Introduction
Pancreatic cancer is a major health problem with a
poor prognosis. In the USA it is the fourth leading cause of
cancer-related mortality in both genders (1) and as many as 55% of patients are
diagnosed at a metastatic stage. Despite the attempts at
management, prognosis of metastatic patients is poor, with a median
survival of ∼3–6 months and a 5-year survival rate of 2% (1).
Non-surgical treatment options, such as chemotherapy
or targeted therapy, have been investigated with regard to whether
they prolong the overall survival of patients with metastatic
pancreatic cancer. Due to the moderate improvement achieved by
chemotherapeutics, recent studies evaluated whether subgroups of
patients may be identified who would benefit the most from specific
treatment strategies (2–4). This may improve the identification of
patients with a poor prognosis and subsequent administration of
supportive care alone, which may help avoid the unnecessary adverse
effects and complications of systemic chemotherapy.
Pancreatic cancer is a disease that mainly affects
the elderly population (2). The
incidence of pancreatic cancer increases with age, with 60% of
patients >65 years (2–5). Patient age has been a well-recognized
prognostic factor in numerous types of cancer treated with
definitive intent. In addition to the pretreatment serum hemoglobin
levels, initial serum carbohydrate antigen 19-9 (CA19-9),
carcinoembryonic antigen (CEA) and lactate dehydrogenase (LDH)
levels have been identified as significant prognostic factors in
different stages of pancreatic cancer. Furthermore, several studies
have demonstrated that patient age is an important independent
prognostic factor affecting survival (6,7).
Moreover, elderly patients usually benefit from single and/or
combination chemotherapy regimens.
In a previous study, we investigated the immediate
and long-term outcome in a limited number of patients with
pancreatic cancer and evaluated the possible impact of different
clinicopathological factors on survival (8). The aim of this study was to identify
and evaluate the same clinicopathological factors in a larger
cohort and elucidate the clinical significance of patient age for
the outcome of metastatic pancreatic cancer.
Materials and methods
Patients
Data from 154 patients with histologically confirmed
diagnosis of metastatic pancreatic cancer, treated and followed-up
in our clinic, were recorded from medical charts. Tumor
localization was determined surgically, endoscopically or
radiologically and pathological confirmation of pancreatic cancer
was obtained by surgery or by fine-needle aspiration biopsy.
Staging of metastatic patients was performed with various imaging
modalities, such as computed tomography (CT), magnetic resonance
imaging and positron emission tomography (PET)/CT scan. Patients
were staged according to the International Union Against Cancer TNM
classification. Written informed consent was obtained from all
patients for their participation in this study. This study was
approved by the Institute of Oncology, University of Istanbul
(Istanbul, Turkey).
Treatment and prognostic variables
Chemotherapy was administered to the majority of the
patients with metastatic disease (n=113, 73%). Patients with
metastatic disease were treated with various single-agent or
combination chemotherapeutic regimens, selected according to the
performance status of the patients and the extent of the disease.
Drug schemes were applied as follows: gemcitabine alone,
combination of gemcitabine with platinum, capecitabine alone or
fluorouracil (5-FU) with folinic acid. Response to chemotherapy was
evaluated radiologically after 2–3 cycles of chemotherapy according
to international criteria. Patients not responding to chemotherapy
were treated with second-line chemotherapy, provided they had a
good performance status. Chemotherapy was continued until disease
progression or unacceptable toxicity.
The possible prognostic variables were selected
based on those identified in previous studies (6–8).
Serum CEA and CA19-9 levels were determined by microparticle enzyme
immunoassay (Abbott Diagnostics, Chicago, IL, USA). Serum LDH,
albumin and hemoglobin levels were measured at presentation in our
biochemical laboratory. Serum LDH activity was determined
immediately after collection by the kinetic method on a Targa-3000
autoanalyzer (Pointe Scientific Inc., Lincoln Park, MI, USA) at
37°C. The laboratory parameters were evaluated at diagnosis within
the normal ranges of our institition.
