Introduction
Breast cancer (BC) is a heterogeneous and complex
disease, with a great variation in clinical outcomes. BC is the
most frequently diagnosed cancer in females and second in causes of
cancer mortality in both sexes, as metastatic BC remains an almost
incurable disease. Incidence of male BC is rare (~1% of numbers of
female BC and 1% of all malignancies in males in Western
countries); however, in previous years, a slight increase in
incidence has been observed in certain countries (1–5). BC in
males has become most frequently diagnosed at an early anatomic
stage (AS), and an improvement in overall survival (OS) has been
observed (6–9). However, the lack of information
regarding male BC and the unviability of screening have contributed
to a persistently high percentage of diagnoses at advanced AS. In
Portugal, the annual male BC gross incidence rates in 2010 and 2011
were 1.23 and 1.77 per 100,000 people, respectively, and the gross
mortality rates were 0.34 and 0.51, respectively (10).
Despite increasing interest, the biology and optimal
management of male BC remain poorly understood, and contradictory
data are often identified. Certain common epidemiological risk
factors, which remain to be identified in either sex, may be
relevant in the understanding and prevention of the disease
(11). Genetic predisposition
appears to be an important contributor to risk and may have
clinical implications (8,12,13).
Family history (FH) is also relevant, and a positive FH in a
masculine family member is strong indication for genetics
consultation (13). In addition,
genetic mutations may be identified in patients without FH and
should be routinely screened in male BC (13). In contrast to those identified in
females, mutations in the BRCA2, DNA repair associated (BRCA2) gene
are predominant in male BC, while BRCA1, DNA repair associated
(BRCA1) gene mutations are infrequent (8,12,14,15).
Obesity is one of the most common causes of
hyperestrogenism in males, and adolescent overweight has been
associated with an increased risk of male BC (2,12,16). In
addition, liver diseases, alcoholism, Klinefelter's syndrome,
exogenous estrogen use (namely for the treatment of prostate
cancer) and androgen deficiency due to testicular disease including
hypogonadism and orchitis, appear to increase the risk of disease
(2,4,7).
Occupational and environmental exposures to radiation, and heat and
electromagnetic fields have also been implicated as potential risk
factors (3,8,12).
Male BC is diagnosed by mammography or
ultrasonography and confirmed by core biopsy, which are always
performed following a suspicious clinical examination. Therapeutic
procedures are based on the recommendations for female BC, but
mastectomy rather than breast-conserving surgery is performed in
the vast majority of cases. In addition, hormone therapy is less
tolerated in males compared with in females, and side effects
including weight gain, depression, deep venous thrombosis,
decreases in libido and impotence, with high rates of
discontinuation, were described (4,5,17,18).
Molecular testing in BC, through the use of
sophisticated techniques including deep sequencing analysis, has
led to an improved understanding of this disease. Concomitantly,
the identification of targeted therapies has reinforced the
requirement for improved stratification in BC subtypes (13). The present study investigated a
retrospective series of male BC cases, assessed the
clinicopathological and molecular characteristics that are
currently the basis for therapeutic strategies, and estimated their
association and significance in disease-free survival (DFS) and OS
of male BC.
Patients and methods
Patient selection and
clinicopathological evaluation
The present retrospective study involved 196 male
patients with BC diagnosed and treated according to therapeutic
protocols from March 1970 to March 2018 (mean follow-up time, 84.9
months), at the Portuguese Institute of Oncology of Lisbon (Lisbon,
Portugal). The institutional Ethical Committee of the Portuguese
Institute of Oncology of Lisbon approved the study. Patient data,
including age, obesity, FH, bilaterality, presence of non-breast
primary neoplasms (NBPN), presence of distant metastasis and
therapeutic modalities were obtained by review of the clinical
records. All slides were reviewed. AS classification included
pathological tumor size (pT), pathological axillary nodal status
(pN) and distant metastasis (M), and was registered according to
the TNM classification system recommended by the 8th edition of the
American Joint Committee on Cancer (AJCC) staging system (19). The histological type was re-evaluated
as per the World Health Organization 2012 classification (20). Histological grade of differentiation
(G) was assessed using the Elston-Ellis grading system criteria
(21).
BRCA status
Nucleic acids were obtained from peripheral blood
nucleated cells. DNA was extracted with the EZ1 Bio Robot and the
EZ1 DNA blood kit (Qiagen GmbH, Hilden, Germany) and RNA was
extracted using TRIzol® (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). A total of 44 patients were pre-screened for the
c.156_157insAlu BRCA2 Portuguese founder mutation, analyzed
for BRCA1/2 point mutations by next generation sequencing
(NGS) with a CE-IVD MASTR BRCA molecular diagnostic assay
(Multiplicom; Agilent Technologies, Inc., Santa Clara, CA, USA) in
a MiSeq Instrument (Illumina, Inc., San Diego, CA, USA) and for
large rearrangements with a Multiplex Ligation-dependent Probe
Amplification (MLPA) assay using P002 BRCA1 and P045 BRCA2/CHEK2
kits (MRC-Holland, Amsterdam, The Netherlands). Variant Studio
v.2.2 (Illumina, Inc, San Diego, CA, USA) and DNAnexus, Inc,
Mountain View, CA, USA were used to analyze the NGS data, and
Coffalyzer (MRC-Holland, Amsterdam, The Netherlands) software was
used for the MLPA data. In addition to the information provided in
the Variant Studio and DNAnexus programs, the Breast Cancer
Information Core database and the Universal Mutation Database were
used to clarify certain variants. Prior to NGS screening, patient
samples were analyzed by conformation sensitive capillary
electrophoresis or conformation sensitive gel electrophoresis, and
samples with different patterns (fragment pattern comparison
between the 44 patient samples analyzed in the same batch and also
comparison with a negative control) were sequenced by Sanger
sequencing using an ABI 3130 instrument as described previously
(22,23).
Estrogen receptor α (ERα),
progesterone receptor (PR), receptor tyrosine kinase erbB-2
[(ERBB2), antigen Ki-67 (Ki-67) immunohistochemistry (IHC) and
ERBB2 in situ hybridization (ISH)]
IHC was performed using a
peroxidase-indirect-polymer technique performed on a Ventana
Benchmark™ ULTRA instrument (Ventana Medical Systems, Inc.; Roche
Diagnostics, Basel, Switzerland) on formalin-fixed paraffin
embedded tumor tissues. All cases were re-analyzed under the same
conditions for all samples within this study and all kits were used
according to the protocol of the manufacturer. The levels of ERα
clone SP1 (Ventana Medical Systems, Inc.; Roche Diagnostics; cat.
no. 790-4324) and PR clone IE2 (Ventana Medical Systems, Inc.;
Roche Diagnostics cat. no. 790-2223) were recorded as the
percentage of positively-stained neoplastic cell nuclei, using a
≥1% cut-off value as the criterion for positivity. The staining
intensity was not evaluated (24).
The ERBB2 clone 4B5 (Ventana Medical Systems, Inc.; Roche
Diagnostics cat. no. 790-2991) was used for ERBB2 evaluation. The
quantification of ERα, PR and ERBB2 oncoprotein overexpression (0,
1+, 2+ and 3+) was based on the American Society of Clinical
Oncology (ASCO)/College of American Pathologists (CAP) guidelines
(25). The Ki-67 index was recorded
as the percentage of positively-stained cells, using the Ki-67
clone 30-9 (Ventana Medical Systems, Inc.; Roche Diagnostics; cat.
no. 790-4286), and ‘hot spots’ were classified as those areas
containing 100 malignant cells. The threshold for high
proliferation was 20%, based on the adaptation of the 2013 St.
