Introduction
Lung cancer is the leading cause of cancer death
worldwide, accounting for 19.4% of cancer deaths in adults
(1). It is essentially divided into
two types: Small-cell lung cancer (approximately 15%) and non-small
cell lung cancer (NSCLC) (approximately 85%). Despite advances in
early detection and standard treatment, NSCLC is still often
diagnosed at an advanced stage and has a poor prognosis (2).
In search of a biomarker to predict the prognosis of
NSCLC patients, there has been accumulating evidence reported of
the usefulness of inflammation- or nutritional factor-based
prognostic scores, such as the Glasgow prognostic score (GPS) based
on C-reactive protein (CRP) and albumin (3). Previously, we reported significance of
the high-sensitivity modified GPS in patients with resectable NSCLC
(4). However, as these scoring
systems require calculation using several variables, a simpler
preoperative prognostic biomarker is needed.
Transthyretin (TTR), also known as prealbumin, has a
relatively short half-life (approximately two days), and is the
earliest laboratory indicator of malnutrition status because it
contains a high percentage of essential amino acids (5). TTR correlates with patient outcome in
various diseases (6–8). With regard to lung cancer, TTR has been
reported to be useful in distinguishing lung cancer from lung
infection (9), and to be synthesized
in lung cancer cells (10). Low
perioperative serum TTR was reported to predict early recurrence
after curative-intent surgery in NSCLC patients (11). Although Alifano et al
(12) reported that TTR was useful
to predict prognosis of NSCLC patients in relation to CRP and CD8+
lymphocyte, its prognostic value remains to be determined. Thus, we
sought to examine the preoperative prognostic impact of serum TTR
in patients with NSCLC in relation to other nutritional and
inflammatory factors.
Patients and methods
Study population
Forty-two NSCLC patients were prospectively enrolled
between September, 2011 and September, 2012 (25 men and 17 women,
mean age 68.3; range 50 to 85 years, 24 patients with
adenocarcinoma and 18 patients with squamous cell carcinoma).
Patient characteristics are summarized in Table I. Among these, 35 patients underwent
curative-intent surgery. Following surgery, cancer stage of the
patients was determined pathologically according to the TNM
classification system of malignant tumors published by the Union
for International Cancer Control, eighth edition (13). All operated patients underwent
lobectomy with lymphadenectomy without preoperative chemotherapy or
radiotherapy. Adjuvant chemotherapy was performed on eight out of
the 35 operated patients. The study protocol was approved by the
Ethics Committee of Fukushima Medical University (Fukushima, Japan;
approval no. 1095) and written informed consent was obtained from
the enrolled patients.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Clinical
characteristics | Total n | % |
|---|
| Age, years |
|
|
|
<75 | 27 | 64.3 |
| ≥75 | 15 | 35.7 |
| Sex |
|
|
| Male | 25 | 59.5 |
|
Female | 17 | 40.5 |
| Histology |
|
|
|
Adeno | 24 | 57.1 |
|
Squamous | 18 | 42.9 |
| T |
|
|
| 1a | 0 | 0.0 |
| 1b | 13 | 31.0 |
| 1c | 13 | 31.0 |
| 2a | 6 | 14.3 |
| 2b | 5 | 11.9 |
| 3 | 3 | 7.1 |
| 4 | 2 | 4.8 |
| N |
|
|
| 0 | 27 | 64.3 |
| 1 | 4 | 9.5 |
| 2 | 9 | 21.4 |
| 3 | 2 | 4.8 |
| M |
|
|
| 0 | 40 | 95.2 |
| 1 | 2 | 4.8 |
| Stage |
|
|
| I | 21 | 50.0 |
| II | 9 | 21.4 |
|
III | 10 | 23.8 |
| IV | 2 | 4.8 |
Measurements of TTR and other
parameters
Blood samples were collected before treatment
initiation. Patient nutritional status was determined by measuring
the serum concentrations of total protein, albumin, retinol binding
protein (RBP), TTR, and transferrin (TF). These parameters were
measured at the Central Clinical Laboratory of Fukushima Medical
University Hospital. As for the inflammatory parameters, CRP, white
blood cell count (WBC), neutrophil, lymphocyte and monocyte counts,
as well as neutrophil-to-lymphocyte ratio (NLR) and
lymphocyte-to-monocyte ratio (LMR), were used. With regard to tumor
markers, carcinoembryonic antigen (CEA) (40 patients) and
cytokeratin-19 fragment (38 patients) were evaluated. Squamous cell
carcinoma antigen was tested when a patient had squamous cell
carcinoma (17 out of the 18 patients with squamous cell
carcinoma).
