Introduction
Angiomyolipoma (AML) is a rare renal tumor
accounting for 2–6.4% of all kidney neoplasms (1,2). AML
originates from mesenchymal tissue and typically consists of three
histopathological components: Fusiform spindle or epithelioid
smooth muscle cells, dysmorphic blood vessels and adipose tissue
(triphasic pattern). AML may be composed mainly or entirely of one
element, such as smooth muscle or adipose tissue. According to the
WHO classification, there are two types of renal AML: Classical and
epithelioid (3). The former is a
benign tumor and composed of the abovementioned three components,
while epithelioid AML (EAML) has a predominant epithelioid
component and potentially malignant behavior (3,4). EAMLs
are part of the perivascular epithelioid cell family of tumors
(PEComas). They mainly consist of a large number of hyperplastic
epithelioid cells arranged in sheets, whereas the proportion of
mature fat cells tends to be <5%. Epithelioid cells are atypical
large cells with abundant cytoplasm, vesicular nuclei and prominent
nucleoli (3–5). EAMLs comprise 5–8% of all surgically
treated renal AMLs (5,6). Since renal AML is frequently managed by
surveillance or selective arterial embolization, the proportion of
EAMLs is probably even smaller. EAML may also be found in the liver
and other organs, albeit infrequently.
In contrast to the benign biological behavior of
classic AML, malignant behavior has been observed in some cases of
EAML. Characteristics of malignancy, such as the presence of tumor
venous extension, distant metastasis and local tumor recurrence
have been reported in such EAML cases (5,7–9). Therefore, it is important to
distinguish EAML from classic AML, as each carries unique
therapeutic and prognostic implications.
We herein report a case of locally recurrent and
metastatic EAML, which was observed 12 years after nephrectomy for
erroneously diagnosed simple AML, along with a review of the
relevant literature.
Case report
A 60-year old male patient presented in September
2013 to the Medical School of Crete University Hospital (Heraklion,
Greece) with dull abdominal pain. The patient had undergone left
nephrectomy with left adrenalectomy and lymphadenectomy for a renal
tumor 12 years earlier. The histology report had revealed an AML of
the left kidney, with a maximal diameter of 17 cm, while the left
adrenal gland and the harvested lymph nodes had been noted to be
normal. No history of tuberous sclerosis syndrome or renal tumors
was recorded for the patient or his family. On physical
examination, a tumor was palpable at the left side of the abdomen.
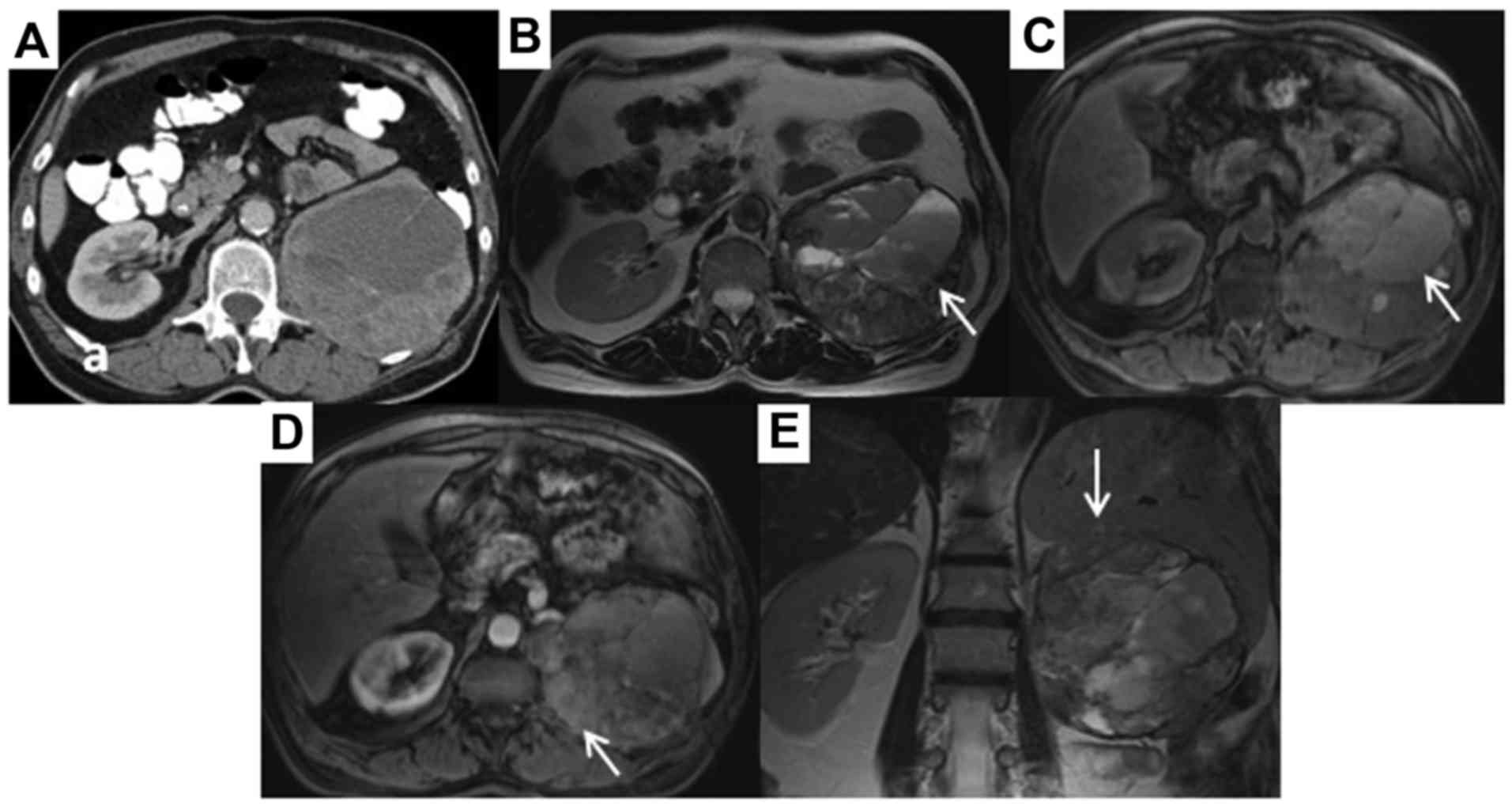
While a computed tomography (CT) examination performed 2 years
