Introduction
The vascular endothelial growth factor (VEGF)/VEGF-
receptor pathway plays a key role in malignant tumor angiogenesis
(1). This has led to the development
of humanized monoclonal antibodies targeted against this pathway to
inhibit tumor angiogenesis. Bevacizumab (BV) was the first approved
anti-VEGF antibody (2) and it has
become a standard part of several combination chemotherapy regimens
used in patients with metastatic colorectal cancer (mCRC), after
having been shown by several clinical trial to exert a
statistically significant favorable effect on overall survival (OS)
and progression-free survival (PFS) (3). Some of the most common adverse events
associated with the use of BV include hypertension, hemorrhage,
proteinuria, delayed wound healing and bowel perforation (4). Some have reported increased risk of
arterial events, mainly cardiovascular and cerebrovascular events
(5). However, pneumothorax (PTX) has
rarely been reported in association with the use of BV (6–12). We
herein report the case of a 68-year-old female patient who was
undergoing third-line chemotherapy with folinic acid, fluorouracil
(5-FU) and oxaliplatin (FOLFOX) plus BV when she presented with
shortness of breath (SOB) and was diagnosed with pneumohydrothorax.
We consider the present case to be important, as it sheds light on
a rare adverse event associated with the use of BV, in an era where
VEGF-inhibitors are being implemented in the treatment of several
malignancies. Clinicians should be aware of this potential adverse
event in order to possess a high clinical index of suspicion for
making this diagnosis.
Case report
A 68-year-old female patient presented in January
2018 to Saint Joseph's Hospital (Chicago, USA) with complaints of
worsening SOB for 1 month, with an associated right-sided pleuritic
chest pain and a dry cough. On physical examination, the patient
was found to be mildly tachycardic to 102 beats per min, she was
not tachypneic and her oxygen saturation was 98% breathing ambient
air. Physical examination was notable for decreased breath sounds
over the right lower lung fields. Otherwise, the physical
examination was unremarkable. This presentation was 1 week after
the completion of the 4th cycle of combination chemotherapy with
FOLFOX and BV for mCRC.
The patient was initially diagnosed with stage IIIB
mCRC 9 years earlier, for which she had undergone sigmoidectomy and
completed 12 cycles of adjuvant chemotherapy with FOLFOX. Four
years later, a positron emission tomography with computed
tomography (PET/CT) scan revealed disease recurrence, with 3
metastatic lung lesions. The patient subsequently underwent
Cyberknife therapy followed by 12 cycles of leucovorin, 5-FU and
irinotecan (FOLFIRI) with cetuximab, followed by maintenance
therapy with cetuximab. Two years later, a CT scan of the abdomen
revealed evidence of a new left adrenal mass, which was treated
with radiofrequency ablation; a biopsy was consistent with
metastatic adenocarcinoma. The patient was then placed on
combination chemotherapy with FOLFIRI and BV followed by
maintenance capecitabine, which she tolerated well, and a PET/CT
scan 1 year later showed no evidence of disease. However, 3 months
later, an elevation in her carcinoembryonic antigen (CEA) levels
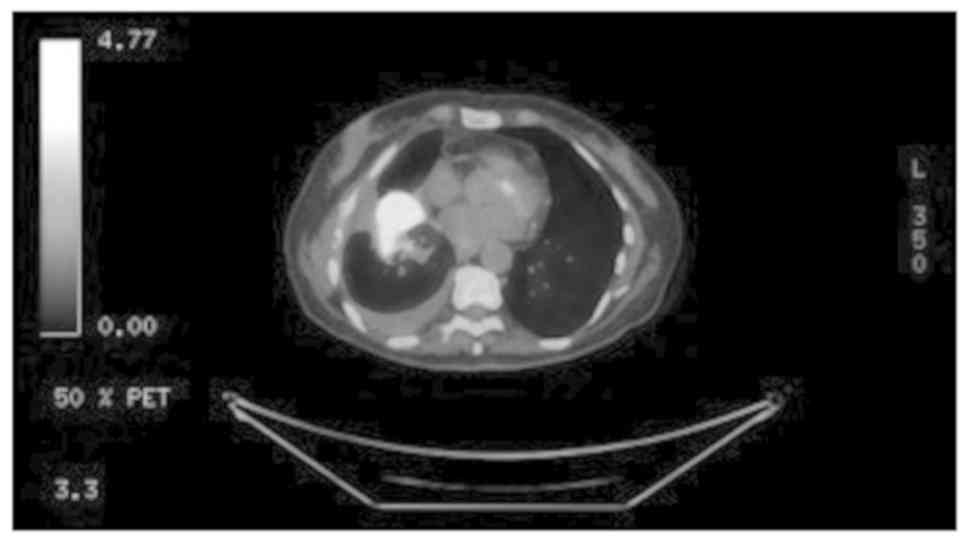
was observed, and a PET/CT scan (Fig.