Gallen consensus guidelines (26).
ERBB2 gene amplification was determined by FISH using a BenchMark™
Ventana® system (Ventana Medical Systems, Inc.; Roche
Diagnostics) or, in samples collected from 2009, by silver in
situ hybridization (SISH) with evaluation of chromosome 17
(Dual-Color SISH red ISH/Benchmark™ ULTRA Ventana®;
Ventana Medical Systems, Inc.; Roche Diagnostics) in 77 cases,
which included 38 IHC-negative (0/1+), 35 equivocal (2+) and 4
positive (3+) cases, according to the latest ASCO/CAP guidelines.
The IHC pattern, complemented with ERBB2 ISH, allowed the
identification of clinically-defined, treatment-oriented subtypes
(CS), according to AJCC, 8th Edition (19), and were as follows: Luminal A-like
(high hormone receptors and low proliferation level), Luminal
B-like (low hormone receptors and high proliferation level),
HER2-like (ERBB2-positive and negative or positive hormone receptor
expression) and Triple negative (TN; ER-, PR- and
ERBB2-negative).
DNA flow cytometry
DNA flow cytometric analysis was performed on
representative paraffin-embedded tissue according to the method
described by Hedley et al (27), with slight modifications (50 µm
sections and propidium iodide DNA staining were used). DNA content
of the neoplastic cells was determined in 79 invasive carcinomas
with no neoadjuvant therapy. The cell cycle analysis of DNA
histograms was assessed using the Multicycle software program
(32-bit version; Phoenix Flow Systems, San Diego, CA, USA). The
male BC cases were also classified as diploid vs. aneuploid
according to their nuclear DNA ploidy status.
Statistical analysis
A survival study with an initial descriptive
analysis and subsequent nonparametric, semiparametric and
parametric statistical techniques was elaborated. The statistical
analysis was performed using the software R Core Team 2018
(28). Pearson's χ2 and
Fisher's exact tests of independence were used to evaluate the
association between categorical variables. Fisher's exact test was
used when the number of observations was small (n<20). Identical
conclusions were obtained following the use of each test. Survival
curves were estimated using Kaplan-Meier analysis, and the
differences between curves were assessed by the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference. The DFS represented the remission time until a relapse
event. The OS was defined as the interval between pathological
diagnosis and the occurrence of mortality due to BC. Patients
without disease recurrence during the study period and those who
succumbed to other causes, or those who were lost to follow-up,
were considered as censored observations. The OS was evaluated in
the subgroup of patients with M1 disease, and the DFS and OS in the
whole series, excluding patients with M1 disease, NBPN and in
situ carcinomas. OS and DFS were also evaluated and compared in
the following groups: Patients with vs. patients without NBPN, and
patients diagnosed in the years 1970–1998 (group A; n=84) vs. those
diagnosed in the years 1996–2018 (group B; n=132) (prior and
subsequent to the introduction of taxane chemotherapy). The
variables describing the type of treatment were excluded due of the
variability of protocols used. A Cox proportional hazards
regression model was employed to assess the independent prognostic
value of the variables. Initially, the model was fitted for each
variable to evaluate their effects on OS time and remission time of
disease, as a simple regression analysis. Following the
determination of significant variables, a Cox regression model was
performed with all variables simultaneously, as a multiple
regression analysis. A backward stepwise procedure based on
Akaike's Information Criterion (29)
was used to select auxiliary variables. The statistical
significance was obtained by the Wald test, and complementary
inference was calculated as relative risk and 95% confidence
intervals for each category. The quality of the fitted models was
assessed using a residual analysis, and a test of proportionality
of risk functions was conducted for Cox regression models
associated with OS and DFS.
Results
Descriptive analysis and
associations
The clinicopathological and therapeutic
characteristics of the series are summarized in Table I. BRCA mutation, hormone receptors
and Ki-67 protein expression, ERBB2 overexpression and
amplification, CS and DNA ploidy data are presented in Table II. Significant associations between
variables are indicated in Table
III.
 | Table I.Clinicopathological and therapeutic
characteristics of the patient cohort. |
Table I.
Clinicopathological and therapeutic
characteristics of the patient cohort.
|
Characteristics | N | N (%) |
|---|
| Age (years) | 196 |
|
|
31–39 |
| 7 (3.6) |
|
40–69 |
| 108 (55.1) |
|
70–89 |
| 81 (41.3) |
| Family history
(FH) | 196 |
|
| No |
| 166 (84.7) |
|
Yes |
| 30 (15.3) |
| Bilaterality | 196 |
|
| No |
| 189 (96.4) |
|
Yes |
| 7 (3.6) |
| Non-breast primary
neoplasms (NBPN) | 196 |
|
| No |
| 168 (85.7) |
|
Yes |
| 28 (14.3) |
| Tumor size
(pT) | 196 |
|
|
pTis |
| 7 (3.6) |
|
pT1 |
| 52 (26.5) |
|
pT2-3 |
| 58 (29.6) |
|
pT4 |
| 79 (40.3) |
| Axillary nodal
status (pN) | 195 |
|
|
pN0 |
| 85 (43.6) |
|
pN1 |
| 110 (56.4) |
| Distant metastasis
(M) | 196 |
|
| M0 |
| 178 (90.8) |
| M1 |
| 18 (9.2) |
| Anatomic stage
(AS) | 196 |
|
| In
situ (is) |
| 7 (3.6) |
| I |
| 77 (39.2) |
| II |
| 88 (44.9) |
|
III |
| 6 (3.1) |
| IV |
| 18 (9.2) |
| Histological type
(HT) | 196 |
|
| In
situ |
| 6 (3.1) |
|
Invasive no special type
(NST) |
| 177 (90.3) |
| Other
invasive subtypes |
| 13 (6.6) |
| Histological grade
(G) | 190 |
|
| G1 |
| 45 (23.7) |
| G2 |
| 110 (57.9) |
| G3 |
| 35 (18.4) |
| Therapy | 196 |
|
|
Surgery |
| 177(90.3) |
|
Radiotherapy |
| 124 (63.3) |
|
Hormonotherapy |
| 118 (60.2) |
|
Chemotherapy |
| 73 (37.2) |
 | Table II.Molecular characteristics of the
series. |
Table II.
Molecular characteristics of the
series.
|
Characteristics | N | N (%) |
|---|
| BRCA2
mutation | 44 |
|
|
Indeterminate |
| 31 (70.5) |
|
Positive |
| 13 (29.5) |
| Estrogen receptors
(ER) | 190 |
|
|
Positive |
| 177 (93.1) |
|
Negative |
| 13 (6.9) |
| Progesterone
receptors (PR) | 190 |
|
|
Positive |
| 143 (75.3) |
|
Negative |
| 47 (24.7) |
| ERBB2 (IHC +
ISH) | 190 |
|
|
Negative |
| 179 (94.2) |
|
Positive |
| 11 (5.8) |
| Ki67 | 190 |
|
|
Low |
| 78 (41.1) |
|
High |
| 112 (58.9) |
| Clinical defined
subtypes (CS) | 190 |
|
| Luminal
A-like |
| 77 (40.5) |
| Luminal
B-like |
| 86 (45.3) |
|
HER2-like |
| 13 (6.8) |
| Triple
negative |
| 14 (7.4) |
| DNA ploidy | 79 |
|
|
Diploid |
| 9 (11.4) |
|
Aneuploid |
| 70 (88.6) |
 | Table III.Significant associations between
clinical and molecular characteristics of the patient cohort
(Pearson's chi-square test). |
Table III.
