Introduction
Salivary duct carcinoma (SDC), an aggressive and
relatively rare tumor arising from the ductal epithelium of the
salivary gland, accounts for approximately 10% of all salivary
gland malignancies (1,2). Nearly 100 cases of SDC are diagnosed
each year in the world, and it arises almost exclusively in the
major salivary glands. SDC is three times more common in men, and
usually occurs in patients over 50 years of age (1–3).
Generally, its prognosis is favorable if the primary lesion is
completely resected and no metastases are present (2–5).
However, local recurrence and distant metastasis represent the most
common forms of treatment failure (2–5). In a
review of 104 cases of SDC, Barnes et al (3) reported that one third of patients
experience local recurrence after curative treatment, with 59%
developing positive regional lymph nodes and 46% showing systemic
metastases (lungs and bones). In addition, 65% of patients die of
their disease usually within 4 years of initial diagnosis. In 141
cases of SDC in Japan, the 3-year overall survival and disease-free
survival rates were 70.5 and 38.2%, respectively (2). Treatment failure after initial surgery
occurred in 78 patients, and 70% of these patients had distant
metastases (2). Thus, the prognosis
of progressive and metastatic diseases is poor, and effective
systemic therapy is therefore important (2–5). Data
regarding the efficacy of cytotoxic chemotherapeutic agents in
cases of advanced and/or metastatic SDC are limited (2–5).
The histological findings of SDC are similar to