earlier had not revealed any abnormalities, a contrast-enhanced CT
scan of the abdomen demonstrated a round heterogeneous mass, sized
12×12×13 cm, in the area of the resected left kidney. The mass lay
adjacent to the psoas muscle and the spleen, and caused elevation
of the left hemidiaphragm (Fig. 1A).
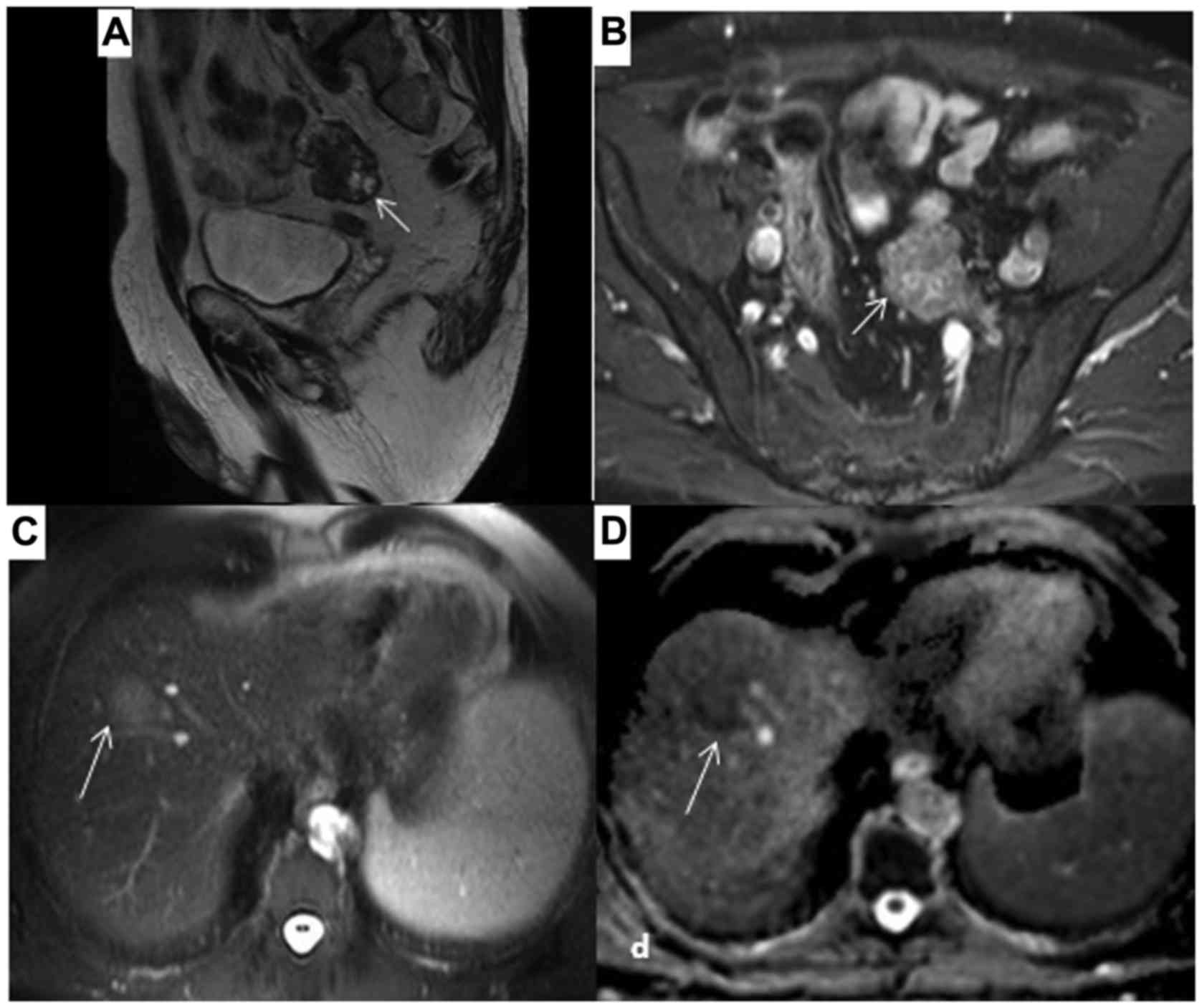
In addition, a tumor 3 cm in greatest diameter was found at the
left side of the pelvis, while a lesion 1.9 cm in greatest
diameter, suspicious for metastasis, was found in segment VIII of
the liver. Multiple small simple liver cysts were also identified;
the right kidney appeared normal. There were no enlarged abdominal
lymph nodes or ascites. A CT scan of the chest did not reveal any
pulmonary abnormalities. CT-guided core needle biopsy of the large
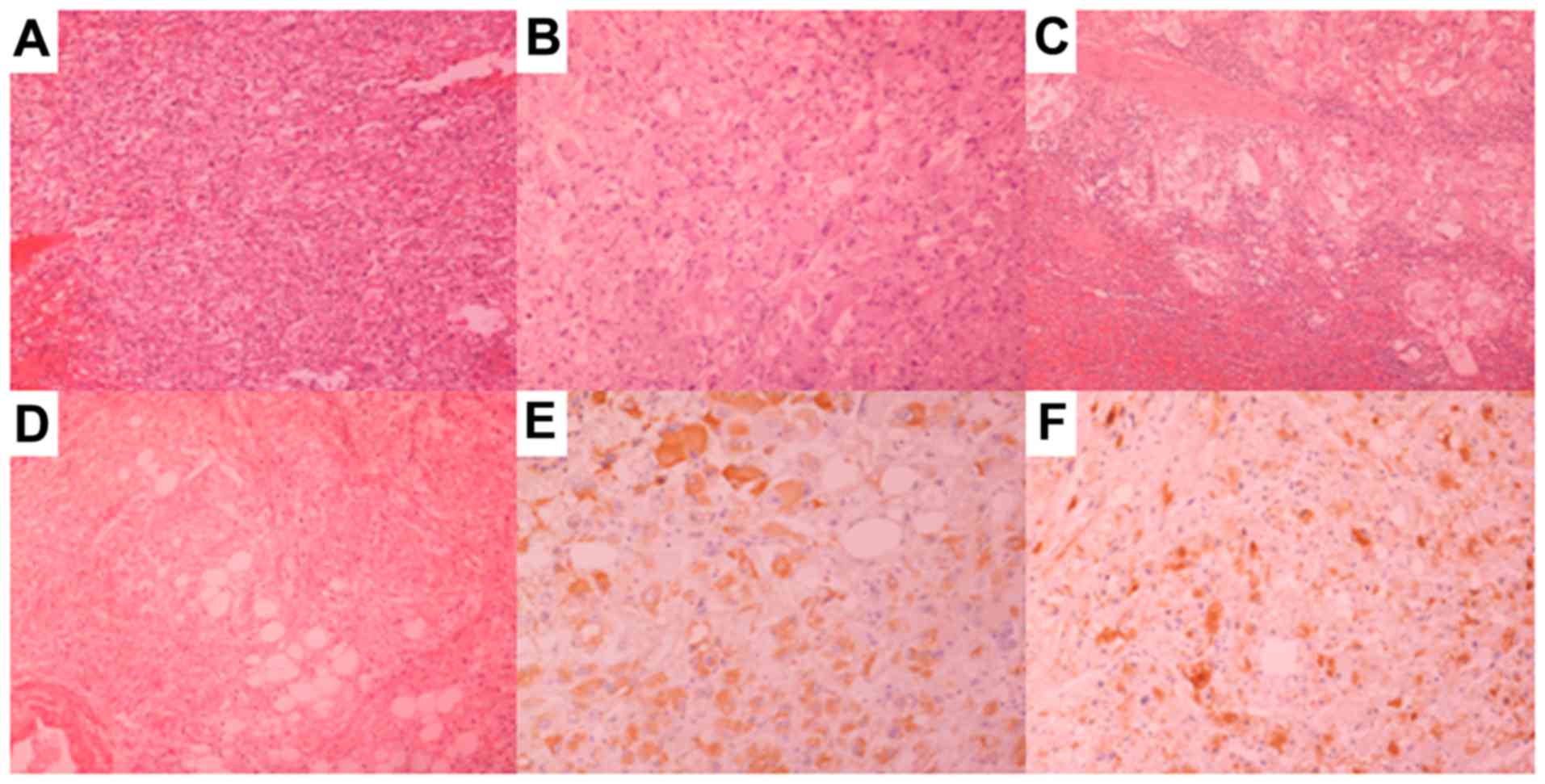
mass and revision of the histology of the nephrectomy revealed an
EAML (Fig. 2A and B). In the primary
tumor, ~10% of the cells were epithelioid. Magnetic resonance
imaging (MRI) of the abdomen was performed to further delineate the
anatomic relations of the mass and the nature of the pelvic and
liver tumors. A relatively circumscribed large mass, sized 14×12×13
cm, was noted at the anatomic site of the resected left kidney
(Fig. 1B, C and D), with evident
infiltration of the spleen (Fig.
