Introduction
Epithelial ovarian cancer (EOC) is the most lethal
gynecological malignancy due to the fact that it is usually
diagnosed at an advanced stage with peritoneal carcinomatosis in
the majority of patients (1). Even
with aggressive primary surgery and adjuvant chemotherapy, the
majority of patients with advanced-stage EOC often suffer relapses
and develop chemotherapeutic resistance (2). The recurrence and progression of
peritoneal carcinomatosis leads to a poor survival and an impaired
quality of life due to marked ascites. Therefore, novel treatment
strategies are required to control peritoneal carcinomatosis.
Following the demonstration of EOC immunogenicity,
multiple immunotherapeutic approaches remain underdeveloped
(3-5). Immune checkpoint inhibitors, such as
anti-cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) or
anti-programmed death 1 (PD-1)/PD-L1 antibodies, have been
considered as innovative treatments for a variety of malignancies.
However, checkpoint inhibitors merely disin- hibit ongoing T-cell
responses. As a consequence, they often fail in tumors with a
paucity of pre-existing tumor-infiltrating cytotoxic T
lymphocytes.
Interleukin (IL)-1 family members are known to alter
host responses to an infectious, inflammatory, or immunological
challenge. IL-33 is a member of the IL-1 family that has been
identified as a potent activator of the immune system. IL-33 is
released by damaged cells, acting as an alarmin (6). IL-33 functions by interacting with
its receptors, ST2 (also known as IL1RL1) and IL-1RAcP (7,8).
IL-33 has been reported to be associated with a number of diseases,
including infections (9,10), asthma (11,12),
autoimmune diseases (13,14), atherosclerosis and cardiovascular
disease (15,16). However, there are only a few
reports available to date on the role of IL-33 signaling in cancer.
Recently, the high expression of IL-33 and its receptor, ST2, were
reported to be poor prognostic factors for survival. IL-33 was
shown to promote gastric cancer cell migration and invasion by
stimulating the secretion of IL-6 and MMP-3 (17). Increased IL-33 protein levels have
been observed in serum and liver tissue from patients with
hepatocellular carcinoma (18).
Furthermore, IL-33 and ST2 expression levels have been shown to be
higher in human breast cancer tissue than in normal breast tissue
(19,20). By contrast, a protective role for
IL-33 has been reported in other studies.
The IL-33/ST2 signaling axis may play a protective
role in colon carcinogenesis through macrophage inhltration
(21), and IL-33 may increase the
death of ST2-expressing lung cancer cells under conditions
mimicking the tumor environment (22). The reports differ depending on the
type of cancer; therefore, the function of IL-33 is
controversial.
In ovarian cancer, a high expression of IL-33 has
been reported to be associated with a poor prognosis of patients
with EOC (23,24); however, to date, at least to the
best of our knowledge, the association between ovarian cancer and
the function of IL-33 has not yet been fully elucidated. We
investigated the correlation between IL-33 and EOC in the
peritoneal carcinomatosis environment, which is likely to be
accessible and refractory in advanced EOC.
Materials and methods
Cells and cell culture
Murine ovarian cancer cell lines (ID8 and its
subclones ID8-T6, ID8-mock and ID8-IL-33) and human EOC cell lines
(SKOV-3/CAOV3/OV90/A2780) were used in this study. The ID8 cell
line was kindly provided by Dr Katherine Roby (University of Kansas
Medical Center). The SKOV-3, CAOV3 and OV90 cell lines were
obtained from the American Type Culture Collection (ATCC). The
A2780 cell line was obtained from the European Collection of Cell
Cultures (ECACC). These cells were maintained in RPMI-1640 medium
(Sigma-Aldrich) supplemented with 10% fetal calf serum, penicillin
(100 U/ml) and streptomycin (100 g/ml) at 37°C in a humidihed
atmosphere containing 5% CO2. All cell lines were
regularly tested for mycoplasma contamination.
Animal and tumor model
A total of 95 C57BL/6 female mice (89 4-5 weeks old
mice; weight range, 13.8-18.0 g; mean weight, 15.6 g and 6 10-12
weeks old mice) and 8 BALB/C nude female mice (5 weeks old; weight
range, 16.8-18.6 g; mean weight, 18.0 g) were purchased from
Charles River Laboratories Japan. Animal care and experimental
procedures were approved by the Animal Experiment Committee of
Nagoya University (approval no. 30065), and all animals were
maintained under specific pathogen-free conditions. Mice were
housed in groups of 3 or 4 in cages and kept in a room maintained
at 23±1°C with free access to food and water, on a 12-h light-dark
cycle throughout the experiments. The mice were allowed to
acclimatize to their conditions for 2 weeks. The C57BL/6 mice and
BALB/C nude mice were intra-abdominally injected with
5.0×106 tumor cells in 0.3 ml of PBS to induce
peritoneal metastasis. Mice with peritoneal carcinomatosis with a
body weight >25 g were sacrificed, and intraperitoneal
dissemination was evaluated. We generated the subclone of ID8 with
higher peritoneal dissemination capacity. Peritoneal dissemination
was harvested and cultured in medium, and tumor cells were
re-implanted intraperitoneally into 3 different C57BL/6 mice. This
re-implantation was performed a total of 5 times to establish the
ID8-T6 mice (Fig. S1). We used 18
C57BL/6 mice in this process (3 mice for initial disseminated tumor
formation and we repeated the re-implantation of 3 mice each 5
times).
The C57BL/6 mice were injected with
5.0×106 tumor cells in 0.2 ml of PBS into the right rear
flank of each mouse to form subcutaneous tumors. Subcutaneously
inoculated mice were sacrificed at 6 weeks following the injection.
Subcutaneous tumor size was measured weekly thereafter using a
digital Vernier caliper. Tumor volumes were calculated using the
following formula: V = (longest diameter x shortest
diameter2)/2.
Humane endpoints
Humane euthanasia using CO2 was performed
when a mouse reached an experimental endpoint, was sampled with
irreversible and persistent pain or became moribund. The flow rate
and container volume for CO2 asphyxiation were 2.0 l/min
and container volume was 10 l, respectively. To confirm mouse
death, we checked for the absence of multiple vital signs (loss of
bladder control, absence of heart rate, lack of a toe-pinch
response and cessation of respiration). In order to obtain a rapid
and sufficient amount of bone marrow cells, they were collected
following euthanasia. To minimize animal suffering and distress at
the time of invasive procedures, the mice were anesthetized by
isofluorane inhalation (2-5%) administered by nose cone to reach a
steady state of anesthesia, which was determined by toe pinch
reflex and slow steady breathing.
Vectors and transductions
pSIREN-RetroQ-ZsGreen-Control
(5′-TTCTCCGAACGTGTCACGT-3′) and -mIL33-2334
(5′-AGGTATAATTGTTTCATTAATTT-3′) are small hairpin RNA (shRNA)
expression vectors encoding non-target shRNA or shRNA against mouse
IL-33 (IL33-2334), respectively. pRetroX-IRES-DsRedExpress-mIL33 is
a mammalian expression vector encoding mouse IL-33. pRetroX-IRES
-DsRe-dExpress-Mock, which is an empty vector, was used as a
control.
The ID8-T6 cells were transduced with
pSIREN-RetroQ-ZsGreen-Control and -mIL33-2334, which were used for
gene silencing. The ID8 cells were transduced with
pRetroX-IRES-DsRedExpress-Mock and -mIL33, which were used for gene
overexpression. The vector transfections were carried out using
Lipofectamine 3000 (Thermo Fisher Scientific). To produce viral for
delivery, 293T cells were transfected with
pSIREN-RetroQ-DsRed-Express (or pRetroX-IRES-DsRed Express), VSV
and gag-pol. After 3 days, the supernatant was collected and
filtrated (0.45 µm). Filtrated supernatant with
polybrene (final 5 mg/ml) was added to ID8-T6 (or ID8) cells and
incubated for 24 h at 37°C.
Both pRetroX-IRES-DsRed Express and pSIREN-RetroQ-
ZsGreen were purchased from Clontech (Takara Bio). All oligo DNA
were synthesized by and purchased from Hokkaido System Science.
