Introduction
Submucosal heterotopic gastric gland (SHGG) is a
rare entity characterized by ectopic proliferation of gastric
glandular elements in the lamina propria and reported in 3.0–20.1%
of gastric cancer cases worldwide (1,2). Gastric
cancer is a common malignant tumor reported to be the seventh
leading cause of cancer mortality worldwide, and the second-most
frequent cause of cancer-related deaths in Japan (3,4). While
SHGG is considered a benign disease, and close follow-up by
endoscopy is the recommended treatment strategy, a few cases of
gastric carcinogenesis associated with SHGG have been reported
(1,2,5). Herein,
we report a case of early gastric cancer with multiple SHGGs
treated by distal gastrectomy.
Case report
An 85-year-old Japanese man with a past history of
right hemicolectomy for an ascending colon cancer 11 years earlier
visited his local doctor for a periodic medical examination.
Esophagogastroduodenoscopy (EGD) revealed an early gastric cancer
diagnosed as adenocarcinoma on biopsy, and the patient was referred
to our hospital. Physical examination on admission was unremarkable
and the laboratory findings were as follows: normal red blood cell
count (441×104/mm3; normal range,
435–555×104/mm3), normal white blood cell
count (5.8×103/mm3; Normal range,
3.3–8.6×103/mm3), and elevated C-reactive
protein levels (0.16 mg/dl; normal range, <0.14 mg/dl). An
analysis of serum tumor markers revealed elevated carcinoembryonic
