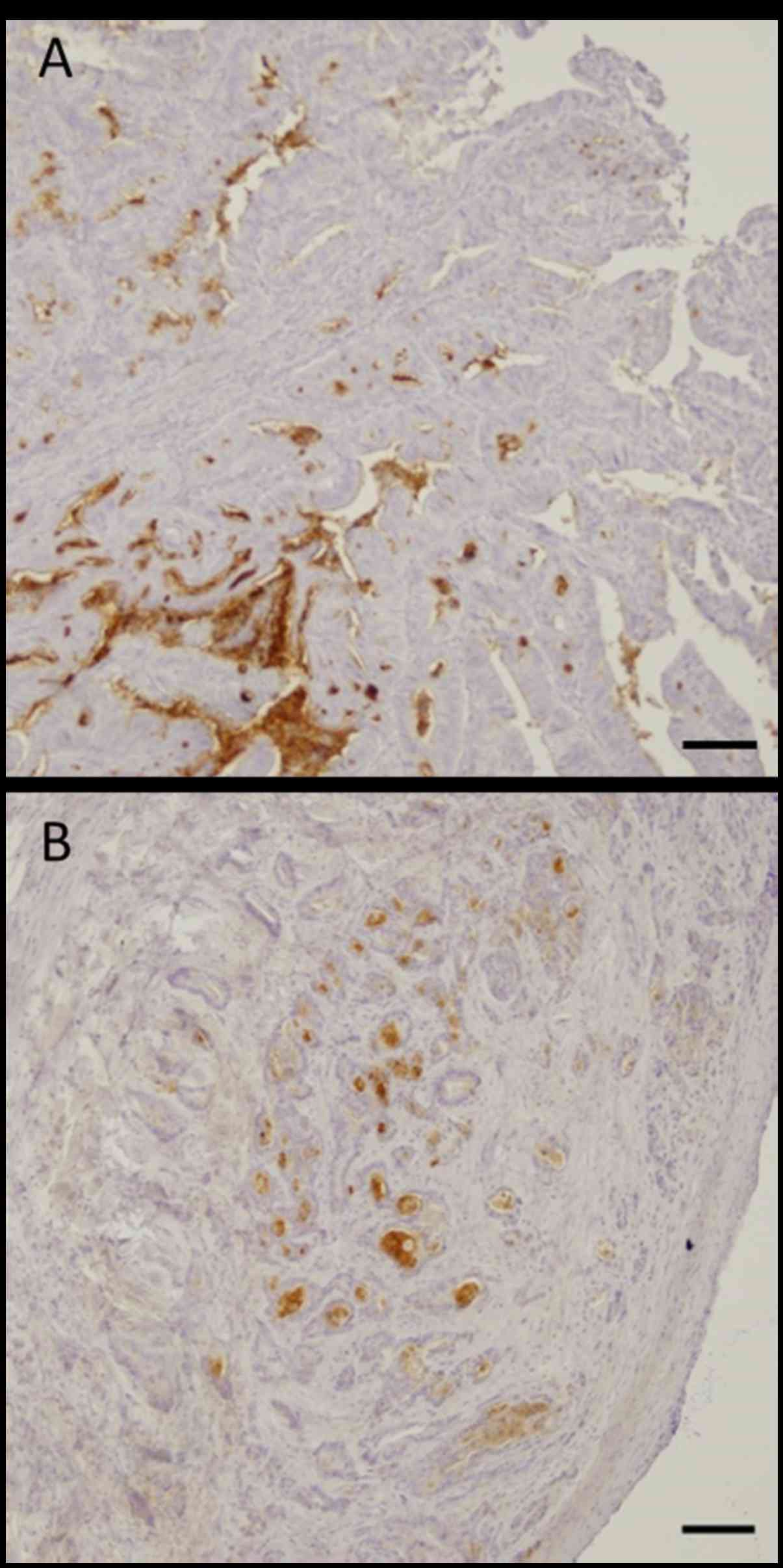
immunohistochemical staining pattern of CD133, ALDH1, and Lgr5 is
shown in Fig. 2. Membranous
immunoreactivity was observed for CD133, and cytoplasmic
immunoreactivity was observed for ALDH1 and Lgr5. The rates of the
positive expression of CD133, ALDH1 and Lgr5 in our primary tumour
samples were 55.6% (79/142), 47.2% (67/142) and 78.9% (112/142),
respectively.
The clinicopathological characteristics of the
patients are shown in Table I.
Concurrent distant metastasis was found in 59 patients. While 33 of
these patients had a single organ metastasis other than the
peritoneum, 20 patients had 2, and 6 patients had more than 3 sites
of metastasis in addition to the peritoneum. The most common site
of metastasis was the liver (35 patients), followed by the lung (20
patients), the distant lymph nodes (19 patients) and the ovary (6
patients). With regards to adjuvant chemotherapy following the
resection of the peritoneal nodules, 5-fluorouracil regimens were
used in 16 patients and cytotoxic doublet regimens were used in 20
patients.
 | Table IClinicopathological characteristics of
the patients from whom primary tumour samples were obtained. |
Table I
Clinicopathological characteristics of
the patients from whom primary tumour samples were obtained.
