Introduction
Renal cell carcinoma (RCC) is a common tumor,
accounting for 21.82% of urinary tumors, second only to bladder and
prostate cancer (1). In China, the
incidence of renal cancer increases every year (2). There are several pathological types of
renal cell carcinoma which include: Clear cell carcinoma, papillary
renal cell carcinoma, chromophobe cell carcinoma, collecting ductal
carcinoma and medullary carcinoma (2). Approximately 30% of patients with renal
cell carcinoma exhibit distant metastasis at the time of initial
diagnosis, and ~50% of patients with localized renal cell carcinoma
exhibit distant metastasis following surgery (3). Sarcomatoid renal cell carcinoma (SRCC)
is a rare type of renal cell carcinoma, with a high malignancy and
a poor prognosis (4). SRCC accounts
for ~5–7% of all RCC cases (5,6). The
incidence of SRCC is increased in males compared with females,
particularly in the unilateral and right kidney (4). The median survival time of patients is
6–12 months amongst those aged 31–81 (median age, 60 years)
(4). Histologically, SRCC is
composed of epithelial (cancerous) and mesenchymal (sarcomatoid)
components, which is in difference to renal sarcoma (7,8). SRCC is
a more aggressive and advanced form of kidney cancer, which has a
shorter overall survival (9). It has
therefore been the subject of many studies.
At present, SRCC treatment includes surgery,
chemotherapy and radiotherapy, in addition to targeted biological
and cytokine therapies (4). Surgery
alone is often an inadequate measure to cure patients (10–12).
Furthermore, SRCC does not respond well to cytotoxic chemotherapy,
cytokines and targeted therapies (9,13). A
higher percentage of sarcomatoid features are also associated with
a poorer survival (14,15).
Considering the multitude of factors that influence
the survival time of patients with SRCC, in the present study a
multivariate Cox's proportional hazards regression model was used
to analyze the factors affecting the survival time of patients with
SRCC.
Materials and methods
Patients
The present study was ethically approved by the
ethical review board of The First Hosptial of Shanxi Medical
University (approval no. 2018-K006). A retrospective review was
performed to identify patients with SRCC whose diagnoses were
confirmed using pathology between January 2000 and September 2017
at The First Hospital of Shanxi Medical University. In total, 21
patients were included (13 males; 8 females; mean age, 57 years;
age range, 30–77 years). Four cases were treated at The First
Hosptial of Shanxi Medical University; the other 17 cases were
retrieved from a reference search. Clinical data were collected,
including sex, age, maximum tumor size, the proportion of sarcoma
elements, lymph node metastasis, distant metastasis, surgery and
drug therapy. The proportion of sarcoma elements was defined as the
proportion of sarcoma elements to the whole tumor observed under a
microscope.
Data analysis
All patient data were analyzed using the
Kaplan-Meier estimate and log-rank test for univariate analysis. In
addition, survival curves between the two groups were compared
using the log-rank method. A Cox's proportional hazard model was
used for multivariate analysis. Data were assigned numbers, which
are presented in Table I. SPSS
version 20 (IBM Corp, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
 | Table I.Factors and assignments of sarcomatoid
renal cell carcinoma. |
Table I.
Factors and assignments of sarcomatoid
renal cell carcinoma.
| Factors | Variable name | Assignments |
|---|
| Age | X1 | (year) |
| Sex | X2 | Female, 0; male,
1 |
| Sarcoma elements | X3 | <50%, 0; ≥50%,
1 |
| Lymph node
metastasis | X4 | No, 0; Yes, 1 |
| Distant
metastasis | X5 | No, 0; Yes, 1 |
| Maximum diameter of
the tumor | X6 | (cm) |
| Treatment method | X7 | Conservative, 0;
surgery, 1 |
| Drug treatment | X8 | No, 0; Yes, 1 |
| Survival time | t | (months) |
| Survival outcome | Y | Censored, 0; death,
1 |
Results
Clinical data
In total, 14 patients had a proportion of sarcoma
elements >50% in their tumor samples, and seven patients with
<50%. Nine patients received drug treatment, whereas 12 patients
did not. Full characteristics of the patient population are
presented in Table II (16–26).
 | Table II.Characteristics of the patient
population. |
Table II.