antigen levels (4.5 ng/ml; normal range, <3.5 ng/m), elevated
carbohydrate antigen (CA) 72-4 levels (7.3 U/ml; normal range,
<5.3 U/ml), Normal CA 125 levels (16.9 U/ml; normal range,
<35 U/ml), normal CA 19-9 levels (19.6 U/ml; normal range,
<37 U/ml), and normal alpha-fetoprotein levels (4.8 ng/ml;
normal range, <100 ng/ml). The carbon-13 urea breath test to
examine Helicobacter pylori infection was positive.
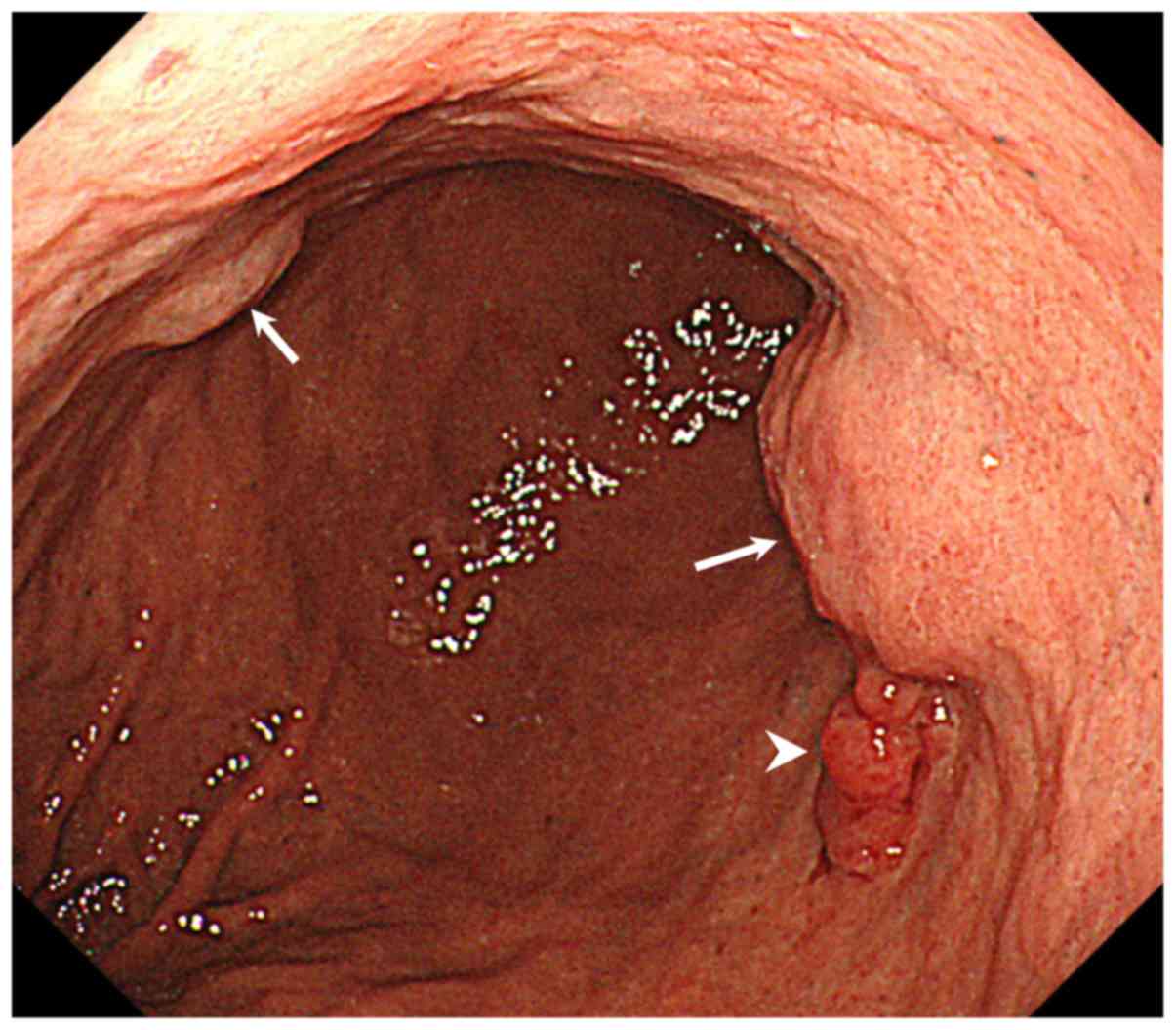
A second EGD showed an irregular nodular elevated
lesion on the greater curvature side of the middle third of the
stomach that was diagnosed on biopsy as well-differentiated
adenocarcinoma. Submucosal tumor (SMT)-like lesions were also
detected in an adjacent area on the anterior wall of the middle
