Introduction
While urothelial carcinoma is a very common tumor
type, involvement of the upper urinary tract is relatively
uncommon, accounting for 5 to 10% of all primary urothelial
carcinomas (1–3). The gold standard for curative treatment
of localized renal pelvis and ureter cancer is open or laparoscopic
surgery (4). Due to a lack of data
about the disease, the exact role of nonsurgical therapies,
including radiation therapy (RT), remains unclear; the primary role
of RT has been considered to be in controlling pain and hemorrhage
as palliation or preventing recurrence after surgery as adjuvant
therapy (5,6). However, delivery of curative doses
safely is challenging, as the small intestine and colon may be
extensively irradiated during photon RT for patients with these
tumors. Ding et al (7),
investigated outcomes of 1,910 patients with primary transitional
cell carcinoma of the ureter using the Surveillance, Epidemiology,
and End Results database, and the 5-year overall survival rates of
surgery only and RT only for stage I–II patients were 59.6 and 0%,
respectively. The patient's backgrounds of the two groups were
unadjusted, but RT exerted a very limited impact on clinical
outcomes as a curative treatment. Therefore, it is necessary for
patients unfit for surgery including patients with unresectable
tumors or medically inoperable condition to devise new approaches
in the curative setting.
Protons have unique physical characteristics called
Bragg peaks, and a region of uniform dose can be fit to the
location and size of a tumor by overlaying several peaks, known as
a spread-out Bragg peak (SOBP) (8,9). Thus,
proton beam therapy (PBT) can deliver conformal high-dose
irradiation to the target while minimizing radiation-induced
complications in surrounding healthy tissue. In recent decades, the
efficacy and feasibility of PBT for urological malignancies
including bladder cancer and prostate cancer have been reported
with an increase of PBT facilities in clinical operation worldwide
(10–14). On the other hand, no reports
regarding PBT for renal pelvis and ureter cancer have been
published. In our institute, we have treated 5 patients treated
with definitive PBT, and herein describe our experience to explore
the potential effectiveness of PBT as a curative treatment for
renal pelvis and ureter cancer patients unfit for surgery.
Patients and methods
Between September 2009 and July 2013, 5 patients
with renal pelvis or ureter cancer were definitively treated with
PBT at our hospital. Before PBT, a cystoscopy was performed for all
patients and confirmed no evidence of malignancy in the bladder and
urethra. Patient characteristics are summarized in Table I. The median age of was 72 years
(range, 59 to 85 years). The initial Eastern Cooperative Oncology
Group performance status was 0 or 1, but 3 patients were unfit for
surgery (medically inoperable, n=1; unresectable, n=2). Primary
tumor sites included the renal pelvis (n=3) and ureter (n=2). In
reference to previous reports for RT for cancer of the upper
urinary tract, clinical staging but not pathological staging was
used in the present study (15,16). Two
patients with stage IV (case 3 and case 5) received systemic
chemotherapy and the response to the chemotherapy was so good that
these patients were sent to our hospital to obtain a control of the
localized residual tumors.
 | Table I.Patient and treatment
characteristics. |
Table I.
Patient and treatment
characteristics.
| No. | Age (years) | PS | Site | Size (cm) | Cytology/Biopsy | TNM | Risk | Fractionation [Gy
(RBE)/fr] | RT field | Chemotherapy | Reason |
|---|
| 1 | 72 | 0 | Renal pelvis | 3 | – | T1/2N0M0 | High | 72.6/22 | Limited | No | Refusal of
surgery |
| 2 | 85 | 1 | Renal pelvis | 2 | Class V | T1/2N0M0 | High | 72.6/22 | Limited | No | Medically
inoperable |
| 3 | 59 | 1 | Ureter | 25 | Class V | T3N2M0 | High | 66/33 | Extended | Yes | Unresectable |
|
|
|
|
|
|
| (ycT4N0M0) |
|
|
| (prior to PBT) |
|
| 4 | 80 | 0 | Ureter | 3 | Urothelial
carcinoma | T1/2N0M0 | High | 66/33 | Extended | No | Refusal of
surgery |
| 5 | 64 | 0 | Renal pelvis | 4 | Class V | T4N2M1 | High | 66/33 | Extended | Yes | Unresectable |
|
|
|
|
|
|
| (ycT3N2M0) |
|
|
| (prior to PBT) |
|
Proton beam therapy
PBT for all 5 patients was performed without
combining it with photon RT. Treatment planning for PBT involved
respiratory-synchronized computed tomography (CT) at 5-mm intervals
in the treatment position during the expiratory phase, and the
images were transferred directly to a treatment planning system
(Hitachi Co., Ltd., Japan). Proton beams were delivered in
double-scattering mode during the expiratory phase using an
end-expiratory gated system controlled by a laser range-finder that
monitors the movement of the patient's body surface caused by
respiratory motion (17). The beams
were synchronized with respiration, and the position was examined
by fluoroscopy during each treatment session. Clinical target
volume (CTV), which was defined as a macroscopic tumor volume that
included visible tumors with 5 to 10-mm margins, was covered by
>95% of the prescribed dose at the isocenter by selection of
appropriate ports and margins. The relative biological effective
(RBE) value for protons was set to be 1.1 in our institute, and the
irradiation dose was expressed in Gy (RBE) [physical proton dose
(Gy)xRBE].