1) revealed disease recurrence with lesions in the right lung
base, subcarinal mediastinal lymph node and a left adrenal mass.
The patient was then started on combination chemotherapy with
FOLFIRI and the endothelial growth factor receptor inhibitor
panitumumab. Disease progression was identified on restaging CT
scan, along with an increase of the CEA levels; hence, the decision
was made to start the patient on FOLFOX and BV. The patient had a
family history of prostate cancer in her father and heart failure
in her mother. The patient was a never smoker, and she did not
drink alcohol or use illicit drugs.
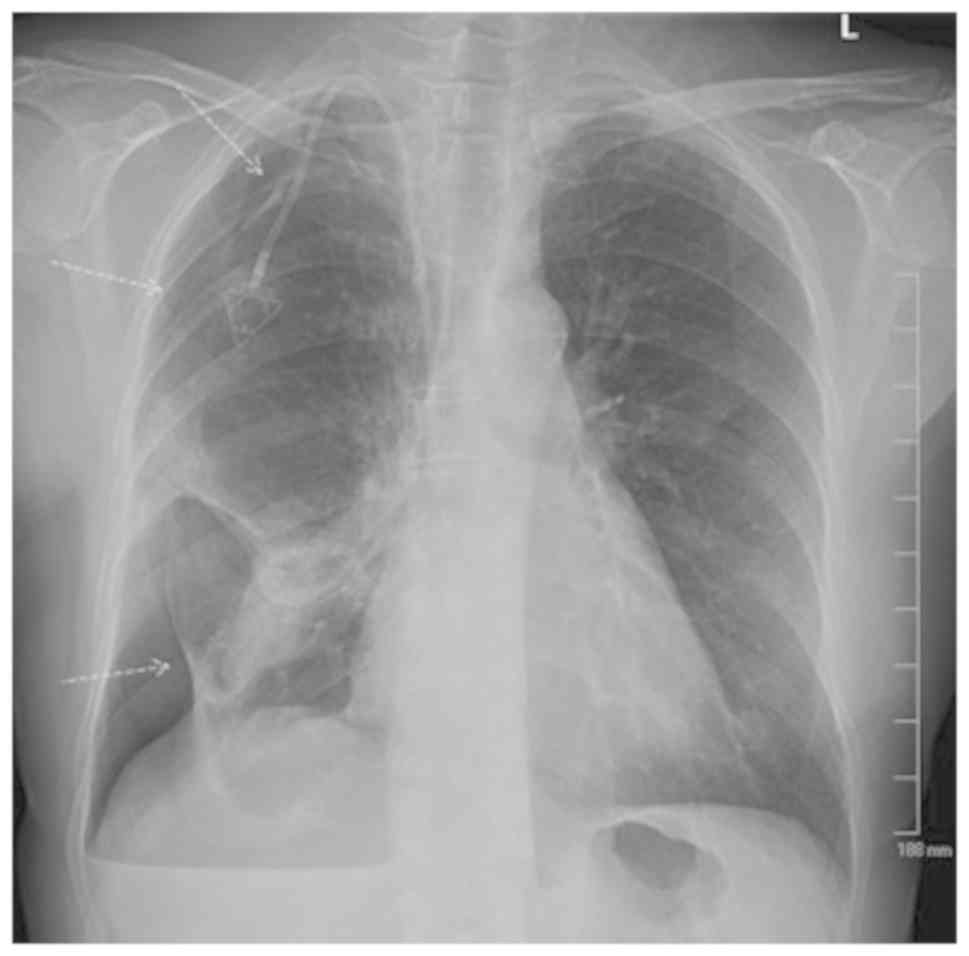
The patient underwent a chest X-ray (Fig. 2), which revealed evidence of a
moderate hydropneumothorax encompassing 40% of the right hemithorax
volume. There was also a stable opacity within the right perihilar
region, which correlated with known metastatic disease.