those of ductal carcinoma of the breast (2,3,6–8). Several
studies indicated that the tumor cells sometimes overexpress human
epidermal growth factor receptor 2 (HER2) in patients with SDC, at
rates ranging from 20 to 77% (6–9). The
humanized monoclonal antibody against HER2, trastuzumab, is
expected to be effective in such cases. Indeed, several cases of
advanced and/or metastatic SDC showed good responses to treatment
with trastuzumab alone or along with chemotherapy (10–21).
Therefore, trastuzumab is increasingly being used in the management
of HER2/erB2-positive SDC. However, there remains insufficient
evidence regarding the efficacy of treatment because of the rarity
of the disease itself, particularly in Japanese patients.
Here, we describe two cases of HER2-overexpressing
and advanced SDC treated with trastuzumab plus paclitaxel. A good
response was obtained in one case, and the primary tumor mass and
lymph node metastasis regressed with combined trastuzumab and
paclitaxel therapy for over 2 years after initiation of treatment.
Here, we report the clinical course of this case along with a
review of the relevant literature focusing on HER-2 gene
amplification.
Case report
Case 1
A 59-year-old man was referred to our hospital for
further examination of a right cervical swelling and pain. Head and
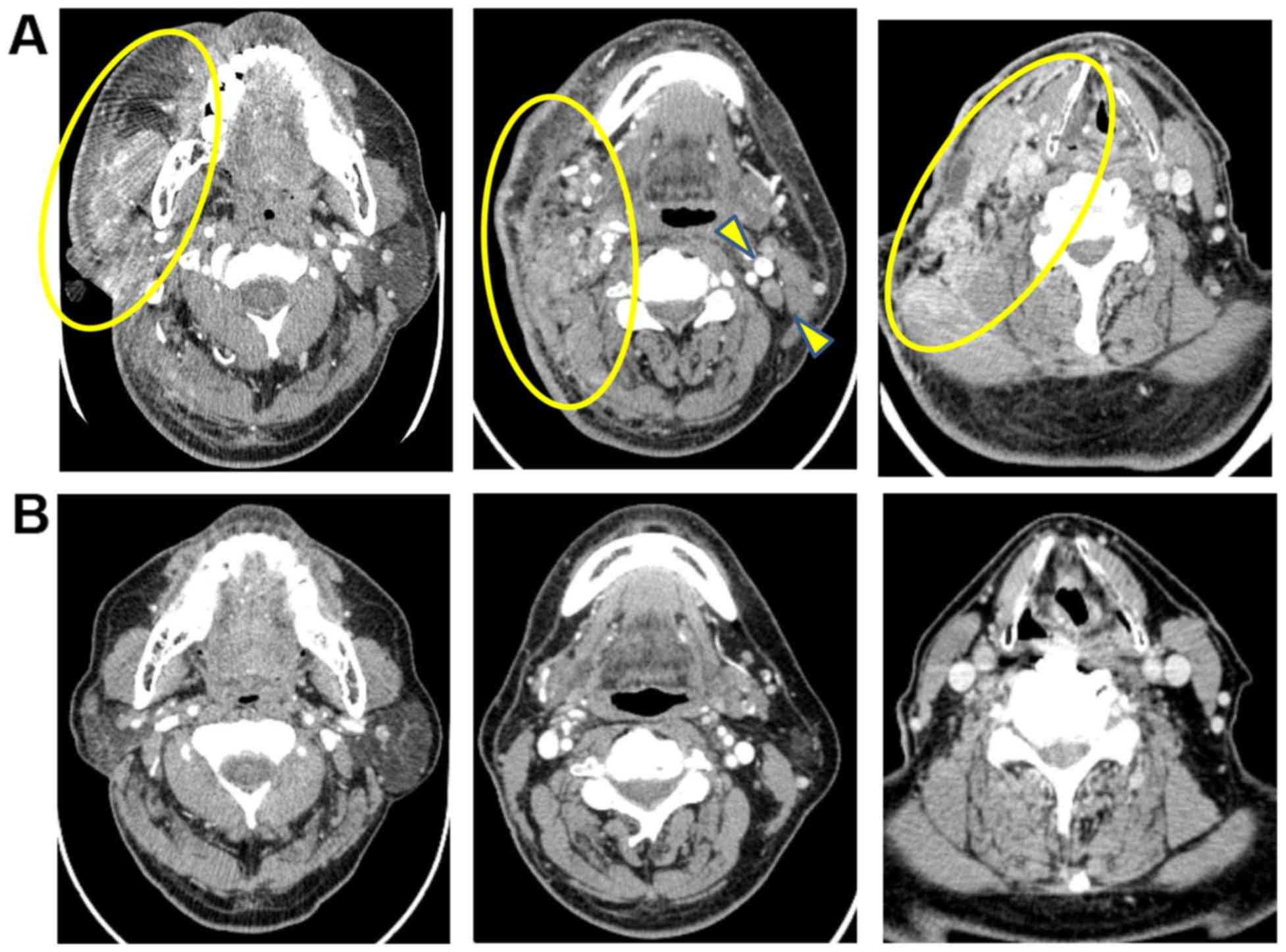
neck computed tomography (CT) (Fig.
1A) and magnetic resonance imaging (MRI) showed a soft tissue
mass shadow in the right cervical region. The soft tissue shadow
spread into the trapezius muscle and broad neck muscles, masseter
