Introduction
Mesothelioma is a relatively rare disease, with
~1,000 cases of mesothelioma-related mortality annually in Japan
(1). However, its incidence has
been on the increase and is predicted to reach a peak in 2030 in
Japan (2). There is currently no
standard diagnostic method for this disease worldwide (3). In most countries, pathological
confirmation from tumor biopsy samples is used for a confirmatory
diagnosis (3). However, a tumor
biopsy is typically performed only if this disease is suspected,
since it is a highly invasive procedure. Thus, numerous patients
with mesothelioma are diagnosed at a late stage. Therefore, the
establishment of a more convenient and non-invasive method for the
early diagnosis of mesothelioma is a pressing issue. Mesothelioma
is highly associated with exposure to asbestos and X-rays are
commonly used in a mass examination for subjects with such a risk
factor (4). However, there are
currently no available studies indicating that an X-ray mass
examination may be used for the early diagnosis of mesothelioma
(5) to improve patient
survival.
We previously reported on the renal carcinoma gene
ERC, which is highly expressed in renal cancer in Eker rats
(6). We also reported that ERC is
a homolog of the human megakaryocyte potentiating factor/mesothelin
gene (7,8). The human mesothelin gene product is a
71-kDa precursor protein, which is cleaved by a furin-like protease
into a 40-kDa C-termimal fragment that remains membrane-bound and a
31-kDa N-terminal fragment (N-ERC/mesothelin) that is secreted into
the bloodstream (9). Therefore,
N-ERC/mesothelin would be expected to serve as a specific and
easily-measured biomarker of mesothelioma. We developed an
enzyme-linked immunosorbent assay (ELISA) system that detects
N-ERC/mesothelin (10) and
recently reported that N-ERC/mesothelin may be useful for the early
diagnosis of mesothelioma (11).
We initiated a 5-year large-scale research screening
in 2007, in order to determine whether this blood tumor marker is
useful for early diagnosis in a mass examination and to recommend
an effective screening method that may be conducted on a large
scale. This is an interim retrospective analysis report of this
research screening for a future prospective study that is currently
under planning.
Materials and methods
Screening population
Adult male or female participants who were members
of the Tokyo General Construction Workers Union and the Tokyo Doken
National Health Insurance Association were invited to participate
in this research screening, since they are or have been at a risk
of asbestos exposure, considering the nature of their occupations,
which included construction or plumbing. When the participants
underwent annual health check-ups, written informed consent was
obtained prior to their participation, regardless of their asbestos
exposure history or the duration of their construction work
history. This being a large-scale screening, the target number of
participants was ~40,000.
Screening method
This research screening was approved by the
Institutional Review Board (IRB) of Juntendo University School of
Medicine, its Affiliated Hospital (Tokyo, Japan)and the
Immuno-Biological Laboratories (Gunma, Japan). This study was
conducted at 85 research sites, which were approved by the IRB.
Blood samples were collected on an annual basis. The high-risk
population was identified based on the results of an annual
N-ERC/mesothelin assessment. The participants who were selected as
members of the high-risk population were advised to visit Juntendo
University or its Affiliated Hospital for further assessments to
achieve early diagnosis. As a control population for data analysis,
a low-risk population was also selected for comparison with the
high-risk population.
Identification of the high-risk
population
As previously reported (12), we initially defined the
participants as high-risk if they exhibited abnormal levels
(>8.0 ng/ml) of N-ERC/mesothelin. However, it was considered
that this criterion was not sufficient for a screening and that
more optimal criteria were required. We therefore set the new
criteria for the high-risk population as follows: i) human
anti-mouse antibody (HAMA) not detected; ii) absence of any
evidence of renal dysfunction based on medical history and a
laboratory test. This criterion was included to consider the effect
of renal failure on this marker (13); iii) age ≥35 years; iv) detection of
abnormal values of N-ERC/mesothelin on more than two occasions
during the annual assessments.
The final confirmation of the high-risk population
was performed by a case review committee, which was held on a
monthly basis.