Statistical analysis
SPSS software version 16 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Quantitative analysis was
summarized by median, minimum and maximum and qualitative analyses
were presented as frequencies and percentages. The Chi-square test
was used to assess the differences in the distribution of the
clinicopathological parameters of the metastatic disease. Overall
survival was determined as the time elapsed between the time of
histological diagnosis and the date of death, the date of the last
follow-up visit or the point of the study at which the patient was
still alive. Time dependent variables and overall survival were
estimated with the Kaplan Meier method and their differences were
evaluated by the log-rank test. Multivariate analysis (Cox
proportional hazards model) was used to determine the variables
with an independent effect on survival. All deaths were considered
as events, regardless of their cause. P≤0.05 was considered to
indicate a statistically significant difference.
Results
Clinicopathological characteristics
The demographic, laboratory and clinicopathological
characteristics of the patients are listed in Table I. In this retrospective study, the
outcome of 154 patients with metastatic pancreatic cancer treated
and followed-up in our clinic was analyzed. Of these, 102 (66%)
were male, with a median age of 58 years (range, 25–88 years). The
majority of the patients had a poor performance status (64%),
weight loss >10% body weight (74%), tumor size of >3 cm (75%)
and elevated tumor markers, including CEA (66%) and CA19-9 (85%).
Moreover, the rate of response to chemoterapy was 24%.
 | Table I.Patient characteristics and
distribution of prognostic factors based on patient age. |
Table I.
Patient characteristics and
distribution of prognostic factors based on patient age.
| Characteristics | Poor prognostic
factor | Patients
| P-value |
|---|
| Total (%) | Younger (<60
years, %) | Older (>60 years,
%) |
|---|
| Gender | Male | 66 | 67 | 65 | 0.810 |
| Performance status
(ECOG) | Poor (2–4) | 64 | 71 | 60 | 0.157 |
| Weight loss | Present (>10%
BW) | 74 | 72 | 76 | 0.640 |
| Jaundice | Present | 42 | 43 | 40 | 0.830 |
| Tumor location | Head | 50 | 45 | 56 | 0.240 |
| Tumor diameter | Large (>3 cm) | 75 | 64 | 90 | 0.002 |
| Hemoglobin
levels | Anemia (<12
g/dl) | 38 | 47 | 28 | 0.048 |
| WBC count | Elevated
(>10000) | 31 | 31 | 31 | 0.990 |
| Platelet count | Elevated
(>450000) | 10 | 16 | 2 | 0.170 |
| Albumin levels | Low (<4 g/dl) | 54 | 55 | 52 | 0.880 |
| LDH levels | Elevated (>450
U/l) | 29 | 24 | 36 | 0.300 |
| CEA levels | Elevated (>4
ng/ml) | 66 | 62 | 71 | 0.400 |
| CA19-9 levels | Elevated (>37
IU/ml) | 85 | 86 | 83 | 0.770 |
| Response to
chemotherapy | Absent
(stable/progression) | 24 | 24 | 24 | 0.870 |
The distributions of prognostic factors depending on
patient age were generally identical (Table I). Specifically, the percentage of
patients with larger tumors was higher among elderly compared to
younger patients (64 vs. 90%, respectively; P=0.002). However,
fewer elderly patients were anemic compared to younger patients (28
vs. 47%, respectively; P=0.048).