The patient's PTX was considered to be secondary to
the presence of an underlying pulmonary metastatic lesion in the
setting of BV use. The patient was a never smoker, with no
underlying obstructive lung disease; she had no chronic pulmonary
conditions other than her metastatic disease that had been present
for several years; she had also not had any recent chest trauma, or
undergone any recent procedures.
The patient underwent ultrasound-guided placement of
a 10 French chest tube with evacuation of 200 ml of air. The chest
tube was kept on water seal for 5 days, then clamped the next day,
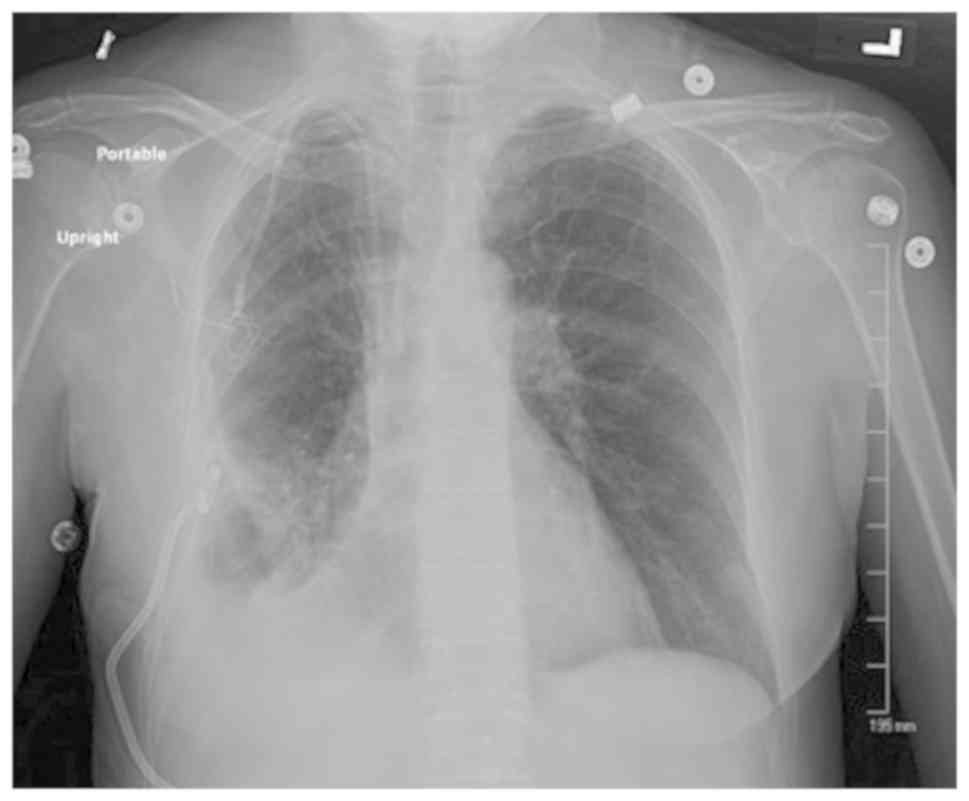
and a repeat X-ray revealed resolution of the PTX (Fig. 3). The chest tube was removed, and the
patient was discharged.