Immunohistochemical (IHC) staining
IHC analyses were performed on human EOC tissues and
intraperitoneally transplanted tumors from mice. The human EOC
tissues were obtained from 100 patients who underwent surgical
treatment at Nagoya University Hospital between 1989 and 2011. All
samples were fixed in 10% formalin and embedded in paraffin. The
sections were cut at a thickness of 5 µm. For heat-induced
epitope retrieval, deparaffinized sections in 0.1 M citrate buffer
were treated at 90°C at 750 W for 15 min using a microwave oven.
Immunostaining was conducted using Histofine Simple Stain MAX PO
(MULTI) (424151, Nichirei Biosciences), followed by incubation at
4°C overnight with the primary antibodies. Histofine Simple Stain
MAX PO is the labeled polymer prepared by combining amino acid
polymers with peroxidase and secondary antibody which is reduced to
Fab' fragment. We applied 2 drops of this detection reagent to each
slide so as to provide a complete cover of the sections. This was
followed by incubation at room temperature for 30 min. For IL-33,
CD4 and CD8, the samples were incubated with rabbit anti-IL-33
(clone Nessy-1, 1:1,000 dilution; ALX-804-840, Enzo Life Sciences),
rabbit anti-CD4 (clone SP35, 1:100 dilution; M3354, Spring
Bioscience) and mouse anti-CD8 (clone C8/144B, 1:100 dilution;
M7103, Dako).
As regards the IHC staining of mouse tumors, tissues
removed from the mice were immersed in OCT compound (Sakura
Finetek) and rapidly frozen and stored at -80°C. Frozen tissue
sections were cut at 5 µm thickness using a cryostat
microtome at -15°C, air-dried and fixed in acetone for 10 min. The
sections were then incubated with rat anti-CD4 (clone GK1.5, 1:100
dilution; 100401, BioLegend), rat anti-CD8a (clone 53-6.7, 1:100
dilution; 100701, BioLegend), rat anti-CD11b (clone M1/70, 1:500
dilution; 101201, BioLegend) and rat anti-F4/80 (clone BM8, 1:500
dilution; 123101, BioLegend) at room temperature overnight. The
sections were then washed 3 times in PBS, and 2 drops of Histofine
Simple Stain Mouse MAX-PO (Rat) (414311, Nichirei Biosciences)
reagent were added for 30 min at room temperature.
Immunohistochemical evaluation
The samples were classified according to the
intensity of IL-33 staining and scored from 0 to 3 as follows: 0,
negative; 1, weak; 2, moderate; and 3, strong. Cases with scores of
0 and 1 belonged to the IL-33 low group and cases with scores of 2
or 3 belonged to the IL-33 high group. Tumor-infiltrating
CD4+ and CD8+ cells were counted at ×400
magnification, CDllb+ and F4/80+ cells were
counted at ×200 magnification in 4 different microscopic fields,
and the average number was calculated (using a ZEISS Axio Imager A1
microscope). The scoring of IL-33 intensity and counting of
tumor-infiltrating immune cells were carried out twice by two
independent gynecologists (each blinded to the other's score)
without any knowledge of the patient clinical parameters or other
prognostic factors. The concordance rate was >90% between the
observers.
Flow cytometric analysis
Flow cytometry (FCM) was carried out to quantify the
expression of CD11b and Gr-1 on the surface of the cells. Mice with
tumor formation were sacrificed by CO2 gas asphyxiation
and their disseminated tumors were rapidly collected. The cells
were stained with the following antibodies for 30 min at 4°C: APC
anti-mouse CD11b (clone M1/70; 101201, BioLegend) and PE anti-mouse
Gr-1 (clone RB6-8C5; 108407, BioLegend). FCM data were acquired
using the Attune Acoustic Focusing Cytometer (Life Technologies;
Thermo Fisher Scientific), and analyzed using Attune cytometric
software version 2.1.0. Gating was implemented on the basis of
negative-control staining profiles.
RNA isolation and gene expression
analysis
Total RNA was isolated from the cells and tissues
using the RNeasy RNA Isolation kit (Qiagen) and cDNA was
synthesized using the ReverTraAce qPCR RT kit (Toyobo). The
approximate size of the PCR products was estimated by the
electrophoresis of µ1 of each PCR reaction on 1.0% agarose gel
containing GelRed (Biotium). Real-time PCR was performed on a
LightCycler using SYBR-Green 1 Master (Roche). The
2-ΔΔCq method was adopted for quantification (25). The primary PCR primer pairs are
listed in Table SI. Microarray
analyses were performed using the Affymetrix GeneChip. The reaction
was performed for 10 min at 30°C, 20 min at 42°C and 5 min at 99°C,
and then stored at 4°C.
Western blot (WB) analysis
WB analysis for IL-33 and receptor ST2 was performed
as previously described (26). In
brief, cells were treated with 10% RIPA lysis buffer (Thermo Fisher
Scientific) in PBS and cOmplete, Mini, EDTA-free (Roche).
Subsequently, 30 µg of total cell lysate were
electrophoresed on a 10% SDS -polyacrylamide gel and transferred
electrophoretically to Immobilon membranes (Millipore). After
blocking in blocking solution (5% non-fat dry milk/0.1%
Tween-20/PBS) at room temperature for 1 h, the membranes were
incubated overnight with a recommended dilution of primary
antibodies. The following antibodies were used at 4°C overnight:
Anti-mouse and human IL-33 (clone Nessy-1; 1:1,000 dilution;
ALX-804-840, Enzo Life Sciences), anti-human ST2 (1:1,000 dilution;
PRS3363-100UG, Sigma-Aldrich) and anti-β-actin (1:5,000 dilution;
017-24573, Wako). The primary antibodies were washed in 0.05%
Tween-20/PBS and then incubated with the appropriate HRP-linked
secondary antibody at room temperature for 1 h (1:10,000; 7074 and
7076, Cell Signaling Technology). Proteins were detected with an
ECL kit (GE Healthcare Life Science) and visualized using the
ImageQuant LAS 4000 Mini system (GE Healthcare Life Science).
Determination of the IL-33
concentration
The IL-33 concentration in the supernatant and
ascites was evaluated by ELISA (murine IL-33; R&D Systems). The
ID8-WT, ID8-T6, ID8-mock and ID8-IL-33 cells (5×105
cells/well) were seeded in 6-well plates and incubated in
appropriate culture medium for 24 h at 37°C. After reaching
confluence, the cells were washed with serum-free medium, and
incubated for a further 48 h at 37°C. Following incubation, the
supernatants were collected for the assay. Ascites were harvested
from ID8-mock or ID8-IL-33 peritoneal tumor-bearing mice and the
cells were removed by centrifugation (500 ×g, 5 min at 4°C) for the
assays.
In vitro cell proliferation assay
The cells were plated in hexaplicate at a density of
1,000 cells in 200 µ1 in 96-well plates, and cultured for 1
to 3 days at 37°C. Cell viability was assayed using a modified
tetrazolium salt MTT assay performed using the CellTiter 96 Aqueous
One Solution Cell Proliferation Assay kit (Promega). The absorbance
was measured at 490 nm using a microplate reader (Labsystems,
Multiskan Bichromatic).
Myeloid-derived cell generation
assay
Bone marrow cells were harvested from the femurs of
6 healthy female C57BL/6 mice (10-12 weeks old). The bone marrow
cells were cultured in RPMI-1640 with 10% FBS in the presence of
ascites fluid that was extracted from ID8-mock or ID8-IL-33
peritoneal tumor-bearing mice from which the cells were removed by
centrifugation (500 × g, 5 min at 4°C). The cells were collected on
day 7 for FCM. For stimulation with IL-33, recombinant mouse IL-33
(R&D Systems) at 20, 200 or 2,000 was added to each well on day
1.
Scratch wound migration assay (wound
healing assay)
Tumor cells were seeded on 6-well plates and
incubated at 37°C overnight. The cell monolayer was then scratched
using a 200-µ1 pipette tip and washed twice with PBS to
ensure the scratch area. The cells were examined 8 h after the
scratch was made using an OLYMPUS IX71 microscope (Olympus).