| CD133
| ALDH1
| Lgr5
|
|---|
(+)
n=79
n (%) | (−)
n=63
n (%) | P-value | (+)
n=67
n (%) | (−)
n=75
n (%) | P-value | (+)
n=112
n (%) | (−)
n=30
n (%) | P-value |
|---|
| Age, years | | | 0.701 | | | 0.218 | | | 0.757 |
| Median
(interquartile range) | 64 (55–75) | 64 (58–74) | | 65 (57–77) | 25 (55–73) | | 65 (55–75) | 64 (58–72) | |
| Sex | | | 0.983 | | | 0.545 | | | 0.963 |
| Male | 45 (57.0) | 36 (57.1) | | 40 (59.7) | 41 (54.7) | | 64 (57.1) | 17 (56.7) | |
| Female | 34 (43.0) | 27 (42.9) | | 27 (40.3) | 34 (45.3) | | 48 (42.9) | 13 (43.3) | |
| Site of primary
tumour | | | 0.707 | | | 0.119 | | | 0.056 |
| Proximal
(right) | 30 (38.0) | 22 (34.9) | | 29 (43.3) | 23 (30.7) | | 46 (41.1) | 6 (20.0) | |
| Distal (left) | 49 (62.0) | 41 (65.1) | | 38 (56.7) | 52 (69.3) | | 66 (59.9) | 24 (80.0) | |
|
Differentiation | | | <0.001a | | | 0.078 | | | 0.340 |
| Well | 32 (40.5) | 25 (39.7) | | 22 (32.9) | 35 (46.7) | | 45 (40.2) | 12 (40.0) | |
| Moderate | 47 (59.5) | 24 (38.1) | | 35 (52.2) | 36 (52.0) | | 58 (51.8) | 13 (33.3) | |
| Poor | 0 (0.0) | 14 (22.2) | | 10 (14.9) | 4 (5.3) | | 9 (8.0) | 5 (16.7) | |
| Histology | | | <0.001a | | | 0.067 | | | 0.020a |
| Tubular
adenocarcinoma | 78 (98.7) | 52 (82.5) | | 58 (86.6) | 72 (96.0) | | 106 (94.6) | 24 (80.0) | |
| Mucinous
adenocarcinoma | 1 (1.3) | 11 (17.5) | | 9 (13.4) | 3 (4.0) | | 6 (5.4) | 6 (20.0) | |
| T category | | | 0.582 | | | 0.200 | | | 0.991 |
| T1–3 | 23 (29.1) | 16 (25.4) | | 22 (32.8) | 17 (22.7) | | 31 (27.7) | 8 (26.6) | |
| T4a | 41 (51.9) | 38 (60.3) | | 32 (47.8) | 47 (62.7) | | 62 (55.4) | 17 (56.7) | |
| T4b | 15 (19.0) | 9 (14.3) | | 13 (19.4) | 11 (14.6) | | 19 (17.0) | 5 (16.7) | |
| N category | | | 0.002a | | | 0.484 | | | 0.631 |
| N0 | 31 (39.2) | 12 (19.1) | | 21 (31.3) | 22 (29.3) | | 36 (32.1) | 7 (23.4) | |
| N1 | 32 (40.5) | 22 (34.9) | | 28 (41.8) | 26 (34.7) | | 41 (36.6) | 13 (43.3) | |
| N2 | 16 (20.3) | 29 (46.0) | | 18 (26.9) | 27 (36.0) | | 35 (31.3) | 10 (33.3) | |
| Examined lymph node
count | | | 0.628 | | | 0.717 | | | 0.708 |
| <12 | 19 (24.1) | 13 (20.6) | | 16 (23.9) | 16 (21.3) | | 26 (23.2) | 6 (20.0) | |
| ≥12 | 60 (75.9) | 50 (79.4) | | 51 (76.1) | 59 (78.7) | | 86 (76.8) | 24 (80.0) | |
| Lymphatic
invasion | | | 0.416 | | | 0.686 | | | 0.830 |
| ly0 | 38 (48.1) | 26 (41.3) | | 29 (43.3) | 35 (46.7) | | 51 (12.2) | 13 (33.3) | |
| ly1 | 41 (51.9) | 37 (58.7) | | 38 (56.7) | 40 (53.3) | | 61 (54.5) | 17 (56.7) | |
| Venous
invasion | | | 0.200 | | | 0.946 | | | 0.080 |
| v0 | 10 (12.7) | 13 (20.6) | | 11 (16.4) | 12 (16.0) | | 15 (13.4) | 8 (26.6) | |
| v1 | 69 (87.3) | 50 (79.4) | | 56 (83.6) | 63 (84.0) | | 97 (86.6) | 22 (73.4) | |
| Concurrent
metastasis | | | 0.276 | | | 0.531 | | | 0.148 |
| Peritoneum
alone | 43 (54.4) | 40 (63.5) | | 41 (61.2) | 42 (56.0) | | 62 (55.4) | 21 (70.0) | |
| Other
metastasis | 36 (45.6) | 23 (36.5) | | 26 (38.8) | 33 (44.0) | | 50 (44.6) | 9 (30.0) | |
| 1 Other
metastatic site | 17 (21.5) | 16 (25.4) | | 13 (19.4) | 20 (26.7) | | 27 (24.1) | 6 (20.0) | |
| 2 Other
metastatic sites | 15 (19.0) | 5 (7.9) | | 10 (14.9) | 10 (13.3) | | 17 (15.2) | 3 (10.0) | |
| ≥3 Metastatic
sites | 4 (5.1) | 2 (3.2) | | 3 (4.5) | 3 (4.0) | | 6 (5.4) | 0 (0.0) | |
| Presentation | | | 0.468 | | | 0.057 | | | 0.674 |
| Synchronous
metastasis | 31 (39.2) | 21 (33.3) | | 30 (44.8) | 22 (29.3) | | 42 (37.5) | 10 (33.3) | |
| Metachronous
metastasis | 48 (60.8) | 42 (66.7) | | 37 (55.2) | 53 (70.7) | | 70 (62.5) | 20 (66.7) | |
| Peritoneal cancer
index | | | 0.156 | | | 0.470 | | | 0.157 |
| <10 | 65 (82.3) | 47 (74.6) | | 50 (74.6) | 62 (82.7) | | 86 (76.8) | 26 (86.6) | |
| 10–20 | 13 (16.4) | 11 (17.5) | | 13 (19.4) | 11 (14.6) | | 22 (19.6) | 2 (6.7) | |
| >20 | 1 (1.3) | 5 (7.9) | | 4 (6.0) | 2 (2.7) | | 4 (35.7) | 2 (6.7) | |
| Status of
metastasectomy | | | 0.627 | | | 0.717 | | | 0.002 |
| Complete
resection | 32 (40.5) | 23 (36.5) | | 27 (40.3) | 28 (37.3) | | 36 (32.1) | 19 (63.3) | |
| Residual
metastasis (+) | 47 (59.5) | 40 (63.5) | | 40 (59.7) | 47 (62.7) | | 76 (67.9) | 11 (36.7) | |
| Chemotherapy after
metastasectomy | | | 0.544 | | | 0.853 | | | 0.795 |
| Adjuvant
chemotherapy (−) | 10 (12.7) | 9 (14.3) | | 9 (13.4) | 10 (13.3) | | 12 (10.7) | 7 (23.4) | |
| Adjuvant
chemotherapy (+) | 22 (27.8) | 14 (22.2) | | 18 (26.9) | 18 (24.0) | | 24 (21.4) | 12 (40.0) | |
| b5-FU | 12 (15.1) | 4 (6.3) | | 4 (6.0) | 12 (16.0) | | 11 (9.8) | 5 (16.7) | |
| cFOLFOX/dCapeOX | 10 (12.7) | 10 (15.9) | | 14 (20.9) | 6 (8.0) | | 13 (11.6) | 7 (23.3) | |
The expression levels of CD133 and Lgr5 were
significantly lower in the mucinous adenocarcinoma samples
(P<0.001 and P=0.020). In addition, the expression of CD133
significantly correlated with differentiation (P<0.001) and the
N category (P=0.002), and the percentage of complete resection was
significantly lower in the Lgr5-negative group (P=0.002) (Table I).
