Introduction
Intrahepatic cholangiocarcinoma (ICC) and
extrahepatic cholangiocarcinoma (EHCC) are two subtypes of
cholangiocarcinoma (CC), which is the most common and deadly
malignant tumor in the biliary tract. ICC originates form the liver
parenchyma, and, worldwide, it is the most common hepatic malignant
tumor after hepatocellular carcinoma (HCC). The incidence and
mortality rates of ICC have increased during the last three decades
(1–3). Unfortunately, there are no effective
molecular targets for ICC, as is the case for other cancers, such
as lung and breast cancer (4).
Thus, it is necessary to identify new biomarkers and therapeutic
targets for ICC.
H2A histone family member Z (H2A.Z) is a variant of
histone H2A, serving an important role in regulating chromatin
remodeling, gene expression and other basic cellular processes. The
expression of H2A.Z was first reported in normal mouse tissues,
human HeLa cells and chicken erythrocytes (5). H2A.Z is a key molecule in DNA
replication, chromosome segregation and maintenance of the
heterochromatic status (6–8), and has a pivotal role in regulating
cell cycle transition in yeast (9). Furthermore, H2A.Z is crucial in the
proliferation and differentiation of distributed stem cells (DSCs)
(10,11), and is essential in the early
development of mammals (12).
H2A.Z is overexpressed in multiple malignant tumors, including
breast cancer (13), prostate
cancer (14), bladder cancer
(15) and malignant melanoma
(16). H2A.Z1, an isoform of
H2A.Z, is upregulated in both HCC specimens and cell lines, and
depletion of H2A.Z1 induces the expression of cell cycle inhibitors
cyclin-dependent kinase inhibitor 1A (CDKN1A, also known as p21)
and cyclin-dependent kinase inhibitor 1B (CDKN1B, also known as
p27) in HCC (17). S-phase
kinase-associated protein 2 (Skp2) is part of the E3 ubiquitin
ligase complex that regulates many substrate proteins, including
p27 and p21, through ubiquitination (18). This protein is encoded by
skp2, an established proto-oncogene in many cancers that
promotes cancer progression and metastasis (19,20).
However, the relationship between ICC and H2A.Z, and whether H2A.Z
regulates p27/p21 via Skp2 in ICC remains unclear.
In the present study, H2A.Z was demonstrated to be
highly expressed in ICC tissues compared with normal tissues and
high expression of H2A.Z correlated with poor prognosis in patients
with ICC. H2A.Z promoted ICC progression by regulating cell cycle
transition, reducing cell apoptosis and inhibiting
epithelial-mesenchymal transition (EMT). The results also
demonstrated that reduction of H2A.Z enhanced the antitumor effect
of cisplatin on ICC cells. The present results suggest that H2A.Z
may serve as a diagnostic biomarker and an effective therapeutic
target in ICC.
Materials and methods
Research involving human participants and
animals
For this type of study, informed consent was
obtained from all patients at the original time of collection
(2009–2012) for the storage and use of their tissue. The Clinical
Specimens Ethics Committee of the First Affiliated Hospital of
Zhejiang University School of Medicine (Hangzhou, China) approved
the present research.
All animal experiments were performed according to
the guidelines of the National Institutes of Health (Guide for the
Care and Use of Laboratory Animals, 2011). All experiments were
approved by the Animal Experimental Ethics Committee of the First
Affiliated Hospital of Zhejiang University School of Medicine
(Hangzhou, China), and all procedures performed on animals were in
accordance with the ethical standards of the First Affiliated
Hospital of Zhejiang University School of Medicine.
ICC tissue samples
In the present study, 28 samples of ICC tissues with
matching peritumoral tissues were collected between 2009 and 2012
at the First Affiliated Hospital, Zhejiang University School of
Medicine (Hangzhou, China). Immunohistochemistry was performed as
previously described (21), and
data obtained were used to perform survival analysis. Eight samples
of ICC tissues with paired peritumoral tissues were randomly
selected to measure the expression of H2A.Z. The assessment of the
staining was based on the following formula: Total score = the
score of the % of positively stained cells over total × the score
of the staining intensity. The scores of the % of positively
stained cells were as follows: 0, ≤5%; 1, 5–25%; 2, 25–50%; 3,
50–75%; 4, >75%. The staining intensity scores were as follows:
1, low intensity; 2, medium intensity; 3, high intensity. A total
score ≤6 was considered indicative of low expression of H2A.Z,
while a total score >6 was considered indicative of high
expression of H2A.Z.
Cell culture
Four ICC cell lines (CCLP-1, HCCC-9810, RBE and
HuCCT-1) and a normal human intrahepatic biliary epithelial cell
line (HIBEC) were purchased from Cell Bank of Type Culture
Collection of Chinese Academy of Sciences, (Shanghai, China). Cells
were cultivated according to the protocols from their supplier. All
cell lines were grown in RPMI-1640 complete medium (Biological
Industries, Kibbutz Beit-Haemek, Israel) supplemented with 10%
fetal bovine serum (FBS; Moregate Biotech, Brisbane, Australia),
and were cultured in an incubator of 37°C and 5%
CO2.
Antibodies
For western blotting and immunohistochemistry
analyses, the following antibodies were purchased from Abcam
(Cambridge, UK): anti-H2A.Z (cat no. ab150402; dilution 1:1,000 for
western blot analysis and dilution 1:300 for immunohistochemistry),
anti-p21/WAF1/Cip1 (cat. no. ab109520), anti-p27/Kip1 (cat. no.
ab32034), anti-Skp2 (cat. no. ab124799), anti-cyclinA (cat. no.
ab181591) and matrix metalloproteinase (MMP)2 (cat. no. ab37150).
The following antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA): anti-cyclin-dependent kinase
(CDK) 4 (cat. no. 12790), anti-CDK6 (cat. no. 3136) and anti-CDK2
(cat. no. 2546). Ki67 (cat. no. ab15580; Abcam) was used for the
immunohistochemical analysis of mouse tumor tissue. The following
antibodies were purchased from Epitomics (Burlingame, CA, USA):
anti-β-actin (cat. no. 1854-1), and anti-GAPDH (cat. no. 5632-1). A
kit with antibodies targeting EMT-associated proteins (E-cadherin,
N-cadherin, Slug, Snail, and Vimentin) was obtained from Cell
Signaling Technology, Inc. (cat, no. 9782S). Antibodies,
recognizing total (#9665) and cleaved caspase-3 (#9664), caspase-9
(#9508), Bcl-2 homologous antagonist/killer (Bak) (#12105) and
B-cell lymphoma 2 (Bcl-2) (#15071) were obtained from Cell
Signaling Technology, Inc.
RNA silencing
H2A.Z short interfering (si) RNA (two different
sequences tested; siA, GGAACAUUCUGCAGUAUAAAGG GAG; and siB,
GGACUCUAAAUACUCUAACAGCUGT) and negative control siRNA
(UUCUCCGAACGUGUCACGU) were purchased from Origene Technologies,
Inc. (Rockville, MD, USA). siRNA (10 nM) was transfected with 5
µl/ml Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Firstly, cells
(2×105 per well) were seeded in 6-well plates and
incubated at 37°C for 24 h. A total of 5 µl siRNA was added
into 250 µl RPMI-1640, and 5 µl Lipofectamine 2000
was added to another 250 µl RPMI-1640, and the two solutions
were incubated at 24°C for 5 min. Next, the two solutions were
mixed together and incubated at 24°C for 20 min. The supernatant of
the wells was removed, and the transfection solution and 1.5 ml
RPMI-1640 medium were added and incubated at 37°C for 8 h. After 8
h, the supernatant was removed and 2 ml RPMI-1640 medium with 10%
FBS were added, and the following experiments were performed at 24
h after transfection.