Statistical analysis
For the results of in vitro and in
vivo experiments, statistical comparisons between groups were
performed using the non-paired Student's t-test. The comparisons
between multiple groups were assessed using one-way ANOVA, followed
by Tukey's test. Overall survival curves were generated using the
Kaplan-Meier method and compared using a log-rank test. The
comparison between the IL-33 expression levels in different groups
was assessed using Chi-squared tests (Table I). Differences between groups were
considered signihcant at P<0.05. Data are expressed as the means
± SD.
 | Table IAssociation between the expression of
IL-33 and the clinicopathological parameters of the patients with
EOC. |
Table I
Association between the expression of
IL-33 and the clinicopathological parameters of the patients with
EOC.
| Parameter | No. | IL-33
expression | P-value |
|---|
| Low, n (%) | High, n (%) |
|---|
| Total | 100 | 33 | 67 | |
| Age (years) | | | | 0.288 |
| ≤55 | 50 | 14 (42.4) | 36 (53.7) | |
| >55 | 50 | 19 (57.6) | 31 (46.3) | |
| FIGO stage | | | | 0.792 |
| I | 31 | 12 (36.4) | 19 (28.4) | |
| II | 20 | 5 (15.2) | 15 (22.4) | |
| III | 43 | 14 (42.4) | 29 (43.3) | |
| IV | 23 | 2 (6.1) | 4 (6.0) | |
| Histological
type | | | | 0.664 |
| Serous | 48 | 16 (48.5) | 32 (47.8) | |
| Clear | 29 | 11 (33.3) | 18 (26.9) | |
| Endometrioid | 23 | 6 (18.2) | 17 (25.4) | |
| Grade | | | | 0.205 |
| G1 | 8 | 4 (12.1) | 4 (6.0) | |
| G2 | 31 | 6 (18.2) | 25 (37.3) | |
| G3 | 57 | 22 (66.7) | 35 (52.2) | |
| Unknown | 4 | 1 (3.0) | 3 (4.5) | |
| CA125 (U/ml) | | | | 0.229 |
| <350 | 49 | 19 (57.6) | 30 (44.8) | |
| >350 | 51 | 14 (42.4) | 37 (55.2) | |
Results
Generation and characterization of
invasive ID8-T6 ovarian tumor cells
The ID8 murine ovarian cancer cell line slowly forms
tumors when injected intraperitoneally in C57BL/6 mice. In order to
investigate the mechanisms of peritoneal dissemination, we prepared
a more invasive subclone of ID8. A syngeneic mouse model was
established by injecting ID8 cells intraperitoneally into C57BL/6
mice. Peritoneal dissemination was harvested and cultured in
medium, and tumor cells were re-implanted intraperitoneally into
different C57BL/6 mice. This re-implantation was repeated 5 more
times to create the invasive ID8 cell line ID8-T6 (Fig. S1).
The ID8-WT and ID8-T6 cell lines were
intraperitoneally injected into C57BL/6 mice, and their survival
was compared. ID8-T6 caused the earlier accumulation of ascites and
the formation of dissemination compared with ID8-WT (Fig. S2A). As shown in Fig. S2B, the ID8-T6 tumor-bearing mice
had a significantly poorer survival than the ID8-WT tumor-bearing
mice (P=0.002). Based on the above, ID8-T6 became a cell line that
more easily formed peritoneal dissemination. In order to confirm
whether there was a difference between ID8-WT and ID8-T6 in
vitro, cell proliferation was examined. No marked differences
were observed in the growth rates between the ID8-WT cells and
ID8-T6 cells (Fig. S2C). We also
examined the effects of ID8-T6 on cell migration by wound healing
assay, in which cells migrate from the edge of a scratch wound.
ID8-T6 cells had spread along the wound edges more rapidly than the
ID8-WT cells at 8 h (Fig.
S3).
To identify which genes promote peritoneal
carcinomatosis, we performed microarray analysis to compare the
ID8-WT cells with ID8-T6 cells (in vitro conditions) and
ID8-WT tumor tissue with ID8-T6 tumor tissue (in vivo
conditions). Among the genes listed, we focused on humoral factors.
IL-33 was found to be upregulated in both the ID8-T6 cells and
tissue as compared with ID8-WT (15.1-fold higher, 50.7-fold higher,
Table SII). To confirm these
results, we performed RT-qPCR on the ID8-WT and ID8-T6 cells for 10
genes [IL-6, IL-8, IL-13, IL-33, interleukin 1 receptor like 1
(IL1RL1), transforming growth factor (TGF)-ß1, heparin binding EGF
like growth factor (HB-EGF), vascular endothelial growth factor
(VEGF) α, C-C motif chemokine ligand 5 (CCL5) and prostaglandin E
receptor 2 (PTGER2)]. Significant differences were found for all
genes. IL-33 and its receptor IL1RL1 were significantly upregulated
(Fig. S2D). In addition, the
increased protein expression of IL-33 was observed by WB and IHC
analyses (Fig. S2E and F).
Furthermore, we confirmed the expression of IL-33 in human ovarian
cancer. We also examined the expression of IL-33 and its receptor
ST2 in human ovarian cancer cell lines (SKOV3, CAOV3, OV90 and
A2780) by WB analysis. No expression of IL-33 was observed in the
A2780 cell line; however, both IL-33 and ST2 were found to be
expressed in the SKOV3, CAOV3 and OV90 cell lines (Fig. S4). This finding suggested that
IL-33 was highly expressed in ID8-T6 cells and may thus be involved
in human ovarian cancer.
In vitro functional experiments on
ID8-IL33 ovarian tumor cells
To examine the effects of IL-33 on peritoneal
carcinomatosis, ID8-WT, a cell line lacking IL-33, was
gene-transferred with IL-33 cDNA, and ID8-IL33 cells expressing
IL-33 and ID8-mock control cells were generated. The expression of
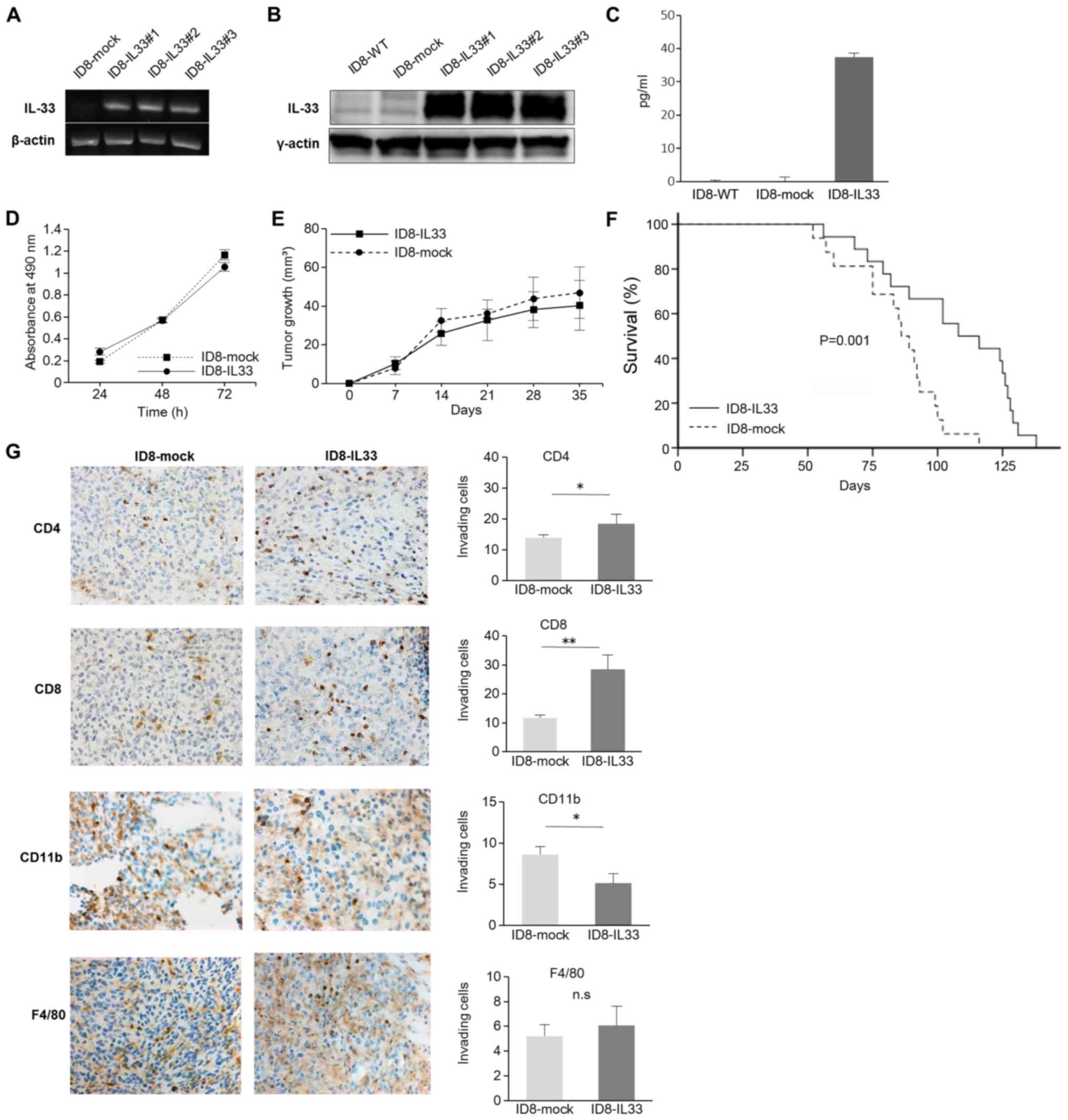
IL-33 following gene transfer was confirmed by semi-quantitative
PCR and WB analysis (Fig. 1A and
B). In addition, the IL-33 concentrations in the cell culture
supernatant were examined. It was confirmed that the IL-33
concentration in the ID8-IL33 cells was high enough (Fig. 1C). We then further examined whether
there was a difference between the ID8-IL33 and ID8-mock cells
in vitro by evaluating cell proliferation. However, the
in vitro growth rates between the ID8-IL33 cells and
ID8-mock cells did not differ (Fig.