1E). The mass included extensive areas of necrosis, while it
did not have a fatty component. The large mass was adjacent to the
psoas muscle, the paravertebral muscles, the pancreatic tail, the
left hemidiaphragm and the aorta, but without signs of infiltration
of these structures. The imaging characteristics of the 4-cm lesion
at the left side of the pelvis were similar to those of the large
mass and it was considered to be a peritoneal metastasis (Fig. 3A and B). A 3.1-cm metastatic lesion
was also found in segment VIII of the liver, along with multiple
small cysts (Fig. 3C and D).
In the absence of a well-established effective
systemic treatment, a two-stage operation was planned for this
locally recurrent and oligometastatic disease, with initial
resection of the large abdominal mass and the pelvic lesion, and
subsequent excision of the liver metastasis. During laparotomy, no
other lesions, apart from the ones identified on preoperative
imaging, were found. The large tumor appeared to infiltrate the
spleen, the mesocolon of the left colonic flexure, part of the left
hemidiaphragm posteriorly, and part of the left psoas muscle. The
mass was resected en bloc along with the spleen, left colonic
flexure, part of the left diaphragm and part of the left psoas
muscle. The lesion in the pelvis was located superficially at the
mesocolon of the sigmoid and was excised. Both lesions were
macroscopically completely resected. The defect in the diaphragm
was closed and an end-to-end colon-colonic anastomosis was
performed. The postoperative course was uneventful. Histological
examination of the specimens revealed recurrent EAML with malignant
characteristics. The large tumor was 17×11×9 cm in size, had large
areas of necrosis and was composed of epithelioid cells with 2
mitoses per 10 high-power fields (Fig.
2C). The tumor infiltrated the spleen (Fig. 2D) and the mesocolon, but not the
colon itself, the diaphragm or the psoas muscle microscopically.
Immunohistochemical staining was negative for cytokeratin (MNF116),
epithelial membrane antigen, CD10, desmin and c-kit, while the
epithelioid cells were focally positive for Melan A (Fig. 2E), human melanoma black (HMB)-45
(Fig. 2F) and S-100. The pelvic
lesion was 5.5×4.5×2 cm in size and exhibited characteristics
similar to those of the large abdominal tumor.
At 3 months postoperatively, a CT scan of the chest
and abdomen did not reveal any other suspicious findings apart from
the solitary liver lesion. The patient subsequently underwent
resection of the liver lesion and cholecystectomy. The
postoperative course was complicated by a biliary fistula and an
abscess of 6 cm in diameter in the resection bed. The fistula was
treated conservatively with removal of the drain when its
production stopped on the 8th postoperative day. The abscess was
successfully drained percutaneously, while a broad-spectrum
antibiotic regimen was administered. Histological examination
confirmed the diagnosis of a liver metastasis originating from
EAML. The lesion had a greatest diameter of 3.5 cm, while the
surgical margins were tumor-free. The patient did not receive any
adjuvant treatment.
Over 4 years (52 months) after two-stage surgery for
recurrent EAML, the patient remains in excellent clinical condition
and free of any symptoms, while physical examination and imaging
studies did not show any evidence of recurrent disease at his last
follow-up visit on July 25, 2018.
Discussion
The development of renal AML may be associated with
the tuberous sclerosis complex (TSC), which is a systemic autosomal
dominant disorder that is usually caused by decreased or absent
expression of TSC1 (hamartin) or TSC2 (tuberin) genes. The products
(hamartin-tuberin complex) of TSC1 and TSC2 are associated with
regulation of the mammalian target of rapamycin (mTOR) signaling
pathway (10,11). Lack of hamartin-tuberin complex
results in the development of tumors in a number of organs,
including AML in the kidneys. The incidence of renal AML is ~80%
among patients with TSC (12).
Similarly, analysis of sporadic AMLs and EAMLs has revealed an
association with TSC2 (13–15). Sporadic AML is at least 2–4 times
more common compared with TSC-associated AML (8,14).
Furthermore, while TSC-associated AMLs are usually multiple,
bilateral and most often first detected in childhood, sporadic AMLs
occur in older patients and are usually single and smaller
(12,16).