Significant associations between
clinical and molecular characteristics of the patient cohort
(Pearson's chi-square test).
|
Characteristics | P-value |
|---|
| Age (<40, 40–69,
≥70 years) |
|
| pT | 0.021 |
| Family history (FH)
(no vs. yes) |
|
| G | 0.009 |
| AS | 0.011 |
| Ki67 index | 0.002 |
| CS | 0.001 |
| BRCA2 mutation | 0.002 |
| Bilaterality (no
vs. yes) |
|
| FH | 0.009 |
| Non-breast primary
neoplasms (NBPN) | <0.001 |
| DNA ploidy | 0.004 |
| BRCA2 mutation | 0.008 |
| Tumour size (pT)
(pT1 vs. pT2-3 vs. pT4) |
|
| pN | <0.001 |
| M | <0.001 |
| AS | <0.001 |
| PR | 0.029 |
| Nodal status (pN)
(pN0 vs. pN1) |
|
| M | 0.002 |
| AS | <0.001 |
| Ki67 index | 0.003 |
| CS | 0.030 |
| Distant metastasis
(M) (M0 vs. M1) |
|
| AS | <0.001 |
| CS | 0.030 |
| ERBB2 | 0.009 |
| Anatomic stage (AS)
(I vs. II/III vs. IV) |
|
| Ki67 index | 0.004 |
| CS | 0.009 |
| ERBB2 | 0.015 |
| Histological type
(HT) (NST vs. others) |
|
| pT | <0.001 |
| pN | 0.012 |
| AS | <0.001 |
| Grade (G) (G1 vs.
G2 vs. G3) |
|
| Ki67 index | <0.001 |
| CS | 0.002 |
| Estrogen receptors
(ER) (positive vs. negative) |
|
| PR | <0.001 |
| Progesterone
receptors (PR) (positive vs. negative) |
|
| BRCA2 mutation | 0.010 |
| Ki-67 (low vs.
high) |
|
| CS | <0.001 |
| ER | 0.004 |
| PR | <0.001 |
| ERBB2 | 0.011 |
| BRCA2 mutation | 0.047 |
| Age |
|
| pT | 0.021 |
| Family history
(FH) |
|
| G | 0.009 |
| AS | 0.011 |
| Ki67 index | 0.002 |
| CS | 0.001 |
| BRCA2 mutation | 0.002 |
| Bilaterality |
|
| FH | 0.009 |
| Non-breast primary
neoplasms (NBPN) | <0.001 |
| DNA ploidy | 0.004 |
| BRCA2 mutation | 0.008 |
| Tumour size
(pT) |
|
| pN | <0.001 |
| M | <0.001 |
| AS | <0.001 |
| PR | 0.029 |
| Nodal status
(pN) |
|
| M | 0.002 |
| AS | <0.001 |
| Ki67 index | 0.003 |
| CS | 0.030 |
| Distant metastasis
(M) |
|
| AS | <0.001 |
| CS | 0.030 |
| ERBB2 | 0.009 |
| Anatomic stage
(AS) |
|
| Ki67 index | 0.004 |
| CS | 0.009 |
| ERBB2 | 0.015 |
| Histological type
(HT) |
|
| pT | <0.001 |
| pN | 0.012 |
| AS | <0.001 |
| Grade (G) |
|
| Ki67 index | <0.001 |
| CS | 0.002 |
| Estrogen receptors
(ER) |
|
| PR | <0.001 |
| Progesterone
receptors (PR) |
|
| BRCA2 mutation | 0.010 |
| Ki-67 |
| CS | <0.001 |
| ER | 0.004 |
| PR | <0.001 |
| ERBB2 | 0.011 |
| BRCA2 mutation | 0.047 |
The mean and median age of patients at diagnosis was
65.2 and 66.5 years (range, 31–89 years), respectively. The
majority of the patients (n=108; 55.1%) were between 40 and 69
years, and older patients (≥70 years) comprised 41.3% of the
sample. Old age (≥70 years) exhibited a significant association
with pT4 (P=0.021). Body mass index was not evaluated in the
majority of the cases; however, from review of the clinical
records, obesity was observed in ~20% of the patients.
A confirmed FH of BC was obtained in 30 patients
(15.3%). FH is significantly associated with G2/G3 carcinomas, high
Ki-67, Luminal B-like subtype, high anatomical stage, presence of
BRCA2 mutations and bilaterality. A total of 7 patients (3.6%)
exhibited bilateral carcinomas, and 1 patient exhibited synchronous
tumors. FH and bilaterality were significantly associated with
BRCA2 mutations. Bilaterality was also associated with the presence
of NBPN. The occurrence of NBPN was identified in 28 patients
(14.3%). One patient exhibited 3 other carcinomas, in the prostate,
colon and kidney, and 2 patients exhibited 2 carcinomas, in the
prostate and bladder and in the prostate and kidney. From the
remaining patients, 12 had prostate carcinomas, 3 presented with
colon-rectal carcinomas, 3 exhibited head and neck squamous cell
carcinomas, 3 had gastric carcinomas, 1 had papillary thyroid
carcinoma, 1 exhibited chronic lymphocytic leukemia, 1 had Hodgkin
disease and 1 exhibited soft tissue histiocytic sarcoma. In the
majority of cases, BC was the first neoplasm recorded. In 5
patients, it was the second neoplasm observed; 2 of these patients
had exhibited lymphoma previously. A total of 2 patients with
prostate carcinoma also had bilateral BC.
A total of 79 patients (40.3%) presented with pT4
carcinomas and 110 (56.4%) with axillary lymph node metastasis. The
majority of the patients (n=178; 90.8%) had no distant metastasis
at presentation (M0). At diagnosis, 88 patients (44.9%) were
diagnosed with AS II disease. Nodal status and AS were
significantly associated with Ki-67. Distant metastases at
presentation (M1) were associated with ERBB2-positive
carcinomas.
Regarding the histological type, 177 (90.3%)
invasive carcinoma of no special type (NST) were identified, ~25%
of which exhibited a range of proportions of in situ
components, and the other most frequent invasive subtypes were
mucinous carcinoma (1 pure and 4 mixed) and papillary carcinoma (4
cases). The case of pure mucinous carcinoma belonged to a 37 years
old patient with no FH, diagnosed with a pT2/pN0/M0 tumor, G2,
ER/PR-positive, ERBB2-negative and BRCA indeterminate, who survived
with no recurrence during a follow-up of 96 months. A total of 2
patients (1%), at 51 and 64 years old, were diagnosed with lobular
invasive carcinoma, one with a FH and the other with a pathogenic
BRCA2 mutation.
The majority of the male BC cases were graded as G2.
A total of 45 cases (23.7%) were classified as G1, and only a
minority of the cases (18.4%) was integrated in the G3 group. High
grades were associated with high Ki-67 levels.
During the present study, therapeutic strategies for
male BC followed the patterns of the recommendations for BC in
females. The majority of patients (90.3%) underwent surgery, but
only 9 patients (4.6%) performed breast-conserving surgery. A total
of 33 patients (16.8%) received neoadjuvant therapy. Adjuvant
radiotherapy was used in 63.3% of the patients, adjuvant
hormonotherapy in 60.2% and chemotherapy in 37.2% of the patients.
ERBB2-targeting agents were used in 2 patients in the cohort.
BRCA2 mutations were identified in 13 (29.5%) of the
44 patients examined and, in addition to the associations with FH
and bilaterality, were also significantly associated with high
Ki-67 and negative PR expression levels. A total of 10 (76.9%)
confirmed BRCA2 mutated carcinomas belonged to the surrogate
Luminal B-like subtype and 2 cases were HER2-like, according to the
AJCC 8th edition classification system (19). No BRCA1 mutations were identified in
the series.
