Introduction
Extranodal natural killer (NK)/T-cell lymphoma
(ENKTL) is an uncommon subclass of non-Hodgkin's lymphoma primarily
encountered in Asian and South American populations (1). These lymphomas are of putative NK-cell
origin, with a minority derived from the T-cell lineage.
Pathologically, 80% of ENKTLs occur in the nose and upper
aerodigestive tract, and rarely present as widespread metastasis
(2). These lymphomas are locally
very invasive and may cause perforation of the hard palate, whereas
they rarely cause widespread distant metastasis involving the skin,
soft tissues, uterus and gastrointestinal tract. The pathogenesis
almost invariably involves Epstein-Barr virus (EBV) infection.
Nicolae et al (3) recently
reported a series of 7 cases of EBV-negative aggressive ENKTL.
These lymphomas are clinically and pathologically indistinguishable
from EBV-positive ENKTLs and they tend to occur in older patients.
Gao et al (4) also reported a
series of 3 patients with EBV-negative ENKTL in the western
hemisphere, which shared similar characteristics with EBV-positive
ENKTL and exhibited a highly aggressive clinical course. The immune
checkpoint protein programmed death ligand 1 (PD-L1) was found to
be overexpressed in all 3 patients. Thus, targeting the PD-L1/PD-1
axis may be a potent mechanism of immune evasion by averting
effector T-cell signaling and inhibiting anti-lymphoma immunity
(5,6). The treatment of these lymphomas is
usually aggressive chemotherapy. Unfortunately, the treatment
options for elderly patients are limited due to their poor
tolerance to chemotherapy. In such cases, physicians tend to
recommend hospice care and/or palliative radiation or palliative
chemotherapy. In the present case, compassionate use of
pembrolizumab was applied. To the best of our knowledge, this case
is the first example of pembrolizumab treatment for naïve
EBV-negative ENKTL.
Case report
A 90-year-old Hispanic female patient presented in
December 2017 to the Saint-Luke's Cancer Institute (Kansas City,
USA) with severe inflammation and ulceration of the hard palate for
the last 2 months. On physical examination, the patient had
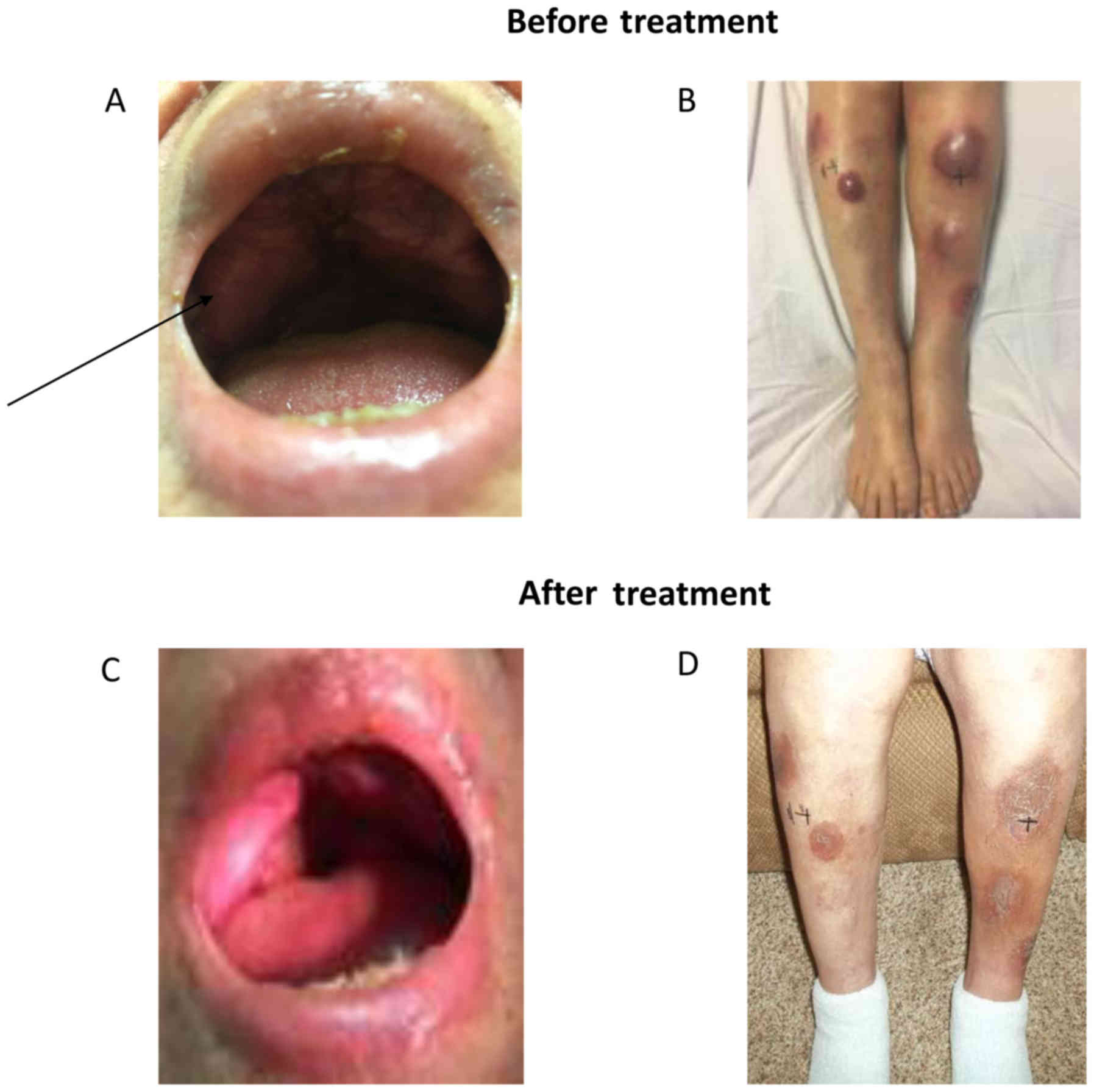