muscle, trapezius muscle, shoulder cap muscle, sternocleidomastoid
muscle, carotid gap, pharyngopharyngeal space, and the subcutaneous
soft tissue. Laboratory findings indicated increased
carcinoembryonic antigen level (59.6 mg/ml). As percutaneous needle
aspiration cytology revealed adenocarcinoma, open biopsy was
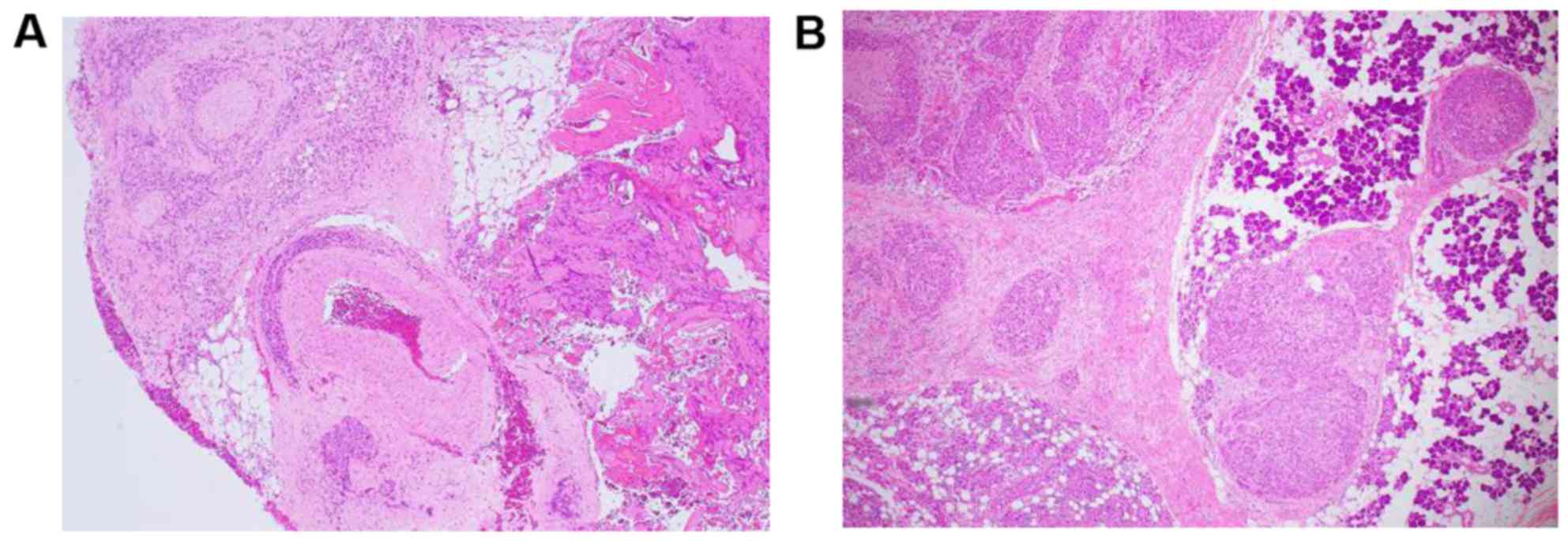
performed and the histological diagnosis of SDC was confirmed
(Fig. 2A). Chemotherapy with
docetaxel (60 mg/m2, day 1), cisplatin (60
mg/m2, day 1), and 5-fluorouracil (700 mg/m2,
days 2–5) was performed with a 28-day cycle. However, four cycles
of chemotherapy failed to reduce the soft tissue mass and symptoms.
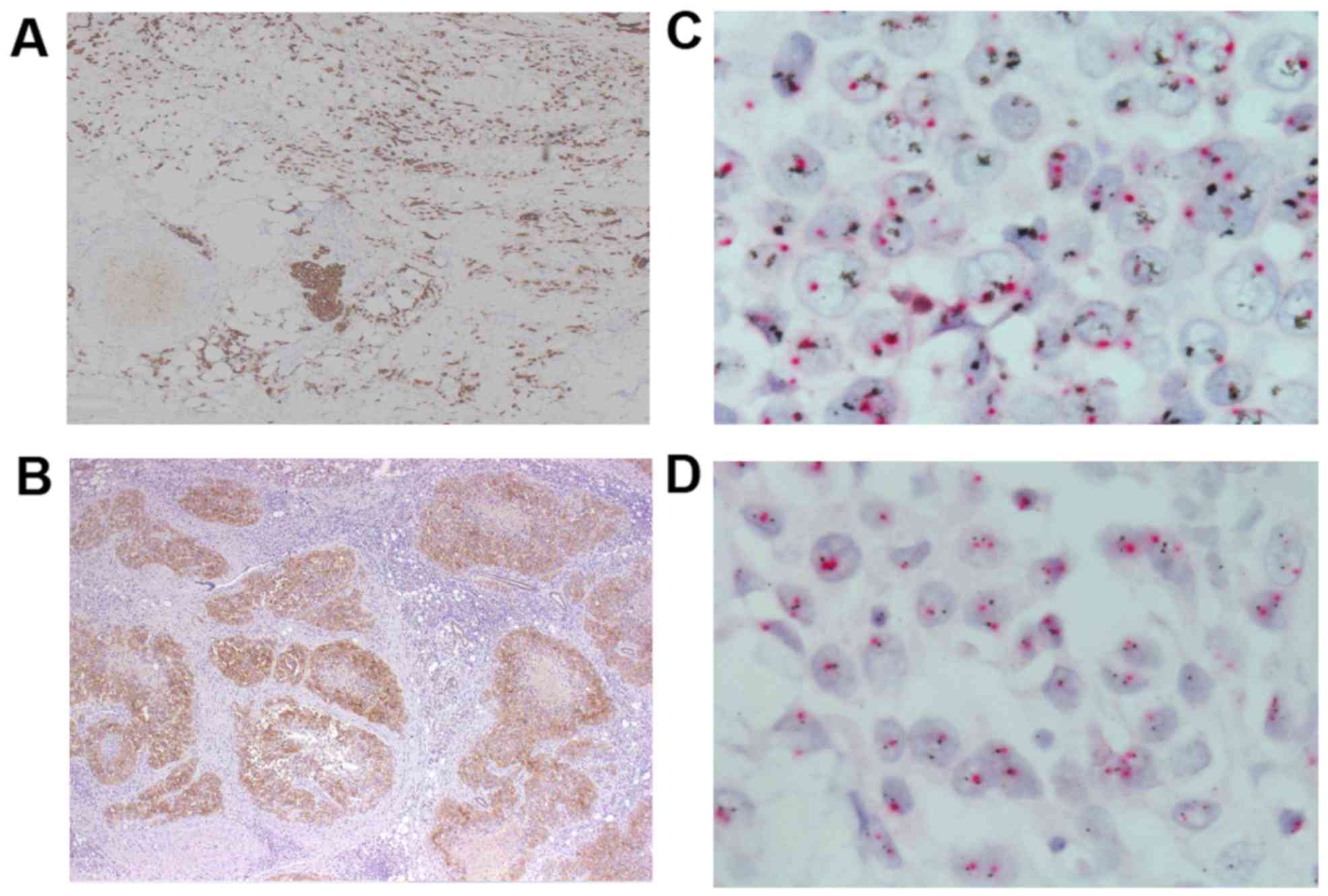
During chemotherapy, immunohistochemistry (IHC) of the biopsy
specimen was performed and revealed that tumor cells were positive
for HER2 (Fig. 3A) and negative for
estrogen and progesterone receptors. The patient was then treated
with trastuzumab plus paclitaxel. Trastuzumab was administered at a
loading dose of 4 mg/kg followed by 2 mg/kg weekly in combination
with weekly paclitaxel at 80 mg/m2. There were no
adverse effects, including cardiotoxicity, neurotoxicity, and
hematotoxicity. The right cervical soft tissue mass decreased in
size and disappeared 10 months after initiation of chemotherapy
(Fig. 1B). Although the therapy was
discontinued due to paclitaxel-induced neurotoxicity, we used
trastuzumab for over 2 years after complete response due to
concerns regarding early relapse. This patient remained well over 2
years after cessation of trastuzumab.