For stable H2A.Z knockdown, H2A.Z short hairpin RNA
(shRNA) lentiviral vectors (lenti-shRNA/H2A.Z) and negative control
lentivirus [expressing green fluorescent protein (GFP)] were
purchased from GeneChem Co., Ltd. (Shanghai, China). Target cells
were infected with lentivirus of shH2A.Z and negative control
groups at the concentration of 1×108 TU/ml. Cells
(2×105 per well) were seeded in 6-well plates and
incubated for 24 h. Next, the supernatant was removed, and 1 ml
RPMI-1640 was mixed with 1×108 TU lentivirus and
1µl polybrene and incubated at 37°C for 6 h. After 6 h, the
supernatant was changed with culture medium. The concentration of
lentivirus and the conditions of infection were according to the
manufacturer's instructions. Cells expressing shH2A.Z were selected
in culture medium containing puromycin (3 µg/ml). The H2A.Z
shRNA target sequence was ACTTGAACTGG CAGGAAAT.
Western blotting
CC tissues and cells were lysed using RIPA lysis
buffer (Beyotime Biotechnology, Shanghai, China) and sonicated.
Lysates containing soluble proteins were collected and stored at
−80°C. Protein concentration was determined by the Bradford assay
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal amounts (30
µg) of proteins were separated by 12% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes for 1.5 h. The
membranes were washed one time with TBS/0.1% Tween-20 (TBST)
buffer, and incubated with a solution containing the primary
antibody (1:1,000) at 4°C overnight. Then, the membranes were
washed three times with TBST, and incubated with a solution
containing the horseradish peroxidase (HRP)-conjugated secondary
antibody (1:3,000) for 1.5 h at 20–25°C. Following incubation, the
membranes were washed three times with TBST. Enhanced
chemiluminescence (ECL) (Guge Biotechnology, China) was used to
detect the immunoreactive bands, according to the manufacturer's
recommendations.
Cell viability assay
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used to assess cell
viability. Cells (5×103 cells/well) were seeded in
96-well plates, and incubated in a humidified incubator for 24, 48,
72 or 96 h. The supernatant in each well was then replaced with 90
µl medium and 10 µl CCK-8 solution, and the cells
were incubated at 37°C for 1 h. The absorbance was detected at 450
nm using a microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA).
Colony formation assay
Cells (1,000 cells/well) were seeded onto 6-well
plates and incubated at 37°C in a humidified incubator. The medium
was changed every 4 days. After two weeks, the cells were washed
with PBS, fixed with 100% methanol for 30 min at room temperature,
and stained with 0.2% crystal violet for 15 min at room
temperature. Following staining, the cells were washed with PBS
three times and colonies were observed under a light microscope and
counted.
Ethynyl deoxyuridine (EdU) assay
Control or H2A.Z knockdown cells (5×104)
were plated in confocal dishes (2 cm diameter), and incubated for
24 h. An EdU cell proliferation assay kit (cat. no. C10310-1;
Ribobio Co., Ltd., Guangzhou, China) was used for the EdU assays,
according to the manufacturer's recommendations.
Cell cycle and cell apoptosis assay
Control or H2A.Z knockdown cells (2×105)
were seeded into 6-well plates, and incubated for 48 h. The cells
were then harvested and fixed in 75% ethanol at −20°C for 24 h.
Following fixation, the cells were resuspended and washed one time
with PBS. Next, 200 µl DNA PREP Stain (Beckman Coulter,
Inc., Brea, CA, USA) was added and the suspension was incubated in
the dark for 20 min at room temperature. Cell cycle analysis was
performed by flow cytometry, using a BD LSR II instrument (BD
Biosciences, San Jose, CA, USA). Modfit LT version 5 was used for
the analysis.
Cell apoptosis assays were performed as previously
described (22). Specifically,
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) assays were performed to evaluate apoptosis, according to
the instructions provided by the manufacturer (Promega, Madison,
WI, USA).
Wound-healing assays
Cells (1×105/well) were seeded in 24-well
plates, and wounds were generated by making a scratch on the plate
using a sterile tip. Cells were washed with PBS and incubated in
culture medium without serum. After the indicated time, the
distance between the two margins of the wound was measured. The
data for the CCLP-1 cells were measured at 0, 48 and 96 h, and the
data for the HCCC-9810 cells were measured at 0, 48 and 120 h,
respectively.
Cell migration and invasion assays
Cell migration and invasion assays were performed
using Millicell Cell Culture Inserts (24-well plates; 8 µm
pore size; Merck KGaA, Darmstadt, Germany). Stably transduced cells
were used for these assays. For the migration assay,
3×104 CCLP-1 cells or 5×104 HCCC-9810 cells
in serum-free medium were seeded on the upper chambers. For the
invasion assays, the membranes of the upper chambers were coated
with 8 µl Matrigel (BD Biosciences) in 32 µl
RPMI-1640 medium for 3 h in a humidified incubator. The cells were
then seeded in the coated upper chambers. RPMI-1640 medium
containing 10% FBS was added into the lower chambers. CCLP-1 cells
and HCCC-9810 cell were incubated for 24 or 48 h for the migration
assays, and 48 or 72 h for the invasion assays, respectively. Then,
the cells on the lower membranes were stained using a Wright-Giemsa
Stain kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) and observed at ×100 magnification. Five fields were
randomly chose and cells were counted upon observation under a
light microscope, the number of cell of average per field was
calculated finally.
Tumor xenograft experiments
A total of 40 BALB/c male nude mice (8 weeks old)
were purchased from Shanghai X-B Animal Ltd., Shanghai, China. All
mice were kept in pathogen-free cages in 26–28°C and 50% humidity.
CCLP-1 expressing shH2A.Z or control cells were resuspended in 100
µl PBS and subcutaneously injected into the right side of
nude mice (5×106 cells/mouse, 10 nude mice per group).
Tumor volumes were measured after 7 days, and every 2 days
afterwards. Tumor volume was calculated using the formula: V
(mm3) = width2 (mm2) × length
(mm)/2. All the mice were sacrificed 3 weeks after the injection
and tumors were harvested for analysis.
To generate a pulmonary metastasis mouse model,
2.5×106 CCLP-1 cells expressing shH2A.Z or control shRNA
were resuspended in 100 µl PBS and injected into another set
of BALB/c nude mice through the tail vein (10 nude mice per group).
The lungs of the mice were collected after 7 weeks. Each lung was
disposed into 30 sections, and H&E staining was performed to
analyze the tumor clusters in the lung tissues. All tumors were
examined.
Cisplatin treatment and H2A.Z knockdown
assays
CCLP-1 cells were used for this experiment. The
cells were divided into four groups: normal control (NC), H2A.Z
knockdown (H2A.Z-KD), NC treated with cisplatin, and H2A.Z-KD
treated with cisplatin. The cells were first seeded in 6-well
plates for 24 h, and then cisplatin (4 µg/ml) was added into
the cell culture medium for 48 h. Cells were then processed as
described.
Statistical analysis
The SPSS 22.0 software (IBM Corp., Armonk, NY, USA)
was used for statistical analyses. Analysis of variance was used
for multiple comparison among groups, and Student-Newman-Keuls test
was used as a post hoc test. Comparisons between two groups were
assessed using a two-tailed Student's t-test. The Kaplan-Meier
method was used to assess the overall survival rate of patients.