The classic renal AMLs are often found incidentally
and are relatively easy to identify on imaging studies due to their
fatty component. Due to their non-aggressive behavior, AMLs are
rarely resected, unless they reach a size where the risk of rupture
and hemorrhage is significant. Even in the latter case, many are
embolized rather than resected (16). Thus, it is not surprising that many
of the resected cases have a predominance of one of the components
with paucity of the others, as they are likely to have atypical
imaging characteristics. Fat-predominant and muscle-predominant
AMLs may mimic liposarcoma and leiomyosarcoma, respectively, the
most common types of retroperitoneal sarcomas. The epithelioid
variant of AML was initially described in the 1990s (17). Focal epithelioid morphology may be
observed in a number of classic AMLs and, to date, there are no
data to suggest that this characteristic alters its benign
behavior. There is no consensus as to the percentage of epithelioid
cells required for diagnosing EAML, with some authors (2,7)
suggesting that only ≥5% of the cells must exhibit epithelioid
histology, while others demanding at least 20% (18,19) or
even 80% (2). In addition to the
epithelioid histology, these cells must also have enlarged
vesicular nuclei with prominent nucleoli. When the epithelioid
component predominates and nuclear atypia is extensive, these
tumors may be erroneously diagnosed as renal carcinoma or sarcoma.
It may be necessary to perform immunohistochemical studies to
confirm the diagnosis of EAML (5–9,17). While staining for epithelial cell
markers is negative, positive staining for HMB-45 and Melan A is
generally observed. The cells often express smooth muscle markers
as well, particularly SMA and, less commonly, desmin. Staining for
S-100 protein is usually negative. The majority of EAML cases
display membranous and cytoplasmic staining of E-cadherin, whereas
classic AML cases demonstrate cytoplasmic staining alone (20). Moreover, in diagnostically
challenging cases, staining for CD68 (PG-M1) (21) and PNL2 (22) may be helpful in distinguishing renal
EAML form other renal tumors.
The mean age of the patients presenting with renal
EAML is ~40–50 years, while there appears to be no sex prevalence
(2,5–9,23). In various series (2,5–8,23), the
size of the resected renal EAMLs varied from 1 to 37 cm, with a
mean size of 7–11 cm. Although EAML may be found incidentally on
imaging, the majority of the patients are symptomatic, similar to
classic AML cases (2,8). Flank pain, hematuria and a palpable
mass may be present, while renal AML may cause hypertension, renal
failure and life-threatening hemorrhage (16,24).
Hemorrhage, which is strongly associated with aneurysm formation,
is the major cause of death from this disease in adults (16), as the aneurysm size increases in
accordance with the expansion of the AML. An AML or aneurysm size
exceeding 4 cm and 5 mm, respectively, is associated with an
imminent risk of rupture and subsequent hemorrhage (24). This major complication is more
frequently observed in TSC-associated rather than sporadic AMLs
(12,16).
The diagnosis of renal EAML is rarely established
preoperatively and this tumor is often misdiagnosed as renal cell
carcinoma, as both are characterized by an insidious onset and
non-specific clinical manifestations. In addition, the amount of
fat in EAML on CT and MRI is markedly lower (<5%) compared with
classic AML and, consequently, EAML may be misdiagnosed as renal
cell carcinoma or retroperitoneal sarcoma (2,25). Renal
EAMLs may exhibit variable morphological characteristics on CT and
MRI. Hypointensity on T-weighed MRI, tumor necrosis, hemorrhage,
cystic changes, infiltrative extrarenal (exophytic) growth, dilated
vessels, extension to the renal sinus and renal vein, and inferior
vena cava tumor thrombus may be helpful in distinguishing renal
EAML (1,25–27). On
dynamic contrast-enhanced MRI, the enhancement patterns are
non-specific, with varying degrees of enhancement (27). In contrast to AML, lymph node and
systemic metastases may be observed on imaging studies in EAML.
While in one series with 41 selected cases (8), 30% of the EAML patients presented with
lymph node or systemic metastases at the time of the initial
diagnosis, this percentage was significantly lower (0–9%) in other
series (5–7,9).
Definitive diagnosis is usually obtained after core needle biopsy
or histological examination of the resected kidney.
The malignant potential of renal EAML may result in
local recurrence and/or metastatic disease, most frequently to the
liver, lymph nodes, lungs and peritoneum (5–9). Recent
series (6–8) have reported extremely varying rates of
such malignant behavior (0–52%), most likely due to the potential
bias by certain studies including patients with a small epithelioid
component and others including many consultation cases in tertiary
referral hospitals. Consultation cases may cause selection bias,
since they are often particularly unusual cases, due to either
their histological characteristics or their clinical behavior. Most
recently, three major centers reported their collected data of EAML
patients, excluding consultation cases and those with an
epithelioid component of <80% (5). After a median follow-up of 52 months
(range, 1–356 months) only 1 of the 20 patients had developed
metastatic disease, while all others remained disease-free. The
authors considered that the incidence of malignant behavior of true
EAML appeared to be in the order of 5% (2). However, since in other series (7,8)
recurrence was observed up to 72 months after initial diagnosis,
and only 8 of the 20 patients in this series were followed up for
>72 months, the true incidence may be slightly higher.