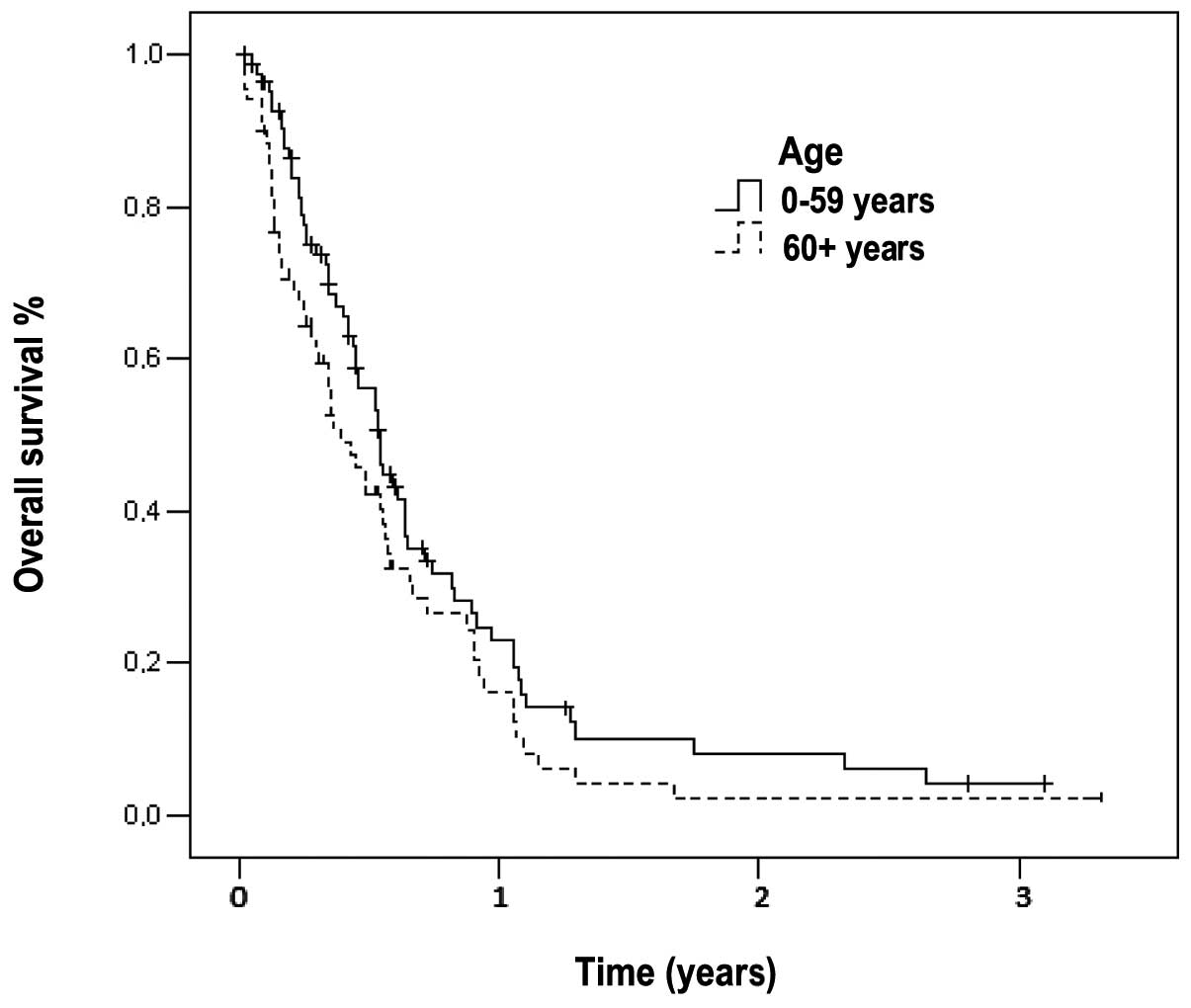
Overall survival
The median follow-up time was 290 days (range, 1–78
months) for all the patients. At the time of the analysis, only 32
(21%) patients were alive. The median survival time of patients
with metastatic disease was 179 days (95% CI: 148–209) and the
1-year survival rate was 7% (Fig.
1). In the subset analysis, we noted that out of the 32
surviving patients, 12 (17%) were elderly and the remaining 20
(24%) belonged to the younger age group. The median survival time
of the elderly patients (144 days, 95% CI: 90–197) was
significantly lower compared to that of younger patients (198 days,
95% CI: 165–230, P=0.039). The 1-year survival rates for elderly
and younger patients were 3 and 10%, respectively (Fig. 1).
In the univariate analysis, elderly patients had
poorer outcomes compared to younger patients (median survival, 114
vs. 198 days, respectively; P=0.04) (Table II). In addition, patients with a
poor performance status, high CA19-9 and CEA levels, jaundice,
leukocytosis and unresponsiveness to chemotherapy exhibited shorter
survival. In the multivariate analysis, similar to other prognostic
factors identified as significantly different in the univariate
analysis, a significant difference was observed in elderly patients
(P=0.048).
 | Table II.Prognostic factors predicting overall
survival in patients with metastatic pancreatic cancer. |
Table II.