Immunohistochemical expression in primary
tumours and prognosis
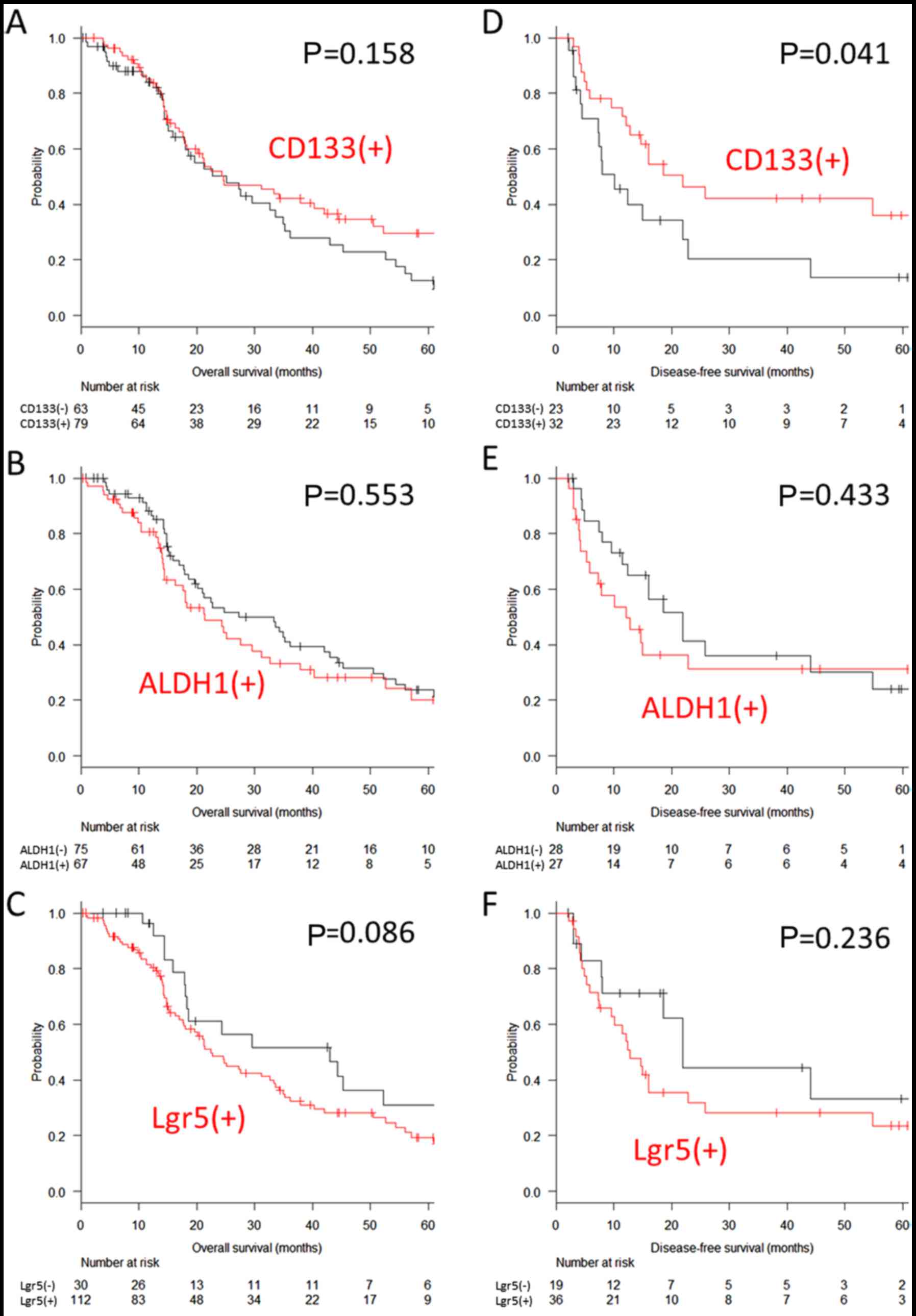
The median overall survival of the patients with
peritoneal metastasis was 24.6 months, and the 5-year overall
survival rate was 22.0% among all patients. The expression of
CD133, ALDH1 and Lgr5 was not associated with overall survival
(29.7 vs. 12.7%, P=0.158 for CD133; 20.2 vs. 23.8%, P=0.553 for
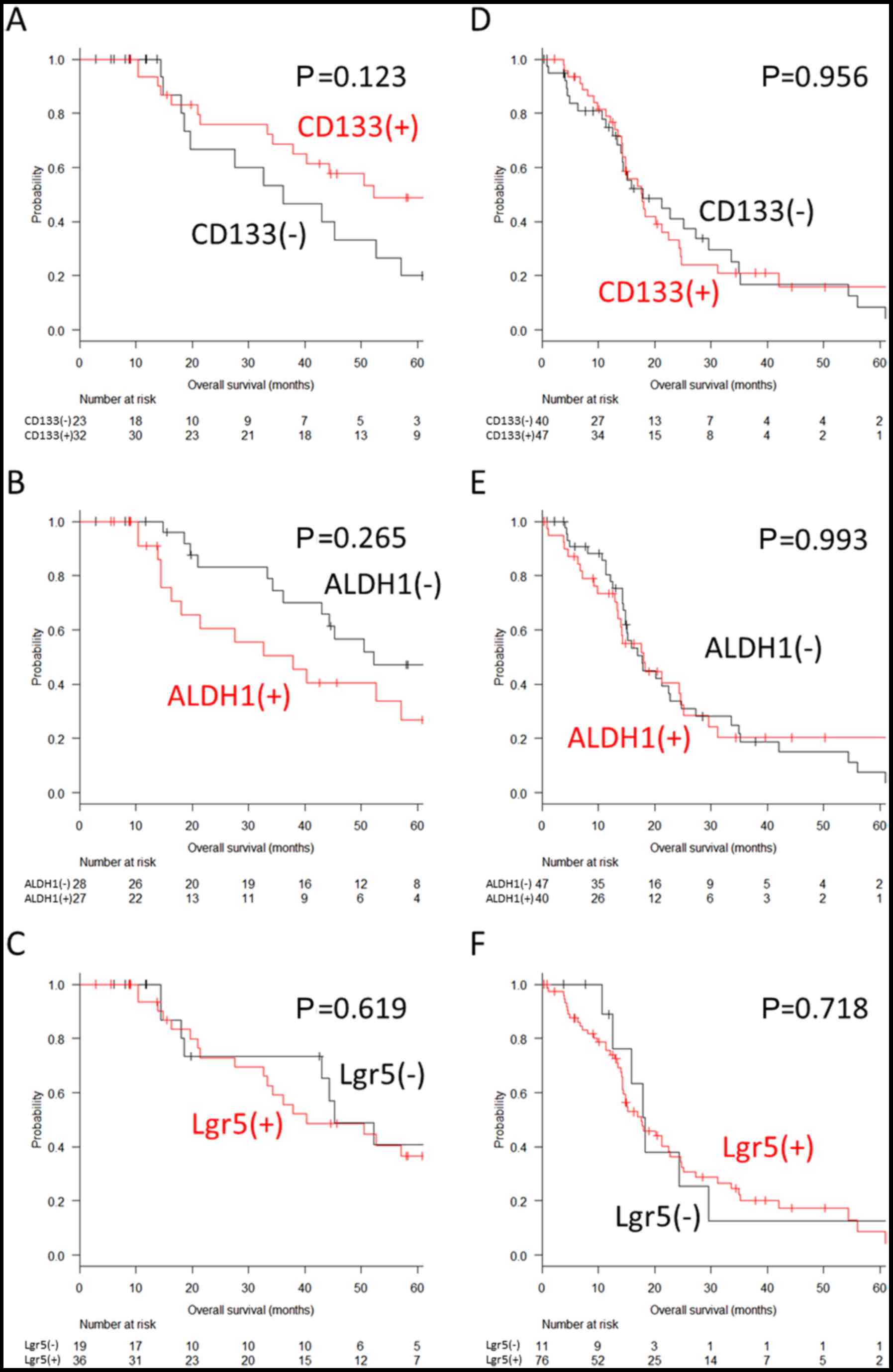
ALDH1; and 19.4 vs. 31.1%, P=0.086 for Lgr5) (Fig. 3A–C). Similar results were observed
when the patient subgroups were stratified according to the
completeness of metastasectomy (P=0.123, P=0.265 and P=0.619 for
CD133, ALDH1 and Lgr5, respectively, in the patients with complete
resection, and P=0.956, P=0.993 and P=0.718 for CD133, ALDH1 and
Lgr5, respectively, in the patients with residual metastasis)
(Fig. 4).