The majority of male breast carcinomas were
ER-positive/PR-positive/ERBB2-negative, and 14 (7.4%) were TN. All
PR-positive cases were ER-positive, and 34 cases (75.6%) of
PR-negative carcinomas were ER-positive (P<0.0001). Using IHC,
35 ERBB2-equivocal (2+) cases and 6 positive (3+) cases were
identified. From the equivocal cases, 5 (14.2%) became positive. In
total, 11 ERBB2-positive cases were identified, 10 cases of which
were triple-positive and 1 case was ER-positive and PR-negative.
Positive ERBB2 expression was significantly associated with M1
carcinomas at presentation, high AS and high Ki-67 expression.
High Ki-67 (n=112; 58.9%) was significantly
associated with positive FH, high grades, pN1, high AS,
ER-negative, PR-negative and ERBB2-positive expression, and the
presence of BRCA2 mutations.
The incidence rates of the 4 clinically-defined CS
using IHC, according to AJCC 8th Edition (19), were as follows: Luminal A-like
(40.5%), Luminal B-like (45.3%), HER2-like (6.8%) and TN
(7.4%).
DNA ploidy pattern was analyzed in 79 cases,
revealing a high percentage of aneuploid carcinomas (88.6%). As
shown in Table III, aneuploid
carcinomas were significantly associated with bilaterality.
Survival analysis
The 5 and 10-year DFS rates of patients, excluding
patients with M1 carcinomas, patients with non-primary breast
neoplasms and in situ carcinomas (n=145) were 65.9 and
58.2%, respectively, and the 5 and 10 year OS rates were 77.5 and
59.2%, respectively. Mean and median remission times were 75.6 and
50 months (range, 0–312), respectively, and mean and median
survival times were 87.8 and 72 months (range, 3–396),
respectively. Of the 18 patients with distant metastasis at
presentation, only 1 was alive with bone metastasis after 34 months
of follow-up. All other 17 patients succumbed to the disease, with
the mean and median survival times for all patients with distant
metastases (M1) being 18.7 and 15.5 months (range, 1–38 months).
The occurrence of NBPN did not decrease OS, as patients with NBPN
exhibited 5 and 10-year OS rates of 92.3 and 92.3% compared with
75.5 and 59.2% of patients without NBPN.
Kaplan-Meier estimates indicated that a longer DFS
and an improved OS were significantly associated with pT1/pN0/stage
I (all P<0.001), low Ki-67 carcinomas (P=0.030 and P=0.010,
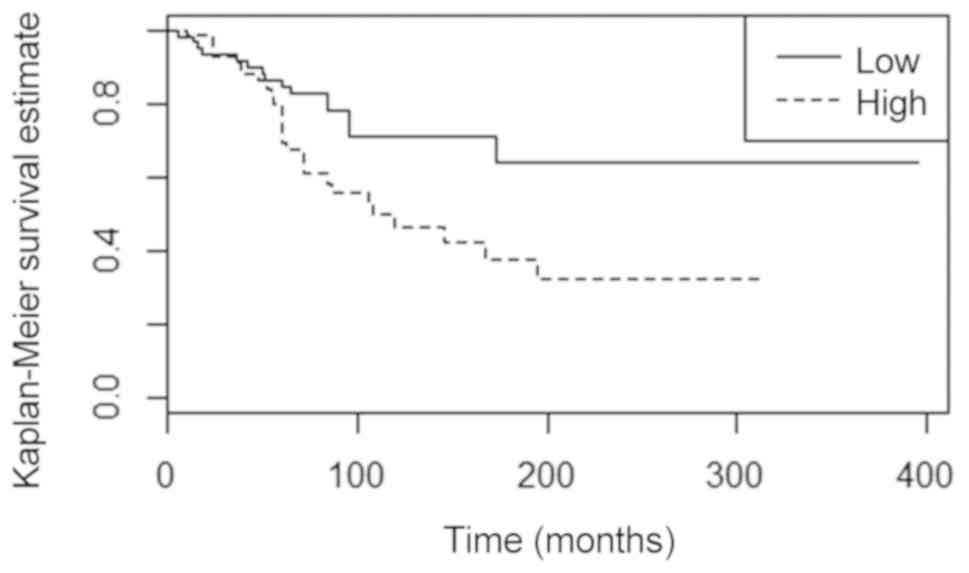
respectively; Fig. 1 and Tables IV and V), while a shorter DFS and poorer OS were
associated with Luminal B-like subtype (P=0.002) and the presence
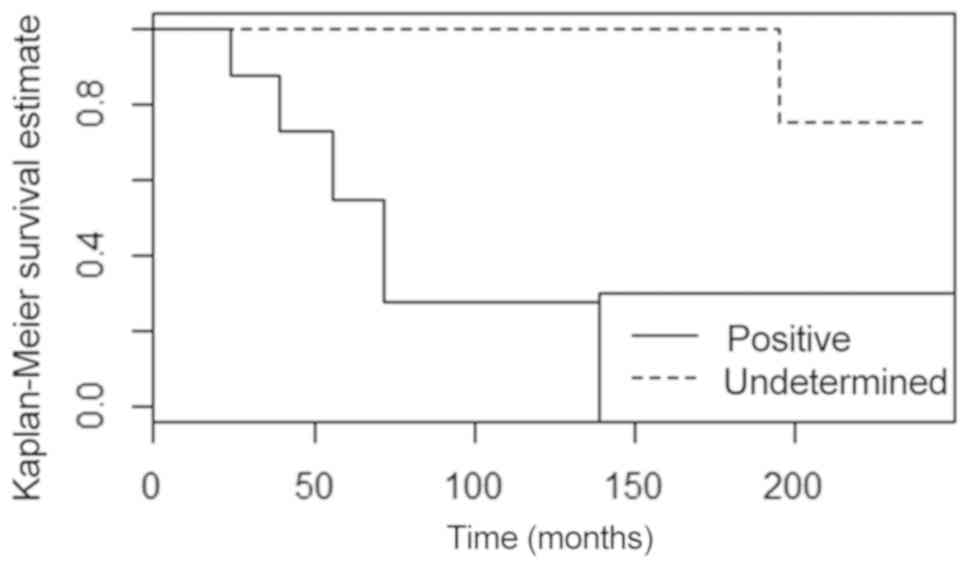
of BRCA2 mutations (P=0.003 and P<0.001, respectively) (Fig. 2). Patients with G3 carcinomas also
exhibited a shorter DFS (P=0.020). Additionally, a longer OS was
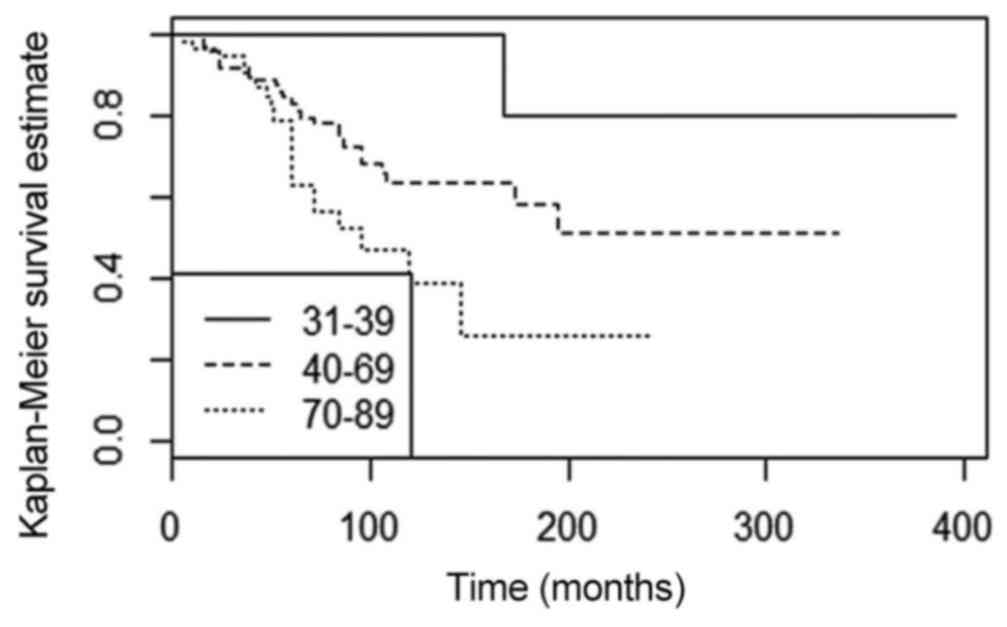
associated with young age (<40 years; P=0.010 and Fig. 3). The patients diagnosed prior to the
introduction of taxane chemotherapy exhibited significantly
decreased 5 and 10-year DFS (P=0.030) and OS (P=0.050) compared
with those diagnosed in the years following the introduction of
taxane chemotherapy.