Data were presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
H2A.Z is highly expressed in ICC tissues
and is associated with poor prognosis in patients with ICC
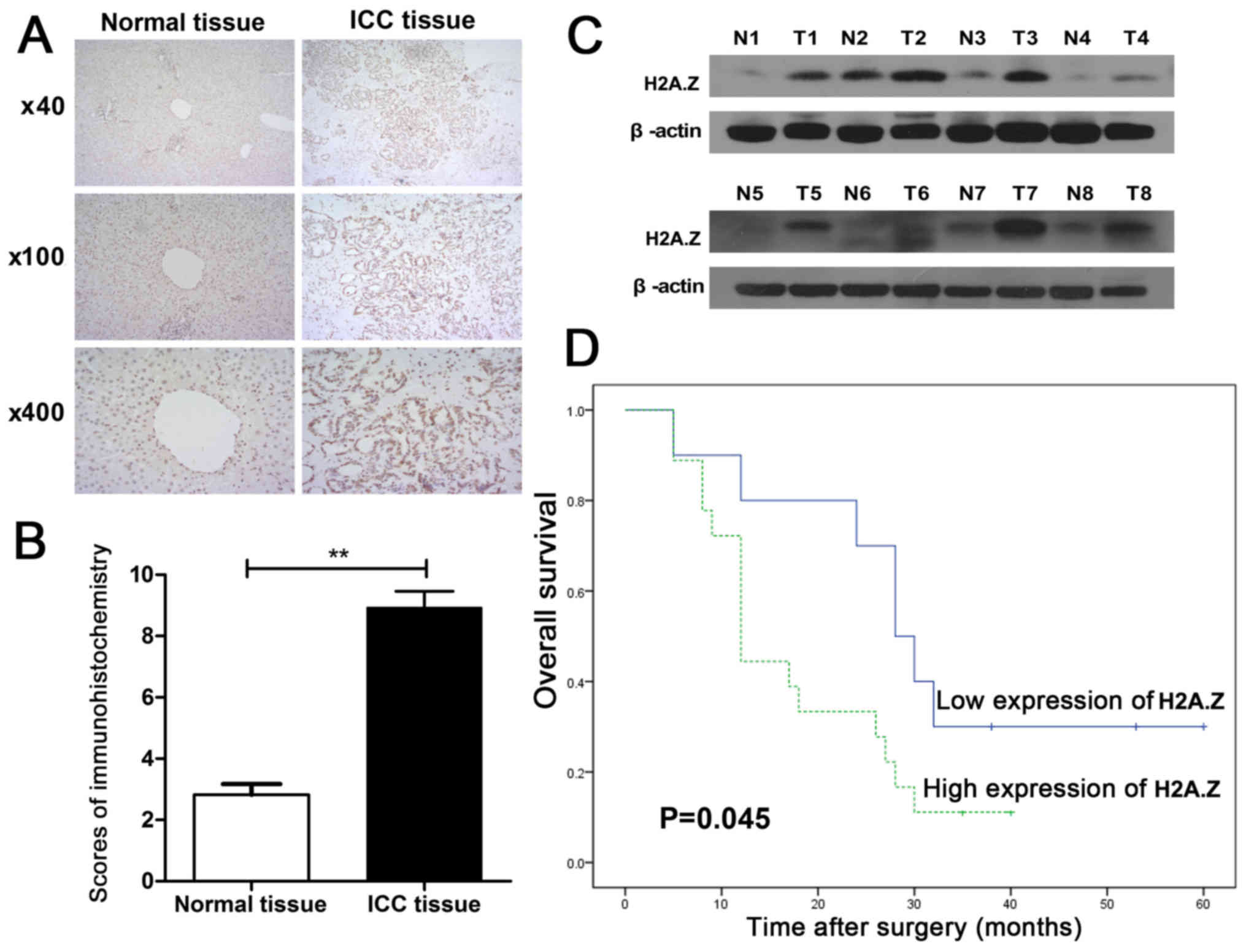
First, immunohistochemistry analysis was performed
on the 28 pairs of ICC specimens. The clinicopathological features
of the patients that the specimens were derived from are listed in
Table I. H2A.Z was overexpressed
in the cancer tissues, compared with their paired non-cancer
samples (Fig. 1A and B).
Additionally, H2A.Z expression was significantly associated with
the tumor, node and metastasis (TNM) stage of the samples (P=0.022;
Table I). Eight pairs of ICC
samples were randomly chosen to determine the protein expression
levels of H2A.Z by western blotting. As expected, the results of
the western blot analysis also revealed that the expression of
H2A.Z in ICC tissues was markedly higher compared with their paired
non-cancerous tissues (Fig. 1C).
To elucidate the association between H2A.Z expression and overall
survival, the 28 patients whose ICC samples were analyzed in the
present study were divided into two groups: H2A.Z high expression
group (n=18) and H2A.Z low expression group (n=10). Kaplan-Meier
analysis indicated that the overall survival rate of the H2A.Z high
expression cluster was higher compared with the H2A.Z low
expression cluster (P=0.045; Fig.
1D).
 | Table IAssociation between H2A.Z expression
and clinicopathological features. |
Table I
Association between H2A.Z expression
and clinicopathological features.
| Variable | H2A.Z expression
| P-value |
|---|
| Low (n=8) | High (n=20) |
|---|
| Sex | | | |
| Male | 1 | 5 | 0.432 |
| Female | 7 | 15 | |
| Age (years) | | | |
| ≤54 | 1 | 8 | 0.17 |
| >54 | 7 | 12 | |
| Tumor size
(cm) | | | |
| ≤5 | 4 | 4 | 0.131 |
| >5 | 4 | 16 | |
| Metastasis | | | |
| Yes | 5 | 13 | 0.615 |
| No | 3 | 7 | |
| TNM stage | | | |
| I–II | 6 | 5 | 0.022 |
| III–IV | 2 | 15 | |
| Tumor number | | | |
| ≤1 | 7 | 15 | 0.432 |
| >1 | 1 | 5 | |
| CA-19-9 (U/ml) | | | |
| ≤40 | 3 | 7 | 0.615 |
| >40 | 5 | 13 | |
Knockdown of H2A.Z inhibits cell
proliferation of ICC cells in vitro
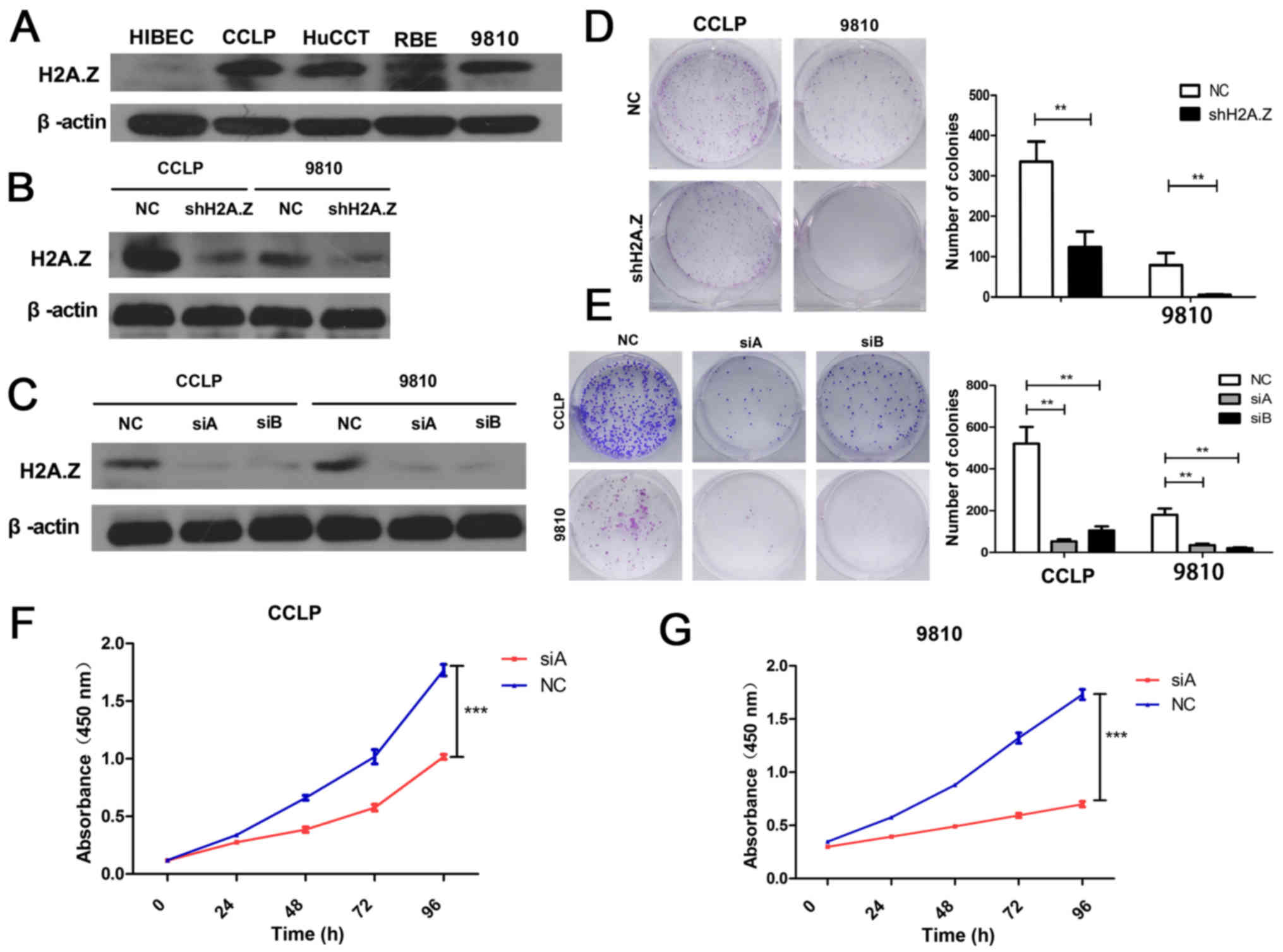
To better understand the role of H2A.Z in ICC, the
expression of H2A.Z was investigated in HIBEC cells and ICC cell
lines (CCLP-1, HuCCT-1, RBE and HCCC-9810). The expression of H2A.Z
in the ICC cell lines was higher compared with the normal HIBEC
cells (Fig. 2A). The two cell
lines with significantly higher H2A.Z expression (CCLP-1 and
HCCC-9810) were selected to perform additional experiments. To
better explore the functional role of H2A.Z in ICC, H2A.Z
expression was silenced in CCLP-1 and HCCC-9810 cells. The effect
of knockdown was confirmed by western blot analysis (Fig. 2B and C). CCK-8 and colony formation
assays were performed to examine the role of H2A.Z in the
proliferation of ICC cells. H2A.Z knockdown inhibited cell
viability and colony formation ability in both CCLP-1 and HCCC-9810
cells (Fig. 2D–G). These results
indicated that H2AZ affects the proliferation of ICC cells.
