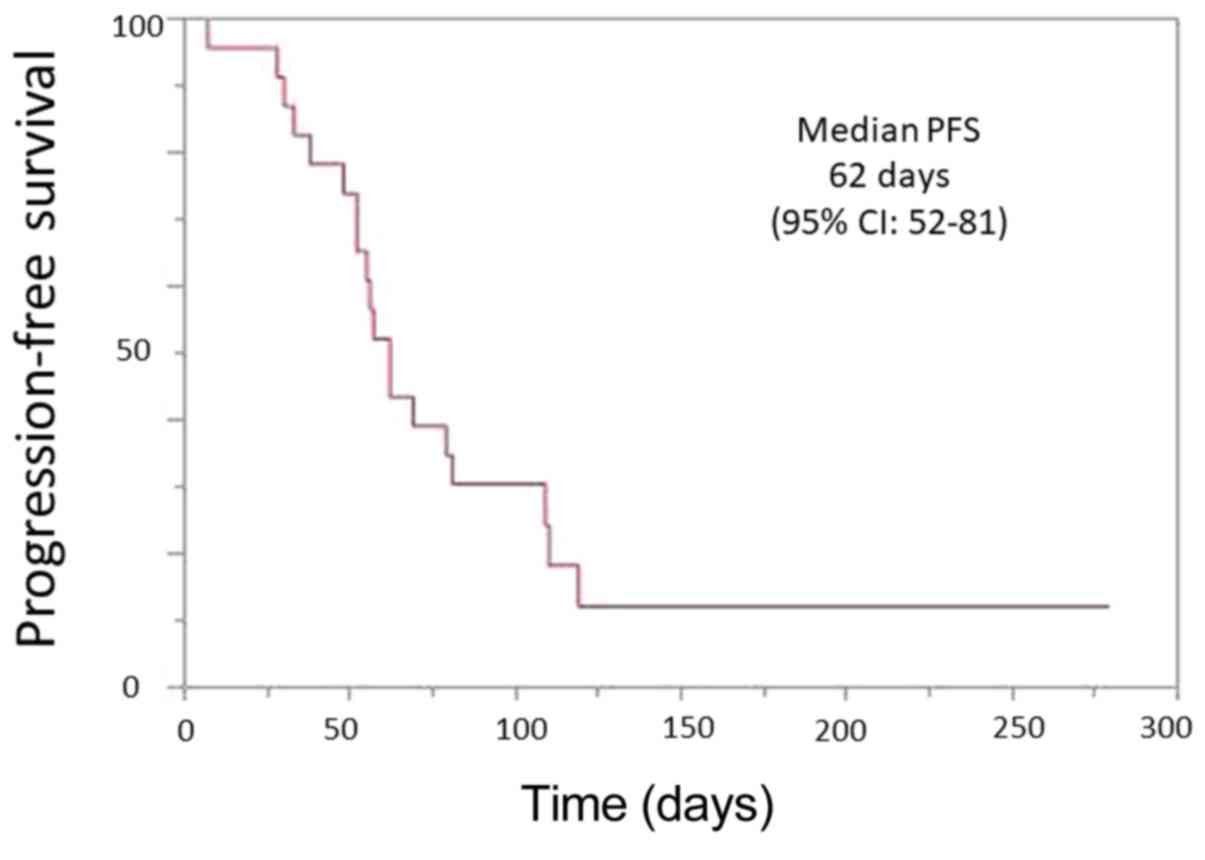
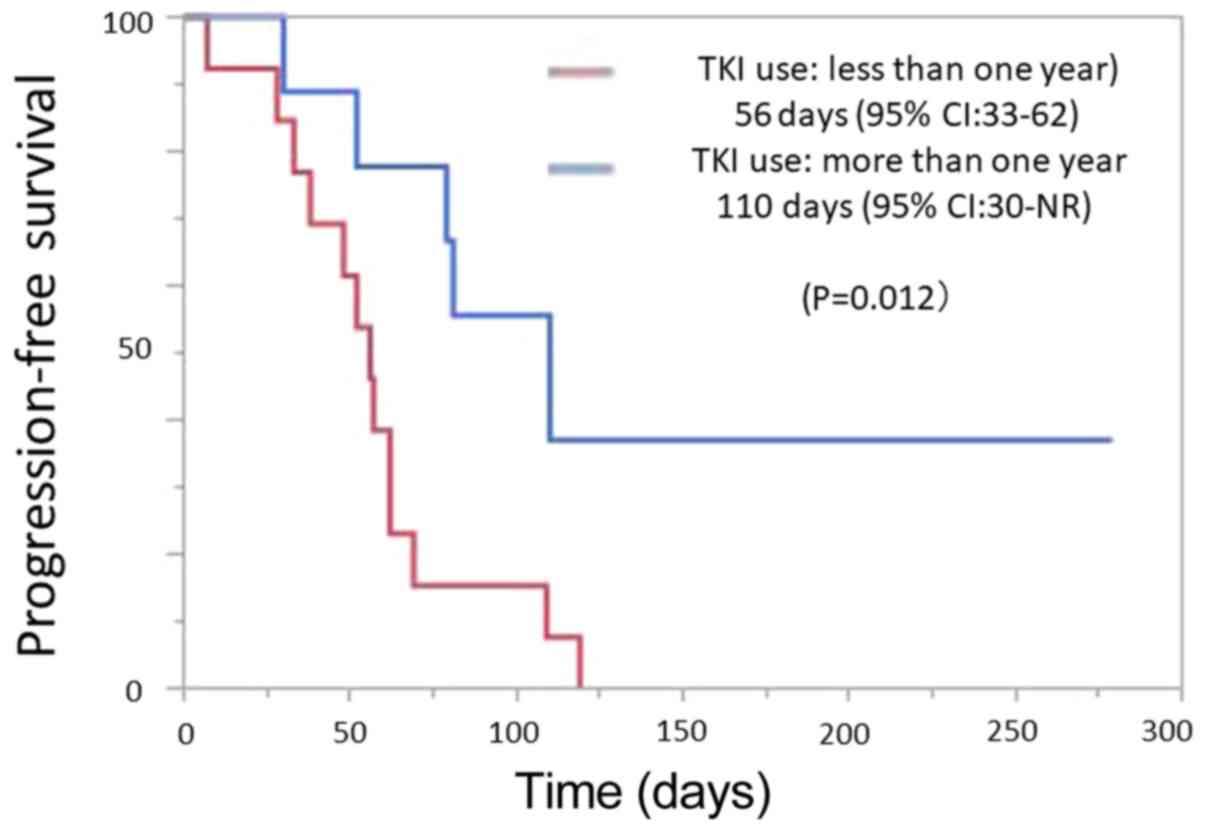
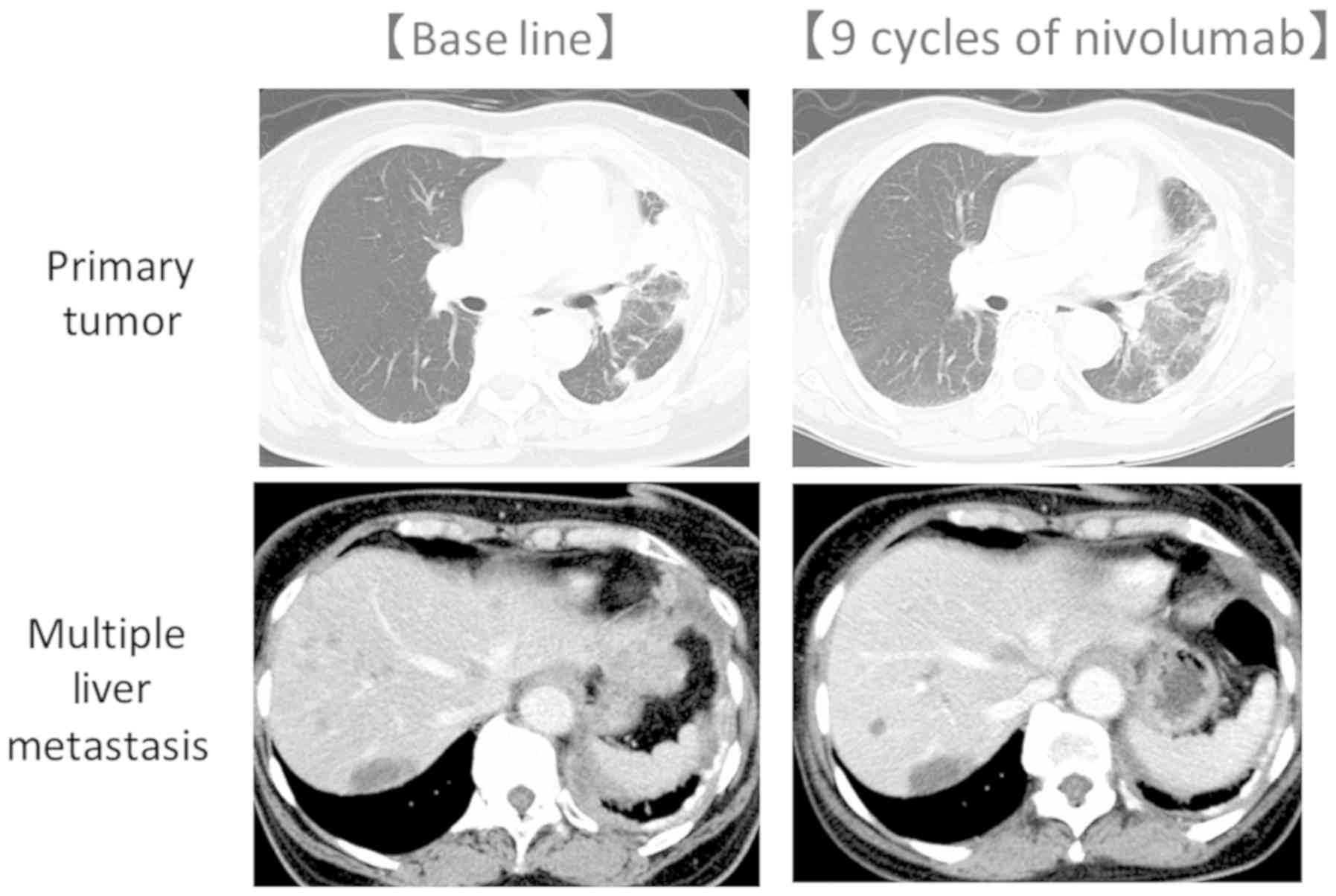
|
1
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita, et
al: Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang
Y, Li W, Hou M, Shi JH, Lee KY, et al: Afatinib versus cisplatin
plus gemcitabine for first-line treatment of Asian patients with
advanced non-small-cell lung cancer harbouring EGFR mutations
(LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet
Oncol. 15:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mok TS, Wu Y-L, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaw AT, Ou SH, Bang YJ, Camidge DR,
Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa
DB, et al: Crizotinib in ROS1-rearranged non-small-cell lung
cancer. N Engl J Med. 371:1963–1971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee CK, Man J, Lord S, Links M, Gebski V,
Mok T and Yang JC: Checkpoint inhibitors in metastatic EGFR-mutated
non-small cell lung cancer-a meta-analysis. J Thorac Oncol.
12:403–407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bylicki O, Paleiron N, Margery J, Guisier
F, Vergnenegre A, Robinet G, Auliac JB, Gervais R and Chouaid C:
Targeting the PD-1/PD-L1 immune checkpoint in EGFR-Mutated or
ALK-Translocated non-small-cell lung cancer. Target Oncol.