The median disease-free survival of 55 patients who
underwent complete resection was 16.0 months and the 5-year
disease-free survival rate for these patients was 27.2%. The most
common site of recurrence was the lung (5 patients), followed by
the peritoneum, liver and distant lymph nodes (4 patients for
each). While no significant difference was observed in the
expression of ALDH1 and Lgr5 (31.2 vs. 24.1%, P=0.433 for ALDH1 and
23.6 vs. 33.3%, P=0.236 for Lgr5), a positive expression of CD133
was associated with a significantly improved 5-year disease-free
survival (36.1 vs. 13.7%, P=0.041) (Fig. 3D–F).
The Cox multivariate model selected using the
Collett’s model selection approach contained age, adjuvant
chemotherapy following peritoneal nodule resection, and the
expression of CD133. A positive CD133 expression was found to be a
significant positive factor for disease-free survival (HR, 0.33;
95% CI, 0.16–0.72, P=0.005) as was adjuvant chemotherapy (HR, 0.23;
95% CI, 0.10–0.52, P<0.001) and older age (HR, 0.24; 95% CI,
0.08–0.63 for patients aged 50–69 years; and HR, 0.16, 95% CI,
0.05–0.48 for patients aged ≥70 years, P=0.003) (Table II).
 | Table IIAssociation between
clinicopathological variables and disease-free survival. |
Table II
Association between
clinicopathological variables and disease-free survival.
| Univariate analysis
| Multivariate
analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | | | 0.174 | | | 0.003 |
| <50 | Reference | | | Reference | | |
| 50–69 | 0.45 | 0.17–1.16 | | 0.23 | 0.09–0.63 | |
| ≥70 | 0.39 | 0.13–1.13 | | 0.16 | 0.05–0.48 | |
| Sex | | | 0.462 | | | |
| Male | Reference | | | | | |
| Female | 1.30 | 0.65–2.60 | | | | |
| Site of primary
tumour | | | 0.254 | | | |
| Proximal
(right) | Reference | | | | | |
| Distal (left) | 0.66 | 0.32–1.35 | | | | |
|
Differentiation | | | 0.086 | | | |
| Well | Reference | | | | | |
| Moderate | 1.54 | 0.74–3.18 | | | | |
| Poor | 3.55 | 1.14–11.05 | | | | |
| Histology | | | 0.465 | | | |
| Tubular
adenocarcinoma | Reference | | | | | |
| Mucinous
adenocarcinoma | 1.56 | 0.47–5.14 | | | | |
| T category | | | 0.937 | | | |
| T3 | Reference | | | | | |
| T4a | 1.08 | 0.49–2.35 | | | | |
| T4b | 0.90 | 0.27–2.91 | | | | |
| N category | | | 0.281 | | | |
| N0 | Reference | | | | | |
| N1 | 1.41 | 0.54–3.70 | | | | |
| N2 | 2.10 | 0.80–5.48 | | | | |
| No. of examined
lymph nodes | | | 0.555 | | | |
| <12 | Reference | | | | | |
| ≥12 | 0.77 | 0.32–1.86 | | | | |
| Lymphatic
invasion | | | 0.291 | | | |
| ly0 | Reference | | | | | |
| ly1 | 1.45 | 0.73–2.88 | | | | |
| Venous
invasion | | | 0.881 | | | |
| v0 | Reference | | | | | |
| v1 | 0.92 | 0.32–2.63 | | | | |
| Concurrent
metastasis | | | 0.548 | | | |
| Peritoneum
alone | Reference | | | | | |
| Other
metastasis | 0.75 | 0.29–1.93 | | | | |
| Presentation of
peritoneal metastasis | | | 0.599 | | | |
| Synchronous | Reference | | | | | |
| Metachronous | 0.83 | 0.42–1.64 | | | | |
| Adjuvant
chemotherapy | | | 0.012a | | | <0.001a |
| Adjuvant (−) | Reference | | | Reference | | |
| Adjuvant (+) | 0.40 | 0.20–0.82 | | 0.23 | 0.10–0.52 | |
| CD133
expression | | | 0.045a | | | 0.005a |
| CD133(−) | Reference | | | Reference | | |
| CD133(+) | 0.50 | 0.25–0.99 | | 0.33 | 0.16–0.72 | |
Immunohistochemical expression in
peritoneal nodules and clinicopathological variables
The rate of the positive expression of CD133, ALDH1
and Lgr5 was 40.0% (30/75), 30.7% (23/75) and 78.6% (59/75),
respectively, in our peritoneal nodule samples. The expression of
CD133 and ALDH1 in the peritoneal nodules was significantly lower
than that in the primary tumour samples (P=0.002 and P=0.001,
respectively), while that of Lgr5 did not differ significantly
(P=0.207). The clinicopathological characteristics of the patients
with peritoneal metastasis are shown in Table III. CD133-negative peritoneal
nodules significantly correlated with differentiation (P=0.0030),
but not with mucinous adenocarcinoma (P=0.392) or the N category
(P=0.119). The number of patients with <12 examined lymph nodes
was higher in those with ALDH1-negative peritoneal nodules
(P=0.016).
 | Table IIIClinicopathological characteristics
of the patients with peritoneal nodules. |
Table III
Clinicopathological characteristics
of the patients with peritoneal nodules.