As for the immunological cytokines, the productivity
of interleukin (IL)-10, 12, and 17 were examined. Peripheral blood
mononuclear cells were separated on Ficoll-Hypaque
(Pharmacia-Biotech, Uppsala, Sweden) columns, and washed twice with
Roswell Park Memorial Institute media (RPMI)-1640 (Wako Pure
Chemical Industries Ltd., Osaka, Japan). The isolated PBMCs were
then incubated in one ml of RPMI-1640 at a concentration of
106 cells/ml with 10% heat-inactivated fetal calf serum
(Gibco BRL, St. Louis, MO, USA) in 5% CO2 at 37°C for 24
h with the following stimulations: 20 µg/ml phytohemagglutinin for
IL-10 and IL-17 production assays, and 0.01% of Staphylococcus
aureus cowan-1 for IL-12 production assays. Aliquots of these
supernatants were then frozen and stored at −80°C until use.
Supernatant samples were subsequently thawed and used for the
measurement of IL-10, IL-12, and IL-17 concentrations using
enzyme-linked immunosorbent assay (R&D Systems, Minneapolis,
MN, USA). Each sample was used only once after thawing, and not all
blood samples were of sufficient volume for all measurements.
Statistical analysis
Data are presented as frequencies or percentages for
categorical variables and mean ± standard deviation for continuous
variables, unless otherwise indicated. For categorical clinical
variables, differences between two groups were evaluated by
chi-square test or Fisher's exact test. The differences in mean
values between the groups were analyzed using the Mann-Whitney U
test. One-way analysis of variance was used for comparisons of
variables between more than two groups with Tukey's post hoc test
for multiple comparisons. Associations between the two variables
were quantified using Spearman's rank correlation coefficient.
With regard to survival analysis, the mean
observation period was 67.9 months (range: 60.6–73.8). The final
assessment of disease status was made on September 30, 2017. The
overall survival rate was calculated using the Kaplan-Meier method
and differences between the groups were assessed using the log-rank
test. The TTR cutoff value for evaluation as a prognostic factor
was set at 22 mg/dl according to its normal range (≥22 mg/dl) at
our institution. According to our previous study (4) the albumin, CRP and NLR cutoff values,
