Malignant tumors, which are characterized by
phenotypic plasticity, metabolic reprogramming and immune evasion,
continuously adapt to conventional treatment strategies,
highlighting the urgent need for the development of novel and more
effective therapeutic approaches (1). Radiotherapy exerts its tumor-killing
effects through two main mechanisms: i) Direct DNA damage induced
by high-energy radiation; and ii) indirect DNA damage via reactive
oxygen species (ROS). Ionizing radiation (IR) releases high-energy
photons that disrupt the DNA structure of tumor cells, leading to
base modifications and single- or double-strand breaks. These DNA
lesions result in cell cycle arrest, apoptosis or necrosis,
directly impacting tumor cell survival (2). In addition to DNA damage, IR induces
the decomposition of intracellular water, generating substantial
hydroxyl radicals (OH·) and other ROS species. IR causes a redox
imbalance within cells, which leads to lipid peroxidation, protein
denaturation and DNA damage, further disrupting normal cellular
processes, particularly mitochondrial function (3). With advancements in computational
modeling, image-guided technologies and biochemical agents,
radiotherapy has been increasingly optimized and currently
demonstrates distinct advantages in combination with other
therapies. While previous studies have primarily focused on the
mechanisms underlying DNA damage from radiotherapy (4,5),
recent research on tumor hypoxia and metabolic reprogramming has
shifted attention toward ROS-induced damage and mitochondrial
dysfunction resulting from redox imbalance, which have become key
areas of investigation (6,7).
Ferroptosis is a newly identified form of programmed
cell death characterized by iron-dependent lipid peroxidation,
accompanied by an immunogenic response. This form of cell death is
intricately linked to the cellular metabolic state and redox
balance, serving a critical role in the regulation of radiotherapy
efficacy (8). IR promotes the
generation of ROS, which directly damage nucleic acids and lipids
in tumor cells, thereby triggering lipid peroxidation and
ferroptosis (9). In the context
of therapeutic resistance, particularly in chemotherapy,
radiotherapy and immunotherapy, tumor cells (and especially those
in a mesenchymal state with higher metastatic potential) become
more susceptible to ferroptosis due to mitochondrial metabolic
alterations dependent on the tumor microenvironment (TME) (10). Ferroptosis emerges as a promising
pathway for enhancing radiation sensitivity, facilitated by the
combined effects of mitochondrial-mediated redox reactions, lipid
peroxidation and dysregulated iron metabolism. This offers notable
clinical potential for overcoming resistance to conventional
therapies (11,12).
Mitochondria are not only the powerhouse of the cell
but also central hubs for redox reactions, iron metabolism and
lipid peroxidation (13).
Mitochondrial involvement in ferroptosis extends beyond regulating
redox imbalance, as they are also crucial in ROS accumulation
(14). As a result, mitochondria
serve a pivotal role in maintaining intracellular redox equilibrium
and are essential for executing ferroptotic cell death. In the
context of radiotherapy, mitochondrial dysfunction and redox
imbalance further drive ferroptosis, while the immunogenic response
triggered by ferroptosis provides new opportunities for enhancing
radioimmunotherapy. Ferroptosis is not merely a form of cell death;
it elicits an immune response that activates immune cells within
the TME, thereby improving the therapeutic efficacy of radiotherapy
(15).
Despite these findings, the reciprocal regulatory
mechanisms between mitochondria and ferroptosis, as well as the
associated immune activation in the context of radiotherapy, remain
incompletely understood. As research into the association between
mitochondria and ferroptosis advances, targeting mitochondrial
ferroptosis may become a key strategy for radiation
immunosensitization. The present review explored the mechanisms
underlying the interaction between ferroptosis and mitochondria
under IR, and discussed the potential for targeting mitochondrial
ferroptosis as a strategy for enhancing radioimmunotherapy, aiming
to uncover the clinical importance of mitochondrial ferroptosis in
radiation-based cancer treatment.
The incidence and mortality rates of cancer continue
to rise, and radiotherapy, a cornerstone in cancer treatment, has
been developed and use in the clinic for >100 years (16). Several common cancer types,
including nasopharyngeal carcinoma and esophageal cancer, can be
effectively treated with radiotherapy, either as a monotherapy or
in combination with chemotherapy, immunotherapy and
targeted-therapy (17). However,
a subset of patients still experiences recurrence or metastasis,
presenting a persistent challenge to the efficacy of clinical
radiotherapy. Radiotherapy dosage is a crucial determinant of tumor
control rates; however, high radiation doses may lead to
significant damage to surrounding normal tissues and organs.
Therefore, optimizing the radiation dose, enhancing tumor
radiosensitivity and minimizing toxic side effects remain critical
challenges yet unresolved in the field of radiotherapy (18,19).
Previous research has indicated that the primary
mechanism of tumor cell death induced by radiotherapy is through
IR-induced proliferative cell death, which includes apoptosis,
autophagy and necrosis (20).
However, increasing attention has been paid to the potential role
of other forms of regulated cell death (RCD) in radiotherapy's
therapeutic effects (21,22). Ferroptosis, a form of
radiation-induced cell death, has emerged as an important
contributor to tumor response, and exhibits characteristics of ICD,
which is intricately linked with radioimmunotherapy (23). The hallmark of ferroptosis is
iron-dependent lipid peroxidation, particularly of polyunsaturated
fatty acid (PUFA)-phospholipids (PLs), accompanied by
characteristic mitochondrial morphological changes such as
shrinkage, loss or reduction of mitochondrial cristae and increased
mitochondrial membrane density. In the context of radiotherapy, IR
induces immune system recognition and activation by releasing
specific immune stimulants, such as High Mobility Group Box 1
Protein (HMGB1, a nuclear protein promotes late-stage
inflammation), ATP and exposed cell membrane antigens, thus
triggering ICD and activating CD8+ T cells. IFN-γ
produced by CD8+ T cells can alter the lipid metabolic
profile of tumor cells, promoting ferroptosis through an
ACSL4-dependent pathway (24).
Notably, ferroptosis exhibits crosstalk with various
forms of RCD, particularly under oxidative stress conditions, a
hallmark of radiation exposure. NADPH oxidase (NOX), a key
regulator of lipid redox signaling, serves dual roles in both
apoptotic pathways and ferroptosis initiation through lipid
peroxidation induction (25).
During bortezomib-induced apoptosis, ACSL4 inactivation may
suppress the incorporation of PUFAs into cell membranes,
consequently diminishing ferroptotic susceptibility. Furthermore,
selective autophagy-mediated GPX4 degradation results in
intracellular accumulation of free iron and lipid peroxides,
ultimately driving ferroptosis progression (26,27).
Radiation-induced ferroptosis not only interacts
with other RCD mechanisms, such as apoptosis and autophagy, to
modulate tumor cell radiosensitivity, but also reshapes the TME by
enhancing the activation and infiltration of immune cells (28). This immune activation, in turn,
further sensitizes the tumor to radiotherapy, especially in
radioimmunotherapy strategies (28). Specifically, radiotherapy-induced
ferroptosis enhances the uptake of tumor antigens by dendritic
cells (DCs), thus boosting tumor-specific T cell responses.
Additionally, ferroptosis can modulate the activity of
myeloid-derived suppressor cells (MDSCs) and regulatory T cells
(Tregs), thus reshaping the immune landscape of the TME and
preventing immune escape (29).
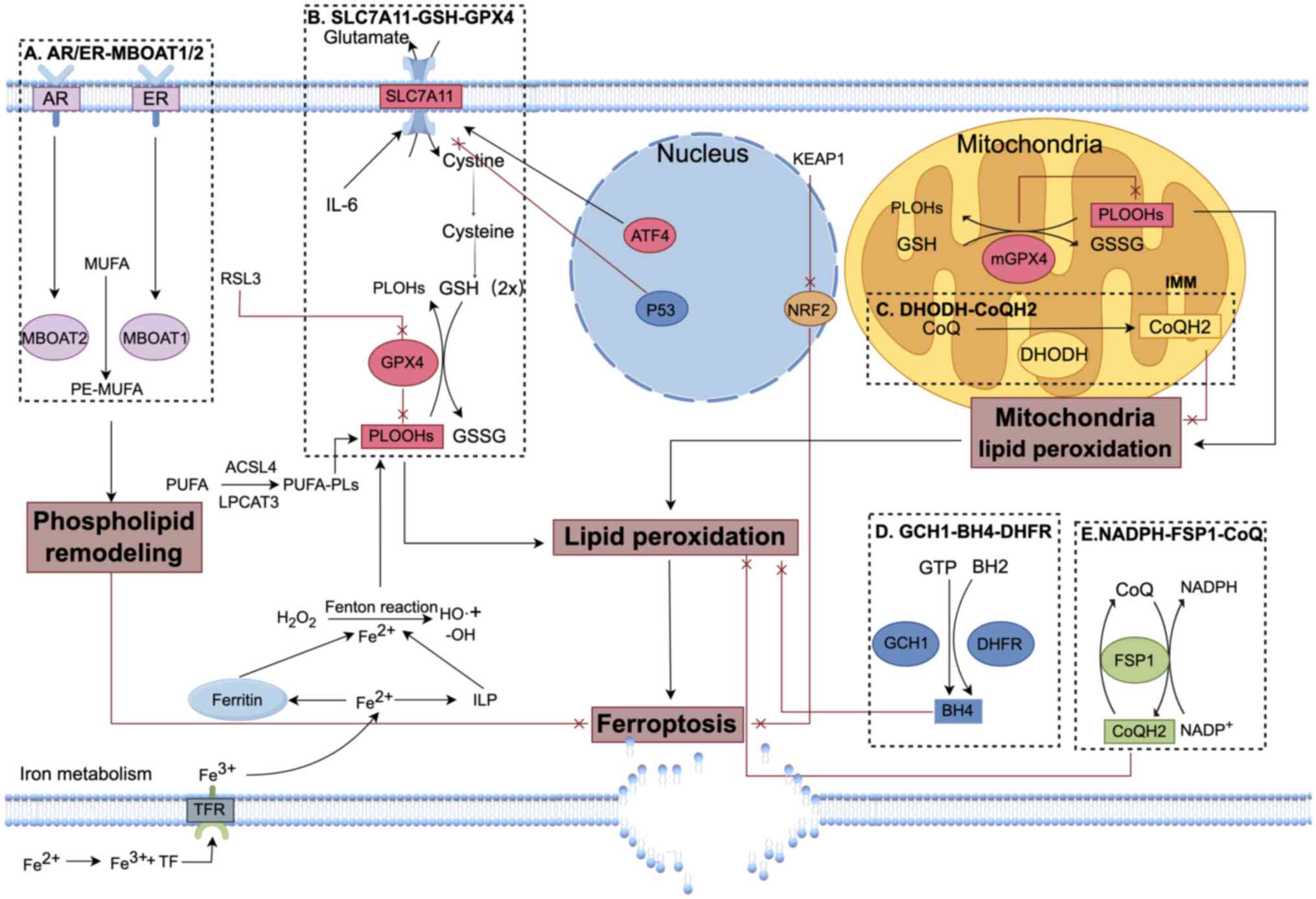
Research into the regulatory pathways of ferroptosis
has identified several defense systems with distinct subcellular
localizations, including Solute Carrier Family 7 Member 11
(SLC7A11, an antiporter that imports cystine for glutathione
synthesis)-glutathione (GSH)-glutathione peroxidase 4 (GPX4),
NAD(P)H-FSP1-CoQ, GCH1-BH4-DHFR, sex hormone-MBOAT1/2 and
mitochondrial-localized dihydroorotate dehydrogenase
(DHODH)-CoQH2 (30).
These defense systems regulate key processes such as iron
metabolism, redox balance and lipid peroxidation in different
cellular compartments, collectively maintaining cellular
homeostasis (Fig. 1). When these
defense mechanisms are disrupted, cells become more vulnerable to
ferroptosis, particularly metabolically active tumor cells
(31). Inhibiting key antioxidant
enzymes and iron homeostasis regulators can significantly enhance
the efficacy of radiotherapy. Consequently, targeting the
ferroptosis defense systems has emerged as an effective strategy
for radiosensitization in malignant tumors.
Mitochondria are dynamic organelles with a
double-membrane structure, serving a pivotal role in maintaining
cellular homeostasis through multiple pathways, including the
kallikrein system, the unfolded protein response, autophagy and
various metabolic processes (32). However, common mitochondrial
defects in tumor cells can significantly alter energy production,
shifting from oxidative phosphorylation (OXPHOS) to a more
simplified, oxygen-independent glycolysis pathway, in order to meet
the high metabolic demands of rapid proliferation and the hypoxic,
acidic microenvironment typical of tumors (33). These mitochondrial defects also
downregulate the mitochondrial apoptosis pathway, a key factor that
enables tumor cells to sustain their malignant phenotype and
develop resistance to radiation. IR induces serious damage to the
mitochondrial membrane, altering its permeability and leading to
mitochondrial swelling, vacuolization and fragmentation of the
cristae. The damage impairs the electron transport chain (ETC) and
results in ROS accumulation (34). The dysfunction of mitochondria
caused by IR can be further amplified through mitochondrial fusion
and fission processes, which help dilute the damaged mitochondria.
This leads to the genetic transmission of oxidative stress damage
within the cell, causing side effects, exacerbating instability in
both mitochondrial and nuclear DNA (35). In response to IR, tumor cells
compensate by increasing the number of mitochondria and activating
repair mechanisms to offset declines in mitochondrial respiratory
efficiency, partially restoring mitochondrial function after
IR-induced damage (36). However,
this compensatory response leads to sustained ROS production,
excessive depletion of mitochondrial antioxidants such as manganese
superoxide dismutase and coenzyme Q, which further aggravates
mitochondrial damage and generates more ROS, creating a vicious
cycle that increases the risk of cell death (37).
In a microenvironment with persistently high ROS
levels, tumor cells undergo long-term damage, leading to cellular
senescence after repeated irradiation and exacerbating secondary
radiation damage (38).
Mitochondria play a critical role in cellular metabolic plasticity
and regulate various forms of RCD, including ferroptosis, following
radiation injury. Irreversible mitochondrial outer membrane
permeabilization (MOMP) results in the release of pro-apoptotic
factors such as cytochrome c from the intermembrane space
into the cytoplasm, which activates the caspase-9-dependent
intrinsic apoptosis pathway (39). Furthermore, mitochondrial DNA
(mtDNA) released into the cytoplasm is recognized by cyclic GMP-AMP
synthase (cGAS), which activates the cGAS-STING inflammatory
pathway, triggering pyroptosis and inhibiting mitochondrial
autophagy, thereby exacerbating mitochondrial damage (40). In addition to acting as a
cytoplasmic DNA sensor that activates innate immune responses, cGAS
also contains a mitochondrial targeting sequence, enabling its
localization to the mitochondrial outer membrane (OMM), where it
plays a protective role against ferroptosis (41). As IR intensifies its effects on
mitochondrial function in tumor cells, structural and metabolic
alterations in mitochondria significantly enhance ferroptosis,
supporting the potential of ferroptosis in radioimmunotherapy
strategies.
Mitophagy, a selective form of macroautophagy that
induces the degradation of dysfunctional and damaged mitochondria
via the autophagy pathway. Mitophagy serves a key role in
maintaining cellular homeostasis (42). Protein disulfide isomerase (PDI),
an enzyme primarily located in the endoplasmic reticulum (ER),
mediates disulfide bond formation and is often highly expressed in
tumor tissues, where its elevated levels correlate with increased
pathological grading (43). In
colorectal cancer (CRC) cells subjected to radiation-induced ER
stress, PDI not only inhibits autophagy initiation by activating
the GRP78-mTOR pathway but also competes with LC3Ⅱ for binding to
the mitochondrial autophagy receptor PHB2, thereby regulating the
mitophagy process and reducing radiation sensitivity (44). Specifically, PDI plays a critical
role in balancing ferroptosis and mitophagy. During ferroptosis,
PDI modulates cellular iron homeostasis and redox reactions by
influencing antioxidant activity. Additionally, through its dual
localization in both the ER and mitochondria, PDI regulates the ER
stress response and impacts mitophagy initiation by interacting
with PHB2 (44). Through these
mechanisms, PDI not only suppresses autophagy initiation but also
plays a pivotal role in regulating ferroptosis.
Functionally intact and structurally complete
mitochondria are crucial in defending against ferroptosis through
various metabolic and antioxidant pathways (45). On the OMM, voltage-dependent anion
channels (VDAC) 2/3, iron transporter proteins Mfrn1/Mfrn2, the
mitochondrial permeability transition pore and the mitochondrial
Ca2+ uniporter facilitate the physiological uptake of
Fe2+, maintaining a labile iron pool (46). Mitochondrial ferritin (FtMt) plays
a pivotal role in regulating iron metabolism within the
mitochondrial matrix. It is involved in the synthesis of
iron-sulfur (Fe/S) cluster proteins, such as NADH dehydrogenase,
cytochrome c and succinate dehydrogenase, thereby
maintaining ATP production and preventing the accumulation of
mitochondrial ROS (mtROS) (47).
FtMt also regulates the availability of free iron in mitochondria,
mitigating iron overload, oxidative stress and ferroptosis under
conditions such as cerebral ischemia-reperfusion and IR injury. The
Fe/S cluster proteins mitoNEET and frataxin on the OMM are
similarly essential for maintaining mitochondrial iron metabolism
balance and inhibiting ferroptosis. In addition to iron metabolism,
cellular energy metabolic pathways, including the tricarboxylic
acid cycle (TCA) and OXPHOS, as well as associated regulatory
factors such as NADPH, GSH and AMPK, all play critical roles in
ferroptosis regulation (48). For
example, the mitochondrial transmembrane protein MTCH1 stabilizes
the mitochondrial OXPHOS cycle and mtROS levels; its deficiency
activates pro-ferroptotic retrograde signaling involving the
FoxO1-GPX4 axis, which synergistically enhances the antitumor
activity of ferroptosis-inducing agents such as sorafenib (49).
Mitochondrial cysteine metabolism also plays a
central role in ferroptosis regulation. The cysteine desulfurase
complex NFS1-ISD11 provides sulfur for Fe-S cluster formation and
promotes GSH synthesis, thus upregulating the activity of the
GSH-GPX4 antioxidant system (50). The hereditary inactivation of
fumarase, a key enzyme in the TCA cycle, induces ferroptosis by
downregulating GPX4 activity in conditions such as smooth muscle
tumors and renal cell carcinoma (51). NADPH, which is critical for GSH
conversion in the mitochondrial TCA cycle, is catalyzed by
NADP+-dependent isocitrate dehydrogenase (IDH), and its
cytoplasmic source can be provided by the reversible oxidative
decarboxylation of malate to pyruvate catalyzed by malic enzyme 1
(ME1). NADPH not only drives ATP release through coupling with
OXPHOS but also maintains the GSH cycle across various metabolic
pathways, thus counteracting mitochondrial lipid peroxidation and
reducing susceptibility to ferroptosis (51).
The ETC complexes (complexes I-IV) and ATP synthase
(complex V) stimulate ATP synthesis through phosphocreatine and
activate the AMPK signaling pathway. Inhibiting acetyl-CoA
carboxylase phosphorylation and PUFA synthesis also negatively
regulates mitochondrial ferroptosis, exerting similar effects to
those of the LKB1-AMPK axis (52). Similarly, enzymes targeting
mitochondrial CoQ reduction, such as DHODH and G3P dehydrogenase 2
(GPD2), inhibit mitochondrial lipid peroxidation and ferroptosis by
reducing CoQ to CoQH2 (53). GPD2 further synergizes with
mtGPX4, leading to the acetylation of mtGPX4 and contributing to
cadmium-induced renal cell ferroptosis (54). The role of mitochondrial
supercomplexes formation and its regulatory factor COX7A2L in
ferroptosis sensitivity has also been confirmed (55). COX7A2L deficiency induces
mitochondrial metabolic reprogramming, enhancing glutamine (GLN)
metabolism, cell proliferation and oxidative defense against
ferroptosis (56). Furthermore,
several regulatory factors that are localized in or translocated to
mitochondria after activation also serve a key role in
mitochondrial ferroptosis. Mitochondrial inner membrane-localized
PDK4 inhibits ferroptosis by blocking pyruvate
dehydrogenase-dependent pyruvate oxidation and fatty acid
synthesis. However, the roles of AMPK and PDK4 in ferroptosis are
cell-type-dependent, with complex interactions between energy
status and ferroptosis induced by different activators (57). The mitochondrial metabolic enzyme
PCK2 phosphorylates ACSL4 and promotes ACSL4-associated PL
remodeling in tumor cells. Cancer stem cells promote PCK2
ubiquitination and degradation, thus maintaining membrane PLs in a
ferroptosis-tolerant state (58).
Activated by PLC kinase, monocarboxylate transporter 1 (MCT1)
translocates to the mitochondrial surface, mediating lactate
uptake, promoting ATP production and inactivating AMPK, which
increases the expression of sterol regulatory element-binding
protein 1, stearoyl-CoA desaturase 1 and the end products
monounsaturated fatty acids, thereby contributing to ferroptosis
resistance (59). Furthermore,
lithium carbonate-induced MCT1 translocation and Ca2+
release activate LA oxidation in mitochondria, reactivating
LA-suppressive CD8+ T effector cells (60). Mitochondria serve a key role in
cysteine deprivation-induced (CDI) ferroptosis, which is distinct
from GPX4 inhibition-induced ferroptosis, with the TCA cycle and
amino acid metabolism pathways closely linked to CDI ferroptosis
(61). GLN, a key respiratory
substrate for energy production and lipid synthesis in tumor cells,
is degraded by mitochondrial glutaminases (GLS) 1/2. Due to their
similar amino acid sequences encoded by unrelated genes, reduction
of GLS2, rather than GLS1, through genetic or pharmacological
mechanisms, inhibits CDI ferroptosis. Aminooxyacetate inhibits the
conversion of GLN to α-ketoglutarate, rescuing CDI ferroptosis in
tumor cells (61).
Mitochondria-associated ER membranes (MAMs) are
highly dynamic and tightly interconnected structures between the
mitochondria and the ER. MAMs serve as a platform for
Ca2+ signaling between the two organelles and serve a
vital role in regulating mitochondrial ferroptosis signaling
(62). Mitochondrial microsomal
glutathione S-transferase 1 limits lipid peroxidation and
ferroptosis by binding to and downregulating ALOX5 activity
(63). The calcium-sensitive
receptor chaperone protein σ1R mediates the inhibitory effect of
the kinase inhibitor CGI1746 on ferroptosis. CGI1746 also slows the
Ca2+ transport rate within MAMs, disrupting the
ferroptosis process (64). The
IP3R-GRP75-VDAC1 axis is a key regulatory pathway for the dynamic
changes in MAMs, and its inhibition leads to the accumulation of
PUFA-containing triacylglycerols, conferring resistance to
ferroptosis (65). Mitofusin-2
(Mfn-2), originating from both the mitochondrial membrane and the
ER surface, mediates physical and biochemical interactions between
the mitochondria and the ER. Mfn-2 serves a key role in
mitochondrial fusion and stabilizing mitochondrial-ER contact sites
(66). Mfn-2 interacts with the
ER-resident protein inositol-requiring enzyme 1α (IRE1α) to
participate in arsenic-induced ferroptosis, while IRE1α also
influences ferroptosis sensitivity by regulating GSH synthesis
(67). Deficiency in the
autophagy regulator WIPI4 increases the localization of ATG2 to
mitochondria and MAMs, which enhances the transfer of
phosphatidylserine within MAMs and the ATG2-dependent synthesis of
mitochondrial phosphatidylethanolamine (PE) by phosphatidylserine
decarboxylase (PISD), ultimately triggering mitochondrial membrane
lipid peroxidation and ferroptosis (68). Inhibition of PISD protects cells
from ferroptosis induced by WIPI4 depletion and other inducers,
suggesting that PE synthesis in mitochondria may represent a
general early step in ferroptosis (69).
