Introduction
In rectal cancer (RC), lymph node (LN) metastasis is
a poor prognostic factor (1,2) and improvement of outcomes using
neoadjuvant chemotherapy (NAC) or neoadjuvant chemoradiotherapy
(NACRT) has previously been reported (3,4).
Appropriate introduction of preoperative treatment requires
accurate prediction of lymph node metastasis prior to surgery. At
present, size-based diagnosis using the maximum short axis diameter
of LNs on computed tomography (CT) or magnetic resonance imaging
(MRI) is predominantly used to predict metastasis (5,6).
However, diagnostic accuracy of size-based diagnosis is
unsatisfactory. Ogawa et al (6) reported diagnostic accuracy of short
axis diameter for pararectal LN (PRLN) was 63.7% (cutoff; 5 mm) and
for laterally pelvic lymph node (LPLN) was 77.6% (cutoff; 5 mm) in
MRI findings. These accuracies were not enough to introduce
preoperative therapy appropriately, so studies focusing on the
‘quality’ of LNs have recently been tried to improve the diagnostic
accuracy. Previously, prediction of PRLN metastasis in RC using
dual-energy CT (DECT) has been reported (7). By contrast, to the best of our
knowledge, prediction of LPLN metastasis in low RC using DECT has
not been previously reported. LPLN dissection has been performed
for locally advanced low RC in Japan, which has been demonstrated
to reduce the rate of local recurrence (8). However, LPLN dissection can cause
complications, including increased blood loss, postoperative
dysuria and sexual dysfunction (8–10);
therefore, selection of patients is necessary. However, the
diagnostic reliability of size-based diagnosis for LPLN metastasis
is unsatisfactory. In a JCOG0212 study (8), the pathological LPLN
metastasis-positive rate was only 7% in patients with a maximum
short axis diameter ≤10 mm on preoperative MRI, and LPLN dissection
was not required for >90% of the patients. These findings
suggest that size-based diagnosis alone is insufficient for the
prediction of LPLN metastasis and a different approach is required
for the selection of patients. The aim of the present study was to
investigate the predictability of DECT for PRLN and LPLN metastasis
in RC.
Patients and methods
Patients
The current study involved 44 patients with RC who
were examined preoperatively using DECT and then underwent surgery
at our department between May 2015 and September 2017. During these
periods, DECT was used as the routine preoperative CT examination
for clinical staging. Patients who underwent preoperative
chemotherapy (n=25) were examined by DECT prior to preoperative
therapy. Samples examined by DECT were analyzed retrospectively.
The present study was approved by the Human Research Ethics
Committee of the Hirosaki University Graduate School of Medicine
(Aomori, Japan; reference no. 2018-1047). The clinical stage was
judged using the 8th edition of the Japanese Classification of
Colorectal Carcinoma (11). LPLN
dissection was performed in 24 patients with lower RC in whom the
clinical invasion depth of tumor was T3 or deeper (deeper than the
muscularis propria), with the lower margin present on the anal side
of the peritoneal reflection. LPLN dissection was performed
bilaterally. The histopathological evaluation of LNs were performed
retrospectively from the results of routine pathological diagnosis
by pathologists.
Dual-energy CT technique
DECT imaging was performed using a Discovery 750 HD
system (GE Healthcare, Chicago, IL, USA) with a fast kilovoltage
switching method, as previously reported by Aoki et al
(12), with a few adjustments.
Briefly, a non-ionic contrast medium dose of 600 mgI/kg body
weight, with an iodine content of 300 mgI/ml (Omnipaque 300;
Daiichi-Sankyo Co. Ltd., Japan), was administered. The total amount
of contrast medium was intravenously injected within 30 sec, and
scanning of the arterial phase (AP) and portal venous phase (PP)
began 35 and 70 sec after initiating the injection of contrast
medium. The thickness of the slice analyzed was 1.25 mm.
Imaging analysis
DECT images were transferred to a workstation
(Advantage workstation 4.6; GE Healthcare) for analysis. One
surgeon (KS) and one medical student (RK) analyzed the images.