Prognostic factors predicting overall
survival in patients with metastatic pancreatic cancer.
| Factor | P-value
|
|---|
| Univariate
analysis | Multivariate
analysis |
|---|
| Performance status
(good vs. poor) | <0.001 | 0.002 |
| CA19-9 level (normal
vs. high) | <0.001 | 0.034 |
| CEA level (normal vs.
high) | 0.001 | 0.034 |
| Jaundice (present vs.
absent) | 0.004 | 0.043 |
| Response to
chemotherapy (present vs. absent) | 0.030 | 0.047 |
| WBC count (normal vs.
high) | 0.035 | - |
| Age (older vs.
younger) | 0.040 | 0.048 |
| Tumor location (head
vs. others) | - | 0.019 |
Clinical significance of patient age
Table III
summarizes the analysis of the association of patient age with
various clinical and laboratory parameters. In both the elderly and
younger patient groups, the univariate analysis identified the same
prognostic factors, such as patient performance status and tumor
markers, including serum CEA and CA19-9 levels, to be associated
with survival. In the multivariate analysis, younger patients with
a poor performance status had a significantly shorter overall
survival compared to those with a good performance status
(P=0.008). However, no significant prognostic factor affecting
outcome was identified in the elderly patients.
 | Table III.Prognostic variables for survival
according to patient age. |
Table III.
Prognostic variables for survival
according to patient age.
| Patients | Univariate
analysis | P-value | Multivariate
analysis | P-value |
|---|
| Younger (<60
years) | Performance status
(poor vs. good) | <0.001 | Performance status
(poor vs. good) | 0.008 |
| CA19-9 (normal vs.
high) | 0.003 |
| Jaundice (present vs.
absent) | 0.014 |
| CEA (normal vs.
high) | 0.018 |
| Tumor location (head
vs. others) | 0.034 |
| Older (>60
years) | Performance status
(poor vs. good) | <0.001 | No independent risk
factor | |
| CA19-9 (normal vs.
high) | 0.033 |
| CEA (normal vs.
high) | 0.041 |
| Response to
chemotherapy (present vs. absent) | 0.049 |
| Gender (female vs.
male) | 0.052 |
Discussion
It has been demonstrated that elderly patients are
underrepresented in cancer clinical trials (2). Despite the fact that elderly patients
account for the majority of cancer patients, they are markedly
underrepresented in cancer clinical treatment trials, constituting
only 30–40% of the number of cancer patients (9). In National Cancer Institute-sponsored
clinical trials, <1% of adults 75–79 years of age are enrolled
(4,10). Possible explanations for this are
the presence of comorbidities and the limited expectations for
long-term benefit of chemotherapy. For these reasons, exclusion of
older individuals is considered acceptable based on non-eligibility
criteria (9). Similarly, the
treatment of elderly pancreatic cancer patients poses a significant
challenge (4).
A recent study conducted on elderly patients with
various malignancies demonstrated that elderly patients benefit
from chemotherapy to a similar extent as younger patients, with
manageable side effects (4). A
meta-analysis of three randomized metastatic colon cancer studies,
comparing combination chemotherapy with 5-FU compared to 5-FU
monotherapy, demonstrated that patients >70 years of age
benefited from the treatment similar to younger patients, with no
apparent greater toxicity (11).
Furthermore, gemcitabine is well-tolerated in elderly patients with
other types of tumors (12).
Chemotherapy is as feasible in the elderly as in the
younger pancreatic cancer patients (2,4,9).
Recent studies suggested that the safety and efficacy of
gemcitabine-based chemotherapy in elderly patients is similar to
that in younger patients (2,9). The
response rate and outcome were similar in elderly and younger
patients (2). Furthermore,
although the majority of very elderly patients (aged >80 years)
with metastatic pancreatic cancer do not receive any treatment, the
administration of chemotherapy in this particular patient
population was associated with improved survival (4).