The regulatory role of mitochondrial metabolic
pathways in ferroptosis varies across different tumor types
(70). For instance, abnormal
NADPH production and regulation may result in notable differences
in ferroptotic effects across malignancies (71). Enzymes involved in NADPH
production, such as IDH and ME1, exhibit mutations or deletions in
different cancer types, leading to an imbalance in the antioxidant
system (72). Specifically, in
cholangiocarcinoma, IDH1 mutations promote ferroptosis by lowering
GPX4 expression and accelerating GSH depletion (73). In synovial sarcoma, ME1 deficiency
alters the mitochondrial NADPH-dependent antioxidant system,
increasing susceptibility to ferroptosis induced by ACXT-3102
(74). MAMs also display
different ferroptosis sensitivities under various treatments within
the same tissue type. mtROS activate the PERK pathway, suppress
Mfn-2 expression and cause MAM dysfunction, contributing to
arsenic-induced ferroptosis in liver cells. Upregulation of PERK-c
fragment expression in MAMs can trigger alcoholic liver
ferroptosis, a process inhibited by quercetin (75). Thus, research into mitochondrial
metabolic pathways and MAMs regulatory mechanisms across tumor
types could offer novel insights into their susceptibility and
resistance to ferroptosis, providing potential therapeutic targets
for clinical treatment.
Overall, the regulatory role of mitochondria in
ferroptosis encompasses iron metabolism, energy metabolism,
antioxidant regulation and transmembrane signaling with the ER.
These pathways and mechanisms exhibit marked variability across
different tumor types. A deeper understanding of these differences
could provide personalized therapeutic strategies for
ferroptosis-related anticancer treatments.
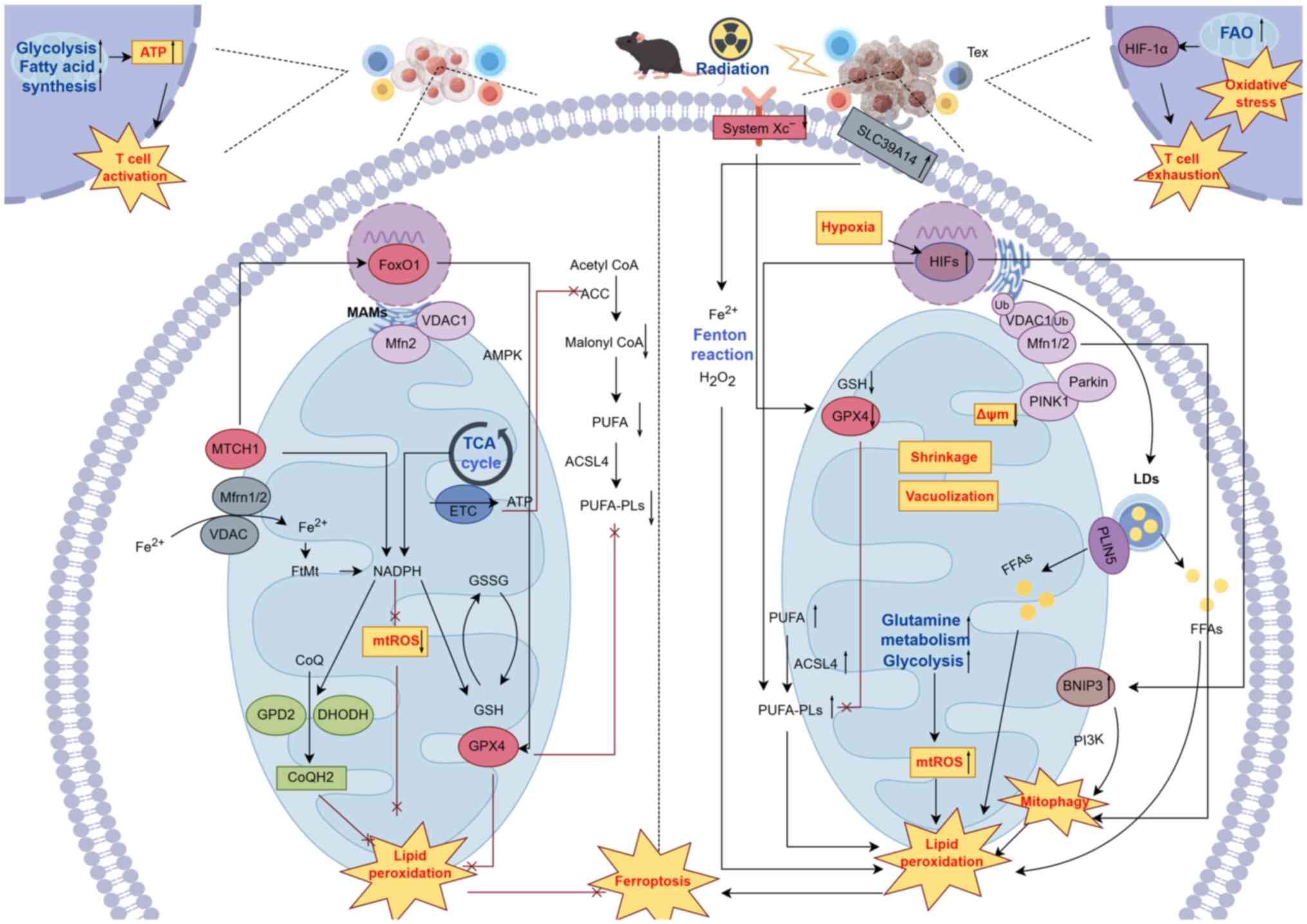
IR induces ferroptosis in tumor cells through
multiple mechanisms, which is closely tied to mitochondrial
metabolic reprogramming (15).
Upon exposure to IR, mitochondria in tumor cells undergo shrinkage
and vacuolization, while key components of the cysteine/glutamate
antiporter system (system XC-), including SLC3A2 and SLC7A11, are
significantly downregulated. This downregulation reduces the
synthesis of mtGPX4, thereby weakening the mitochondria's ability
to counter lipid peroxidation (76). IR also enhances tumor cell glucose
and GLN metabolism, dose-dependently increasing ROS production,
which leads to mitochondrial energy stress. In addition, IR
upregulates the expression of the iron transporter SLC39A14 and the
key enzyme ACSL4, which is involved in PUFA-PLs metabolism, thus
inducing lipid peroxidation (77). Furthermore, a reduction in
mitochondrial membrane potential and the proteolytic cleavage of
PTEN-induced kinase 1 (PINK1) by the inner membrane protease,
presenilin-associated rhomboid-like protease, leads to the
stabilization of full-length PINK1 on the OMM. This stabilization
recruits the E3 ubiquitin ligase Parkin from the cytoplasm,
promoting the ubiquitination of OMM proteins, including VDAC1 and
mitochondrial fusion proteins, Mfn-1/2. p62 is subsequently
recruited to the mitochondrial surface, initiating mitophagy
(78). Lipid droplets (LDs),
which serve as cellular organelles for fatty acid storage, are
degraded by lysosomes via autophagy under radiation stress,
increasing the release of free fatty acids (FFAs) and the risk of
lipid peroxidation (79). IR also
induces an increase in LD volume and their proximity to
mitochondria, eventually forming mitochondrial-LD complexes that
facilitate fatty acid transfer to mitochondria (80,81). Key components of this process,
such as the LD-anchor protein PLIN5 and carnitine
palmitoyltransferase 1, help mitigate lipid accumulation in LDs,
thus enhancing mitochondrial fatty acid oxidation (FAO) and the
risk of ferroptosis (82).
Through the PINK1/Parkin-mediated mitophagy pathway, damaged
mitochondrial-LD complexes are engulfed and degraded by lysosomes,
and the released FFAs further accelerate the ferroptosis process
(83). Additionally, the
PINK1/Parkin pathway promotes the ubiquitin-mediated degradation of
the mitochondrial iron transporters SLC25A37 and SLC25A28, which
enhances mitochondrial sensitivity to ferroptosis. Inhibition of
this pathway leads to disrupted mitochondrial iron-dependent immune
metabolic function (84).
Hypoxic microenvironments markedly diminish the
radiosensitivity of tumor cells and are a major factor contributing
to radiation resistance (85).
Hypoxia induces the expression of BCL2-interacting protein 3
(BNIP3), which integrates into and anchors to the OMM. By
competitively binding to the BH3 domain and activating the PI3K
pathway, BNIP3 promotes the initiation of mitophagy (86). Under hypoxic conditions, ROS
production increases, promoting the formation of PUFA-PLs through
the hypoxia inducible factors (HIFs)-hypoxia inducible lipid
droplet associated (HILPDA) axis. The LD-associated protein HILPDA
remodels the cellular lipidome, particularly altering the levels of
mitochondrial cardiolipin (CL). The HILPDA-CLS1 axis further
enhances the accumulation of CL, which promotes mitophagy induced
by IR (87). Therefore,
mitophagy-dependent ferroptosis may represent a key pathway for
enhancing radiosensitivity in hypoxic solid tumors. Furthermore, IR
activates multiple signaling pathways that regulate mitochondrial
metabolic reprogramming and ferroptosis. These pathways, including
AMPK/NF-κB, JAK2/STAT3 and PI3K/Akt/mTOR, activate the
reprogramming of mitochondrial glycolysis and lipid metabolism,
thereby enhancing ferroptosis susceptibility (3).
Mitochondrial activity-driven innate and adaptive
immune responses also serve a key role in the IR-mediated
enhancement of ferroptosis sensitivity in tumor cells (15). Upon IR exposure, the mitochondrial
metabolism of immune cells, such as T cells, monocytes, macrophages
and B cells, becomes increasingly reliant on FAO as a substrate.
This metabolic shift serves as a critical endogenous trigger for T
cell exhaustion (88). Elevation
of FAO leads to mitochondrial depolarization, impaired biosynthesis
and excessive ROS production, thereby inducing oxidative stress.
Furthermore, FAO inhibits the proteasomal degradation of HIF1α,
mediating the glycolytic reprogramming of precursor exhausted T
cells. This reprogramming drives their transcriptional and
metabolic transformation into terminally exhausted T cells
(89).
The newly identified metabolic reprogramming and
autophagy pathways offer valuable insights into how modulation of
mitochondrial function and ferroptosis mechanisms can enhance the
efficacy of clinical radiotherapy for radiation-resistant tumors.
These findings potentially provide novel therapeutic targets and
sensitization strategies for radioimmunotherapy (Fig. 2).
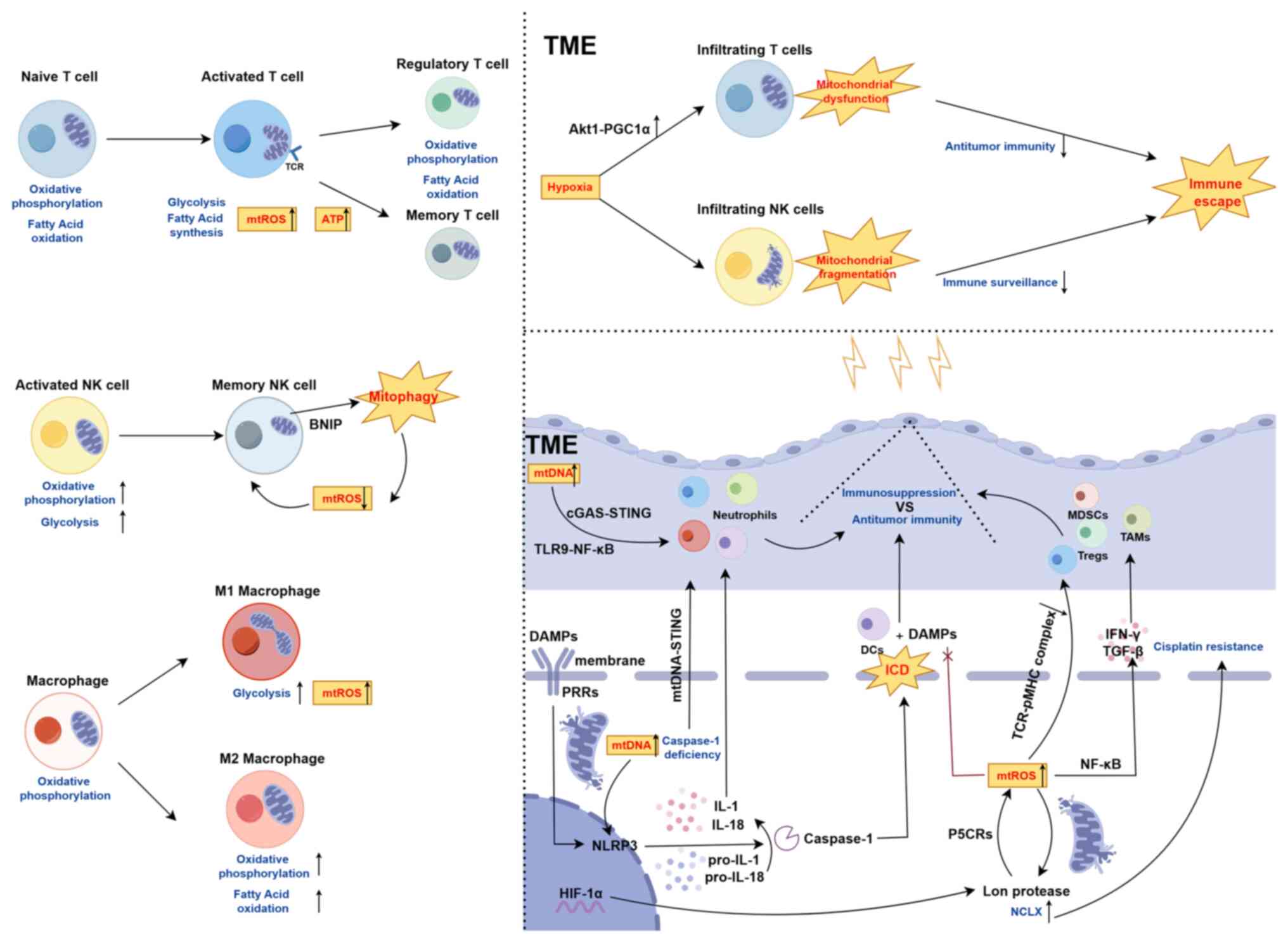
Mitochondrial dysfunction in various cell types,
including tumor cells and immune cells within the TME, serves a
pivotal role in tumor progression and immune evasion (90). Specifically, during T cell
activation, naïve T cells undergo a metabolic shift from OXPHOS and
FAO to aerobic glycolysis and fatty acid synthesis. During this
mitochondrial reprogramming, mitochondria accumulate beneath the
activated T cell receptor clusters, promoting mitochondrial fission
and concurrent relaxation of the inner membrane cristae. This
process stimulates mitochondrial activity, including increased
production of mtROS and ATP synthesis (91). This metabolic transition is
essential for T cell activation and supports their short-term
immune responses. However, as T cells differentiate into memory T
cells or Tregs, mitochondrial metabolism gradually shifts back to
OXPHOS and FAO to support cell survival, maintain cellular
phenotype and ensure immune tolerance or immunosuppressive
functions (76). In the TME,
tumor-infiltrating T cells exposed to chronic hypoxia environments
sustained mitochondrial dysfunction due to aberrant activation of
the Akt1-PGC1α signaling pathway, leading to persistent
mitochondrial damage. This metabolic dysfunction weakens T cell
anti-tumor immunity, inhibits cytokine secretion and ultimately
facilitates tumor immune evasion (92). Similarly, the immune response of
natural killer (NK) cells is significantly influenced by
mitochondrial metabolic alterations. During NK cell activation,
glycolytic capacity and basal OXPHOS rates increase remarkably.
However, in the transition to memory NK cells, damaged mitochondria
are removed via mitophagy-related proteins such as BNIP3(L),
thereby reducing mtROS production and supporting the formation of
memory NK cells (93). By
contrast, within a hypoxic TME, tumor-infiltrating NK cells exhibit
severe mitochondrial fragmentation, which impairs their
tumor-killing capacity and immune surveillance function, thus
promoting tumor immune evasion (94). Furthermore, mitochondrial
metabolic states profoundly impact macrophage polarization and
function within the TME. Upon M1 polarization, macrophages shift
their mitochondrial metabolism from OXPHOS to aerobic glycolysis,
accompanied by increased mitochondrial fission and elevated mtROS
production. By contrast, M2 polarization is characterized by
enhanced mitochondrial fusion, increased OXPHOS and FAO (95). These metabolic reprogramming
processes not only govern macrophage polarization but also directly
regulate their immune functions and responses to tumor progression.
Therefore, dynamic alterations in mitochondrial metabolic states
and morphology have a direct impact on immune cell polarization and
immune efficacy within the TME, serving a crucial role in tumor
immune evasion (Fig. 3).
IR-induced mitochondrial stress triggers the release
of mtDNA into the cytoplasm and extracellular space, where it
functions as a damage-associated molecular pattern (DAMP) that
activates pattern recognition receptors such as Toll-like receptors
(TLRs) and NOD-like receptors (NLRs). This activation sets off a
cascade of innate immune signaling (27). Notably, NLRP3 recognizes cytosolic
mtDNA, initiating caspase-1 activation, and promoting the cleavage
and maturation of the inflammatory cytokines IL-1 and IL-18. These
cytokines recruit T lymphocytes, macrophages and neutrophils,
thereby amplifying the immune response (96). In normal cells, caspase-dependent
mitophagy serves to mitigate immune signaling induced by mtDNA by
clearing damaged mitochondria. However, under pathological
conditions, impaired mitophagy or caspase-dependent mtDNA release
may convert immunologically silent cell death into ICD. In the
absence of caspases, MOMP activates the mtDNA-STING pathway,
initiating antitumor immune responses (97). IR also upregulates the expression
of mitochondrial fission protein 1 Drp1, contributing to
mitochondrial dysfunction and subsequent mtDNA release. This
extracellular mtDNA activates immune cells via the cGAS-STING and
TLR9-NF-κB pathways, modulating the polarization and function of
immune cells, including T lymphocytes, macrophages and DCs
(98). Thus, IR-induced ICD and
released mtDNA serves a dual role in tumor immune evasion and
activation. On one hand, mtDNA enhances the host's antitumor immune
response by stimulating immune reactions. On the other hand,
IR-induced ICD may also foster tumor immune evasion. Therefore, IR
not only activates immune responses through mtDNA release but also
modulates immune evasion via mitophagy regulation (99).
ROS serve a pivotal role in tumorigenesis,
particularly in the inflammatory TME, where they contribute to the
formation of immune-suppressive environments (100). Elevated ROS levels inhibit the
formation of TCR-antigen peptide-MHC complexes on the surface of
infiltrating T cells, thereby impairing T cell activation and
facilitating tumor immune escape (101). mtROS regulate immune cell
phenotypes and functions, thus supporting the establishment of an
immune-suppressive TME (102).
For instance, HIF-1α-induced mitochondrial Lon protease promotes
mtROS production through interaction with pyrroline-5-carboxylate
reductase (P5CRs), which in turn activates the NF-κB pathway,
leading to the secretion of immune-suppressive cytokines such as
IFN-γ and TGF-β by tumor cells (103). In addition, mtROS can further
enhance Lon protease expression, activate the mitochondrial
Na+/Ca2+ exchanger and regulate mitochondrial
calcium efflux, thus improving tumor cell resistance to cisplatin
(24). The central role of mtROS
in immune evasion underscores the importance of understanding the
complex regulatory mechanisms between mtROS and immune cell
activation in the TME. It is crucial for enhancing
radioimmunotherapy through mitochondrial ferroptosis sensitization.
Tumor-associated fibroblasts amplify oxidative stress in
surrounding monocytes, promoting their differentiation into MDSCs,
which in turn inhibit CD8+ T cell proliferation
(104). Elevated ROS levels,
through pathways involving HIF-1α and Lon protease, contribute to
the generation of immune-suppressive cells such as MDSCs,
tumor-associated macrophages (TAMs) and Tregs, thereby further
promoting immune suppression (105). ROS also alter the immunogenicity
of antigen peptides in antigen-presenting cells, affecting the
interaction between damage-associated molecular patterns (DAMPs)
and DC receptors, thus inhibiting antitumor immunity triggered by
ICD. Conversely, in the antitumor immune response, activated T
lymphocytes and NK cells utilize ROS to recruit neutrophils and
macrophages, thereby enhancing tumor cell killing. In reduced
GSH-deficient Tregs, abnormal serine metabolism leads to oxidative
stress, which downregulates Foxp3 expression and impairs the
immune-suppressive function of Tregs (106). Therefore, the levels and
sustained production of ROS in both tumor cells and immune cell
subsets are critical factors in determining the occurrence of ICD
and its capacity to induce effective antitumor immunity.
Macrophages are key immune cells within the TME,
serving a crucial role in maintaining immune homeostasis during
tumor progression. Tumor cells remodel the surrounding and distal
microenvironments by secreting tumor-derived factors that activate
monocytes and macrophages in circulation or local tissues, thereby
accelerating tumor progression (107,108). While mtROS were once regarded as
harmful byproducts of mitochondrial metabolism, recent research
underscores their signaling roles in preventing excessive immune
responses, particularly in regulating macrophage immune functions
(109). A previous study has
shown that mitochondrial Lon protease expression is elevated in
M2-type macrophages, suggesting that, during tumorigenesis,
macrophages regulate Lon expression through multiple signals to
induce mtROS generation, thereby serving a critical role in the
differentiation of TAMs (110).
Radiotherapy enhances antitumor immunity through
multiple mechanisms, notably inducing tumor ICD, which activates
adaptive anti-tumor immune responses. IR promotes the release of
double-stranded DNA (dsDNA) from the nucleus, leading to its
accumulation in tumor-derived exosomes and increasing OMM
permeability, thereby exposing mtDNA in the cytoplasm (111). As potent mediators, dsDNA and
mtDNA initiate the cGAS-STING pathway, upregulating IFN I
transcription, which activates the innate immune system, promotes
T-cell activation and suppresses tumor growth. Additionally, IR
induces the expression of MHC class I molecules and
tumor-associated antigens on tumor cell surfaces, while increasing
the exposure of calreticulin, HMGB1 and ATP, expanding the antigen
pool for presentation. This further enhances tumor immunogenicity
and promotes immune responses within the TME (112). Furthermore, IR stimulates the
release of inflammatory mediators and chemokines, such as CXCL9,
CXCL10 and CXCL11, from tumor and stromal cells. These factors
increase the infiltration of DCs, macrophages and T cells,
triggering both local and systemic antitumor immune responses
(113). These immune-enhancing
mechanisms suggest that radiotherapy not only exerts direct
cytotoxic effects on tumor cells but also significantly activates
the host immune system by reshaping the TME. When combined with
anti-PD-L1 immunotherapy, IR further reduces the accumulation of
MDSCs in the TME, creating a more inflammatory environment and
effectively reversing immunosuppression (114).
However, under certain dosing schedules and time
gradients, radiotherapy may also induce immunosuppressive effects
(115). These effects, driven by
alterations in the tumor hypoxic microenvironment, particularly
metabolic reprogramming and expansion of immune-suppressive cells
such as Tregs, may impair therapeutic efficacy and promote tumor
immune escape. IR can activate the GPR81/mTOR/HIF-1α/STAT3 pathway
by enhancing tumor cell glycolysis, which in turn promotes MDSC
activation in the TME (116).
Additionally, tumor metabolic reprogramming increases mitochondrial
FAO, providing ATP through mitochondrial catabolism, thus
facilitating tumor cell radioresistance. FAO upregulates CD47
transcription via the citrate-acetyl-CoA-RelA pathway, further
contributing to immunosuppression by protecting radioresistant
tumor cells from macrophage-mediated attack (116). The sustained activation of
HIF-1α by IR not only maintains the hypoxic microenvironment but
also drives macrophage subtypes (such as Macro-HK2, Macro-HSP and
Macro-MT) toward a pro-angiogenic phenotype. These macrophages
secrete additional chemokines, which recruit immunosuppressive
neutrophils after radiotherapy (117).
Under conventional fractionated radiotherapy,
sufficient radiotoxicity depletes circulating peripheral blood
monocytes and induces tumor cells to secrete Gal-1, which promotes
T-cell apoptosis (118). Tumor
cells subjected to repeated irradiation induce chronic expression
of FN I and IFN-γ, upregulating PD-L1 and IDO, leading to T-cell
exhaustion. This process is further enhanced by the IR-activated
cGAS-STING signaling pathway, which mobilizes Tregs and MDSCs while
inhibiting effector T-cell function (119). In local radiotherapy, IR
upregulates the secretion of CCL2 and CCL5, recruiting monocytes
and activating Tregs in a TNF-α-dependent manner, further
diminishing the therapeutic effect of radiotherapy (120). In later stages of radiotherapy,
the proportion of terminally exhausted CD8+ T cells increases,
along with upregulated exhaustion markers such as Pdcd1, Lag3 and
Tigit. This is accompanied by a limited maturation of DCs,
impairing their ability to activate CD4+/CD8+
T cells and exacerbating T-cell dysfunction (121).