Knockdown of H2A.Z induces cell cycle
arrest in ICC cells in vitro
Cell cycle arrest, apoptosis or cellular senescence
could explain the effect mediated by the H2A.Z knockdown. In EdU
proliferation assays, the nuclei were weakly stained in the H2A.Z
knockdown group in both CCLP-1 and HCCC-9810 cells (Fig. 3A). In the flow cytometric cell
cycle analyses, the results demonstrated a significant increase in
the % of cells arrested in the G1 and a decrease of the % of cells
in S and G2 phases, in the H2A.Z-silenced group compared with
control group (P<0.05; Fig. 3B and
C). Through western blot analysis, CDK2, CDK4, cyclinA and Skp2
were demonstrated to be downregulated following H2A.Z knockdown,
whereas p21 and p27 were upregulated in both CCLP-1 and HCCC-9810
cells; however, no change was observed in the level of MMP2 after
H2A.Z knockdown (Fig. 3D and E).
These data strongly suggested that H2A.Z knockdown results in the
suppression of Skp2, upregulation of p21 and p27, and
downregulation of CDK2, CDK4, CDK6 and cyclinA in ICC cells.
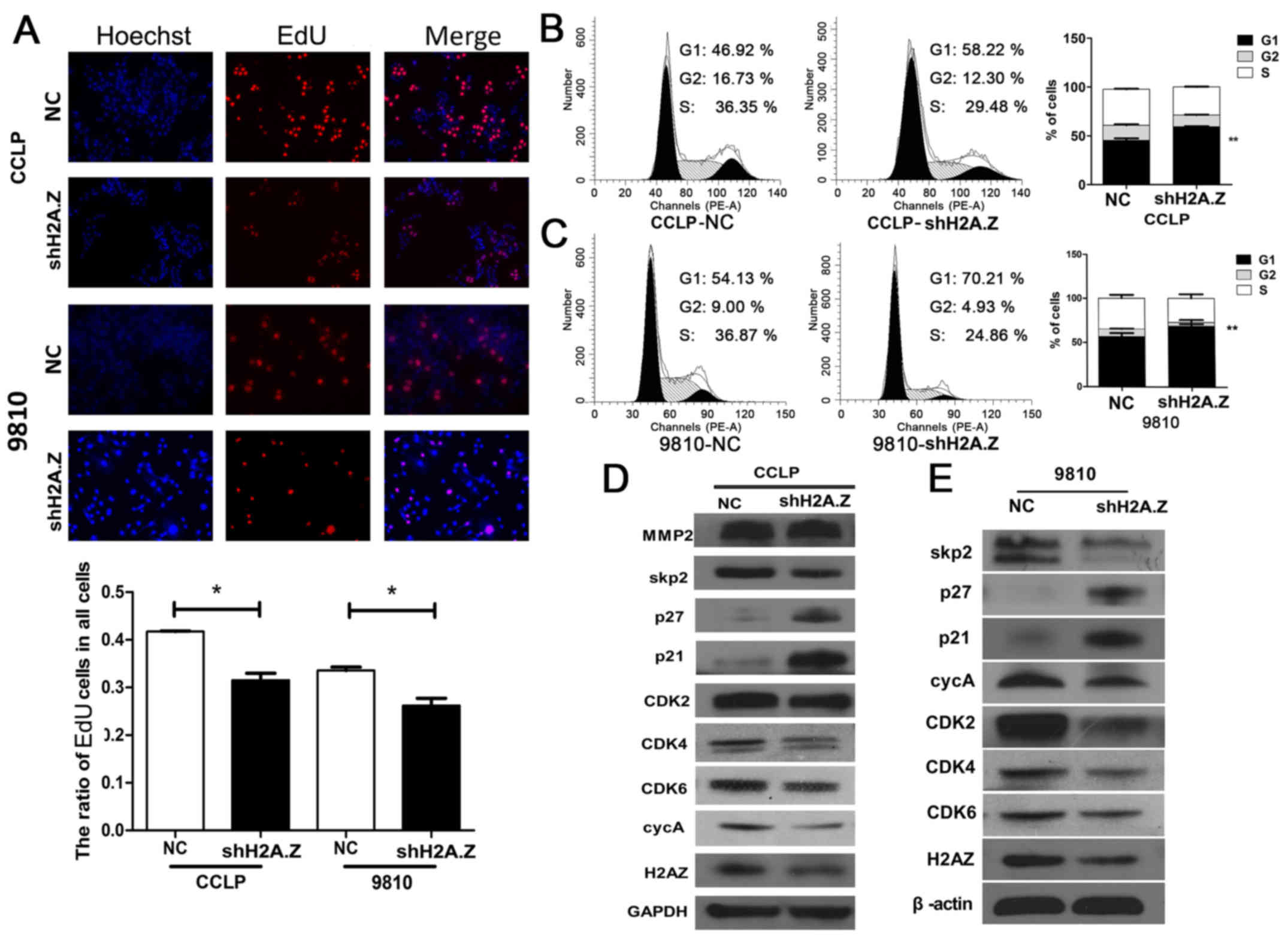 | Figure 3H2A.Z knockdown induces cell cycle
arrest in ICC cells. (A) Cell proliferation was detected by EdU
assay (red signal). Hoechst (blue signal) was used to counterstain
the cell nuclei. Representative images and quantification are
shown. (B and C) The effect of H2A.Z knockdown on cell cycle arrest
was examined by flow cytometry. (D and E) CCLP-1 and HCCC-9810
cells were transfected with H2A.Z shRNA by lentivirus. The cell
lysates were used to perform western blot analysis with the
indicated antibodies and β-actin was used as a loading control.