were set at 3.5 g/dl, 0.3 mg/dl and 4.5, respectively. Prognostic
factor candidates were subjected to univariate and multivariate
analysis using a Cox proportional hazard model to identify
independent predictors of prognosis. When a P-value of a factor was
under 0.05 in the univariate analysis, the factor was also analyzed
in the multivariate analysis. A two-sided P<0.05 was considered
to indicate a statistically significant difference. All statistical
calculations were performed using SPSS version 24 software (IBM,
Corp., Armonk, NY, USA).
Results
Relationship between TTR and
clinicopathological features
As shown in Fig. 1,
serum TTR levels exhibited significant correlations with serum
albumin (r=0.710, P<0.001), RBP (r=0.792,
P<0.001), TF (r=0.480, P=0.002) and LMR (r=0.391,
P=0.010), but also showed significantly inverse correlations
with age (r=−0.606, P<0.001), CRP (r=−0.414,
P=0.007) and NLR (r=−0.341, P=0.027). When TTR levels
were compared with T categories (T4 was excluded due to its small
number), T categories had a statistically significant effect on TTR
levels using one-way ANOVA {F (2, 37)=6.533, P=0.004} (Fig. 2A). In an analysis of the post hoc
test using Turkey's method, TTR levels of the patients with T3
showed statistically lower than those with T1 (P=0.003) or T2
(P=0.005). In addition, IL-12-productivity, serum RBP, albumin and
TF levels, and LMR were significantly lower in the patients with
TTR <22 mg/dl than those in the patients with TTR ≥22 mg/dl
(Fig. 2B).
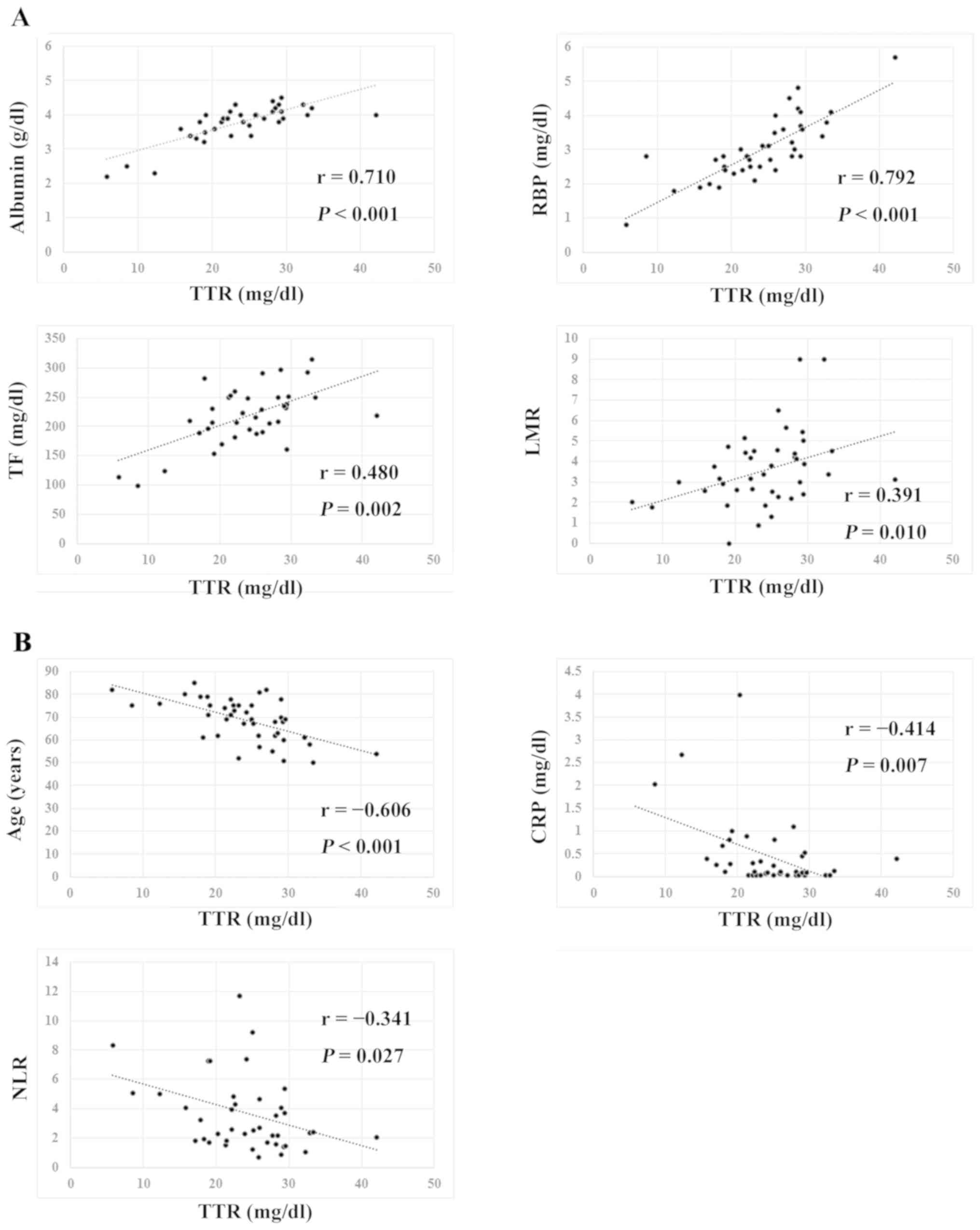 | Figure 1.Transthyretin correlation. (A) Serum
transthyretin levels exhibited significant correlations with serum
albumin (r=0.710, P<0.001), RBP (r=0.792, P<0.001), TF
(r=0.480, P=0.002) and LMR (r=0.391, P=0.010). (B) Serum
transthyretin levels exhibited significantly inverse correlations
with age (r=−0.606, P<0.001), CRP (r=−0.414, P=0.007) and NLR
(r=−0.341, P=0.027). TTR, transthyretin; RBP, retinol binding
protein; TF, transferrin; LMR, lymphocyte-to-monocyte ratio; CRP,
C-reactive protein; NLR, neutrophil-to-lymphocyte ratio. |
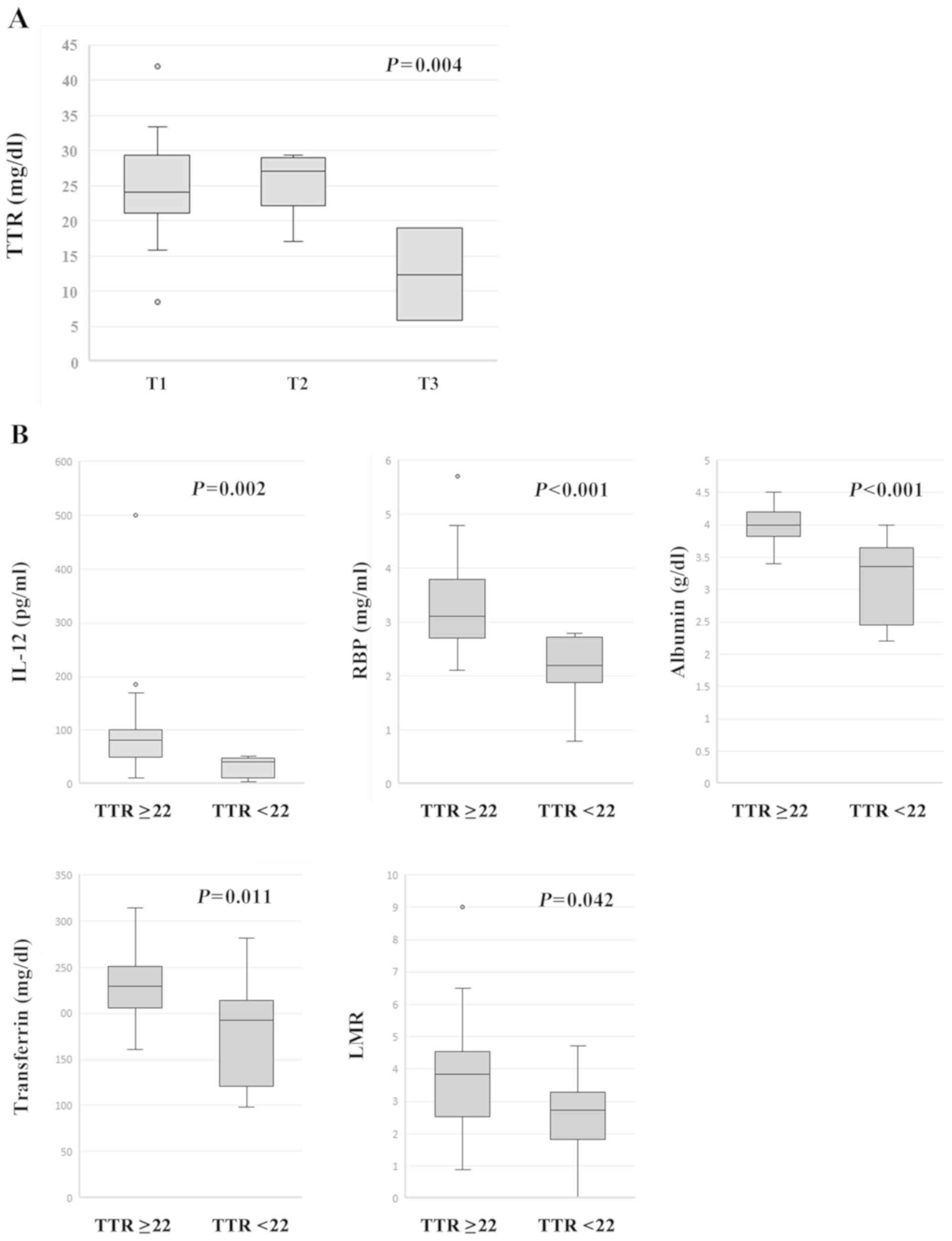 | Figure 2.Associations between serum TTR and
clinicopathological features. (A) When the TTR levels were compared