The first 2 cases with T1-2N0M0 disease were treated
with local PBT at a total dose of 66.0/72.6 Gy (RBE) in 22
fractions with a fractional dose of 3.0/3.3 Gy (RBE). Since a
designed seamless irradiation technique called the ‘patch-fields
technique’ for extended whole mediastinal PBT for esophageal cancer
was developed in 2010 (18,19), our treatment policy for PBT for renal
pelvis and ureter cancer has changed. Namely, total doses of 60/66
Gy (RBE) in 33 fractions with conventional fractionation were
administered with prophylactic lymph node irradiation, including
the bilateral paraaortic lymph nodes area and ipsilateral common
iliac nodes, to the entire ureter and through the renal pelvis to
the ureteral orifice using extended PBT fields. After 36.4/40 Gy
(RBE) of irradiation was administered, shrunken PBT fields covering
gross tumor volumes were used for an additional boost to 60/66 Gy
(RBE).
Results
This retrospective analysis was approved by the
ethical committee of our hospital (H29-300). Before start of PBT, a
written informed consent for their treatment was obtained from each
patient, but the consent for this retrospective analysis was
waived. All patients were followed up for >3 years or until
death. For the first 2 years after PBT, all patients were followed
every 3 months. Thereafter, the follow-up period was extended for 3
to 6 months. All patients regularly examined urine cytology,