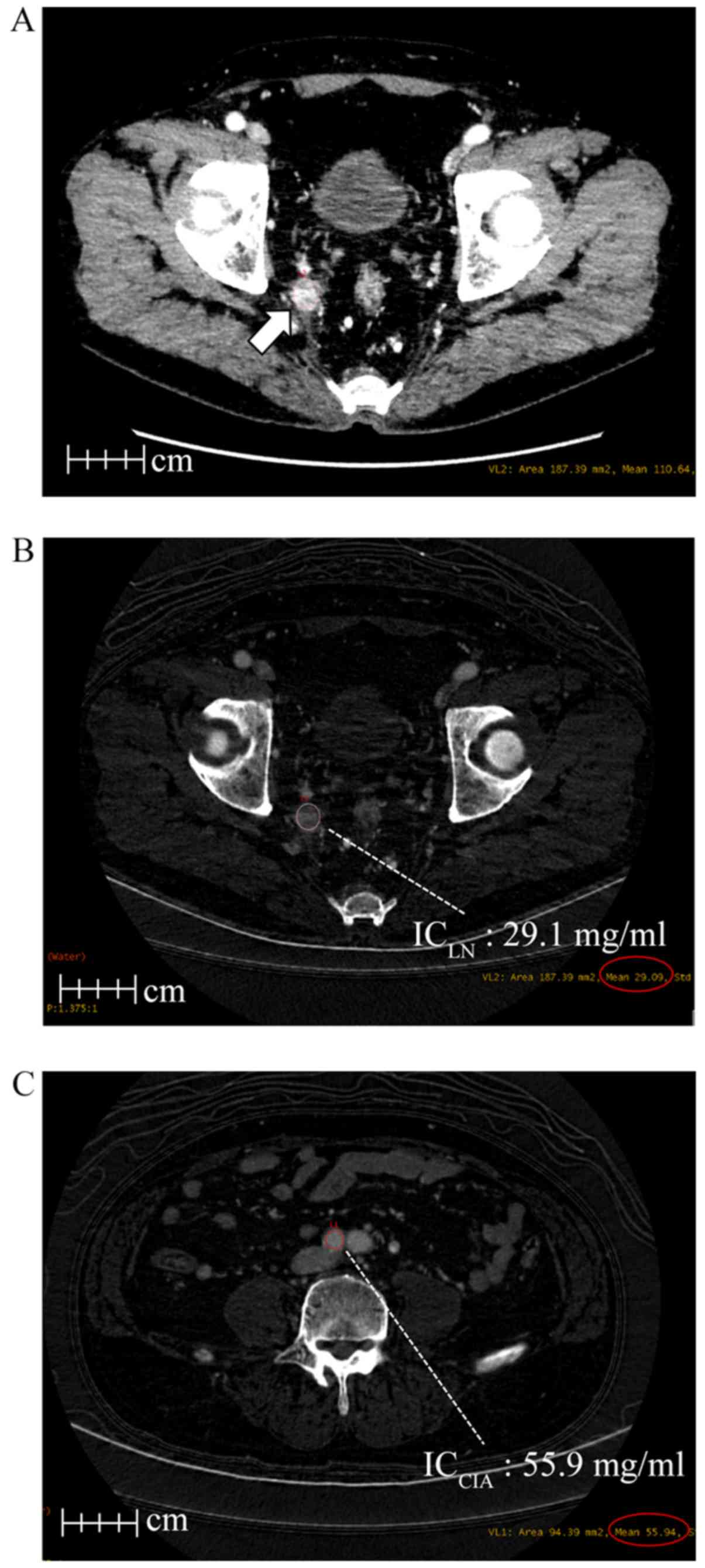
Using iodine overlay images, the iodine concentration of LNs
(ICLN) was measured by a circular region of interest
(ROI) using the extracted maximum short axis diameter of the PRLN
and LPLN in the AP and PP (Fig. 1A and
B). The iodine concentration of the common iliac artery
(ICCIA) was measured for the right common iliac artery
in the AP and PP (Fig. 1C). The
normalized iodine concentration (nIC) value was calculated by the
following formula, nIC = ICLN (mg/ml)/ICCIA
(mg/ml), as previously described by Liu et al (7).
Selection of evaluated LNs and
radiological-histopathological comparison
The largest PRLN and the largest LPLN inside the
dissection area were selected to evaluate nIC value by DECT. LPLN
dissection was performed bilaterally, and larger side LPLN was
selected for evaluation. Patients with a maximum short axis
diameter ≤3 mm LNs were excluded because of difficulty in
extracting the ROI. Following the exclusion, the nIC values for
PRLNs and LPLNs were calculated in 43/44 and 22/24 patients,
respectively. Pathological PRLN and LPLN metastasis were evaluated
from the results of the routine pathological diagnosis for the
staging of RC.
Methods of radiological-histopathological comparison
was following; when pathological metastatic-positive LNs (at least
≥1) existed in the pathologically-examined LNs,
radiologically-selected LNs by DECT were metastasis-positive. When
there were no pathologically-metastatic LNs,
radiologically-selected LNs by DECT were metastasis-negative.