Characteristics of the patient
population.
| Patient | Sex | Age (years) | Maximum tumor
size | Sarcoma ≥50% | Lymph node
metastasis | Distant
metastasis | Surgery | Drug treatment | Survival time
(months) |
|---|
| 1 | Male | 65 | 9 | Yes | No | Yes | Yes | No | 2 |
| 2 | Male | 53 | 5.3 | Yes | Yes | Yes | Yes | No | 3 |
| 3 | Male | 56 | 6 | No | Yes | No | Yes | No | 48 |
| 4 | Male | 61 | 8 | No | Yes | Yes | Yes | No | 14 |
| 5 | Male | 44 | 4.2 | Yes | No | Yes | Yes | Yes | 21 |
| 6 | Female | 30 | 8.2 | Yes | Yes | Yes | No | No | 6 |
| 7 | Female | 47 | 5 | No | No | No | Yes | No | 24 |
| 8 | Male | 70 | 6.5 | Yes | No | Yes | Yes | No | 5 |
| 9 | Male | 35 | 19 | Yes | Yes | Yes | Yes | Yes | 14 |
| 10 | Male | 43 | 6 | Yes | No | No | Yes | No | 12 |
| 11 | Male | 40 | 10 | Yes | No | No | Yes | No | 12 |
| 12 | Female | 62 | 13 | Yes | Yes | Yes | Yes | Yes | 71 |
| 13 | Male | 47 | 6 | Yes | No | No | Yes | No | 12 |
| 14 | Male | 55 | 9 | No | No | Yes | Yes | Yes | 35 |
| 15 | Male | 59 | 2.5 | Yes | No | Yes | Yes | Yes | 19 |
| 16 | Female | 77 | 7 | Yes | Yes | No | Yes | Yes | 24 |
| 17 | Female | 66 | 7 | Yes | No | No | Yes | No | 9 |
| 18 | Female | 68 | 8 | No | Yes | Yes | Yes | Yes | 60 |
| 19 | Male | 30 | 12 | No | No | No | No | Yes | 14 |
| 20 | Female | 77 | 7 | Yes | Yes | Yes | Yes | Yes | 12 |
| 21 | Female | 64 | 5.8 | No | No | No | Yes | No | 18 |
Univariate analysis
There was a significant negative association between
the proportion of sarcoma elements and survival time (P<0.02).
In addition, there was a significant positive association between
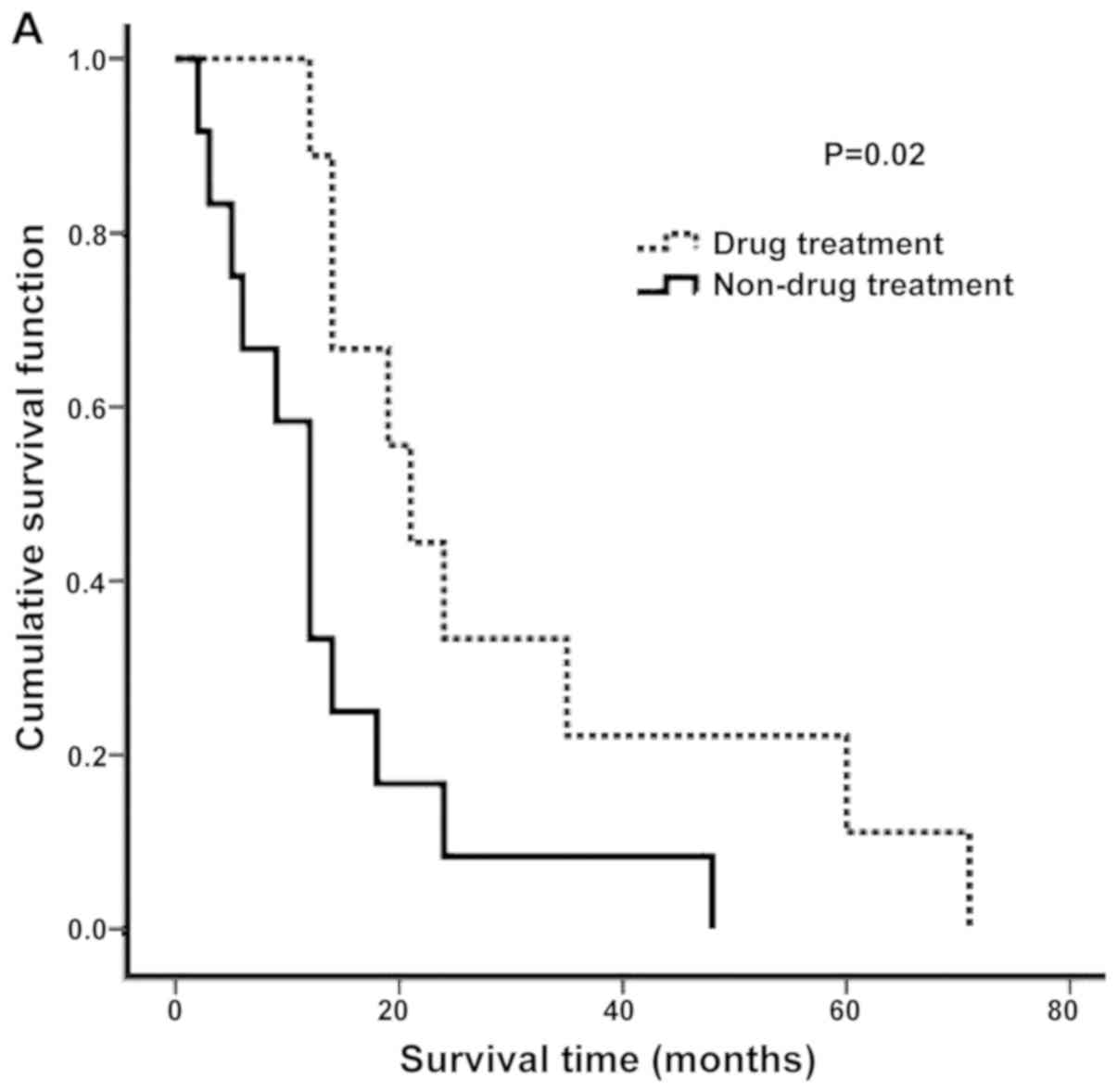
drug treatment and survival time (P<0.02; Table III). The Kaplan-Meier curves
revealed that the post-operative non-drug treatment group had a