ultrasonography and CT during the follow-up period. The median
follow-up time was 51.2 months (range, 4.6–97.5 months). Local
recurrences were observed at 36 and 57 months after PBT in 2
patients, but primary tumors were controlled in the other 3
patients. Distant metastases developed in 2 patients. Two patients
died of cancer recurrence, and another died due to lung cancer
recurrence. The remaining 3 patients are still alive at last
follow-up. Thus, 4 patients (80%) survived for >3 years after
PBT (Table II).
 | Table II.Summary of treatment outcomes. |
Table II.
Summary of treatment outcomes.
| No. | Recurrence | Recurrence site | Time to recurrence
(months) | Status | Survival
(months) |
|---|
| 1 | No |
|
| Alive without
disease | 97.5 |
| 2 | Yes | Local (within RT
field: Primary site) | 36 | Dead with
recurrence | 66.3 |
| 3 | Yes | Liver | 1 | Dead with
recurrence | 4.6 |
| 4 | Yes | Local (out of RT
field: Bladder) | 48 | Alive with
disease | 62.2 |
| 5 | Yes | Lung | 28 | Alive with
disease | 40.3 |
Table III
summarizes treatment-related toxicities according to the National
Cancer Institute Common Terminology Criteria for Adverse Effects
(CTCAE), version 4.0. With respect to acute toxicity, dermatitis
was common but manageable. Non-severe hematological toxicity was
observed in all patients who received prophylactic irradiation
using extend PBT fields, including 2 patients who received