Case 2
A 68-year-old man was referred to our hospital for
further chemotherapy as treatment for relapsed SDC. He underwent
total right parotidectomy with cervical lymphadenectomy and facial
nerve reconstruction using the femoral nerve. The pathological
diagnosis was SDC. However, he developed pulmonary metastasis 6
months after the operation and received several chemotherapeutic
regimens, including platinum compounds plus docetaxel or
5-fluorouracil, and cetuximab plus paclitaxel. However, the disease
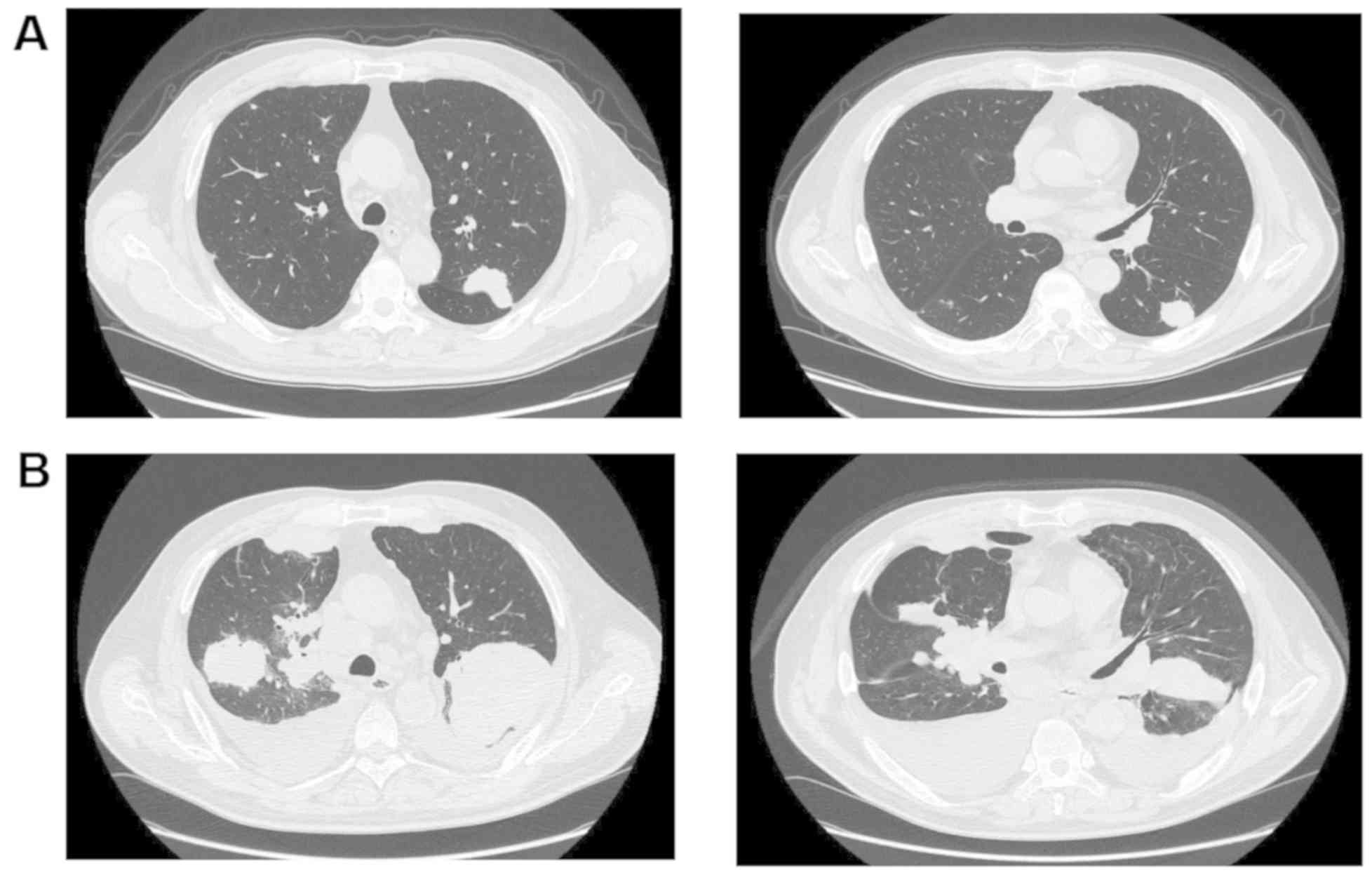
was progressive and chest CT showed multiple metastatic nodules in
both lungs (Fig. 4A). IHC analysis
of the resected primary tumor indicated that the tumor cells were
positive for HER2 (Fig. 3B).
Therefore, the patient was treated with trastuzumab plus paclitaxel
at the same dose and according to the same schedule as in case 1.
However, the therapy (six cycles of trastuzumab plus paclitaxel)
failed to improve radiographic findings in the lungs (Fig. 4B), and the patient was followed up by
best supportive care. The patient died due to respiratory failure 6
months later.
The treatment in both cases was approved by the
institutional review board of Shinshu University Hospital (approval
number: B0290) and was conducted in accordance with the principles
of the Declaration of Helsinki. Dual fluorescence in situ
hybridization was performed after initiation of trastuzumab
combined with chemotherapy in both cases. Case 1 was positive for
HER2 gene amplification (Fig. 3C),
while the findings were negative in case 2 (Fig. 3D).