Radiological-histopathological comparison for LPLN was performed
using only one side selected for calculating nIC by DECT. For
example, when the nIC was calculated from the right side LPLN by
DECT, pathological evaluation was performed using only right side
LPLNs.
Statistical analysis
Associations of nIC and short axis diameter for
PRLNs and LPLNs with metastasis were investigated statistically.
Cut-off values for these associations were determined using
receiver operating curve (ROC) analysis, and the area under the
curve (AUC), sensitivity, specificity, positive predictive value
(PPV), negative predictive value (NPV) and accuracy at the cut-off
were determined. Statistical analysis was performed by Mann-Whitney
U-test and χ2 test. P<0.05 was considered to indicate a
statistically significant difference. These analyses were performed
using Easy R software (13).
Results
Clinical characteristics
The clinical characteristics of the patients are
presented in Table I. PRLN
metastasis was detected in 34.1% of the 44 patients. LPLN
dissection was performed in 24 patients (54.5%) and LPLN metastasis
was detected in 3 of these patients (12.5%).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Value |
|---|
| Male, n (%) | 34 (77.2) |
| Median Age, years
(range) | 65 (36–82) |
| Median BMI,
kg/m2 (range) | 21.6 (16.0–31.0) |
| Median distance of
tumor from AV, cm (range) | 5 (2–15) |
| Location of tumor:
Rb, P, n (%) | 33 (75) |
| Clinical T stage, n
(%) |
|
| 1
(M-SM) | 2 (4.5) |
| 2
(MP) | 6 (13.6) |
| 3 (SS,
A) | 23 (52.3) |
| 4 (SE,
SI, AI) | 13 (29.6) |
| Clinical N stage, n
(%) |
|
| 0 | 23 (52.3) |
| 1 (number
of metastatic LNs; 1–3) | 5 (11.4) |
| 2 (number
of metastatic LNs; ≥4) | 6 (13.6) |
| 3 (with
LPLN metastasis) | 10 (22.7) |
| Preoperative
chemotherapy, n (%) | 25 (56.8) |
| Laparoscopic, robot,
n (%) | 40 (90.1) |
| Median operation
time, min (range) | 294.5 (121–487) |
