Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide and in China (1,2).
Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer
(3). Despite advances in cancer
research and treatment, the prognosis of NSCLC remains poor, with
the 5-year survival rate being only 15% (4). Although novel drugs including
epithelial growth factor receptor have been demonstrated to be
beneficial, particularly in patients with sensitive target
mutations, the survival and outcome have not altered dramatically
(5). Therefore, it is important to
elucidate the pathogenesis of NSCLC and identify novel treatment
targets.
Copines are a family of calcium-dependent
phospholipid-binding proteins that are evolutionally conserved in
various eukaryotic organisms and protists (6). Currently, nine family members have
been identified (7,8). It has been reported that Copine 1,
encoded by CPNE1, is a soluble membrane-binding protein, which
includes two tandem C2 domains at the N-terminus and an A domain at
the C-terminus (6). The C2 domains
act as calcium-dependent phospholipid binding motifs and may be
associated with cell signalling and/or membrane trafficking
pathways (9). The A domain is
named from von Willebrand Factor, a plasma and extracellular matrix
protein, and have been studied in integrins and several
extracellular matrix proteins and appear to function as
protein-binding domains (10). It
was previously reported that Copine 1 binds with various
intracellular proteins via its A domain (7,8). To
date, the role of CPNE1 in lung cancer remains unclear.
In the present study, the expression of CPNE1 in 128
lung adenocarcinoma patient tissues was initially investigated by
immunohistochemical staining. Higher expression of CPNE1 was
observed to be significantly associated with advanced tumor, node
and metastasis (TNM) stage, lymph node metastasis and distant
metastasis in lung adenocarcinoma. In addition, the results of the
present study indicated that knockdown of CPNE1 inhibits cell cycle
progression, cell growth and migration of NSCLC cells. In
conclusion, it was hypothesized that CPNE1 serves an important role
in tumorigenesis of human lung adenocarcinoma.
Materials and methods
Tissue samples
Following approval by the Ethics Committee of The
First Affiliated Hospital of Soochow University (Suzhou, China), a
group of 128 patients with lung adenocarcinoma were recruited
consecutively from The First Affiliated Hospital of Soochow
University, from March 2009 to December 2012. Those patients
included 67 males and 61 females, and their ages ranged from 20-79
years. Patients had not received chemotherapy or radiotherapy prior
to diagnosis and tissue sampling. Patients were diagnosed with
NSCLC based on their histological and pathological characteristics
according to the Revised International System for Staging Lung
Cancer (11). Lung adenocarcinoma
and adjacent normal tissues from the 128 patients were snap-frozen
and stored at −80°C. All participants provided written informed
consent for the whole study.
Cell culture
The human lung adenocarcinoma A549, H1299, SPC-A1
and HCC827 cell lines and human lung squamous carcinoma cell line
H226 and human immortalized normal epithelial cell line BEAS-2B and
the 293T (293) cell line were obtained from the Cell Bank of
Chinese Academy of Sciences (Shanghai, China). H1299 and A549 cells
were identified by short tandem repeat method (12). Both A549 and H1299 cells were grown
in Dulbecco's modified Eagle's medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) containing 10% fetal bovine serum (FBS)
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). All
cells were maintained in a humidified atmosphere with 5%
CO2 at 37°C. Beijing Microread Genetics Co., Ltd.
(Beijing, China) determined the genetic characteristics of the
cells using a Goldeneye™ 20A kit (Peoplespot, Beijing, China) and
an ABI 3100 genetic analyser (Thermo Fisher Scientific, Inc.). All
cell lines were passaged for <3 months and tested in February
2017.
Immunohistochemical assay
Immunohistochemical analyses of 128 lung
adenocarcinoma and normal tissues were conducted as described in
the present authors' previous study (13). The sections were incubated with
anti-Copine 1 antibodies (1:100; cat. no. ab155675; Abcam,
Cambridge, UK) overnight at 4°C. Then incubated with pre-diluted
biotinylated secondary antibodies for 40 min at 37°C (SV0002; Wuhan
Boster Biological Technology, Ltd., Wuhan, China) according to the
manufacturer's protocol. The reactions were developed using the DAB
Substrate Kit (cat. no. 550880; BD Biosciences, San Jose, CA, USA),
and the sections were counterstained for 2 min at room temperature
with hematoxylin.
The scoring of immunostaining was evaluated on the
basis of staining intensity and percentages of three positively
stained areas at random by two pathologists in a double-blinded
manner. Briefly, the proportion of positive cells in each specimen
was quantitatively evaluated and scored as follows: 0, Staining in
0% of the cells examined; 1, staining in 0.01-10% of the cells
examined; 2, staining in 10.01-50% of the cells examined; 3,
staining in 50.01-75% of the cells examined; and 4, staining in
>75% of the cells examined. The staining intensity was graded as
follows: 0, No signal; 1, weak; 2, moderate; and 3, strong. The
histological score for each section was computed using the
following formula: Histological score = proportion score x
intensity score. A total score with a possible range of 0-12 was
calculated and graded as follows: Negative (-; score, 0), weak (+;
score, 1-4), moderate (++; score, 5-8) or strong (+++; score,
9-12). Scores of - and + were considered to indicate low expression
levels, whereas scores of ++ and +++ were considered to indicate
high expression levels.
Establishment of CPNE1-silenced stable
cell lines
The human CPNE1 (GenBank accession no. NM_003915)
specific small interfering RNA (siRNA) sequence, which was designed
with BLOCK-iT RNAi Designer (Thermo Fisher Scientific, Inc.) was
5′-CACACAACTGGTCTCATACTT-3′. The non-silencing sequence generated
by Shanghai GeneChem Co., Ltd. (Shanghai, China;
5′-TTCTCCGAACGTGTCACGT-3′) was used as a scrambled (scr) control.
Pairs of complementary oligonucleotides were synthesized, annealed,
and ligated into linear pGCSIL-green fluorescent protein (GFP)
lentiviral plasmids generated by Shanghai GeneChem Co., Ltd. and
digested by enzyme AgeI and EcoRI. Subsequently,
lentiviral vectors that expressed the CPNE1-specific siRNA or
negative control siRNA (Shanghai GeneChem Co., Ltd.) were generated
in 293T packaging cells by co-transfection of the recombinant
pGCSIL-GFP vector (20 μg), pHelper 1.0 (15 μg;
Shanghai GeneChem Co., Ltd.) and pHelper 2.0 plasmids (10
μg; Shanghai GeneChem Co., Ltd.) using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Viral particles from
the media were harvested at 48 h following transfection and
purified by ultracentrifugation, centrifugation for 2 h at 21,000 x
g and 4°C. The viral titer was determined by counting the numbers
of infected green cells under fluorescence microscopy following
transfection of 293T cells with serial dilutions of concentrated
lentivirus.