12:563–569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gainor JF, Shaw AT, Sequist LV, Fu X,
Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et
al: EGFR mutations and ALK rearrangements are associated with low
response rates to PD-1 pathway blockade in non-small cell lung
cancer: A retrospective analysis. Clin Cancer Res. 22:4585–4593.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Azuma K, Ota K, Kawahara A, Hattori S,
Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, et
al: Association of PD-L1 overexpression with activating EGFR
mutations in surgically resected nonsmall-cell lung cancer. Ann
Oncol. 25:1935–1940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi K, Nakachi I, Naoki K, Satomi R,
Nakamura M, Inoue T, Tateno H, Sakamaki F, Sayama K, Terashima T,
et al: Real-world efficacy and safety of nivolumab for advanced
non-small-cell lung cancer: A retrospective multicenter analysis.
Clin Lung Cancer. 19:e349–e358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al: First-line nivolumab in stage IV or recurrent non-small-cell
lung cancer. N Engl J Med. 376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gandara DR, Paul SM, Kowanetz M,
Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G,
Malboeuf C, et al: Blood-based tumor mutational burden as a
predictor of clinical benefit in non-small-cell lung cancer
patients treated with atezolizumab. Nat Med. 24:1441–1448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rozenblum AB, Ilouze M, Dudnik E, Dvir A,
Soussan-Gutman L, Geva S and Peled N: Clinical impact of hybrid
capture-based next-generation sequencing on changes in treatment
decisions in lung cancer. J Thorac Oncol. 12:258–268. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hatakeyama K, Nagashima T, Urakami K,
Ohshima K, Serizawa M, Ohnami S, Shimoda Y, Ohnami S, Maruyama K,
Naruoka A, et al: Tumor mutational burden analysis of 2,000
Japanese cancer genomes using whole exome and targeted gene panel
sequencing. Biomed Res. 39:159–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spiegel DR, Schrock AB, Fabrizio D,
Frampton GM, Sun J and He J: Total mutation burden (TMB) in lung
cancer (LC) and relationship with response to PD-1/PD-L1 targeted
therapies. J Clin Oncol. 34 (Suppl 15):S90172016. View Article : Google Scholar
|
|
22
|
Yoneshima Y, Ijichi K, Anai S, Ota K,
Otsubo K, Iwama E, Tanaka K, Oda Y, Nakanishi Y and Okamoto I:
PD-L1 expression in lung adenocarcinoma harboring EGFR mutations or
ALK rearrangements. Lung Cancer. 118:36–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen N, Fang W, Zhan J, Hong S, Tang Y,
Kang S, Zhang Y, He X, Zhou T, Qin T, et al: Upregulation of PD-L1
by EGFR activation mediates the immune escape in EGFR-Driven NSCLC:
Implication for optional immune targeted therapy for NSCLC Patients
with EGFR mutation. J Thorac Oncol. 10:910–923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Omori S, Kenmotsu H, Abe M, Watanabe R,
Sugino T, Kobayashi H, Nakashima K, Wakuda K, Ono A, Taira T, et
al: Changes in programmed death ligand 1 expression in non-small
cell lung cancer patients who received anticancer treatments. Int J
Clin Oncol. 23:1052–1059. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han JJ, Kim DW, Koh J, Keam B, Kim TM,
Jeon YK, Lee SH, Chung DH and Heo DS: Change in PD-L1 expression
after acquiring resistance to gefitinib in EGFR-Mutant
non-small-cell lung cancer. Clin Lung Cancer. 17:263–270. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin SY, Yang CY, Liao BC, Ho CC, Liao WY,
Chen KY, Tsai TH, Hsu CL, Hsu WH, Su KY, et al: Tumor PD-L1
expression and clinical outcomes in advanced-stage non-small cell
lung cancer patients treated with nivolumab or pembrolizumab:
Real-world data in Taiwan. J Cancer. 9:1813–1820. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haratani K, Hayashi H, Tanaka T, Kaneda H,
Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, et
al: Tumor immune microenvironment and nivolumab efficacy in EGFR
mutation-positive non-small-cell lung cancer based on T790M status
after disease progression during EGFR-TKI treatment. Ann Oncol.
28:1532–1539. 2017. View Article : Google Scholar : PubMed/NCBI
|