Age as a prognostic factor has been investigated in
numerous studies with controversial results. A previous study
described age as an independent prognostic factor (6) whereas others did not identify age as
such a factor (2,9). The majority of studies on prognostic
factors are questionable in terms of sample size and statistical
methods, being largely based on small retrospective analyses
(7). Data consisting a total of 34
possible prognostic factors from 653 advanced pancreatic cancer
patients were analyzed and the log-rank analysis indicated that age
was a potentially important factor affecting survival (7). In our study, we observed that age
appears to affect survival. This result is consistent with those
found in the literature.
Patients of identical age may greatly differ in
their functional status (4). The
performance status, which provides a useful guide in making
treatment decisions for younger patients, is often insufficient to
assess the overall status of elderly patients (4). The performance status is an
independent negative prognostic factor for elderly patients
(9).
In conclusion, almost all of the patients with
metastatic pancreatic cancer have a poor prognosis and establishing
definitive prognostic variables during the initial diagnosis may
help physicians determine which patients should be considered for
supportive care alone, single-agent chemotherapy, combination
chemotherapy or multimodality treatment options. In this study, we
demonstrated that patient age is a major prognostic factor
affecting survival in metastatic pancreatic cancer. Age should not
preclude patients from receiving chemotherapy and treatment
decisions should be based on the physiological rather than the
chronological age (4). The
evaluation of factors including functional status, co-morbidity and
cognition in elderly patients is necessary (4).
Therefore, elderly patients may be eligible for
treatment options, provided they exhibit no weight loss, have a
good performance status and favorable prognostic factors, such as
normal tumor marker serum levels.
References
|
1.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2.
|
Hentic O, Dreyer C, Rebours V, et al:
Gemcitabine in elderly patients with advanced pancreatic cancer.
World J Gastroenterol. 17:3497–3502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Shore S, Vimalachandran D, Raraty MG and
Ghaneh P: Cancer in the elderly: pancreatic cancer. Surg Oncol.
13:201–210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Aldoss IT, Tashi T, Gonsalves W, et al:
Role of chemotherapy in the very elderly patients with metastatic
pancreatic cancer - a Veterans Affairs Cancer Registry analysis. J
Geriatr Oncol. 2:209–214. 2011. View Article : Google Scholar
|
|
5.
|
Sener SF, Fremgen A, Menck HR and
Winchester DP: Pancreatic cancer: a report of treatment and
survival trends for 100,313 patients diagnosed from 1985–1995,
using the National Cancer Database. J Am Coll Surg. 189:1–7.
1999.PubMed/NCBI
|
|
6.
|
Park JK, Yoon YB, Kim YT, Ryu JK, Yoon WJ
and Lee SH: Survival and prognostic factors of unresectable
pancreatic cancer. J Clin Gastroenterol. 42:86–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Stocken DD, Hassan AB, Altman DG, et al:
Modelling prognostic factors in advanced pancreatic cancer. Br J
Cancer. 99:883–893. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Tas F, Aykan F, Alici S, Kaytan E, Aydiner
A and Topuz E: Prognostic factors in pancreatic carcinoma: serum
LDH levels predict survival in metastatic disease. Am J Clin Oncol.
24:547–550. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Marechal R, Demols A, Gay F, et al:
Tolerance and efficacy of gemcitabine and gemcitabine-based
regimens in elderly patients with advanced pancreatic cancer.
Pancreas. 36:e16–e21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hutchins LF, Unger JM, Crowley JJ, Coltman
CA Jr and Albain KS: Underrepresentation of patients 65 years of
age or older in cancer treatment trials. N Engl J Med.
341:2061–2067. 1999.PubMed/NCBI
|
|
11.
|
Folprecht G, Rougier P, Saltz L, et al:
Irinotecan in first line therapy of elderly and non-elderly
patients with metastatic colorectal cancer: Meta-analysis of four
trials investigating 5-FU and irinotecan. J Clin Oncol.
24:35782006.
|
|
12.
|
Ricci S, Antonuzzo A, Galli L, et al:
Gemcitabine monotherapy in elderly patients with advanced
non-small-cell lung cancer: a multicenter phase II study. Lung
Cancer. 27:75–80. 2000. View Article : Google Scholar : PubMed/NCBI
|