third of the stomach (Fig. 1).
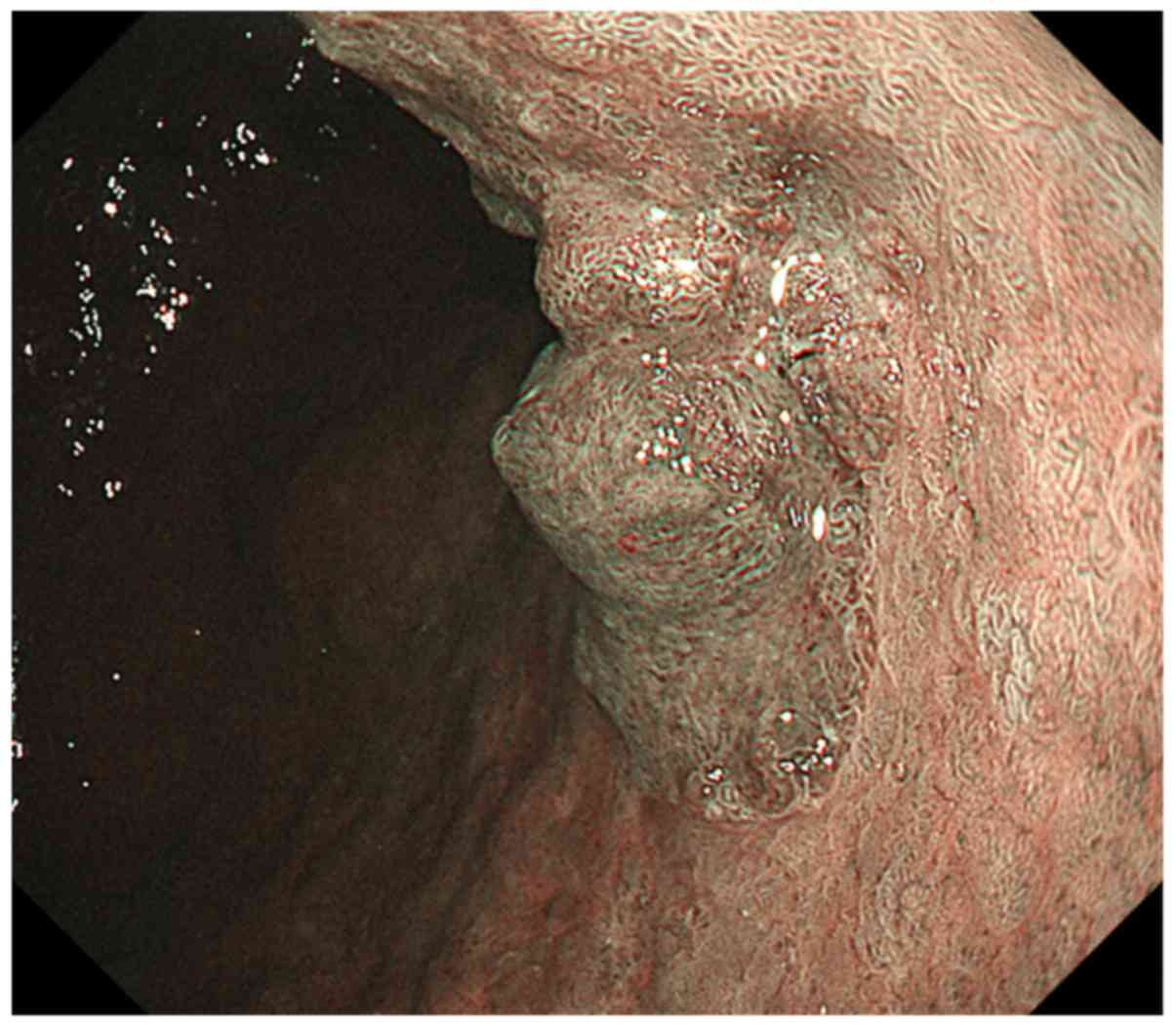
Magnifying endoscopy with narrow-band imaging showed irregular
microvascular and microsurface pattern in nodular elevated lesion,
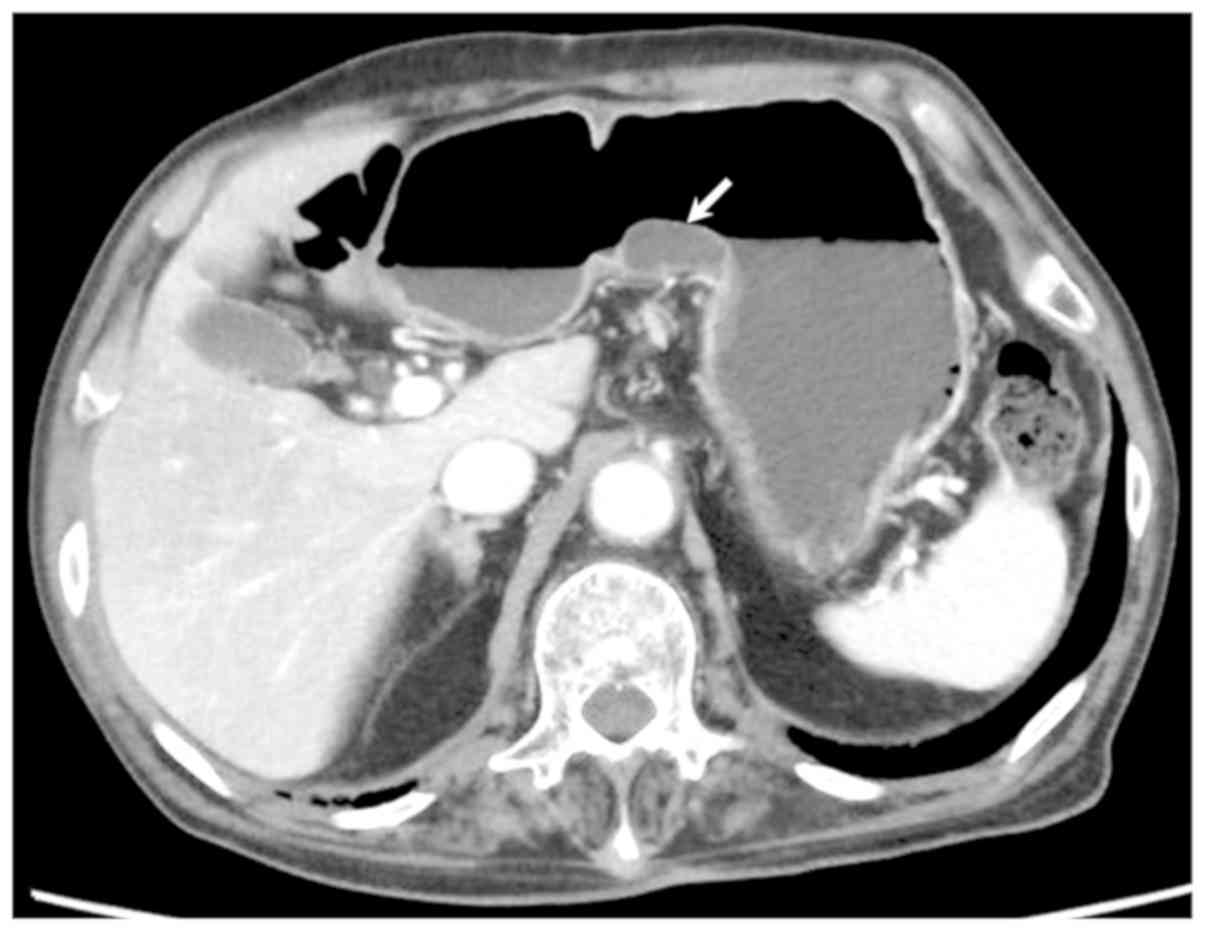
and normal pattern in SMT-like lesion (Fig. 2). Abdominal contrast-enhanced
computed tomography (CT) showed cystic lesions in the middle part
of the stomach, but no enlarged perigastric lymph nodes or mass
lesions in the liver (Fig. 3). Under
a clinical diagnosis of T1N0M0, stage IA according to the 8th
International Union Against Cancer (UICC) TNM classification
(6), the patient underwent distal
gastrectomy with regional lymphadenectomy followed by Billroth I
reconstruction.