1D). We also assessed whether IL-33 promotes the migratory
ability of the tumor cells by scratch wound migration assay;
however, there was no difference in the migratory ability between
the ID8-IL33 and IL33-mock cells (Fig. S5). Thus, the overexpression of
IL-33 did not affect tumor cell proliferation or migration in
vitro.
IL-33 does not promote tumor progression,
but increases tumor immunity by promoting the infiltration of
immune cells into the tumor
As IL-33 had no effect on tumor cells in
vitro, we decided to assess the role of IL-33 in tumor growth
in vivo. First, we established a subcutaneous syngeneic
mouse model. Similar tumor growth rates were observed between the
ID8-mock and ID8-IL33 cells in this model (Fig. 1E). The volumes calculated in
Fig. 1E were for one tumor per
mouse. We then established a syngeneic mouse model of peritoneal
carcinomatosis and compared their survival. The survival rate of
the ID8-IL33 tumor-bearing mice was not shortened, but was
significantly prolonged compared with that of the ID8-mock
tumor-bearing mice (P=0.001; Fig.
1F). The effects of IL-33 on tumors is controversial, and there
are some reports stating that IL-33 enhances antitumor immunity and
suppresses tumor growth (27-29).
Considering the possibility that IL-33 acts on tumor
immunity, we examined the localization of immune cells (CD4, CD8,
CD11b and F4/80) within the disseminated tumor tissues by IHC
analyses (Fig. 1G). The numbers of
infiltrating CD4+ cells and CD8+ cells were
significantly increased in the ID8-IL33 tumors compared with the
ID8-mock tumors. By contrast, the numbers of infiltrating
CD11b+ cells were decreased in ID8-IL33 tumors. No
marked no difference was observed in the numbers of infiltrating
F4/80+ cells. These results suggested that IL-33
inhibits tumor growth by promoting antitumor immunity.
IL-33 in ascites directly inhibits
myeloid-derived cell differentiation
IL-33 increased the number of infiltrating
CD4+ and CD8+ cells, and decreased the amount
of infiltrating CD11b+ cells. Based on these results, we
considered the involvement of myeloid-derived suppressor cells
(MDSCs). MDSCs are characterized as
CD11b+Gr-1+ cells in mice. In cancer, MDSCs
have a prominent immunosuppressive ability that enables them to
control immune responses, leading to tumor immune escape and
disease progression (30).
Therefore, in this study, we examined intratumoral
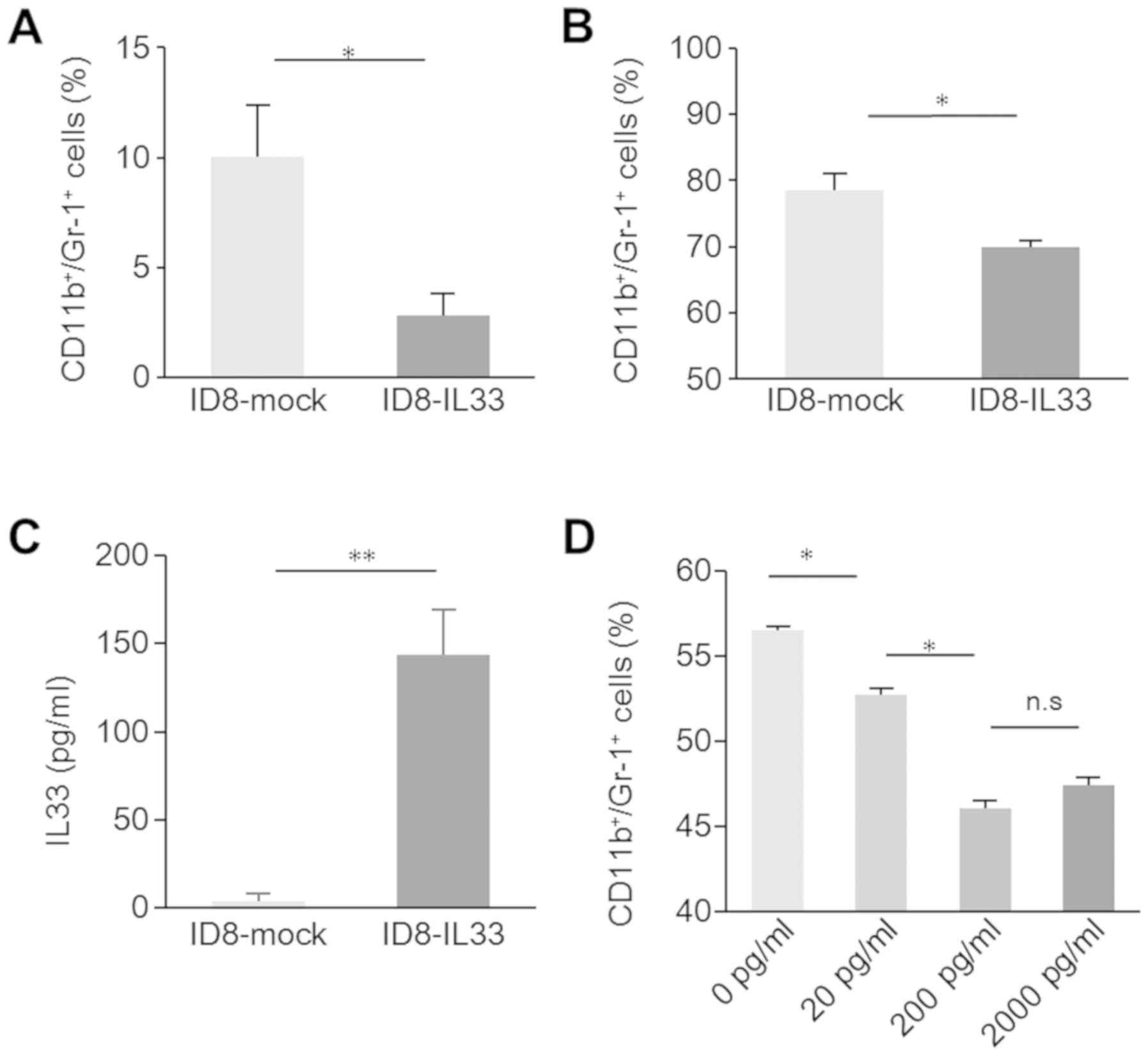
CD11b+Gr-1+ cells by FCM. The number of
intratumoral CD11b+Gr-1+ cells was
significantly decreased in the IL33-overexpressing tumors (Fig. 2A). This suggested that immune
activation was also mediated through MDSC inhibition, although it
was likely due to the direct effects of IL-33 on lymphocytes. No
difference was observed in tumor growth in the subcutaneous model;
but a difference was observed in the model of peritoneal
dissemination. Thus, we considered that cancerous ascites may be
involved in the reduction of intratumoral
CD11b+Gr-1+ cells, as ovarian cancer ascitic
fluids contain several cytokines, such as IL-6, IL-10 and GM-CSF,
which induce differentiation into MDSCs (31-33).