with T categories (T4 was excluded due to its small number), the T
categories were revealed to have a statistically significant effect
on TTR levels as determined by one-way analysis of variance {F (2,
37)=6.533, P=0.004}. Furthermore, following post hoc Tukey test
comparisons, the TTR levels of the patients with T3 were
significantly lower than those with T1 (P=0.003) or T2 (P=0.005).
(B) IL-12-productivity (P=0.002), serum RBP (P<0.001), albumin
(P<0.001) and transferrin levels (P=0.011) and LMR (P=0.042)
were significantly lower in the patients with TTR <22 mg/dl than
those in the patients with TTR ≥22 mg/dl. TTR, transthyretin;
IL-12, interleukin-12; RBP, retinol binding protein; LMR,
lymphocyte-to-monocyte ratio. |
Serum TTR level and prognosis
Candidates for independent predictors of prognostic
factors (age, <75 vs. ≥75; sex, male vs. female; T1 vs. ≥ T2;
lymph node metastasis negative vs. positive; lymphatic invasion
negative vs. positive; blood vessel invasion negative vs. positive;
serum albumin, <3.5 mg/dl vs. ≥3.5 mg/dl; CRP, <0.3 mg/dl vs.
≥0.3 mg/dl; NLR: <4.5 vs. ≥4.5; TTR, ≥22 mg/dl vs. <22 mg/dl)
were subjected to univariate and multivariate analysis using a Cox
proportional hazard model.
Patient characteristics according to the serum TTR
level are summarized in Table II.
The mean age of the patients with TTR <22 mg/dl (66.6±9.0 years)
was significantly higher than that of the patients with TTR ≥22
mg/dl (75.0±7.7 years) (P=0.005). In addition, the incidence of
abnormal CEA levels, albumin <3.5 mg/dl and CRP >0.3 mg/dl
was significantly higher in the patients with TTR <22 mg/dl than
in the patients with TTR ≥22 mg/dl (P=0.042, 0.004 and 0.022,
respectively). There were no other significant differences in the
demographics between the patients with TTR ≥22 mg/dl and TTR <22
mg/dl. Regarding the operated 35 patients, the mean age of the
patients with TTR <22 mg/dl (74.9±8.6 years) was higher than
that of the patients with TTR ≥22 mg/dl (67.4±8.8 years) (P=0.031),
and the incidence of abnormal CEA levels, albumin <3.5 mg/dl and
CRP >0.3 mg/dl was significantly higher in the patients with TTR
<22 mg/dl than in the patients with TTR ≥22 mg/dl (P=0.034,
0.006 and 0.007, respectively).
 | Table II.Patient characteristics according to
transthyretin level. |
Table II.