scattered erythematous nodular skin lesions (Fig. 1). A biopsy from the hard palate
lesions revealed an atypical population of intermediate to large
lymphoid cells with a diffuse growth pattern. Immunohistochemical
examination revealed positive staining for CD3 (membranous and
cytoplasmic), CD43, CD56, multiple myeloma oncogene 1, perforin,
granzyme B and T-cell intracellular antigen. The tumor cells were
negative for CD4, CD5, CD7, CD8, CD15, CD20, CD30, anaplastic
lymphoma kinase-1, B-cell lymphoma (BCL)-2, BCL-6 and EBV-in
situ hybridization. Ki-67 was positive in 90% of the neoplastic
cells. Serum EBV polymerase chain reaction was negative. Based on
morphology and immunophenotypic characteristics, the findings were
consistent with EBV-negative ENKTL. Position emission
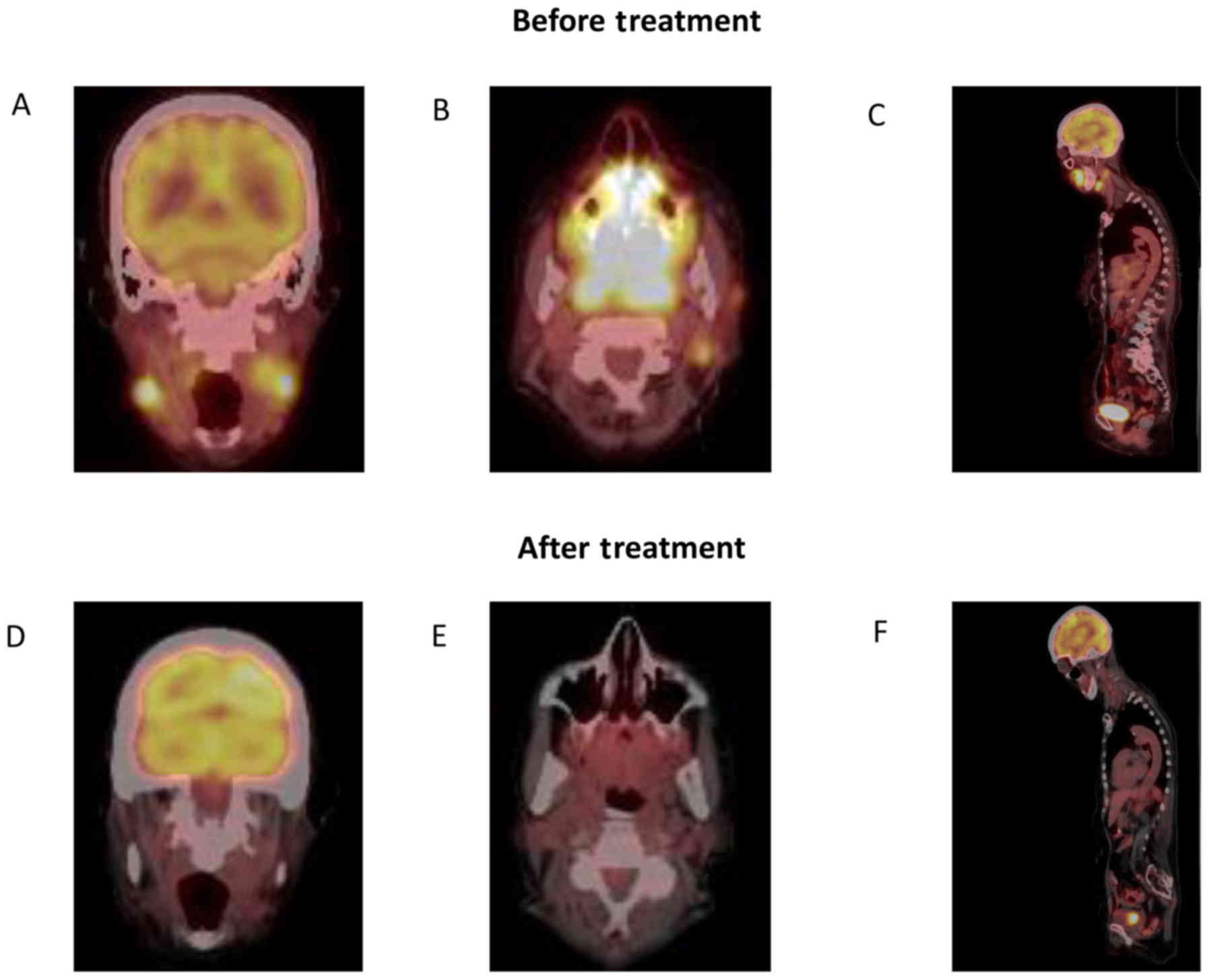
tomography-computed tomography (PET-CT) examination demonstrated a
nasopharyngeal mass measuring 4.5×3.5 cm, a left submandibular mass
measuring 2.6×2 cm, as well as multiple fluorodeoxyglucose-avid
cervical lymph nodes, several bilateral infiltrative breast masses
and subcutaneous nodules in the gluteal region of the left leg and
right calf; these findings were consistent with disseminated stage
IV ENKTL (Fig. 2A). PD-L1 staining
was positive in 25% of the tumor cells. Given the patient's
advanced age and Eastern Cooperative Oncology Group performance
status score of 3, she was not considered a candidate for
aggressive chemotherapy. Therefore, treatment was selected based on
the published experience of Kwong et al (6) on 7 patients with refractory ENKTL
treated with pembrolizumab. The patient received 200 mg
pembrolizumab every 3 weeks with concurrent radiation to the hard
palate and skin nodules over the left leg, followed by maintenance
pembrolizumab 200 mg every 3 weeks as compassionate use, and she
tolerated the treatment well. The main treatment-related side
effect in our patient was hypophosphatemia, which persisted for 3
months and responded to IV phosphate treatment. The lesions of the
palate and skin responded to this treatment (Fig. 1B), and a PET scan at 3 months showed
a notable response to treatment (Fig.
2B). Unfortunately, at the end of the 6th cycle, the patient