To investigate the effects of cancerous ascites on MDSCs, we
collected bone marrow cells from the femurs of healthy C57Bl/6 mice
and incubated them with ascites from ID8-IL33 and ID8-mock
tumor-bearing mice. The number of CD11b+Gr-1+
cells induced by ascites from the ID8-IL33 mice was significantly
lower than that induced by ascites from ID8-mock mice (Fig. 2B). Ascites from the ID8-IL33
tumor-bearing mice inhibited myeloid-derived cell differentiation.
Subsequently, we investigated what factor in the ascites acts to
prevent differentiation into CD11b+Gr-1+
cells. First, the concentration of IL-33 in the mouse ascites was
measured by ELISA. The IL-33 concentration in ascites from the
ID8-IL33 tumor-bearing mice was significantly higher, whereas
ascites from ID8-mock tumor-bearing mice were almost free of IL-33
(Fig. 2C). IL-33-responsive innate
cells have been previously reported in mouse bone marrow (34). Therefore, we considered that IL-33
in ascites may directly inhibit the differentiation of
lineage-negative progenitor bone marrow cells into
CD11b+Gr-1+ cells. We added recombinant IL-33
to IL-33-free ascites from ID8-mock tumor-bearing mice and cultured
with bone marrow cells from healthy C57BL/6 mice. The addition of
recombinant IL-33 reduced myeloid-derived cell differentiation.
However, the effect plateaued when the rIL33 concentration reached
a certain level (Fig. 2D). These
results suggested that IL-33 in ascites inhibited MDSC
differentiation induced by cytokines, thereby promoting tumor
immunity.
Silencing of IL-33 suppresses tumor
immunity and promotes tumor progression
Although IL-33 was found to be overexpressed in
ID8-T6 cells, ID8-T6 significantly shortened the survival of mice
in a murine model of peritoneal carcinomatosis compared with ID8-WT
(Fig. S2B). To clarify this
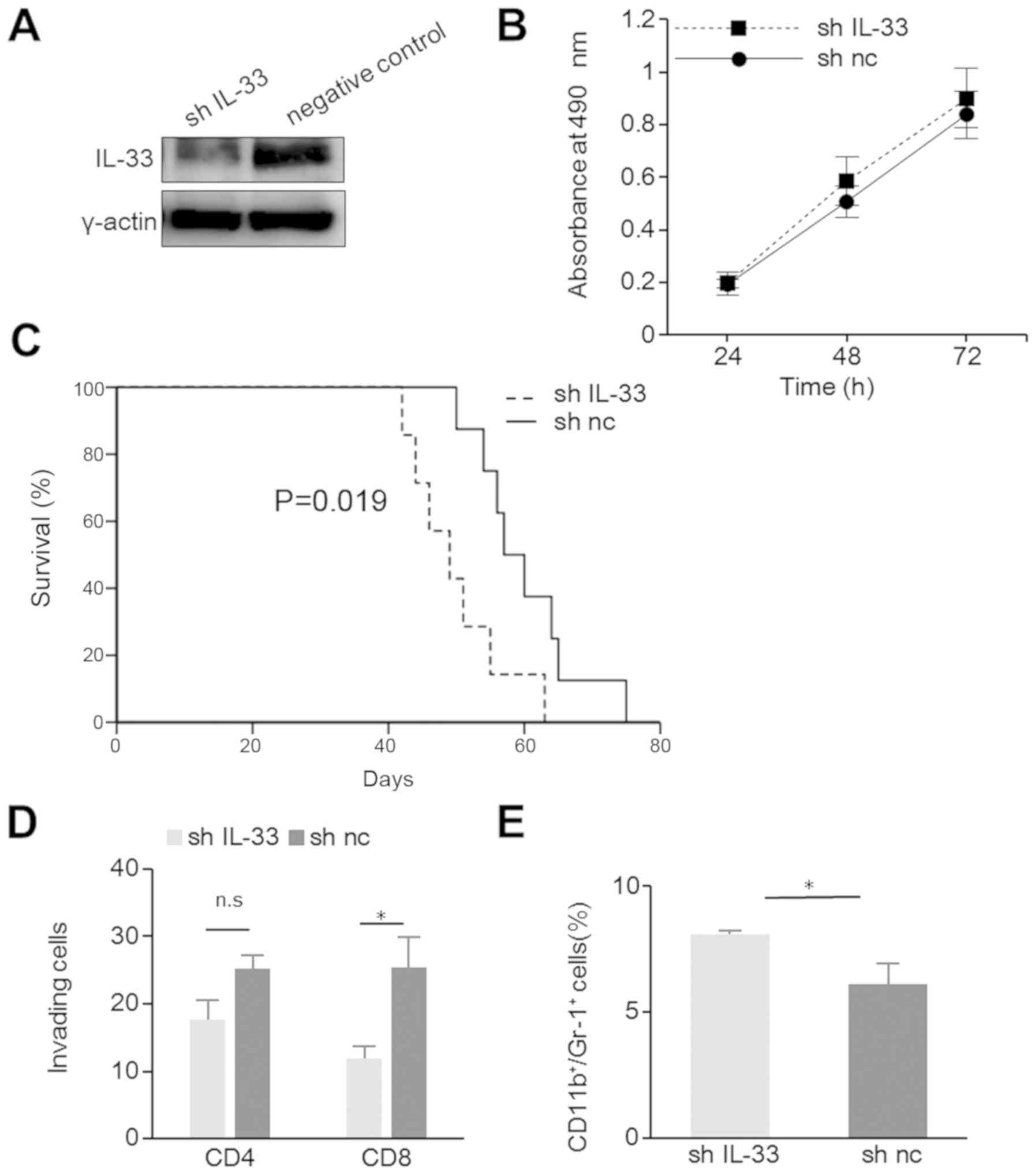
contradiction, we generated IL33-a-silenced ID8-T6 cells, shIL-33
and a negative control. The knockdown of IL-33 in the shIL-33 cells
was confirmed by WB analysis (Fig.
3A). Cell proliferation in vitro was similar between the
shIL-33 and negative control-transfected cells (Fig. 3B). In a murine model of peritoneal
carcinomatosis, the shIL-33 tumor-bearing mice exhibited
significantly shorter survival times (P=0.019; Fig. 3C). Furthermore, the silencing of
IL-33 reduced the number of infiltrating CD8+ cells, and
increased the number of CD11b+Gr-1+ cells
(Fig. 3D and E). This result
suggested that IL-33 also suppressed tumor progression through
tumor immunity in ID8-T6 cells and conhrmed the antitumor effects
of IL-33.