In one of the abovementioned series (8), the presence of ≥3 of the following
factors was highly prognostic for aggressive biological behavior:
Presence of tuberous sclerosis syndrome, tumor size >7.7 cm,
tumor necrosis, extrarenal extension or renal vein invasion and
carcinoma-like histology. In another series (7), ≥70% of atypical epithelioid cells,
>2 mitoses/10 high-power fields, atypical mitoses and necrosis
were considered as adverse prognostic factors; the presence of ≥3
of these factors was highly associated with malignant behavior. In
another study (20), tumor size,
necrosis and invasive growth differed significantly between
favorable and adverse prognostic groups of renal EAML patients. In
the present case, the primary tumor manifested a large size (i.e.,
17 cm in greatest diameter), but none of the other abovementioned
adverse prognostic factors was observed.
The main local treatment options for classic renal
AML are active surveillance, selective arterial embolization,
nephron-sparing surgery or nephrectomy (28,29).
Primary indications for intervention include symptoms, such as pain
or bleeding, or suspicion of malignancy (28). Prophylactic intervention is
justifiable for large AML tumors, in women of childbearing age or
in patients in whom follow-up or access to emergency care may be
inadequate (28,29). The treatment of choice for primary
and locally recurrent EAML is surgical resection. Primary surgery
may be either nephrectomy or, less frequently, nephron-sparing
surgery. However, due to its rarity, there are currently no
treatment guidelines for metastatic disease. In the absence of
highly effective systemic treatment, surgery appears to be a
reasonable treatment option for resectable oligometastatic disease
(30), as in the present case.
Response to chemotherapy has been sporadically reported (31–33).
Recently, targeted agents against mammalian target of rapamycin
(mTOR), such as sirolimus and everolimus, have been used
successfully to treat TSC-associated renal AML, particularly in
cases with bilateral tumors or when tumor progression is expected
to lead to significant morbidity (12,34,35).
Since sporadic as well as TSC-associated EAMLs harbor similar
germline mutations that interfere with the mTOR pathway (10,11,13,14),
mTOR inhibitors may also be effective in metastatic disease
consequent to sporadic renal EAML. In case reports of metastatic
renal EAML, treatment with mTOR inhibitors has demonstrated
clinical effectiveness (36–39). Therefore, the correct diagnosis of
renal EAML can guide the clinicians, particularly in patients with
extensive disease, to select a more effective systemic treatment.
To date, there is lack of sufficient evidence for adjuvant
treatment following resection of the primary tumor. However, the
administration of mTOR inhibitors, such as sirolimus and
everolimus, either as neoadjuvant or adjuvant targeted therapy may
lead to a better clinical outcome in selected high-risk EAML
patients (35,36). However, the expected benefits should
be weighed against the potentially serious adverse effects. No
systemic treatment was administered in the present case, in view of
the complete surgical resection of locally recurrent and
oligometastatic disease and the absence of robust scientific data
supporting its effectiveness.
Collective data of a total of 130 EAML patients from
various series (5,7–9) with a
mean follow-up of 33–52 months, demonstrated that the median time
to local recurrence was 15 months (n=9; range, 8–72 months) and the
median time to lymph node or systemic recurrence was 14 months
(n=12; range, 6–72 months). It is noteworthy that the present case
was characterized by a very late local, peritoneal and systemic
recurrence, i.e., 12 years after the initial resection of the
tumor. Although in one series (8)
33% of the 33 selected renal EAML patients succumbed to the
disease, in other such series (5,6,8,9) with
similar follow-up periods this rate was significantly lower, with
percentages ranging from 0 to 11%. The mortality rate may be
slightly higher with longer follow-up, since patients developing
late recurrence, as in the present case, have also been
reported.
In conclusion, our limited knowledge of the
potentially malignant behavior of renal EAML may be attributed to
its rarity. The diagnosis is usually established by histological
examination of the resected tumor. Correct diagnosis of this
subtype of AML is crucial for its management. The mainstay of
treatment is surgery, while for metastatic disease encouraging
results have been reported with targeted agents. The role of these
agents in the neoadjuvant or adjuvant setting is yet unknown. Due
to the risk of recurrent disease, which may occur even very late,
and the presence of effective surgical and other emerging medical
treatment options, long-term follow-up is indicated for renal
EAMLs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
EDB: Concept, design, literature search, manuscript
preparation. DS: Literature search, manuscript preparation,
manuscript editing and review. EC: Performance, analysis and
interpretation of imaging methods, manuscript review. DM:
Manuscript preparation, manuscript review. MT: Performance,
analysis and interpretation of histological examinations,
manuscript review. All authors read and approved the final version
of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Consent has been obtained from the patient for the
publication of the case details and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Game X, Soulie M, Moussouni S, Roux D,
Escourrou G, Chevreau C and Aziza R: Renal angiomyolipoma
associated with rapid enlargement and inferior vena caval tumor
thrombus. J Urol. 170:918–919. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hassan M, El-Hefnawy AS, Elshal AM, Mosbah
A, El-Baz M and Shaaban A: Renal epithelioid angiomyolipoma: A rare
variant with unusual behavior. Int Urol Nephrol. 46:317–322. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moch H, Humphrey PA, Ulbright TM and
Reuter VE: WHO classification of tumours of the urinary system and
male genital organs. (4th). IARC Press. (Lyon). 2016.