neoadjuvant chemotherapy prior to PBT, and grade 3 myelosuppression
in 1 patient who received 6 cycles of neoadjuvant cisplatin and
gemcitabine chemotherapy that was not completely resolved before
initiation of PBT.
 | Table III.Summary of treatment morbidities. |
Table III.
Summary of treatment morbidities.
|
| Acute |
|
|---|
|
|
|
|
|---|
| No. | Hematological
(grade) | Non-hematological
(grade) | Late (grade) |
|---|
| 1 | None (0) | Dermatitis
(2) | None |
| 2 | None (0) | Dermatitis
(1) | Hematuria (2) |
| 3 | Anemia (1) | Dermatitis
(1), urinary frequency (1) | None |
| 4 | Thrombocytopenia
(1) | None (0) | GI bleeding
(1) |
| 5 | Anemia (1), thrombocytopenia (1) | Dermatitis
(1), diarrhea (1) | None |
With respect to late toxicities, hematuria and
gastrointestinal (GI) bleeding occurred in each case, but no grade
≥3 toxicities were observed.
Case presentation
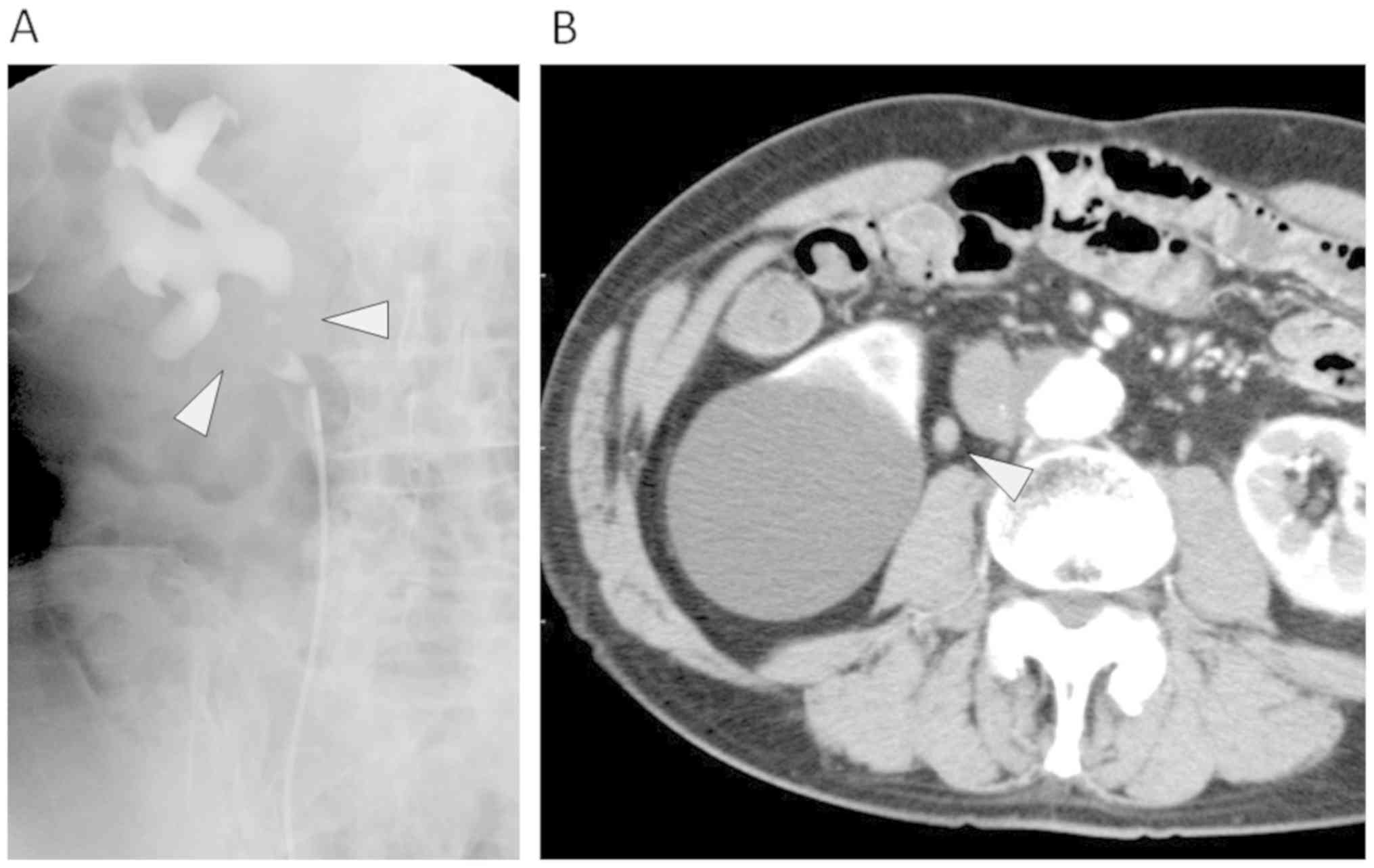
Case 1: An 80-year-old man (No. 4, Table I), who had a history of surgery for
colon cancer and an aneurysm, experienced gross hematuria.
Cystoscopic examination revealed hemorrhage from the tumor located
at the right ureter, and urine cytology was positive. Retrograde
pyelography showed an irregular filling defect of the right
proximal ureter and hydronephrosis (Fig. 1A). Computed tomography (CT) revealed
a right ureter tumor, and a diagnosis of cT1/2N0M0 right ureter