Data on a history of asbestos exposure were not
collected unless the participants developed mesothelioma, since the
scope of our research was a mass scale screening of subjects with a
risk factor.
Case review committee
This committee was comprised of at least one
physician, one data manager, one technician familiar with
N-ERC/mesothelin and one administrator from the Tokyo General
Construction Workers Union. The role of the committee was to
ultimately identify the high-risk population.
Selection of the low-risk population
In order to characterize the high-risk population, a
low-risk population was also selected as a control prior to the
initiation of data analysis. In total, 7,850 participants from 17
approved research sites were selected as the low-risk population,
who underwent N-ERC/mesothelin measurements at least twice and
exhibited no abnormal values.
Confirmation of the development of
mesothelioma in the high-risk population
The confirmatory diagnosis of mesothelioma was
determined via tumor biopsy in the context of appropriate clinical
and radiological findings.
Preparation of anti-ERC/mesothelin
antibodies
The anti-N-ERC/mesothelin monoclonal antibody (mAb)
clones 7E7 and 16K16 were described previously (10,11).
N-ERC/mesothelin, expressed in Escherichia coli as a
glutathione-S-transferase and histidine-tagged fusion protein, was
purified and used as an immunogen. Splenocytes from immunized mice
were fused with the X-73-Ag8.653 mouse myeloma cells. Using ELISA,
the supernatants of the hybridoma cells were screened by their
reactivity to the immunogen and several positive clones were
selected with the limiting dilution method. The 7E7 and 16K16
clones were selected for ELISA.
ELISA
Blood N-ERC/mesothelin levels were determined with
the sandwich ELISA system using 7E7 and 16K16 mAbs. The details of
this analysis were previously described (10,11).
Briefly, microtiter plates (96 wells) were coated with 100
μl/well of 100 mmol/l carbonate buffer (pH 9.5) containing
7E7 mAb. The plates were washed with phosphate-buffered saline with
0.1% Tween-20 (PBS-T) and blocked with 200 μl/well 1% (w/v)
bovine serum albumin (BSA) in PBS containing 0.05% NaN3.
After washing with PBS-T, 100-μl aliquots of test samples or
recombinant N-ERC/mesothelin as a standard, serially diluted in
PBS-T with 1% BSA, were added in duplicate to the wells, followed
by incubation at 37°C for 1 h. After washing with PBS-T, 100
μl horseradish peroxidase (HRP)-conjugated 16K16 mAb was
added to each well, followed by incubation for 30 min at 4°C. The
wells were washed with PBS-T and 100 μl of freshly prepared
tetramethyl benzidine solution was added as a substrate. The
resulting mixture was incubated in the dark for 30 min at room
temperature. The reaction was terminated by the addition of 100
μl of 1 N H2SO4. The absorbance of the
solution was measured at 450 nm in an ELISA reader (EMax; Molecular
Devices Co. Ltd., Sunnyvale, CA, USA). In the case of samples with
extra value due to HAMA, they were re-measured with sample diluents
containing normal mouse IgG in PBS-T with 1% BSA as substitute for
PBS-T with 1% BSA.
Statistical analysis
A longitudinal data analysis model was used to
estimate the mean change from Time 1 (the first assessment) over
time. The model included factors for group, time and group-by-time
interaction as fixed effects. An unstructured covariance matrix was
used to model the correlation among repeated assessments.
Ninety-five percent confidence intervals (95% CIs) were provided
for the difference in change from Time 1 values over time between
the high-risk and low-risk groups, using an appropriate contrast of
least squares means and referencing a t-distribution. P values were
also computed based on the contrast statistics. A step-down closed
testing procedure was employed to test the differences in change
from the Time 1 value. The procedure started at Time 5 and
continued in a descending order in time until the lower limit of
the 95% CI for difference in change from Time 1 at a particular
time point failed to exceed 0 ng/ml. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of the high-risk
population
As of September 26, 2010, ~40,000 participants were
enrolled in this research screening and a total of 124,288 blood
samples were collected and analyzed for N-ERC/mesothelin. Among
these, 1,603 samples exhibited abnormal values, defined as >8.00
ng/ml of N-ERC/mesothelin, which was rarely detected among healthy
volunteers (10,11). These samples were re-analyzed, in
order to exclude HAMA detection. Following re-analysis, 714 samples
still exhibited high values. Based on the decision of the case
review committee, 62 participants were finally identified as the
high-risk population. According to X-ray findings, there were no
reports indicating suspected mesothelioma among the high-risk
population in this interim assessment. The mean age of the
high-risk subjects was 56 years (range, 35–76 years).