Patient characteristics according to
transthyretin level.
|
| Total patients
(n=42) | Operated patients
(n=35) |
|---|
|
|
|
|
|---|
|
Characteristics | TTR≥22 (n=31) | TTR<22
(n=11) | P-value (n=26) | TTR≥22 (n=9) | TTR<22 | P-value |
|---|
| Age | 66.6±9.0 | 75±7.7 | 0.005 | 64.7±8.8 | 74.9±8.6 | 0.031 |
| Gender |
|
| 0.151 |
|
| 0.121 |
|
Male | 16 | 9 |
| 11 | 7 |
|
|
Female | 15 | 2 |
| 15 | 2 |
|
| Histology |
|
| 0.483 |
|
| 0.451 |
|
Adeno | 19 | 5 |
| 16 | 4 |
|
|
Squamous | 12 | 6 |
| 10 | 5 |
|
| Size | 25.7±9.6 | 36.3±17.4 | 0.078 | 25.5±8.8 | 36.6±16.6 | 0.061 |
| T |
|
| 0.281 |
|
| 0.220 |
| T1 | 21 | 5 |
| 19 | 4 |
|
|
≥T2 | 10 | 6 |
| 7 | 5 |
|
| N |
|
| 1.000 |
|
| 1.000 |
|
Negative | 20 | 7 |
| 16 | 6 |
|
|
Positive | 11 | 4 |
| 10 | 3 |
|
| Stage |
|
| 0.437 |
|
| 0.694 |
|
I+II | 24 | 7 |
| 19 | 6 |
|
|
III+IV | 7 | 4 |
| 7 | 3 |
|
| CEA |
|
| 0.042 |
|
| 0.034 |
|
<5.0 | 24 | 5 |
| 21 | 4 |
|
|
≥5.0 | 5 | 6 |
| 4 | 5 |
|
| SCC |
|
| 0.206 |
|
| 0.400 |
|
<1.5 | 9 | 0 |
| 9 | 0 |
|
|
≥1.5 | 6 | 2 |
| 5 | 1 |
|
| Cyfra21-2 |
|
| 1.000 |
|
| 1.000 |
|
<3.5 | 26 | 9 |
| 22 | 8 |
|
|
≥3.5 | 2 | 1 |
| 2 | 0 |
|
| ALB |
|
| 0.004 |
|
| 0.006 |
|
≥3.5 | 25 | 5 |
| 24 | 4 |
|
|
<3.5 | 2 | 6 |
| 2 | 5 |
|
| CRP |
|
| 0.022 |
|
| 0.007 |
|
<0.3 | 23 | 3 |
| 22 | 3 |
|
|
≥0.3 | 8 | 7 |
| 4 | 6 |
|
| NLR |
|
| 0.243 |
|
| 0.396 |
|
<4.5 | 24 | 6 |
| 21 | 6 |
|
|
≥4.5 | 7 | 5 |
| 5 | 3 |
|
| Ly |
|
|
|
|
| 0.711 |
|
Negative | NA | NA |
| 14 | 4 |
|
|
Positive | NA | NA |
| 12 | 5 |
|
| V |
|
|
|
|
| 0.432 |
|
Negative | NA | NA |
| 9 | 5 |
|
|
Positive | NA | NA |
| 17 | 4 |
|
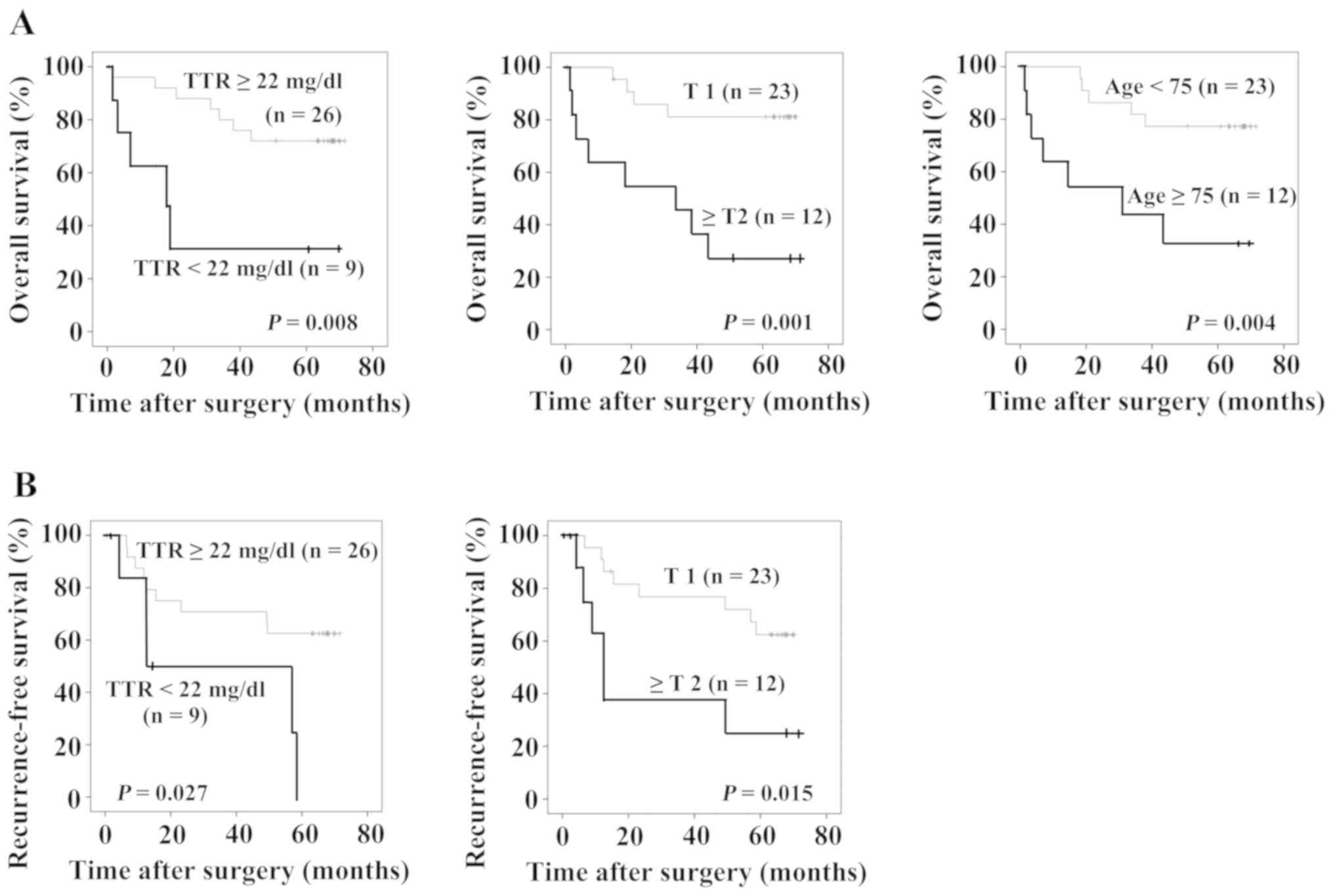
Fig. 3A shows overall
survival and Fig. 3B shows
recurrence-free survival of the patients. The patients with TTR
<22 mg/dl (P=0.008), the patients with T ≥2, and the patients
who were aged 75 years or older showed significantly poorer overall
prognoses (Fig. 3A). Furthermore,
the patients with TTR <22 mg/dl (P=0.027) and those with T ≥2
showed significantly poorer recurrence-free survival rates
(Fig. 3B). Table III shows the results of prognostic
factor analysis using a Cox proportional hazard model. TTR <22
mg/dl, ≥T2 and age ≥75 years were statistically significant in the
univariate analysis. In the multivariate analysis, ≥T2 (HR=7.161,
95% confidence interval, 1.959–26.172, P=0.003) and age ≥75 years
(HR=3.775, 95% confidence interval, 1.098–12.982, P=0.035) were
independent prognostic factors of overall survival. With regard to
recurrence-free survival, TTR <22 mg/dl and ≥T2 were
statistically significant in the univariate analysis. In the