BV was indefinitely discontinued and substituted
with cetuximab. After the 7th cycle of chemotherapy, a restaging
PET/CT scan revealed enlargement of the right basilar lung mass
with new right hilar and mediastinal lymphadenopathy, indicating
progression of disease (POD). The patient completed 12 cycles of
the current chemotherapy regimen, and POD occurred again, after
which time she was started on trifluridine and tipiracil. The
patient was then admitted to the hospital with acute encephalopathy
and was found to have sepsis secondary to pneumonia. A CT scan of
the head revealed a 0.9-cm metastatic lesion in the left frontal
lobe. Palliative and hospice care were recommended with services
initiated during the same hospitalization. The patient succumbed to
the disease 1 week after admission.
Discussion
BV is a recombinant humanized monoclonal antibody
that exerts antitumor effects by binding to VEGF and inhibiting
tumor angiogenesis (1). BV has been
widely used in the treatment of solid tumors, including CRC, renal
cell carcinoma, ovarian carcinoma and several others (4). BV has several side effects pertaining
to its antiangiogenic effects. However, PTX as a complication of BV
use has rarely been reported. MEDLINE was searched using two
keywords, namely ‘BV’ and ‘pneumothorax’; the search yielded a
total of 15 results, 7 of which were case reports relevant to the
topic, and 6 of which had text available in English (Table I).
 | Table I.Case reports of PTX as a complication
of BV. |
Table I.
Case reports of PTX as a complication
of BV.
| Case report,
year | Patient no. | Age (years)/sex | Cancer
type | Disease on the side
of the PTX | Chemotherapy | No. of preceding
cycles (days since last cycle) | Management | (Refs.) |
|---|
| Zhang et al,
2012 | 1 | 23/M | Fibrosarcoma | No | BV + DP | 3 | Small-caliber chest
tube | (6) |
| Yang et al,
2011 | 1 | 45/M | CRC | Yes | BV + FOLFOXIRI | 2 | Small-caliber chest
tube | (7) |
| Koh et al,
2013 | 1 | 54/M | NSCLC | Unknown | BV + carboplatin +
paclitaxel | Unknown | Conservative
management | (9) |
| Iida et al,
2016 | 1 | 57/M | CRC | Yes | BV + XELOX followed
by BV + FOLFIRI | 8 of first regimen
and 5 of second regimen | Chest drainage with
aspiration followed by pleurodesis with blood 4 weeks later
followed by surgery | (10) |
| Bazan et al,
2014 | 1 | 38/M | Synovial sarcoma | Yes | BV +
temozolomide | 3 | Conservative
management | (11) |
| Ueda et al,
2015 | 1 | 56/F | Breast cancer | Yes | BV + paclitaxel | NA | Endobronchial
Watanabe spigot | (12) |
In 5 of the reviewed cases, including the present
case, pulmonary metastases were present. However, Zhang et
al reported a case of PTX following treatment of fibrosarcoma
where no lung lesions were present. Hence, PTX may occur even in
the absence of metastatic disease. Patients with mCRC were more
likely to develop PTX with BV (n=3), as compared with fibrosarcoma
(n=1), synovial sarcoma (n=1) and breast cancer (n=1). It remains
unclear whether this observation is a matter of chance, considering
that mCRC is the second most common malignancy to metastasize to
the lung (13), and the fact that
mCRC is one of the most common indications for BV treatment. PTX in
association with BV was observed following a variable number of
therapy cycles.
Srinivas and Varadhachary proposed that
malignancy-associated PTX may result from tumor compression of the
bronchial wall, leading to the formation of a one-way valve,
resulting in air trapping and eventual rupture. Another potential
cause is bronchopleural fistula formation as a result of effective
chemotherapy, and spontaneous vascular occlusion within the tumor
itself (14). Hence, it is possible
that the antiangiogenic effect of BV, which leads to distortion of
the tumor vasculature, may lead to PTX in peripherally located
tumors. In addition to the suggested tumor-related mechanisms of
PTX development, a study by Kasahara et al in animal models
found that chronic treatment with VEGF inhibitors led to the
distortion of the alveolar structure through the induction of cell
apoptosis, suggesting that this may contribute to the development
of emphysema (15), which is a risk
factor for PTX.