Data analysis of N-ERC/mesothelin in the
high- and low-risk population
Mean N-ERC/mesothelin value by risk group, gender
and assessment time are presented in Table I. The mean N-ERC/mesothelin level
in the low-risk population were ~2.20–4.33 ng/ml, similar to that
in healthy volunteers and the mean N-ERC/mesothelin level in the
high-risk population was 9.83–17.28 ng/ml, similar to that in
patients with mesothelioma, as we previously reported (11). No significant differences in the
mean N-ERC/mesothelin level by age (data not shown in Table I) or gender were identified between
the two populations, although the number of data points in the
high-risk population was limited.
 | Table I.Mean N-ERC/mesothelin (ng/ml) in the
low- and high-risk populations by gender and by assessment. |
Table I.
Mean N-ERC/mesothelin (ng/ml) in the
low- and high-risk populations by gender and by assessment.
| Risk group | Gender | Assessment time | No. | Mean | SD | Max | Median | Min |
|---|
| High | F | Time 1 | 26 | 9.34 | 3.30 | 19.39 | 9.26 | 3.23 |
| High | F | Time 2 | 26 | 10.46 | 5.39 | 28.54 | 9.13 | 2.49 |
| High | F | Time 3 | 15 | 9.90 | 2.40 | 15.81 | 9.90 | 5.23 |
| High | F | Time 4 | 5 | 11.03 | 1.24 | 12.63 | 10.90 | 9.22 |
| High | M | Time 1 | 36 | 10.17 | 3.80 | 27.95 | 9.25 | 3.99 |