| CD133
| ALDH1
| Lgr5
|
|---|
(+)
n=30
n (%) | (−)
n=45
n (%) | P-value | (+)
n=23
n (%) | (−)
n=52
n (%) | P-value | (+)
n=59
n (%) | (−)
n=16
n (%) | P-value |
|---|
| Age, years | | | 0.095 | | | 0.936 | | | 0.969 |
| Median
(interquartile range) | 68 (60–72) | 61 (53–70) | | 62 (58–71) | 65 (55–71) | | 64 (57–71) | 64 (55–75) | |
| Sex | | | 0.850 | | | 0.773 | | | 0.886 |
| Male | 16 (53.3) | 25 (55.6) | | 12 (52.2) | 29 (55.8) | | 32 (54.2) | 9 (56.3) | |
| Female | 14 (46.7) | 20 (44.4) | | 11 (47.8) | 23 (44.2) | | 27 (45.8) | 7 (43.7) | |
| Site of primary
tumour | | | 0.104 | | | 0.205 | | | 0.726 |
| Proximal
(right) | 9 (30.0) | 22 (48.9) | | 12 (52.2) | 19 (36.5) | | 25 (42.4) | 6 (37.5) | |
| Distal (left) | 21 (70.0) | 23 (51.1) | | 11 (47.8) | 33 (63.5) | | 34 (57.6) | 10 (62.5) | |
|
Differentiation | | | 0.030a | | | 0.719 | | | 0.177 |
| Well | 12 (40.0) | 18 (40.0) | | 8 (34.8) | 22 (42.3) | | 22 (37.3) | 8 (50.0) | |
| Moderate | 18 (60.0) | 19 (42.2) | | 13 (56.5) | 24 (46.2) | | 32 (54.2) | 5 (31.2) | |
| Poor | 0 (0.0) | 8 (17.8) | | 2 (8.7) | 6 (11.5) | | 5 (8.5) | 3 (18.8) | |
| Histology | | | 0.392 | | | 1.000 | | | 0.602 |
| Tubular
adenocarcinoma | 29 (96.7) | 40 (88.9) | | 21 (91.3) | 48 (92.3) | | 55 (93.2) | 14 (88.5) | |
| Mucinous
adenocarcinoma | 1 (3.3) | 5 (11.1) | | 2 (8.7) | 4 (7.7) | | 4 (6.8) | 2 (12.5) | |
| T category | | | 0.625 | | | 0.449 | | | 0.523 |
| T1–3 | 9 (30.0) | 10 (22.2) | | 8 (34.8) | 11 (21.1) | | 15 (25.4) | 4 (25.0) | |
| T4a | 16 (53.3) | 24 (53.3) | | 11 (47.8) | 29 (55.8) | | 33 (55.9) | 7 (43.7) | |
| T4b | 5 (16.7) | 11 (24.4) | | 4 (17.4) | 12 (23.1) | | 11 (18.6) | 5 (31.3) | |
| N category | | | 0.119 | | | 0.734 | | | 0.636 |
| N0 | 11 (36.6) | 10 (22.2) | | 8 (34.8) | 13 (25.0) | | 18 (30.5) | 3 (18.8) | |
| N1 | 14 (46.7) | 18 (40.0) | | 9 (39.1) | 23 (44.2) | | 25 (42.4) | 7 (43.7) | |
| N2 | 5 (16.7) | 17 (37.8) | | 6 (26.1) | 16 (30.8) | | 16 (27.1) | 6 (37.5) | |
| Examined lymph
count | | | 0.828 | | | 0.016a | | | 1.000 |
| <12 | 8 (26.7) | 11 (24.4) | | 10 (43.5) | 9 (17.3) | | 15 (25.4) | 4 (25.0) | |
| ≥12 | 22 (73.3) | 34 (75.6) | | 13 (56.5) | 43 (82.7) | | 44 (74.6) | 12 (75.0) | |
| Lymphatic
invasion | | | 0.336 | | | 0.540 | | | 0.135 |
| ly0 | 14 (46.7) | 16 (35.6) | | 8 (34.8) | 22 (42.3) | | 21 (35.6) | 9 (56.3) | |
| ly1 | 16 (53.3) | 29 (64.4) | | 15 (65.2) | 30 (57.7) | | 38 (64.4) | 7 (43.7) | |
| Venous
invasion | | | 0.746 | | | 1.000 | | | 0.692 |
| v0 | 5 (16.7) | 6 (13.3) | | 3 (13.0) | 8 (15.4) | | 8 (13.6) | 3 (18.8) | |
| v1 | 25 (83.3) | 39 (86.7) | | 20 (87.0) | 44 (84.6) | | 51 (86.4) | 13 (81.2) | |
| Concurrent
metastasis | | | 0.840 | | | 0.465 | | | 0.242 |
| Peritoneum
alone | 20 (66.7) | 31 (68.9) | | 17 (74.0) | 34 (65.4) | | 38 (64.4) | 13 (81.2) | |
| Other
metastasis | 10 (33.3) | 14 (31.1) | | 6 (26.0) | 18 (34.6) | | 21 (35.6) | 3 (18.8) | |
| Presentation | | | 0.134 | | | 0.215 | | | 0.842 |
| Synchronous
metastasis | 17 (56.7) | 33 (73.3) | | 13 (56.5) | 37 (71.2) | | 39 (66.1) | 11 (68.7) | |
| Metachronous
metastasis | 13 (43.3) | 12 (26.7) | | 10 (43.5) | 15 (28.8) | | 20 (33.9) | 5 (31.3) | |
| Peritoneal cancer
index | | | 0.511 | | | 0.263 | | | 0.788 |
| <10 | 24 (80.0) | 31 (68.9) | | 17 (74.0) | 38 (73.1) | | 42 (71.2) | 13 (81.3) | |
| 10–20 | 4 (13.3) | 11 (24.4) | | 3 (13.0) | 12 (23.1) | | 13 (22.0) | 2 (12.5) | |
| >20 | 2 (6.7) | 3 (6.7) | | 3 (13.0) | 2 (3.8) | | 4 (6.8) | 1 (6.2) | |
| Status of
metastasectomy | | | 0.419 | | | 0.465 | | | 0.499 |
| Complete
resection | 22 (73.3) | 29 (64.4) | | 17 (74.0) | 34 (65.4) | | 39 (66.1) | 12 (75.0) | |
| Residual
metastasis (+) | 8 (26.7) | 16 (35.6) | | 6 (26.0) | 18 (34.6) | | 20 (33.9) | 4 (25.0) | |
| Chemotherapy after
metastasectomy | | | 0.689 | | | 0.358 | | | 1.000 |
| Adjuvant
chemotherapy (−) | 8 (26.7) | 9 (20.0) | | 4 (17.4) | 13 (25.0) | | 13 (22.0) | 4 (25.0) | |
| Adjuvant
chemotherapy (+) | 14 (46.7) | 20 (44.4) | | 13 (56.5) | 21 (40.4) | | 26 (44.1) | 8 (50.0) | |
Immunohistochemical expression in
peritoneal nodules and prognosis
The impact of immunohistochemical expression in
peritoneal nodules on prognosis was similar to that of the primary
tumour. The expression of CD133, ALDH1 and Lgr5 was not associated
with overall survival (46.4 vs. 20.9%, P=0.617 for CD133; 35.7 vs.