cancer was made (Fig. 1B).
Urologists considered radical surgery, but he had a high surgical
risk because of his history of abdominal surgery and advanced age.
Consequently, he selected nonsurgical treatment and was referred to
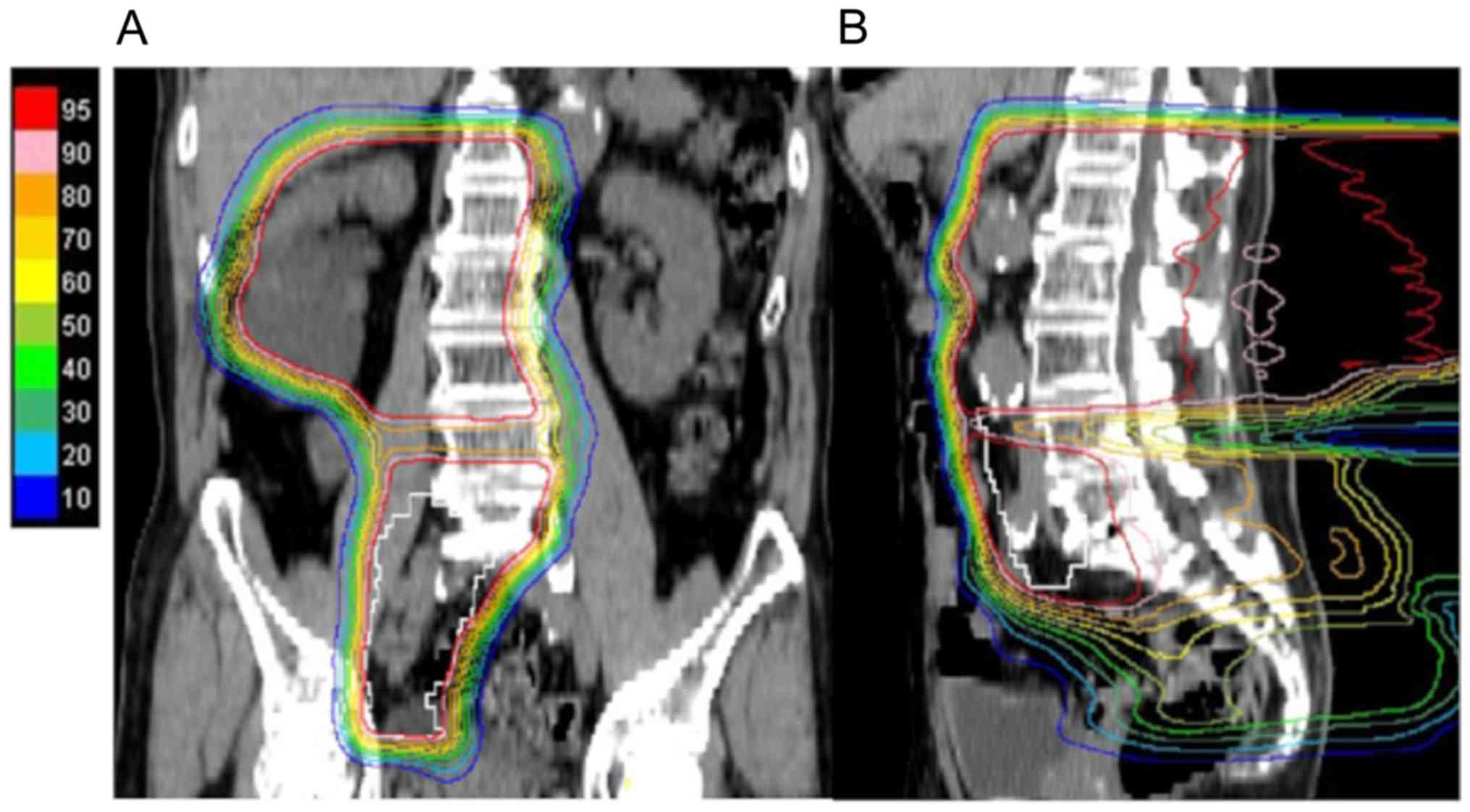
our hospital to receive PBT. The patient underwent PBT at a total
dose of 60/66 Gy (RBE) in 33 fractions over 7 weeks; prophylactic
irradiation at a total dose of 36.4/40 Gy (RBE) in 20 fractions
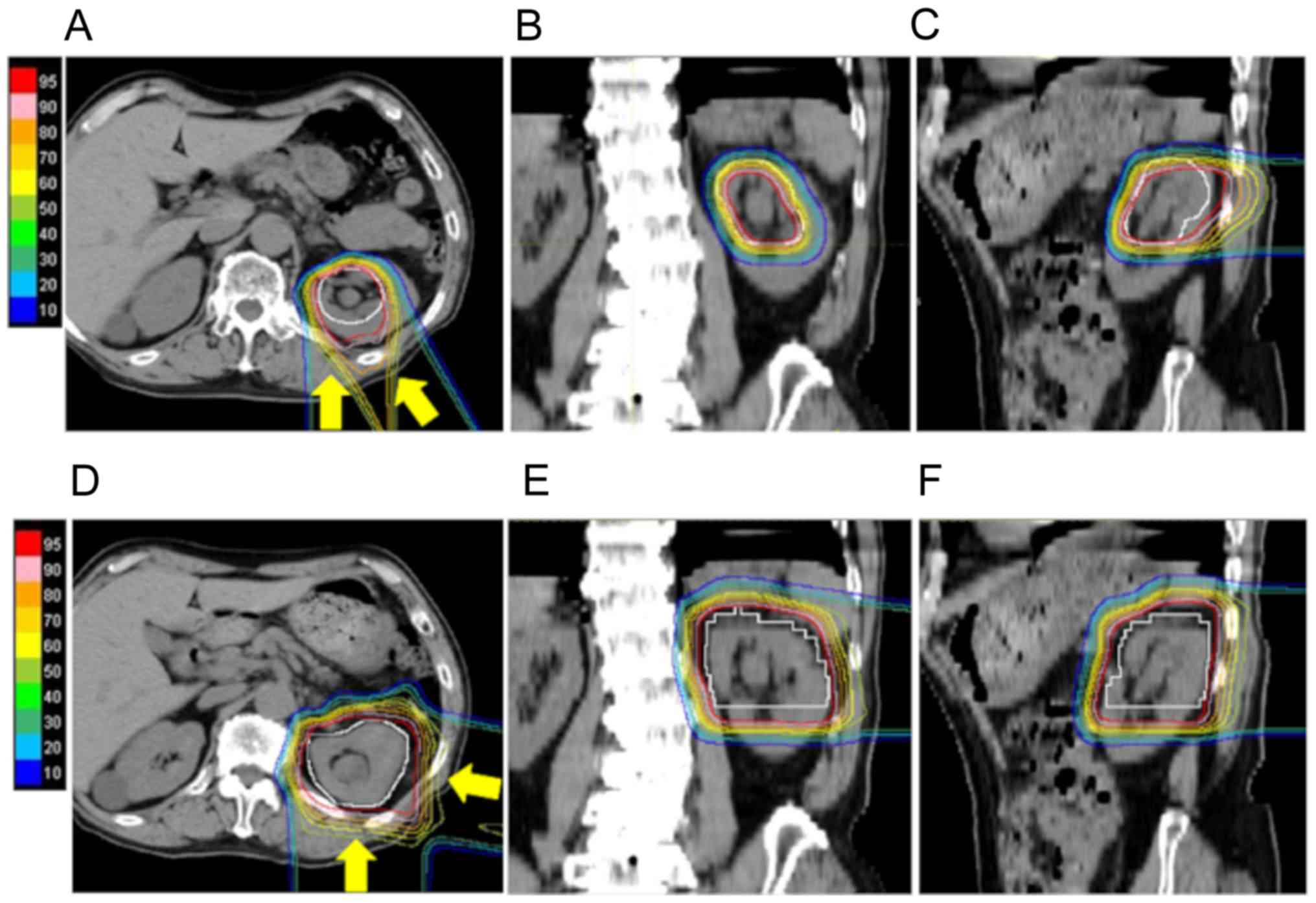
using extended PBT fields (Fig. 2A and
B) and boost therapy of 23.6/26 Gy (RBE) in 13 fractions was
performed with small PBT fields. The scheduled treatment was
completed without any severe complications. He developed melena
possibly caused by grade 2 small intestinal bleeding at 18 months
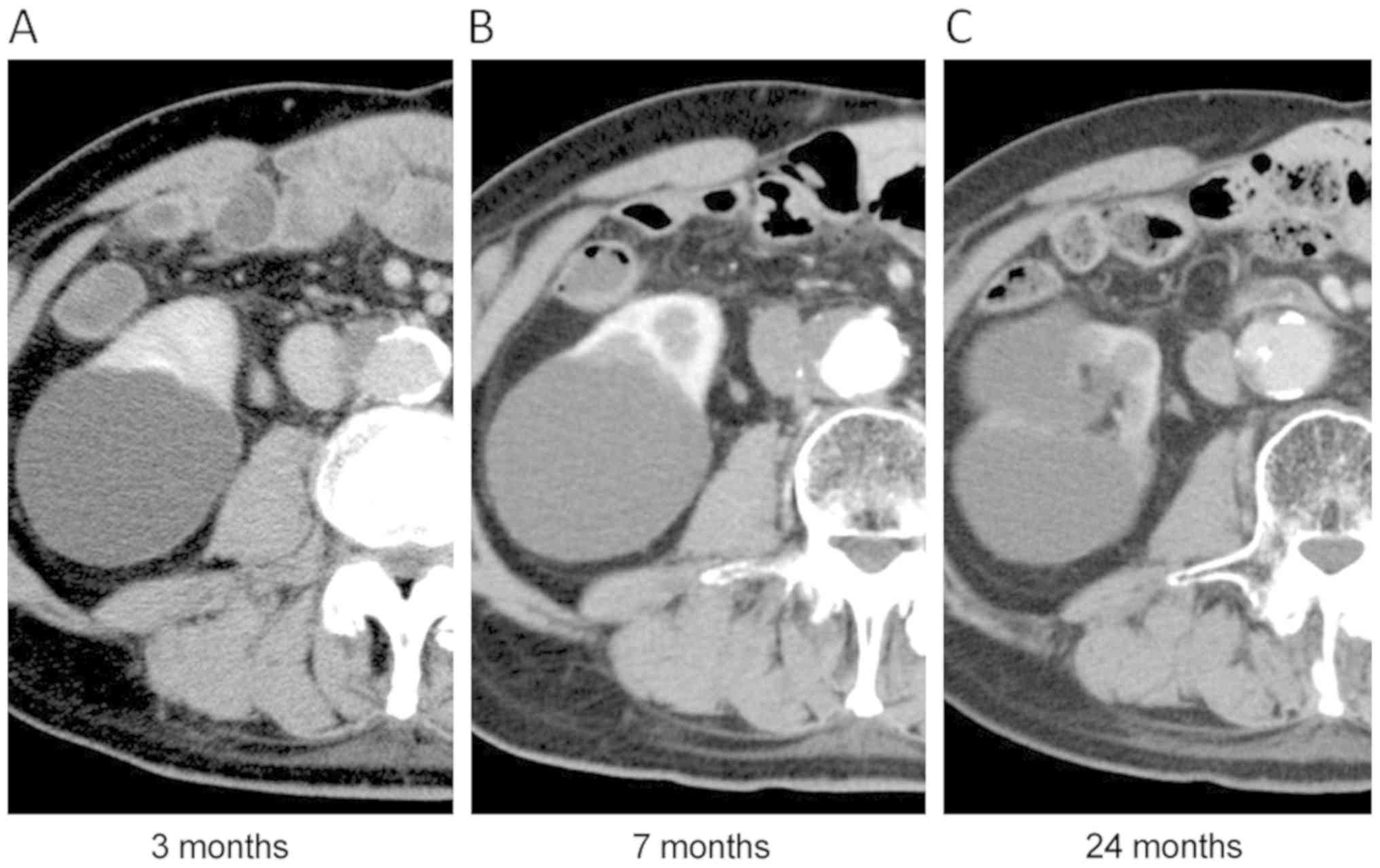
after PBT, but this was resolved with medication. The primary tumor
gradually decreased in size (Fig.
3), but he experienced non-muscle invasive bladder cancer and
underwent transurethral tumor resection 48 months after PBT. CT
revealed further recurrent tumors in the right ureter at the common
iliac level, but not recurrence of the primary lesion, 57 months
after PBT. Because he was 86 years old, best supportive care was
selected. The patient remains alive at last follow-up and he has
enjoyed his daily life for more than 7 months without any
treatment.