 | Table IV.Univariate Cox regression analysis in
relation to DFS and OS. |
Table IV.
Univariate Cox regression analysis in
relation to DFS and OS.
|
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Variables | RR | 95% CI | P value | RR | 95% CI | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
31–39 | 1 | – | – | 1 | – | – |
|
40–69 | 4.37 | 0.59–32.3 | 0.148 | 4.05 | 0.54–30.1 | 0.172 |
|
70–89 | 6.35 | 0.84–48.2 | 0.074 | 8.02 | 1.06–60.8 | 0.044 |
| Grade (G) |
|
|
|
|
|
|
|
G1-2 | 1 | – | – |
|
|
|
| G3 | 2.14 | 1.12–4.05 | 0.020 |
|
|
|
| Tumor size
(pT) |
|
|
|
|
|
|
|
pT1 | 1 | – | – | 1 | – | – |
|
pT2-3 | 3.19 | 1.23–8.27 | 0.017 | 3.55 | 1.14–11.0 | 0.029 |
|
pT4 | 6.10 | 2.50–14.9 | <0.001 | 9.10 | 3.19–26.0 | <0.001 |
| Nodal status
(pN) |
|
|
|
|
|
|
|
pN0 | 1 | – | – | 1 | – | – |
|
pN1 | 6.32 | 2.93–13.6 | <0.001 | 6.40 | 2.84–14.4 | <0.001 |
| Anatomic stage
(AS) |
|
|
|
|
|
|
| I | 1 | – | – | 1 | – | – |
| II | 6.71 | 3.11–14.5 | <0.001 | 7.81 | 3.28–18.6 | <0.001 |
|
III | 4.80 | 1.01–22.7 | 0.048 | 11.1 | 2.75–44.9 | <0.001 |
| Ki-67 |
|
|
|
|
|
|
|
Low | 1 | – | – | 1 | – | – |
|
High | 1.89 | 1.05–3.39 | 0.033 | 2.14 | 1.16–3.96 | 0.015 |
| Clinical subtype
(CS) |
|
|
|
|
|
|
| Luminal
A-like | 1 | – | – | 1 | – | – |
| Luminal
B-like | 2.67 | 1.45–4.90 | 0.002 | 2.97 | 1.58–5.60 | <0.001 |
|
HER2-like | 0.81 | 0.11–6.08 | 0.837 | 1.25 | 0.16–9.51 | 0.828 |
| Triple
negative | 0.52 | 0.12–2.23 | 0.379 | 0.64 | 0.15–2.80 | 0.556 |
| BRCA2 mutation |
|
|
|
|
|
|
|
Indeterminate | 1 | – | – | 1 | – | – |
|
Positive | 0.11 | 0.02–0.63 | 0.013 | 0.06 | 0.01–0.52 | 0.011 |
 | Table V.Multivariate Cox regression analysis
in relation to DFS and OS. |
Table V.
Multivariate Cox regression analysis
in relation to DFS and OS.
|
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Variables | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Bilaterality |
|
|
|
|
|
|
| No | 1 | – | – |
|
|
|
|
Yes | 6.16 | 1.30–29.3 | 0.022 |
|
|
|
| Family history
(FH) |
|
|
|
|
|
|
| No |
|
|
|
|
|
|
|
Yes |
|
|
| 0.33 | 0.13–0.90 | 0.030 |
| Grade (G) |
|
|
|
|
|
|
|
G1-2 | 1 | – | – | 1 | – | – |
| G3 | 2.20 | 1.10–4.42 | 0.026 | 2.06 | 1.0–4.24 | 0.051 |
| Anatomic stage |
|
|
|
|
|
|
| I | 1 | – | – | 1 | – | – |
| II | 4.08 | 1.53–10.9 | 0.005 | 8.95 | 3.65–21.9 | <0.001 |
|
III | 6.79 | 1.15–40.2 | 0.035 | 45.7 | 9.92–211 | <0.001 |
| Clinical
subtype |
|
|
|
|
|
|
| Luminal
A-like | 1 | – | – | 1 | – | – |
| Luminal
B-like | 1.72 | 0.92–3.23 | 0.091 | 2.05 | 1.07–3.93 | 0.030 |
The results of the univariate Cox model analysis
(Table IV) were consistent with the
Kaplan-Meier analysis. The categories pT2-3 and 4, pN1, AS II and
III, high Ki-67, Luminal B-like and BRCA2 mutations were
significantly associated with shorter DFS and OS. In addition, G3
and ages >70 years were significantly associated with lower DFS
and poorer OS, respectively.
In the multivariate Cox regression analysis
(Table V), bilaterality, G3 and AS
II and III carcinomas were the significant factors associated with
a higher risk of disease recurrence. The presence of FH, AS II and
III and Luminal B-like subtype were the significant characteristics
associated with low OS.
Discussion
BC is a complex disease that affects females and
males, and the primary known difference between sexes is incidence.
According to recognized biological heterogeneity, studies comparing
female and male BC have demonstrated similarities and differences
(1,3,7,30,31). The
understanding of the effects of the clinicopathological, molecular
and genomic features associated with therapy and prognosis is
progressing continuously in male BC and, as more data become
available, the hypothesis that males only exhibit
endocrine-associated BC identical to that in postmenopausal females
becomes less plausible, and male BC emerges as a distinctive
subtype of BC lacking its own guidelines (15,32–36).
Survival has been a controversial issue in male BC.
The majority of studies have demonstrated a poorer outcome in male
compared with female patients, but others revealed that there was
no difference in the prognosis of the two sexes, when paired
according to specific groups (15,30,32–38). A
similar DFS and OS to pre/peri-menopausal females, but poorer
compared with post-menopausal female BC, was described in male
patients with BC (32), and even a
lower risk of mortality compared with comparable females, despite
the frequent presentation in elderly and more advanced disease in
male BC (1). M1 patients have
incurable disease and, in the patient cohort in the present study,
all but 1 succumbed to the disease, with a median survival time of
15.5 months. The present study encompassed a long time period, with
the 10-year OS rates of cases (stages I–III; 59.2%) measuring
slightly longer compared with those demonstrated by Leone et
al (53.7%) (6), Chen et
al (40.1%) (38) and Tural et
al (52.5%) (39).