| Median blood loss, ml
(range) | 50 (0–2160) |
| pT3, T4, n (%) | 26 (59.1) |
| LNND, n (%) | 24 (54.5) |
| PRLN metastasis, n
(%) | 15 (34.1) |
| LPLN metastasis, n
(%) | 3 (12.5) |
| Rate of anal
preservation, n (%) | 33 (75) |
Associations of size and nIC of PRLNs
for PRLN metastasis
The associations of the maximum short axis diameter
of PRLNs and nIC in the AP and PP in cases with and without PRLN
metastasis are presented in Table
II. The median maximum short axis diameter of PRLNs was
insignificantly different between PRLN metastasis-positive and
metastasis-negative cases (7.6 vs. 6.4 mm; P=0.33). The median nIC
of the maximum-size PRLN was significantly lower in the PRLN
metastasis-positive cases compared with the PRLN
metastasis-negative cases in the AP (0.18 vs. 0.25; P=0.01) and in
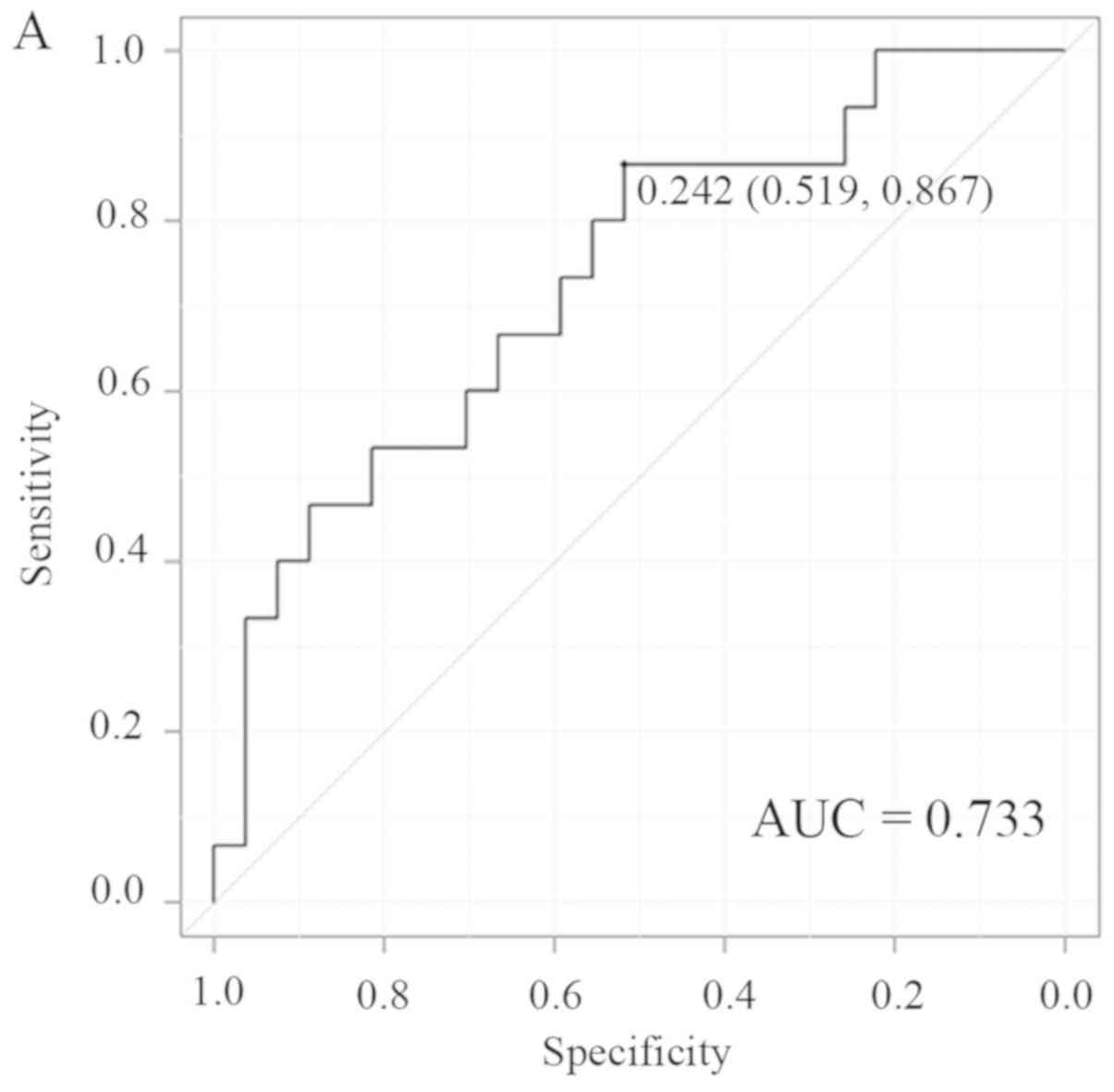
the PP (0.47 vs. 0.61; P=0.03). The cut-off values of nIC for PRLNs
in ROC analysis were 0.24 (AUC, 0.733) and 0.59 (AUC, 0.701) in the
AP and PP, respectively (Fig. 2A and
B), and these cut-off values provided a sensitivity,
specificity, PPV, NPV and accuracy of 86.7, 51.9, 48.1, 83.3, and
62.8% for the AP, respectively, and 80, 55.6, 48.0, 83.3 and 62.8%
for the PP, respectively, for prediction of metastasis to PRLNs
(Table III).
 | Table II.Association between PRLN metastasis
and short axis diameter of PRLNs, nIC value of PRLNs. |
Table II.
Association between PRLN metastasis
and short axis diameter of PRLNs, nIC value of PRLNs.
| Parameter | PRLN metastasis (−)
(n=29) | PRLN metastasis (+)
(n=15) | P-value |
|---|
| Median size of PRLN
(mm) |
|
|
|
| Short
axis | 6.4 (3.4–11.1) | 7.6 (4.0–17.0) | 0.33 |
| Median nIC
value |
|
|
|
| AP | 0.25
(0.10–0.41) | 0.18
(0.05–0.27) | 0.01 |
| PP | 0.61
(0.16–0.96) | 0.47
(0.17–0.68) | 0.03 |
 | Table III.Cut-off value of nIC value in PRLN
metastasis and diagnostic performance to PRLN metastasis. |
Table III.