Every experiment was repeated three times. *P<0.05
and, **P<0.01 compared with NC. H2A.Z, H2A histone
family member Z; ICC, intrahepatic cholangiocarcinoma; EdU, ethynyl
deoxyuridine; sh, short hairpin; NC, negative control; Skp2,
S-phase kinase-associated protein 2; p27, cyclin-dependent kinase
inhibitor 1B; p21, cyclin-dependent kinase inhibitor 1A; CDK,
cyclin-dependent kinase. |
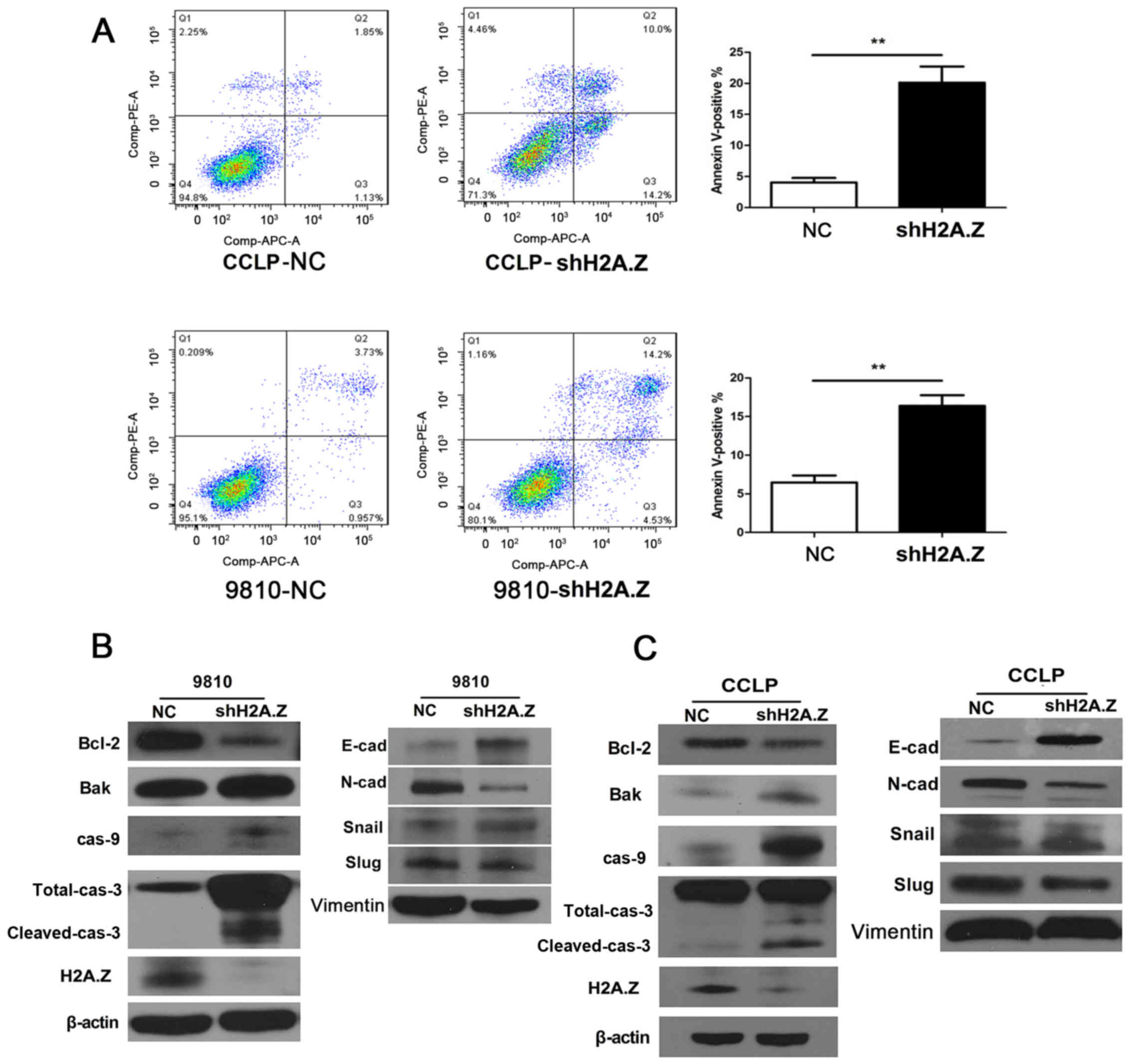
To elucidate the association between H2A.Z and ICC
cell apoptosis, flow cytometry analyses were conducted in CCLP-1
and HCCC-9810 cells. Compared with the negative control group, the
cells in which H2A.Z was knocked down displayed a significantly
increased percentage of apoptotic cells (Fig. 4A). The analysis of expression of
apoptosis-related proteins by western blotting revealed that H2A.Z
knockdown resulted in the downregulation of Bcl-2, and the
upregulation of Bak, caspase-9, and both total and cleaved
caspase-3 (Fig. 4B and C).
Knockdown of H2A.Z inhibits EMT in ICC
cells in vitro
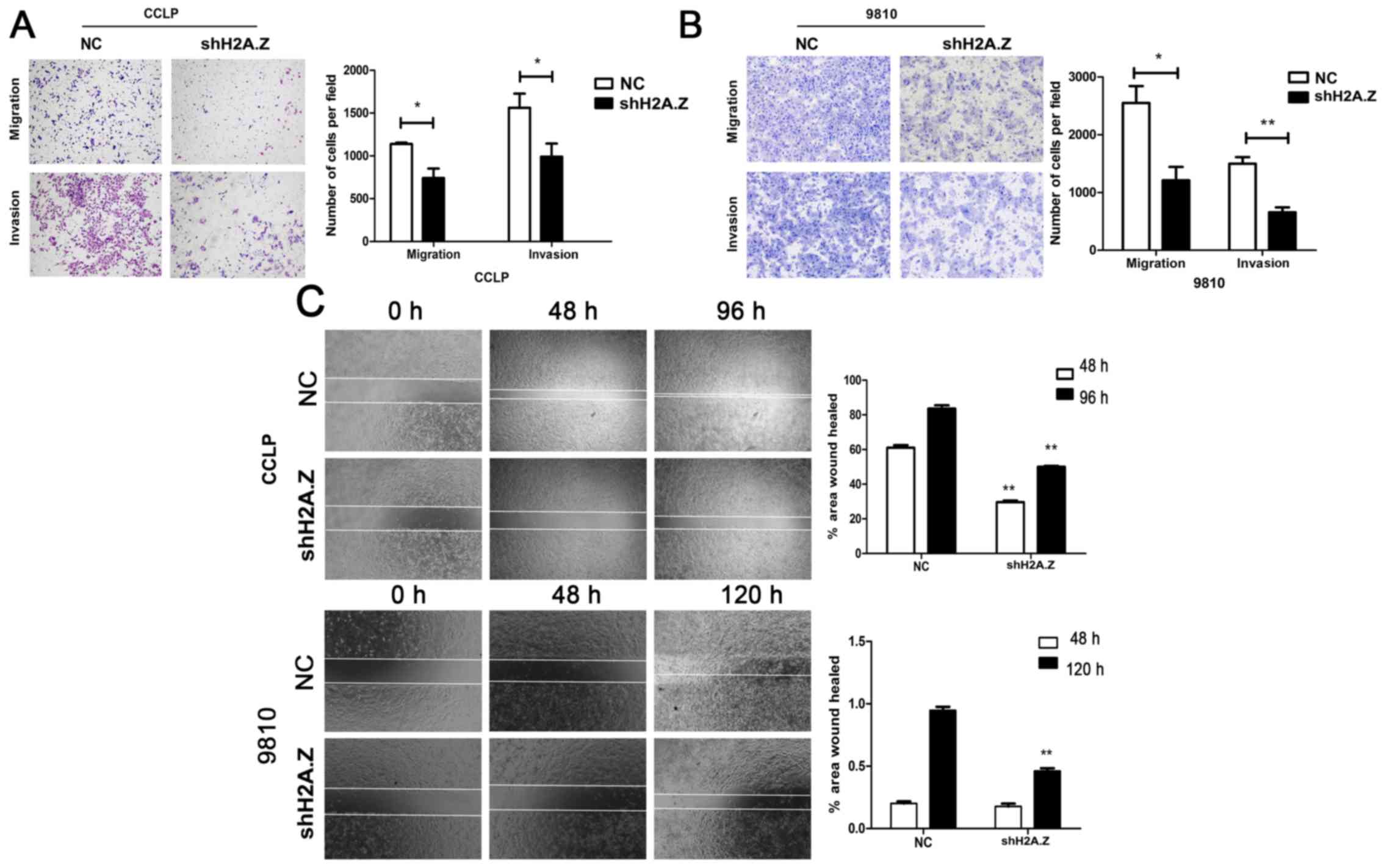
To investigate whether H2A.Z has a role in cell
migration and invasion, Transwell assays were performed. H2A.Z
knockdown significantly decreased the motility and invasion ability
of CCLP-1 and HCCC-9810 cells (Fig. 5A
and B). Similarly, wound healing assays revealed that H2A.Z
silencing was associated with decreased wound healing in ICC cells
(Fig. 5C). To elucidate the
molecular mechanism of H2A.Z in EMT, western blot analysis was
performed for EMT-associated proteins in ICC cells. Notably, H2A.Z
knockdown induced E-cadherin expression, while it inhibited the
expression of N-cadherin, Slug and Snail in CCLP-1 and HCCC-9810
cells (Fig. 4B and C).