| High | M | Time 2 | 39 | 10.16 | 5.33 | 37.37 | 8.95 | 5.25 |
| High | M | Time 3 | 18 | 12.09 | 6.54 | 31.49 | 10.37 | 6.30 |
| High | M | Time 4 | 12 | 17.40 | 19.57 | 78.60 | 12.35 | 6.80 |
| High | M | Time 5 | 5 | 12.10 | 3.40 | 16.48 | 12.47 | 8.69 |
| Low | F | Time 1 | 1834 | 2.41 | 1.15 | 7.91 | 2.14 | 0.56 |
| Low | F | Time 2 | 1842 | 3.05 | 1.43 | 7.95 | 2.76 | 0.05 |
| Low | F | Time 3 | 753 | 3.44 | 1.49 | 7.92 | 3.12 | 0.52 |
| Low | F | Time 4 | 220 | 3.62 | 1.53 | 7.88 | 3.42 | 0.48 |
| Low | F | Time 5 | 37 | 3.82 | 1.60 | 7.32 | 3.84 | 0.05 |
| Low | M | Time 1 | 5988 | 2.14 | 1.15 | 7.87 | 1.84 | 0.05 |
| Low | M | Time 2 | 5980 | 2.63 | 1.38 | 7.96 | 2.33 | 0.05 |
| Low | M | Time 3 | 2956 | 2.92 | 1.47 | 7.96 | 2.66 | 0.05 |
| Low | M | Time 4 | 1107 | 3.15 | 1.44 | 7.96 | 2.92 | 0.05 |
| Low | M | Time 5 | 220 | 2.88 | 1.32 | 7.52 | 2.56 | 0.28 |
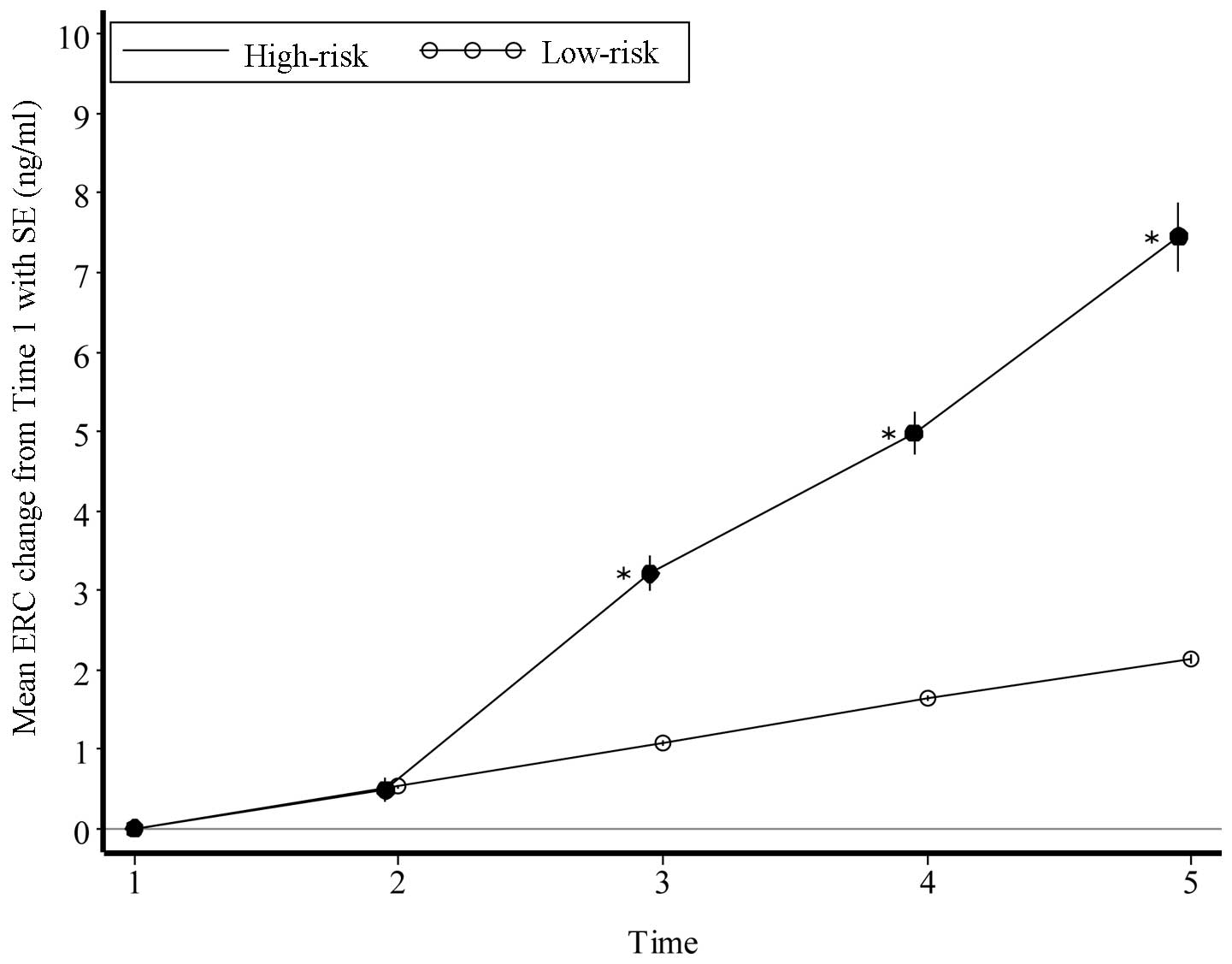
We also calculated the mean ± SE of N-ERC/mesothelin
value and the mean change in N-ERC/mesothelin value from the first
assessment in the two populations (Table II). In the high-risk population,
the level increased by ~2.0 points annually, whereas it was
increasing by only 0.4 points annually in the low-risk population.