26.9%, P=0.576 for ALDH1; and 29.0 vs. 34.8%, P=0.679 for Lgr5)
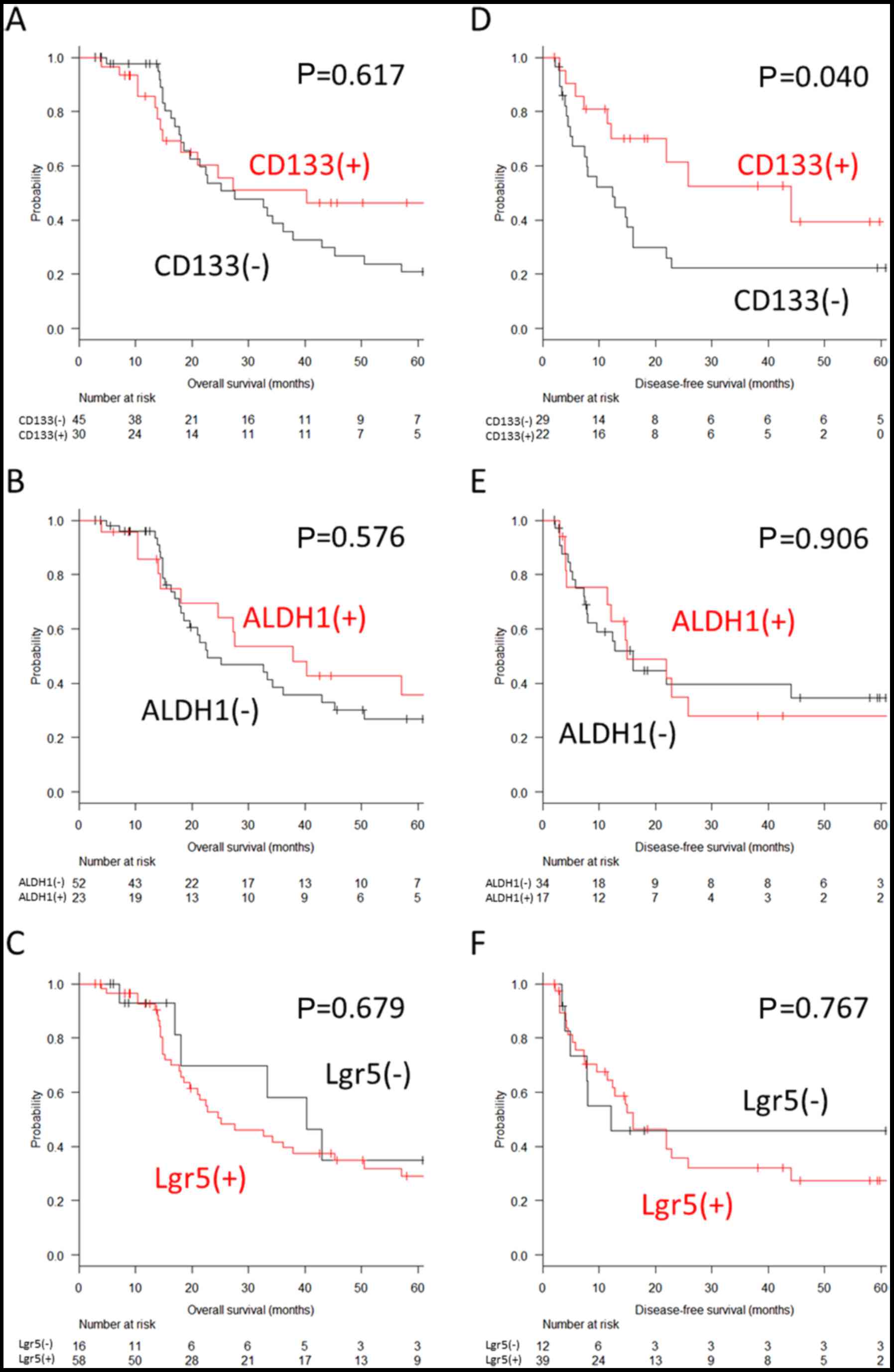
(Fig. 5A–C). By contrast, the
5-year disease-free survival after complete surgery was
significantly superior in the patients with CD133-positive
peritoneal nodules (39.5 vs. 22.4%, P=0.040). However, no
significant difference was observed in the expression of ALDH1 and
Lgr5 in peritoneal nodules (27.9 vs. 34.7%, P=0.906 for ALDH1; and
27.5 vs. 45.8%, P=0.767 for Lgr5) (Fig. 5D–F).
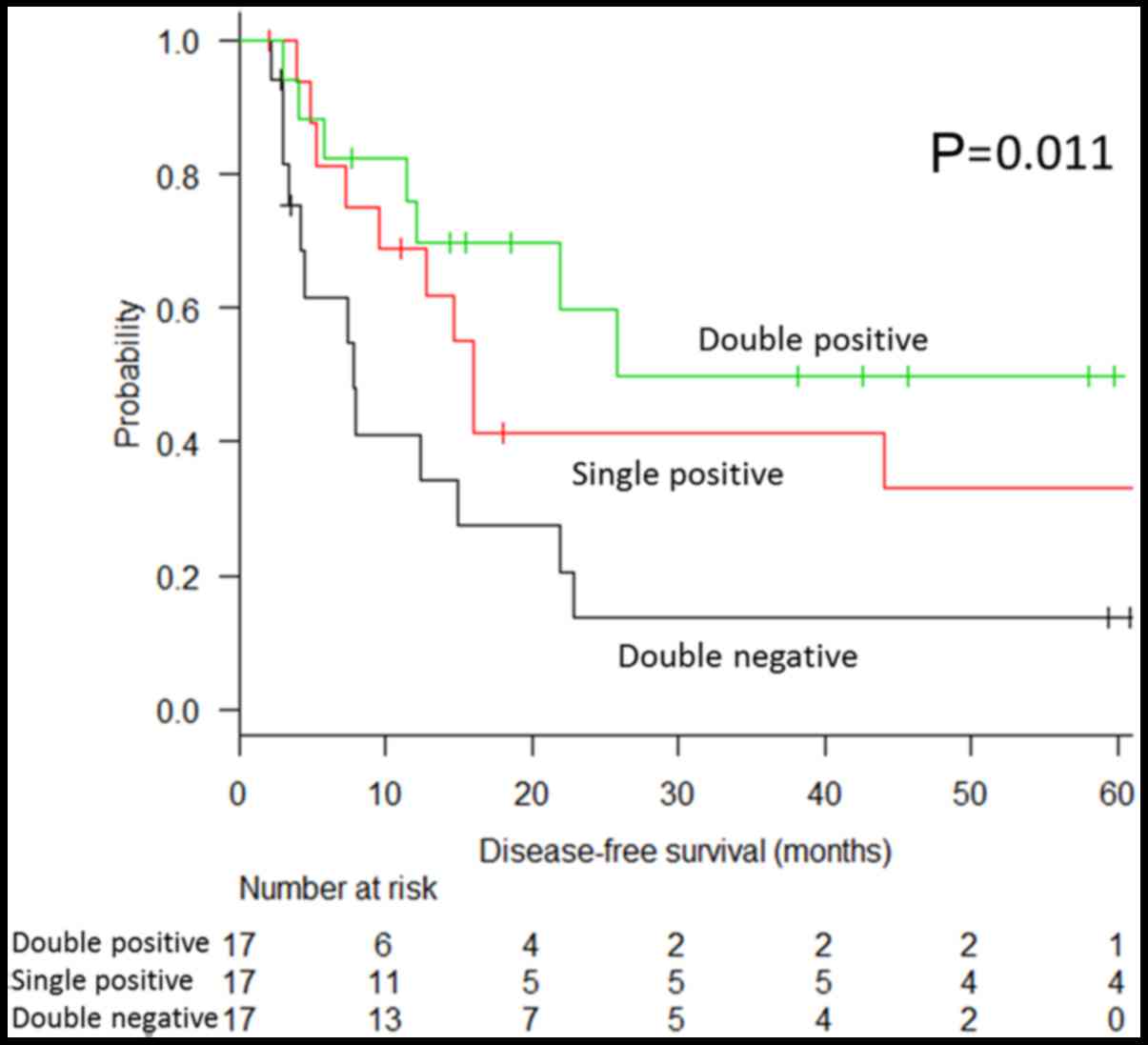
The CD133 expression patterns were clearly
associated with disease-free survival. The optimal survival was
observed when both the primary tumour and peritoneal nodules were
CD133-positive (double positive, Fig.