Although radiotherapy combined with anti-PD-1
antibodies enhances tumor control via a CD8+
T-cell-dependent mechanism in certain therapeutic regimens,
low-dose fractionated radiotherapy (such as 2 Gy x10) may inhibit
IFN-γ expression in tumor-specific CD8+ T cells within
draining LNs. This effect is closely linked to radiation-induced
lymphopenia and the immunosuppressive TME (122). In localized radiotherapy,
selective lymph node irradiation (which is effective in addressing
subclinical lymph node micrometastases) may contribute to an
immunosuppressive TME when combined with anti-CTLA-4 antibodies.
This combination upregulates PD-L1 expression, leading to T-cell
exhaustion and radioresistance (123).
Among the immunomodulatory mechanisms of
radiotherapy, ferroptosis has emerged as a critical effector
pathway. Ferroptosis is not only a form of cell death but also a
potential mechanism through which radiotherapy induces ICD, thereby
activating antitumor immunity (124). IR induces ICD, prompting
activated CD8+ T cells in the TME to release IFN-γ. This
process inhibits system XC-(SLC3A2 and SLC7A11) expression, reduces
cysteine uptake by tumor cells and impairs the chelation of
GSH-cisplatin complexes, thus diminishing the ferroptotic defense
system and potentially reversing cisplatin-based chemoresistance
(125). Furthermore, IR remodels
the lipid profile of tumor cells, increasing the incorporation of
linoleic acid into C16- and C18-acyl PLs in an ACSL4-dependent
manner, thereby inducing ferroptosis (126). IFN-γ secreted by Th1, NK and NKT
cells can further promote ferroptosis by interacting with specific
fatty acids in the TME, thereby enhancing antitumor immunity
(127). This suggests a complex
interplay between ferroptosis, radiotherapy and immune responses,
presenting novel therapeutic targets for radioimmunotherapy
strategies. Within tumor cells, DNA damage sensors (including
ATRIP, Rad24p, γH2AX, NBS1, BRCA1/2, Ku70/80 and RNA polymerases)
recognize radiation-induced damage signals and recruit core DNA
damage response kinases ATM, ATR and DNA-dependent protein kinase
(DNA-PK) to DNA break sites, initiating repair mechanisms (128). Previous research has shown that
IR-activated ATM kinase, together with IFN-γ, synergistically
suppresses SLC7A11 expression and cysteine uptake, promoting lipid
peroxidation and ferroptosis, thus enhancing CD8+
T-cell-mediated antitumor immunity (129,130). This highlights the dual role of
ATM in radiation biology. Inhibition of ferroptosis diminishes the
efficacy of immune checkpoint inhibitors (ICIs) in combination with
radiotherapy, while inactivation of SLC7A11 enhances the
synergistic effects of ICIs and IR (131). Additionally, resistance to
ferroptosis mediated by lysosomal exocytosis pathways has been
negatively correlated with the efficacy of tumor
radioimmunotherapy. Although previous studies suggest that
tumor-derived CD8+ T cells exhibit elevated levels of
lipid peroxidation within the TME, thereby increasing their
susceptibility to ferroptosis (an important factor influencing
radiotherapy efficacy), the exact mechanism by which DC function
and antitumor immunity are influenced by radiotherapy-induced
ferroptosis warrants further investigation (132-134). Ferroptosis in immune cell
subsets can also modulate immune responses within the TME. Tumor
cells damaged by radiation typically exhibit dysregulated lipid
metabolism, while tumor-infiltrating T cells accumulate lipids in a
CD36 receptor-dependent manner, increasing intracellular oxidized
low-density lipoprotein levels, which induces lipid peroxidation
and ferroptosis. Inhibition of CD36 reduces ferroptosis in
CD8+ T cells, preserves antitumor immunity while
maintaining radiation-induced damage and enhances the efficacy of
immune checkpoint blockade (135). Activation of the ER stress
response factor X-box binding protein 1 (XBP1) in DCs inhibits T
cell-DC interactions by driving abnormal lipid accumulation.
Silencing XBP1 improves the antitumor and immune-stimulatory
functions of DCs, suggesting that targeting the ER stress response
could enhance antitumor immunity and promote ferroptosis in tumor
cells (136).
The role of granulocytes in ferroptosis within the
TME is still under extensive investigation. Granulocytes isolated
from mouse glioma models have been shown to transfer
myeloperoxidase to tumor cells via cytoplasmic vesicles, thereby
inducing lipid peroxidation products and triggering ferroptosis in
tumor cells (137). Granulocytic
myeloid-derived suppressor cells (G-MDSCs) in the TME are highly
sensitive to ferroptosis. They release oxidized lipids that
suppress T-cell activity, thereby contributing to immune evasion.
Beyond G-MDSCs, T-cell subsets may also undergo ferroptosis under
different environmental conditions (138,139). In Treg cells, the deletion of
GPX4 following TCR signaling leads to lipid peroxidation and
ferroptosis, which inhibits tumor growth and enhances antitumor
immunity (140). By contrast,
the deletion of GPX4 in CD8+ T cells results in the
failure of antigen-stimulated T-cell proliferation, attributed to
the rapid accumulation of membrane lipid peroxides and ferroptosis
within the cells. Furthermore, the suppression of GPX4 expression
in naïve CD4+ T cells prevents the generation of
follicular helper T (Tfh) cells and impedes germinal center
responses, limiting antibody production (141). Although T-cell proliferation
in vitro depends on system XC-, this transporter is not an
essential factor for T-cell proliferation or primary/memory immune
responses in vivo, with cysteine's role potentially
compensated by other mechanisms. Ferroptotic cells release
surface-exposed oxidized lipid species, such as SAPE-OOH, which
deliver key phagocytic signals, enhancing macrophage-mediated
phagocytosis via TLR2 (142).
However, the regulatory mechanisms of SAPE-OOH and the
immunogenicity of the TLR pathway remain incompletely understood.
Therefore, the composition of the TME serves a decisive role in
determining whether ferroptosis induces immune suppression or
activation. Before utilizing ferroptosis induction as a strategy to
sensitize radioimmunotherapy, targeted therapies for specific
immune cell populations may be required.
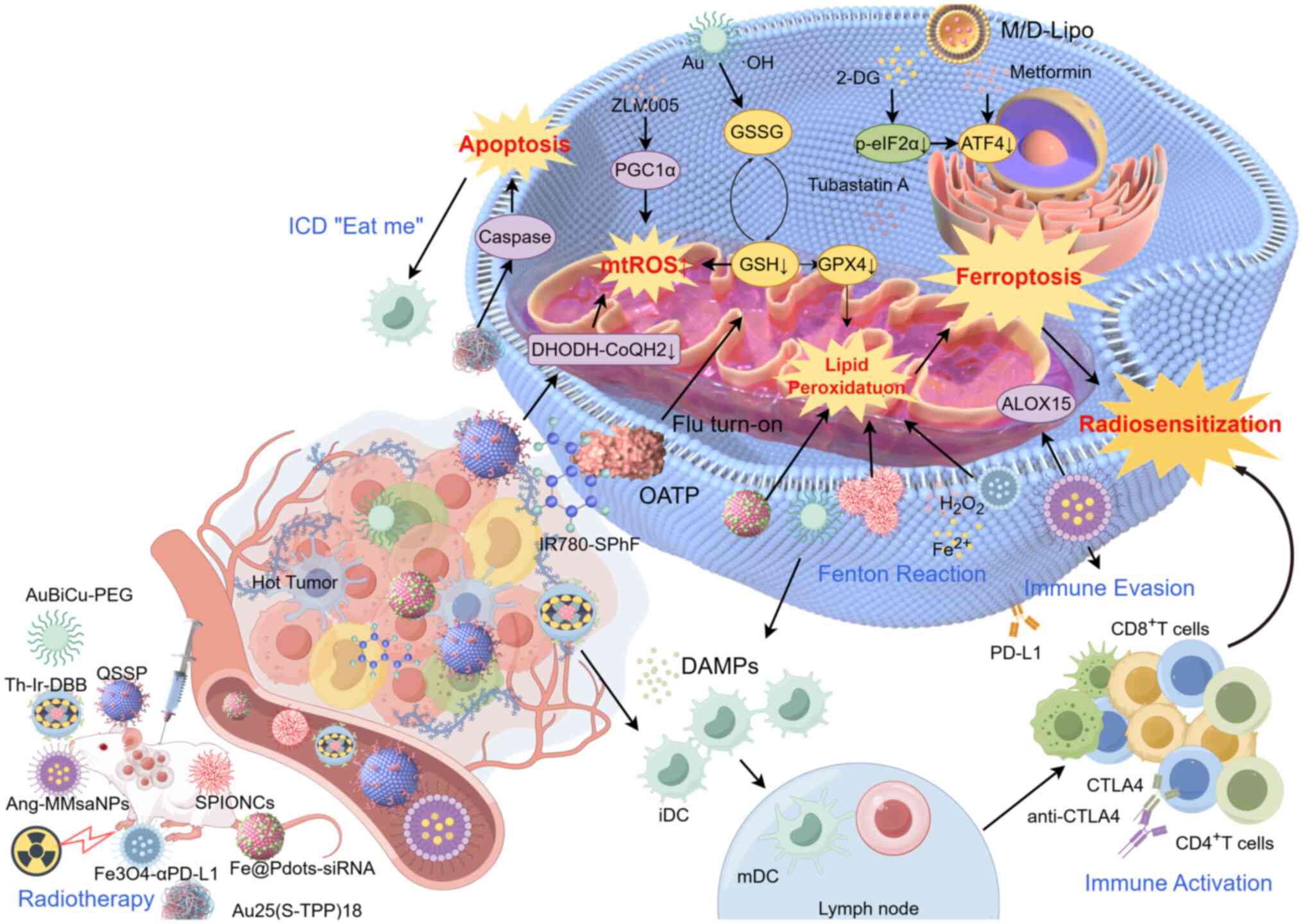
Inducing mitochondrial ferroptosis has emerged as a
promising approach to enhance tumor radiosensitivity in hypoxic
microenvironments (143).
IR-induced incomplete MOMP is a critical factor contributing to
radiation resistance. This process leads to the upregulation of
phosphorylayed-eIF2α and ATF4, which exacerbates tumor
radioresistance (144).
Liposomal formulations containing metformin and 2-deoxyglucose, by
targeting ATF4, effectively reverse radioresistance in CRC,
preventing T lymphocyte exhaustion and enhancing the efficacy of
fractionated radiotherapy through ferroptosis sensitization
(145). PGC1α, a key regulator
of mitochondrial metabolism, is recognized by DNA-PK under IR
conditions, promoting its ubiquitination and degradation. This
contributes to radioresistance in glioma subtypes. Therefore, the
use of the PGC1α activator ZLM005 can reactivate mitochondrial ROS
(mtROS) production and upregulate various forms of cell death,
including ferroptosis, thereby enhancing radiosensitivity (146). Additionally,
subcellular-targeted nanostructures, including DHODH inhibitors
such as QSSP, can enhance radiosensitivity by disrupting the
ferroptotic defense system. The mitochondria-targeted ferroptosis
inducer IR780-SPhF, which exhibits specific GSH-responsive
properties, induces mitochondrial redox imbalance, offering a novel
treatment strategy for breast cancer (147). Tubastatin A inhibits GPX4 enzyme
activity in a concentration- and time-dependent manner,
synergistically enhancing ferroptosis and sensitizing breast cancer
cells to radiotherapy (148).
Apart from targeting the ferroptosis defense system,
regulating the concentration of metal ions such as Fe2+
within mitochondria serves a key role in enhancing
ferroptosis-mediated radiosensitization (48). In nasopharyngeal carcinoma,
previous research has demonstrated that nanocarriers targeting
circular (circ) RNA circADARB1 can elevate intracellular iron ion
concentrations, thereby promoting radiotherapy-induced ferroptosis
and increasing radiosensitivity. Self-assembled pH-sensitive
superparamagnetic iron oxide nanoclusters enhance
mitochondrial-localized ferroptosis and apoptosis, synergistically
sensitizing tumors to radiotherapy (149). Cationic nanometal-organic
frameworks such as Th-Ir-DBB/digoxin, developed for ICD-induced
ferroptosis, utilize mitochondrial targeting and cholesterol
depletion to enhance the antitumor efficacy of
radiotherapy-radiodynamic therapy (150).
In the era of radioimmunotherapy, radiation
resistance and immune suppression within the TME often limit the
effectiveness of radiation-induced antitumor immune responses.
Ferroptosis has emerged as an effective strategy to enhance
antitumor immunity in this context (Fig. 4) (151). Targeting macrophage
membrane-coated biomimetic nanoparticles enables immune camouflage
to evade phagocytosis by the mononuclear phagocyte system, damaging
mitochondria and inducing ferroptosis in glioblastoma cells
(152). The
mitochondrial-targeting synthetic cluster Au25(S-TPP)18 inhibits
the thioredoxin reductase system, enhancing mtROS production. This
cluster exhibits potent radiosensitizing effects, induces a
bystander effect and activates the immune response (153). Tri-metallic nanoparticles
(AuBiCu-PEG NPs) with dual enzymatic activities not only promote
ICD induced by radiotherapy and enhance antitumor immunity, but
also effectively reverse radiation-induced PD-L1 upregulation.
These NPs suppress tumor immune evasion, reshape the immune
microenvironment and synergistically sensitize radiotherapy through
X-ray deposition, hypoxia alleviation and the induction of
ferroptosis and cuproptosis (154). Fe3O4-αPD-L1 nanoprobes, when
combined with radiotherapy, specifically target MDSCs, modulating
lipid and amino acid metabolism, particularly unsaturated fatty
acids and antioxidant metabolites associated with ferroptosis. This
approach reprograms the tumor immune-suppressive microenvironment,
promoting both local and systemic antitumor immune responses
(155). Equally important,
iron-containing hyaluronic acid (HA) NPs, which internalize by
high-affinity interactions with overexpressed CD44 receptors
through HA, generate lipid ROS. When combined with radiotherapy,
these nanoparticles induce both mitochondrial apoptosis and
ferroptosis (156).
Additionally, CSM nanoparticles loaded with single-atom
nanocatalysts (SAzyme), and manganese carbon monoxide enhance FLASH
radioimmunotherapy by inducing ferroptosis through multiple
pathways, including GSH depletion and CD8+ T-cell
infiltration (157). Other
ferroptosis-inducing NPs, such as BZAMH NPs, 8HSA8 NPs, Au/CuNDs
and Cu2WS4-PEG, also serve crucial roles in sensitization of
radioimmunotherapy (158).
Radiopharmaceutical therapy (RPT), or targeted
radionuclide therapy, directly treats malignant tumors by
delivering radioactive atoms (β- or α-particles) to the tumor site
(159). Compared with
X-ray-mediated IR, RPT allows for precise delivery of radiation to
cancer cells or the TME, maximizing tumor cell killing while
sparing surrounding healthy tissues (160). For instance, 3-nm
monocrystalline iron NPs (iRGD-bcc-USINP), when combined with a
PD-L1 blocking agent, target ferroptosis and synergistically exert
antitumor immune effects. However, ultra-small NPs (<6 nm)
demonstrate poor tumor accumulation (161). Particle size-optimized NPs,
assembled with radiopharmaceuticals such as USINP-131I-aPD-L1,
promote ROS production and enhance ferroptosis induced by USINP,
activating immune pathways and synergizing with PD-L1 blockade to
enhance antitumor immune responses. Furthermore, the 124I-P/C@
CMLvs system, combined with PET imaging tracer, can synergistically
enhance ferroptosis and necroptosis, inducing lung cancer
regression and improving the efficacy of anti-PD-L1 immunotherapy
(162).
In addition to enhancing radiotherapy, mitochondrial
ferroptosis can also sensitize emerging tumor treatments, such as
photothermal therapy (PTT), photodynamic therapy (PDT), and
sonodynamic therapy (SDT) (163). The IR780/Ce@ EGCG/APT
nanoreactor, by combining ferroptosis with PTT, improves the
immune-suppressive microenvironment in breast cancer and activates
systemic immune responses, leading to long-term immune memory.
Mitochondria-specific type I PDT mediated by organic NIR-II
photosensitizers (TPEQM-DMA) and two-photon nanosensitizers
(IR-g-C3N4) disrupt the lipid repair system, resulting in
mitochondrial dysfunction and induction of both apoptosis and
ferroptosis, providing an effective phototherapy strategy for
hypoxic tumors (164).
Furthermore, the mitochondria-specific organic-metal ultrasound
sensitizer IRCur-Pt enhances the synergistic effect of ferroptosis
and SDT, further activating immune pathways. Pancreatic
cancer-specific antibody NRP2-guided MOFs@ COF nanocarriers target
and disrupt mitochondrial and ER homeostasis, inducing
autophagy-dependent ferroptosis while sensitizing chemotherapy and
SDT (165). In summary, inducing
mitochondrial ferroptosis through the disruption of iron ion
homeostasis and peroxidation intervention has emerged as an
effective strategy to improve the radiosensitivity of malignant
tumors and enhance the efficacy of radioimmunotherapy.
The present review summarized the research
advancements in novel radioimmunotherapy strategies, focusing on
the transition from 'ferroptosis' to 'mitochondrial dysfunction'.
Ferroptosis, a form of RCD, has recently been implicated in tumor
resistance to radiotherapy. Inducing ferroptosis by modulating iron
metabolism and redox imbalance has emerged as a promising strategy
to enhance the efficacy of radiotherapy [Table SI (166-276)]. Concurrently, radiation-induced
mitochondrial dysfunction serves as a critical pathway for ICD in
tumor cells. Radiotherapy-ICIs has shown considerable therapeutic
benefits in clinical studies across various cancer types. These
studies underscore the complex interplay between ferroptosis and
mitochondrial dysfunction in tumor treatment responses,
highlighting the potential of combination strategies that integrate
radiotherapy, immunotherapy and ferroptosis modulation.
Although the potential of ferroptosis and
mitochondrial dysfunction in enhancing radioimmunotherapy is
becoming increasingly apparent, the research presently described
demonstrates several unresolved paradoxes in the regulation of
ferroptosis. Specifically, i) in hepatocytes, while mtROS-activated
PERK signaling promotes arsenite-induced ferroptosis by
downregulating Mfn-2 and impairing MAMs function, its cleaved
C-terminal fragment paradoxically exerts protective effects against
alcoholic hepatocyte ferroptosis (75); ii) the complex role of cGAS-STING
in either promoting cell death (via pyroptosis) (40) or supporting cell survival (by
suppressing ferroptosis) (41);
iii) the TME presents even more complex dichotomies: Although
hypoxia generally diminishes radiosensitivity, hypoxia-induced
BNIP3 protein can stimulate mitophagy and elevate ROS production,
mechanisms known to potentiate ferroptosis and consequently improve
radiation response (86); and iv)
radiation-enhanced FAO exhibits dual effects: While increasing
tumor cell vulnerability to ferroptosis, it simultaneously induces
T cell exhaustion, ultimately attenuating anti-tumor immunity and
compromising radioimmunotherapy efficacy (88).
The current research still faces other challenges.
First, the immunogenicity of ferroptosis in different contexts
remains unclear. Second, whether DAMPs are released during
ferroptosis, and how these DAMPs mediate immune responses within
the TME, require further investigation. Additionally, the
interactions between various cell death pathways and the
optimization of drugs that either promote or inhibit ferroptosis
are critical issues that need to be addressed. Resolving these
questions will help clarify the precise role of ferroptosis in the
TME and provide new insights for cancer treatment.
These challenges collectively indicate that the
current understanding remains incomplete regarding the molecular
mechanisms of ferroptosis are not fully understood, particularly in
terms of its heterogeneous effects across different tumor types and
subcellular organelles, which warrants further exploration
[Table SII (277-430)] such as the impact of TME factors
(oxidative stress, hypoxia and lipid metabolism) on ferroptosis
susceptibility and the precise mechanisms by which ferroptosis
synergizes with radioimmunotherapy, including the efficacy of
radioimmunotherapy combined with ferroptosis modulation may vary
significantly depending on the clinical context. Therefore,
accurately assessing patient responses and resistance mechanisms
remains a key area of research. Furthermore, while the combination
of ICIs and radiotherapy has shown promise, the interactions
between immune-related side effects and radiotherapy-induced
toxicity have not been thoroughly evaluated. Balancing therapeutic
efficacy with safety and optimizing treatment regimens remains a
major challenge in clinical applications.
Future research should focus on several pivotal
areas. First, a deeper exploration of the molecular mechanisms
underpinning ferroptosis and mitochondrial dysfunction,
particularly their roles within different TMEs, is essential. By
utilizing molecular targeting technologies, the development of
novel drugs or small molecule modulators that specifically induce
ferroptosis or restore mitochondrial function could offer new
strategies to enhance radiotherapy efficacy. Second, there is a
need for the advancement of immune monitoring techniques,
particularly those aimed at precisely assessing the association
between immune system responses and tumor cell death. This will
provide the foundation for personalized treatment strategies.
Furthermore, combining modern gene-editing technologies with
immunotherapeutic approaches to overcome the current resistance to
radiotherapy will be a major focus of future research.
In conclusion, the combination of radiotherapy and
immunotherapy holds significant promise in cancer treatment.
However, to achieve widespread clinical application, several
mechanistic and clinical challenges must be overcome. Future
research will deepen the current understanding of the interactions
between ferroptosis, mitochondrial dysfunction and immunotherapy.
Based on these insights, more precise and safer treatment regimens
will be developed, offering patients with cancer a broader range of
therapeutic options.
Not applicable.
TW, XZ and XY conceived the study and wrote the
manuscript. AZ, YF, KC, HT, ZT and PZ conducted literature
search/selection and data extraction. XH and LY revised and edited
the manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present work was supported by grants from the National
Natural Science Foundation of China (grant no. 82172804), the
Yishan Research Project of Jiangsu Cancer Hospital (grant no.
YSPY202407) and Clinical Research Foundation of collaborative
innovation center for cancer personalized medicine (grant no.
JZ21449020210616).
|
1
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petroni G, Cantley LC, Santambrogio L,
Formenti SC and Galluzzi L: Radiotherapy as a tool to elicit
clinically actionable signalling pathways in cancer. Nat Rev Clin
Oncol. 19:114–131. 2022. View Article : Google Scholar :
|
|
3
|
Azzam EI, Jay-Gerin JP and Pain D:
Ionizing radiation-induced metabolic oxidative stress and prolonged
cell injury. Cancer Lett. 327:48–60. 2012. View Article : Google Scholar
|
|
4
|
Su W, Wang H, Zhao S, Ji X, Jiang H, Li K,
Zuo C, Zheng J, Ni D and Hu J: Lysosome-escaping and
nucleus-targeting nanomedicine for enhanced cancer catalytic
internal radiotherapy. Eur J Nucl Med Mol Imaging. July 1–2025.Epub
ahead of print. View Article : Google Scholar
|
|
5
|
Yuan P, Wang Y, Wang A, Lin J, Chen X, Liu
X, Zhang Y, Gao W, Wang P, Zhang G and Liu L: Synergistic cancer
treatment with PCuLu nanocatalysts by inducing cuproptosis and
enhancing radiotherapy. J Colloid Interface Sci. 699:1382602025.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu H, Tang Y, Singh A, Vong J, Cordero J,
Mathes A, Gao R, Jia Y, Garvalov BK, Acker T, et al: RNF20 links
the DNA damage response and metabolic rewiring in lung cancer
through HIF1α. Nat Commun. 16:49292025. View Article : Google Scholar
|
|
7
|
Shankar V, Wilhelmy J, Curtis EJ, Michael
B, Cervantes L, Mallajosyula V, Davis RW, Snyder M, Younis S,
Robinson WH, et al: Oxidative stress is a shared characteristic of
ME/CFS and Long COVID. Proc Natl Acad Sci USA. 122:e24265641222025.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng J and Conrad M: The metabolic
underpinnings of ferroptosis. Cell Metab. 32:920–937. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24:4492022. View Article : Google Scholar
|
|
10
|
Wang M, Li M, Jiang Y, Wang S, Yang X,
Naseem A, Algradi AM, Hao Z, Guan W, Chen Q, et al: Saponins from
Astragalus membranaceus (Fisch.) bge alleviated neuronal
ferroptosis in Alzheimer's disease by regulating the NOX4/Nrf2
signaling pathway. J Agric Food Chem. 73:7725–7740. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Q, Liu J, Zhang Y, Li Z, Zhao Z,
Jiang W, Zhao J, Hou L and Wang Q: Microglial CR3 promotes neuron
ferroptosis via NOX2-mediated iron deposition in rotenone-induced
experimental models of Parkinson's disease. Redox Biol.