For lentivirus transduction, H1299 and A549 cells
were subcultured into 6-well culture plates at 70-80% confluence
and infected with CPNE1-specific siRNA lentivirus or control
lentivirus at a multiplicity of infection of 20. The infection
efficiency was detected using fluorescence microscopy to observe
cells expressing GFP after 5 days. Each cell line was divided into
the following two groups: The sh-NC group and the sh-CPNE1
group.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
RNA isolation, cDNA synthesis and RT-qPCR analysis
were performed as previously described (13). The primer sequences used for CPNE1
mRNA detection were 5′-ACCCACTCTGCGTC CTT-3′ (forward) and
5′-TGGCGTCTTGTTGTCTATG-3′ (reverse), Cq values for CPNE1 mRNA were
equilibrated to ACTB mRNA, for which the following primer sequences
were used: 5′-CACAGAGCCTCGCCTTTGCC-3′ (forward) and
5′-ACCCATGCCCACCATCACG-3′ (reverse), which were used as internal
controls. The 2−ΔΔCq method (14) was applied to calculate the relative
expression of these gene.
Western blotting assay
Western blot analysis was performed as previously
described (13). The antibodies
used in the analysis were anti-CPNE1 (1:500; Z6; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); anti-phosphorylated
(p)-protein kinase B (AKT; Ser473; 1:1,000 D9E), anti-AKT (1:1,000;
9272s; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-extracellular signal-regulated kinase (ERK; 1:1,000; 137F5),
anti-p-ERK (Thr202/Tyr202; 1:1,000; D13.14.4E), anti-matrix
metalloproteinase MMP2 (1:1,000; D8N9Y), anti-MMP9 (1:1,000; 603H),
anti-Snail (1:1,000; C15D3; Cell Signaling Technology, Inc.);
anti-cyclin A1 (1:300; ab53699; Abcam), anti-cyclin B1 (1:300;
Ab-147; Abcam), anti-cyclin E1 (1:300; ab33911, Abcam) and
anti-GAPDH (1:5,000; D4C6R; Cell Signaling Technology, Inc.)
primary antibodies, and peroxidase conjugated anti-mouse or
anti-rabbit secondary antibodies (1:5,000; 14709 and 14708; Cell
Signaling Technology, Inc.).
Cell proliferation analysis
Cell proliferation was examined using a Cell
Counting Kit-8 (CCK-8; Wuhan Boster Biological Technology, Ltd.),
and the A549 sh-CPNE1 cells or the corresponding negative control
cells were seeded in 96-well plates at a density of
2×103 cells per well and further grown under normal
culture conditions for 24, 48 and 72 h. Cell viability was
determined according to the manufacturer's protocol and the
experiments were performed in triplicate. Cell proliferation was
also detected using a clonogenic assay. Briefly, stable cells were
diluted in complete culture medium and 2000 cells were reseeded in
a 60 mm plate. Following incubation for 7-14 days, depending on
cell growth rate, foci formed by at least 50 cells were stained
with Giemsa at room temperature for 2 h and counted via light
microscopy (CKX41; Olympus Corporation, Tokyo, Japan) at ×200. Cell
viability was measured according to the manufacturer's protocol at
several time points (24, 48 and 72 h). Each experiment was
performed in triplicate.
Migration and invasion assays
The motility of cells analysis was evaluated as
previously described (11).
According to the manufacturer's protocol (BD Biosciences),
5×104 cells were seeded with medium containing 1% FBS
into the upper chamber of a Transwell insert, and 800 μl
medium containing 10% FBS was added to the lower chamber and then
incubated at 37°C for 24 h. Cells that migrated to the lower
chamber were stained with 1% crystal violet overnight at room
temperature then washed with 1X PBS two times. For the invasion
assay, the inserts were coated with Matrigel matrix (BD
Biosciences) diluted in serum-free medium and incubated at 37°C for
2 h. The cells from three microscopic fields were imaged and
counted via microscopy (CKX41; Olympus Corporation, Tokyo, Japan).
The results were determined from three repeated experiments.
Cell cycle analysis
According to the protocol of the Cell Cycle and
Apoptosis Analysis kit (Beyotime Institute of Biotechnology,
Haimen, China), cells were cultured in 6-well plates for 72 h. The
cells were subsequently collected, washed with cold PBS, fixed in
70% ethanol at 4°C for 24 h, washed with cold PBS again and stained
in a propidium iodide/RNase A mixture. Following incubation in the
dark at 37°C for 30 min, the cells were analyzed using a
FACSCalibur flow cytometer (Beckman Coulter, Inc., Brea, CA, USA)
and with MultiCycle 6-16-03-F32 (Beckman Coulter, Inc.).
Animal experiments and
immunocytochemistry staining
A total of 12 female BALB/c athymic nude mice (age,
4-6 weeks old; weight, 16-20 g) were purchased from the
Experimental Animal Center of Soochow University and bred under
pathogen-free conditions. Mice were maintained in exhaust
ventilated closed system cages in a specific pathogen-free
environment, with 55±5% humidity, at 23±2°C, ad libitum access to
food and water, and a 14/10 h light/dark cycle. To establish the
lung carcinoma xenograft model, control A549 cells or
CPNE1-silenced A549 cells were suspended at a concentration of
2×106 cells/100 μl PBS and inoculated
subcutaneously into the flanks of nude mice. Mice were randomly
divided into control group and sh-CPNE1 croup (n=6/group).
Following 20 days, the tumor volume (V) was determined by measuring
the length (L) and width (W) with a vernier caliper
and applying the following formula: V =
(LxW2)×0.5. All experiments were performed
in accordance with protocols approved by the Animal Ethical and
Welfare Committee of the First Affiliated Hospital of Soochow
University.
Statistical analysis
The differences in CPNE1 expression between NSCLC
tissues and normal tissues were analyzed using a paired Student's
t-test (two-tailed). For cell lines, differences between two groups
were assessed using an unpaired Student's t-test (two-tailed).
Expression levels of mRNA and the clinicopathologic characteristics
in the NSCLC samples were compared using non-parametric tests
(Mann-Whitney U test for comparison between two groups, and the
Kruskall-Wallis test for comparison between three or more groups).
A two-way analysis of variance was used to determine the difference
in cell growth between two groups. In all cases, P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using GraphPad Prism 5.02
(GraphPad Software, Inc., La Jolla, CA, USA) and SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA).