The risk of developing BC increases with age,
similar to the majority of carcinomas at all sites. In the present
study, the percentage of the patients aged ≥70 years (41.3%)
confirms the high incidence of BC in older males, and also that the
average age at diagnosis is ~5-10 years older compared with in
females (1,12,36). The
high frequency in the elderly population is important, as the
therapeutic approach in older male patients is based on studies
performed in females of different ages, and comorbidities in the
elderly population may result in inadequate treatment. In the
present study, elderly patients exhibited larger carcinomas and
higher Ki-67 expression levels compared with younger patients, and
old age was a prognostic factor significantly associated with low 5
and 10-year OS in Kaplan-Meier estimates, which were concordant
with data from previous studies (6,7,33,38,39).
Poorer prognoses in older males may be associated with tumor
biology, late diagnosis, comorbidities and/or inadequate
therapeutic management, and constitutes a persistent clinical
problem (33,39). Similar to older patients, obese
patients have unknown risk factors affecting the accurate
prediction of toxicity of treatments and prognosis (35). Obesity is an important risk factor
and the proportion of obese male patients with BC observed in the
present study was similar to that identified by Gargiulo et
al (8,40).
FH appears to be particularly relevant in male BC.
Bouchardy et al (41)
identified a positive FH in ~20% of male patients with BC, but no
significant differences in OS in patients with FH compared with
sporadic cases were observed. As the present study included
patients diagnosed from 1970 onwards, the majority of patients had
no information regarding FH in their clinical records. However, a
confirmed family history of BC in a first-degree relative was
significantly associated with BRCA mutations, and also to high AS,
high grade, high Ki-67 and Luminal B-like subtype. Additionally, in
the multivariate analysis, a positive FH was associated with OS. In
concordance with previous data (41), positive FH was also associated with
bilateral male BC. Bilaterality occurred in 3.6% of the patients in
the cohort within the present study, and was significantly
associated with BRCA2 mutations and with the presence of NBPN. Male
patients with BC also have an increased risk of NPBN, and the long
survival times currently observed should be observed cautiously
(42–44). A total of ~14% of the patients in the
present study exhibited NBPN, and 2 with bilaterality and prostate
carcinoma. As described previously (45), prostate carcinoma is the most
frequently observed non-breast primary tumor. The risk of head and
neck, colon and thyroid carcinomas were demonstrated to be high in
male BC (43,44), and their occurrence was also observed
in the present study. A total of 2 patients had previous lymphoma,
supporting the observation that males who survive lymphoma may have
an increased risk of developing BC (44,46).
Among the factors identified to be responsible for causing a second
neoplasia, genetic factors appear to represent an important
contribution. These data suggest the requirement of a genetic
consultation in male BC. BRCA2 is one of the most frequently
mutated genes in male BC, ranging between 4 and 40% depending on
the population studied (15); 29.5%
of the 44 patients included in the present study exhibited this
mutation, while BRCA1 mutations are infrequent; none were observed
in the present study, suggesting a dissimilar genetic etiology
between sexes (13). As described
previously (8,16), the majority of BRCA2-mutated
carcinomas in the present study belonged to the Luminal B-like
subtype, and a significant association between BRCA2 mutations and
poorer prognosis was observed.
The AS classification systems represents one of the
most important established prognostic tools for male BC, as
demonstrated in the present study and in previous studies (6,8,34). Male BC is increasingly diagnosed
earlier (6,7), and a predominance of early stages was
observed in the present study. However, high rates of advanced
stages are frequently observed (1,6,8,12,33,36,37).
The unviability of screening due to low incidence rates, the high
occurrence of gynecomastia that may exhibit identical presentation
symptoms, the fact that males are less likely to report symptoms
that would lead to early diagnosis, the absence of
publicly-available information about the disease, the incidence in
old age with an associated suboptimal access to healthcare, and
anatomic and biological differences, may explain the number of
diagnoses at high stages observed (4,36,39).
The proportion of pure in situ carcinomas,
one-half with papillary morphology, varies in previous studies, but
is significantly decreased compared with the proportion described
in females (30,33,47). The
relative frequency of papillary morphology, either in situ
or invasive carcinomas, may be associated with the common
subareolar localization in male BC. The heterogeneous histological
type of invasive carcinoma NST, with an occurrence between 85 and
95% described in previous studies (5,37), was
diagnosed in 90.3% of cases in the present study. The percentage of
associated in situ components was similar to the proportion
demonstrated in females (48).
Mucinous carcinoma accounts for 1–4% of male BC, has a favorable
prognosis in the pure form, but the pathogenesis is not understood
(30,39,49,50). In
the present study, 5 cases (2.5% of all cases) were observed, one
being the patient with a pure form, unusually young for the
described in mucinous carcinomas. Invasive lobular carcinoma, the
second most frequent histological type in females (10% of the
cases), is exceptionally rare in males (1%) and its etiology
remains unexplained considering the lack of development of terminal
lobules in males (6,51). A total of 2 invasive lobular
carcinomas (1%) were identified in the present study, both with
negative epithelial-cadherin staining.
G2 carcinomas were predominant in the present study,
similar to other previous studies (5,7,30,33,52).
High histological grade (G3) is commonly associated with poor
prognosis, but this is not always statistically significant
(6,7). In the present study, G3 carcinomas
occurred in 18.4% of the cases, and were demonstrated to be
significantly associated with FH, high Ki-67 expression, Luminal B
clinical subtype and poorer prognosis in univariate and
multivariate Cox regression analyses.
The lack of randomized trials in male BC explains
why therapy is based on the guidelines for BC in females. However,
due to primarily anatomical and hormonal reasons, the management is
not exactly the same, highlighting the requirement to improve the
personalized care of male BC (17,38).
Breast-conserving surgery vs. mastectomy may be performed in early
stages, but is rarely used due to the paucity of breast tissue and
the frequent subareolar location of carcinomas associated with the
distribution of epithelial breast tissue (6–8,49). Tamoxifen is the most frequently
employed systemic treatment (38,49), but
low tolerance, side effects and high rates of discontinuation have
been described (4,17,38,49). The
relatively low rate of hormonotherapy compared with the high
percentage of ER-positive carcinomas identified in the present
study, and demonstrated in previous studies (7,31), may
be associated with the fact that the use of tamoxifen in males was
only recently standardized (7).
Different chemotherapy agents and regimens have been used and the
introduction of taxanes marked a significant advance in the
treatment of metastatic disease in females, but there are no
specific evidence-based guidelines for male BC (33,35,49). In
addition to the therapeutic effects of the treatment, the
improvement in DFS and OS observed in the present study when
comparing the groups of patients diagnosed prior and subsequent to
taxane chemotherapy may be associated with early diagnosis,
standardized clinicopathological evaluation and improved follow-up
observed in recent years (1).
Biomarker evaluation by IHC has resulted in
differing data among male BC studies, primarily due to different
methodologies, the development of scoring systems and the range of
cut-off values used, but the high frequency of
ER-positive/PR-positive expression and the low frequency of TN
carcinomas are concordant (7,8,38,45,52).
ER-positive expression is associated with improved prognoses at
5-year OS (6), but certain
clinically aggressive male BC cases do not appear to have an active
ER pathway (16). In the present
study, negative PR expression status was associated with BRCA
mutations.
Despite the different estimates described, ERBB2
positivity has a low frequency in males (7,51). Using
IHC and ISH, 6.8% of the cases in the present study were identified
as HER2-like clinical subtypes, according to AJCC 8th Edition
(19), and significantly associated
with a high Ki-67 expression level and high AS. ERBB2 positivity is
generally associated with aggressive phenotypes, but survival of
HER2-like carcinomas has improved in previous years, due to
specific treatment with associated ERBB2-targeting agents (19,33).