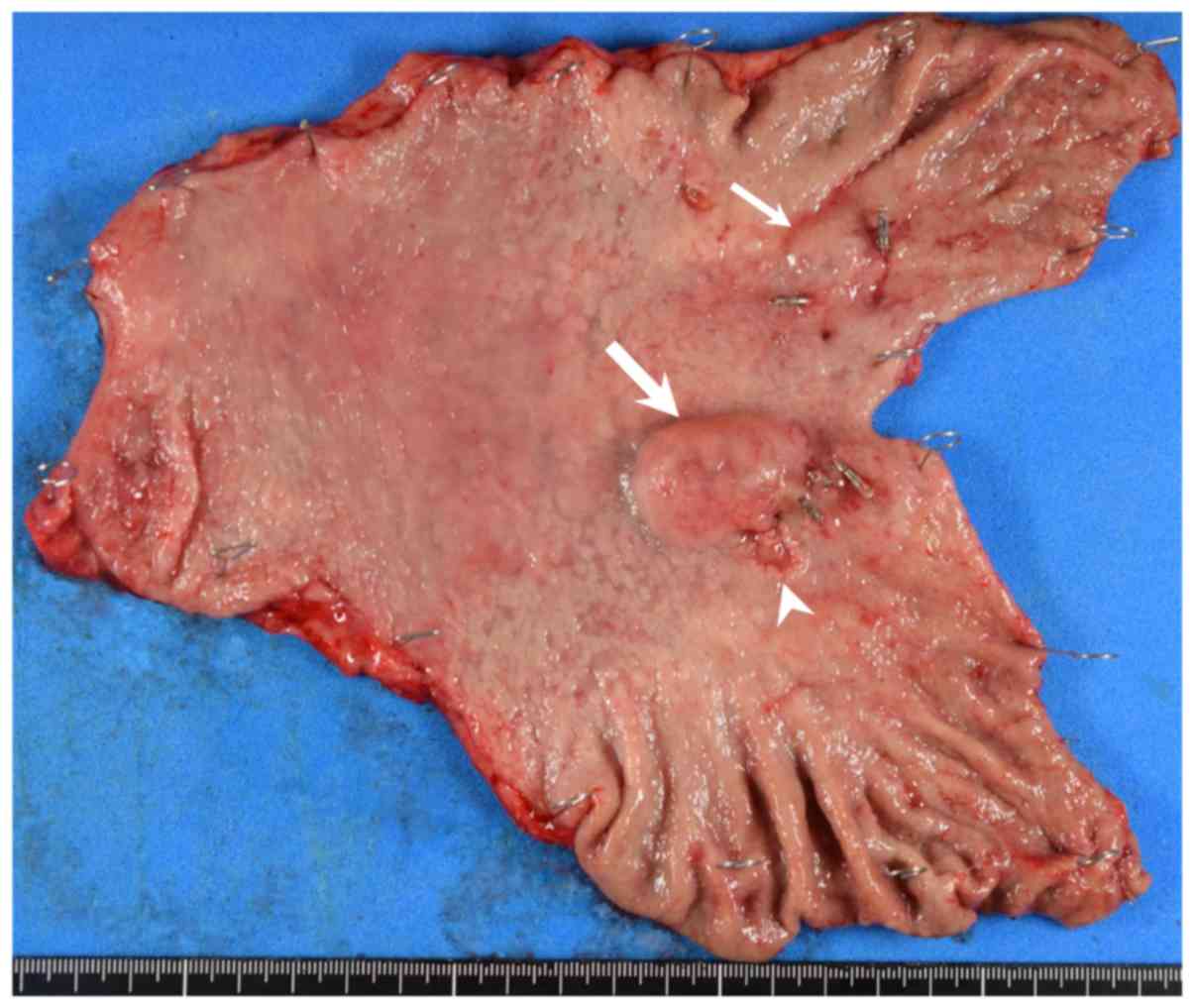
Macroscopic examination of the resected specimen
showed an SMT-like lesion measuring 2.8×2.6 cm in contact with a
superficial depressed lesion measuring 1.7×0.9 cm in the posterior
wall of the middle third of the stomach, and another SMT-like
lesion measuring 1.5×1.4 cm in the anterior wall of the middle
third of the stomach (Fig. 4). The
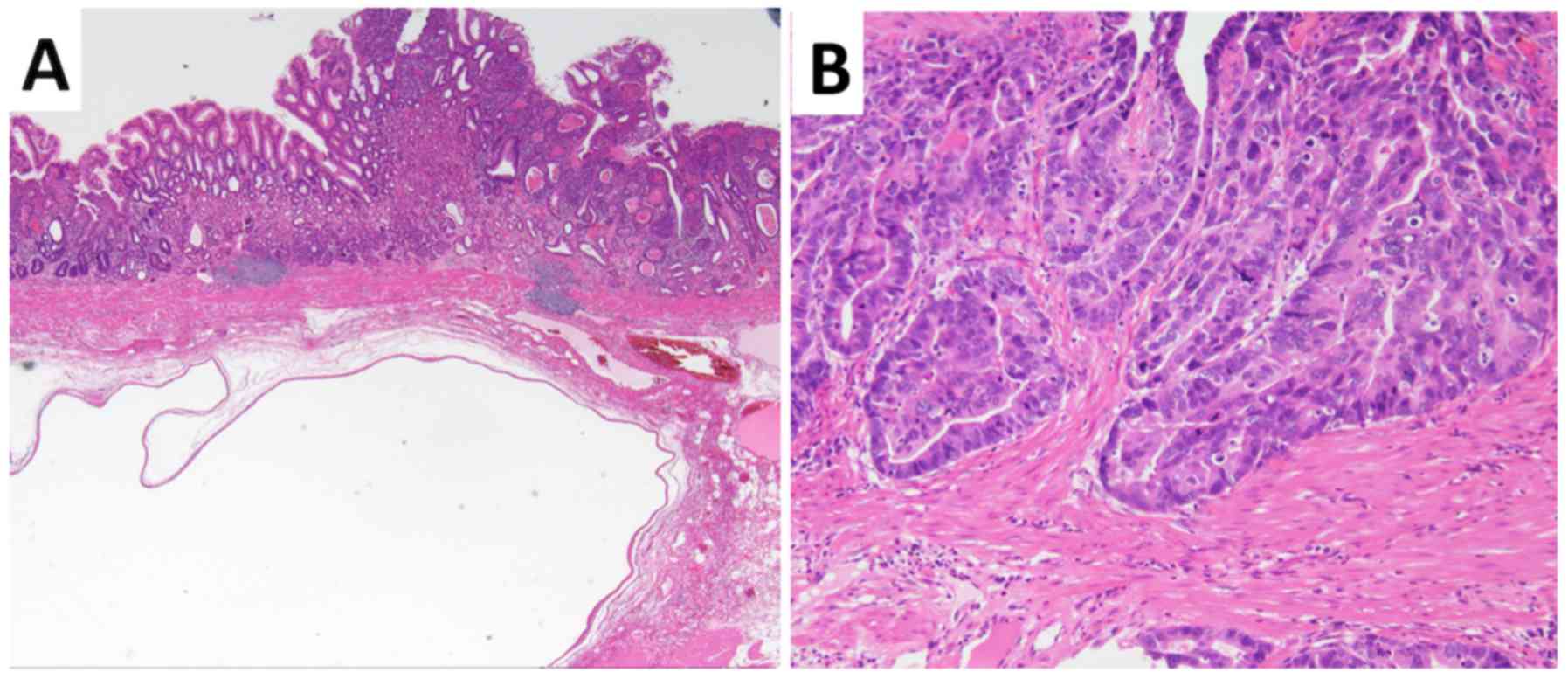
superficial depressed lesion was diagnosed pathologically as
well-differentiated tubular adenocarcinoma invading the gastric
submucosal layer (Fig. 5). The
SMT-like lesions consisted of glandular structures, showing
irregular branching and cystic formation with no dysplasia, and
were diagnosed as SHGG (Fig. 5).