Results
CPNE1 is frequently overexpressed in lung
adenocarcinoma tissues and cell lines
It has been previously reported that Copines I, II
and III are expressed ubiquitously in humans using cDNA probes
(15), although the actual content
of Copine proteins in human tissues has not been systematically
investigated. A recent study demonstrated that the expression level
of CPNE1 is increased in prostate cancer compared with normal
prostate tissue (16); however,
the expression of CPNE1 in NSCLC is unknown. Lung tumor tissue
samples were surgically resected from 128 lung adenocarcinoma
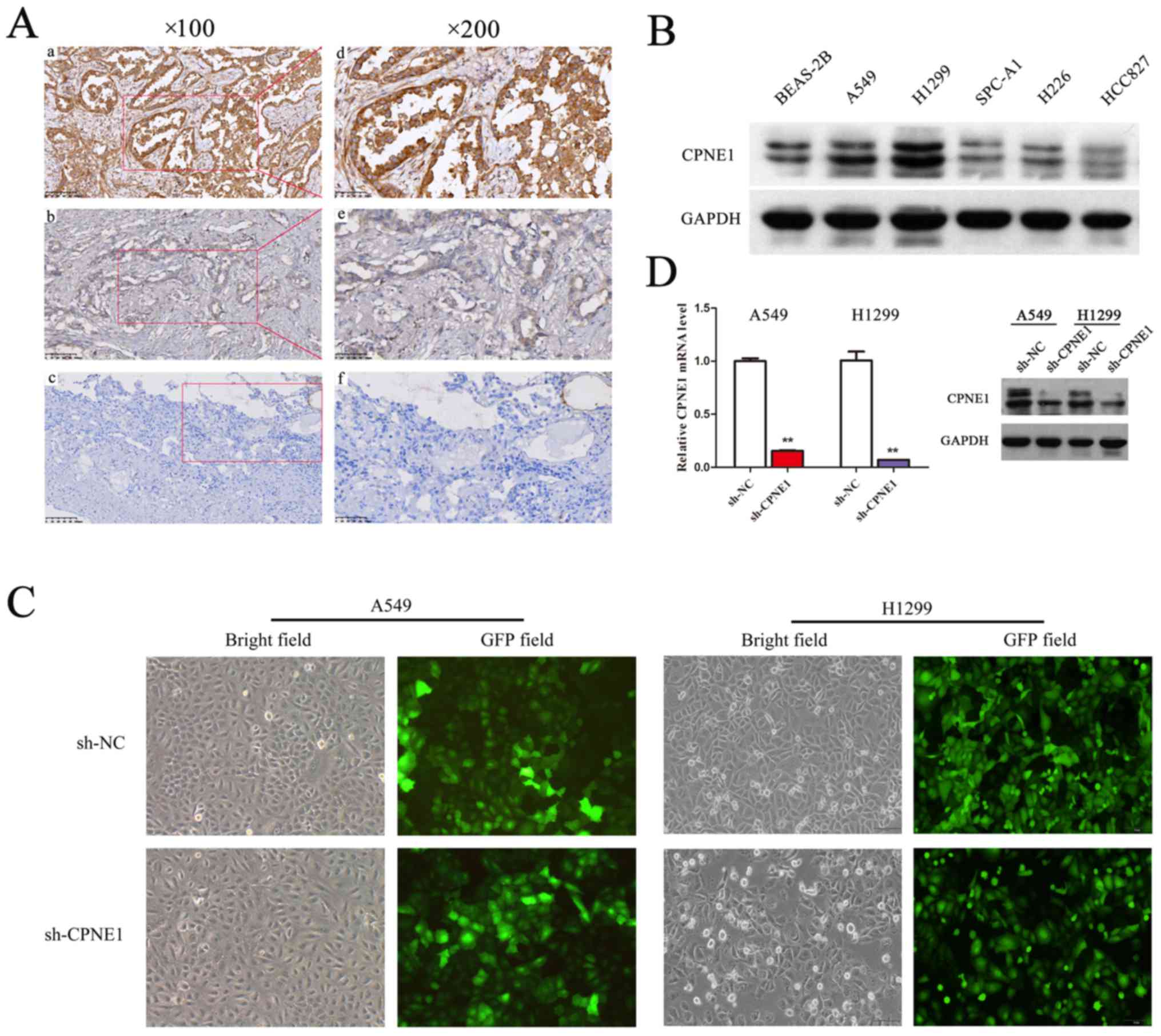
patients, and then were detected via immunohistochemistry (Fig. 1A). In the present study, higher
expression of CPNE1 was observed to be significantly associated
with advanced TNM stage, lymph node metastasis and distant
metastasis in lung adenocarcinoma (17). CPNE1 expression level was
demonstrated to be positively associated with stage (P=0.002) and
significantly associated with lymph node status (P= 0.011) and
distant metastasis (P=0.042; Table
I). In addition, the results demonstrated that expression of
CPNE1 in advanced patients was more frequently increased (III+IV
vs. I+II; P<0.001). However, no significant association was
observed between CPNE1 expression and other clinicopathological
features, including age, sex, tumor size and tumor differentiation
in the present study. In addition to the above, CPNE1 protein
expression were detected in 5 NSCLC cell lines and BEAS-2B cells by
western blot analysis (Fig. 1B).
Therefore, it was demonstrated that CPNE1 expression was frequently
increased in NSCLC tissues and cell lines compared with the
control.
 | Table IDistribution of CPNE1 status in NSCLC
according to clinicopathological characteristics. |
Table I
Distribution of CPNE1 status in NSCLC
according to clinicopathological characteristics.
| Characteristic | CPNE1 expression
| P-value |
|---|
| Low | High | χ2 |
|---|
| Sex | | | | |
| Male | 25 (37.31%) | 42 (62.69%) | 0.056 | 0.813 |
| Female | 24 (39.34%) | 37 (60.66%) | | |
| Age, years) (mean ±
SD) | 57.98±11.12 | 61.27±8.88 | −1.845 | 0.067 |
| Tumor size, cm | | | | |
| <3 | 26 (48.15%) | 28 (51.85%) | 5.082 | 0.079 |
| ≥3, <5 | 18 (35.29%) | 33 (64.71%) | | |
| ≥5 | 5 (21.74%) | 18 (78.26%) | | |
|
Differentiation | | | | |
| Low | 22 (33.85%) | 43 (66.15%) | 1.099 | 0.294 |
| Medium, high | 27 (42.86%) | 36 (57.14%) | | |
| Nodal status | | | | |
| N0 | 32 (50.00%) | 32 (50.00%) | 8.986 | 0.011 |
| N1 | 9 (36.00%) | 16 (64.00%) | | |
| N2/N3 | 8 (20.51%) | 31 (79.49%) | | |
| Distant
metastasis | | | | |
| No | 46 (41.82%) | 64 (58.18%) | 4.142 | 0.042 |
| Yes | 3 (16.67%) | 15 (83.33%) | | |
| TNM stage | | | | |
| I–II | 40 (57.97%) | 29 (42.03%) | 24.563 | <0.001 |
| III–V | 9 (15.25%) | 50 (84.75%) | | |
Lentivirus-mediated RNA interference
efficiently inhibits CPNE1 expression in lung adenocarcinoma
cells
In order to evaluate the association between CPNE1
and lung adenocarcinoma, lentivirus-delivered CPNE1-siRNA and
scr-siRNA vector were successfully constructed. The results of
transfection demonstrated that >90% of the A549 and H1299 cells
exhibited green fluorescence indicating successful infection
(Fig. 1C). To determine the
silencing efficiency, the mRNA and protein expression levels of
CPNE1 were detected by RT-qPCR and western blot analysis,
respectively. The results demonstrated that the mRNA and protein
expression levels of CPNE1 were significantly decreased in the
sh-CPNE1 group compared with the sh-NC group (P<0.01; Fig. 1D). These data suggest that CPNE1
silencing may effectively downregulate CPNE1 expression.