significantly shorter survival time compared with the drug
treatment group (P<0.05; Fig.
1A). Patients with a proportion of sarcoma components ≥50%
exhibited a significantly shorter survival time compared with the
<50% group (P<0.05; Fig.
1B).
 | Table III.Univariate analyses of factors
affecting cancer-specific survival time. |
Table III.
Univariate analyses of factors
affecting cancer-specific survival time.
| Factors | Cases | Median survival
(months) | 1-year survival
rate (%) | P-value |
|---|
| Age (years) |
|
|
| 0.551 |
|
<60 | 12 | 14 | 83.3 |
|
|
≥60 | 9 | 14 | 66.7 |
|
| Sex |
|
|
| 0.148 |
|
Female | 8 | 14 | 75 |
|
|
Male | 13 | 18 | 76.9 |
|
| Maximum tumor
diameter (cm) |
|
|
| 0.565 |
|
<7 | 9 | 18 | 77.8 |
|
| ≥7 | 12 | 14 | 75 |
|
| Sarcoma components
(%) |
|
|
| 0.02a |
|
<50 | 7 | 12 | 100 |
|
|
≥50 | 14 | 24 | 64.2 |
|
| Surgery |
|
|
| 0.25 |
|
Yes | 19 | 14 | 78.9 |
|
| No | 2 | 6 | 50 |
|
| Drug treatment |
|
|
| 0.02a |
|
Yes | 9 | 21 | 66.7 |
|
| No | 12 | 12 | 58.3 |
|
| Lymph node
metastasis |
|
|
| 0.12 |
|
Yes | 9 | 14 | 77.8 |
|
| No | 12 | 12 | 75 |
|
| Distant
metastasis |
|
|
| 0.61 |
|
Yes | 11 | 14 | 72.7 |
|
| No | 10 | 12 | 60 |
|
Multivariate analyses
The multivariate Cox's proportional hazard model
revealed that drug treatment [risk ratio (RR), 0.171; 95%
confidence interval (CI), 0.049–0.591; P=0.005] was a significant
protective factor in survival time. Mortality risk in the
post-operative non-drug therapy patients following surgery was
5.822 times greater compared with that of the post-operative drug
therapy group. It also revealed that a ≥50% proportion of sarcoma
elements (RR, 4.682; 95% CI, 1.345–16.299; P=0.015) was a
significant risk factor for survival time. Mortality risk in the
≥50% group was 4.682 times higher compared with those in the
<50% group (Table IV).