multivariate analysis, TTR <22 mg/dl (HR=3.866, 95% confidence
interval, 1.223–12.220, P=0.021) and ≥T2 (HR=4.035, 95% confidence
interval, 1.341–12.143, P=0.013) were independent prognostic
factors of recurrence-free survival.
 | Table III.Cox proportional hazard model. |
Table III.
Cox proportional hazard model.
| A, Overall
survival |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Categories | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | 4.797 | 1.505–15.286 | 0.008b | 3.775 | 1.098–12.982 | 0.035a |
| Sex | 0.301 | 0.081–1.113 | 0.072 |
|
|
|
| T | 5.823 | 1.745–19.435 | 0.004b | 7.161 | 1.959–26.172 | 0.003b |
| N | 0.975 | 0.311–3.096 | 0.975 |
|
|
|
| Ly | 0.813 | 0.262–2.522 | 0.720 |
|
|
|
| V | 1.003 | 0.302–3.335 | 0.996 |
|
|
|
| ALB | 1.227 | 0.268–5.624 | 0.792 |
|
|
|
| CRP | 1.048 | 0.283–3.882 | 0.944 |
|
|
|
| NLR | 1.731 | 0.468–6.411 | 0.411 |
|
|
|
| TTR | 3.513 | 1.029–11.993 | 0.049a | 4.100 | 0.990–16.985 | 0.052 |
|
| B, Recurrence
free survival |
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
Categories | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Age, years | 1.444 | 0.452–4.613 | 0.535 | 1.444 | 0.452–4.613 |
|
| Sex | 0.888 | 0.311–2.536 | 0.825 | 0.888 | 0.311–2.536 |
|
| T | 3.482 | 1.194–10.154 | 0.022a | 3.482 | 1.194–10.154 | 0.013a |
| N | 2.080 | 0.721–6.002 | 0.176 | 2.080 | 0.721–6.002 |
|
| Ly | 1.958 | 0.613–6.253 | 0.257 | 1.958 | 0.613–6.253 |
|
| V | 1.245 | 0.390–3.974 | 0.711 | 1.245 | 0.390–3.974 |
|
| ALB | 0.428 | 0.056–3.278 | 0.414 | 0.428 | 0.056–3.278 |
|
| CRP | 1.726 | 0.574–5.192 | 0.311 |
|
|
|
| NLR | 0.718 | 0.160–3.215 | 0.665 |
|
|
|
| TTR | 3.254 | 1.070–9.896 | 0.038a | 3.866 | 1.223–12.220 | 0.021a |
Discussion
In the present study, the NSCLC patients with serum
TTR levels of <22 mg/dl showed a poorer overall and
recurrence-free survival than those with serum TTR levels of ≥22
mg/dl. Although TTR <22 mg/dl was not an independent prognostic
factor for overall survival, TTR <22 mg/dl was an independent
prognostic factors for recurrence-free survival. Alifano et
al (12) reported that TTR
<28 mg/dl was one of the independent prognostic factors for
overall survival in patients with NSCLC. However, they did not
assess recurrence-free survival. Moreover, Kawai and Ota (11) reported that preoperative TTR <23
mg/dl and postoperative TTR <15 mg/dl was an independent
prognostic factor for 2-year recurrence free survival. Thus, the
results of the current study revealed, for the first time, that
preoperative TTR was an independent prognostic factor for 5-year
recurrence-free survival in NSCLC patients.