Knockdown of CPNE1 inhibits in vitro cell
growth, cell cycle progression and migration of NSCLC cells
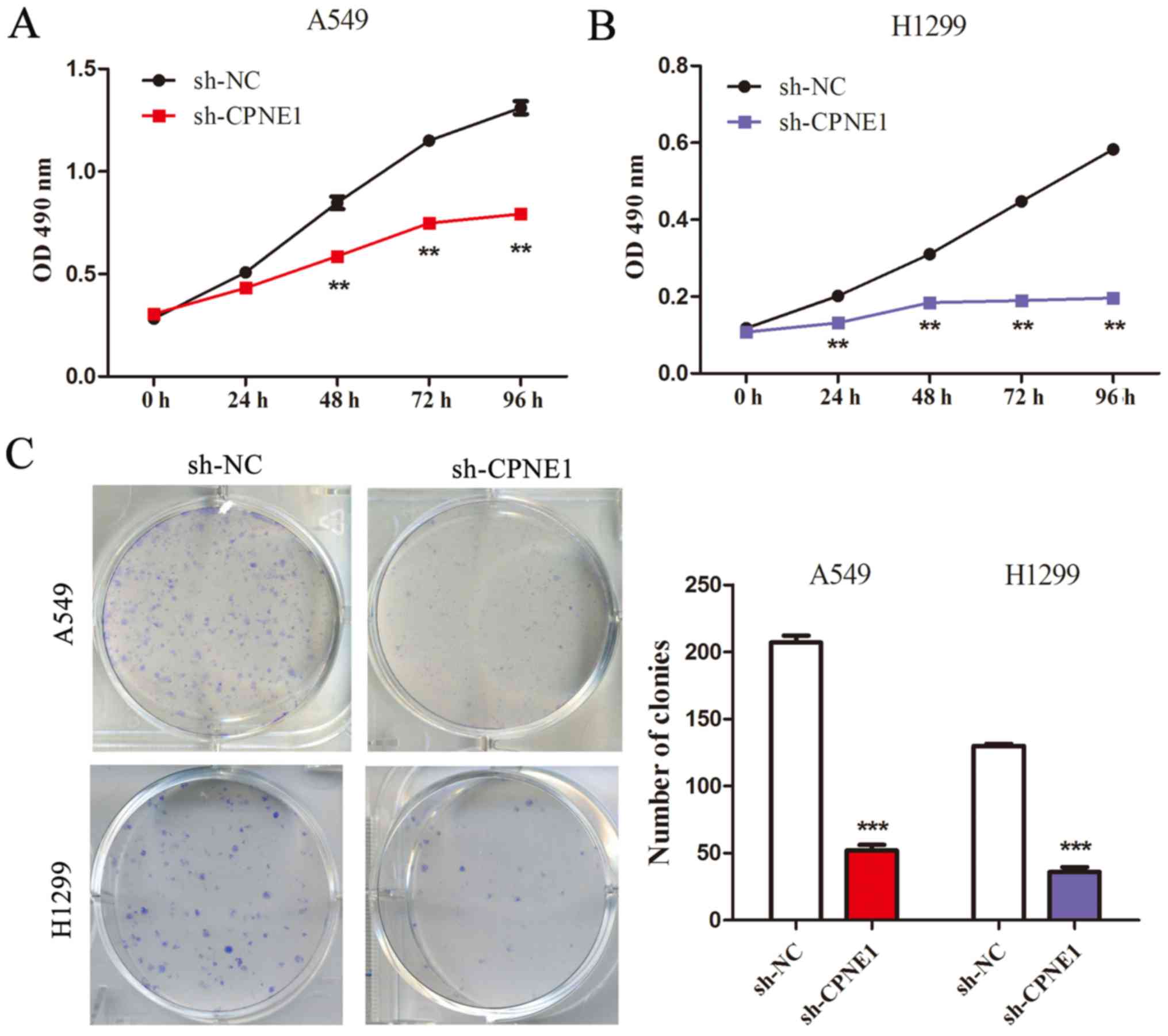
The CCK-8 assay demonstrated that compared with the
control cells, cell growth was significantly inhibited in cells
with stable knockdown of CPNE1 at 48 and 72 h following
transfection (P<0.01; Fig. 2A and
B). These results were also confirmed by performing a
clonogenic assay (P<0.001; Fig.
2C). These results indicate that CPNE1 can promote cell growth
via its effects on the cell cycle and apoptosis in NSCLC cells.
Furthermore, flow cytometric results indicated that
transfection with sh-CPNE1 in NSCLC cells had an effect on the cell
cycle, as the proportion of cells in the S phase was significantly
decreased and the proportion of cells in the G0/G1 phase was
significantly increased in CPNE1-silenced cells compared with the
control cells (P<0.05; Fig. 3A and
B).
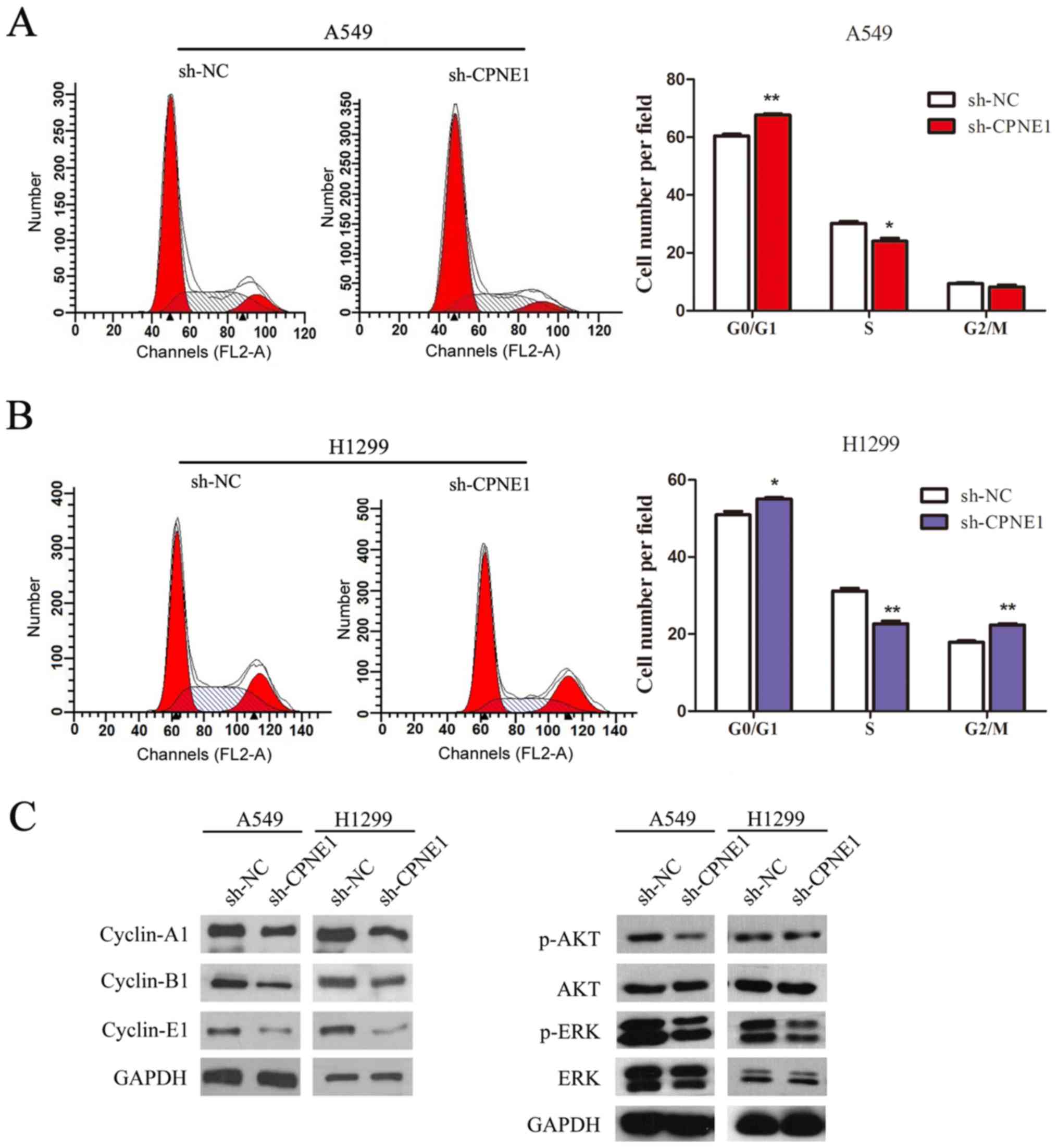 | Figure 3Knockdown of CPNE1 decelerates the
cell cycle in NSCLC cells. (A and B) Flow cytometric analysis of
A549 and H1299 cells. Cells were harvested and stained with
propidium iodide. The percentage of cells in each cell cycle phase
is presented in the inset of each panel, in which the values
represent the mean ± standard deviation of three measurements. (C)
Signaling molecules were detected, and the data demonstrated that
p-AKT, p-ERK, cyclin-A1, cyclin-B1 and cyclin-E1 levels were