Association of IL-33 with clinical
outcome in human ovarian cancer
Taken together, our preclinical results demonstrated
that IL-33 improves prognosis by enhancing tumor immunity. To
assess whether this finding is clinically relevant, we examined the
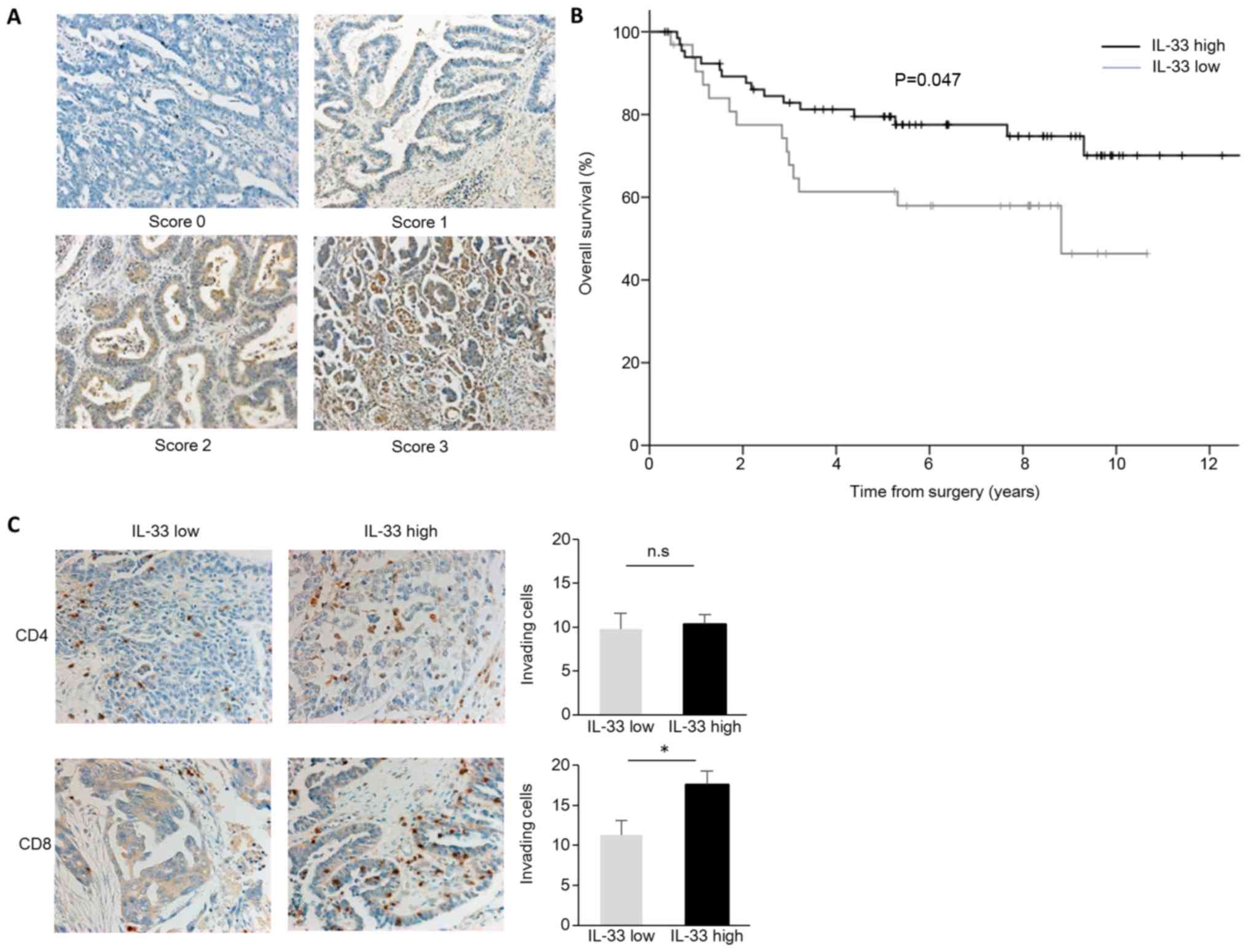
expression of IL-33 in 100 paraffin-fixed human primary ovarian
cancer tissues (Table I). We found
different expression levels of IL-33 in ovarian cancer cells. We
classified scores 0/1 as IL-33 low and 2/3 as IL-33 high. In total,
67 of the samples expressed high levels of IL-33 (IL-33 high) in
the ovarian cancer cells (Table I
and Fig. 4A). Cases with high
staining of IL-33 had significantly longer overall survival times
(P=0.047; Fig. 4B). To confirm the
association of tumor IL-33 expression with antitumor immunity, we
performed additional IHC staining for CD4 and CD8. Although no
differences were observed in the number of inhitrating
CD4+ cells, the numbers of inhitrating CD8+
cells were significantly increased in the IL-33 high expression
group (Fig. 4C). These findings
suggest that IL-33 enhances tumor immunity and prolongs
survival.
Discussion
The importance of interactions in both cancer and
tumor microenvironments for the malignant phenotype and genotype of
EOC has been demonstrated in recent studies (35-37).
These complex interactions may be due to the accumulation of
spatiotemporal signaling events that function in tumor progression
or regression. Due to the heterogeneity of EOC, the underlying
mechanisms altering gene expression remain unclear. These
alterations involve a number of secreted components, such as
cytokines, chemokines, growth factors, metabolites and exosomal
miRNAs (38). These non-cellular
components under peritoneal carcinomatosis conditions may have a
greater influence on the acquisition of malignancy by providing
energy, growth signals, drug resistance tumor microenvironment,
evasion of immune surveillance, and metastatic and angiogenesis
cues. Peritoneal carcinomatosis is a devastating metastatic form of
EOC. Thus, switching the function of these secreted components from
tumor acceleration to antitumor activity has been considered as a
new target for EOC therapy.
Based on this, we searched for potential factors
involved in peritoneal carcinomatosis. We focused on IL-33, which
is a humoral factor that has recently been reported to be
associated with cancer. We initially hypothesized that IL-33
promotes tumorigenesis; however, the overexpression of IL-33
significantly prolonged the survival of mice in a murine model of
peritoneal carcinomatosis. By contrast, the knockdown of IL-33 in
ID8-T6 cells significantly shortened the survival of mice. Thus,
IL-33 enhanced tumor immunity and suppressed tumor growth in a
murine model of peritoneal carcinomatosis. As to why ID8-T6
acquired a high dissemination ability, several progression factors
that have a greater influence on peritoneal dissemination than
IL-33 are likely to be expressed in ID8-T6 cells. Thus, the
antitumor effects of IL-33 are likely subverted due to interactions
with other tumor promoting factors. Accordingly, we
intraperitoneally injected ID8-WT or ID8-T6 cells into
immunodeficient mice (BALB/C nude). Similar as in immunocompetent
mice, peritoneal dissemination formation by ID8-T6 cells was
earlier than that by ID8-WT cells (data not shown). We considered a
number of potent progression factors, regardless of immunity, to be
expressed in ID8-T6 cells, and that the antitumor effects of IL-33
were masked.
Although IL-33 was highly expressed in ID8-T6 cells,
IL-33 is a naturally acquired tumor suppressor in the process of
ID8 with recurrent peritoneal dissemination. Therefore, the
expression of IL-33 in the tumor may finally increase as the tumor
progresses to a certain extent in humans. Indeed, it was previously
reported that the expression levels of IL-33 were further increased
in tumor tissues at the metastatic site compared with at the
primary site (23). It may
therefore be difficult to use IL-33 as a prognostic marker, even
though it significantly prolonged human survival in this study.
In this study, we demonstrated that IL-33
overexpression promoted the infiltration of CD4+ and
CD8+ cells into tumors in a syngeneic mouse model of
peritoneal carcinomatosis. Infiltrating CD8+ cells
isolated from the disseminated tumors have the ability of
functional cytokine production. Although tumor-infiltrating
CD8+ T cell function was similar between ID8-mock and
ID8-IL33 peritoneal tumor settings, the number of infiltrating
CD8+ cells differed (data not shown).
Furthermore, in 100 clinical cases of ovarian
cancer, CD8+ cells were significantly increased in the
IL-33 high group. The expression of ST2 was detected on Th2 cells
(39,40). Recently, activated Th1 cells
(41) and cytotoxic T cells
(42) were found to have ST2
expression and IL-33 signaling. Thus, it is possible that IL-33
directly acts on CD4+ and CD8+ cells to
enhance tumor immunity. However, as a significant difference was
observed in the number of CD11b+ cells, as opposed to
CD4+ or CD8+ cells, in this study, we
hypothesized that some other signaling mechanism is involved.