The normal function of TTR is to transport thyroxine
and RBP/retinol complex in the blood (14). TTR exists in the blood as a
homotetramer and contains four tryptophans which are essential
amino acids (5). These
characteristics make TTR useful in assessing acute phase
nutritional condition. As TTR is also a member of rapid turnover
proteins, its level is affected by the presence of infections and
inflammation. Therefore, serum albumin in combination with CRP, has
been used to evaluate a prognosis of cancer patients (4). Recently, however, serum TTR level has
been reported to be a prognostic biomarker in various malignancies
such as esophageal (15), gastric
(16), colorectal (17), pancreatic (18), and renal cancers (19), as well as lung cancer (11,12).
Taken together, TTR might be associated with tumor
microinflammation, resulting in a poorer prognosis.
With regard to the immunological status in the
present study, IL-12 productivity and LMR were lower in the
patients with TTR <22 mg/dl. IL-12 is mainly secreted from
antigen presenting cells in response to pathogens, promoting CD4+
cell differentiation into Th1 cells, and enhances the cytotoxic
ability of both natural killer and CD8+ T cells (20). On the other hand, lower LMR has been
reported to be a useful independent prognostic marker in patients
with NSCLC (21). Although higher
LMR was firstly reported to be related with favorable prognosis in
some hematology malignancies (22),
a low pre-treatment LMR seems to represent an unfavorable prognosis
in solid tumors (23). In the
present study, lymphocyte and monocyte counts showed no
statistically significant differences between the patients with TTR
≥22 mg/dl and TTR <22 mg/dl, while LMR did exhibit a statistical
difference. Taken together, decreased IL-12 productivity and lower
LMR represent suppressed anti-tumor immunity in the patients with
TTR <22 mg/dl.
A major limitation of the present study is that it
includes a small number of NSCLC patients in a retrospective
setting. Secondly, not all blood samples were of sufficient volume
for all measurements for interleukin productivity. Further research
is required to explore the putative association between serum TTR
level and prognosis of patients with NSCLS. In conclusion, serum
TTR levels, as well as T category, can be useful for predicting the
5-year recurrence-free survival of NSCLC patients. Further studies
aimed at discovering nutritional treatments that improve outcomes
of patients with NSCLC are warranted.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author upon reasonable
request.
Authors' contributions
TS, MS and HS contributed to the concept, design and
integrity of the present study. TI, YO, TY, SM, TH and YS acquired,
analyzed and interpreted the data. TS and MS drafted the manuscript
and critically revised it for important intellectual content.