Discussion
We reported two cases of advanced and metastatic
HER2-overexpressing SDC (3+) treated with trastuzumab and
paclitaxel chemotherapy. One patient, who was positive for HER2
gene amplification, showed a complete response lasting for over 2.5
years after commencement of therapy. However, the other patient,
who was negative for HER2 gene amplification, showed no response to
trastuzumab combined therapy.
Recently, Ghazali et al (13) reported a patient with HER2-positive
metastatic salivary adenocarcinoma who showed complete response to
trastuzumab, and summarized 15 published papers, including a
prospective study and a case report, presenting details of 56 cases
treated with trastuzumab monotherapy or combined chemotherapy.
Although the dose and schedule of trastuzumab and combined
chemotherapy agents were different between the reports, 22 patients
achieved good response. In addition, there were disparities
regarding the criteria for HER2 overexpression in these reports. A
phase II trial of trastuzumab enrolled 14 patients with salivary
grand carcinoma overexpressing HER2 over 2+ as determined by IHC,
and showed only one case of partial response lasting longer than 2
years (16). HER2 was a potential
therapeutic target in salivary grand carcinoma. However, HER2
overexpression evaluated by IHC was not always associated with
significant efficacy of tumor response to trastuzumab.
In recent case reports showing the usefulness of
trastuzumab in SDC, overexpression of HER2 was evaluated by both
IHC and gene amplification (9–11,14–22).