Tumor-derived cytokines and inflammatory cytokines, including VEGF,
prostaglandin E2 (PGE2), TGF-β, stem cell factor (SCF), IL-1β,
IL-4, IL-6, IL-10, IL-12, IL-13, matrix metalloproteinase (MMP)-9,
granulocyte-macrophage colony-stimulating factor (GM-CSF) and
granulocyte-colony stimulating factor (G-CSF), have been reported
to play a role in MDSC differentiation (43,44).
Furthermore, it was recently reported that IL-33 effectively
reduces MDSC-dependent immune suppression and improves antitumor
responses (45). Therefore, we
assumed that the increase in infiltrating CD4+ and
CD8+ cells was mediated by MDSCs. Indeed, we
demonstrated that IL-33 in cancerous ascites was able to inhibit
the differentiation of bone marrow cells into
CD11b+Gr-1+ cells. Although we used CD11b and
Gr-1 as markers to identify MDSC, these markers are also expressed
by other cells. Indeed, the only way to definitively identify cells
as MDSCs is to demonstrate that they have immune suppressive
activity. However, there was a limitation in our technical ability
to evaluate the T cell-suppressive activity of tumor infiltrating
CD11b+Gr-1+ cells, as we were unable to use
the FACS cell sorter to purify CD11b/Gr-1 double-positive cells. It
is possible that other mechanisms are involved and influenced the
survival, but it is certain that this mechanism is involved.
In this study, no significant difference was
observed in tumor growth by the overexpression of IL-33 in cancer
cells in a subcutaneous syngeneic mouse model, whereas the
overexpression of IL-33 significantly prolonged the survival of
mice in a murine model of peritoneal carcinomatosis. We considered
this to be a unique result from peritoneal dissemination. IL-33
expressed by the tumor acts only locally in the subcutaneous model;
however, IL-33 expressed by tumor cells may act extensively via
cancerous ascites in a murine model of peritoneal carcinomatosis.
Even in hepatocellular carcinoma that is likely to develop
peritoneal carcinomatosis, such as ovarian cancer, the infiltration
of cancer cells by IL-33+ and CD8+ cells is
independently associated with prolonged patient survival (46). In the experiment using IL-33
transgenic mice, IL-33 promoted the proliferation, activation and
infiltration of CD8+ T cells, and the inhibition of
pulmonary metastasis in the B16 melanoma and Lewis lung carcinoma
metastatic mice models (28). It
is believed that IL-33 enhances tumor immunity and exhibits
antitumor effects only when it functions systemically.
In conclusion, we demonstrated that IL-33 reduces
tumor immune suppression and inhibits tumor progression in ovarian
cancer. Furthermore, we suggest that the reduction of MDSCs by
IL-33 reduced immune suppression. Further the elucidation of the
antitumorigenic role of IL-33 will aid in developing novel
therapeutic approaches for the treatment of EOC with peritoneal
carcinomatosis in the future.
Supplementary Materials
Funding
This study was supported by JSPS KAKENHI (grant no.
15K20138) (research funding).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
AS and SS conceived the study, analyzed and
interpreted data, performed statistical analyses and wrote the
manuscript. AT, SH and YS participated in data collection and the
provision of patient samples. NY, YK and HK aided in the design of
the study, and collected and assembled the data. FK was involved in
the conception and design of the study, coordinated the study over
the entire period and participated in editing and proofreading.
All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board at Nagoya University School of Medicine (approval no. for the
use of human samples: 2017-0053). Informed consent was obtained in
the form of opt-out on the web-site (https://www.med.nagoya-u.ac.jp/medical_J/ethics/pdf/1024_2017-0053.pdf).
Those who rejected were excluded. Animal care and experimental
procedures were approved by the Animal Experiment Committee of
Nagoya University (approval no. 30065).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
EOC
|
epithelial ovarian cancer
|
|
FCM
|
flow cytometry
|
|
IL
|
interleukin
|
|
IHC
|
immunohistochemical
|
|
MDSC
|
myeloid-derived suppressor cell
|
|
WB
|
western blot
|
Acknowledgments
Not applicable.
References
|
1
|
Ebell MH, Culp MB and Radke TJ: A
Systematic review of symptoms for the diagnosis of ovarian cancer.
Am J Prev Med. 50:384–394. 2016. View Article : Google Scholar
|
|
2
|
Coleman RL, Monk BJ, Sood AK and Herzog
TJ: Latest research and treatment of advanced-stage epithelial
ovarian cancer. Nat Rev Clin Oncol. 10:211–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chae CS, Teran-Cabanillas E and
Cubillos-Ruiz JR: Dendritic cell rehab: New strategies to unleash
therapeutic immunity in ovarian cancer. Cancer Immunol Immunother.
66:969–977. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki S, Sakata J, Utsumi F, Sekiya R,
Kajiyama H, Shibata K, Kikkawa F and Nakatsura T: Efficacy of
glypican-3-derived peptide vaccine therapy on the survival of
patients with refractory ovarian clear cell carcinoma.
OncoImmunology. 5:e12385422016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu X, Cai H, Zhao L, Ning L and Lang J:
CAR-T cell therapy in ovarian cancer: From the bench to the
bedside. Oncotarget. 8:64607–64621. 2017.PubMed/NCBI
|
|
6
|
Haraldsen G, Balogh J, Pollheimer J,
Sponheim J and Küchler AM: Interleukin-33 - cytokine of dual
function or novel alarmin? Trends Immunol. 30:227–233. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liew FY, Pitman NI and McInnes IB:
Disease-associated functions of IL-33: The new kid in the IL-1
family. Nat Rev Immunol. 10:103–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-asso-
ciated cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oboki K, Ohno T, Kajiwara N, Saito H and
Nakae S: IL-33 and IL-33 receptors in host defense and diseases.
Allergol Int. 59:143–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sattler S, Smits HH, Xu D and Huang FP:
The evolutionary role of the IL-33/ST2 system in host immune
defence. Arch Immunol Ther Exp (Warsz). 61:107–117. 2013.
View Article : Google Scholar
|
|
11
|
Kurowska-Stolarska M, Kewin P, Murphy G,
Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y,
Niedbala W, et al: IL-33 induces antigen-specific IL-5+
T cells and promotes allergic-induced airway inflammation
independent of IL-4. J Immunol. 181:4780–4790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurowska-Stolarska M, Stolarski B, Kewin
P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B,
van Rooijen N, et al: IL-33 amplifies the polarization of
alternatively activated macrophages that contribute to airway
inflammation. J Immunol. 183:6469–6477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pei C, Barbour M, Fairlie-Clarke KJ, Allan
D, Mu R and Jiang HR: Emerging role of interleukin-33 in autoimmune
diseases. Immunology. 141:9–17. 2014. View Article : Google Scholar :
|
|
14
|
Wang S, Ding L, Liu SS, Wang C, Leng RX,
Chen GM, Fan YG, Pan HF and Ye DQ: IL-33: A potential therapeutic
target in autoimmune diseases. J Investig Med. 60:1151–1156. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miller AM, Xu D, Asquith DL, Denby L, Li
Y, Sattar N, Baker AH, McInnes IB and Liew FY: IL-33 reduces the
development of atherosclerosis. J Exp Med. 205:339–346. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sanada S, Hakuno D, Higgins LJ, Schreiter
ER, McKenzie AN and Lee RT: IL-33 and ST2 comprise a critical
biomechanically induced and cardioprotective signaling system. J
Clin Invest. 117:1538–1549. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu XX, Hu Z, Shen X, Dong LY, Zhou WZ and
Hu WH: IL-33 promotes gastric cancer cell invasion and migration
via ST2 ERK1/2 pathway. Dig Dis Sci. 60:1265–1272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang P, Liu XK, Chu Z, Ye JC, Li KL,
Zhuang WL, Yang DJ and Jiang YF: Detection of interleukin-33 in
serum and carcinoma tissue from patients with hepatocellular
carcinoma and its clinical implications. J Int Med Res.