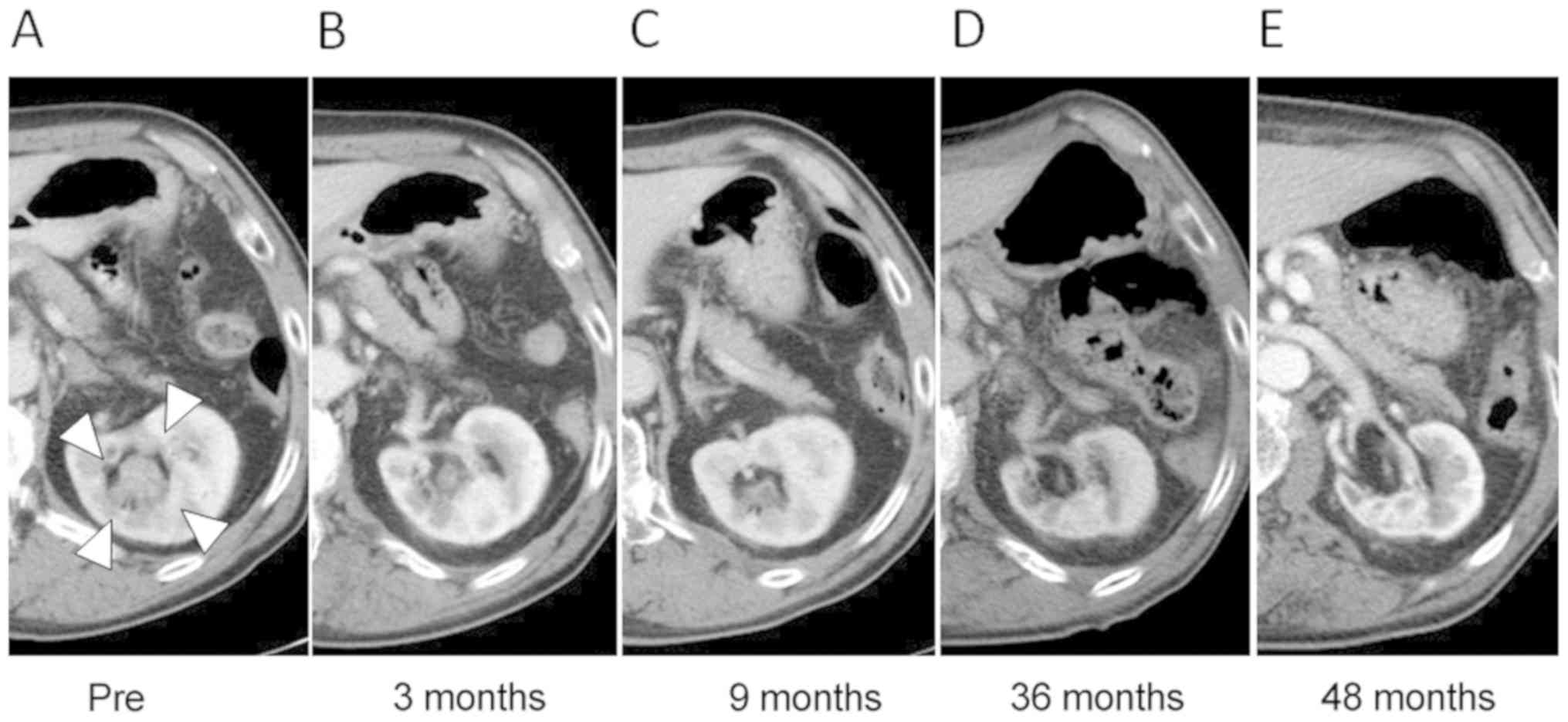
Case 2: A 72-year-old man (No. 1 in Table I) presented with visible hematuria,
and CT revealed a tumor in the left renal pelvis (Fig. 4). He was the first case of PBT for
renal pelvis and ureter cancer in our institute. Surgery was
proposed, but he refused to receive it. He underwent local PBT at a
total dose of 66.0/72.6 Gy (RBE) in 22 fractions (Fig. 5). For initial treatment planning, the
clinical target volume (CTV) was defined as the visible tumor plus
10-mm margins in all except caudal direction (15-mm margin).
Although PBT was performed using the respiratory synchronization
system, we realized that his left kidney moved approximately 3 cm
in the craniocaudal direction during treatment. After 9.0/9.9 Gy
(RBE), the target volume (2nd-CTV) was therefore modified to cover
his kidney at the levels of the initial CTV and >95% of the
prescribed dose completely covered for the 2nd-CTV in the remaining
19 fractions [57.0/62.7 Gy (RBE)]. Although he developed grade 2
acute dermatitis, treatment was completed on schedule. The tumor
progressively decreased in size, and finally disappeared (Fig. 4). The patient remains alive, and the
tumor has been well-controlled for 8 years. Although atrophic
changes of the irradiated kidney were observed, his renal function
has been maintained.
Discussion
In the present study, we treated 5 patients with
renal pelvis or ureter cancer and experienced two important
clinical issues. First, all treatments were completed on schedule,
and no severe complications were experienced, even though 3 of the
5 patients were treated using extended RT fields and 2 of them
received neoadjuvant chemotherapy due to T4 disease. Second, total
PBT doses of 66.0/72.6 Gy (RBE) in 22 fractions (PBT alone;
hypofractionation schedule) for T1-2N0 disease (n=2) and 60.0/66.0
Gy (RBE) in 33 fractions with prophylactic lymph node irradiation
for advanced disease (n=3) were delivered without concurrent
chemotherapy, per our treatment policy. In 2 cases, local
recurrence was observed at 36 months (outside the RT field;
bladder) and 48 months (primary site) after PBT; however, the
tumors of the remaining 3 patients were locally controlled.
Consequently, 4 patients (80%) survived for >3 years, and 1 has
experienced no recurrence 97 months after PBT.