|
|
4
|
Lane BR, Aydin H, Danforth TL, Zhou M,
Remer EM, Novick AC and Campbell SC: Clinical correlates of renal
angiomyolipoma subtypes in 209 patients: Classic, fat poor,
tuberous sclerosis associated and epithelioid. J Urol. 180:836–843.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He W, Cheville JC, Sadow PM, Gopalan A,
Fine SW, Al-Ahmadie HA, Chen YB, Oliva E, Russo P, Reuter VE, et
al: Epithelioid angiomyolipoma of the kidney: Pathological features
and clinical outcome in a series of consecutively resected tumors.
Mod Pathol. 26:1355–1364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aydin H, Magi-Galluzzi C, Lane BR, Sercia
L, Lopez JI, Rini BI and Zhou M: Renal angiomyolipoma:
Clinicopathologic study of 194 cases with emphasis on the
epithelioid histology and tuberous sclerosis association. Am J Surg
Pathol. 33:289–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brimo F, Robinson B, Guo C, Zhou M, Latour
M and Epstein JI: Renal epithelioid angiomyolipoma with atypia: A
series of 40 cases with emphasis on clinicopathologic prognostic
indicators of malignancy. Am J Surg Pathol. 34:715–722.
2010.PubMed/NCBI
|
|
8
|
Nese N, Martignoni G, Fletcher CD, Gupta
R, Pan CC, Kim H, Ro JY, Hwang IS, Sato K, Bonetti F, et al: Pure
epithelioid PEComas (so-called epithelioid angiomyolipoma) of the
kidney: A clinicopathologic study of 41 cases: detailed assessment
of morphology and risk stratification. Am J Surg Pathol.
35:161–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei JH, Liu LR, Wei Q, Song TR, Yang L,
Yuan HC, Jiang Y, Xu H, Xiong SH and Han P: A four-year follow-up
study of renal epithelioid angiomyolipoma: A multi-center
experience and literature review. Sci Rep. 5:100302015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sancak O, Nellist M, Goedbloed M,
Elfferich P, Wouters C, Maat-Kievit A, Zonnenberg B, Verhoef S,
Halley D and van den Ouweland A: Mutational analysis of the TSC1
and TSC2 genes in a diagnostic setting: Genotype - phenotype
correlations and comparison of diagnostic DNA techniques in
Tuberous Sclerosis Complex. Eur J Hum Genet. 13:731–741. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crino PB, Nathanson KL and Henske EP: The
tuberous sclerosis complex. N Engl J Med. 355:1345–1356. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bissler JJ and Kingswood JC: Optimal
treatment of tuberous sclerosis complex associated renal
angiomyolipomata: A systematic review. Ther Adv Urol. 8:279–290.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henske EP, Neumann HP, Scheithauer BW,
Herbst EW, Short MP and Kwiatkowski DJ: Loss of heterozygosity in
the tuberous sclerosis (TSC2) region of chromosome band 16p13
occurs in sporadic as well as TSC-associated renal angiomyolipomas.
Genes Chromosomes Cancer. 13:295–298. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chuang CK, Lin HCA, Tasi HY, Lee KH, Kao
Y, Chuang FL, Chang YH, Lin PH, Liu CY and Pang ST: Clinical
presentations and molecular studies of invasive renal epithelioid
angiomyolipoma. Int Urol Nephrol. 49:1527–1536. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kenerson H, Folpe AL, Takayama TK and
Yeung RS: Activation of the mTOR pathway in sporadic
angiomyolipomas and other perivascular epithelioid cell neoplasms.
Hum Pathol. 38:1361–1371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bissler JJ and Kingswood JC: Renal
angiomyolipomata. Kidney Int. 66:924–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mai KT, Perkins DG and Collins JP:
Epithelioid cell variant of renal angiomyolipoma. Histopathology.