There were no signs of malignancy in the SHGG, and neither
lymphovascular invasion nor lymph node metastasis was evident.
Following an uneventful postoperative course, the
patient was discharged on postoperative day 10 and has been well
with no evidence of recurrence for 3 months following the
operation. Written informed consent was obtained from the patient
for publication of this Case Report and any accompanying
images.
Discussion
We presented a rare case of SHGG in a patient with
gastric cancer who underwent distal gastrectomy. SHGG is a
relatively rare entity that is considered benign and is rarely
associated with malignant transformation (7). To the best of our knowledge, this is
the first reported case of multiple SHGG presenting as SMT-like
lesions accompanying gastric cancer.
SHGG is thought to arise from gastric glands
existing congenitally in the submucosa, or from aberration of the
epithelium into the submucosa as a result of repeated erosion and
regeneration of the mucosa (1,8). The
present case showed no obvious pathological continuity between the
SHGG and gastric cancer, despite the two lesion types being in
close proximity, and indeed, little is known about the possibly
carcinogenesis of SHGG (1,2,5,9). The dominant purported mechanism
regarding an association between SHGG and gastric cancer is that
both entities develop in coincidence with repeated erosion and
regeneration of the mucosa, and that SHGG are paracancerous lesions
(8,9). In support of this, the histological
characteristics of SHGG with cystic expansion are similar to
gastritis cystic profunda (GCP), which can occur at anastomotic
sites after gastrectomy (10). GCP
is an uncommon, hyperplastic, benign lesion characterized by cystic
dilatation of the gastric glands extending into the submucosa of
the stomach that follows mucosal injury caused by a surgical
procedure or suturing technique promoting mucosal prolapse and
herniation of glands into the submucosa (10,11). GCP
therefore usually occurs at a gastroenterostomy site, although it
can also be found in undisturbed stomach, and it is considered a
potentially precancerous lesion (10).
As other similar potentially precancerous lesion,
Barrett's esophagus is thought to be a premalignant condition,
which has strong association with esophageal adenocarcinoma. Abbasi
et al reported that Barrett's epithelial metaplasia that
completely eliminated after several years of treatment with proton
pump inhibitor (12). A reduction of
cancer risk might be anticipated if treatment could induce a
regression of Barrett's epithelial metaplasia.
SHGG can present as elevated lesions covered with
normal mucosa such as SMT in EGD (13). In the case of gastric cancer
originated from SHGG is difficult to diagnose, because cancer
exists at the submucosa, and cancer components are not exposed on
the surface (14). Endoscopic
ultrasonography (EUS) is considered a useful modality for both
detecting SHGG and judging the depth of lesion invasion, because it
can reveal hypoechoic scattered cystic lesions within a
heterogeneous area (13,15). In the present case, the pathologic
evaluation led to a diagnosis of SHGG after surgery. Based on the
EUS findings, fine needle biopsy might be useful to diagnose this
entity preoperatively.
There are no previous reports of gastric cancer with
multiple SHGG presented as SMT-like lesions in the English
literature; however, Tsuji et al (16) reported multiple early gastric tumors
of varied histological types associated with GCP, and speculated
that repeated erosion and regeneration induced by chronic
inflammation causes multicentric carcinogenesis as well as an
aberration of the gastric glands. Accordingly, multiple SHGG as
well as GCP might be risk factors for gastric cancer.