markedly decreased in sh-CPNE1 compared with those in sh-NC.
*P<0.05 and **P<0.01 vs. sh-NC. CPNE1,
Copine 1; NSCLC, non-small cell lung cancer; p, phosphorylated;
AKT, protein kinase B; ERK, extracellular signal regulated kinase;
sh-NC, negative control cells; sh-CPNE1, CPNE1-silenced cells. |
Furthermore, the molecular alterations and
associated pathways in CPNE1-silenced cells were measured by
western blotting. As illustrated in Fig. 3C, the results of the present study
demonstrated that cell cycle associated proteins (cyclin-A1,
cyclin-B1 and cyclin-E1), p-AKT and p-ERK levels were markedly
decreased in the CPNE1-silenced cells compared with the control
cells.
In addition, as presented in Fig. 4, a Transwell assay of the NSCLC
stable cells lines further indicated that the migratory ability of
NSCLC cells was significantly suppressed as a result of the loss of
CPNE1 (P<0.001). In line with it, it was observed that Snail,
MMP2, MMP9 were markedly decreased in the CPNE1-silenced cells
compared with those in the control cells (Fig. 4B and D).
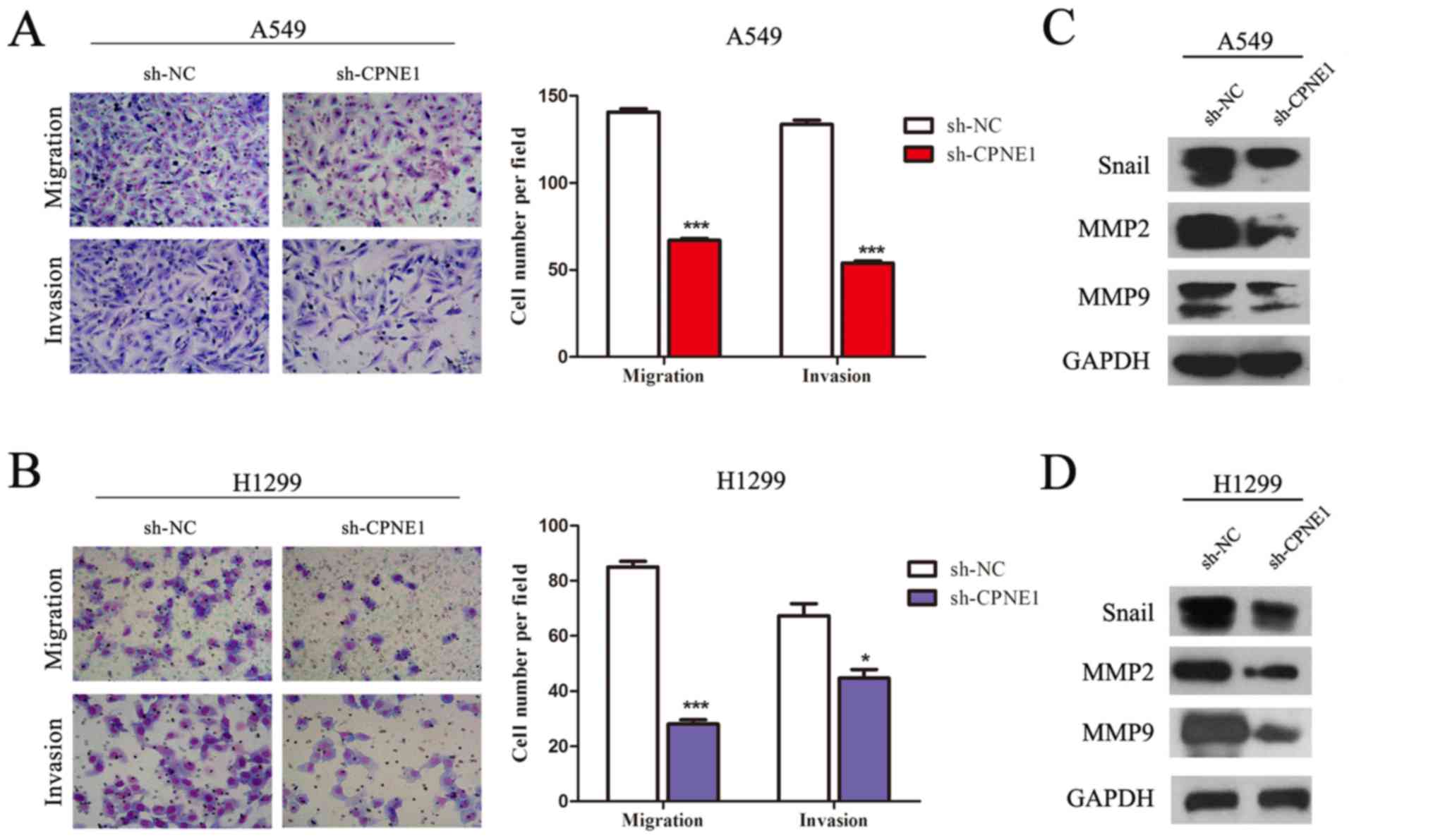 | Figure 4Silencing of CPNE1 inhibits the
migratory and invasive abilities of NSCLC cells, and its associated
pathways. (A and B) CPNE1 silencing inhibits invasion and migration
of NSCLC cells. Assays were performed in a Transwell plate. The
cells that had migrated were stained with crystal violet and
counted in at least three microscopic fields (magnification, ×100).