Limaye et al (17) reported
five cases of SDC positive for both HER2 on IHC and for HER2 gene
amplification, treated with trastuzumab combined with chemotherapy.
All patients showed good response and the median duration of
response was 18 months (range, 8–52 months). Thus, the response to
trastuzumab chemotherapy in patients may be dependent on HER2
overexpression and gene amplification. Our case 1 showed good
response, and was also positive for both HER2 overexpression and
gene amplification. Based on these findings and our experience, we
emphasize that both HER2 protein overexpression and gene
amplification should be examined as therapeutic biomarkers in
SDC.
There have been several studies regarding the
coexistence of HER2 IHC and gene amplification in SDC. Thirteen of
14 Japanese cases of HER2 IHC (3+) were positive on FISH (6). Cornolti et al (7) reported eight cases of FISH positivity
among 10 with grade 3+ IHC. Dagrada et al (8) reported a coexistence rate of 73% (8/11
cases) on IHC and FISH assessment. In addition, Skalova et
al (11) reported three cases of
FISH positivity among seven with IHC positivity over grade 3 (3+)
in salivary gland carcinoma. These concordance rates were lower
than that in breast adenocarcinomas (>90%) with high (3+) HER2
expression (22). Further case
studies presenting details of HER2 examination and treatment for
advanced salivary malignancies are warranted.
In addition, the therapeutic effects of trastuzumab
are related to the immunological backgrounds (23). White blood cell and lymphocyte counts
were 7,660 and 1,800 in case 1 and 6,140 and 1,600 in case 2 before
trastuzumab therapy, respectively. Although we did not examine the
specific immunological parameters in both cases, we should pay
attention to the immunological examination in patients with HER-2
positive SDC.
In summary, our experiences indicated that HER2
should be examined in patients with advanced and/or metastatic SDC,
and that findings of both HER2 overexpression and gene
amplification define a group of patients with SDC who would benefit
from targeted therapy with trastuzumab.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TG, NS and TKoi wrote the manuscript and all authors
TG, NS, DG, TN, TF, TKob, TO, SY and TKoi treated the patients. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committees of Shinshu University Hospital
approved the treatment of two cases (approval number: B0290).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McHugh JB, Visscher DW and Barnes EL:
Update on selected salivary gland neoplasms. Arch Pathol Lab Med.
133:1763–1774. 2009.PubMed/NCBI
|
|
2
|
Otsuka K, Imanishi Y, Tada Y, Kawakita D,
Kano S, Tsukahara K, Shimizu A, Ozawa H, Okami K, Sakai A, et al:
Clinical outcomes and prognostic factors for salivary duct
carcinoma: A multi-institutional analysis of 141 patients. Ann Surg
Oncol. 23:2038–2045. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barnes L, Rao U, Krause J, Contis L,
Schwartz A and Scalamogna P: Salivary duct carcinoma. Part I. A
clinicopathologic evaluation and DNA image analysis of 13 cases
with review of the literature. Oral Surg Oral Med Oral Pathol.
78:64–73. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnston ML, Huang SH, Waldron JN, Atenafu
EG, Chan K, Cummings BJ, Gilbert RW, Goldstein D, Gullane PJ, Irish
JC, et al: Salivary duct carcinoma: Treatment, outcomes, and
patterns of failure. Head Neck. 38:E820–E826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lagha A, Chraiet N, Ayadi M, Krimi S,
Allani B, Rifi H, Raies H and Mezlini A: Systemic therapy in the
management of metastatic or advanced salivary gland cancers. Oral
Oncol. 48:948–957. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masubuchi T, Tada Y, Maruya S, Osamura Y,
Kamata SE, Miura K, Fushimi C, Takahashi H, Kawakita D, Kishimoto S
and Nagao T: Clinicopathological significance of androgen receptor,
HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int J
Clin Oncol. 20:35–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cornolti G, Ungari M, Morassi ML,
Facchetti F, Rossi E, Lombardi D and Nicolai P: Amplification and
overexpression of HER2/neu gene and HER2/neu protein in salivary
duct carcinoma of the parotid gland. Arch Otolaryngol Head Neck
Surg. 133:1031–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dagrada GP, Negri T, Tamborini E, Pierotti
MA and Pilotti S: Expression of HER-2/neu gene and protein in
salivary duct carcinomas of parotid gland as revealed by
fluorescence in-situ hybridization and immunohistochemistry.