Ethics approval and consent to
participate
The present retrospective study was carried out in
accordance with the ethical standards of the Ethics Committee of
Fukushima Medical University (no. 1095) and with the 1964 Helsinki
declaration and its later amendments or ethical standards. Written
informed consent was obtained from all enrolled patients. All
patient data were treated in accordance with the local privacy
regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
GPS
|
Glasgow prognostic score
|
|
CRP
|
C-reactive protein
|
|
TTR
|
transthyretin
|
|
RBP
|
retinol binding protein
|
|
TF
|
transferrin
|
|
WBC
|
white blood cell count
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
LMR
|
lymphocyte-to-monocyte ratio
|
|
CEA
|
carcinoembryonic antigen
|
|
IL
|
interleukin
|
|
RPMI
|
Roswell Park Memorial Institute
media
|
References
|
1
|
Yoon HI, Kwon OR, Kang KN, Shin YS, Shin
HS, Yeon EH, Kwon KY, Hwang I, Jeon YK, Kim Y and Kim CW:
Diagnostic value of combining tumor and inflammatory markers in
lung cancer. J Cancer Prev. 21:187–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McMillan DC: The systemic
inflammation-based Glasgow prognostic score: A decade of experience
in patients with cancer. Cancer Treat Rev. 39:534–540. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Osugi J, Muto S, Matsumura Y, Higuchi M,
Suzuki H and Gotoh M: Prognostic impact of the high-sensitivity
modified Glasgow prognostic score in patients with resectable
non-small cell lung cancer. J Cancer Res Ther. 12:945–951. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spiekerman AM: Nutritional assessment
(protein nutriture). Anal Chem. 67:429R–436R. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Caccialanza R, Palladini G, Klersy C, Cena
H, Vagia C, Cameletti B, Russo P, Lavatelli F and Merlini G:
Nutritional status of outpatients with systemic immunoglobulin
light-chain amyloidosis 1. Am J Clin Nutr. 83:350–354. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rambod M, Kovesdy CP, Bross R, Kopple JD
and Kalantar-Zadeh K: Association of serum prealbumin and its
changes over time with clinical outcomes and survival in patients
receiving hemodialysis. Am J Clin Nutr. 88:1485–1494. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Devakonda A, George L, Raoof S, Esan A,
Saleh A and Bernstein LH: Transthyretin as a marker to predict
outcome in critically ill patients. Clin Biochem. 41:1126–1130.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding H, Liu J, Xue R, Zhao P, Qin Y, Zheng
F and Sun X: Transthyretin as a potential biomarker for the
differential diagnosis between lung cancer and lung infection.
Biomed Rep. 2:765–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hao S, Sun S, Xiao X, He D and Liu L:
Selective expression of transthyretin in subtypes of lung cancer. J
Mol Histol. 47:239–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawai H and Ota H: Low perioperative serum
prealbumin predicts early recurrence after curative pulmonary
resection for non-small-cell lung cancer. World J Surg.
36:2853–2857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alifano M, Mansuet-Lupo A, Lococo F, Roche
N, Bobbio A, Canny E, Schussler O, Dermine H, Régnard JF, Burroni
B, et al: Systemic inflammation, nutritional status and tumor
immune microenvironment determine outcome of resected non-small
cell lung cancer. PLoS One. 9:e1069142014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asamura H: Lung, pleural, and thymic
tumors. In: TNM Classification of Malignant Tumours. (8th edition.
Brierley JD, Gospodarowicz MK and Wittekind C (ed.) John Wiley
& Sons, Ltd., West Sussex). 105–118. 2017.
|
|
14
|
Sun X, Dyson HJ and Wright PE:
Fluorotryptophan incorporation modulates the structure and
stability of transthyretin in a site-specific manner. Biochemistry.
56:5570–5581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kelly P, Paulin F, Lamont D, Baker L,
Clearly S, Exon D and Thompson A: Pre-treatment plasma proteomic
markers associated with survival in oesophageal cancer. Br J
Cancer. 106:955–961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han WX, Chen ZM, Wei ZJ and Xu AM:
Preoperative pre-albumin predicts prognosis of patients after
gastrectomy for adenocarcinoma of esophagogastric junction. World J
Surg Oncol. 14:2792016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujii T, Sutoh T, Morita H, Katoh T,
Yajima R, Tsutsumi S, Asao T and Kuwano H: Serum albumin is
superior to prealbumin for predicting short-term recurrence in
patients with operable colorectal cancer. Nutr Cancer.
64:1169–1173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Chen LJ, Xia YL, Zhou HC, Yang RB,
Wu W, Lu Y, Hu LW and Zhao Y: Identification and verification of
transthyretin as a potential biomarker for pancreatic ductal
adenocarcinoma. J Cancer Res Clin Oncol. 139:1117–1127. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai W, Kong W, Dong B, Zhang J, Chen Y,
Xue W, Huang Y, Zhou L and Huang J: Pretreatment serum prealbumin
as an independent prognostic indicator in patients with metastatic
renal cell carcinoma using tyrosine kinase inhibitors as first-line
target therapy. Clin Genitourin Cancer. 15:e437–e446. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smyth MJ, Taniguchi M and Street SE: The
anti-tumor activity of IL-12: Mechanisms of innate immunity that
are model and dose dependent. J Immunol. 165:2665–2670. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu P, Shen H, Wang G, Zhang P, Liu Q and
Du J: Prognostic significance of systemic inflammation-based
lymphocyte-monocyte ratio in patients with lung cancer: Based on a
large cohort study. PLoS One. 9:e1080622014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Porrata LF, Ristow K, Colgan JP, Habermann
TM, Witzig TE, Inwards DJ, Ansell SM, Micallef IN, Johnston PB,
Nowakowski GS, et al: Peripheral blood lymphocyte/monocyte ratio at
diagnosis and survival in classical Hodgkin's lymphoma.
Haematologica. 97:262–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nishijima TF, Muss HB, Shachar SS, Tamura
K and Takamatsu Y: Prognostic value of lymphocyte-to-monocyte ratio
in patients with solid tumors: A systematic review and
meta-analysis. Cancer Treat Rev. 41:971–978. 2015. View Article : Google Scholar : PubMed/NCBI
|