Subsequently, the cells were treated as above and allowed to invade
through the Matrigel-coated membrane in Transwell plates. Invasive
cells were stained with crystal violet and counted under a light
microscope (magnification, ×100). (C and D) Snail, MMP2, MMP9 were
markedly decreased in sh-CPNE1 compared with sh-NC.
*P<0.05 and ***P<0.001 vs. sh-NC.
CPNE1, Copine 1; NSCLC, non-small cell lung cancer; MMP, matrix
metalloproteinase; sh-NC, negative control cells; sh-CPNE1,
CPNE1-silenced cells. |
Knockdown of CPNE1 inhibits tumor growth
in vivo
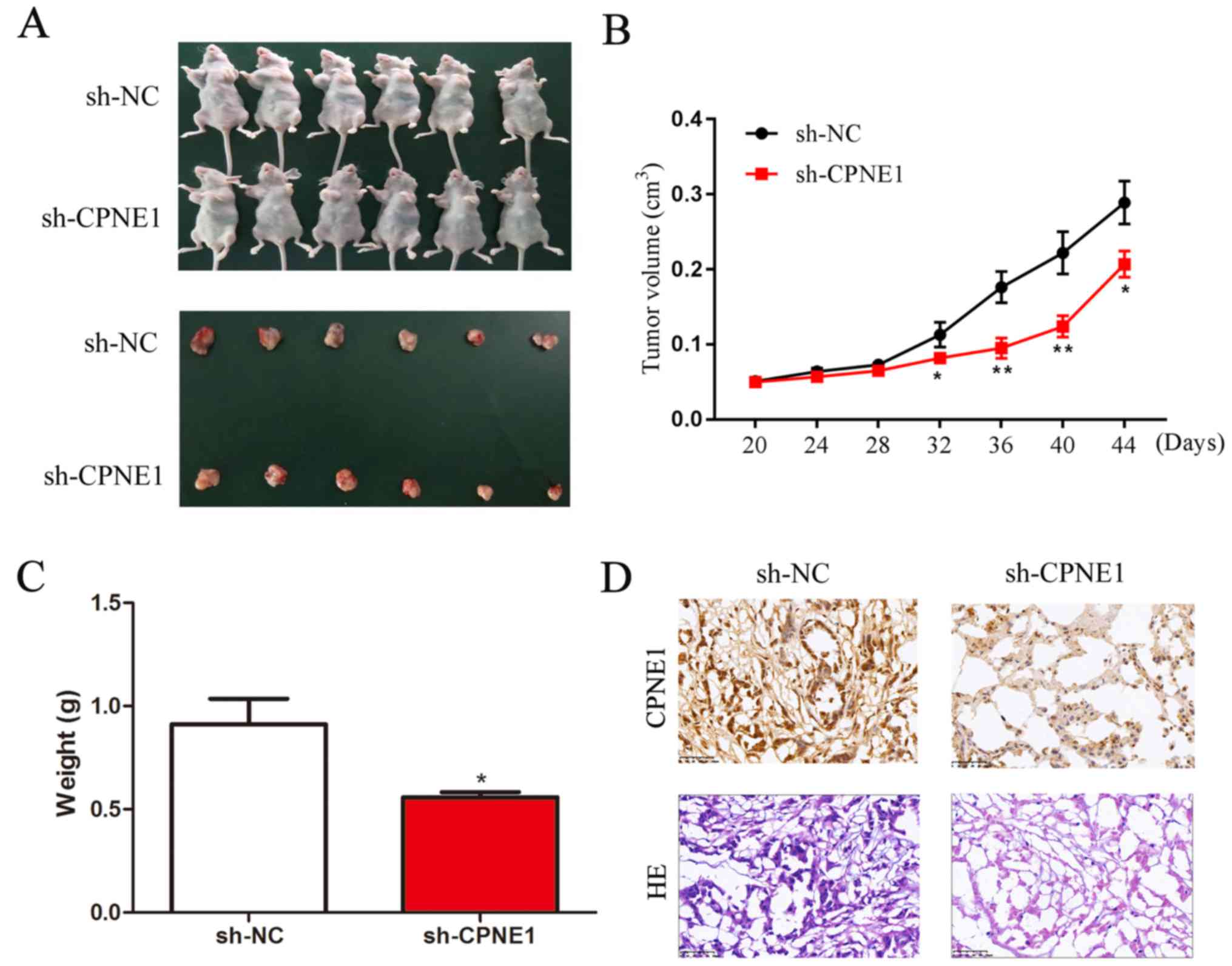
To further assess the effect of CPNE1-silenced on
NSCLC cells in vivo, a xenograft model was used to determine
the role of CPNE1 in the tumorigenicity and development of A549
cells. As demonstrated in Fig. 5A and
B, tumor growth in the sh-CPNE1 group was significantly
decreased compared with the sh-NC group. The final tumor volume at
44 days was 0.222±0.059 cm3 in the CPNE1-siRNA group,
whereas the volume in the scr-siRNA group was 0.400±0.142
cm3 (P<0.01). The mean tumor weight in the
CPNE1-siRNA group was significantly decreased compared with the
scr-siRNA group (0.60±0.10 g vs. 0.90±0.30 g; Fig. 5C). Consequently, these data
demonstrate that silencing CPNE1 expression may suppress tumor
growth of human lung adenocarcinoma cells in nude mice. The tissues
resected from the xenograft tumors were analyzed to verify CPNE1
expression using hematoxylin and immunohistochemistry (Fig. 5D).
Discussion
Despite improvements in therapeutic strategies, the
survival and outcome of patients with NSCLC have not been markedly
affected (4). The majority of
patients have progressed to the advanced stage of the disease when
the diagnosis is confirmed, which is beyond the optimal treatment
period (18). Therefore, it is
essential to elucidate the pathogenesis of NSCLC and to identify
novel treatment targets.
It has been reported that Copine 1, encoded by
CPNE1, is a soluble membrane-binding protein, which includes two
tandem C2 domains at the N-terminus and an A domain at the
C-terminus (6,9). Copine 1 can bind with different
intracellular proteins via its A domain at the C-terminus (7). Further study has demonstrated that
this A domain has been identified in several integrins and
extracellular matrix proteins and may serve as a protein-binding
domain (10). The C2 domain was
demonstrated to be important for calcium and phospholipid binding
(19,20). In addition, multiple proteins have
been identified as Copine targets, and a number of these are
proteins associated with intracellular signalling pathways
including dual specificity mitogen-activated protein kinase kinase
1/ERK, protein phosphatase 5, and CDC42-regulated protein kinase
(7,8). Notably, Park et al (9) previously reported that CPNE1 serves a
vital role in regulating neuronal differentiation of HiB5 cells,
which may be associated with activating AKT signalling via
phosphorylating on the residue 473 (S473) of AKT. Recently, another
study demonstrated that CPNE1 may promote the development and
progression of prostate cancer via its C2 domain (16). Although CPNE1 was demonstrated to
bind several intracellular proteins with diverse biological
functions, the role of CPNE1 in regulating biological processes is
not well understood.