Our patient had also undergone radiosurgery with
Cyberknife 4 years prior to her current presentation. PTX has been
reported with Cyberknife therapy following CT-guided fiducial
placement (16), an acute
complication that was not observed in our patient. CT scan of the
chest following Cyberknife therapy did not show evidence of lung
damage that would be attributable to that therapy. However, it is
possible that our patient developed lung parenchymal damage
secondary to radiation that was undetectable on imaging modalities
and may have contributed to the development of PTX. However, we
consider the PTX that she developed to be associated with her most
recent treatment with BV in the setting of lung metastatic disease,
as the PTX developed after the 4th dose of BV, and the fact that
this patient had also received several cycles of chemotherapy
following Cyberknife therapy (12 cycles of FOLFIRI and cetuximab,
and 24 cycles of FOLFIRI and panitumumab), which were
well-tolerated. Our patient had received BV in the past, which she
also tolerated well; however, it is worth noting that, at the time,
the patient did not display evidence of lung metastatic disease on
imaging. Therefore, underlying lung parenchymal disease, including
lung metastatic disease, may place patients at an increased risk of
developing BV-associated PTX.
In a recent study of breast cancer patients
published by Lodola et al, a finding suggested that the
intracellular Ca2+ toolkit, which is responsible for the
pro-angiogenic effect of VEGF, is remodeled in cancer patients and
rendered insensitive to VEGF (17).
This suggests that these tumor cells are resistant to the
angiogenic effect of VEGF, and may consequently be resistant to
VEGF inhibitors as anti-angiogenic agents. This finding suggests
that VEGF inhibitors (including BV), may not have as important a
role in tumor vascularization as previously thought, and raises the
possibility that PTX, in addition to the other adverse effects of
these agents, may be a result of an off-target effect rather than
an anti-angiogenic effect. Further in vitro studies that
investigate this off-target effect are required. These studies may
also uncover, as the abovementioned study, potential novel targets,
such as the store-operated Ca2+ entry mechanism.
It is difficult to ascertain the exact frequency of
BV-associated PTX, given the scarcity of reported cases, and the
inability to ascertain the number of patients receiving BV therapy
annually. However, Interiano et al conducted a retrospective
analysis of selected pediatric patients with recurrent or
refractory solid malignancies who had undergone combination therapy
with BV and sorafenib with low-dose cyclophosphamide therapy. The
goal of the analysis was to assess the risk of developing PTX. The
study reported an unexpectedly high incidence of PTX in 11 of the
44 subjects (25%) (18). Although
that study was conducted in pediatric patients, its results are
significant and suggest that BV-associated PTX may be occurring at
higher rates than reported as compared to healthy individuals. In
comparison, primary spontaneous PTX (PSP) in healthy individuals is
estimated to occur at a rate of 7.4–18 cases per 100,000 amongst
males, and 6 cases per 100,000 amongst females. In addition, PSP
rarely occurs after the age of 40 years (19).
It is possible that the lack of a significant number
of case reports describing PTX in association with BV therapy is
due to the lack of widespread knowledge of this association; hence,
there may be a number of unreported such cases. Therefore, it is
important to report these cases to spread awareness amongst
clinicians to this potentially life-threatening collateral effect
of BV. There is also the need for further studies to establish a
causal association between BV and PTX and elucidate the mechanisms
underlying this effect.
Acknowledgements
The authors would like to thank Dr Asem Al-Refaie
for his comments on the manuscript.
Funding
No funding was received.
Availability of data and materials
All data generated/analyzed in the present study are
included in the published manuscript.