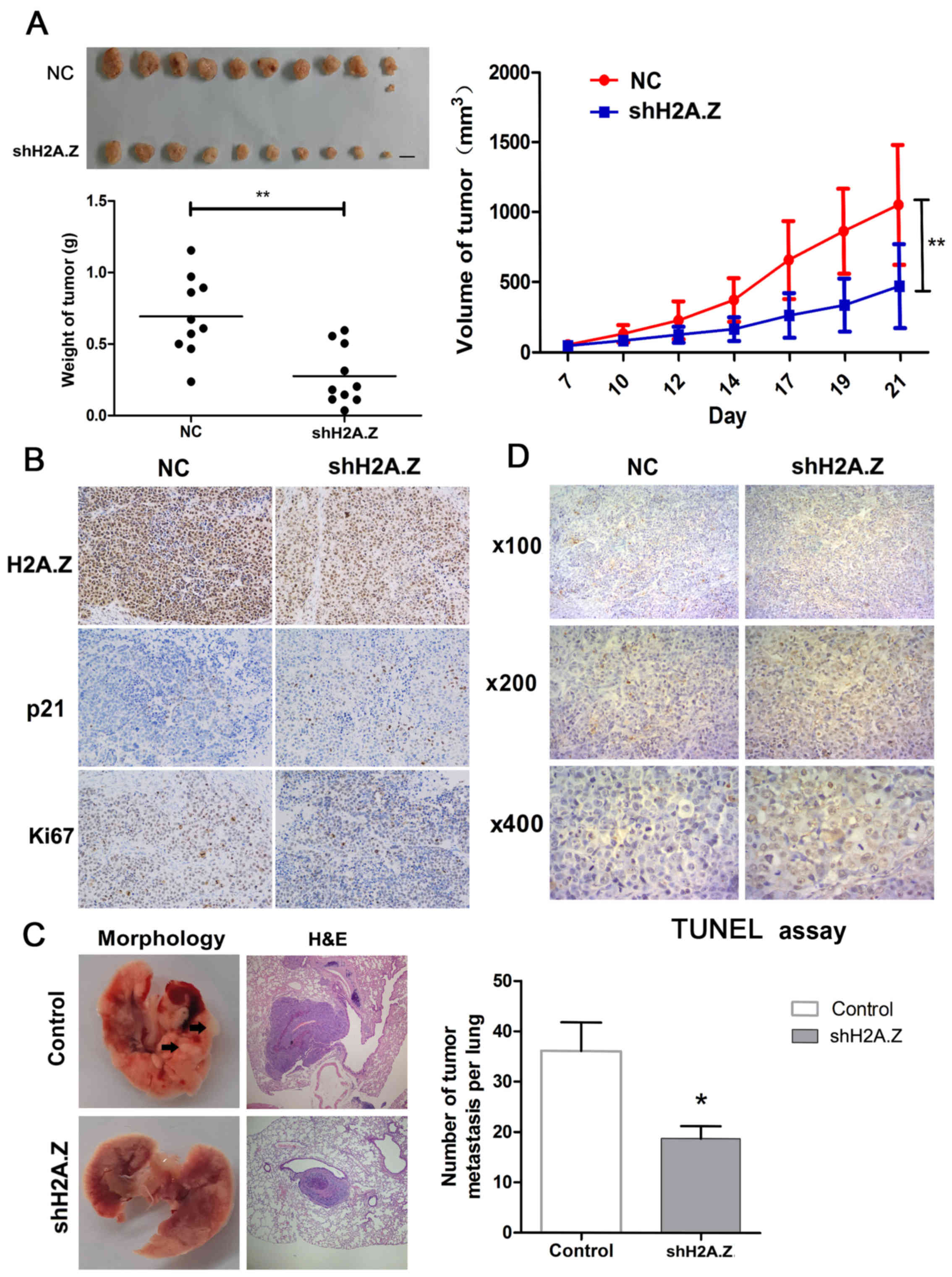
H2A.Z knockdown inhibits tumor growth and
metastasis in vivo
Next, to demonstrate the effect of H2A.Z on ICC
growth in vivo, H2A.Z-silenced or negative control CCLP-1
cells were injected subcutaneously into nude mice (10 mice per
group). Compared with control, tumors derived from the H2A.Z
knockdown cells had significantly reduced tumor growth rate,
average volume and weight (Fig.
6A).
Tumors from the two experimental groups were then
immunostained for H2A.Z, Ki67 and p21. H2A.Z knockdown tumors
exhibited decreased Ki67 staining, but increased p21 staining
(Fig. 6B). In addition, in a
pulmonary experimental metastasis assay, it was demonstrated that
H2A.Z knockdown cells resulted in decreased number of metastases in
the lung (Fig. 6C). H2A.Z
knockdown induced apoptosis in ICC cell lines (Fig. 4A). Therefore, TUNEL assays were
performed in sections from the harvested tumors in order to
investigate whether H2A.Z had the same effect on cell apoptosis
in vivo. As expected, H2A.Z knockdown was associated with a
higher percentage of TUNEL-positive cells in the tumors, compared
with the control group (Fig.
6D).