6), and this was followed by the survival of patients with
CD133 positivity either in the primary tumour or peritoneal nodules
(single positive). The worst survival was observed when CD133
expression was negative in both the primary tumour and peritoneal
nodule (double negative) (49.8, 33.0 and 13.7%, respectively;
P-value for the trend, P=0.011) (Fig.
7).
CD133 expression and
chemosensitivity
We further evaluated the sensitivity to chemotherapy
according to CD133 expression. Systemic chemotherapy significantly
improved the overall survival of the CD133-negative group (HR,
0.19; 95% CI, 0.07–0.50, P<0.001) while the risk reduction in
the CD133-positive group was not statistically significant (HR,
0.61; 95% CI, 0.27–1.38, P=0.231). Furthermore, the positive impact
of systemic chemotherapy in the CD133-negative group was
significantly greater than that in the CD133-positive group
(P-value for the interaction, P=0.039).
With regard to adjuvant chemotherapy following
resection, the risk reduction in disease-free survival was
statistically significant only in the CD133-negative group (HR,
0.48; 95% CI, 0.18–1.25, P=0.132 in the CD133-positive group; and
HR, 0.24; 95% CI, 0.07–0.80, P=0.021 in the CD133-negative group).
However, the impact was not statistically significant between the 2
groups (P-value for the interaction, P=0.345).
Discussion
In this study, we investigated the expression of 3
cancer stem cell markers in patients with colon cancer with
peritoneal metastasis, and revealed that CD133-negative patients
had a reduced disease-free survival following resection. To the
best of our knowledge, this is the first study to examine the
impact of stem cell marker expression on the prognosis of patients
with colon cancer with peritoneal metastasis. Additionally, we
found that patients with CD133-negative disease were more sensitive
to chemotherapy than those with CD133-positive disease. Therefore,
our data suggest that CD133 may be a useful clinical biomarker for
the selection of patients who may receive optimal benefits from
adjuvant chemotherapy following metastasectomy.
The impact of CD133 on survival has not yet been
fully clarified. Based on the cancer stem cell theory,
CD133-positive tumours should have a worse prognosis. In fact, a
number of studies, including 2 meta-analyses, have shown reduced
overall survival in patients with CD133-positive colorectal cancer
(14–17). However, studies on liver metastasis
of colorectal origin have reported contradictory results,
suggesting that the absence of CD133 expression is associated with
a poor prognosis (7–9), which is consistent with our
finding.
In the present study, we demonstrated that the
CD133-negative group had a higher risk of recurrence following the
resection of peritoneal metastasis. We employed 2 other stem cell
markers, ALDH1 and Lgr5, in order to evaluate the impact of
stemness, as CD133 cannot be designated as a definitive marker of
stemness. For instance, while CD133-positive cells have been
reported to have higher tumour initiation ability, CD133-negative
subsets can also initiate tumours (18). Considering that a similar impact
was not recognised in the expression of ALDH1 and Lgr5, the
significance of CD133 identified in this study was presumed to be
caused by CD133-specific factors other than stemness.
Although the findings on the function of CD133 are
limited, in vitro studies have reported that E-cadherin
expression is significantly lower (8) and that β1-integrin expression is
higher (19) in CD133-negative
cells than in CD133-positive cells. Other studies have found that
CD133 is mainly expressed in well- and moderately-differentiated
adenocarcinomas, and that CD133 negativity may reflect a more
undifferentiated state (20,21),
which is consistent with our findings. Therefore, CD133-negative
cells may have a greater capacity to induce metastasis and
recurrence than CD133-positive cells.
By contrast, we demonstrated in this study that the
benefit of systemic chemotherapy was more evident in CD133-negative
disease than in CD133-positive disease, which was consistent with
the findings of previous studies (22,23).
This difference in chemosensitivity may explain why the
CD133-negative group was not inferior to the CD133-positive group
in terms of overall survival, despite the higher risk of
recurrence.
To the best of our knowledge, this is the first
study to examine the expression of cancer stem cell markers in
nodules of peritoneal metastasis. We found that the expression rate
of CD133 and ALDH1 was lower in peritoneal nodules than in primary
tumours. The result was contrary to our assumption prior to the
study, in that we had considered that the expression of cancer stem
cell marker would be higher in peritoneal nodules, since each
nodule should have a tumour initiation ability and peritoneal
metastasis was known to be resistant to chemotherapy. The reason
for the low expression is not clear; however, it may be related to
the characteristics of CD133-negative cells that we described
earlier. In particular, β1-integrin is known to be a key factor for
developing peritoneal metastasis (24). ALDH1, in contrast, is a detoxifying
enzyme contributing to differentiation and proliferation (25). It may represent the slow-growing
nature of peritoneal nodules of colon cancer.
A major limitation of the present study was that
none of our patients underwent peritonectomy or HIPEC, which is
performed in specialised centres as an effective treatment for
peritoneal metastasis of colorectal origin (26). Since the procedure has not been
popular in the Japanese clinical setting, we have been performing
macroscopic peritoneal nodule resection and have reported the
survival benefit (27). Although
our findings cannot be directly applied to patients who underwent
peritonectomy and HIPEC, we speculate that the CD133 expression
pattern may also be a useful indicator of recurrence risk in
patients who undergo peritonectomy and HIPEC.
Another limitation is that the evaluation method of
CD133 expression widely varies according to different reports,
particularly in terms of the anti-CD133 antibody and the cut-off
value (14,15). We believe that the
immunohistochemical evaluation of CD133 is clinically practicable
since the antibody is easily available and the analysis can be
performed in many medical facilities. However, as variation in the
evaluation method can cause inconsistent outcomes, standardised
assessment criteria should be established. In addition, due to the
small sample size of the study, the significance of ALDH1 and Lgr5
may be underestimated. Therefore, the findings of this study
require confirmation in a larger patient cohort.
In conclusion, in this study, we demonstrated that a
negative CD133 expression was a significant risk factor for
post-operative recurrence following resection in patients with
colon cancer with peritoneal metastasis. In addition, we also found
that CD133-negative tumours were more sensitive to chemotherapy
than CD133-positive ones. Our data suggest that CD133 expression
may be a useful clinical biomarker in the treatment of peritoneal
metastasis of colon cancer; however, the oncological function of
CD133 requires further clarifications in future studies.