Patients with carcinoma of the renal pelvis or
ureter are usually treated with surgery. While nephron-sparing
approaches are used to treat early-stage disease (3), radical nephroureterectomy, in which
Gerota's fascia with the ipsilateral ureter and the bladder cuff
are removed, is required for treatment of advanced cases (20). Furthermore, systemic chemotherapy is
often provided in both the neoadjuvant and adjuvant settings. In
this report, we defined CTV as the primary tumor plus margins for
T1-2N0 disease, whereas we used a patch-fields technique to cover
large CTV, including the renal pelvis, entire ureter, and regional
lymph nodes for 3 cases with advanced disease (Fig. 2). Therefore, the treatment area in
this study was similar to that targeted by surgery.
RT for renal pelvis or ureter cancer is usually used
with palliative intent or as adjuvant treatment in the
postoperative setting. Because locoregional failures after surgery
have been observed in 9–15% of patients with low-grade, low-stage
disease but in 30–50% of patients with high-grade and/or advanced
disease (21,22), postoperative RT to eliminate
microscopic residual disease appears to significantly reduce local
failure risk of advanced disease compared to the surgery alone
(5). However, damage to healthy,
radiosensitive tissue, such as the GI tract, close to the RT target
are concerns in the curative treatment setting, which requires high
irradiation doses for tumor control. To our knowledge, no
guidelines exist for management of inoperable patients with upper
urinary tract cancer due to extensive disease burden, a solitary
kidney, poor performance status, or patient refusal to undergo
surgery. Therefore, new RT approaches that allow sparing of organs
at risk from high RT dose areas are required for successful
curative treatment of this disease.
PBT is used as highly-conformal RT and possesses the
possibility to deliver curative doses not only to the tumor sites
but also prophylactically to lymph node areas while avoiding toxic
doses to healthy tissues. Protons from posterior beams are directed
at the primary site and regional lymph nodes, while they are
withheld from the GI tract. Furthermore, the irradiation doses
administered to the spinal cord are also acceptable (Fig. 3). As a result, we were able to
deliver sufficient irradiation doses to locally control not only
small (T1-2) but also advanced (T3-T4) tumors. In the present
study, local failure was observed in 2 cases, but 1 recurrence
occurred within the prophylactic RT field at 2 years after initial
recurrence in the bladder. Furthermore, the local recurrence in the
other patient developed 5 years after hypofractionated PBT.
Therefore, our treatment strategy appears to improve patient
survival compared to chemotherapy alone or best supportive care.
Recently, some studies have suggested the utility of stereotactic
RT for localized renal pelvis and/or ureter cancer. Maehata et
al reported 3 cases of inoperable localized T2N0M0 ureter
carcinoma treated with stereotactic body radiotherapy (SBRT). In
their study, no acute adverse events were observed, and tumor
control was obtained in 2 of the 3 patients (15). When high-dose local irradiation is
delivered to T1-2 tumors, local tumor control can be achieved.
Considering the outcomes of a series of renal pelvis
and ureter cancer surgeries, treatment of regional lymph nodes
appears to be necessary for T3-4 disease, irrespective of the
presence of lymph node metastasis (21–23). In
the present study, 3 patients who received PBT with prophylactic
lymph node irradiation using extended fields did not develop
further lymph node metastasis, although 1 patient had T4N2 disease.
Therefore, our treatment policy for advanced tumors appears to be
reasonable. However, distant metastases in the lung and liver were
observed in two patients with T4 disease, despite administration of
systematic chemotherapy prior to PBT and prophylactic lymph node
irradiation. The utility of adjuvant chemotherapy following PBT
should be further evaluated in future prospective studies.
There were several limitations to this case report.
This report was retrospective observation and of a limited number
of patients. Staging of renal pelvis and urether cancer was in
accordance with clinical findings, not with pathological findings.
Further studies including a larger number of patients are also
needed to validate the effectiveness of definitive PBT for the
disease.
In conclusion, this is the first report of curative
PBT for localized renal pelvis and ureter cancers. The present
results demonstrate that PBT may be effective and feasible as a
curative treatment modality for the disease, and it probably has
the potential to become a good candidate as an alternative
radiotherapy for inoperable patients with early or locally advanced
upper urinary tract cancer patients. Prospective studies are
required to confirm the efficacy of PBT in this setting.
Acknowledgements
Not applicable.
Funding
The present study work was supported by JSPS KAKENHI
(grant no. JP17K10467).
Availability of data and materials
The datasets generated during and/or analysed during
the present study are not publicly available for maintaining the
privacy of the patients but are available from the corresponding
author on reasonable request.
Authors' contributions
TI made substantial contributions to acquisition of
data and drafting the manuscript. HI made substantial contributions
to conception, design and revising the manuscript. YS, KO, MM, and
TN made substantial contributions to acquisition, analysis and
interpretation of data. HS also made substantial contributions to
interpretation of data, revising the manuscript, and the final
approval of the version to be published. All authors read, approved
the final manuscript and agreed to be accountable for all aspects
of the work in ensuring that questions related to the accuracy or
integrity of any part of the work were appropriately investigated
and resolved.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Tsukuba University Hospital (H29-300). For this
type of study formal consent was not required. Information about
the current study was disclosed to patients instead of obtaining
their written informed consent, and patients who declined to
participate were excluded.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kirkali Z and Tuzel E: Transitional cell
carcinoma of the ureter and renal pelvis. Crit Rev Oncol Hematol.