77:1033692024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martínez-Reyes I and Chandel NS: Cancer
metabolism: Looking forward. Nat Rev Cancer. 21:669–680. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang YP, Sharda A, Xu SN, van Gastel N,
Man CH, Choi U, Leong WZ, Li X and Scadden DT: Malic enzyme 2
connects the Krebs cycle intermediate fumarate to mitochondrial
biogenesis. Cell Metab. 33:1027–1041.e8. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miwa S, Kashyap S, Chini E and von
Zglinicki T: Mitochondrial dysfunction in cell senescence and
aging. J Clin Invest. 132:e1584472022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lei G, Mao C, Yan Y, Zhuang L and Gan B:
Ferroptosis, radiotherapy, and combination therapeutic strategies.
Protein Cell. 12:836–857. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bray F, Laversanne M, Weiderpass E and
Soerjomataram I: The ever-increasing importance of cancer as a
leading cause of premature death worldwide. Cancer. 127:3029–3030.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI
|
|
18
|
Liermann-Wooldrik KT, Kosmacek EA,
McDowell JA, Takkar S, Murthy D, Singh PK, Schott MB, Ponnusamy MP
and Oberley-Deegan RE: Radiation promotes acute and chronic damage
to adipose tissue. Int J Mol Sci. 26:56262025. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wungcharoen P, Prayongrat A and
Tangjaturonrasme N: Hearing loss and middle ear effusion in
nasopharyngeal carcinoma following radiotherapy: Dose-response
relationship and normal tissue complication probability modeling.
Int Arch Otorhinolaryngol. 29:1–9. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ning J, Chen L, Zeng Y, Xiao G, Tian W, Wu
Q, Tang J, He S, Tanzhu G and Zhou R: The scheme, and regulative
mechanism of pyroptosis, ferroptosis, and necroptosis in radiation
injury. Int J Biol Sci. 20:1871–1883. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao S, Huang J, Zhao R, He H, Zhang J and
Wen X: Comprehensive analysis of multiple regulated cell death risk
signatures in lung adenocarcinoma. Heliyon. 10:e386412024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuwahara Y, Tomita K, Urushihara Y, Sato
T, Kurimasa A and Fukumoto M: Association between radiation-induced
cell death and clinically relevant radioresistance. Histochem Cell
Biol. 150:649–659. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang S, Zhang J, Fan X, Liu H, Zhu M,
Yang M, Zhang X, Zhang H and Yu F: Ionizing radiation-induced
ferroptosis based on nanomaterials. Int J Nanomedicine.
17:3497–3507. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J,
Zhang Z and Wang X: Membrane damage during ferroptosis is caused by
oxidation of phospholipids catalyzed by the oxidoreductases POR and
CYB5R1. Mol Cell. 81:355–369.e10. 2021. View Article : Google Scholar
|
|
25
|
Kim JH, Rho JR and Choi JH: Marine
sponge-derived gukulenin A sensitizes ovarian cancer cells to PARP
inhibition via ferroptosis induction. Mar Drugs. 23:1382025.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Y, Ji Y, Jin X, Xu G, Wang X, Song S,
Li R, Wang Y, Liu R and Li Z: YLKTT, a potent biopeptide that
ameliorates oxygen-glucose deprivation-induced neuronal cell
ferroptosis by ACSL4/GPX4 and SLC7A11/GPX4 axis. J Pharm Pharmacol.
rgaf0372025. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong J, Qi F, Qie H, Du S, Li L, Zhang Y,
Xu K, Li D and Xu Y: Oleic acid inhibits SDC4 and promotes
ferroptosis in lung cancer through GPX4/ACSL4. Clin Respir J.
18:e700142024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lei G, Zhang Y, Koppula P, Liu X, Zhang J,
Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H and Gan B: The role of
ferroptosis in ionizing radiation-induced cell death and tumor
suppression. Cell Res. 30:146–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu T, Zhu C, Chen X, Guan G, Zou C, Shen
S, Wu J, Wang Y, Lin Z, Chen L, et al: Ferroptosis, as the most
enriched programmed cell death process in glioma, induces
immunosuppression and immunotherapy resistance. Neuro Oncol.
24:1113–1125. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Hu J, Wu S, Fleishman JS, Li Y, Xu
Y, Zou W, Wang J, Feng Y, Chen J and Wang H: Targeting epigenetic
and posttranslational modifications regulating ferroptosis for the
treatment of diseases. Signal Transduct Target Ther. 8:4492023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Galy B, Conrad M and Muckenthaler M:
Mechanisms controlling cellular and systemic iron homeostasis. Nat
Rev Mol Cell Biol. 25:133–155. 2024. View Article : Google Scholar
|
|
32
|
Zhang H, Tsui CK, Garcia G, Joe LK, Wu H,
Maruichi A, Fan W, Pandovski S, Yoon PH, Webster BM, et al: The
extracellular matrix integrates mitochondrial homeostasis. Cell.
187:4289–4304.e26. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zong WX, Rabinowitz JD and White E:
Mitochondria and cancer. Mol Cell. 61:667–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim S, Piao S, Lee I, Nagar H, Choi SJ,
Shin N, Kim DW, Shong M, Jeon BH and Kim CS: CR6 interacting factor
1 deficiency induces premature senescence via SIRT3 inhibition in
endothelial cells. Free Radic Biol Med. 150:161–171. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rucheton B, Jardel C, Filaut S, Amador
MDM, Maisonobe T, Serre I, Romero NB, Leonard-Louis S, Haraux F and
Lombès A: Homoplasmic deleterious MT-ATP6/8 mutations in adult
patients. Mitochondrion. 55:64–77. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bo T, Yamamori T, Yamamoto K, Fujimoto M,
Yasui H and Inanami O: Mitochondrial fission promotes
radiation-induced increase in intracellular Ca2+ level
leading to mitotic catastrophe in mouse breast cancer EMT6 cells.
Biochem Biophys Res Commun. 522:144–150. 2020. View Article : Google Scholar
|
|
37
|
Okon IS and Zou MH: Mitochondrial ROS and
cancer drug resistance: Implications for therapy. Pharmacol Res.
100:170–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kam WW and Banati RB: Effects of ionizing
radiation on mitochondria. Free Radic Biol Med. 65:607–619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Saunders TL, Windley SP, Gervinskas G,
Balka KR, Rowe C, Lane R, Tailler M, Nguyen TN, Ramm G, Lazarou M,
et al: Exposure of the inner mitochondrial membrane triggers
apoptotic mitophagy. Cell Death Differ. 31:335–347. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xia L, Yan X and Zhang H: Mitochondrial
DNA-activated cGAS-STING pathway in cancer: Mechanisms and
therapeutic implications. Biochim Biophys Acta Rev Cancer.
1880:1892492025. View Article : Google Scholar
|
|
41
|
Qiu S, Zhong X, Meng X, Li S, Qian X, Lu
H, Cai J, Zhang Y, Wang M, Ye Z, et al: Mitochondria-localized cGAS
suppresses ferroptosis to promote cancer progression. Cell Res.
33:299–311. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qiu YH, Zhang TS, Wang XW, Wang MY, Zhao
WX, Zhou HM, Zhang CH, Cai ML, Chen XF, Zhao WL and Shao RG:
Mitochondria autophagy: A potential target for cancer therapy. J
Drug Target. 29:576–591. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu S, Sankar S and Neamati N: Protein
disulfide isomerase: A promising target for cancer therapy. Drug
Discov Today. 19:222–240. 2014. View Article : Google Scholar
|
|
44
|
Wang R, Shang Y, Chen B, Xu F, Zhang J,
Zhang Z, Zhao X, Wan X, Xu A, Wu L and Zhao G: Protein disulfide
isomerase blocks the interaction of LC3II-PHB2 and promotes mTOR
signaling to regulate autophagy and radio/chemo-sensitivity. Cell
Death Dis. 13:8512022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao M, Yi J, Zhu J, Minikes AM, Monian P,
Thompson CB and Jiang X: Role of mitochondria in ferroptosis. Mol
Cell. 73:354–363.e353.e3. 2019. View Article : Google Scholar :
|
|
46
|
Richardson DR, Lane DJ, Becker EM, Huang
ML, Whitnall M, Suryo Rahmanto Y, Sheftel AD and Ponka P:
Mitochondrial iron trafficking and the integration of iron
metabolism between the mitochondrion and cytosol. Proc Natl Acad
Sci USA. 107:10775–10782. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang P, Cui Y, Liu Y, Li Z, Bai H, Zhao Y
and Chang YZ: Mitochondrial ferritin alleviates apoptosis by
enhancing mitochondrial bioenergetics and stimulating glucose
metabolism in cerebral ischemia reperfusion. Redox Biol.
57:1024752022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun K, Zhi Y, Ren W, Li S, Zhou X, Gao L
and Zhi K: The mitochondrial regulation in ferroptosis signaling
pathway and its potential strategies for cancer. Biomed
Pharmacother. 169:1158922023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang X, Ji Y, Qi J, Zhou S, Wan S, Fan C,
Gu Z, An P, Luo Y and Luo J: Mitochondrial carrier 1 (MTCH1)
governs ferroptosis by triggering the FoxO1-GPX4 axis-mediated
retrograde signaling in cervical cancer cells. Cell Death Dis.
14:5082023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Adam AC, Bornhövd C, Prokisch H, Neupert W
and Hell K: The Nfs1 interacting protein Isd11 has an essential
role in Fe/S cluster biogenesis in mitochondria. EMBO J.
25:174–183. 2006. View Article : Google Scholar
|
|
51
|
Kerins MJ, Milligan J, Wohlschlegel JA and
Ooi A: Fumarate hydratase inactivation in hereditary leiomyomatosis
and renal cell cancer is synthetic lethal with ferroptosis
induction. Cancer Sci. 109:2757–2766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang J, Lee Y and Hwang CS: The
ubiquitin-proteasome system links NADPH metabolism to ferroptosis.
Trends Cell Biol. 33:1088–1103. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jonckheere AI, Smeitink JA and Rodenburg
RJ: Mitochondrial ATP synthase: Architecture, function and
pathology. J Inherit Metab Dis. 35:211–225. 2012. View Article : Google Scholar :
|
|
54
|
Gawargi FI and Mishra PK: MMP9 drives
ferroptosis by regulating GPX4 and iron signaling. iScience.
27:1106222024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang K, Chen L, Wang B, Chen D, Ye X, Han
X, Fang Q, Yu C, Wu J, Guo S, et al: Mitochondrial supercomplex
assembly regulates metabolic features and glutamine dependency in
mammalian cells. Theranostics. 13:3165–3187. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lobo-Jarne T, Nývltová E, Pérez-Pérez R,
Timón-Gómez A, Molinié T, Choi A, Mourier A, Fontanesi F, Ugalde C
and Barrientos A: Human COX7A2L regulates complex III biogenesis
and promotes supercomplex organization remodeling without affecting
mitochondrial bioenergetics. Cell Rep. 25:1786–1799.e4. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Song X, Liu J, Kuang F, Chen X, Zeh HJ
III, Kang R, Kroemer G, Xie Y and Tang D: PDK4 dictates metabolic
resistance to ferroptosis by suppressing pyruvate oxidation and
fatty acid synthesis. Cell Rep. 34:1087672021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li Z, Xu ZM, Chen WP, Du XJ, Ou CX, Luo
ZK, Wang R, Zhang CQ, Ge CD, Han M, et al: Tumor-repopulating cells
evade ferroptosis via PCK2-dependent phospholipid remodeling. Nat
Chem Biol. 20:1341–1352. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z,
Cai K, Zhao Y and Luo Z: HCAR1/MCT1 regulates tumor ferroptosis
through the lactate-mediated AMPK-SCD1 activity and its therapeutic
implications. Cell Rep. 33:1084872020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ma J, Tang L, Tan Y, Xiao J, Wei K, Zhang
X, Ma Y, Tong S, Chen J, Zhou N, et al: Lithium carbonate
revitalizes tumor-reactive CD8+ T cells by shunting
lactic acid into mitochondria. Nat Immunol. 25:552–561. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gao M, Monian P, Quadri N, Ramasamy R and
Jiang X: Glutaminolysis and transferrin regulate ferroptosis. Mol
Cell. 59:298–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Missiroli S, Patergnani S, Caroccia N,
Pedriali G, Perrone M, Previati M, Wieckowski MR and Giorgi C:
Mitochondria-associated membranes (MAMs) and inflammation. Cell
Death Dis. 9:3292018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kuang F, Liu J, Xie Y, Tang D and Kang R:
MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic
cancer cells. Cell Chem Biol. 28:765–775.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pontisso I and Combettes L: Role of
sigma-1 receptor in calcium modulation: Possible involvement in
cancer. Genes (Basel). 12:1392021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu Y, Ma X, Fujioka H, Liu J, Chen S and
Zhu X: DJ-1 regulates the integrity and function of ER-mitochondria
association through interaction with IP3R3-Grp75-VDAC1. Proc Natl
Acad Sci USA. 116:25322–25328. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rieusset J: The role of endoplasmic
reticulum-mitochondria contact sites in the control of glucose
homeostasis: An update. Cell Death Dis. 9:3882018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wei S, Qiu T, Wang N, Yao X, Jiang L, Jia
X, Tao Y, Zhang J, Zhu Y, Yang G, et al: Ferroptosis mediated by
the interaction between Mfn2 and IREα promotes arsenic-induced
nonalcoholic steatohepatitis. Environ Res. 188:1098242020.
View Article : Google Scholar
|
|
68
|
Zhu Y, Fujimaki M, Snape L, Lopez A,
Fleming A and Rubinsztein DC: Loss of WIPI4 in neurodegeneration
causes autophagy-independent ferroptosis. Nat Cell Boil.
26:542–551. 2024. View Article : Google Scholar
|
|
69
|
Liu Y and Yang H: WIPI4 loss linked to
ferroptosis. Nat Cell Biol. 26:506–507. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zheng J and Conrad M: Ferroptosis: When
metabolism meets cell death. Physiol Rev. 105:651–706. 2025.
View Article : Google Scholar
|
|
71
|
Ju HQ, Lin JF, Tian T, Xie D and Xu RH:
NADPH homeostasis in cancer: Functions, mechanisms and therapeutic
implications. Signal Transduct Target Ther. 5:2312020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pirozzi CJ and Yan H: The implications of
IDH mutations for cancer development and therapy. Nat Rev Clin
Oncol. 18:645–661. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sarcognato S, Sacchi D, Fabris L, Zanus G,
Gringeri E, Niero M, Gallina G and Guido M: Ferroptosis in
intrahepatic cholangiocarcinoma: IDH1105GGT single
nucleotide polymorphism is associated with its activation and
better prognosis. Front Med (Lausanne). 9:8862292022. View Article : Google Scholar
|
|
74
|
Brashears CB, Prudner BC, Rathore R,
Caldwell KE, Dehner CA, Buchanan JL, Lange SES, Poulin N, Sehn JK,
Roszik J, et al: Malic enzyme 1 absence in synovial sarcoma shifts
antioxidant system dependence and increases sensitivity to
ferroptosis induction with ACXT-3102. Clin Cancer Res.
28:3573–3589. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chen J, Li X, Ge C, Min J and Wang F: The
multifaceted role of ferroptosis in liver disease. Cell Death
Differ. 29:467–480. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang X, Li X, Zheng C, Yang C, Zhang R,
Wang A, Feng J, Hu X, Chang S and Zhang H: Ferroptosis, a new form
of cell death defined after radiation exposure. Int J Radiat Biol.
98:1201–1209. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ye LF, Chaudhary KR, Zandkarimi F, Harken
AD, Kinslow CJ, Upadhyayula PS, Dovas A, Higgins DM, Tan H, Zhang
Y, et al: Radiation-induced lipid peroxidation triggers ferroptosis
and synergizes with ferroptosis inducers. ACS Chem Biol.
15:469–484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yao N, Wang C, Hu N, Li Y, Liu M, Lei Y,
Chen M, Chen L, Chen C, Lan P, et al: Inhibition of
PINK1/Parkin-dependent mitophagy sensitizes multidrug-resistant
cancer cells to B5G1, a new betulinic acid analog. Cell Death Dis.
10:2322019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Singh R, Kaushik S, Wang Y, Xiang Y, Novak
I, Komatsu M, Tanaka K, Cuervo AM and Czaja MJ: Autophagy regulates
lipid metabolism. Nature. 458:1131–1135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xiao Y, Hu X, Rudolphi CF, Nollet EE,
Nederlof R, Wang Q, Bakker D, Nikolaou PE, Knol JC, Haas RRG, et
al: The innate immune receptor NLRX1 is a novel required modulator
for mPTP opening: Implications for cardioprotection. Basic Res
Cardiol. June 19–2025.Epub ahead of print. View Article : Google Scholar :
|
|
81
|
Zhong Z, Hou Y, Zhou C, Wang J, Gao L, Wu
X, Zhou G, Liu S, Xu Y and Yang W: FL3 mitigates cardiac
ischemia-reperfusion injury by promoting mitochondrial fusion to
restore calcium homeostasis. Cell Death Discov. 11:3042025.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fan H and Tan Y: Lipid
droplet-mitochondria contacts in health and disease. Int J Mol Sci.
25:68782024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li J, Yang D, Li Z, Zhao M, Wang D, Sun Z,
Wen P, Dai Y, Gou F, Ji Y, et al: PINK1/Parkin-mediated mitophagy
in neurodegenerative diseases. Ageing Res Rev. 84:1018172023.
View Article : Google Scholar
|
|
84
|
Springer W and Kahle PJ: Regulation of
PINK1-Parkin-mediated mitophagy. Autophagy. 7:266–278. 2011.
View Article : Google Scholar
|
|
85
|
Telarovic I, Wenger RH and Pruschy M:
Interfering with tumor hypoxia for radiotherapy optimization. J Exp
Clin Cancer Res. 40:1972021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li Y, Zheng W, Lu Y, Zheng Y, Pan L, Wu X,
Yuan Y, Shen Z, Ma S, Zhang X, et al: BNIP3L/NIX-mediated
mitophagy: Molecular mechanisms and implications for human disease.
Cell Death Dis. 13:142021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bensaad K, Favaro E, Lewis CA, Peck B,
Lord S, Collins JM, Pinnick KE, Wigfield S, Buffa FM, Li JL, et al:
Fatty acid uptake and lipid storage induced by HIF-1α contribute to
cell growth and survival after hypoxia-reoxygenation. Cell Rep.
9:349–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tapio S, Little MP, Kaiser JC, Impens N,
Hamada N, Georgakilas AG, Simar D and Salomaa S: Ionizing
radiation-induced circulatory and metabolic diseases. Environ Int.
146:1062352021. View Article : Google Scholar
|
|
89
|
Rao D, Verburg F, Renner K, Peeper DS,
Lacroix R and Blank CU: Metabolic profiles of regulatory T cells in
the tumour microenvironment. Cancer Immunol Immunother.
70:2417–2427. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Biswas SK: Metabolic reprogramming of
immune cells in cancer progression. Immunity. 43:435–449. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Buck MD, O'Sullivan D, Klein Geltink RI,
Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O, Braas D, van der
Windt GJ, et al: Mitochondrial dynamics controls T cell fate
through metabolic programming. Cell. 166:63–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Dumauthioz N, Tschumi B, Wenes M, Marti B,
Wang H, Franco F, Li W, Lopez-Mejia IC, Fajas L, Ho PC, et al:
Enforced PGC-1α expression promotes CD8 T cell fitness, memory
formation and anti-tumor immunity. Cell Mol Immunol. 18:1761–1771.
2021. View Article : Google Scholar
|
|
93
|
Miao L, Lu C, Zhang B, Li H, Zhao X, Chen
H, Liu Y and Cui X: Advances in metabolic reprogramming of NK cells
in the tumor microenvironment on the impact of NK therapy. J Transl
Med. 22:2292024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Meybodi SM, Ejlalidiz M, Manshadi MR,
Raeisi M, Zarin M, Kalhor Z, Saberiyan M and Hamblin MR: Crosstalk
between hypoxia-induced pyroptosis and immune escape in cancer:
From mechanisms to therapy. Crit Rev Oncol Hematol. 197:1043402024.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Galván-Peña S and O'Neill LA: Metabolic
reprograming in macrophage polarization. Front Immunol.
5:4202014.PubMed/NCBI
|
|
96
|
Qiu Y, Huang Y, Chen M, Yang Y, Li X and
Zhang W: Mitochondrial DNA in NLRP3 inflammasome activation. Int
Immunopharmacol. 108:1087192022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yan C, Liu X, Xu H and Wang L: Cytoplasmic
mtDNA clearance suppresses inflammatory immune responses. Trends
Cell Biol. 34:897–900. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bai R and Cui J: Mitochondrial immune
regulation and anti-tumor immunotherapy strategies targeting
mitochondria. Cancer Lett. 564:2162232023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Vinay DS, Ryan EP, Pawelec G, Talib WH,
Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, et
al: Immune evasion in cancer: Mechanistic basis and therapeutic
strategies. Semin Cancer Biol. 35(Suppl): S185–S198. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kuo CL, Ponneri Babuharisankar A, Lin YC,
Lien HW, Lo YK, Chou HY, Tangeda V, Cheng LC, Cheng AN and Lee AY:
Mitochondrial oxidative stress in the tumor microenvironment and
cancer immunoescape: foe or friend? J Biomed Sci. 29:742022.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chen X, Song M, Zhang B and Zhang Y:
Reactive oxygen species regulate T cell immune response in the
tumor microenvironment. Oxid Med Cell Longev. 2016:15809672016.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hopfner KP and Hornung V: Molecular
mechanisms and cellular functions of cGAS-STING signalling. Nat Rev
Mol Cell Biol. 21:501–521. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Taneja N, Chauhan A, Kulshreshtha R and
Singh S: HIF-1 mediated metabolic reprogramming in cancer:
Mechanisms and therapeutic implications. Life Sci. 352:1228902024.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yang D, Liu J, Qian H and Zhuang Q:
Cancer-associated fibroblasts: from basic science to anticancer
therapy. Exp Mol Med. 55:1322–1332. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Mortezaee K and Majidpoor J: The impact of
hypoxia on immune state in cancer. Life Sci. 286:1200572021.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kim J, Kim J and Bae JS: ROS homeostasis
and metabolism: A critical liaison for cancer therapy. Exp Mol Med.
48:e2692016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
He L, Tam PK and Deng CX: Orchestration of
tumor-associated macrophages in the tumor
cell-macrophage-CD8+ T cell loop for cancer
immunotherapy. Int J Biol Sci. 21:4098–4116. 2025. View Article : Google Scholar :
|
|
108
|
Li Y, You J, Zou Z, Sun G, Shi Y, Sun Y,
Xu S and Zhang X: Decoding the tumor microenvironment:
Exosome-mediated macrophage polarization and therapeutic frontiers.
Int J Biol Sci. 21:4187–4214. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ricchi F, Kenno S, Pedretti N, Brenna G,
De Seta F, Ardizzoni A and Pericolini E: Cutibacterium acnes lysate
improves cellular response against Candida albicans, Escherichia
coli and Gardnerella vaginalis in an in vitro model of vaginal
infection. Front Cell Infect Microbiol. 15:15788312025. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Matsushima Y, Takahashi K, Yue S,
Fujiyoshi Y, Yoshioka H, Aihara M, Setoyama D, Uchiumi T, Fukuchi S
and Kang D: Mitochondrial Lon protease is a gatekeeper for proteins
newly imported into the matrix. Commun Biol. 4:9742021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li JY, Zhao Y, Gong S, Wang MM, Liu X, He
QM, Li YQ, Huang SY, Qiao H, Tan XR, et al: TRIM21 inhibits
irradiation-induced mitochondrial DNA release and impairs
antitumour immunity in nasopharyngeal carcinoma tumour models. Nat
Commun. 14:8652023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Liu S, Wang W, Hu S, Jia B, Tuo B, Sun H,
Wang Q, Liu Y and Sun Z: Radiotherapy remodels the tumor
microenvironment for enhancing immunotherapeutic sensitivity. Cell
Death Dis. 14:6792023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Seo YN, Baik JS, Lee SM, Lee JE, Ahn HR,
Lim MS, Park MT and Kim SD: Ionizing radiation selectively
increases CXC ligand 10 level via the DNA-damage-induced p38
MAPK-STAT1 pathway in murine J774A.1 macrophages. Cells.