experienced worsening of the lower extremity nodules and appearance
of new cutaneous masses, and received a modified second-line
regimen including pegaspargase, gemcitabine and oxaliplatin
(P-GEMOX). After the first cycle, the lactate dehydrogenase level
was normalized, and the cutaneous and visceral masses
regressed.
However, P-GEMOX was poorly tolerated and patient
clinical course was complicated by pseudomonas sepsis. On July 30,
2018, she had no evidence of lymphoma progression but due to poor
performance status the patient's family eventually decided to
pursue hospice care.
Discussion
The present case report demonstrated that PD-1
blockade concurrently with radiation and as maintenance therapy may
be a viable treatment option for aggressive NK-cell lymphomas.
Treatment with pembrolizumab appears to be feasible as well as
effective in clinically naïve patients.
PD-1, a member of the CD28/CTLA4 family expressed on
activated T-cells, is normally involved in immune tolerance and
prevention of tissue damage associated with chronic inflammation.
Importantly, inhibition of PD-1/PD-L1 has demonstrated high
response rates in patients with classical Hodgkin's lymphoma
(7). Pembrolizumab is an IgG4
isotype antibody that targets the PD-1 receptor on lymphocytes.
Antibody binding to the ligand PD-L1 delivers an inhibitory signal,
reducing cytokine production and proliferation. Kwong et al
(6) recently published their
experience on 7 patients with relapsed EBV-positive ENKTL. Of the 7
patients, 6 received 2 mg/kg pembrolizumab every 3 weeks and 1
patient received 2 mg/kg every 2 weeks. All the patients responded
to pembrolizumab. In that study, strong PDL1 expression was
associated with higher response rates. Li et al (8) administered pembrolizumab to 7 patients
at 100 mg every 3 weeks and reported a 57% overall response rate,
but did not observe a direct correlation between the expression of
PD-L1 and clinical response. As our patient had EBV-negative ENKTL,
PD-L1 expression on tumor tissue (25% in this case) was used to
guide pembrolizumab therapy. Second, pembrolizumab 200 mg every 3
weeks was used concurrently with radiation and then as maintenance
treatment, which was well-tolerated in the present case.