Cut-off value of nIC value in PRLN
metastasis and diagnostic performance to PRLN metastasis.
| Parameter | AUC | 95% CI | Cutoff | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % |
|---|
| AP | 0.733 | 0.57–0.89 | 0.24 | 86.7 | 51.9 | 48.1 | 87.5 | 62.8 |
| PP | 0.701 | 0.54–0.87 | 0.59 | 80 | 55.6 | 48 | 83.3 | 62.8 |
Associations of size and nIC of LPLNs
for LPLN metastasis
The associations of the maximum short axis diameter
of LPLNs and nIC in the AP and PP in cases with and without LPLN
metastasis are presented in Table
IV. The median maximum short axis diameter of the LPLNs was
significantly larger in LPLN metastasis-positive cases compared
with LPLN metastasis-negative cases (9.1 vs. 4.8 mm; P=0.03). The
median nIC of the maximum-size LPLN was insignificantly different
between LPLN metastasis-positive and -negative cases in the AP
(0.15 vs. 0.21; P=0.19), but was significantly lower in LPLN
metastasis-positive cases compared with LPLN metastasis-negative
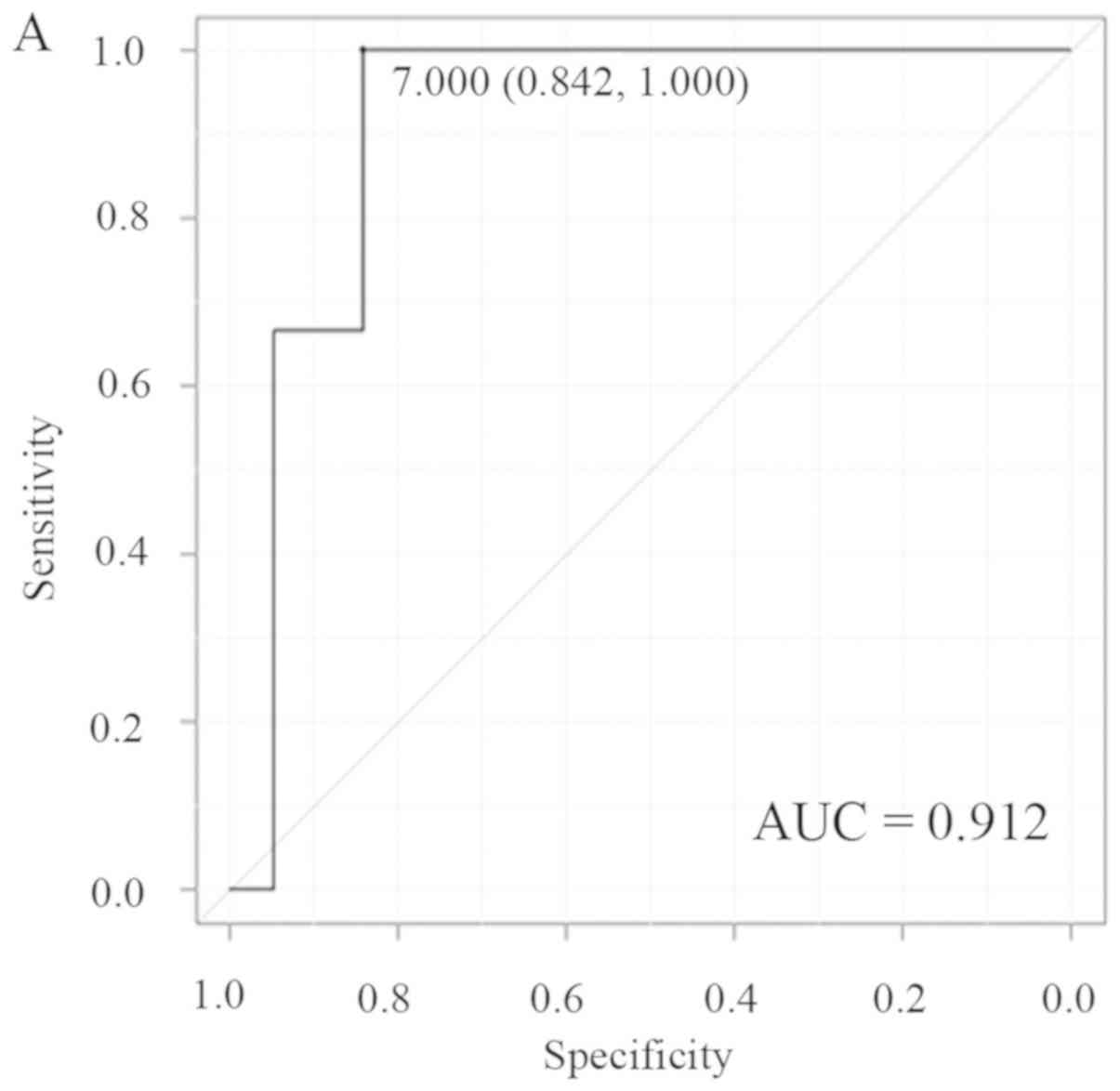
cases in the PP (0.29 vs. 0.55; P=0.04). The cut-off values for
nICs of LPLNs were 7.0 (AUC, 0.912) and 0.29 mm (AUC, 0.877) in the
AP and PP, respectively (Fig. 3A and
B), and these cut-off values provided a sensitivity,
specificity, PPV, NPV and accuracy of 100, 84.2, 50, 100 and 86.4%
for the AP, and 66.7, 100, 100, 95.2 and 95.7% for the PP,
respectively, for prediction of metastasis to LPLNs (Table V).