Abbreviations:
|
ALDH1
|
aldehyde dehydrogenase-1
|
|
Lgr5
|
leucine-rich repeating G-protein
coupled receptor-5
|
|
HIPEC
|
hyperthermic intraperitoneal
chemotherapy
|
|
CT
|
computed tomography
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
Acknowledgments
This study was supported by Grants-in-Aid for
Scientific Research from Japan Society for the Promotion of Science
(grant nos. 16H02672, 16K07143, 16K07161, 17K10620, 17K10621 and
17K10623) and the Project for Cancer Research and Therapeutic
Evolution from the Japan Agency for Medical Research and
Development (grant no. 16cm0106502h0001). The authors would like to
thank Editage (http://www.editage.com) for editing
and reviewing this manuscript for English language.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Watanabe T, Muro K, Ajioka Y, Hashiguchi
Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S,
et al Japanese Society for Cancer of the Colon and Rectum: Japanese
Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016
for the treatment of colorectal cancer. Int J Clin Oncol. Mar
27–2017.Epub ahead of print. View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steele SR, Chang GJ, Hendren S, Weiser M,
Irani J, Buie WD and Rafferty JF; Clinical Practice Guidelines
Committee of the American Society of Colon and Rectal Surgeons:
Practice guideline for the surveillance of patients after curative
treatment of colon and rectal cancer. Dis Colon Rectum. 58:713–725.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vermeulen L, de Sousa e Melo F, Richel DJ
and Medema JP: The developing cancer stem-cell model: Clinical
challenges and opportunities. Lancet Oncol. 13:e83–e89. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kishikawa J, Kazama S, Oba K, Hasegawa K,
Anzai H, Harada Y, Abe H, Matsusaka K, Hongo K, Oba M, et al: CD133
Expression at the metastatic site predicts patients’ outcome in
colorectal cancer with synchronous liver metastasis. Ann Surg
Oncol. 23:1916–1923. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto S, Tanaka K, Takeda K, Akiyama H,
Ichikawa Y, Nagashima Y and Endo I: Patients with CD133-negative
colorectal liver metastasis have a poor prognosis after
hepatectomy. Ann Surg Oncol. 21:1853–1861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pitule P, Cedikova M, Daum O, Vojtisek J,
Vycital O, Hosek P, Treska V, Hes O, Kralickova M and Liska V:
Immunohistochemical detection of cancer stem cell related markers
CD44 and CD133 in metastatic colorectal cancer patients. BioMed Res
Int. 2014:4321392014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th. Wiley-Blackwell;
West Sussex: 2010
|
|
11
|
Maeda S, Shinchi H, Kurahara H, Mataki Y,
Maemura K, Sato M, Natsugoe S, Aikou T and Takao S: CD133
expression is correlated with lymph node metastasis and vascular
endothelial growth factor-C expression in pancreatic cancer. Br J
Cancer. 98:1389–1397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saigusa S, Inoue Y, Tanaka K, Toiyama Y,
Matsushita K, Kawamura M, Okugawa Y, Hiro J, Uchida K, Mohri Y, et
al: Clinical significance of LGR5 and CD44 expression in locally
advanced rectal cancer after preoperative chemoradiotherapy. Int J
Oncol. 41:1643–1652. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collett D: Modelling Survival Data in
Medical Research. 3rd. CRC Press; London: 2015
|
|
14
|
Chen S, Song X, Chen Z, Li X, Li M, Liu H
and Li J: CD133 expression and the prognosis of colorectal cancer:
A systematic review and meta-analysis. PLoS One. 8:e563802013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Peng J, Zhang E, Jiang N, Li J,
Zhang Q, Zhang X and Niu Y: CD133 expression may be useful as a
prognostic indicator in colorectal cancer, a tool for optimizing
therapy and supportive evidence for the cancer stem cell
hypothesis: A meta-analysis. Oncotarget. 7:10023–10036.
2016.PubMed/NCBI
|
|
16
|
Horst D, Scheel SK, Liebmann S, Neumann J,
Maatz S, Kirchner T and Jung A: The cancer stem cell marker CD133
has high prognostic impact but unknown functional relevance for the
metastasis of human colon cancer. J Pathol. 219:427–434. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kemper K, Versloot M, Cameron K, Colak S,
de Sousa e Melo F, de Jong JH, Bleackley J, Vermeulen L, Versteeg
R, Koster J, et al: Mutations in the Ras-Raf Axis underlie the
prognostic value of CD133 in colorectal cancer. Clin Cancer Res.
18:3132–3141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shmelkov SV, Butler JM, Hooper AT, Hormigo
A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, et
al: CD133 expression is not restricted to stem cells, and both
CD133+ and CD133− metastatic colon cancer
cells initiate tumors. J Clin Invest. 118:2111–2120.
2008.PubMed/NCBI
|
|
19
|
Hongo K, Tanaka J, Tsuno NH, Kawai K,
Nishikawa T, Shuno Y, Sasaki K, Kaneko M, Hiyoshi M, Sunami E, et
al: CD133(−) cells, derived from a single human colon cancer cell
line, are more resistant to 5-fluorouracil (FU) than CD133(+)
cells, dependent on the β1-integrin signaling. J Surg Res.
175:278–288. 2012. View Article : Google Scholar
|
|
20
|
Horst D, Kriegl L, Engel J, Kirchner T and
Jung A: CD133 expression is an independent prognostic marker for
low survival in colorectal cancer. Br J Cancer. 99:1285–1289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ueno H, Murphy J, Jass JR, Mochizuki H and
Talbot IC: Tumour ‘budding’ as an index to estimate the potential
of aggressiveness in rectal cancer. Histopathology. 40:127–132.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ong CW, Kim LG, Kong HH, Low LY, Iacopetta
B, Soong R and Salto-Tellez M: CD133 expression predicts for
non-response to chemotherapy in colorectal cancer. Mod Pathol.
23:450–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stanisavljević L, Myklebust MP, Leh S and
Dahl O: LGR5 and CD133 as prognostic and predictive markers for
fluoropyrimidine-based adjuvant chemotherapy in colorectal cancer.
Acta Oncol. 55:1425–1433. 2016. View Article : Google Scholar
|
|
24
|
Sluiter N, de Cuba E, Kwakman R, Kazemier
G, Meijer G and Te Velde EA: Adhesion molecules in peritoneal
dissemination: Function, prognostic relevance and therapeutic
options. Clin Exp Metastasis. 33:401–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen J, Xia Q, Jiang B, Chang W, Yuan W,
Ma Z, Liu Z and Shu X: Prognostic value of cancer stem cell marker
ALDH1 expression in colorectal cancer: A systematic review and
meta-analysis. PLoS One. 10:e01451642015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Verwaal VJ, van Ruth S, Witkamp A, Boot H,
van Slooten G and Zoetmulder FA: Long-term survival of peritoneal
carcinomatosis of colorectal origin. Ann Surg Oncol. 12:65–71.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagata H, Ishihara S, Hata K, Murono K,
Kaneko M, Yasuda K, Otani K, Nishikawa T, Tanaka T, Kiyomatsu T, et
al: Survival and prognostic factors for metachronous peritoneal
metastasis in patients with colon cancer. Ann Surg Oncol.
24:1269–1280. 2017. View Article : Google Scholar
|