Cell proliferation is also an important biological
factor, usually associated with poor outcome (53). Ki-67, a nuclear protein present
during all phases of the cell cycle, is the most commonly used
marker to evaluate proliferation, although it lacks standardized
methodology or generally accepted cut-offs. With a cut-off of ≥20%,
previous studies identified a predominance of low Ki-67 values
(7,8). By contrast, the present study
identified a slight predominance of high Ki-67 values,
significantly associated with old age, positive FH, high grade,
pN1, high AS, CS and poor prognosis in the univariate Cox
regression analysis.
The criteria for the definition of BC molecular
subtypes used clinically for decisions regarding therapy are
continuously progressing, resulting in difficulties when comparing
data (5,7,15,30,38,52).
Using IHC surrogates, Luminal A-like and Luminal B-like subtypes
were identified in the majority of BC in males and females, usually
with a poorer outcome for Luminal B-like (7,15,26). As
PR, ERBB2 and Ki-67 are important prognostic and predictive
factors, the inclusion of carcinomas with positive or negative PR
and/or ERBB2 expression statuses and different Ki-67 cut-offs in
the same subtype are key factors contributing to discordant
results. In the present study, according to the AJCC 8th edition
(19), Luminal B-like subtype
exhibited the poorest OS.
Bezić et al (54) observed aneuploidy in 78% of 31 male
patients with BC. In a previous comparative study between sexes (50
cases each), our group demonstrated a significantly higher
frequency of DNA aneuploid tumors in males compared with females
(80 vs. 46%) (55). The high
percentage of aneuploidy, which was observed to be increased in the
present study, suggested a distinctive genomic instability in the
carcinogenesis of male BC.
The present study has the limitations of a
retrospective study from a single institution conducted over a long
time period. However, the results are consistent with those of
large and/or multi-institutional studies, confirming that studies
involving smaller, but well-characterized clinicopathological and
molecular subgroups, diagnosed and followed in multidisciplinary
departments within single institutions, are important in improving
the understanding of this disease.
In conclusion, the present study demonstrated that
male BC was more likely to be diagnosed in older patients (with
consequent associated comorbidities and suboptimal therapy), and
exhibited poorer prognosis in elderly and in high anatomical
stages. BRCA2 mutations were frequent, associated with FH,
bilaterality, high Ki-67, PR negativity and Luminal B-like subtype,
and with shorter DFS and OS in univariate analysis. In addition,
male patients with BC were at high risk for NBPN. In the
multivariate analysis, FH and Luminal B carcinomas were associated
with poorer OS. These data underline the importance of early
diagnosis and genetic screening in male BC. As sex may be a crucial
feature to improve personalized care, additional studies
investigating male BC are warranted and may lead to the development
of relevant management approaches for BC in males and females.
Acknowledgements
Not applicable.
Funding
Professor Giovani Silva was partially funded by
FCT-Portugal project (grant no. UID/MAT/00006/2019).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SA and AEP discussed experimental design,
interpreted and discussed the data and wrote the manuscript. TP and
FS performed IHC experiments. PM and FV performed BRCA analysis. MA
and GLS analyzed and interpreted statistical data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of the Portuguese Institute of Oncology Lisbon
Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miao H, Verkooijen HM, Chia KS, Bouchardy
C, Pukkala E, Larønningen S, Mellemkjaer L, Czene K and Hartman M:
Incidence and outcome of male breast cancer: An international
population-based study. J Clin Oncol. 29:4381–4386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Speirs V and Shaaban AM: The rising
incidence of male breast cancer. Breast Cancer Res Treat.
115:429–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ly D, Forman D, Ferlay J, Brinton LA and
Cook MB: An international comparison of male and female breast
cancer incidence rates. Int J Cancer. 132:1918–1926. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
White J, Kearins O, Dodwell D, Horgan K,
Hanby AM and Speirs V: Male breast carcinoma: Increased awareness
needed. Breast Cancer Res. 13:2192011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yalaza M, İnan A and Bozer M: Male breast
cancer. J Breast Health. 12:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leone JP, Zwenger AO, Iturbe J, Leone J,
Leone BA, Vallejo CT and Bhargava R: Prognostic factors in male
breast cancer: A population-based study. Breast Cancer Res Treat.
156:539–548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cardoso F, Bartlett JMS, Slaets L, van
Deurzen CHM, van Leeuwen-Stok E, Porter P, Linderholm B, Hedenfalk
I, Schröder C, Martens J, et al: Characterization of male breast
cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG international
male breast cancer program. Ann Oncol. 29:405–417. 2018.PubMed/NCBI
|
|
8
|
Gargiulo P, Pensabene M, Milano M, Arpino
G, Giuliano M, Forestieri V, Condello C, Lauria R and De Placido S:
Long-term survival and BRCA status in male breast cancer: A
retrospective single-center analysis. BMC Cancer. 16:3752016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson WF, Jatoi I, Tse J and Rosenberg
PS: Male breast cancer: A population-based comparison with female
breast cancer. J Clin Oncol. 28:232–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miranda AC: National Oncological Registry
South (ROR-South) 2010–2011. Incidence. (Survival and Mortality for
cancer in the Southern region of Portugal - ISM2010/2011.
ROR-South. Portuguese Institute of Oncology of Lisbon). 2017.
|
|
11
|
Kreiter E, Richardson A, Potter J and
Yasui Y: Breast cancer: Trends in international incidence in men
and women. Br J Cancer. 110:1891–1897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferzoco RM and Ruddy KJ: The epidemiology
of male breast cancer. Curr Oncol Rep. 18:12016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deb S, Lakhani SR, Ottini L and Fox SB:
The cancer genetics and pathology of male breast cancer.
Histopathology. 68:110–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pritzlaff M, Summerour P, McFarland R, Li
S, Reineke P, Dolinsky JS, Goldgar DE, Shimelis H, Couch FJ, Chao
EC and LaDuca H: Male breast cancer in a multi-gene panel testing
cohort: Insights and unexpected results. Breast Cancer Res Treat.
161:575–586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johansson I, Killander F, Linderholm B and
Hedenfalk I: Molecular profiling of male breast cancer-lost in
translation? Int J Biochem Cell Biol. 53:526–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keinan-Boker L, Levine H, Leiba A, Derazne
E and Kark JD: Adolescent obesity and adult male breast cancer in a
cohort of 1,382,093 men. Int J Cancer. 142:910–918. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan MH, Allerton R and Pettit L: Hormone
therapy for breast cancer in men. Clin Breast Cancer. 15:245–50.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pemmaraju N, Munsell MF, Hortobagyi GN and
Giordano SH: Retrospective review of male breast cancer patients:
Analysis of tamoxifen-related side-effects. Ann Oncol.
23:1471–1474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual (eighth). 2017.
View Article : Google Scholar
|
|
20
|
Lakhani SR, Ellis IO, Schnitt SJ, Hoon Tan
PH and van de Vijver MJ: World health organization classification
of tumors of the breast. (lyon). IARC. WHO Classification of
Tumours. 2012.
|
|
21
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. the value of histological
grade in breast cancer: Experience from a large study with long
follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Machado PM, Brandão RD, Cavaco BM, Eugénio
J, Bento S, Nave M, Rodrigues P, Fernandes A and Vaz F: Screening
for a BRCA2 rearrangement in high-risk breast/ovarian cancer
families: Evidence for a founder effect and analysis of the
associated phenotypes. J Clin Oncol. 25:2027–2034. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Freitas AC, Opinião A, Fragoso S, Nunes H,
Santos M, Clara A, Bento S, Luís A, Silva J, Moura C, et al: Men
seeking counselling in a breast cancer risk evaluation clinic.