40:1654–1661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JY, Lim SC, Kim G, Yun HJ, Ahn SG and
Choi HS: Interleukin-33/ST2 axis promotes epithelial cell
transformation and breast tumorigenesis via upregulation of COT
activity. Oncogene. 34:4928–4938. 2015. View Article : Google Scholar
|
|
20
|
Liu J, Shen JX, Hu JL, Huang WH and Zhang
GJ: Significance of interleukin-33 and its related cytokines in
patients with breast cancers. Front Immunol. 5:1412014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Donnell C, Mahmoud A, Keane J, Murphy C,
White D, Carey S, O'Riordain M, Bennett MW, Brint E and Houston A:
An antitumorigenic role for the IL-33 receptor, ST2L, in colon
cancer. Br J Cancer. 114:37–43. 2016. View Article : Google Scholar :
|
|
22
|
Akimoto M, Hayashi JI, Nakae S, Saito H
and Takenaga K: Interleukin-33 enhances programmed oncosis of
ST2L-positive low-metastatic cells in the tumour microenvironment
of lung cancer. Cell Death Dis. 7:e20572016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tong X, Barbour M, Hou K, Gao C, Cao S,
Zheng J, Zhao Y, Mu R and Jiang HR: Interleukin-33 predicts poor
prognosis and promotes ovarian cancer cell growth and metastasis
through regulating ERK and JNK signaling pathways. Mol Oncol.
10:113–125. 2016. View Article : Google Scholar
|
|
24
|
Saied EM and El-Etreby NM: The role and
prognostic value of inducible nitric oxide synthase (iNOS) and
interleukin-33 (IL-33) in serous and mucinous epithelial ovarian
tumours. Ann Diagn Pathol. 27:62–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
26
|
Suzuki S, Terauchi M, Umezu T, Kajiyama H,
Shibata K, Nawa A and Kikkawa F: Identification and
characterization of cancer stem cells in ovarian yolk sac tumors.
Cancer Sci. 101:2179–2185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Villarreal DO, Wise MC, Walters JN,
Reuschel EL, Choi MJ, Obeng-Adjei N, Yan J, Morrow MP and Weiner
DB: Alarmin IL-33 acts as an immunoadjuvant to enhance
antigen-specific tumor immunity. Cancer Res. 74:1789–1800. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao K, Li X and Zhang L, Bai L, Dong W,
Gao K, Shi G, Xia X, Wu L and Zhang L: Transgenic expression of
IL-33 activates CD8(+) T cells and NK cells and inhibits tumor
growth and metastasis in mice. Cancer Lett. 335:463–471. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao X, Wang X, Yang Q, Zhao X, Wen W, Li
G, Lu J, Qin W, Qi Y, Xie F, et al: Tumoral expression of IL-33
inhibits tumor growth and modifies the tumor microenvironment
through CD8+ T and NK cells. J Immunol. 194:438–445.
2015. View Article : Google Scholar
|
|
30
|
Millrud CR, Bergenfelz C and Leandersson
K: On the origin of myeloid-derived suppressor cells. Oncotarget.
8:3649–3665. 2017. View Article : Google Scholar :
|
|
31
|
Kolomeyevskaya N, Eng KH, Khan AN,
Grzankowski KS, Singel KL, Moysich K and Segal BH: Cytokine
profiling of ascites at primary surgery identifies an interaction
of tumor necrosis factor-α and interleukin-6 in predicting reduced
progression-free survival in epithelial ovarian cancer. Gynecol
Oncol. 138:352–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morales JK, Kmieciak M, Knutson KL, Bear
HD and Manjili MH: GM-CSF is one of the main breast tumor-derived
soluble factors involved in the differentiation of
CD11b-GrL- bone marrow progenitor cells into
myeloid-derived suppressor cells. Breast Cancer Res Treat.
123:39–49. 2010. View Article : Google Scholar
|
|
33
|
Wu L, Deng Z, Peng Y, Han L, Liu J, Wang
L, Li B, Zhao J, Jiao S and Wei H: Ascites-derived IL-6 and IL-10
synergistically expand CD14+HLA-DR-/low
myeloid-derived suppressor cells in ovarian cancer patients.
Oncotarget. 8:76843–76856. 2017.PubMed/NCBI
|
|
34
|
Brickshawana A, Shapiro VS, Kita H and
Pease LR: Lineage(-) Sca1+c-Kit(-)CD25+ cells
are IL-33-responsive type 2 innate cells in the mouse bone marrow.
J Immunol. 187:5795–5804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mitsui H, Shibata K, Suzuki S, Umezu T,
Mizuno M, Kajiyama H and Kikkawa F: Functional interaction between
peritoneal mésothélial cells and stem cells of ovarian yolk sac
tumor (SC-OYST) in peritoneal dissemination. Gynecol Oncol.
124:303–310. 2012. View Article : Google Scholar
|
|
36
|
Lau TS, Chan LK, Wong EC, Hui CW, Sneddon
K, Cheung TH, Yim SF, Lee JH, Yeung CS, Chung TK, et al: A loop of
cancer-stroma-cancer interaction promotes peritoneal metastasis of
ovarian cancer via TNFα-TGFα-EGFR. Oncogene. 36:3576–3587. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fujikake K, Kajiyama H, Yoshihara M,
Nishino K, Yoshikawa N, Utsumi F, Suzuki S, Niimi K, Sakata J,
Mitsui H, et al: A novel mechanism of neovascularization in
peritoneal dissemination via cancer-associated mesothelial cells
affected by TGF-β derived from ovarian cancer. Oncol Rep.
39:193–200. 2018.
|
|
38
|
Yokoi A, Yoshioka Y, Yamamoto Y, Ishikawa
M, Ikeda SI, Kato T, Kiyono T, Takeshita F, Kajiyama H, Kikkawa F,
et al: Malignant extracellular vesicles carrying MMP1 mRNA
facilitate peritoneal dissemination in ovarian cancer. Nat Commun.
8:144702017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lohning M, Stroehmann A, Coyle AJ, Grogan
JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A and Kamradt
T: T1/ST2 is preferentially expressed on murine Th2 cells,
independent of interleukin 4, interleukin 5, and interleukin 10,
and important for Th2 effector function. Proc Natl Acad Sci USA.
95:6930–6935. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu D, Chan WL, Leung BP, Huang F, Wheeler
R, Piedrahta D, Robinson JH and Liew FY: Selective expression of a
stable cell surface molecule on type 2 but not type 1 helper T
cells. J Exp Med. 187:787–794. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baumann C, Bonilla WV, Fröhlich A,
Helmstetter C, Peine M, Hegazy AN, Pinschewer DD and Löhning M:
T-bet- and STAT4-dependent IL-33 receptor expression directly
promotes antiviral Th1 cell responses. Proc Natl Acad Sci USA.
112:4056–4061. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bonilla WV, Fröhlich A, Senn K, Kallert S,
Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon
PG, et al: The alarmin interleukin-33 drives protective antiviral
CD8+ T cell responses. Science. 335:984–989. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ribechini E, Greifenberg V, Sandwick S and
Lutz MB: Subsets, expansion and activation of myeloid-derived
suppressor cells. Med Microbiol Immunol (Berl). 199:273–281. 2010.
View Article : Google Scholar
|
|
45
|
Lim HX, Choi S, Cho D and Kim TS: IL-33
inhibits the differentiation and immunosuppressive activity of
granulocytic myeloid-derived suppressor cells in tumor-bearing
mice. Immunol Cell Biol. 95:99–107. 2017. View Article : Google Scholar
|
|
46
|
Brunner SM, Rubner C, Kesselring R, Martin
M, Griesshammer E, Ruemmele P, Stempfl T, Teufel A, Schlitt HJ and
Fichtner-Feigl S: Tumor-infiltrating, interleukin-33-producing
effector-memory CD8(+) T cells in resected hepatocellular carcinoma
prolong patient survival. Hepatology. 61:1957–1967. 2015.
View Article : Google Scholar : PubMed/NCBI
|