12:10092023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Gong J, Le TQ, Massarelli E, Hendifar AE
and Tuli R: Radiation therapy and PD-1/PD-L1 blockade: The clinical
development of an evolving anticancer combination. J Immunother
Cancer. 6:462018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Donlon NE, Power R, Hayes C, Reynolds JV
and Lysaght J: Radiotherapy, immunotherapy, and the tumour
microenvironment: Turning an immunosuppressive milieu into a
therapeutic opportunity. Cancer Lett. 502:84–96. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang H, Li S, Wang D, Liu S, Xiao T, Gu
W, Yang H, Wang H, Yang M and Chen P: Metabolic reprogramming and
immune evasion: The interplay in the tumor microenvironment.
Biomark Res. 12:962024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX
and Weissman IL: Phagocytosis checkpoints as new targets for cancer
immunotherapy. Nat Rev Cancer. 19:568–586. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cagnoni AJ, Giribaldi ML, Blidner AG,
Cutine AM, Gatto SG, Morales RM, Salatino M, Abba MC, Croci DO,
Mariño KV and Rabinovich GA: Galectin-1 fosters an
immunosuppressive microenvironment in colorectal cancer by
reprogramming CD8+ regulatory T cells. Proc Natl Acad
Sci USA. 118:e21029501182021. View Article : Google Scholar
|
|
119
|
Guo S, Yao Y, Tang Y, Xin Z, Wu D, Ni C,
Huang J, Wei Q and Zhang T: Radiation-induced tumor immune
microenvironments and potential targets for combination therapy.
Signal Transduct Target Ther. 8:2052023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Spiotto M, Fu YX and Weichselbaum RR: The
intersection of radiotherapy and immunotherapy: Mechanisms and
clinical implications. Sci Immunol. 1:EAAG12662016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kang K, Lin X, Chen P, Liu H, Liu F, Xiong
W, Li G, Yi M, Li X, Wang H and Xiang B: T cell exhaustion in human
cancers. Biochim Biophys Acta Rev Cancer. 1879:1891622024.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhou S, Zhu M, Wei X, Mu P, Shen L, Wang
Y, Wan J, Zhang H, Xia F and Zhang Z: Low-dose radiotherapy
synergizes with iRGD-antiCD3-modified T cells by facilitating T
cell infiltration. Radiother Oncol. 194:1102132024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lumniczky K and Sáfrány G: The impact of
radiation therapy on the antitumor immunity: Local effects and
systemic consequences. Cancer Lett. 356:114–125. 2015. View Article : Google Scholar
|
|
124
|
Lynch C, Pitroda SP and Weichselbaum RR:
Radiotherapy, immunity, and immune checkpoint inhibitors. Lancet
Oncol. 25:e352–e362. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Liu T, Pei P, Shen W, Hu L and Yang K:
Radiation-induced immunogenic cell death for cancer
radioimmunotherapy. Small Methods. 7:e22014012023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Liao P, Wang W, Wang W, Kryczek I, Li X,
Bian Y, Sell A, Wei S, Grove S, Johnson JK, et al: CD8+
T cells and fatty acids orchestrate tumor ferroptosis and immunity
via ACSL4. Cancer Cell. 40:365–378.e6. 2022. View Article : Google Scholar
|
|
127
|
Xu H, Ye D, Ren M, Zhang H and Bi F:
Ferroptosis in the tumor microenvironment: Perspectives for
immunotherapy. Trends Mol Med. 27:856–867. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Goldstein M and Kastan MB: The DNA damage
response: implications for tumor responses to radiation and
chemotherapy. Annu Rev Med. 66:129–143. 2015. View Article : Google Scholar
|
|
129
|
Yu X, Zhu D, Luo B, Kou W, Cheng Y and Zhu
Y: IFNγ enhances ferroptosis by increasing JAK-STAT pathway
activation to suppress SLCA711 expression in adrenocortical
carcinoma. Oncol Rep. 47:972022. View Article : Google Scholar
|
|
130
|
Liang B, Khan M, Storts H, Zhang EH, Zheng
X, Xing X, Claybon H, Wilson J, Li C, Jin N, et al: Riluzole
enhancing anti-PD-1 efficacy by activating cGAS/STING signaling in
colorectal cancer. Mol Cancer Ther. 24:131–140. 2025. View Article : Google Scholar :
|
|
131
|
Li H, Li X, Kong Y and Sun W:
Ubiquitin-specific protease 34 in macrophages limits CD8 T
cell-mediated onset of vitiligo in mice. Immunobiology.
228:1523832023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Machado E, White-Gilbertson S, van de
Vlekkert D, Janke L, Moshiach S, Campos Y, Finkelstein D, Gomero E,
Mosca R, Qiu X, et al: Regulated lysosomal exocytosis mediates
cancer progression. Sci Adv. 1:e15006032015. View Article : Google Scholar
|
|
133
|
Han C, Ge M, Xing P, Xia T, Zhang C, Ma K,
Ma Y, Li S, Li W, Liu X, et al: Cystine deprivation triggers
CD36-mediated ferroptosis and dysfunction of tumor infiltrating
CD8+ T cells. Cell Death Dis. 15:1452024. View Article : Google Scholar
|
|
134
|
Mai LT, Swaminathan S, Nguyen TH, Collette
E, Charpentier T, Carmona-Pérez L, Loucif H, Lamarre A, Heinonen
KM, Langlais D, et al: Transcription factor IRF-5 regulates lipid
metabolism and mitochondrial function in murine CD8+
T-cells during viral infection. EMBO J. June 10–2025.Epub ahead of
print.
|
|
135
|
Deschler S, Pohl-Topcu J, Ramsauer L,
Meiser P, Erlacher S, Schenk RP, Maurer HC, Shen P, Kager J, Zink
J, et al: Polyunsaturated fatty acid-induced metabolic exhaustion
and ferroptosis impair the anti-tumour function of MAIT cells in
MASLD. J Hepatol. June 18–2025.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Anderson DA III, Murphy KM and Briseño CG:
Development, diversity, and function of dendritic cells in mouse
and human. Cold Spring Harb Perspect Biol. 10:a0286132018.
View Article : Google Scholar
|
|
137
|
Powell DR and Huttenlocher A: Neutrophils
in the tumor micro-environment. Trends Immunol. 37:41–52. 2016.
View Article : Google Scholar
|
|
138
|
Zhang C, Liu X, Jin S, Chen Y and Guo R:
Ferroptosis in cancer therapy: A novel approach to reversing drug
resistance. Mol Cancer. 21:472022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Lou X, Gao D, Yang L, Wang Y and Hou Y:
Endoplasmic reticulum stress mediates the myeloid-derived immune
suppression associated with cancer and infectious disease. J Transl
Med. 21:12023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Shi H, Dong G, Yan F, Zhang H, Li C, Ma Q,
Zhang J, Ning Z, Li Z, Dai J, et al: Arctigenin ameliorates
inflammation by regulating accumulation and functional activity of
MDSCs in endotoxin shock. Inflammation. 41:2090–2100. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Li J, Liu J, Zhou Z, Wu R, Chen X, Yu C,
Stockwell B, Kroemer G, Kang R and Tang D: Tumor-specific GPX4
degradation enhances ferroptosis-initiated antitumor immune
response in mouse models of pancreatic cancer. Sci Transl Med.
15:eadg30492023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Yang Y, Wang Y, Guo L, Gao W, Tang TL and
Yan M: Interaction between macrophages and ferroptosis. Cell Death
Dis. 13:3552022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Luo L, Wang H, Tian W, Zeng J, Huang Y and
Luo H: Targeting ferroptosis for cancer therapy: Iron metabolism
and anticancer immunity. Am J Cancer Res. 11:5508–5525.
2021.PubMed/NCBI
|
|
144
|
Ma S, Fu X, Liu L, Liu Y, Feng H, Jiang H,
Liu X, Liu R, Liang Z, Li M, et al: Iron-dependent autophagic cell
death induced by radiation in MDA-MB-231 breast cancer cells. Front
Cell Dev Biol. 9:7238012021. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Shukla SK, Kulkarni NS, Chan A,
Parvathaneni V, Farrales P, Muth A and Gupta V:
Metformin-encapsulated liposome delivery system: An effective
treatment approach against breast cancer. Pharmaceutics.
11:5592019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Abu Shelbayeh O, Arroum T, Morris S and
Busch KB: PGC-1α is a master regulator of mitochondrial lifecycle
and ros stress response. Antioxidants (Basel). 12:10752023.
View Article : Google Scholar
|
|
147
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Liu S, Zhang HL, Li J, Ye ZP, Du T, Li LC,
Guo YQ, Yang D, Li ZL, Cao JH, et al: Tubastatin A potently
inhibits GPX4 activity to potentiate cancer radiotherapy through
boosting ferroptosis. Redox Biol. 62:1026772023. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wang D, Tang L, Chen M, Gong Z, Fan C, Qu
H, Liu Y, Shi L, Mo Y, Wang Y, et al: Nanocarriers targeting
circular RNA ADARB1 boost radiosensitivity of nasopharyngeal
carcinoma through synergically promoting ferroptosis. ACS Nano.
18:31055–31075. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhong XF and Sun X: Nanomedicines based on
nanoscale metal-organic frameworks for cancer immunotherapy. Acta
Pharmacol Sin. 41:928–935. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Lin J, Lai Y, Lu F and Wang W: Targeting
ACSLs to modulate ferroptosis and cancer immunity. Trends
Endocrinol Metab. 36:677–690. 2025. View Article : Google Scholar
|
|
152
|
Zhang Y, Cai K, Li C, Guo Q, Chen Q, He X,
Liu L, Zhang Y, Lu Y, Chen X, et al: Macrophage-membrane-coated
nanoparticles for tumor-targeted chemotherapy. Nano Lett.
18:1908–1915. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Liu N, Lin Q, Huang Z, Liu C, Qin J, Yu Y,
Chen W, Zhang J, Jiang M, Gao X, et al: Mitochondria-targeted
prodrug nanoassemblies for efficient ferroptosis-based therapy via
devastating ferroptosis defense systems. ACS Nano. 18:7945–7958.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zeng L, Gowda BHJ, Ahmed MG, Abourehab
MAS, Chen ZS, Zhang C, Li J and Kesharwani P: Advancements in
nanoparticle-based treatment approaches for skin cancer therapy.
Mol Cancer. 22:102023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Jiang Q, Wang K, Zhang X, Ouyang B, Liu H,
Pang Z and Yang W: Platelet membrane-camouflaged magnetic
nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small.
16:e20017042020. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Yang M, Zhang Y, Hu Z, Xie H, Tian W and
Liu Z: Application of hyaluronic acid-based nanoparticles for
cancer combination therapy. Int J Pharm. 646:1234592023. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Liu Q, Zhao Y, Zhou H and Chen C:
Ferroptosis: Challenges and opportunities for nanomaterials in
cancer therapy. Regen Biomater. 10:rbad0042023. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Shao C, Li Z, Zhang C, Zhang W, He R, Xu J
and Cai Y: Optical diagnostic imaging and therapy for thyroid
cancer. Mater Today Bio. 17:1004412022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Mulford DA, Scheinberg DA and Jurcic JG:
The promise of targeted {alpha}-particle therapy. J Nucl Med.
46(Suppl 1): 199S–204S. 2005.PubMed/NCBI
|
|
160
|
Seidl C: Radioimmunotherapy with
α-particle-emitting radionuclides. Immunotherapy. 6:431–458. 2014.
View Article : Google Scholar
|
|
161
|
Xu J, Song M, Fang Z, Zheng L, Huang X and
Liu K: Applications and challenges of ultra-small particle size
nanoparticles in tumor therapy. J Control Release. 353:699–712.
2023. View Article : Google Scholar
|
|
162
|
Vangijzegem T, Stanicki D and Laurent S:
Magnetic iron oxide nanoparticles for drug delivery: Applications
and characteristics. Expert Opin Drug Deliv. 16:69–78. 2019.
View Article : Google Scholar
|
|
163
|
Wang H, Qiao C, Guan Q, Wei M and Li Z:
Nanoparticle-mediated synergistic anticancer effect of ferroptosis
and photodynamic therapy: Novel insights and perspectives. Asian J
Pharm Sci. 18:1008292023.PubMed/NCBI
|
|
164
|
Chang Q, Wang P, Zeng Q and Wang X: A
review on ferroptosis and photodynamic therapy synergism: Enhancing
anticancer treatment. Heliyon. 10:e289422024. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Zhang L, Yi H, Song J, Huang J, Yang K,
Tan B, Wang D, Yang N, Wang Z and Li X: Mitochondria-targeted and
ultrasound-activated nanodroplets for enhanced deep-penetration
sonodynamic cancer therapy. ACS Appl Mater Interfaces.
11:9355–9366. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Ding Y, Liu Z, Dai X, Ruan R, Zhong H, Wu
Z, Yao Y, Chen J, Deng J and Xiong J: Ubiquitin-specific peptidase
49 promotes adenocarcinoma of the esophagogastric junction
malignant progression via activating SHCBP1-β-catenin-GPX4 axis.
Carcinogenesis. 46:bgae0602025. View Article : Google Scholar
|
|
167
|
Rittase WB, Slaven JE, Suzuki YJ, Muir JM,
Lee SH, Rusnak M, Brehm GV, Bradfield DT, Symes AJ and Day RM: Iron
deposition and ferroptosis in the spleen in a murine model of acute
radiation syndrome. Int J Mol Sci. 23:110292022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Wu Z, Chen T, Qian Y, Luo G, Liao F, He X,
Xu W, Pu J and Ding S: High-dose ionizing radiation accelerates
atherosclerotic plaque progression by regulating P38/NCOA4-mediated
ferritinophagy/ferroptosis of endothelial cells. Int J Radiat Oncol
Biol Phys. 117:223–236. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Fu S, Li Y, Shen L, Chen Y, Lu J, Ran Y,
Zhao Y, Tang H, Tan L, Lin Q and Hao Y:
Cu2WS4-PEG nanozyme as multifunctional
sensitizers for enhancing immuno-radiotherapy by inducing
ferroptosis. Small. 20:e23095372024. View Article : Google Scholar
|
|
170
|
Duan Q, Cui Z, Wang M, Li R, Han F and Ma
J: Ginkgetin enhances breast cancer radiotherapy sensitization by
suppressing NRF2-HO-1 axis activity. Toxicol Appl Pharmacol.
495:1171992025. View Article : Google Scholar
|
|
171
|
Shi J, Cui G, Jin Y, Mi B, Liu K, Zhao L,
Bao K, Lu Z, Liu J, Wang Y, et al: Glutathione-depleted
photodynamic nanoadjuvant for triggering nonferrous ferroptosis to
amplify radiotherapy of breast cancer. Adv Healthc Mater.
13:e24024742024. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Hu H, Zheng S, He C, Zheng Y, Wei Q, Chen
S, Wu Z, Xu Y, Zhao B and Yan C: Radiotherapy-sensitized cancer
immunotherapy via cGAS-STING immune pathway by activatable
nanocascade reaction. J Nanobiotechnology. 22:2342024. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Yu J, Zhang Y, Li L, Xiang Y, Yao X, Zhao
Y, Cai K, Li M, Li Z and Luo Z: Coordination-driven FBXW7
DNAzyme-Fe nanoassembly enables a binary switch of breast cancer
cell cycle checkpoint responses for enhanced
ferroptosis-radiotherapy. Acta Biomater. 169:434–450. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Zheng S, Hu H, Hou M, Zhu K, Wu Z, Qi L,
Xia H, Liu G, Ren Y, Xu Y, et al: Proton pump inhibitor-enhanced
nanocatalytic ferroptosis induction for stimuli-responsive
dual-modal molecular imaging guided cancer radiosensitization. Acta
Biomater. 162:72–84. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Wei M, Bai J, Shen X, Lou K, Gao Y, Lv R,
Wang P, Liu X and Zhang G: Glutathione-exhausting nanoprobes for
NIR-II fluorescence imaging-guided surgery and boosting radiation
therapy efficacy via ferroptosis in breast cancer. ACS Nano.
17:11345–11361. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Zhang Z, Lo H, Zhao X, Li W, Wu K, Zeng F,
Li S and Sun H: Mild photothermal/radiation therapy potentiates
ferroptosis effect for ablation of breast cancer via MRI/PA imaging
guided all-in-one strategy. J Nanobiotechnology. 21:1502023.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
El-Benhawy SA, Abdelrhman IG, Sadek NA,
Fahmy EI, AboGabal AA, Elmasry H, Saleh SAM, Sakr OA, Elwany MN and
Rabie MAF: Studying ferroptosis and iron metabolism pre- and
post-radiotherapy treatment in breast cancer patients. J Egypt Natl
Canc Inst. 35:42023. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Hou YK, Zhang ZJ, Li RT, Peng J, Chen SY,
Yue YR, Zhang WH, Sun B, Chen JX and Zhou Q: Remodeling the tumor
microenvironment with core-shell nanosensitizer featuring
dual-modal imaging and multimodal therapy for breast cancer. ACS
Appl Mater Interfaces. 15:2602–2616. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Liu L, Zhang C, Qu S, Liu R, Chen H, Liang
Z, Tian Z, Li L, Ma S and Liu X: ESR1 inhibits ionizing
radiation-induced ferroptosis in breast cancer cells via the
NEDD4L/CD71 pathway. Arch Biochem Biophys. 725:1092992022.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Liu R, Liu L, Bian Y, Zhang S, Wang Y,
Chen H, Jiang X, Li G, Chen Q, Xue C, et al: The dual regulation
effects of ESR1/NEDD4L on SLC7A11 in breast cancer under ionizing
radiation. Front Cell Dev Biol. 9:7723802022. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Long Q, Tao H, Wang P, Wu B, Zhu Q, Chen
H, Lao G, Yang Y, Liu G, Liu S and Wu Y: Fludarabine enhances
radiosensitivity by promoting ferroptosis in B-cell lymphoma.
Radiat Res. 201:224–239. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Chen H, Zhu P, Zhu D, Jin J, Yang Q and
Han X: Role and mechanism of KIAA1429 in regulating cellular
ferroptosis and radioresistance in colorectal cancer. Biomol
Biomed. 24:1669–1681. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Zhang Z, Ye B, Lin Y, Liu W, Deng J and Ji
W: LncRNA OTUD6B-AS1 overexpression promoted GPX4-mediated
ferroptosis to suppress radioresistance in colorectal cancer. Clin
Transl Oncol. 25:3217–3229. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Shen D, Luo J, Chen L, Ma W, Mao X, Zhang
Y, Zheng J, Wang Y, Wan J, Wang S, et al: PARPi treatment enhances
radiotherapy-induced ferroptosis and antitumor immune responses via
the cGAS signaling pathway in colorectal cancer. Cancer Lett.
550:2159192022. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Singhal R, Mitta SR, Das NK, Kerk SA,
Sajjakulnukit P, Solanki S, Andren A, Kumar R, Olive KP, Banerjee
R, et al: HIF-2α activation potentiates oxidative cell death in
colorectal cancers by increasing cellular iron. J Clin Invest.
131:e1436912021. View Article : Google Scholar
|
|
186
|
Chen C, Gu W, Shao Y, Zheng P and Jiang J:
Expression of glutathione peroxidase4 in patients with esophageal
squamous carcinoma and its impact on the radiosensitivity of
esophageal squamous carcinoma. Ann Clin Lab Sci. 54:160–169.
2024.PubMed/NCBI
|
|
187
|
Jin Y, Pan S, Wang M, Huang S, Ke Y, Li D,
Luo H, Kou Z, Shi D, Kou W, et al: The m6A modification
of ACSL4 mRNA sensitized esophageal squamous cell carcinoma to
irradiation via accelerating ferroptosis. Cell Biol Int.
48:1877–1890. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Yan L, Hu H, Feng L, Li Z, Zheng C, Zhang
J, Yin X and Li B: ML385 promotes ferroptosis and radiotherapy
sensitivity by inhibiting the NRF2-SLC7A11 pathway in esophageal
squamous cell carcinoma. Med Oncol. 41:3092024. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Chen J, Ying K, Sun J, Wang Y, Ji M and
Sun Y: NEDD4L affects KLF5 stability through ubiquitination to
control ferroptosis and radiotherapy resistance in oesophageal
squamous cell carcinoma. J Cell Mol Med. 28:e700622024. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Heng J, Li Z, Liu L, Zheng Z, Zheng Y, Xu
X, Liao L, Xu H, Huang H, Li E and Xu L: Acetyl-CoA
acetyltransferase 2 confers radioresistance by inhibiting
ferroptosis in esophageal squamous cell carcinoma. Int J Radiat
Oncol Biol Phys. 117:966–978. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zhao M, Lu T, Bi G, Hu Z, Liang J, Bian Y,
Feng M and Zhan C: PLK1 regulating chemoradiotherapy sensitivity of
esophageal squamous cell carcinoma through pentose phosphate
pathway/ferroptosis. Biomed Pharmacother. 168:1157112023.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Jiang K, Yin X, Zhang Q, Yin J, Tang Q, Xu
M, Wu L, Shen Y, Zhou Z, Yu H and Yan S: STC2 activates PRMT5 to
induce radioresistance through DNA damage repair and ferroptosis
pathways in esophageal squamous cell carcinoma. Redox Biol.
60:1026262023. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Luo H, Wang X, Song S, Wang Y, Dan Q and
Ge H: Targeting stearoyl-coa desaturase enhances radiation induced
ferroptosis and immunogenic cell death in esophageal squamous cell
carcinoma. Oncoimmunology. 11:21017692022. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Feng L, Zhao K, Sun L, Yin X, Zhang J, Liu
C and Li B: SLC7A11 regulated by NRF2 modulates esophageal squamous
cell carcinoma radiosensitivity by inhibiting ferroptosis. J Transl
Med. 19:3672021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Xue Y, Lu F, Chang Z, Li J, Gao Y, Zhou J,
Luo Y, Lai Y, Cao S, Li X, et al: Intermittent dietary methionine
deprivation facilitates tumoral ferroptosis and synergizes with
checkpoint blockade. Nat Commun. 14:47582023. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Wang Z, Wang Y, Shen N, Liu Y, Xu X, Zhu
R, Jiang H, Wu X, Wei Y and Tang J: AMPKα1-mediated ZDHHC8
phosphorylation promotes the palmitoylation of SLC7A11 to
facilitate ferroptosis resistance in glioblastoma. Cancer Lett.
584:2166192024. View Article : Google Scholar
|
|
197
|
Li H, Qi X, He L, Yang H and Ju H: PRMT1
promotes radiotherapy resistance in glioma stem cells by inhibiting
ferroptosis. Jpn J Radiol. 43:129–137. 2025. View Article : Google Scholar
|
|
198
|
Liu X, Cao Z, Wang W, Zou C, Wang Y, Pan
L, Jia B, Zhang K, Zhang W, Li W, et al: Engineered extracellular
vesicle-delivered CRISPR/Cas9 for radiotherapy sensitization of
glioblastoma. ACS Nano. 17:16432–16447. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Cirotti C, Taddei I, Contadini C, Di
Girolamo C, Pepe G, De Bardi M, Borsellino G, Helmer-Citterich M
and Barilà D: NRF2 connects Src tyrosine kinase to ferroptosis
resistance in glioblastoma. Life Sci Alliance. 7:e2023022052023.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Koike N, Kota R, Naito Y, Hayakawa N,
Matsuura T, Hishiki T, Onishi N, Fukada J, Suematsu M, Shigematsu
N, et al: 2-Nitroimidazoles induce mitochondrial stress and
ferroptosis in glioma stem cells residing in a hypoxic niche.
Commun Biol. 3:4502020. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
He J, Li M, Bao J, Peng Y, Xue W, Chen J
and Zhao J: β-Elemene promotes ferroptosis and reverses
radioresistance in gastric cancer by inhibiting the OTUB1-GPX4
interaction. Front Pharmacol. 15:14691802024. View Article : Google Scholar
|
|
202
|
Wang H, Niu H, Luo X, Zhu N, Xiang J, He
Y, Chen Z, Li G and Hu Y: Radiosensitizing effects of
pyrogallol-loaded mesoporous or-ganosilica nanoparticles on gastric
cancer by amplified ferroptosis. Front Bioeng Biotechnol.