Ecancermedicalscience. 12:8042018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hammond ME, Hayes DF, Wolff AC, Mangu PB
and Temin S: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Oncol Pract. 6:195–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American society of
clinical oncology/college of American pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the St gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hedley DW, Friedlander ML, Taylor IW, Rugg
CA and Musgrove EA: Method for analysis of cellular DNA content of
paraffin-embedded pathological material using flow cytometry. J
Histochem Cytochem. 31:1333–1335. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schäler J, Thaller G, Hinrichs D and R
Core Team R: A Language and environment for statistical computing.
R Foundation for statistical computing (Vienna, Austria).
Agricultural Sciences. 9:2018.
|
|
29
|
Akaike H: A new look at the statistical
model identification. IEEE Transactions on Automatic Control.
19:716–723. 1974. View Article : Google Scholar
|
|
30
|
Shaaban AM, Ball GR, Brannan RA, Cserni G,
Di Benedetto A, Dent J, Fulford L, Honarpisheh H, Jordan L, Jones
JL, et al: Characterization of male breast cancer: Results of the
EORTC 10085/TBCRC/BIG/NABCG international male breast cancer
program. Ann Oncol. 29:405–417. 2018.PubMed/NCBI
|
|
31
|
Greif JM, Pezzi CM, Klimberg VS, Bailey L
and Zuraek M: Gender differences in breast cancer: Analysis of
13,000 breast cancers in men from the national cancer data base.
Ann Surg Oncol. 19:3199–3204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu XF, Yang HJ, Yu Y, Zou DH and Miao LL:
A prognostic analysis of male breast cancer (MBC) compared with
post-menopausal female breast cancer (FBC). PLoS One.
10:e01366702015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Q, Li J, Zhu S, Wu J, Li X, Liu Q, Wei
W and Sun S: Poorer breast cancer survival outcomes in males than
females might be attributable to tumor subtype. Oncotarget.
7:87532–87542. 2016.PubMed/NCBI
|
|
34
|
Rushton M, Kwong A, Visram H, Graham N,
Petrcich W and Dent S: Treatment outcomes for male breast cancer: A
single-centre retrospective case-control study. Curr Oncol.
21:e400–e407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu E, Stitt L, Vujovic O, Joseph K,
Assouline A, Younus J, Perera F and Tai P: Male breast cancer
prognostic factors versus female counterparts with propensity
scores and matched-pair analysis. Cureus. 7:e3552015.PubMed/NCBI
|
|
36
|
Gnerlich JL, Deshpande AD, Jeffe DB,
Seelam S, Kimbuende E and Margenthaler JA: Poorer survival outcomes
for male breast cancer compared with female breast cancer may be
attributable to in-stage migration. Ann Surg Oncol. 18:1837–1844.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Yang J, Krishnamurti U, Huo L, Ward
KC, O'Reagan R and Peng L: Hormone receptor positive breast cancer
has a worse prognosis in male than in female patients. Clin Breast
Cancer. 17:356–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen X, Liu X, Zhang L, Li S, Shi Y and
Tong Z: Poorer survival of male breast cancer compared with female
breast cancer patients may be due to biological differences. Jpn J
Clin Oncol. 43:954–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tural D, Ukbiricik F, Aydogan F, Bese N,
Yetmen O, Ilvan S, Buyukunal E and Sergendeçti S: Male breast
cancers behave differently in elderly patients. Jpn J Clin Oncol.
43:22–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Freedman RA and Partridge AH: Emerging
data and current challenges for young, old, obese, or male patients
with breast cancer. Clin Cancer Res. 23:2647–2654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bouchardy C, Rapiti E, Fioretta G,
Schubert H, Chappuis P, Vlastos G and Benhamou S: Impact of family
history of breast cancer on tumor characteristics, treatment, risk
of second cancer and survival among men with breast cancer. Swiss
Med Wkly. 143:w138792013.PubMed/NCBI
|
|
42
|
Zheng G, Yu H, Hemminki A, Försti A,
Sundquist K and Hemminki K: Familial associations of male breast
cancer with other cancers. Breast Cancer Res Treat. 166:897–902.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hemminki K, Scélo G, Boffetta P,
Mellemkjaer L, Tracey E, Andersen A, Brewster DH, Pukkala E,
McBride M, Kliever EV, et al: Second primary malignancies in
patients with male breast cancer. Br J Cancer. 92:1288–1292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hung MH, Liu CJ, Teng CJ, Hu YW, Yeh CM,
Chen SC, Chien SH, Hung YP, Shen CC, Chen TJ, et al: Risk of second
non-breast primary cancer in male and female breast cancer
patients: A population-based cohort study. PLoS One.
11:e01485972016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Masci G, Caruso M, Caruso F, Salvini P,
Carnaghi C, Giordano L, Miserocchi V, Losurdo A, Zuradelli M,
Torrisi R, et al: Clinicopathological and immunohistochemical
characteristics in male breast cancer: A retrospective case series.
Oncologist. 20:586–592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Farr DE, Thomas A, Khan SA and Schroeder
MC: Male breast cancer as a second primary cancer: Increased risk
following lymphoma. Oncologist. 22:895–900. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brents M and Hancock J: Ductal carcinoma
in situ of the male breast. Breast Care (Basel). 11:288–290. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kuhl CK, Strobel K, Bieling H, Wardelmann
E, Kuhn W, Maass N and Schrading S: Impact of preoperative breast
MR imaging and MR-guided surgery on diagnosis and surgical outcome
of women with invasive breast cancer with and without DCIS
component. Radiology. 284:645–655. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bradley KL, Tyldesley S, Speers CH, Woods
R and Villa D: Contemporary systemic therapy for male breast
cancer. Clin Breast Cancer. 14:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ishida M, Umeda T, Kawai Y, Mori T, Kubota
Y, Abe H, Iwai M, Yoshida K, Kagotani A, Tani T and Okabe H:
Mucinous carcinoma occurring in the male breast. Oncol Lett.
7:378–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Senger JL, Adams SJ and Kanthan R:
Invasive lobular carcinoma of the male breast-a systematic review
with an illustrative case study. Breast Cancer (Dove Med Press).
9:337–345. 2017.PubMed/NCBI
|
|
52
|
Abreu MH, Afonso N, Abreu PH, Menezes F,
Lopes P, Henrique R, Pereira D and Lopes C: Male breast cancer:
Looking for better prognostic subgroups. Breast. 26:18–24. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nilsson C, Koliadi A, Johansson I, Ahlin
C, Thorstensen S, Bergkvist L, Hedenfalk I and Fjallskog ML: High
proliferation is associated with inferior outcome in male breast
cancer patients. Mod Pathol. 26:87–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bezić J, Šamija Projić I, Projić P,
Ljubkovic J, Zekic Tomas S, Meljanac Salopek K, Pilgic Burazer M
and Tomic S: Flow cytometric DNA hipertetraploid tends to be more
frequent in male than in female breast cancers. Virchows Arch.
466:185–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
André S, Pinto AE, Laranjeira C, Quaresma
M and Soares J: Male and female breast cancer-differences in DNA
ploidy, p21 and p53 expression reinforce the possibility of
distinct pathways of oncogenesis. Pathobiology. 74:323–327. 2007.
View Article : Google Scholar : PubMed/NCBI
|