 | Table IV.Analytical results by Cox's
proportional hazard model. |
Table IV.
Analytical results by Cox's
proportional hazard model.
| Factors | bj | Sbj | Wald value | P-value | Exp (B) | 95% confidence
interval |
|---|
| Sarcoma
components | 1.544 | 0.636 | 5.885 | 0.015 | 4.682 | (1.345,
16.299) |
|
Chemotherapy/Biological drug therapy | −1.768 | 0.634 | 7.786 | 0.005 | 0.171 | (0.049, 0.591) |
Discussion
Identifying the factors that impact the biological
behavior of SRCC is essential for understanding the natural course
of the disease in patients. Drug treatment induces adverse effects
in almost every patient (27).
Therefore, it is necessary to avoid treating patients who will not
ultimately benefit from drug therapy. It is evident that the
clinical behavior of SRCC results from complex interactions between
multiple prognostic factors (27).
The reported effects of chemotherapy and immunotherapy on SRCC
differentiation are contradictory (27). Therefore, determining the prognostic
factors of survival may be helpful in selecting patients for drug
treatment.
It was hypothesized that drug treatment would have a
beneficial effect on survival time. There are certain rationales
for administering drug treatment for SRCC. Firstly, improved
survival time was revealed in patients receiving high-dose
interleukin-2 (IL-2) therapy, compared with patients treated with
surgery alone or any other form of immunotherapy. The relative risk
of mortality is 10.4 times higher in patients not receiving
high-dose IL-2 therapy (28).
Furthermore, surgical resection and high-dose IL-2-based
immunotherapy may serve a function in the successful treatment of
SRCC (28). Another report suggested
that treatment with interferon-α, compared with vinblastine or
medroxyprogesterone, achieves a small improvement in survival time
(29). Secondly, a recent study has
revealed that the use of multiple targeted tyrosine kinase
inhibitors, including sorafenib and sulinitinib, have produced a
positive effect on patient survival time (30). Thirdly, chemotherapy with doxorubicin
and gemcitabine may reverse clinical deterioration in certain
patients, and cause the stabilization or regression of metastases
(31). In addition, the combination
of doxorubicin and gemcitabine has antitumor activity in patients
with SRCC (11). However, in the
present study, a significant beneficial effect of drug treatment on
survival time was observed compared with the non-drug treatment
group (P<0.05). The survival time of patients with postoperative
drug treatment was 21 months, which was significantly higher
compared with 12 months in the postoperative non-drug treatment
group (Table III; P<0.05). Drug
treatment was much more commonly administered in Japan compared
with China, consequently resulting in a longer survival time in
Japan (26).
It was confirmed the survival rate of patients with
≥50% sarcoma elements was worse compared with the <50% group.
This is consistent with other reports demonstrating that
sarcomatoid architecture predicts poor survival (32,33). The
mean survival time of the patients in the ≥50% group was only 14
months, whereas those in the <50% group had an mean survival
time of 27 months, in other words that the higher the proportion of
the sarcoma elements, the worse the prognosis of the patients
(34). Numerous studies have
demonstrated that sarcomatoid differentiation is associated with a
poor prognosis (30,35–38).