Authors' contributions
TA contributed to the conception of the work. TA,
RS, DR, KK and SP contributed to the drafting of the manuscript,
critical review of the article, and approval of the final version.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent to publication
Verbal informed consent was obtained from the
patient for the publication of this case′ details and associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shibuya M: Vascular endothelial growth
factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A
crucial target for anti- and pro-angiogenic therapies. Genes
Cancer. 2:1097–1105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ilic I, Jankovic S and Ilic M: Bevacizumab
combined with chemotherapy improves survival for patients with
metastatic colorectal cancer: Evidence from meta analysis. PLoS
One. 11:e01619122016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keating GM: Bevacizumab: A review of its
use in advanced cancer. Drugs. 74:1891–1925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Totzeck M, Mincu RI and Rassaf T:
Cardiovascular adverse events in patients with cancer treated with
bevacizumab: A meta-analysis of more than 20 000 patients. J Am
Heart Assoc. 6:e0062782017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Yang H, Zhao M and He J:
Bilateral pneumothorax after bevacizumab-containing chemotherapy in
fibrosarcoma. J Thorac Dis. 4:229–231. 2012.PubMed/NCBI
|
|
7
|
Yang SH, Lin JK, Chen WS, Lin TC, Yang SH,
Jiang JK, Chang SC, Lan YT, Chao TC, Yen CC, et al: Pneumothorax
after bevacizumab-containing chemotherapy: A case report. Jpn J
Clin Oncol. 41:269–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Makino T, Kudo S and Ogata T: Pneumothorax
after treatment with bevacizumab-containing chemotherapy for breast
cancer-a case report. Gan To Kagaku Ryoho. 41:233–235. 2014.(In
Japanese). PubMed/NCBI
|
|
9
|
Koh H, Kamiishi N, Kimura Y, Tajima A,
Yagami T and Mukai M: A rare case of persistent pneumothorax in
non-small cell lung cancer on bevacizumab therapy. J Pulm Respir
Med. Mar 26–2013.(Epub ahead of print). doi:
10.4172/2161-105X.S14-001. View Article : Google Scholar
|
|
10
|
Iida T, Yabana T, Nakagaki S, Adachi T and
Kondo Y: A: rupture of a lung metastatic lesion of colon cancer,
leading to pneumothorax caused by bevacizumab. Intern Med.
55:3125–3129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bazan F, Vollmer I and Gayete A:
Chemotherapy-induced secondary pneumothorax. Arch Bronconeumol.
50:442014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ueda Y, Huang CL, Itotani R and Fukui M:
Endobronchial watanabe spigot placement for a secondary
pneumothorax. J Bronchology Interv Pulmonol. 22:278–280. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu M, Hu J, Yang D, Cosgrove DP and Xu R:
Pattern of distant metastases in colorectal cancer: A SEER based
study. Oncotarget. 6:38658–38666. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srinivas S and Varadhachary G: Spontaneous
pneumothorax in malignancy: A case report and review of the
literature. Ann Oncol. 11:887–889. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kasahara Y, Tuder RM,
Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK,
Waltenberger J and Voelkel NF: Inhibition of VEGF receptors causes
lung cell apoptosis and emphysema. J Clin Invest. 106:1311–1319.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Collins BT, Vahdat S, Erickson K, Collins
SP, Suy S, Yu X, Zhang Y, Subramaniam D, Reichner CA, Sarikaya I,
et al: Radical cyberknife radiosurgery with tumor tracking: An
effective treatment for inoperable small peripheral stage I
non-small cell lung cancer. J Hematol Oncol. 2:12009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lodola F, Laforenza U, Cattaneo F,
Ruffinatti FA, Poletto V, Massa M, Tancredi R, Zuccolo E, Khdar DA,
Riccardi A, et al: VEGF-induced intracellular Ca2+
oscillations are down-regulated and do not stimulate angiogenesis
in breast cancer-derived endothelial colony forming cells.
Oncotarget. 8:95223–95246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Interiano RB, McCarville MB, Wu J,
Davidoff AM, Sandoval J and Navid F: Pneumothorax as a complication
of combination antiangiogenic therapy in children and young adults
with refractory/recurrent solid tumors. J Pediatr Surg.
50:1484–1489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sahn SA and Heffner JE: Spontaneous
pneumothorax. N Engl J Med. 342:868–874. 2000. View Article : Google Scholar : PubMed/NCBI
|