Histopathology. 44:301–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alotaibi AM, Alqarni MA, Alnobi A and
Tarakji B: Human epidermal growth factor receptor 2 (HER2/neu) in
salivary gland carcinomas: A review of literature. J Clin Diagn
Res. 9:ZE04–ZE08. 2015.PubMed/NCBI
|
|
10
|
Nabili V, Tan JW, Bhuta S, Sercarz JA and
Head CS: Salivary duct carcinoma: A clinical and histologic review
with implications for trastuzumab therapy. Head Neck. 29:907–912.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Skálová A, Stárek, Kucerová V, Szépe P and
Plank L: Salivary duct carcinoma-A highly aggressive salivary gland
tumor with HER-2/neu oncoprotein overexpression. Pathol Res Pract.
197:621–626. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Block K, Vander Poorten V, Dormaar T,
Nuyts S, Hauben E, Floris G, Deroose CM, Schöffski P and Clement
PM: Metastatic HER-2-positive salivary gland carcinoma treated with
trastuzumab and a taxane: A series of six patients. Acta Clin Belg.
71:383–388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghazali N, Parker L, Settle K and Lubek
JE: Sustained response of HER2-positive metastatic salivary
adenocarcinoma, not otherwise specified, treated with trastuzumab.
Oral Surg Oral Med Oral Pathol Oral Radiol. 122:292–299. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kadowaki S, Yatabe Y, Hirakawa H, Komori
A, Kondoh C, Hasegawa Y and Muro K: Complete response to
trastuzumab-based chemotherapy in a patient with human epidermal
growth factor receptor-2-positive metastatic salivary duct
carcinoma ex pleomorphic adenoma. Case Rep Oncol. 6:450–455. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iqbal MS, Shaikh G, Chatterjee S, Cocks H
and Kovarik J: Maintenance therapy with trastuzumab in her2
positive metastatic parotid ductal adenocarcinoma. Case Rep Oncol
Med. 2014:162516342014.
|
|
16
|
Haddad R, Colevas AD, Krane JF, Cooper D,
Glisson B, Amrein PC, Weeks L, Costello R and Posner M: Herceptin
in patients with advanced or metastatic salivary gland carcinomas.
A phase II study. Oral Oncol. 39:724–727. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Limaye SA, Posner MR, Krane JF, Fonfria M,
Lorch JH, Dillon DA, Shreenivas AV, Tishler RB and Haddad RI:
Trastuzumab for the treatment of salivary duct carcinoma. Oncol.
18:294–300. 2013.
|
|
18
|
Thorpe LM, Schrock AB, Erlich RL, Miller
VA, Knost J, Le-Lindqwister N, Jujjavarapu S, Ali SM and Liu JJ:
Significant and durable clinical benefit from trastuzumab in 2
patients with HER2-amplified salivary gland cancer and a review of
the literature. Head Neck. 39:E40–E44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Corrêa TS, Matos GDR, Segura M and Dos
Anjos CH: Second-line treatment of HER2-positive salivary gland
tumor: Ado-Trastuzumab emtansine (T-DM1) after progression on
trastuzumab. Case Rep Oncol. 11:252–257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Boxtel W, Boon E, Weijs WLJ, van den
Hoogen FJA, Flucke UE and van Herpen CML: Combination of docetaxel,
trastuzumab and pertuzumab or treatment with trastuzumab-emtansine
for metastatic salivary duct carcinoma. Oral Oncol. 72:198–200.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iguchi F, Taniguchi Z, Kusano J, Takahashi
Y and Murai N: A case of metastatic salivary duct carcinoma
successfully treated with trastuzumab-based targeted therapy. Nihon
Jibiinkoka Gakkai Kaiho. 117:1108–1114. 2014.(Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giltnane JM, Molinaro A, Cheng H, Robinson
A, Turbin D, Gelmon K, Huntsman D and Rimm DL: Comparison of
quantitative immunofluorescence with conventional methods for
HER2/neu testing with respect to response to trastuzumab therapy in
metastatic breast cancer. Arch Pathol Lab Med. 132:1635–1647.
2008.PubMed/NCBI
|
|
23
|
Ethier JL, Desautels D, Templeton A, Shah
PS and Amir E: Prognostic role of neutrophil-to-lymphocyte ratio in
breast cancer: A systematic review and meta-analysis. Breast Cancer
Res. 19:22017. View Article : Google Scholar : PubMed/NCBI
|