Patients with sarcomatoid differentiation have more aggressive
tumor characteristics compared with those without sarcomatoid
differentiation. Furthermore, it has been reported that sarcomatoid
differentiation is an independent prognostic predictor of overall
survival time (26). Furthermore,
the 2-year survival rate of patients in the ≥50% group was 7.1%,
whereas the survival rate for the <50% group was 42%. In
conclusion, the higher the proportion of sarcoma elements, the
worse the prognosis and the shorter the survival time.
The present study has a number of limitations. The
number of studied SRCC cases was relatively small, due to the
limited cases allotted. It was not possible to further analyze the
types of drug treatment, so univariate and multivariate analyses
could only be performed from the perspective of whether drug
treatment was or was not used.
Analyzing the prognostic factors of survival for
patients with SRCC would be valuable in guiding treatment. In the
present study, the multivariate analysis of SRCC survival revealed
that drug treatment was a protective factor that affected survival
time (RR=0.172). Additionally, the proportion of sarcoma elements
≥50% was a risk factor that affected survival time (RR=4.682).
Therefore, when a patient is diagnosed with SRCC, drug therapy may
extend the patient's survival time to a certain extent. In
addition, judging the proportion of sarcomatoid components may
provide a reference to judge a patient's condition. However, SRCC
is rare in clinical practice and the number of cases is limited.
The present study has certain limitations and further cases will be
collected for further analysis.
In the present study, the multivariate analysis of
the survival time with SRCC revealed that postoperative drug
treatment is a protective factor that affects survival time
(RR=0.172). The proportion of sarcoma elements ≥50% is a risk
factor that affects survival time (RR=4.682). Therefore, when a
patient is diagnosed with SRCC, postoperative drug therapy may
extend the patient's survival time to a certain extent. In
addition, judging the proportion of sarcomatoid components on time
may provide a certain reference to understanding the patient's
condition.
Ultimately, the present study has identified that
postoperative drug treatment and sarcoma elements were protective
and risk factors for SRCC. However, due to the fact that SRCC is
rare in clinical practice, the present research was not able to
further analyze the effects of different drugs on the survival of
patients with SRCC.
To the best of our knowledge, until now, there has
not been any research analyzing the survival time of patients with
SRCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW collected clinical data and wrote the manuscript.
WS designed and supervised the current study. KY, XT, MX and HY
collected and interpreted clinical data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the ethical standards of the Institutional Review Board of the
First Hospital of Shanxi Medical University (approval no.
2018-K006), in addition to the 1964 Helsinki declaration and its
later amendments or comparable ethical standards. The Institutional
Review Board of the First Hospital of Shanxi Medical University
waived the requirement for informed consent due to the
retrospective design of this study.
Patient consent for publication
All patients provided their consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dingwei YE and Zhang H: Current status and
development trend of renal cancer diagnosis and treatment in China.
Chine J Urol. 35:401–405. 2014.(In Chinese).
|
|
3
|
Xian WEI, Xinwen KE, Zhiquan HU, et al:
Clinical efficacy and safety of sunitinib in the treatment of
advanced renal cell carcinoma. J Mod Reprod Oncol. 9:133–136.
2017.(In Chinese).
|
|
4
|
Shuang WB, Zhang YH and Tong XN: Recent
researches on sarcomatoid renal cell carcinoma. E J Transl Med.
2:6–8. 2018.(In Chinese).
|
|
5
|
Zhang L, Wu B, Zha Z, Zhao H and Feng Y:
The prognostic value and clinicopathological features of
sarcomatoid differentiation in patients with renal cell carcinoma:
A systematic review and meta-analysis. Cancer Manag Res.
10:1687–1703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang BY, Thompson RH, Lohse CM, Leibovich
BC, Boorjian SA, Cheville JC and Costello BA: A novel prognostic
model for patients with sarcomatoid renal cell carcinoma. BJU Int.