A recent study demonstrated that CPNE3 is
upregulated, and can enhance cell metastasis in NSCLC (21). Further study demonstrated that
CPNE3 can activate downstream ErbB2 signalling and promote
migration in SKBr3 breast cancer cells (22). In accordance with these findings,
Heinrich et al (23) also
demonstrated that CPNE3 can interact with ErbB2 and promote tumor
cell migration. The AKT serine/threonine kinase serves essential
roles in regulating cell growth, cell migration, invasion,
survival, and glycolysis. Furthermore, aberrant activation of AKT
signalling is associated with the pathogenesis of cancer and poor
prognosis (24,25). Among the AKT feedback signalling
molecules, ERK is generally activated with AKT in tumor cells and
is pivotal for cell proliferation and evasion of cell apoptosis
(26). In specific cases, AKT and
ERK signalling pathways are compensatory for each other (27,28).
Notably, it was demonstrated in the present study that p-AKT and
p-ERK levels were decreased in the CPNE1-silenced cells compared
with the control cells.
Cyclin B1 is a key regulator in the cell cycle
progression from G2 to M phase. It has been demonstrated that
cyclin B1 serves a pivotal role in tumorigenesis and tumor
development: Deregulation of cyclin B1 can frequently lead to
unrestricted cell-cycle progression and malignant transformation
(29-31), and cyclin B1 overexpression has
been detected in various types of human cancer (32,33).
Cyclin E1 is a key regulator of the cell cycle and serves an
important role in tumorigenesis and angiogenesis (34). Previous studies have demonstrated
that overexpression of cyclin E1 was important in the growth of
ovarian cancer cells and strongly associated with poor prognosis
(35,36). In the present study, the results
demonstrated that transfection with sh-CPNE1 in NSCLC cells had an
effect on the cell cycle, and cyclin-A1, cyclin-B1 and cyclin-E1
levels were lower in the CPNE1-silenced cells than those in the
control cells.
Metastasis and relapse is the major cause of
mortality for lung cancer patients (37). Epithelial-mesenchymal transition is
a critical step for morphogenesis during embryonic development and
the conversion of early-stage tumors into invasive malignancies
(38,39), which is marked by induction of
Snail and MMPs (40,41). In the present study, it was also
demonstrated that Snail, MMP2, MMP9 were decreased in the
CPNE1-silenced cells compared with those in the control cells.
In conclusion, to the best of our knowledge, the
present study reported for the first time that CPNE1 expression is
upregulated in NSCLC and it was observed that increased expression
of CPNE1 is associated with advanced TNM stage, lymph node
metastasis and distant metastasis in lung adenocarcinoma.
Furthermore, the function of CPNE1 in regulation of cell growth,
migration and invasion was investigated, and it was demonstrated
that knockdown of CPNE1 inhibits the cell cycle in NSCLC cells.
Collectively, these data strongly suggest that CPNE1 is an oncogene
in NSCLC and serves an important role in tumorigenesis of NSCLC
progression.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from The
National Natural Science Foundation of China (grant no. 81201575),
The Science and Technology Plan Projects of Suzhou (grant no.
SYS201612), Jiangsu Provincial Medical Youth Talent (grant no.
QNRC2016746), Medicine and Technology Projects of Zhejiang province
(grant no. 2017KY646), The Societal and Developmental Project of
Suzhou (grant no. SS201630), The Suzhou Key Laboratory for
Respiratory Medicine (grant no. SZS201617), The Clinical Medical
Center of Suzhou (grant no. Szzx201502), Jiangsu Provincial Key
Medical Discipline (grant no. ZDXKB2016007) and The Clinical Key
Specialty Project of China.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SLL, HCT, JJZ, HGD, YYZ, WWD, ZLD, PTS and YZ
participated in data collection and analysis. SLL, JAH and ZYL
participated in the design of the study. ZYL participated in the
writing of the manuscript and data interpretation. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Experiments using tissue samples from human subjects
were approved by the Ethics Committee of The First Affiliated
Hospital of Soochow University (Suzhou, China). All participants
provided written informed consent for the whole study. Experiments
on animals were performed following approval from the Animal
Ethical and Welfare Committee of The First Affiliated Hospital of
Soochow University.
Patient consent for publication
All participants provided written informed consent
for the whole study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dai X, Guo G, Zou P, Cui R, Chen W, Chen
X, Yin C, He W, Vinothkumar R, Yang F, et al: (S)-crizotinib
induces apoptosis in human non-small cell lung cancer cells by
activating ROS independent of MTH1. J Exp Clin Cancer Res.
36:1202017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mulshine JL and Sullivan DC: Clinical
practice. Lung cancer screening. N Engl J Med. 352:2714–2720. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petschnigg J, Kotlyar M, Blair L, Jurisica
I, Stagljar I and Ketteler R: Systematic Identification of
Oncogenic EGFR Interaction Partners. J Mol Biol. 429:280–294. 2017.
View Article : Google Scholar :
|
|
6
|
Creutz CE, Tomsig JL, Snyder SL, Gautier
MC, Skouri F, Beisson J and Cohen J: The copines, a novel class of
C2 domain-containing, calcium-dependent, phospholipid-binding
proteins conserved from Paramecium to humans. J Biol Chem.
273:1393–1402. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomsig JL, Snyder SL and Creutz CE:
Identification of targets for calcium signaling through the copine
family of proteins. Characterization of a coiled-coil
copine-binding motif. J Biol Chem. 278:10048–10054. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomsig JL and Creutz CE: Biochemical
characterization of copine: A ubiquitous Ca2+-dependent,
phospholipid-binding protein. Biochemistry. 39:16163–16175. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park N, Yoo JC, Lee YS, Choi HY, Hong SG,
Hwang EM and Park JY: Copine1 C2 domains have a critical
calcium-independent role in the neuronal differentiation of
hippocampal progenitor HiB5 cells. Biochem Biophys Res Commun.