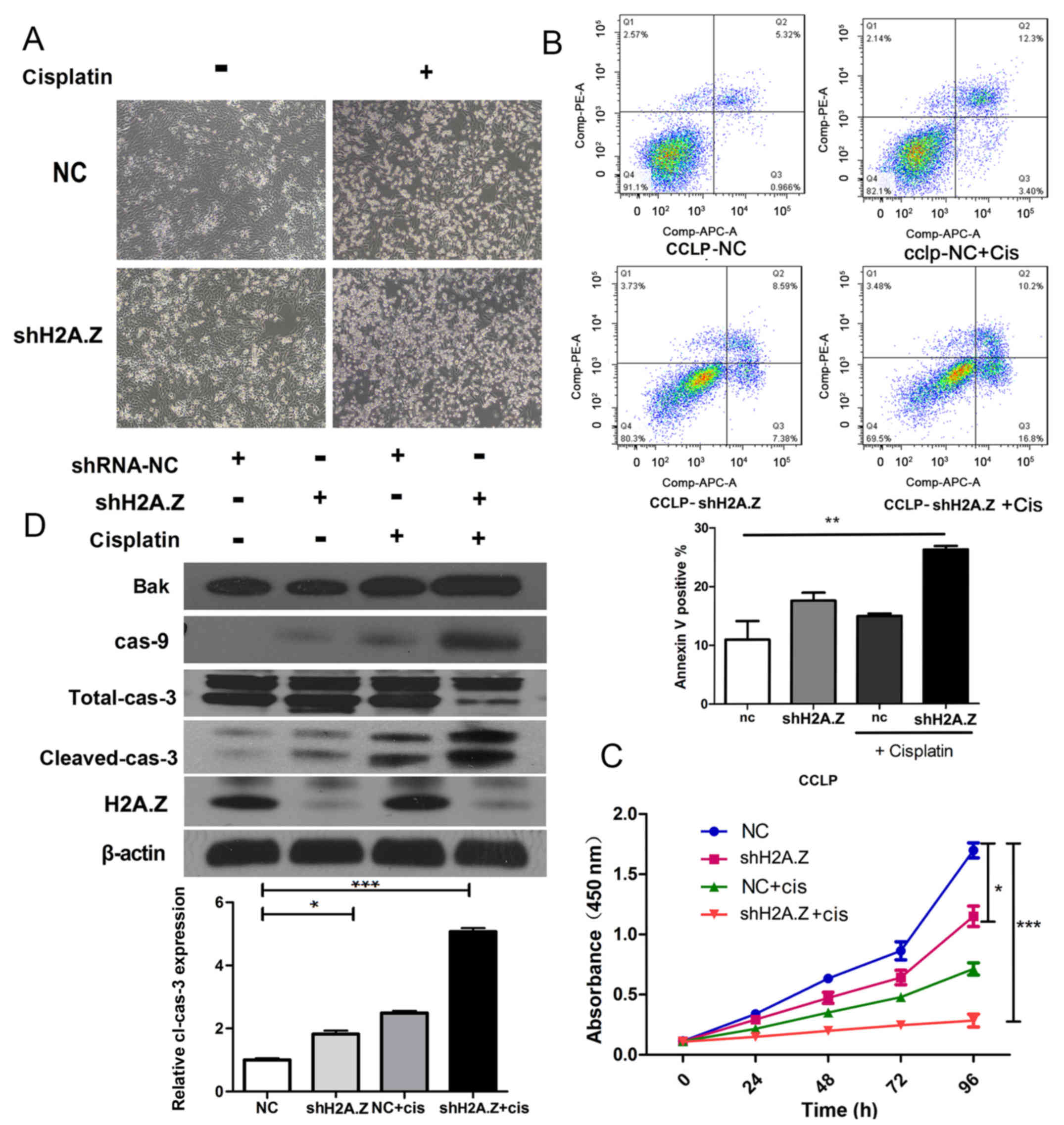
H2A.Z knockdown strengthens the antitumor
effect of cisplatin in ICC cells
Unresectable primary CC is fatal, and the median
survival of these patients without treatment is ~3–6 months
(23). Cisplatin is an effective
antineoplastic drug in the treatment of multiple neoplasms
(24,25). In specific, the hydrolysis product
of cisplatin can react with DNA, producing crosslinks which can
destroy transcription and replication of DNA. Combination of
gemcitabine and cisplatin is the first line chemotherapy for
unresectable CC (26).
Because H2A.Z knockdown induced cell cycle arrest
and apoptosis in ICC cell lines, it was hypothesized that H2A.Z
knockdown might synergistically act with cisplatin in the therapy
of ICC. Indeed, treatment with cisplatin in the H2A.Z-silenced
CCLP-1 cells resulted in increased apoptosis compared with the
normal control group (Fig. 7A and
B). In cell proliferation assays, treatment with cisplatin
combined with H2A.Z knockdown inhibited the proliferation of CCLP-1
cells more powerfully than the single treatments of either
cisplatin or shRNA (P<0.01; Fig.
7C). Next, western blot analysis was performed to evaluate the
molecular mechanism underlying the synergetic effect of H2A.Z
knockdown and cisplatin in ICC cells. Separately, both H2A.Z
knockdown and cisplatin treatment induced the expression of
apoptotic markers. However, the treatment with cisplatin in
H2A.Z-silenced cells resulted in a synergistically increased
expression of apoptosis-related proteins (Fig. 7C). Taken together, these results
suggested that H2A.Z knockdown increased the sensitivity of ICC
cells to cisplatin.
Discussion
In eukaryotes, the nucleosome is the smallest
subunit of chromatin, consisting of histones H2A, H2B, H3, H4 and a
segment of DNA. Nucleosome composition is highly variable and
controls DNA transcription and gene silencing (27). Histone H2A.Z is a variant of
histone H2A; it is an indispensable component of nucleosomes in a
wide variety of organisms, especially vertebrates. H2A.Z is
considered a key chromatin component that regulates DNA
double-strand breaks, chromatin structure formation and DNA
transcription (6,28,29).
H2A.Z also regulates cell proliferation and viability of cancer
cells (17,30,31).
The present study demonstrated that H2A.Z regulated cell cycle and
apoptosis and promoted the proliferation of ICC. Additionally,
H2A.Z was demonstrated to promote ICC metastasis by inducing
EMT.
Skp2 is an oncogene. According to a previous report,
Skp2 is associated with the development of lymphomas (32). Skp2 contains an F-box domain, which
is a component of the SKP1-cullin-F-box (SCF) complex, an E3
ubiquitin ligase complex that catalyzes the ubiquitination of
multiple proteins (33). The
SCF-Skp2 complex specifically controls the ubiquitination-mediated
proteasomal degradation of p21 and p27. Thus, Skp2 controls the
G1/S phase transition of the cell cycle (34,35).
In the present study, knockdown of H2A.Z reduced the expression of
Skp2, followed by upregulation of p21 and p27. These data confirmed
that H2A.Z controls the cell cycle by regulating the expression of
Skp2.
Although the therapeutic effect of chemotherapy is
not always satisfactory, chemotherapy is the treatment of choice
following the surgical resection of a primary tumor (4). Combination of cisplatin and
gemcitabine is considered the standard chemotherapy for the
treatment of ICC. However, cisplatin treatment is associated with
renal toxicity and other side-effects. Therefore, it would be
beneficial to increase the antitumor effects of cisplatin while
reducing its side effects. Cisplatin induces cancer cell apoptosis
and inhibits cell proliferation. In the present study, knockdown of
H2A.Z upregulated the expression of caspase-3 and caspase-9, and
enhanced the effect of cisplatin on ICC cells.
In conclusion, H2A.Z was demonstrated to be highly
expressed in ICC and to correlate with overall survival in patients
with ICC. H2A.Z knockdown inhibited tumor growth and metastasis
in vivo via regulation of cell proliferation and EMT.
Furthermore, H2A.Z knockdown improved the efficacy of cisplatin
treatment in a ICC xenograft mouse model. These findings suggest
that H2A.Z may be a novel biomarker and therapeutic target for
ICC.
Glossary
Abbreviations
Abbreviations:
|
Bak
|
Bcl-2 homologous antagonist/killer
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
CC
|
cholangiocarcinoma
|
|
CDK
|
cyclin-dependent kinase
|
|
DSCs
|
distributed stem cells
|
|
EHCC
|
extrahepatic cholangiocarcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
|
HCC
|
hepatocellular carcinoma
|
|
HIBEC
|
normal human intrahepatic biliary
epithelial cell
|
|
ICC
|
intrahepatic cholangiocarcinoma
|
|
Skp2
|
S-phase kinase-associated protein
2
|
|
TNM
|
tumor, node and metastasis
|
Acknowledgments
The authors would like to thank Dr Heng Xiao and Dr
Meng Li for their technical assistance.
Notes
[1]
Funding
This study was supported by the Innovative Research
Groups of National Natural Science Foundation of China (grant no.
81421062), the National Health and Family Planning Commission of
China (grant no. 20161388643), the Science and Technology
Department of Zhejiang Province (grant no. 2015C03034), the
Zhejiang Provincial Natural Science Foundation (grant no.
LQ15H160002) and the General Project Plan of Zhejiang Medical
Technology (grant no. 2016148875).
[2] Availability
of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
[3] Authors'
contributions
BY, JW and SZ designed the study. BY, RT, HL, JW and
CD performed the experiments. BY, ZX and DC performed the tumor
xenograft experiments. BY and ZX drafted the manuscript. BY, HX and
LZ analyzed the results. All authors have read and approved the
final manuscript.