11:11714502023. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Huang W, He Y, Yang S, Xue X, Qin H, Sun T
and Yang W: CA9 knockdown enhanced ionizing radiation-induced
ferroptosis and radiosensitivity of hypoxic glioma cells. Int J
Radiat Biol. 99:1908–1924. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Sun S, Qi G, Chen H, He D, Ma D, Bie Y, Xu
L, Feng B, Pang Q, Guo H and Zhang R: Ferroptosis sensitization in
glioma: Exploring the regulatory mechanism of SOAT1 and its
therapeutic implications. Cell Death Dis. 14:7542023. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Qiu L, Ji H, Wang K, Liu W, Huang Q, Pan
X, Ye H, Li Z, Chen G, Xing X, et al: TLR3 activation enhances
abscopal effect of radiotherapy in HCC by promoting tumor
ferroptosis. EMBO Mol Med. 16:1193–1219. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Chen Q, Zheng W, Guan J, Liu H, Dan Y, Zhu
L, Song Y, Zhou Y, Zhao X, Zhang Y, et al: SOCS2-enhanced
ubiquitination of SLC7A11 promotes ferroptosis and
radiosensitization in hepatocellular carcinoma. Cell Death Differ.
30:137–151. 2023. View Article : Google Scholar :
|
|
207
|
Feng H, Liu Y, Gan Y, Li M, Liu R, Liang
Z, Liu L, Li L, Chen H, Li G, et al: AdipoR1 regulates ionizing
radiation-induced ferroptosis in HCC cells through Nrf2/xCT
pathway. Oxid Med Cell Longev. 2022:80914642022. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Dang W, Chen WC, Ju E, Xu Y, Li K, Wang H,
Wang K, Lv S, Shao D, Tao Y and Li M: 3D printed hydrogel scaffolds
combining glutathione depletion-induced ferroptosis and
photothermia-augmented chemodynamic therapy for efficiently
inhibiting postoperative tumor recurrence. J Nanobiotechnology.
20:2662022. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Yang M, Wu X, Hu J, Wang Y, Wang Y, Zhang
L, Huang W, Wang X, Li N, Liao L, et al: COMMD10 inhibits HIF1α/CP
loop to enhance ferroptosis and radiosensitivity by disrupting
Cu-Fe balance in hepatocellular carcinoma. J Hepatol. 76:1138–1150.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Yuan Z, Liu T, Huo X, Wang H, Wang J and
Xue L: Glutamine transporter SLC1A5 regulates ionizing
radiation-derived oxidative damage and ferroptosis. Oxid Med Cell
Longev. 2022:34030092022. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Yuan Y, Cao W, Zhou H, Qian H and Wang H:
CLTRN, regulated by NRF1/RAN/DLD protein complex, enhances
radiation sensitivity of hepatocellular carcinoma cells through
ferroptosis pathway. Int J Radiat Oncol Biol Phys. 110:859–871.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Huang P, Ning X, Kang M and Wang R:
Ferroptosis-related genes are associated with radioresistance and
immune suppression in head and neck cancer. Genet Test Mol
Biomarkers. 28:100–113. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Song A, Wu L, Zhang BX, Yang QC, Liu YT,
Li H, Mao L, Xiong D, Yu HJ and Sun ZJ: Glutamine inhibition
combined with CD47 blockade enhances radiotherapy-induced
ferroptosis in head and neck squamous cell carcinoma. Cancer Lett.
588:2167272024. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Reinema FV, Hudson N, Adema GJ, Peeters
WJM, Neuzil J, Stursa J, Werner L, Sweep FCGJ, Bussink J and Span
PN: MitoTam induces ferroptosis and increases radiosensitivity in
head and neck cancer cells. Radiother Oncol. 200:1105032024.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Chen L, Lin W, Zhang H, Geng S, Le Z, Wan
F, Huang Q, Chen H, Liu X, Lu JJ and Kong L: TRIB3 promotes
malignancy of head and neck squamous cell carcinoma via inhibiting
ferroptosis. Cell Death Dis. 15:1782024. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Noh JK, Lee MK, Lee Y, Bae M, Min S, Kong
M, Lee JW, Kim SI, Lee YC, Ko SG, et al: Targeting ferroptosis for
improved radiotherapy outcomes in HPV-negative head and neck
squamous cell carcinoma. Mol Oncol. 19:540–557. 2025. View Article : Google Scholar
|
|
217
|
Liao W, Chen X, Zhang S, Chen J, Liu C, Yu
K, Zhang Y, Chen M, Chen F, Shen M, et al: Megakaryocytic IGF1
coordinates activation and ferroptosis to safeguard hematopoietic
stem cell regeneration after radiation injury. Cell Commun Signal.
22:2922024. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Song H, Sun H, He N, Xu C, Du L, Ji K,
Wang J, Zhang M, Gu Y, Wang Y and Liu Q: Glutathione
depletion-induced versatile nanomedicine for potentiating the
ferroptosis to overcome solid tumor radioresistance and enhance
immunotherapy. Adv Healthc Mater. 13:e23034122021. View Article : Google Scholar
|
|
219
|
Almahi WA, Yu KN, Mohammed F, Kong P and
Han W: Hemin enhances radiosensitivity of lung cancer cells through
ferroptosis. Exp Cell Res. 410:1129462022. View Article : Google Scholar
|
|
220
|
Li Y, Yang J, Gu G, Guo X, He C, Sun J,
Zou H, Wang H, Liu S, Li X, et al: Pulmonary delivery of
theranostic nanoclusters for lung cancer ferroptosis with enhanced
chemodynamic/radiation synergistic therapy. Nano Lett. 22:963–972.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Zhang W, Sun Y, Bai L, Zhi L, Yang Y, Zhao
Q, Chen C, Qi Y, Gao W, He W, et al: RBMS1 regulates lung cancer
ferroptosis through translational control of SLC7A11. J Clin
Invest. 131:e1520672021. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Rai A, Patwardhan RS, Jayakumar S,
Pachpatil P, Das D, Panigrahi GC, Gota V, Patwardhan S and Sandur
SK: Clobetasol propionate, a Nrf-2 inhibitor, sensitizes human lung
cancer cells to radiation-induced killing via mitochondrial
ROS-dependent ferroptosis. Acta Pharmacol Sin. 45:1506–1519. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Liang J, Bi G, Huang Y, Zhao G, Sui Q,
Zhang H, Bian Y, Yin J, Wang Q, Chen Z and Zhan C: MAFF confers
vulnerability to cisplatin-based and ionizing radiation treatments
by modulating ferroptosis and cell cycle progression in lung
adenocarcinoma. Drug Resist Updat. 73:1010572024. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Gotorbe C, Segui F, Echavidre W, Durivault
J, Blanchard T, Vial V, Pagnuzzi-Boncompagni M, Villeneuve R,
Amblard R, Garnier N, et al: Exploiting integrin-αVβ3 to enhance
radiotherapy efficacy in medulloblastoma via ferroptosis. Curr
Oncol. 31:7390–7402. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Zhou H, Wang YX, Wu M, Lan X, Xiang D, Cai
R, Ma Q, Miao J, Fang X, Wang J, et al: FANCD2 deficiency
sensitizes SHH medulloblastoma to radiotherapy via ferroptosis. J
Pathol. 262:427–440. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Lin Y, Chen X, Yu C, Xu G, Nie X, Cheng Y,
Luan Y and Song Q: Radiotherapy-mediated redox
homeostasis-controllable nanomedicine for enhanced ferroptosis
sensitivity in tumor therapy. Acta Biomater. 159:300–311. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Yang F, Gong H, Chen S, Li J, Huang N and
Wang M: Depletion of SLC7A11 sensitizes nasopharyngeal carcinoma
cells to ionizing radiation. Protein Pept Lett. 31:323–331. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Amos A, Jiang N, Zong D, Gu J, Zhou J, Yin
L, He X, Xu Y and Wu L: Depletion of SOD2 enhances nasopharyngeal
carcinoma cell radiosensitivity via ferroptosis induction modulated
by DHODH inhibition. BMC Cancer. 23:1172023. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Huang WM, Li ZX, Wu YH, Shi ZL, Mi JL, Hu
K and Wang RS: m6A demethylase FTO renders radioresistance of
nasopharyngeal carcinoma via promoting OTUB1-mediated
anti-ferroptosis. Transl Oncol. 27:1015762023. View Article : Google Scholar
|
|
230
|
Han F, Chen S, Zhang K, Zhang K, Wang M
and Wang P: Targeting Nrf2/PHKG2 axis to enhance radiosensitivity
in NSCLC. NPJ Precis Oncol. 8:1832024. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Ma Y, Peng Y, Cheng S and Jin L: Targeting
MGST1 makes non-small cell lung cancer cells sensitive to
radiotherapy by epigenetically enhancing ALOX15-mediated
ferroptosis. Curr Cancer Drug Targets. September 27–2024.Epub ahead
of print. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Zhang Y, Liu X, Zeng L, Zhao X, Chen Q,
Pan Y, Bai Y, Shao C and Zhang J: Exosomal protein
angiopoietin-like 4 mediated radioresistance of lung cancer by
inhibiting ferroptosis under hypoxic microenvironment. Br J Cancer.
127:1760–1772. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Bao Y, Pan Z, Zhao L, Qiu J, Cheng J, Liu
L and Qian D: BIBR1532 combined with radiotherapy induces
ferroptosis in NSCLC cells and activates cGAS-STING pathway to
promote anti-tumor immunity. J Transl Med. 22:5192024. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Koppula P, Lei G, Zhang Y, Yan Y, Mao C,
Kondiparthi L, Shi J, Liu X, Horbath A, Das M, et al: A targetable
CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1
inactive lung cancers. Nat Commun. 13:22062022. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Xiao H, Jiang N, Zhang H, Wang S, Pi Q,
Chen H, He X, Luo W, Lu Y, Deng Y and Zhong Z: Inhibitors of APE1
redox and ATM synergistically sensitize osteosarcoma cells to
ionizing radiation by inducing ferroptosis. Int Immunopharmacol.
139:1126722024. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Lee HM, Kuo PC, Chen WH, Chen PJ, Lam SH,
Su YC and Chen CH: Diterpenoid from croton tonkinensis as a
potential radiation sensitizer in oral squamous cell carcinoma: An
in vitro study. Int J Mol Sci. 25:118392024. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Liu J, An W, Zhao Q, Liu Z, Jiang Y, Li H
and Wang D: Hyperbaric oxygen enhances X-ray induced ferroptosis in
oral squamous cell carcinoma cells. Oral Dis. 30:116–127. 2024.
View Article : Google Scholar
|
|
238
|
Guo SS, Chen MM, Yang YH, Zhang YY, Pang
X, Shi YP, Zhuang YC, Fan DD, Bao JF and Ji ZY: Magnetic-vortex
nanodonuts enhance ferroptosis effect of tumor ablation through an
imaging-guided hyperthermia/radiosensitization strategy. iScience.
27:1105332024. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Cai J, Xu X and Saw PE: Nanomedicine
targeting ferroptosis to overcome anticancer therapeutic
resistance. Sci China Life Sci. 67:19–40. 2024. View Article : Google Scholar
|
|
240
|
Mao C, Lei G, Horbath A, Wang M, Lu Z, Yan
Y, Liu X, Kondiparthi L, Chen X, Cheng J, et al: Unraveling ETC
complex I function in ferroptosis reveals a potential
ferroptosis-inducing therapeutic strategy for LKB1-deficient
cancers. Mol Cell. 84:1964–1979.e6. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Zhang HL, Hu BX, Ye ZP, Li ZL, Liu S,
Zhong WQ, Du T, Yang D, Mai J, Li LC, et al: TRPML1 triggers
ferroptosis defense and is a potential therapeutic target in
AKT-hyperactivated cancer. Sci Transl Med. 16:eadk03302024.
View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Wu A, Han M, Ni Z, Li H, Chen Y, Yang Z,
Feng Y, He Z, Zhen H and Wang X: Multifunctional Sr/Se co-doped
ZIF-8 nanozyme for chemo/chemodynamic synergistic tumor therapy via
apoptosis and ferroptosis. Theranostics. 14:1939–1955. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Deng Z, Li B, Yang M, Lu L, Shi X, Lovell
JF, Zeng X, Hu W and Jin H: Irradiated microparticles suppress
prostate cancer by tumor microenvironment reprogramming and
ferroptosis. J Nanobiotechnology. 22:2252024. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Bi G, Liang J, Bian Y, Shan G, Huang Y, Lu
T, Zhang H, Jin X, Chen Z, Zhao M, et al: Polyamine-mediated
ferroptosis amplification acts as a targetable vulnerability in
cancer. Nat Commun. 15:24612024. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Bae C, Hernández Millares R, Ryu S, Moon
H, Kim D, Lee G, Jiang Z, Park MH, Kim KH, Koom WS, et al:
Synergistic effect of ferroptosis-inducing nanoparticles and X-ray
irradiation combination therapy. Small. 20:e23108732024. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Da Silva J, Bienassis C, Schmitt P,
Berjaud C, Guedj M and Paris S: Radiotherapy-activated NBTXR3
nanoparticles promote ferroptosis through induction of lysosomal
membrane permeabilization. J Exp Clin Cancer Res. 43:112024.
View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Zhu W, Cheng X, Xu P, Gu Y, Xu H, Xu J and
Wang Y, Zhang LW and Wang Y: Radiotherapy-driven nanoprobes
targeting for visualizing tumor infiltration dynamics and inducing
ferroptosis in myeloid-derived suppressor cells. J Am Chem Soc.
146:22455–22468. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Zhao J, Chen Y, Xiong T, Han S, Li C, He
Y, He Y, Zhao G, Wang T, Wang L, et al: Clustered cobalt nanodots
initiate ferroptosis by upregulating heme oxygenase 1 for
radiotherapy sensitization. Small. 19:e22064152023. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Zhang Q, Wang X, Zhao Y, Cheng Z, Fang D,
Liu Y, Tian G, Li M and Luo Z: Nanointegrative in situ
reprogramming of tumor-intrinsic lipid droplet biogenesis for
low-dose radiation-activated ferroptosis immunotherapy. ACS Nano.
17:25419–25438. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Yang P, Li J, Zhang T, Ren Y, Zhang Q, Liu
R, Li H, Hua J, Wang WA, Wang J and Zhou H: Ionizing
radiation-induced mitophagy promotes ferroptosis by increasing
intracellular free fatty acids. Cell Death Differ. 30:2432–2445.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Jiang W, Wei L, Chen B, Luo X, Xu P, Cai J
and Hu Y: Platinum prodrug nanoparticles inhibiting tumor
recurrence and metastasis by concurrent chemoradiotherapy. J
Nanobiotechnology. 20:1292022. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Zhao C, Yu D, He Z, Bao L, Feng L, Chen L,
Liu Z, Hu X, Zhang N, Wang T and Fu Y: Endoplasmic reticulum
stress-mediated autophagy activation is involved in cadmium-induced
ferroptosis of renal tubular epithelial cells. Free Radic Biol Med.
175:236–248. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Tomita K, Nagasawa T, Kuwahara Y, Torii S,
Igarashi K, Roudkenar MH, Roushandeh AM, Kurimasa A and Sato T:
MiR-7-5p is involved in ferroptosis signaling and radioresistance
Thru the generation of ROS in radioresistant HeLa and SAS cell
lines. Int J Mol Sci. 22:83002021. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Takashi Y, Tomita K, Kuwahara Y, Roudkenar
MH, Roushandeh AM, Igarashi K, Nagasawa T, Nishitani Y and Sato T:
Mitochondrial dysfunction promotes aquaporin expression that
controls hydrogen peroxide permeability and ferroptosis. Free Radic
Biol Med. 161:60–70. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Chen M, Li X, Du B, Chen S and Li Y:
Upstream stimulatory factor 2 inhibits erastin-induced ferroptosis
in pancreatic cancer through transcriptional regulation of pyruvate
kinase M2. Biochem Pharmacol. 205:1152552022. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Zhu Y, Fang S, Fan B, Xu K, Xu L, Wang L,
Zhu L, Chen C, Wu R, Ni J and Wang J: Cancer-associated fibroblasts
reprogram cysteine metabolism to increase tumor resistance to
ferroptosis in pancreatic cancer. Theranostics. 14:1683–1700. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Feng Y, Luo X, Li Z, Fan X, Wang Y, He RR
and Liu M: A ferroptosis-targeting ceria anchored halloysite as
orally drug delivery system for radiation colitis therapy. Nat
Commun. 14:50832023. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Wang X, Li W, Dong Y, Zhang Y, Huo Q, Lu
L, Zhang J, Zhao Y, Fan S, Dong H and Li D: Ferrostatin-1 mitigates
ionizing radiation-induced intestinal injuries by inhibiting
apoptosis and ferroptosis: An in vitro and in vivo study. Int J
Radiat Biol. 99:1607–1618. 2023. View Article : Google Scholar
|
|
259
|
Tang LF, Ma X, Xie LW, Zhou H, Yu J, Wang
ZX and Li M: Perillaldehyde mitigates ionizing radiation-induced
intestinal injury by inhibiting ferroptosis via the Nrf2 signaling
pathway. Mol Nutr Food Res. 67:e23002322023. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Zhang F, Liu T, Huang HC, Zhao YY, He M,
Yuan W, Li L, Li J, Wu DM and Xu Y: Activation of pyroptosis and
ferroptosis is involved in radiation-induced intestinal injury in
mice. Biochem Biophys Res Commun. 631:102–109. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Zhou H, Zhou YL, Mao JA, Tang LF, Xu J,
Wang ZX, He Y and Li M: NCOA4-mediated ferritinophagy is involved
in ionizing radiation-induced ferroptosis of intestinal epithelial
cells. Redox Biol. 55:1024132022. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Deng S, Wu D, Li L, Li J and Xu Y: TBHQ
attenuates ferroptosis against 5-fluorouracil-induced intestinal
epithelial cell injury and intestinal mucositis via activation of
Nrf2. Cell Mol Biol Lett. 26:482021. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Xie LW, Cai S, Zhao TS, Li M and Tian Y:
Green tea derivative (−)-epigallocatechin-3-gallate (EGCG) confers
protection against ionizing radiation-induced intestinal epithelial
cell death both in vitro and in vivo. Free Radic Biol Med.
161:175–186. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Ning X, Zhao W, Wu Q, Wang C and Liang S:
Therapeutic potential of dihydroartemisinin in mitigating
radiation-induced lung injury: Inhibition of ferroptosis through
Nrf2/HO-1 pathways in mice. Immun Inflamm Dis. 12:e11752024.
View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Li X, Chen J, Yuan S, Zhuang X and Qiao T:
Activation of the P62-Keap1-NRF2 pathway protects against
ferroptosis in radiation-induced lung injury. Oxid Med Cell Longev.
2022:89735092022. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Li L, Wu D, Deng S, Li J, Zhang F, Zou Y,
Zhang T and Xu Y: NVP-AUY922 alleviates radiation-induced lung
injury via inhibition of autophagy-dependent ferroptosis. Cell
Death Discov. 8:862022. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Guo XW, Zhang H, Huang JQ, Wang SN, Lu Y,
Cheng B, Dong SH, Wang YY, Li FS and Li YW: PIEZO1 ion channel
mediates ionizing radiation-induced pulmonary endothelial cell
ferroptosis via Ca2+/calpain/VE-cadherin signaling.
Front Mol Biosci. 8:7252742021. View Article : Google Scholar
|
|
268
|
Cao J, Wu M, Mo W, Zhao M, Gu L, Wang X,
Zhang B and Cao J: Upregulation of PRRX2 by silencing Marveld3 as a
protective mechanism against radiation-induced ferroptosis in skin
cells. Mol Med. 30:1822024. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Zhao J, Tang M, Tang H, Wang M, Guan H,
Tang L and Zhang H: Sphingosine 1-phosphate alleviates
radiation-induced ferroptosis in ovarian granulosa cells by
upregulating glutathione peroxidase 4. Reprod Toxicol. 115:49–55.
2023. View Article : Google Scholar
|
|
270
|
Zhang L, Xu Y, Cheng Z, Zhao J, Wang M,
Sun Y, Mi Z, Yuan Z and Wu Z: The EGR1/miR-139/NRF2 axis
orchestrates radiosensitivity of non-small-cell lung cancer via
ferroptosis. Cancer Lett. 595:2170002024. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Yang X, Zhang M, Xia W, Mai Z, Ye Y, Zhao
B and Song Y: CHAC1 promotes cell ferroptosis and enhances
radiation sensitivity in thyroid carcinoma. Neoplasma. 70:777–786.
2023. View Article : Google Scholar
|
|
272
|
Guo Y, Wang H, Wang X, Chen K and Feng L:
Enhancing radiotherapy in triple-negative breast cancer with
hesperetin-induced ferroptosis via AURKA targeting nanocomposites.
J Nanobiotechnology. 22:7442024. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Chen R, Jiang Z, Cheng Y, Ye J, Li S, Xu
Y, Ye Z, Shi Y, Ding J, Zhao Y, et al: Multifunctional
iron-apigenin nanocomplex conducting photothermal therapy and
triggering augmented immune response for triple negative breast
cancer. Int J Pharm. 655:1240162024. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Zeng L, Ding S, Cao Y, Li C, Zhao B, Ma Z,
Zhou J, Hu Y, Zhang X, Yang Y, et al: A MOF-based potent
ferroptosis inducer for enhanced radiotherapy of triple negative
breast cancer. ACS Nano. 17:13195–13210. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Sun H, Cai H, Xu C, Zhai H, Lux F, Xie Y,
Feng L, Du L, Liu Y, Sun X, et al: AGuIX nanoparticles enhance
ionizing radiation-induced ferroptosis on tumor cells by targeting
the NRF2-GPX4 signaling pathway. J Nanobiotechnology. 20:4492022.
View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Zhang Z, Lu M, Chen C, Tong X, Li Y, Yang
K, Lv H, Xu J and Qin L: Holo-lactoferrin: The link between
ferroptosis and radiotherapy in triple-negative breast cancer.
Theranostics. 11:3167–3182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Tan H, Shen Z, Wang X, Shu S, Deng J, Lu
L, Fan Z, Hu D, Cheng P, Cao X and Huang Q: Endoplasmic
reticulum-targeted biomimetic nanoparticles induce apoptosis and
ferroptosis by regulating endoplasmic reticulum function in colon
cancer. J Control Release. 375:422–437. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
278
|
de la Harpe A, Beukes N and Frost C:
Mitochondrial calcium overload contributes to cannabinoid-induced
paraptosis in hormone-responsive breast cancer cells. Cell Prolif.
57:e136502024. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Qian Y, Ma S, Qiu R, Sun Z, Liu W, Wu F,
Lam SM, Xia Z, Wang K, Fang L, et al: Golgi protein ACBD3
downregulation sensitizes cells to ferroptosis. Cell Biol Int.
48:1559–1572. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao
H, Kang R, Wang X, Tang D and Dai E: Lipid storage and lipophagy
regulates ferroptosis. Biochem Biophys Res Commun. 508:997–1003.
2019. View Article : Google Scholar
|
|
281
|
Lee H, Horbath A, Kondiparthi L, Meena JK,
Lei G, Dasgupta S, Liu X, Zhuang L, Koppula P, Li M, et al: Cell
cycle arrest induces lipid droplet formation and confers
ferroptosis resistance. Nat Commun. 15:792024. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Wang Y, Song Y, Xu L, Zhou W, Wang W, Jin
Q, Xie Y, Zhang J, Liu J, Wu W, et al: A membrane-targeting
aggregation-induced emission probe for monitoring lipid droplet
dynamics in ischemia/reperfusion-induced cardiomyocyte ferroptosis.
Adv Sci (Weinh). 11:e23099072024. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Zhao J, Wang Q, Liu Z, Zhang M, Li J, Fu
ZF, Zhao L and Zhou M: Neuroinvasive virus facilitates viral
replication by employing lipid droplets to reduce arachidonic
acid-induced ferroptosis. J Biol Chem. 300:1071682024. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Ferrada L, Barahona MJ, Vera M, Stockwell
BR and Nualart F: Dehydroascorbic acid sensitizes cancer cells to
system xc− inhibition-induced ferroptosis by
promoting lipid droplet peroxidation. Cell Death Dis. 14:6372023.