115:405–411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He H and Magi-Galluzzi C:
Epithelial-to-mesenchymal transition in renal neoplasms. Adv Anat
Pathol. 21:174–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kyriakopoulos CE, Chittoria N, Choueiri
TK, Kroeger N, Lee JL, Srinivas S, Knox JJ, Bjarnason GA, Ernst SD,
Wood LA, et al: Outcome of patients with metastatic sarcomatoid
renal cell carcinoma: Results from the international metastatic
renal cell carcinoma database consortium. Clin Genitourin Cancer.
13:e79–e85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lebacle C, Pooli A, Bessede T, Irani J,
Pantuck AJ and Drakaki A: Epidemiology, biology and treatment of
sarcomatoid RCC: Current state of the art. World J Urol.
37:115–123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Appleman LJ and Marachie JK: Systemic
therapy following metastasectomy for renal cell carcinoma: Using
insights from other clinical settings to address unanswered
questions. Urol Oncol. 36:17–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nanus DM, Garino A, Milowsky MI, Larkin M
and Dutcher JP: Active chemotherapy for sarcomatoid and rapidly
progressing renal cell carcinoma. Cancer. 101:1545–1551. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rashid MH, Welsh CT, Bissada NK and
Chaudhary UB: Complete response to adriamycin and ifosfamide in a
patient with sarcomatoid renal cell carcinoma. Am J Clin Oncol.
28:107–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Keskin SK, Msaouel P, Hess KR, Yu KJ,
Matin SF, Sircar K, Tamboli P, Jonasch E, Wood CG, Karam JA and
Tannir NM: Outcomes of patients with renal cell carcinoma and
sarcomatoid dedifferentiation treated with nephrectomy and systemic
therapies: Comparison between the cytokine and targeted therapy
eras. J Urol. 198:530–537. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shuch B, Bratslavsky G, Shih J, Vourganti
S, Finley D, Castor B, Treat E, Linehan WM, Pantuck AJ, Said JW and
Belldegrun AS: Impact of pathological tumour characteristics in
patients with sarcomatoid renal cell carcinoma. BJU Int.
109:1600–1606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim T, Zargar-Shoshtari K, Dhillon J, Lin
HY, Yue B, Fishman M, Sverrisson EF, Spiess PE, Gupta S, Poch MA
and Sexton WJ: Using percentage of sarcomatoid differentiation as a
prognostic factor in renal cell carcinoma. Clin Genitourin Cancer.
13:225–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen JH, Liu XD and Yang LI: Sarcomatoid
renal cell carcinoma: One case report. J Kun Ming Med Univ.
29:483–484. 2007.
|
|
17
|
Pei XK, Jiang W, Yang SB, Yang QS and Long
W: A case of ipsilateral renal sarcomatoid renal cell carcinoma
with angiomyolipoma at the same time and literature review. J
Contemp Urol Reproduct Oncol. 304–305. 2014.
|
|
18
|
Yin MX, Zhang Y, Ye XQ, Lin B and Xiao C:
Sarcomatoid renal cell carcinoma: Two case reports. Chin J Clin Exp
Pathol. 26:767–768. 2010.(In Chinese).
|
|
19
|
Li L and Zhou GY: Sarcomatoid renal cell
carcinoma: Two case reports. Chin J Clin Exp Pathol. 20:3842004.(In
Chinese).
|
|
20
|
Hu YC, Huang XY, Li D, Leng J, Bo JJ and
Wang YX: Sarcomatoid renal cell carcinoma: One case report. Chin J
Urol. 23:2172002.(In Chinese).
|
|
21
|
Komeya M, Sano F, Kagota M, Murakami T,
Makiyama K, Miyoshi Y, Nakaigawa N, Ogawa T, Uemura H, Yao M, et
al: Case of sarcomatoid renal cell carcinoma developed in the
chalked kidney (putty kidney). Hinyokika Kiyo. 55:253–257. 2009.(In
Japanese). PubMed/NCBI
|
|
22
|
Ni XH, Yu GH, Ruan ZY, Lin J and Cai FB:
Sarcomatoid renal cell carcinoma: Three cases report. Chin J Urol.