28:277–280. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martignoni G, Pea M, Bonetti F, Zamboni G,
Carbonara C, Longa L, Zancanaro C, Maran M, Brisigotti M and
Mariuzzi GM: Carcinomalike monotypic epithelioid angiomyolipoma in
patients without evidence of tuberous sclerosis: A
clinicopathologic and genetic study. Am J Surg Pathol. 22:663–672.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi N, Kitahara R, Hishimoto Y,
Ohguro A, Hashimoto Y and Suzuki T: Malignant transformation of
renal angiomyolipoma. Int J Urol. 10:271–273. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bi XG, Guo L, Wang XL, Wei Q, Du Q, Jiang
WH, Zheng GY, Zhang HT, Ma JH and Zheng S: Distinct subcellular
localization of E-cadherin between epithelioid angiomyolipoma and
triphasic angiomyolipoma: A preliminary case-control study. Oncol
Lett. 14:695–704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caliò A, Brunelli M, Segala D, Pedron S,
Tardanico R, Remo A, Gobbo S, Meneghelli E, Doglioni C, Hes O, et
al: t(6;11) renal cell carcinoma: A study of seven cases including
two with aggressive behavior, and utility of CD68 (PG-M1) in the
differential diagnosis with pure epithelioid PEComa/epithelioid
angiomyolipoma. Mod Pathol. 31:474–487. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gulavita P, Fletcher CDM and Hirsch MS:
PNL2: An adjunctive biomarker for renal angiomyolipomas and
perivascular epithelioid cell tumours. Histopathology. 72:441–448.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng S, Bi XG, Song QK, Yuan Z, Guo L,
Zhang H and Ma JH: A suggestion for pathological grossing and
reporting based on prognostic indicators of malignancies from a
pooled analysis of renal epithelioid angiomyolipoma. Int Urol
Nephrol. 47:1643–1651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamakado K, Tanaka N, Nakagawa T,
Kobayashi S, Yanagawa M and Takeda K: Renal angiomyolipoma:
Relationships between tumor size, aneurysm formation, and rupture.
Radiology. 225:78–82. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schieda N, Kielar AZ, Al Dandan O, McInnes
MD and Flood TA: Ten uncommon and unusual variants of renal
angiomyolipoma (AML): Radiologic-pathologic correlation. Clin
Radiol. 70:206–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Froemming AT, Boland J, Cheville J,
Takahashi N and Kawashima A: Renal epithelioid angiomyolipoma:
Imaging characteristics in nine cases with radiologic-pathologic
correlation and review of the literature. AJR Am J Roentgenol.
200:W178–W186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong Y, Shen Y, Pan J, Wang Y, An Y, Guo
A, Ma L, Ye H and Wang H: Renal epithelioid angiomyolipoma: MRI
findings. Radiol Med (Torino). 122:814–821. 2017. View Article : Google Scholar
|
|
28
|
Nelson CP and Sanda MG: Contemporary
diagnosis and management of renal angiomyolipoma. J Urol.
168:1315–1325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ouzaid I, Autorino R, Fatica R, Herts BR,
McLennan G, Remer EM and Haber GP: Active surveillance for renal
angiomyolipoma: Outcomes and factors predictive of delayed
intervention. BJU Int. 114:412–417. 2014.PubMed/NCBI
|
|
30
|
Vicens RA, Jensen CT, Korivi BR and
Bhosale PR: Malignant renal epithelioid angiomyolipoma with liver
metastasis after resection: A case report with multimodality
imaging and review of the literature. J Comput Assist Tomogr.
38:574–577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cibas ES, Goss GA, Kulke MH, Demetri GD
and Fletcher CD: Malignant epithelioid angiomyolipoma (‘sarcoma ex
angiomyolipoma’) of the kidney: A case report and review of the
literature. Am J Surg Pathol. 25:121–126. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ferry JA, Malt RA and Young RH: Renal
angiomyolipoma with sarcomatous transformation and pulmonary
metastases. Am J Surg Pathol. 15:1083–1088. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lowe BA, Brewer J, Houghton DC, Jacobson E
and Pitre T: Malignant transformation of angiomyolipoma. J Urol.
147:1356–1358. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
El-Hashemite N, Zhang H, Henske EP and
Kwiatkowski DJ: Mutation in TSC2 and activation of mammalian target
of rapamycin signalling pathway in renal angiomyolipoma. Lancet.
361:1348–1349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng ZF, Yang L, Wang TT, Han P, Liu ZH
and Wei Q: Efficacy and safety of sirolimus for renal
angiomyolipoma in patients with tuberous sclerosis complex or
sporadic lymphangioleiomyomatosis: A systematic review. J Urol.
192:1424–1430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kohno J, Matsui Y, Yamasaki T, Shibasaki
N, Kamba T, Yoshimura K, Sumiyoshi S, Mikami Y and Ogawa O: Role of
mammalian target of rapamycin inhibitor in the treatment of
metastatic epithelioid angiomyolipoma: A case report. Int J Urol.
20:938–941. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shitara K, Yatabe Y, Mizota A, Sano T,
Nimura Y and Muro K: Dramatic tumor response to everolimus for
malignant epithelioid angiomyolipoma. Jpn J Clin Oncol. 41:814–816.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wolff N, Kabbani W, Bradley T, Raj G,
Watumull L and Brugarolas J: Sirolimus and temsirolimus for
epithelioid angiomyolipoma. J Clin Oncol. 28:e65–e68. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wagner AJ, Malinowska-Kolodziej I, Morgan
JA, Qin W, Fletcher CD, Vena N, Ligon AH, Antonescu CR, Ramaiya NH,
Demetri GD, et al: Clinical activity of mTOR inhibition with
sirolimus in malignant perivascular epithelioid cell tumors:
Targeting the pathogenic activation of mTORC1 in tumors. J Clin
Oncol. 28:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|