 | Table IV.Association between LPLN metastasis
and short axis diameter of LPLNs, nIC value of LPLNs. |
Table IV.
Association between LPLN metastasis
and short axis diameter of LPLNs, nIC value of LPLNs.
| Parameter | LPLN metastasis (−)
(n=21) | LPLN metastasis (+)
(n=3) | P-value |
|---|
| Median size of LPLN
(mm) |
|
|
|
| Short
axis | 4.8 (3.0–19.5) | 9.1 (7.0–12.1) | 0.03 |
| Median nIC
value |
|
|
|
| AP | 0.21
(0.1–0.32) | 0.15
(0.06–0.21) | 0.19 |
| PP | 0.55
(0.32–0.73) | 0.29
(0.23–0.48) | 0.04 |
 | Table V.Cut-off value of short axis diameter
of LPLN and nIC value in LPLN metastasis and diagnostic performance
to LPLN metastasis. |
Table V.
Cut-off value of short axis diameter
of LPLN and nIC value in LPLN metastasis and diagnostic performance
to LPLN metastasis.
| Parameter | AUC | 95% CI | Cutoff | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % |
|---|
| Size of LPLN
(mm) |
|
|
|
|
|
|
|
|
| Short axis | 0.912 | 0.78-1 | 7.0 mm | 100 | 84.2 | 50 | 100 | 86.4 |
| nIC value |
|
|
|
|
|
|
|
|
| PP | 0.877 | 0.63-1 | 0.29 | 66.7 | 100 | 100 | 95.2 | 95.7 |
Discussion
The cut-off value of size-based diagnosis for rectal
cancer is inconsistent; for example, Akiyoshi et al
(5) reported that 8 mm is the
optimum cut-off for prediction of LPLN metastasis on MRI, whereas
Ogawa et al (6) proposed a
cut-off of 5 mm. Therefore, the accuracy of size-based diagnosis is
uncertain and other methods to predict metastasis have been
examined. Akiyoshi et al (5)
suggested that a mixed signal intensity (a mixture of various
intensities) was frequent in cases with LPLN metastasis; however,
no significant difference was revealed in multivariate analysis and
prediction was based on a subjective qualitative judgment by
radiologists so this method required advanced expertise. The nIC on
DECT may provide a solution to this problem. DECT uses two tubular
bulbs for fast switching and density-based analysis of materials,
including iodine, is possible (14,15). In
RC, in addition to evaluation of the primary lesion (16), DECT has been used to predict PRLN
metastasis by Liu et al (7)
and Kato et al (17), who
identified a significantly lower nIC in pathological
metastasis-positive cases compared with negative cases. The nIC was
also lower in LN metastasis-positive cases in the current study.
Histopathologically, Naresh et al (18) identified that the number of blood
vessels was smaller in metastatic LNs in head and neck cancer, and
the nIC on DECT may reflect this pathological feature. Since fewer
blood vessels enter metastatic LNs, the nIC may decrease compared
with that in non-metastatic lymph nodes.
Disease control by surgery alone is limited for
advanced rectal cancer with LN metastasis, and increased
preoperative treatment is apparent in recent studies following the
prediction of LN metastasis of lower RC. This includes a recent
introduction of preoperative chemoradiotherapy, including NACRT,
and chemotherapy, including NAC, in Japan (19–21), and
a reduction of local recurrence has been reported. Prediction of LN
metastasis prior to surgery is important for appropriate use of
preoperative treatment. Furthermore, improving the diagnostic
reliability of LPLN metastasis is also important to select the
patients appropriate for LPLN dissection.