21:506–507. 2000.(In Chinese).
|
|
23
|
Gao Y, Shuang WB, Tong XN, Li S and Zhang
YH: Bilateral sarcomatoid renal cell carcinoma: An uncommon case in
young femal patient. Transl Surg. 2:74–77. 2017. View Article : Google Scholar
|
|
24
|
Takada T, Kinouchi T, Kinoshita T, Hatano
K, Kobayashi M, Inoue H, Hara T and Yamaguchi S: Four cases of
sarcomatoid renal cell carcinoma. Hinyokika Kiy. 55:93–97. 2009.(In
Japanese).
|
|
25
|
Tsuchiyama K, Ito H, Ishida H, Itoh H and
Yokoyama O: Advanced sarcomatoid renal cell carcinoma effectively
treated with sunitinib: Report of a case. Hinyokika Kiy.
57:615–618. 2011.
|
|
26
|
Minagawa T, Nishizawa S, Kamigaito M,
Nakayama T and Okaneya T: Sarcomatoid renal cell carcinoma with von
Hippel-Lindau disease: A case report. Nihon Hinyokika Gakkai
Zasshi. 98:723–726. 2007.(In Japanese). PubMed/NCBI
|
|
27
|
Kwak C, Park YH, Jeong CW, Jeong H, Lee
SE, Moon KC and Ku JH: Sarcomatoid differentiation as a prognostic
factor for immunotherapy in metastatic renal cell carcinoma. J Surg
Oncol. 95:317–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cangiano T, Liao J, Naitoh J, Dorey F,
Figlin R and Belldegrun A: Sarcomatoid renal cell carcinoma:
Biologic behavior, prognosis, and response to combined surgical
resection and immunotherapy. J Clin Oncol. 17:523–528. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stadler WM, Huo D, George C, Yang X, Ryan
CW, Karrison T, Zimmerman TM and Vogelzang NJ: Prognostic factors
for survival with gemcitabine plus 5-fluorouracil based regimens
for metastatic renal cancer. J Urol. 170:1141–1145. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Motzer RJ and Russo P: Systemic therapy
for renal cell carcinoma. J Urol. 163:408–417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Motzer RJ, Rini BI, Bukowski RM, Curti BD,
George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G,
et al: Sunitinib in patients with metastatic renal cell carcinoma.
JAMA. 295:2516–2624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dodd PM, McCaffrey JA, Hilton S, Mazumdar
M, Herr H, Kelly WK, Icasiano E, Boyle MG and Bajorin DF: Phase I
evaluation of sequential doxorubicin gemcitabine then ifosfamide
paclitaxel cisplatin for patients with unresectable or metastatic
transitional-cell carcinoma of the urothelial tract. J Clin Oncol.
18:840–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bono AV and Lovisolo JA: Renal cell
carcinoma-diagnosis and treatment: State of the art. Eur Urol. 31
(Suppl 1):S47–S55. 1997. View Article : Google Scholar
|
|
34
|
Zhang L, Shi HY and Hong BF: The
clinicopathological analysis of 16 cases of sarcomatoid renal cell
carcinoma. Med J Chin PLA. 356–358. 2006.
|
|
35
|
Grabowski M, Huzarski T, Lubinski J and
Sikorski A: Survival in patients with rare subtypes of renal cell
carcinoma. BJU Int. 89:599–600. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leibovich BC, Han KR, Bui MH, Pantuck AJ,
Dorey FJ, Figlin RA and Belldegrun A: Scoring algorithm to predict
survival after nephrectomy and immunotherapy in patients with
metastatic renal cell carcinoma: A stratification tool for
prospective clinical trials. Cancer. 98:2566–2575. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Escudier B, Szczylik C, Eisen T, Stadler
WM, Schwartz B and Bukowski MSM: Randomized phase III trial of the
Raf kinase and VEGFR inhibitor sorafenib (BAY 43-9006) in patients
with advanced renal cell carcinoma (RCC). J Clin Oncol.
23:45102005. View Article : Google Scholar
|
|
38
|
Culine S, Bkradda M, Terrier-Lacombe MJ
and Droz JP: Treatment of sarcomatoid renal cell carcinoma: Is
there a role for chemotherapy? Eur Urol. 27:138–141. 1995.
View Article : Google Scholar : PubMed/NCBI
|