View Article : Google Scholar
|
|
285
|
Wei D, Dai Y, Cao J and Fu N: A novel
fluorescent probe for visualizing viscosity changes in lipid
droplets during chemotherapy-induced ferroptosis. Anal Chim Acta.
1299:3424222024. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Silva MJSA, Zhang Y, Vinck R, Santos FMF,
António JPM, Gourdon-Grünewaldt L, Zaouter C, Castonguay A, Patten
SA, Cariou K, et al: BASHY dyes are highly efficient lipid
droplet-targeting photosensitizers that induce ferroptosis through
lipid peroxidation. Bioconjug Chem. 34:2337–2344. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
287
|
Swanda RV, Ji Q, Wu X, Yan J, Dong L, Mao
Y, Uematsu S, Dong Y and Qian SB: Lysosomal cystine governs
ferroptosis sensitivity in cancer via cysteine stress response. Mol
Cell. 83:3347–3359.e9. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Armenta DA, Laqtom NN, Alchemy G, Dong W,
Morrow D, Poltorack CD, Nathanson DA, Abu-Remalieh M and Dixon SJ:
Ferroptosis inhibition by lysosome-dependent catabolism of
extracellular protein. Cell Chem Biol. 29:1588–1600.e7. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Bhardwaj M, Lee JJ, Versace AM, Harper SL,
Goldman AR, Crissey MAS, Jain V, Singh MP, Vernon M, Aplin AE, et
al: Lysosomal lipid peroxidation regulates tumor immunity. J Clin
Invest. 133:e1645962023. View Article : Google Scholar : PubMed/NCBI
|
|
290
|
Mai TT, Hamaï A, Hienzsch A, Cañeque T,
Müller S, Wicinski J, Cabaud O, Leroy C, David A, Acevedo V, et al:
Salinomycin kills cancer stem cells by sequestering iron in
lysosomes. Nat Chem. 9:1025–1033. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
291
|
Wang ZX, Ma J, Li XY, Wu Y, Shi H, Chen Y,
Lu G, Shen HM, Lu GD and Zhou J: Quercetin induces p53-independent
cancer cell death through lysosome activation by the transcription
factor EB and reactive oxygen species-dependent ferroptosis. Br J
Pharmacol. 178:1133–1148. 2021. View Article : Google Scholar
|
|
292
|
Feng Y, Wei H, Lyu M, Yu Z, Chen J, Lyu X
and Zhuang F: Iron retardation in lysosomes protects senescent
cells from ferroptosis. Aging (Albany NY). 16:7683–7703.
2024.PubMed/NCBI
|
|
293
|
Feng J, Wang ZX, Bin JL, Chen YX, Ma J,
Deng JH, Huang XW, Zhou J and Lu GD: Pharmacological approaches for
targeting lysosomes to induce ferroptotic cell death in cancer.
Cancer Lett. 587:2167282024. View Article : Google Scholar : PubMed/NCBI
|
|
294
|
Ralhan I, Chang J, Moulton MJ, Goodman LD,
Lee NYJ, Plummer G, Pasolli HA, Matthies D, Bellen HJ and Ioannou
MS: Autolysosomal exocytosis of lipids protect neurons from
ferroptosis. J Cell Biol. 222:e2022071302023. View Article : Google Scholar : PubMed/NCBI
|
|
295
|
Wei X, Li Y, Chen H, Gao R, Ning P, Wang
Y, Huang W, Chen E, Fang L, Guo X, et al: A lysosome-targeted
magnetic nanotorquer mechanically triggers ferroptosis for breast
cancer treatment. Adv Sci (Weinh). 11:e23020932024. View Article : Google Scholar
|
|
296
|
Peng S, Chen G, Yu KN, Feng Y, Zhao L,
Yang M, Cao W, Almahi WAA, Sun M, Xu Y, Zhao Y, et al: Synergism of
non-thermal plasma and low concentration RSL3 triggers ferroptosis
via promoting xCT lysosomal degradation through ROS/AMPK/mTOR axis
in lung cancer cells. Cell Commun Signal. 22:1122024. View Article : Google Scholar : PubMed/NCBI
|
|
297
|
Liu Y, Pang Z, Wang Y, Liu J, Wang G and
Du J: Targeting PKD2 aggravates ferritinophagy-mediated ferroptosis
via promoting autophagosome-lysosome fusion and enhances efficacy
of carboplatin in lung adenocarcinoma. Chem Biol Interact.
387:1107942024. View Article : Google Scholar
|
|
298
|
Yang YH, Li W, Ren LW, Yang H, Zhang YZ,
Zhang S, Hao Y, Yu DK, Tong RS, Du GH, et al: S670, an amide
derivative of 3-O-acetyl-11-keto-β-boswellic acid, induces
ferroptosis in human glioblastoma cells by generating ROS and
inhibiting STX17-mediated fusion of autophagosome and lysosome.
Acta Pharmacol Sin. 45:209–222. 2024. View Article : Google Scholar
|
|
299
|
Li C, Sun S, Zhuang Y, Luo Z, Ji G and Liu
Z: CTSB nuclear translocation facilitates DNA damage and lysosomal
stress to promote retinoblastoma cell death. Mol Biotechnol.
66:2583–2594. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
300
|
Zhu X, Wang L, Wang K, Yao Y and Zhou F:
Erdafitinib promotes ferroptosis in human uveal melanoma by
inducing ferritinophagy and lysosome biogenesis via modulating the
FGFR1/mTORC1/TFEB signaling axis. Free Radic Biol Med. 222:552–568.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
301
|
Chen Y, Yang Z, Wang S, Ma Q, Li L, Wu X,
Guo Q, Tao L and Shen X: Boosting ROS-mediated lysosomal membrane
permeabilization for cancer ferroptosis therapy. Adv Healthc Mater.
12:e22021502023. View Article : Google Scholar
|
|
302
|
An S and Hu M: Quercetin promotes TFEB
nuclear translocation and activates lysosomal degradation of
ferritin to induce ferroptosis in breast cancer cells. Comput
Intell Neurosci. 2022:52992182022. View Article : Google Scholar : PubMed/NCBI
|
|
303
|
You P, Lu F, Ouyang C, Yu J,
González-García J, Song J, Ni W, Wang J, Yin C and Zhou CQ: Acidic
lysosome-anchoring croconium-based nanoplatform for enhanced
triple-mode bioimaging and Fe3+-triggered tumor
synergistic therapy. ACS Appl Mater Interfaces. 16:46066–46078.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
304
|
Zhang Z, Xiang J, Guan L, Chen P, Li C,
Guo C, Hu Y, Huang S, Cai L and Gong P: Inducing tumor ferroptosis
via a pH-responsive NIR-II photothermal agent initiating lysosomal
dysfunction. Nanoscale. 15:19074–19078. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
305
|
Liang Q, Yu F, Cai H, Wu X, Ma M, Li Z,
Tedesco AC, Zhu J, Xu Q and Bi H: Photo-activated
autophagy-associated tumour cell death by lysosome impairment based
on manganese-doped graphene quantum dots. J Mater Chem B.
11:2466–2477. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
306
|
Turcu AL, Versini A, Khene N, Gaillet C,
Cañeque T, Müller S and Rodriguez R: DMT1 inhibitors kill cancer
stem cells by blocking lysosomal iron translocation. Chemistry.
26:7369–7373. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
307
|
Torii S, Shintoku R, Kubota C, Yaegashi M,
Torii R, Sasaki M, Suzuki T, Mori M, Yoshimoto Y, Takeuchi T and
Yamada K: An essential role for functional lysosomes in ferroptosis
of cancer cells. Biochem J. 473:769–777. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
308
|
Li W, Yin S, Shen Y, Li H, Yuan L and
Zhang XB: Molecular engineering of pH-responsive NIR oxazine
assemblies for evoking tumor ferroptosis via triggering lysosomal
dysfunction. J Am Chem Soc. 145:3736–3747. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
309
|
Jiang L, Zheng H, Lyu Q, Hayashi S, Sato
K, Sekido Y, Nakamura K, Tanaka H, Ishikawa K, Kajiyama H, et al:
Lysosomal nitric oxide determines transition from autophagy to
ferroptosis after exposure to plasma-activated Ringer's lactate.
Redox Biol. 43:1019892021. View Article : Google Scholar : PubMed/NCBI
|
|
310
|
Lan Y, Zhang K, Wang F, Zhang Y, Yan M and
Zuo Y: Polysiloxane-based hyperbranched fluorescent probe for
dynamic visualization of HClO in lysosomes and vivo. Spectrochim
Acta A Mol Biomol Spectrosc. 294:1225272023. View Article : Google Scholar : PubMed/NCBI
|
|
311
|
Sun J, Cheng X, Pan S, Wang L, Dou W, Liu
J and Shi X: Dichloroacetate attenuates the stemness of colorectal
cancer cells via trigerring ferroptosis through sequestering iron
in lysosomes. Environ Toxicol. 36:520–529. 2021. View Article : Google Scholar
|
|
312
|
Hirata Y, Oka K, Yamamoto S, Watanabe H,
Oh-Hashi K, Hirayama T, Nagasawa H, Takemori H and Furuta K:
Haloperidol prevents oxytosis/ferroptosis by targeting lysosomal
ferrous ions in a manner independent of dopamine D2 and sigma-1
receptors. ACS Chem Neurosci. 13:2719–2727. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
313
|
Fang X, Wang H, Han D, Xie E, Yang X, Wei
J, Gu S, Gao F, Zhu N, Yin X, et al: Ferroptosis as a target for
protection against cardiomyopathy. Proc Natl Acad Sci USA.
116:2672–2680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
314
|
Kalkavan H, Chen MJ, Crawford JC, Quarato
G, Fitzgerald P, Tait SWG, Goding CR and Green DR: Sublethal
cytochrome c release generates drug-tolerant persister cells. Cell.
185:3356–3374.e22. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
315
|
Wei S, Qiu T, Yao X, Wang N, Jiang L, Jia
X, Tao Y, Wang Z, Pei P, Zhang J, et al: Arsenic induces pancreatic
dysfunction and ferroptosis via mitochondrial
ROS-autophagy-lysosomal pathway. J Hazard Mater. 384:1213902020.
View Article : Google Scholar
|
|
316
|
Ahola S, Rivera Mejías P, Hermans S,
Chandragiri S, Giavalisco P, Nolte H and Langer T: OMA1-mediated
integrated stress response protects against ferroptosis in
mitochondrial cardiomyopathy. Cell Metab. 34:1875–1891.e7. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
317
|
Sun L, Wang H, Yu S, Zhang L, Jiang J and
Zhou Q: Herceptin induces ferroptosis and mitochondrial dysfunction
in H9c2 cells. Int J Mol Med. 49:172022. View Article : Google Scholar :
|
|
318
|
Yao S, Pang M, Wang Y, Wang X, Lin Y, Lv
Y, Xie Z, Hou J, Du C, Qiu Y, et al: Mesenchymal stem cell
attenuates spinal cord injury by inhibiting mitochondrial quality
control-associated neuronal ferroptosis. Redox Biol. 67:1028712023.
View Article : Google Scholar : PubMed/NCBI
|
|
319
|
Zhang Z, Zhou H, Gu W, Wei Y, Mou S, Wang
Y, Zhang J and Zhong Q: CGI1746 targets σ1R to modulate
ferroptosis through mitochondria-associated membranes. Nat Chem
Biol. 20:699–709. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
320
|
Park MW, Cha HW, Kim J, Kim JH, Yang H,
Yoon S, Boonpraman N, Yi SS, Yoo ID and Moon JS: NOX4 promotes
ferroptosis of astrocytes by oxidative stress-induced lipid
peroxidation via the impairment of mitochondrial metabolism in
Alzheimer's diseases. Redox Biol. 41:1019472021. View Article : Google Scholar : PubMed/NCBI
|
|
321
|
Liang FG, Zandkarimi F, Lee J, Axelrod JL,
Pekson R, Yoon Y, Stockwell BR and Kitsis RN: OPA1 promotes
ferroptosis by augmenting mitochondrial ROS and suppressing an
integrated stress response. Mol Cell. 84:3098–3114.e6. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
322
|
Akiyama H, Zhao R, Ostermann LB, Li Z,
Tcheng M, Yazdani SJ, Moayed A, Pryor ML II, Slngh S, Baran N, et
al: Mitochondrial regulation of GPX4 inhibition-mediated
ferroptosis in acute myeloid leukemia. Leukemia. 38:729–740. 2024.
View Article : Google Scholar
|
|
323
|
Deshwal S, Onishi M, Tatsuta T, Bartsch T,
Cors E, Ried K, Lemke K, Nolte H, Giavalisco P and Langer T:
Mitochondria regulate intracellular coenzyme Q transport and
ferroptotic resistance via STARD7. Nat Cell Biol. 25:246–257.
2023.PubMed/NCBI
|
|
324
|
Jang S, Chapa-Dubocq XR, Tyurina YY, St
Croix CM, Kapralov AA, Tyurin VA, Bayır H, Kagan VE and Javadov S:
Elucidating the contribution of mitochondrial glutathione to
ferroptosis in cardiomyocytes. Redox Biol. 45:1020212021.
View Article : Google Scholar : PubMed/NCBI
|
|
325
|
Wang P, Cui Y, Ren Q, Yan B, Zhao Y, Yu P,
Gao G, Shi H, Chang S and Chang YZ: Mitochondrial ferritin
attenuates cerebral ischaemia/reperfusion injury by inhibiting
ferroptosis. Cell Death Dis. 12:4472021. View Article : Google Scholar : PubMed/NCBI
|
|
326
|
Chen T, Majerníková N, Marmolejo-Garza A,
Trombetta-Lima M, Sabogal-Guáqueta AM, Zhang Y, Ten Kate R, Zuidema
M, Mulder PPMFA, den Dunnen W, et al: Mitochondrial transplantation
rescues neuronal cells from ferroptosis. Free Radic Biol Med.
208:62–72. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
327
|
Wu S, Mao C, Kondiparthi L, Poyurovsky MV,
Olszewski K and Gan B: A ferroptosis defense mechanism mediated by
glycerol-3-phosphate dehydrogenase 2 in mitochondria. Proc Natl
Acad Sci USA. 119:e21219871192022. View Article : Google Scholar : PubMed/NCBI
|
|
328
|
Chen Y, Li S, Yin M, Li Y, Chen C, Zhang
J, Sun K, Kong X, Chen Z and Qian J: Isorhapontigenin attenuates
cardiac microvascular injury in diabetes via the inhibition of
mitochondria-associated ferroptosis through PRDX2-MFN2-ACSL4
pathways. Diabetes. 72:389–404. 2023. View Article : Google Scholar
|
|
329
|
Li MD, Fu L, Lv BB, Xiang Y, Xiang HX, Xu
DX and Zhao H: Arsenic induces ferroptosis and acute lung injury
through mtROS-mediated mitochondria-associated endoplasmic
reticulum membrane dysfunction. Ecotoxicol Environ Saf.
238:1135952022. View Article : Google Scholar : PubMed/NCBI
|
|
330
|
Chen J, Fu CY, Shen G, Wang J, Xu L, Li H,
Cao X, Zheng MZ, Shen YL, Zhong J, et al: Macrophages induce
cardiomyocyte ferroptosis via mitochondrial transfer. Free Radic
Biol Med. 190:1–14. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
331
|
Guo J, Wang S, Wan X, Liu X, Wang Z, Liang
C, Zhang Z, Wang Y, Yan M, Wu P, et al: Mitochondria-derived
methylmalonic acid aggravates ischemia-reperfusion injury by
activating reactive oxygen species-dependent ferroptosis. Cell
Commun Signal. 22:532024. View Article : Google Scholar : PubMed/NCBI
|
|
332
|
Li Z, Zhao B, Zhang Y, Fan W, Xue Q, Chen
X, Wang J and Qi X: Mitochondria-mediated ferroptosis contributes
to the inflammatory responses of bovine viral diarrhea virus (BVDV)
in vitro. J Virol. 98:e01880232024. View Article : Google Scholar : PubMed/NCBI
|
|
333
|
Tan Q, Zhang X, Li S, Liu W, Yan J, Wang
S, Cui F, Li D and Li J: DMT1 differentially regulates
mitochondrial complex activities to reduce glutathione loss and
mitigate ferroptosis. Free Radic Biol Med. 207:32–44. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
334
|
Li Y, Zhou Y, Liu D, Wang Z, Qiu J, Zhang
J, Chen P, Zeng G, Guo Y, Wang X, et al: Glutathione Peroxidase 3
induced mitochondria-mediated apoptosis via AMPK/ERK1/2 pathway and
resisted autophagy-related ferroptosis via AMPK/mTOR pathway in
hyperplastic prostate. J Transl Med. 21:5752023. View Article : Google Scholar
|
|
335
|
Xiao Q, Yan L, Han J, Yang S, Tang Y, Li
Q, Lao X, Chen Z, Xiao J, Zhao H, et al: Metabolism-dependent
ferroptosis promotes mitochondrial dysfunction and inflammation in
CD4+ T lymphocytes in HIV-infected immune
non-responders. EBioMedicine. 86:1043822022. View Article : Google Scholar
|
|
336
|
Wu T, Liang X, Liu X, Li Y, Wang Y, Kong L
and Tang M: Induction of ferroptosis in response to graphene
quantum dots through mitochondrial oxidative stress in microglia.
Part Fibre Toxicol. 17:302020. View Article : Google Scholar : PubMed/NCBI
|
|
337
|
Sun L, Wang H, Xu D, Yu S, Zhang L and Li
X: Lapatinib induces mitochondrial dysfunction to enhance oxidative
stress and ferroptosis in doxorubicin-induced cardiomyocytes via
inhibition of PI3K/AKT signaling pathway. Bioengineered. 13:48–60.
2022. View Article : Google Scholar :
|
|
338
|
Qian B, Che L, Du ZB, Guo NJ, Wu XM, Yang
L, Zheng ZX, Gao YL, Wang MZ, Chen XX, et al: Protein phosphatase
2A-B55β mediated mitochondrial p-GPX4 dephosphorylation promoted
sorafenib-induced ferroptosis in hepatocellular carcinoma via
regulating p53 retrograde signaling. Theranostics. 13:4288–4302.
2023. View Article : Google Scholar :
|
|
339
|
Zeng Y, He Y and Wang L, Xu H, Zhang Q,
Wang Y, Zhang J and Wang L: Dihydroquercetin improves experimental
acute liver failure by targeting ferroptosis and
mitochondria-mediated apoptosis through the SIRT1/p53 axis.
Phytomedicine. 128:1555332024. View Article : Google Scholar : PubMed/NCBI
|
|
340
|
Yang H, Li Q, Chen X, Weng M, Huang Y,
Chen Q, Liu X, Huang H, Feng Y, Zhou H, et al: Targeting SOX13
inhibits assembly of respiratory chain supercomplexes to overcome
ferroptosis resistance in gastric cancer. Nat Commun. 15:42962024.
View Article : Google Scholar : PubMed/NCBI
|
|
341
|
Du C, Liu C, Yu K, Zhang S, Fu Z, Chen X,
Liao W, Chen J, Zhang Y, Wang X, et al: Mitochondrial serine
catabolism safeguards maintenance of the hematopoietic stem cell
pool in homeostasis and injury. Cell Stem Cell. 31:1484–1500.e9.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
342
|
Ding Q, Tang W, Li X, Ding Y, Chen X, Cao
W, Wang X, Mo W, Su Z, Zhang Q and Guo H: Mitochondrial-targeted
brequinar liposome boosted mitochondrial-related ferroptosis for
promoting checkpoint blockade immunotherapy in bladder cancer. J
Control Release. 363:221–234. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
343
|
Reichardt C, Brandt S, Bernhardt A, Krause
A, Lindquist JA, Weinert S, Geffers R, Franz T, Kahlfuss S, Dudeck
A, et al: DNA-binding protein-A promotes kidney
ischemia/reperfusion injury and participates in mitochondrial
function. Kidney Int. 106:241–257. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
344
|
Wu X, Pan J, Yu JJ, Kang J, Hou S, Cheng
M, Xu L, Gong L and Li Y: DiDang decoction improves mitochondrial
function and lipid metabolism via the HIF-1 signaling pathway to
treat atherosclerosis and hyperlipidemia. J Ethnopharmacol.
308:1162892023. View Article : Google Scholar : PubMed/NCBI
|
|
345
|
Dong S, Zhang M, Cheng Z, Zhang X, Liang
W, Li S, Li L, Xu Q, Song S, Liu Z, et al: Redistribution of
defective mitochondria-mediated dihydroorotate dehydrogenase
imparts 5-fluorouracil resistance in colorectal cancer. Redox Biol.
73:1032072024. View Article : Google Scholar : PubMed/NCBI
|
|
346
|
Guo YY, Liang NN, Zhang XY, Ren YH, Wu WZ,
Liu ZB, He YZ, Zhang YH, Huang YC, Zhang T, et al: Mitochondrial
GPX4 acetylation is involved in cadmium-induced renal cell
ferroptosis. Redox Biol. 73:1031792024. View Article : Google Scholar : PubMed/NCBI
|
|
347
|
Yang M, Chen X, Cheng C, Yan W, Guo R,
Wang Y, Zhang H, Chai J, Cheng Y and Zhang F: Cucurbitacin B
induces ferroptosis in oral leukoplakia via the
SLC7A11/mitochondrial oxidative stress pathway. Phytomedicine.
129:1555482024. View Article : Google Scholar : PubMed/NCBI
|
|
348
|
Lu C, Zhang Z, Fan Y, Wang X, Qian J and
Bian Z: Shikonin induces ferroptosis in osteosarcomas through the
mitochondrial ROS-regulated HIF-1α/HO-1 axis. Phytomedicine.
135:1561392024. View Article : Google Scholar
|
|
349
|
Hai Y, Fan R, Zhao T, Lin R, Zhuang J,
Deng A, Meng S, Hou Z and Wei G: A novel mitochondria-targeting
DHODH inhibitor induces robust ferroptosis and alleviates immune
suppression. Pharmacol Res. 202:1071152024. View Article : Google Scholar : PubMed/NCBI
|
|
350
|
Yang B, Xu Y, Yu J, Wang Q, Fan Q, Zhao X,
Qiao Y, Zhang Z, Zhou Q, Yin D, et al: Salidroside pretreatment
alleviates ferroptosis induced by myocardial ischemia/reperfusion
through mitochondrial superoxide-dependent AMPKα2 activation.
Phytomedicine. 128:1553652024. View Article : Google Scholar
|
|
351
|
Lv T, Xiong X, Yan W, Liu M, Xu H and He
Q: Mitochondrial general control of amino acid synthesis 5 like 1
promotes nonalcoholic steatohepatitis development through
ferroptosis-induced formation of neutrophil extracellular traps.
Clin Transl Med. 13:e13252023. View Article : Google Scholar : PubMed/NCBI
|
|
352
|
Qi Y, Chen L, Ding S, Shen X, Wang Z, Qi H
and Yang S: Neutrophil extracellular trap-induced ferroptosis
promotes abdominal aortic aneurysm formation via SLC25A11-mediated
depletion of mitochondrial glutathione. Free Radic Biol Med.
221:215–224. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
353
|
Zhou Q, Dian Y, He Y, Yao L, Su H, Meng Y,
Sun Y, Li D, Xiong Y, Zeng F, et al: Propafenone facilitates
mitochondrial-associated ferroptosis and synergizes with
immunotherapy in melanoma. J Immunother Cancer. 12:e0098052024.
View Article : Google Scholar : PubMed/NCBI
|
|
354
|
Tang J, Zhu J, Xie H, Song L, Xu G, Li W,
Cai L and Han XX: Mitochondria-specific molecular crosstalk between
ferroptosis and apoptosis revealed by in situ raman spectroscopy.
Nano Lett. 24:2384–2391. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
355
|
Zhang X, Zhang M, Zhang Z and Zhou S:
Salidroside induces mitochondrial dysfunction and ferroptosis to
inhibit melanoma progression through reactive oxygen species
production. Exp Cell Res. 438:1140342024. View Article : Google Scholar : PubMed/NCBI
|
|
356
|
Yang C, Yang X, Harrington A, Potts C,
Kaija A, Ryzhova L and Liaw L: Notch signaling regulates mouse
perivascular adipose tissue function via mitochondrial pathways.