Interestingly, complete response in the hard palate was sustained
right up to the time when the patient transitioned to hospice care.
This may be attributed to concurrent radiation and
immunotherapy.
In advanced solid tumors, the combination of
pembrolizumab and radiation has been proven to be safe and has been
shown to enhance T-cell response and augment the abscopal effect on
these tumors (9).
This was hypothesized to be the reason for our
patient achieving complete response in the hard palate. However,
after progression of the lymphoma at other sites, including soft
tissue and skin, the treatment was changed to modified P-GEMOX for
1 cycle. There was radiographic response to this treatment, but the
patient's course was complicated by pseudomonas pneumonia.
Eventually, the patient's family decided to pursue hospice care.
Taking into consideration all available treatments, our experience
suggests that first-line pembrolizumab may be a feasible option in
elderly patients who have limited treatment options, and it may be
preferred over palliative chemotherapy.
Acknowledgements
The authors would like to thank Merck & Co.,
Inc., (Whitehouse Station, NJ, USA) for providing pembrolizumab
through their expanded access program.
Funding
No funding was received.
Availability of data and materials
The datasets used/or analyzed during the present
study are available from the corresponding author upon request.
Authors' contributions
SA, JB, RF and SR designed the study and drafted the
manuscript. SA also retrieved pathology images. JB, RF and SA
reviewed the patient's history, clinical and imaging data. SR
supervised the entire project. MB and AM reviewed and drafted the
manuscript. All the authors have read and approved the final
version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided informed consent in advance via
the formal institutional form for the publication of this report
and any accompanying images.
Competing interests
SR is a member of advisory board and speaker bureau
for Takeda, Novartis, Janssen and Amgen. SR received honoraria, has
a consulting/advisory role and participates in the speaker's
bureaus of Takeda, Novartis, Janssen, Amgen and Celgene. SR has no
competing interests regarding the preparation of this manuscript
and did not receive any financial grants. AM received honoraria,
has a consulting/advisory role and participates in the speaker's
bureaus of Bristol-Myers Squibb, Boehringer Ingelheim and Biocept.
SA, MB, JB and RF declare that they have no competing interests to
disclose.
References
|
1
|
Sun J, Yang Q, Lu Z, He M, Gao L, Zhu M,
Sun L, Wei L, Li M, Liu C, et al: Distribution of lymphoid
neoplasms in China: Analysis of 4,638 cases according to the World
Health Organization classification. Am J Clin Pathol. 138:429–434.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tse E and Kwong YL: The diagnosis and
management of NK/T-cell lymphomas. J Hematol Oncol. 10:852017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nicolae A, Ganapathi KA, Pham TH, Xi L,
Torres-Cabala CA, Nanaji NM, Zha HD, Fan Z, Irwin S, Pittaluga S,
et al: EBV-negative aggressive NK-cell leukemia/lymphoma: Clinical,
pathologic, and genetic features. Am J Surg Pathol. 41:67–74. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao J, Behdad A, Ji P, Wolniak KL,
Frankfurt O and Chen YH: EBV-negative aggressive NK-cell
leukemia/lymphoma: A clinical and pathological study from a single
institution. Mod Pathol. 30:1100–1115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vari F, Arpon D, Keane C, Hertzberg MS,
Talaulikar D, Jain S, Cui Q, Han E, Tobin J, Bird R, et al: Immune
evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more
prominent in Hodgkin lymphoma than DLBCL. Blood. 131:1809–1819.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwong YL, Chan TSY, Tan D, Kim SJ, Poon
LM, Mow B, Khong PL, Loong F, Au-Yeung R, Iqbal J, et al: PD1
blockade with pembrolizumab is highly effective in relapsed or
refractory NK/T-cell lymphoma failing l-asparaginase. Blood.
129:2437–2442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Cui Y, Sun Z, Zhang L, Li L, Wang X,
Wu J, Fu X, Ma W, Zhang X, et al: DDGP versus SMILE in newly
diagnosed advanced natural killer/T-cell lymphoma: A randomized
controlled, multicenter, open-label study in China. Clin Cancer
Res. 22:5223–5228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Cheng Y, Zhang M, Yan J, Li L, Fu X,
Zhang X, Chang Y, Sun Z, Yu H, et al: Activity of pembrolizumab in
relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 11:152018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Dong Y, Kong L, Shi F, Zhu H and Yu
J: Abscopal effect of radiotherapy combined with immune checkpoint
inhibitors. J Hematol Oncol. 11:1042018. View Article : Google Scholar : PubMed/NCBI
|