454:228–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Whittaker CA and Hynes RO: Distribution
and evolution of von Willebrand/integrin A domains: Widely
dispersed domains with roles in cell adhesion and elsewhere. Mol
Biol Cell. 13:3369–3387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asamura H, Chansky K, Crowley J, Goldstraw
P, Rusch VW, Vansteenkiste JF, Watanabe H, Wu YL, Zielinski M, Ball
D, et al: The International Association for the Study of Lung
Cancer Lung Cancer Staging Project: Proposals for the revision of
the N descriptors in the forthcoming 8th edition of the TNM
Classification for Lung Cancer. J Thorac Oncol. 10:1675–1684. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang H, Kirkness EF, Lippert C, Biggs WH,
Fabani M, Guzman E, Ramakrishnan S, Lavrenko V, Kakaradov B, Hou C,
et al: Profiling of short-tandem-repeat disease alleles in 12,632
human whole genomes. Am J Hum Genet. 101:700–715. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu J, Zeng Y, Li W, Qin H, Lei Z, Shen D,
Gu D, Huang JA and Liu Z: CD73/NT5E is a target of miR-30a-5p and
plays an important role in the pathogenesis of non-small cell lung
cancer. Mol Cancer. 16:342017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Tomsig JL and Creutz CE: Copines: A
ubiquitous family of Ca(2+)-dependent phospholipid-binding
proteins. Cell Mol Life Sci. 59:1467–1477. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang J, Zhang J, Ruan J, Mi Y, Hu Q, Wang
Z and Wei B: CPNE1 is a useful prognostic marker and is associated
with TNF receptor-associated factor 2 (TRAF2) expression in
prostate cancer. Med Sci Monit. 23:5504–5514. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu C, Chen K, Zheng H, Guo X, Jia W, Li M,
Zeng M, Li J and Song L: Overexpression of astrocyte elevated
gene-1 (AEG-1) is associated with esophageal squamous cell
carcinoma (ESCC) progression and pathogenesis. Carcinogenesis.
30:894–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Ju Q, Qian B, Zhang F and Shi H: The
effectiveness of PD-1 inhibitors in non-small cell lung cancer
(NSCLC) patients of different ages. Oncotarget. 9:7942–7948.
2017.
|
|
19
|
Nalefski EA and Falke JJ: The C2 domain
calcium-binding motif: Structural and functional diversity. Protein
Sci. 5:2375–2390. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rizo J and Südhof TC: C2-domains,
structure and function of a universal Ca2+-binding
domain. J Biol Chem. 273:15879–15882. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin HC, Zhang FL, Geng Q, Yu T, Cui YQ,
Liu XH, Li J, Yan MX, Liu L, He XH, et al: Quantitative proteomic
analysis identifies CPNE3 as a novel metastasis-promoting gene in
NSCLC. J Proteome Res. 12:3423–3433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi HY, Park N, Na JB, Ko ES, Park JY and
Yoo JC: Direct binding of Copine3 with Jab1 activates downstream
ErbB2 signaling and motility in SKBr3 breast cancer cells. Oncol
Rep. 35:1147–1152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heinrich C, Keller C, Boulay A, Vecchi M,
Bianchi M, Sack R, Lienhard S, Duss S, Hofsteenge J and Hynes NE:
Copine-III interacts with ErbB2 and promotes tumor cell migration.
Oncogene. 29:1598–1610. 2010. View Article : Google Scholar
|
|
24
|
Ciruelos Gil EM: Targeting the
PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer.
Cancer Treat Rev. 40:862–871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miller TW, Rexer BN, Garrett JT and
Arteaga CL: Mutations in the phosphatidylinositol 3-kinase pathway:
Role in tumor progression and therapeutic implications in breast
cancer. Breast Cancer Res. 13:2242011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu Z, Yang H, Sutton MN, Yang M, Clarke
CH, Liao WS and Bast RC Jr: ARHI (DIRAS3) induces autophagy in
ovarian cancer cells by downregulating the epidermal growth factor
receptor, inhibiting PI3K and Ras/MAP signaling and activating the
FOXo3a-mediated induction of Rab7. Cell Death Differ. 21:1275–1289.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saini KS, Loi S, de Azambuja E,
Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE and
Piccart-Gebhart MJ: Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK
pathways in the treatment of breast cancer. Cancer Treat Rev.
39:935–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Britten CD: PI3K and MEK inhibitor
combinations: Examining the evidence in selected tumor types.
Cancer Chemother Pharmacol. 71:1395–1409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niméus-Malmström E, Koliadi A, Ahlin C,
Holmqvist M, Holmberg L, Amini RM, Jirström K, Wärnberg F,
Blomqvist C, Fernö M, et al: Cyclin B1 is a prognostic
proliferation marker with a high reproducibility in a
population-based lymph node negative breast cancer cohort. Int J
Cancer. 127:961–967. 2010.
|
|
30
|
Kedinger V, Meulle A, Zounib O, Bonnet ME,
Gossart JB, Benoit E, Messmer M, Shankaranarayanan P, Behr JP,
Erbacher P, et al: Sticky siRNAs targeting survivin and cyclin B1
exert an antitumoral effect on melanoma subcutaneous xenografts and
lung metastases. BMC Cancer. 13:3382013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matthess Y, Raab M, Sanhaji M, Lavrik IN
and Strebhardt K: Cdk1/cyclin B1 controls Fas-mediated apoptosis by
regulating caspase-8 activity. Mol Cell Biol. 30:5726–5740. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soria JC, Jang SJ, Khuri FR, Hassan K, Liu
D, Hong WK and Mao L: Overexpression of cyclin B1 in early-stage
non-small cell lung cancer and its clinical implication. Cancer
Res. 60:4000–4004. 2000.PubMed/NCBI
|
|
33
|
Zhou L, Li J, Zhao YP, Cui QC, Zhou WX,
Guo JC, You L, Wu WM and Zhang TP: The prognostic value of Cyclin
B1 in pancreatic cancer. Med Oncol. 31:1072014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang F, Fu XD, Zhou Y and Zhang Y:
Down-regulation of the cyclin E1 oncogene expression by
microRNA-16-1 induces cell cycle arrest in human cancer cells. BMB
Rep. 42:725–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakayama N, Nakayama K, Shamima Y,
Ishikawa M, Katagiri A, Iida K and Miyazaki K: Gene amplification
CCNE1 is related to poor survival and potential therapeutic target
in ovarian cancer. Cancer. 116:2621–2634. 2010.PubMed/NCBI
|
|
36
|
Schraml P, Bucher C, Bissig H, Nocito A,
Haas P, Wilber K, Seelig S, Kononen J, Mihatsch MJ, Dirnhofer S, et
al: Cyclin E overexpression and amplification in human tumours. J
Pathol. 200:375–382. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu
Z, Zhao J and Zhang HT: JAK/STAT3 signaling is required for
TGF-β-induced epithelial-mesenchymal transition in lung cancer
cells. Int J Oncol. 44:1643–1651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ren F, Tang R, Zhang X, Madushi WM, Luo D,
Dang Y, Li Z, Wei K and Chen G: Overexpression of MMP family
members functions as prognostic biomarker for breast cancer
patients: A systematic review and meta-analysis. PLoS One.
10:e01355442015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ying TH, Lee CH, Chiou HL, Yang SF, Lin
CL, Hung CH, Tsai JP and Hsieh YH: Knockdown of Pentraxin 3
suppresses tumorigenicity and metastasis of human cervical cancer
cells. Sci Rep. 6:293852016. View Article : Google Scholar : PubMed/NCBI
|