[4] Ethics
approval and consent to participate
For the human specimens, the Clinical Specimens
Ethics Committee of the First Affiliated Hospital of Zhejiang
University School of Medicine (Hangzhou, China) approved the
present research. Informed consent was obtained from all patients
for the storage and use of their tissue. All animal experiments
were approved by the Animal Experimental Ethics Committee of the
First Affiliated Hospital of Zhejiang University School of Medicine
(Hangzhou, China), and all procedures performed on animals were in
accordance with the ethical standards of the First Affiliated
Hospital of Zhejiang University School of Medicine.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
McGlynn KA, Tarone RE and El-Serag HB: A
comparison of trends in the incidence of hepatocellular carcinoma
and intrahepatic cholangiocarcinoma in the United States. Cancer
Epidemiol Biomarkers Prev. 15:1198–1203. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel T: Increasing incidence and
mortality of primary intra-hepatic cholangiocarcinoma in the United
States. Hepatology. 33:1353–1357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel T: Worldwide trends in mortality
from biliary tract malignancies. BMC Cancer. 2:102002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sia D, Tovar V, Moeini A and Llovet JM:
Intrahepatic cholangiocarcinoma: Pathogenesis and rationale for
molecular therapies. Oncogene. 32:4861–4870. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
West MH and Bonner WM: Histone 2A, a
heteromorphous family of eight protein species. Biochemistry.
19:3238–3245. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rangasamy D, Greaves I and Tremethick DJ:
RNA interference demonstrates a novel role for H2A.Z in chromosome
segregation. Nat Struct Mol Biol. 11:650–655. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dhillon N, Oki M, Szyjka SJ, Aparicio OM
and Kamakaka RT: H2A.Z functions to regulate progression through
the cell cycle. Mol Cell Biol. 26:489–501. 2006. View Article : Google Scholar :
|
|
8
|
Meneghini MD, Wu M and Madhani HD:
Conserved histone variant H2A.Z protects euchromatin from the
ectopic spread of silent heterochromatin. Cell. 112:725–736. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kafer GR, Lehnert SA, Pantaleon M, Kaye PL
and Moser RJ: Expression of genes coding for histone variants and
histone-associated proteins in pluripotent stem cells and mouse
preimplantation embryos. Gene Expr Patterns. 10:299–305. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huh YH, Noh M, Burden FR, Chen JC, Winkler
DA and Sherley JL: Sparse feature selection identifies H2A.Z as a
novel, pattern-specific biomarker for asymmetrically self-renewing
distributed stem cells. Stem Cell Res (Amst). 14:144–154. 2015.
View Article : Google Scholar
|
|
11
|
Noh M, Smith JL and Huh YH: A resource for
discovering specific and universal biomarkers for distributed stem
cells. PLoS One. 6:e220772011.PubMed/NCBI
|
|
12
|
Faast R, Thonglairoam V, Schulz TC, Beall
J, Wells JR, Taylor H, Matthaei K, Rathjen PD, Tremethick DJ and
Lyons I: Histone variant H2A.Z is required for early mammalian
development. Curr Biol. 11:1183–1187. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hua S, Kallen CB, Dhar R, Baquero MT,
Mason CE, Russell BA, Shah PK, Liu J, Khramtsov A, Tretiakova MS,
et al: Genomic analysis of estrogen cascade reveals histone variant
H2A.Z associated with breast cancer progression. Mol Syst Biol.
4:1882008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slupianek A, Yerrum S, Safadi FF and
Monroy MA: The chromatin remodeling factor SRCAP modulates
expression of prostate specific antigen and cellular proliferation
in prostate cancer cells. J Cell Physiol. 224:369–375. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim K, Punj V, Choi J, Heo K, Kim JM,
Laird PW and An W: Gene dysregulation by histone variant H2A.Z in
bladder cancer. Epigenetics Chromatin. 6:342013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vardabasso C, Gaspar-Maia A, Hasson D,
Pünzeler S, Valle-Garcia D, Straub T, Keilhauer EC, Strub T, Dong
J, Panda T, et al: Histone variant H2A.Z.2 mediates proliferation
and drug sensitivity of malignant melanoma. Mol Cell. 59:75–88.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang HD, Kim PJ, Eun JW, Shen Q, Kim HS,
Shin WC, Ahn YM, Park WS, Lee JY and Nam SW: Oncogenic potential of
histone-variant H2A.Z.1 and its regulatory role in cell cycle and
epithelial-mesenchymal transition in liver cancer. Oncotarget.
7:11412–11423. 2016.PubMed/NCBI
|
|
18
|
Oh M, Lee JH, Moon H, Hyun YJ and Lim HS:
A chemical inhibitor of the Skp2/p300 interaction that promotes
p53-mediated apoptosis. Angew Chem Int Ed Engl. 55:602–606. 2016.
View Article : Google Scholar
|
|
19
|
Skaar JR, Pagan JK and Pagano M: SCF
ubiquitin ligase-targeted therapies. Nat Rev Drug Discov.
13:889–903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Liu P, Inuzuka H and Wei W: Roles
of F-box proteins in cancer. Nat Rev Cancer. 14:233–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao H, Tong R, Cheng S, Lv Z, Ding C, Du
C, Xie H, Zhou L, Wu J and Zheng S: BAG3 and HIF-1 α coexpression
detected by immunohistochemistry correlated with prognosis in
hepatocellular carcinoma after liver transplantation. BioMed Res
Int. 2014:5165182014. View Article : Google Scholar
|
|
22
|
Xiao H, Tong R, Yang B, Lv Z, Du C, Peng
C, Ding C, Cheng S, Zhou L, Xie H, et al: TAZ regulates cell
proliferation and sensitivity to vitamin D3 in intrahepatic
cholangiocarcinoma. Cancer Lett. 381:370–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farley DR, Weaver AL and Nagorney DM:
'Natural history' of unresected cholangiocarcinoma: Patient outcome
after noncurative intervention. Mayo Clin Proc. 70:425–429. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pabla N and Dong Z: Cisplatin
nephrotoxicity: Mechanisms and renoprotective strategies. Kidney
Int. 73:994–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nat Rev Drug Discov. 4:307–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: ABC-02 Trial Investigators: Cisplatin plus gemcitabine
versus gemcitabine for biliary tract cancer. N Engl J Med.
362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ausió J: Histone variants - the structure
behind the function. Brief Funct Genomics Proteomics. 5:228–243.
2006. View Article : Google Scholar
|
|
28
|
Jin C and Felsenfeld G: Nucleosome
stability mediated by histone variants H3.3 and H2A.Z. Genes Dev.
21:1519–1529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Y, Ayrapetov MK, Xu C, Gursoy-Yuzugullu
O, Hu Y and Price BD: Histone H2A.Z controls a critical chromatin
remodeling step required for DNA double-strand break repair. Mol
Cell. 48:723–733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gévry N, Chan HM, Laflamme L, Livingston
DM and Gaudreau L: p21 transcription is regulated by differential
localization of histone H2A.Z. Genes Dev. 21:1869–1881. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taty-Taty GC, Courilleau C, Quaranta M,
Carayon A, Chailleux C, Aymard F, Trouche D and Canitrot Y: H2A.Z
depletion impairs proliferation and viability but not DNA
double-strand breaks repair in human immortalized and tumoral cell
lines. Cell Cycle. 13:399–407. 2014. View Article : Google Scholar :
|
|
32
|
Latres E, Chiarle R, Schulman BA,
Pavletich NP, Pellicer A, Inghirami G and Pagano M: Role of the
F-box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci USA.
98:2515–2520. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ang XL and Wade Harper J: SCF-mediated
protein degradation and cell cycle control. Oncogene. 24:2860–2870.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barr AR, Cooper S, Heldt FS, Butera F,
Stoy H, Mansfeld J, Novák B and Bakal C: DNA damage during S-phase
mediates the proliferation-quiescence decision in the subsequent G1
via p21 expression. Nat Commun. 8:147282017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barr AR, Heldt FS, Zhang T, Bakal C and
Novák B: A dynamical framework for the all-or-none G1/S transition.
Cell Syst. 2:27–37. 2016. View Article : Google Scholar : PubMed/NCBI
|