In conclusion, the association of gastric cancer and
SHGG remains controversial with respect to both carcinogenesis and
patient treatment strategies. Nevertheless, it is important to take
SHGG into consideration for the differential diagnosis of SMT of
the stomach, and further assessments by accumulation of additional
cases are needed to understand the various presentations of this
rare entity.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
TN contributed to the writing of the manuscript. MK
and KH supervised the study. NI, KY, EM, JI, MM, SU, HM and HK
served as the attending physicians for the presented patient. All
the authors have read and approved that final version of this
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient has given consent for the publication of
the case details and associated images.
Competing interests
The authors declare that they have no competing
interests to disclose.
References
|
1
|
Hagiwara T, Kakushima N, Imai K, Tanaka M,
Takao T, Hotta K, Yamaguchi Y, Takizawa K, Matsubayashi H, Ono H,
et al: Early gastric cancer with spreading to heterotopic gastric
glands in the submucosa: A case report and review of the
literature. Clin J Gastroenterol. 7:123–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kosugi S, Kanda T and Hatakeyama K:
Adenocarcinoma arising from heterotopic gastric mucosa in the
stomach. J Gastroenterol Hepatol. 21:483–484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nashimoto A, Akazawa K, Isobe Y, Miyashiro
I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, et
al: Gastric cancer treated in 2002 in Japan: 2009 annual report of
the JGCA nationwide registry. Gastric Cancer. 16:1–27. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fidler MM, Gupta S, Soerjomataram I,
Ferlay J, Steliarova-Foucher E and Bray F: Cancer incidence and
mortality among young adults aged 20–39 years worldwide in 2012: A
population-based study. Lancet Oncol. 18:1579–1589. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Imamura T, Komatsu S, Ichikawa D,
Kobayashi H, Miyamae M, Hirajima S, Kawaguchi T, Kubota T, Kosuga
T, Okamoto K, et al: Gastric carcinoma originating from the
heterotopic submucosal gastric gland treated by laparoscopy and
endoscopy cooperative surgery. World J Gastrointest Oncol.
7:118–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours. 8th. New
York: John Wiley & Sons; 2017
|
|
7
|
Rubio CA and Mandai K: Gastric
adenocarcinomas in displaced mucosal glands. Anticancer Res.
19:2381–2385. 1999.PubMed/NCBI
|
|
8
|
Iwanaga T, Koyama H, Takahashi Y,
Taniguchi H and Wada A: Diffuse submucosal cysts and carcinoma of
the stomach. Cancer. 36:606–614. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manabe S, Mukaisho KI, Yasuoka T, Usui F,
Matsuyama T, Hirata I, Boku Y and Takahashi S: Gastric
adenocarcinoma of fundic gland type spreading to heterotopic
gastric glands. World J Gastroenterol. 23:7047–7053. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Namikawa T, Kawanishi Y, Fujisawa K,
Munekage E, Munekage M, Maeda H, Kitagawa H, Kobayashi M and
Hanazaki K: Gastric adenocarcinoma at the anastomotic site 50 years
after gastrojejunostomy: A case report. Mol Clin Oncol. 7:249–251.
2017.PubMed/NCBI
|
|
11
|
Lee TH, Lee JS and Jin SY: Gastritis
cystica profunda with a long stalk. Gastrointest Endosc.
77:821–822; discussion 822. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abbasi BA, Rubaye AA and Ahmed N: Complete
disappearance of Barrett's epithelial metaplasia following long
term proton pump inhibitor therapy. J Med Discov.
2:jmd170162017.
|
|
13
|
Hizawa K, Matsumoto T, Kouzuki T, Suekane
H, Esaki M and Fujishima M: Cystic submucosal tumors in the
gastrointestinal tract: Endosonographic findings and endoscopic
removal. Endoscopy. 32:712–714. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itoh K, Tsuchigame T, Matsukawa T,
Takahashi M, Honma K and Ishimaru Y: Unusual gastric polyp showing
submucosal proliferation of glands: Case report and literature
review. J Gastroenterol. 33:720–723. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hashimoto R, Hamamoto H, Omori Y and
Tanuma T: Early gastric cancer on submucosal heterotopic gastric
glands. Gastrointest Endosc. 85:851–852. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsuji T, Iwahashi M, Nakamori M, Ueda K,
Ishida K, Naka T, Ojima T, Akamatsu H and Yamaue H: Multiple early
gastric cancer with gastritis cystica profunda showing various
histological types. Hepatogastroenterology. 55:1150–1152.
2008.PubMed/NCBI
|