In the current study, the efficiency of DECT was
investigated for both PRLNs and LPLNs. For PRLNs, the median
maximum short axis diameter of PRLNs was insignificantly different
between metastasis-positive and -negative cases; however, the nIC
in the AP and PP on DECT was significantly lower for metastatic
PRLNs. This suggests that DECT is more useful compared with the
size of the LNs for prediction of metastasis. By' contrast, for
LPLNs, the maximum short axis diameter of the LNs and nIC in the PP
were both useful predictors of metastasis. A cut-off for the
maximum short axis of the LNs of 7.0 mm based on ROC analysis
provided an AUC of 0.912 and accuracy of 86.4%, and a cut-off nIC
in the PP of 0.29 provided an AUC of 0.877 and accuracy of 95.7%.
This suggests that a high preoperative diagnostic accuracy may be
obtained using a combination of size-based diagnosis and nIC on
DECT for LPLNs. To the best of our knowledge, preoperative
prediction of LPLN metastasis by DECT has not been previously
reported, and further accumulation and investigation of metastatic
LN samples is required. Since numerous PRLNs are dissected, there
is likely to be inconsistency between LNs identified on imaging and
metastatic LNs, and this may explain the low diagnostic accuracy of
DECT for PRLNs compared with that for LPLNs.
The present study had a number of limitations.
Firstly, the sample size of 44 patients, including 24 with LPLN
dissection, was small and the analysis was retrospective. Only one
largest PRLN and LPLN were studied in each case, and it is unclear
whether this LN was consistent with the pathological
metastasis-positive LN. The probability of inconsistency was high,
particularly for PRLNs, as aforementioned. To increase the
accuracy, a method is required to match the LN identified on DECT
with the LN in the resected specimen. Establishing a cut-off value
of nIC in a large-scale prospective study using standard
measurement methods is also required for clinical application.
Within these limitations, it can be concluded that DECT may be
useful for preoperative prediction of metastasis to PRLNs and
LPLNs. For LPLNs, high diagnostic accuracy may be achieved by
combination with size-based diagnosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and analyzed in the present study
are included in this published article.
Authors' contributions
KS, HM, FT, YS, TM, HF, KU, TS, ST, RK, SO, MO and
KH authors contributed to the conception and design of the study.
KS and RK analyzed the images. FT and HF advised the analysis of
images. YS, HM, TM, KU, TS and ST performed the surgeries. SO, MA
and KH supervised the study. All authors participated in the
interpretation of the results and the writing of the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
Human Research Ethics Committee of the Hirosaki University Graduate
School of Medicine (approval no. 2018-1047).
Patient consent for publication
Consent was obtained from the patients, who had the
option to withdraw from the present study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DECT
|
dual energy computed tomography
|
|
RC
|
rectal cancer
|
|
LN
|
lymph node
|
|
CIA
|
common iliac artery
|
|
PRLN
|
pararectal lymph node
|
|
LPLN
|
lateral pelvic lymph node
|
|
nIC
|
normalized iodine concentration
|
|
NAC
|
neoadjuvant chemotherapy
|
|
NACRT
|
neoadjuvant chemoradiotherapy
|
|
AP
|
arterial phase
|
|
PP
|
portal venous phase
|
|
ROI
|
region of interest
|
|
PPV
|
positive predictive value
|
|
NPV
|
negative predictive value
|
|
AUC
|
area under the curve
|
References
|
1
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2014 for treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
MERCURY Study Group, ; Shihab OC, Taylor
F, Bees N, Blake H, Jeyadevan N, Bleehen R, Blomqvist L, Creagh M,
George C, et al: Relevance of magnetic resonance imaging detected
pelvic sidewall lymph node involvement in rectal cancer. Br J Surg.
98:1798–1804. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC: EORTC radiotherapy group trial 22921: Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Medicine.