Genes (Basel). 14:19642023. View Article : Google Scholar : PubMed/NCBI
|
|
357
|
Hirata Y, Yamada Y, Taguchi S, Kojima R,
Masumoto H, Kimura S, Niijima T, Toyama T, Kise R, Sato E, et al:
Conjugated fatty acids drive ferroptosis through chaperone-mediated
autophagic degradation of GPX4 by targeting mitochondria. Cell
Death Dis. 15:8842024. View Article : Google Scholar : PubMed/NCBI
|
|
358
|
Chen H, Nie P, Li J, Wu Y, Yao B, Yang Y,
Lash GE and Li P: Cyclophosphamide induces ovarian granulosa cell
ferroptosis via a mechanism associated with HO-1 and ROS-mediated
mitochondrial dysfunction. J Ovarian Res. 17:1072024. View Article : Google Scholar : PubMed/NCBI
|
|
359
|
Jiang J, Zhou X, Chen H, Wang X, Ruan Y,
Liu X and Ma J: 18β-Glycyrrhetinic acid protects against
deoxynivalenol-induced liver injury via modulating ferritinophagy
and mitochondrial quality control. J Hazard Mater. 471:1343192024.
View Article : Google Scholar
|
|
360
|
Wang X, Wei T, Luo J, Lang K, Song Y, Ning
X, Chao Y, Gu Z, Wang L, Chen C, et al: Iron overload-dependent
ferroptosis aggravates LPS-induced acute lung injury by impairing
mitochondrial function. Inflammation. 47:2013–2026. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
361
|
Liang NN, Guo YY, Zhang XY, Ren YH, He YZ,
Liu ZB, Xu DX and Xu S: Mitochondrial dysfunction-evoked DHODH
acetylation is involved in renal cell ferroptosis during
cisplatin-induced acute kidney injury. Adv Sci (Weinh).
11:e24047532024. View Article : Google Scholar : PubMed/NCBI
|
|
362
|
Tang S, Fuß A, Fattahi Z and Culmsee C:
Drp1 depletion protects against ferroptotic cell death by
preserving mitochondrial integrity and redox homeostasis. Cell
Death Dis. 15:6262024. View Article : Google Scholar : PubMed/NCBI
|
|
363
|
Hu H, Zhang F, Sheng Z, Tian S, Li G, Tang
S, Niu Y, Yang J and Liu Y: Synthesis and mitochondria-localized
iridium (III) complexes induce cell death through pyroptosis and
ferroptosis pathways. Eur J Med Chem. 268:1162952024. View Article : Google Scholar : PubMed/NCBI
|
|
364
|
Song X, Hao X and Zhu BT: Role of
mitochondrial reactive oxygen species in chemically-induced
ferroptosis. Free Radic Biol Med. 223:473–492. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
365
|
Lin YS, Tsai YC, Li CJ, Wei TT, Wang JL,
Lin BW, Wu YN, Wu SR and Lin SC and Lin SC: Overexpression of
NUDT16L1 sustains proper function of mitochondria and leads to
ferroptosis insensitivity in colorectal cancer. Redox Biol.
77:1033582024. View Article : Google Scholar : PubMed/NCBI
|
|
366
|
Deng J, Zhuang H, Shao S, Zeng X, Xue P,
Bai T, Wang X, Shangguan S, Chen Y, Yan S and Huang W:
Mitochondrial-targeted copper delivery for cuproptosis-based
synergistic cancer therapy. Adv Healthc Mater. 13:e23045222024.
View Article : Google Scholar : PubMed/NCBI
|
|
367
|
Lyamzaev KG, Panteleeva AA, Simonyan RA,
Avetisyan AV and Chernyak BV: Mitochondrial lipid peroxidation is
responsible for ferroptosis. Cells. 12:6112023. View Article : Google Scholar : PubMed/NCBI
|
|
368
|
Zhong S, Chen W, Wang B, Gao C, Liu X,
Song Y, Qi H, Liu H, Wu T, Wang R and Chen B: Energy stress
modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated
ferroptosis. Redox Biol. 63:1027602023. View Article : Google Scholar : PubMed/NCBI
|
|
369
|
Li C, Liu J, Hou W, Kang R and Tang D:
STING1 promotes ferroptosis through MFN1/2-dependent mitochondrial
fusion. Front Cell Dev Biol. 9:6986792021. View Article : Google Scholar : PubMed/NCBI
|
|
370
|
Wang X, Chen J, Tie H, Tian W, Zhao Y, Qin
L, Guo S, Li Q and Bao C: Eriodictyol regulated ferroptosis,
mitochondrial dysfunction, and cell viability via Nrf2/HO-1/NQO1
signaling pathway in ovarian cancer cells. J Biochem Mol Toxicol.
37:e233682023. View Article : Google Scholar : PubMed/NCBI
|
|
371
|
Yamashita SI, Sugiura Y, Matsuoka Y, Maeda
R, Inoue K, Furukawa K, Fukuda T, Chan DC and Kanki T: Mitophagy
mediated by BNIP3 and NIX protects against ferroptosis by
downregulating mitochondrial reactive oxygen species. Cell Death
Differ. 31:651–661. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
372
|
Ward NP, Yoon SJ, Flynn T, Sherwood AM,
Olley MA, Madej J and DeNicola GM: Mitochondrial respiratory
function is preserved under cysteine starvation via glutathione
catabolism in NSCLC. Nat Commun. 15:42442024. View Article : Google Scholar : PubMed/NCBI
|
|
373
|
Zheng J, Wang Q, Chen J, Cai G, Zhang Z,
Zou H, Zou JX, Liu Q, Ji S, Shao G, et al: Tumor mitochondrial
oxidative phosphorylation stimulated by the nuclear receptor RORγ
represents an effective therapeutic opportunity in osteosarcoma.
Cell Rep Med. 5:1015192024. View Article : Google Scholar
|
|
374
|
Luo Y, Zhang Y, Pang S, Min J, Wang T, Wu
D, Lin C, Xiao Z, Xiang Q, Li Q and Ma L: PCBP1 protects bladder
cancer cells from mitochondria injury and ferroptosis by inducing
LACTB mRNA degradation. Mol Carcinog. 62:907–919. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
375
|
Wang Z, Tang S, Cai L, Wang Q, Pan D, Dong
Y, Zhou H, Li J, Ji N, Zeng X, et al: DRP1 inhibition-mediated
mitochondrial elongation abolishes cancer stemness, enhances
glutaminolysis, and drives ferroptosis in oral squamous cell
carcinoma. Br J Cancer. 130:1744–1757. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
376
|
Kumar A, Ye C, Nkansah A, Decoville T,
Fogo GM, Sajjakulnukit P, Reynolds MB, Zhang L, Quaye O, Seo YA, et
al: Iron regulates the quiescence of naive CD4 T cells by
controlling mitochondria and cellular metabolism. Proc Natl Acad
Sci USA. 121:e23184201212024. View Article : Google Scholar : PubMed/NCBI
|
|
377
|
Wang W, Xu X, Zhao L, Ye K, Wang S and Lin
C: 3,5-diCQA suppresses colorectal cancer cell growth through
ROS/AMPK/mTOR mediated mitochondrial dysfunction and ferroptosis.
Cell Cycle. 22:1951–1968. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
378
|
Feng Y, Xu J, Shi M, Liu R, Zhao L, Chen
X, Li M, Zhao Y, Chen J, Du W and Liu P: COX7A1 enhances the
sensitivity of human NSCLC cells to cystine deprivation-induced
ferroptosis via regulating mitochondrial metabolism. Cell Death
Dis. 13:9882022. View Article : Google Scholar : PubMed/NCBI
|
|
379
|
Wang J, Zhuang H, Yang X, Guo Z, Zhou K,
Liu N, An Y, Chen Y, Zhang Z, Wang M, et al: Exploring the
mechanism of ferroptosis induction by sappanone A in cancer:
Insights into the mitochondrial dysfunction mediated by
NRF2/xCT/GPX4 axis. Int J Biol Sci. 20:5145–5161. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
380
|
Li H, Guo Y, Su W, Zhang H, Wei X, Ma X,
Gong S, Qu G, Zhang L, Xu H, et al: The mitochondria-targeted
antioxidant MitoQ ameliorates inorganic arsenic-induced
DCs/Th1/Th2/Th17/Treg differentiation partially by activating
PINK1-mediated mitophagy in murine liver. Ecotoxicol Environ Saf.
277:1163502024. View Article : Google Scholar : PubMed/NCBI
|
|
381
|
Lin J, Ou H, Luo B, Ling M, Lin F, Cen L,
Hu Z, Ye L and Pan L: Capsaicin mitigates ventilator-induced lung
injury by suppressing ferroptosis and maintaining mitochondrial
redox homeostasis through SIRT3-dependent mechanisms. Mol Med.
30:1482024. View Article : Google Scholar : PubMed/NCBI
|
|
382
|
Vaishampayan P and Lee Y: Redox-active
vitamin C suppresses human osteosarcoma growth by triggering
intracellular ROS-iron-calcium signaling crosstalk and
mitochondrial dysfunction. Redox Biol. 75:1032882024. View Article : Google Scholar : PubMed/NCBI
|
|
383
|
Fu X, Qu L, Xu H and Xie J: Ndfip1
protected dopaminergic neurons via regulating mitochondrial
function and ferroptosis in Parkinson's disease. Exp Neurol.
375:1147242024. View Article : Google Scholar : PubMed/NCBI
|
|
384
|
He M, Cai Y, Yuan Z, Zhang L and Lu H:
Upregulation of SOCS2 causes mitochondrial dysfunction and promotes
ferroptosis in pancreatic cancer cells. Acta Biochim Pol.
70:163–168. 2023.PubMed/NCBI
|
|
385
|
Zhu ZH, Xu XT, Shen CJ, Yuan JT, Lou SY,
Ma XL, Chen X, Yang B and Zhao HJ: A novel sesquiterpene lactone
fraction from Eupatorium chinense L. suppresses hepatocellular
carcinoma growth by triggering ferritinophagy and mitochondrial
damage. Phytomedicine. 112:1546712023. View Article : Google Scholar : PubMed/NCBI
|
|
386
|
Neitemeier S, Jelinek A, Laino V, Hoffmann
L, Eisenbach I, Eying R, Ganjam GK, Dolga AM, Oppermann S and
Culmsee C: BID links ferroptosis to mitochondrial cell death
pathways. Redox Biol. 12:558–570. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
387
|
Mu M, Chen B, Li H, Fan R, Yang Y, Zhou L,
Han B, Zou B, Chen N and Guo G: Augmented the sensitivity of
photothermal-ferroptosis therapy in triple-negative breast cancer
through mitochondria-targeted nanoreactor. J Control Release.
375:733–744. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
388
|
Geroyska S, Mejia I, Chan AA, Navarrete M,
Pandey V, Kharpatin S, Noguti J, Wang F, Srole D, Chou TF, et al:
N-Myristoytransferase inhibition causes mitochondrial iron overload
and parthanatos in TIM17A-dependent aggressive lung carcinoma.
Cancer Res Commun. 4:1815–1833. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
389
|
Li LG, Zhang D, Huang Q, Yan M, Chen NN,
Yang Y, Xiao RC, Liu H, Han N, Qureshi AM, et al: Mitochondrial
disruption resulting from Cepharanthine-mediated TOM inhibition
triggers ferroptosis in colorectal cancer cells. J Cancer Res Clin
Oncol. 150:4602024. View Article : Google Scholar : PubMed/NCBI
|
|
390
|
Vu NT, Kim M, Stephenson DJ, MacKnight HP
and Chalfant CE: Ceramide kinase inhibition drives ferroptosis and
sensitivity to cisplatin in mutant KRAS lung cancer by
dysregulating VDAC-mediated mitochondria function. Mol Cancer Res.
20:1429–1442. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
391
|
Shen J, He Y, Zhou B, Qin H, Zhang S,
Huang Z and Zhang X: TFAP2C/FLT3 axis reduces ferroptosis in breast
cancer cells by inhibiting mitochondrial autophagy. Int J Biochem
Cell Biol. 177:1066912024. View Article : Google Scholar : PubMed/NCBI
|
|
392
|
He LW, Lin CJ, Zhuang LJ, Sun YH, Li YC
and Ye ZY: Targeting hepatocellular carcinoma: Schisandrin A
triggers mitochondrial disruption and ferroptosis. Chem Biol Drug
Des. 104:e700102024. View Article : Google Scholar : PubMed/NCBI
|
|
393
|
Sun X, Sun P, Zhen D, Xu X, Yang L, Fu D,
Wei C, Niu X, Tian J and Li H: Melatonin alleviates
doxorubicin-induced mitochondrial oxidative damage and ferroptosis
in cardiomyocytes by regulating YAP expression. Toxicol Appl
Pharmacol. 437:1159022022. View Article : Google Scholar : PubMed/NCBI
|
|
394
|
Zhao B, Xu X, Wen X, Liu Q, Dong C, Yang
Q, Fan C, Yoon J and Lu Z: Ratiometric near-infrared fluorescent
probe monitors ferroptosis in HCC Cells by imaging HClO in
mitochondria. Anal Chem. 96:5992–6000. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
395
|
Lei L, Dong Z, Xu L, Yang F, Yin B, Wang
Y, Yue R, Guan G, Xu J, Song G and Zhang XB: Metal-fluorouracil
networks with disruption of mitochondrion enhanced ferroptosis for
synergistic immune activation. Theranostics. 12:6207–6222. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
396
|
Yu H, Li JM, Deng K, Zhou W, Li KH, Wang
CX, Wang Q, Wu M and Huang SW: GPX4 inhibition synergistically
boosts mitochondria targeting nanoartemisinin-induced
apoptosis/ferroptosis combination cancer therapy. Biomater Sci.
11:5831–5845. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
397
|
Fang H, Chen Y, Geng S, Yao S, Guo Z and
He W: Super-resolution imaging of mitochondrial HClO during cell
ferroptosis using a near-infrared fluorescent probe. Anal Chem.
94:17904–17912. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
398
|
Negi M, Kaushik N, Lamichhane P, Patel P,
Jaiswal A, Choi EH and Kaushik NK: Nitric oxide water-driven
immunogenic cell death: Unfolding mitochondrial dysfunction's role
in sensitizing lung adenocarcinoma to ferroptosis and autophagic
cell death. Free Radic Biol Med. 222:1–15. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
399
|
Ye RR, Chen BC, Lu JJ, Ma XR and Li RT:
Phosphorescent rhenium(I) complexes conjugated with artesunate:
Mitochondrial targeting and apoptosis-ferroptosis dual induction. J
Inorg Biochem. 223:1115372021. View Article : Google Scholar : PubMed/NCBI
|
|
400
|
Guo L: Mitochondrial permeability
transition mediated by MTCH2 and F-ATP synthase contributes to
ferroptosis defense. FEBS Lett. 599:352–366. 2025. View Article : Google Scholar
|
|
401
|
Liang Z, Zhang N, Wang X, Zhang J, Li K
and Lei T: Epothilone B inactivation of Sirtuin1 promotes
mitochondrial reactive oxygen species to induce dysfunction and
ferroptosis of Schwann cells. Eur J Pharm Sci. 181:1063502023.
View Article : Google Scholar
|
|
402
|
Yang F, Yu W, Yu Q, Liu X, Liu C, Lu C,
Liao X, Liu Y and Peng N: Mitochondria-targeted nanosystem with
reactive oxygen species-controlled release of CO to enhance
photodynamic therapy of PCN-224 by sensitizing ferroptosis. Small.
19:e22061242023. View Article : Google Scholar : PubMed/NCBI
|
|
403
|
Huang Y, Liu G, Zheng F, Chen J, Lin Y,
Wang J, Huang Y and Peng Y: Asymmetric silicon phthalocyanine based
nanoparticle with spatiotemporally targeting of mitochondria for
synergistic apoptosis-ferroptosis antitumor treatment. Colloids
Surf B Biointerfaces. 238:1138902024. View Article : Google Scholar : PubMed/NCBI
|
|
404
|
Wang J, Zhao Z, Liu Y, Cao X, Li F, Ran H,
Cao Y and Wu C: 'Mito-Bomb': A novel mitochondria-targeting
nanosystem for ferroptosis-boosted sonodynamic antitumor therapy.
Drug Deliv. 29:3111–3122. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
405
|
Yin Y, Chen S, Li H, Pang X, Wang C, Wang
L, Liu P, Xu S and Luo X: Exogenous and endogenous dual-activated
nanoladder for precise imaging of mitochondrial ferroptosis-related
inhibition miRNA with tumor cell specificity. Anal Chem.
96:7550–7557. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
406
|
Lee YS, Kalimuthu K, Park YS, Luo X,
Choudry MHA, Bartlett DL and Lee YJ: BAX-dependent mitochondrial
pathway mediates the crosstalk between ferroptosis and apoptosis.
Apoptosis. 25:625–631. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
407
|
Nikoo A, Roudkenar MH, Sato T, Kuwahara Y,
Tomita K, Pourmohammadi-Bejarpasi Z, Najafi-Ghalehlou N and
Roushandeh AM: Mitochondrial transfer in PC-3 cells fingerprinted
in ferroptosis sensitivity: A brand new approach targeting cancer
metabolism. Hum Cell. 36:1441–1450. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
408
|
Tian L, Ji H, Wang W, Han X, Zhang X, Li
X, Guo L, Huang L and Gao W: Mitochondria-targeted pentacyclic
triterpenoid carbon dots for selective cancer cell destruction via
inducing autophagy, apoptosis, as well as ferroptosis. Bioorg Chem.
130:1062592023. View Article : Google Scholar
|
|
409
|
Xia T, Xia Z, Tang P, Fan J and Peng X:
Light-driven mitochondrion-to-nucleus DNA cascade fluorescence
imaging and enhanced cancer cell photoablation. J Am Chem Soc.
146:12941–12949. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
410
|
Asperti M, Bellini S, Grillo E, Gryzik M,
Cantamessa L, Ronca R, Maccarinelli F, Salvi A, De Petro G, Arosio
P, et al: H-ferritin suppression and pronounced mitochondrial
respiration make Hepatocellular carcinoma cells sensitive to
RSL3-induced ferroptosis. Free Radic Biol Med. 169:294–303. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
411
|
Tan M, Duan J, Chen S, Chen Y, Wang J, Xu
X and Ke F: Construction of a mitochondria-targeted probe to
monitor cysteine levels in cancer cells and zebrafish. Photochem
Photobiol Sci. 23:1425–1434. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
412
|
Xie Y, Zhou X, Li J, Yao XC, Liu WL, Kang
FH, Zou ZX, Xu KP, Xu PS and Tan GS: Identification of a new
natural biflavonoids against breast cancer cells induced
ferroptosis via the mitochondrial pathway. Bioorg Chem.
109:1047442021. View Article : Google Scholar : PubMed/NCBI
|
|
413
|
Karmi O, Sohn YS, Zandalinas SI, Rowland
L, King SD, Nechushtai R and Mittler R: Disrupting CISD2 function
in cancer cells primarily impacts mitochondrial labile iron levels
and triggers TXNIP expression. Free Radic Biol Med. 176:92–104.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
414
|
Fedotcheva TA and Fedotcheva NI:
Protectors of the mitochondrial permeability transition pore
activated by iron and doxorubicin. Curr Cancer Drug Targets.
21:514–525. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
415
|
Ogor P, Yoshida T, Koike M and Kakizuka A:
VCP relocalization limits mitochondrial activity, GSH depletion and
ferroptosis during starvation in PC3 prostate cancer cells. Genes
Cells. 26:570–582. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
416
|
Wei G, Sun J, Hou Z, Luan W, Wang S, Cui
S, Cheng M and Liu Y: Novel antitumor compound optimized from
natural saponin Albiziabioside A induced caspase-dependent
apoptosis and ferroptosis as a p53 activator through the
mitochondrial pathway. Eur J Med Chem. 157:759–772. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
417
|
DeHart DN, Fang D, Heslop K, Li L,
Lemasters JJ and Maldonado EN: Opening of voltage dependent anion
channels promotes reactive oxygen species generation, mitochondrial
dysfunction and cell death in cancer cells. Biochem Pharmacol.
148:155–162. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
418
|
Li Y, Xia J, Shao F, Zhou Y, Yu J, Wu H,
Du J and Ren X: Sorafenib induces mitochondrial dysfunction and
exhibits synergistic effect with cysteine depletion by promoting
HCC cells ferroptosis. Biochem Biophys Res Commun. 534:877–884.
2021. View Article : Google Scholar
|
|
419
|
Zheng Z, Zeng Y, Bao X, Huang C, Guo F, Xu
F and Luo Z: OTULIN confers cisplatin resistance in osteosarcoma by
mediating GPX4 protein homeostasis to evade the mitochondrial
apoptotic pathway. J Exp Clin Cancer Res. 43:3302024. View Article : Google Scholar : PubMed/NCBI
|
|
420
|
Sun Y, Deng R and Zhang C: Erastin induces
apoptotic and ferroptotic cell death by inducing ROS accumulation
by causing mitochondrial dysfunction in gastric cancer cell HGC-27.
Mol Med Rep. 22:2826–2832. 2020.PubMed/NCBI
|
|
421
|
Ren J, Zhou J, Liu H, Jiao X, Cao Y, Xu Z,
Kang Y and Xue P: Ultrasound (US)-activated redox dyshomeostasis
therapy reinforced by immunogenic cell death (ICD) through a
mitochondrial targeting liposomal nanosystem. Theranostics.
11:9470–9491. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
422
|
Liu S, Shang M, Gong J, Sun H and Hu B:
WTAP regulates mitochondrial damage and Lipid oxidation in HCC by
NOA1 mediated m6A modification. J Cancer. 16:315–330. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
423
|
Yagi M, Do Y, Hirai H, Miki K, Toshima T,
Fukahori Y, Setoyama D, Abe C, Nabeshima YI, Kang D and Uchiumi T:
Improving lysosomal ferroptosis with NMN administration protects
against heart failure. Life Sci Alliance. 6:e2023021162023.
View Article : Google Scholar : PubMed/NCBI
|
|
424
|
Zhao Z, Wu Y, Liang X, Liu J, Luo Y, Zhang
Y, Li T, Liu C, Luo X, Chen J, et al: Sonodynamic therapy of NRP2
monoclonal antibody-guided MOFs@COF targeted disruption of
mitochondrial and endoplasmic reticulum homeostasis to induce
autophagy-dependent ferroptosis. Adv Sci (Weinh). 10:e23038722023.
View Article : Google Scholar : PubMed/NCBI
|
|
425
|
Rykowski S, Gurda-Woźna D,
Fedoruk-Wyszomirska A, Orlicka-Płocka M, Kowalczyk A, Stączek P,
Denel-Bobrowska M, Biniek-Antosiak K, Rypniewski W, Wyszko E and
Olejniczak AB: Carboranyl-1,8-naphthalimide intercalators induce
lysosomal membrane permeabilization and ferroptosis in cancer cell
lines. J Enzyme Inhib Med Chem. 38:21710282023. View Article : Google Scholar : PubMed/NCBI
|
|
426
|
Zhang G, Wang Y, Lin J, Wang B, Mohsin A,
Cheng Z, Hao W, Gao WQ, Xu H and Guo M: Biological activity
reduction and mitochondrial and lysosomal dysfunction of
mesenchymal stem cells aging in vitro. Stem Cell Res Ther.
13:4112022. View Article : Google Scholar : PubMed/NCBI
|
|
427
|
Zou Y, Henry WS, Ricq EL, Graham ET,
Phadnis VV, Maretich P, Paradkar S, Boehnke N, Deik AA, Reinhardt
F, et al: Plasticity of ether lipids promotes ferroptosis
susceptibility and evasion. Nature. 585:603–608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
428
|
Tang D and Kroemer G: Peroxisome: The new
player in ferroptosis. Signal Transduct Target Ther. 5:2732020.
View Article : Google Scholar : PubMed/NCBI
|
|
429
|
Gan Y, Deng J, Hao Q, Huang Y, Han T, Xu
JG, Zhao M, Yao L, Xu Y, Xiong J, et al: UTP11 deficiency
suppresses cancer development via nucleolar stress and ferroptosis.
Redox Biol. 62:1027052023. View Article : Google Scholar : PubMed/NCBI
|
|
430
|
Li Z, Ferguson L, Deol KK, Roberts MA,
Magtanong L, Hendricks JM, Mousa GA, Kilinc S, Schaefer K, Wells
JA, et al: Ribosome stalling during selenoprotein translation
exposes a ferroptosis vulnerability. Nat Chem Biol. 18:751–761.
2022. View Article : Google Scholar : PubMed/NCBI
|