47:155–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huben RP, Mounzer AM and Murphy GP: Tumor
grade and stage as prognostic variables in upper tract urothelial
tumors. Cancer. 62:2016–2020. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hutchinson R, Haddad A, Saqalowsky A and
Margulis V: Upper tract urothelial carcinoma: Special
considerations. Clin Adv Hematol Oncol. 14:101–109. 2016.PubMed/NCBI
|
|
4
|
Soderdahl DW, Fabrizio MD, Rahman NU,
Jarrett TW and Bagley DH: Endoscopic treatment of upper tract
transitional cell carcinoma. Urol Oncol. 23:114–122. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cozad SC, Smalley SR, Austenfeld M, Noble
M, Jennings S and Raymond R: Adjuvant radiotherapy in high stage
transitional cell carcinoma of the renal pelvis and ureter. Int J
Radiat Oncol Biol Phys. 24:743–745. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Czito B, Zietman A, Kaufman D, Skowronski
U and Shipley W: Adjuvant radiotherapy with and without concurrent
chemotherapy for locally advanced transitional cell carcinoma of
the renal pelvis and ureter. J Urol. 172:1271–1275. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding T, Zheng Z, Xu R and Zhou C:
Prognostic factors and outcomes of primary transitional cell
carcinoma of the ureter: A population-based study. Oncotarget.
8:65983–65996. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DeLaney TF, Trofimov AV, Engelsman M and
Suit HD: Advanced-technology radiation therapy in the management of
bone and soft tissue sarcomas. Cancer Control. 12:27–35. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inada T, Hayakawa Y, Tada J, Takada Y and
Maruhashi A: Characteristics of proton beams after field shaping at
PMRC. Med Biol Eng Comput. 31 (Suppl):S44–S48. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwata H, Ishikawa H, Takagi M, Okimoto T,
Murayama S, Akimoto T, Wada H, Arimura T, Sato Y, Araya M, et al:
Long-term outcomes of proton therapy for prostate cancer in Japan:
A multi-institutional survey of the Japanese Radiation Oncology
Study Group. Cancer Med. 7:677–689. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ha B, Cho KH, Lee KH, Joung JY, Kim YJ,
Lee SU, Kim H, Suh YG, Moon SH, Lim YK, et al: Long-term results of
a phase II study of hypofractionated proton therapy for prostate
cancer: Moderate versus extreme hypofractionation. Radiat Oncol.
14:42019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verma V, Simone CB II and Mishra MV:
Quality of life and patient-reported outcomes following proton
radiation therapy: A systematic review. J Natl Cancer Inst.
110:2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takaoka EI, Miyazaki J, Ishikawa H, Kawai
K, Kimura T, Ishitsuka R, Kojima T, Kanuma R, Takizawa D, Okumura
T, et al: Long-term single-institute experience with trimodal
bladder-preserving therapy with proton beam therapy for
muscle-invasive bladder cancer. Jpn J Clin Oncol. 47:67–73. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hata M, Miyanaga N, Tokuuye K, Saida Y,
Ohara K, Sugahara S, Kagei K, Igaki H, Hashimoto T, Hattori K, et
al: Proton beam therapy for invasive bladder cancer: A prospective
study of bladder-preserving therapy with combined radiotherapy and
intra-arterial chemotherapy. Int J Radiat Oncol Biol Phys.
64:1371–1379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maehata Y, Kuriyama K, Aoki S, Araya M,
Marino K and Onishi H: Stereotactic body radiotherapy for localized
ureter transitional cell carcinoma: Three case reports. Case Rep
Urol. 2015:5198972015.PubMed/NCBI
|
|
16
|
Evans JD, Hansen CC, Tollefson MK and
Hallemeier CL: Stereotactic body radiation therapy for medically
inoperable, clinically localized, urothelial carcinoma of the renal
pelvis: A case report. Adv Radiat Oncol. 3:57–61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oshiro Y, Mizumoto M, Okumura T, Fukuda K,
Fukumitsu N, Abei M, Ishikawa H, Takizawa D and Sakurai H: Analysis
of repeated proton beam therapy for patients with hepatocellular
carcinoma. Radiother Oncol. 123:240–245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okonogi N, Hashimoto T, Ishida M, Ohno T,
Terunuma T, Okumura T, Sakae T and Sakurai H: Designed-seamless
irradiation technique for extended whole mediastinal proton-beam
irradiation for esophageal cancer. Radiat Oncol. 7:1732012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishikawa H, Hashimoto T, Moriwaki T, Hyodo
I, Hisakura K, Terashima H, Ohkohchi N, Ohno T, Makishima H,
Mizumoto M, et al: Proton beam therapy combined with concurrent
chemotherapy for esophageal cancer. Anticancer Res. 35:1757–1762.
2015.PubMed/NCBI
|
|
20
|
Rouprêt M, Babjuk M, Compérat E, Zigeuner
R, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW,
Kaasinen E, et al: European association of urology guidelines on
upper urinary tract urothelial cell carcinoma: 2015 update. Eur
Urol. 68:868–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Charbit L, Gendroau MC, Mee S and Cukier
J: Tumors of the upper urinary tract: 10 years of experience. J
Urol. 146:1243–1246. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyao N, Masumori N, Takahashi A, Sasai M,
Hisataki T, Kitamura H, Satoh M and Tsukamoto T: Lymph nodde
metastasis in patients with carcinomas of the renal pelvis and
ureter. Eur Urol. 33:180–185. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang D, Chen Q, Song X, Wang J, Che X, Zhu
Z, Zheng W and Wang L: Effect of lymph node dissection on the
outcomes of upper tract urothelial carcinomas: A meta-analysis.
Expert Rev Anticancer Ther. 14:667–675. 2014. View Article : Google Scholar : PubMed/NCBI
|