355:1114–1123. 2006. View Article : Google Scholar
|
|
4
|
Schrag D, Weiser MR, Goodman KA, Gonen M,
Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem
JG, et al: Neoadjuvant chemotherapy without routine use of
radiation therapy for patients with locally advanced rectal cancer:
A pilot trial. J Clin Oncol. 32:513–518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akiyoshi T, Matsueda K, Hiratsuka M, Unno
T, Nagata J, Nagasaki T, Konishi T, Fujimoto Y, Nagayama S,
Fukunaga Y and Ueno M: Indications for lateral pelvic lymph node
dissection based on magnetic resonance imaging before and after
preoperative chemoradiotherapy in patients with advanced low-rectal
cancer. Ann Surg Oncol. 3 (Suppl 22):S614–S620. 2015. View Article : Google Scholar
|
|
6
|
Ogawa S, Hida JI, Ike H, Kinugasa T, Ota
M, Shinto E, Itabashi M, Okamoto T, Yamamoto M, Sugihara K and
Watanabe T: Prediction of lateral pelvic lymph node metastasis from
lower rectal cancer using magnetic resonance imaging and risk
factors for metastasis: Multicenter study of the lymph node
committee of the japanese society for cancer of the colon and
rectum. Int J Colorectal Dis. 32:1479–1487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu H, Yan F, Pan Z, Lin X, Luo X, Shi C,
Chen X, Wang B and Zhang H: Evaluation of dual energy spectral CT
in differentiating metastatic from non-metastatic lymph nodes in
rectal cancer: Initial experience. Eur J Radiol. 84:228–234. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujita S, Mizusawa J, Kanemitsu Y, Ito M,
Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, et al:
Mesorectal excision with or without lateral lymph node dissection
for clinical stage II/III lower rectal cancer (JCOG0212): A
multicenter, randomized controlled, noninferiority trial. Ann Surg.
266:201–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobayashi H, Mochizuki H, Kato T, Mori T,
Kameoka S, Shirouzu K and Sugihara K: Outcomes of surgery alone for
lower rectal cancer with and without pelvic sidewall dissection.
Dis Colon Rectum. 52:567–576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akasu T, Sugihara K and Moriya Y: Male
urinary and sexual functions after mesorectal excision alone or in
combination with extended lateral pelvic lymph node dissection for
rectal cancer. Ann Surg Oncol. 16:2779–2786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Japanese Society for Cancer of the Colon
Rectum, . Japanese Classification of Colorectal Carcinoma (8th).
Kanehara Shuppan. Tokyo, Japan: 2013.
|
|
12
|
Aoki M, Takai Y, Narita Y, Hirose K, Sato
M, Akimoto H, Kawaguchi H, Hatayama Y, Miura H and Ono S:
Correlation between tumor size and blood volume in lung tumors: A
prospective study on dual energy gemstone spectral CT imaging. J
Radiat Res. 55:917–923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplantat. 48:452–458. 2013. View Article : Google Scholar
|
|
14
|
Matsumoto K, Jinzaki M, Tanami Y, Ueno A,
Yamada M and Kuribayashi S: Virtual monochromatic spectral imaging
with fast kilovoltage switching: Improved image quality as compared
with that obtained with conventional 120-kVp CT. Radiology.
259:257–262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang D, Li X and Litl B: Objective
characterization of GE discovery CT750 HD scanner: Gemstone
spectral imaging mode. Med Phys. 38:1178–1188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morohashi H, Sakamoto Y, Ichinohe D, Jin
H, Miura T, Tsushima F, Ono S and Hakamada K: Evaluation of the
therapeutic effect of using dual-energy CT for rectal cancer after
neoadjuvant chemotherapy. Gan To Kagaku Ryoho. 43:1482–1484.
2016.(In Japanese). PubMed/NCBI
|
|
17
|
Kato T, Uehara K, Ishigaki S, Nihashi T,
Arimoto A, Nakamura H, Kamiya T, Oshiro T, Ebata T and Nagino M:
Clinical significance of dual-energy CT-derived iodine
quantification in the diagnosis of metastatic LN in colorectal
cancer. Eur J Surg Oncol. 41:1464–1470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Naresh KN, Nerurkar AY and Borges AM:
Angiogenesis is redundant for tumour growth in lymph node
metastases. Histopathology. 38:466–470. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamiya T, Uehara K, Nakayama G, Ishigure
K, Kobayashi S, Hiramatsu K, Nakayama H, Yamashita K, Sakamoto E,
Tojima Y, et al: Early results of multicenter phase II trial of
perioperative oxaliplatin and capecitabine without radiotherapy for
high-risk rectal cancer: CORONA I study. Eur J Surg Oncol.
42:829–835. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hasegawa S, Goto S, Matsumoto T, Hida K,
Kawada K, Matsusue R, Yamaguchi T, Nishitai R, Manaka D, Kato S, et
al: A multicenter phase 2 study on the feasibility and efficacy of
neoadjuvant chemotherapy without radiotherapy for locally advanced
rectal cancer. Ann Surg Oncol. 24:3587–3595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakamura T, Yamashita K, Sato T, Ema A,
Naito M and Watanabe M: Neoadjuvant chemoradiotherapy using S-1 and
irinotecan in rectal cancer: Impact on long-term clinical outcomes
and prognostic factors. Int J Radiat Oncol Biol Phys. 89:547–555.
2014. View Article : Google Scholar : PubMed/NCBI
|