Therefore, there was a tendency for N-ERC/mesothelin levels to
increase significantly during this study period in the high-risk
population, compared to those in the low-risk population (Fig. 1). In addition, two participants in
the high-risk population ultimately developed mesothelioma. The
background information and outcome of the participants who
developed mesothelioma is summarized below:
 | Table II.Mean N-ERC/mesothelin level and mean
change from Time 1 assessment in the low- and high-risk populations
(ng/ml). |
Table II.
Mean N-ERC/mesothelin level and mean
change from Time 1 assessment in the low- and high-risk populations
(ng/ml).
| Assessment time | Low-risk population
| High-risk population
| Difference in mean
change from Time 1 (95% CI) |
|---|
| Mean ± SE | Mean change from
Time1 (95% CI) | Mean ± SE | Mean change from Time
1 (95% CI) |
|---|
| Time 1 | 2.20±0.01 | | 9.83±0.15 | | |
| Time 2 | 2.73±0.02 | 0.53 (0.50–0.55) | 10.31±0.19 | 0.48 (0.20–0.76) | −0.05 (−0.33,
0.23) |
| Time 3 | 3.27±0.02 | 1.07 (1.04–1.11) | 13.03±0.25 | 3.21 (2.80–3.61) | 2.13 (1.72,
2.54) |
| Time 4 | 3.83±0.03 | 1.63 (1.58–1.69) | 14.80±0.30 | 4.98 (4.48–5.48) | 3.34 (2.84,
3.85) |
| Time 5 | 4.33±0.06 | 2.13
(2.02–2.25) | 17.28±0.47 | 7.45
(6.62–8.23) | 5.32 (4.47,
6.16) |
Mesothelioma case 1
We previously reported a summary of this participant
in the form of a case report (13).
Mesothelioma case 2
The participant was a 72-year-old male, with a
50-year history of asbestos exposure. This participant submitted
his blood samples for N-ERC/mesothelin analysis annually during the
first 3 years and the values were abnormally high on all three
measurements (9.3, 8.8 and 11.1 ng/ml). However, since the
N-ERC/mesothelin values were stable, the participant did not
receive a N-ERC/mesothelin test in the 4th year, although the site
staff advised him to receive a test several times. When the patient
visited the research site in the 5th year, the N-ERC/mesothelin
levels had suddenly escalated to 78.6 ng/ml and he was diagnosed
with epithelioid-type mesothelioma.
Notably, two other participants from the high-risk
population developed other types of cancer (lung and appendiceal
cancer). Since other cancer types were beyond the scope of this
research screening, we were not able to compare the incidence of
other types of cancer between the high-risk and the entire
populations.
Discussion
Since the mean N-ERC/mesothelin levels in the
high-risk population were similar to those among patients with
mesothelioma and two participants from the high-risk population
developed mesothelioma, N-ERC/mesothelin levels may be a useful
marker for the early diagnosis of this disease in a large-scale
setting. We plan to follow up on all the participants of the
high-risk population and prospectively assess how many ultimately
develop mesothelioma, while continuing to investigate for early
signs of mesothelioma. For this purpose, we plan to encourage the
high-risk population to visit the hospital annually for a check
up.
One participant from the high-risk population failed
to undergo the annual N-ERC/mesothelin test and was diagnosed with
mesothelioma in the following year. Had the patient not missed this
annual test, he may have been diagnosed earlier and may have been a
candidate for surgery. Therefore, we strongly recommend encouraging
the participants of the high-risk population to receive an
additional health check-up at least annually, even if there are no
clinical symptoms. The screening system reported herein currently
appears to be efficient; however, we hope to provide a more
credible screening system after all the analyses are completed.
Acknowledgements
The authors would like to thank the
participants and the investigators, the members of the Tokyo
General Construction Workers Union and the Tokyo Doken National
Health Insurance Association for their cooperation in the
conduction of this study. We would also like to thank Mr Akira
Wakana for providing advice on statistical analyses. We are also
grateful to the Vehicle Racing Commemorative Foundation and the
Ministry of Education, Culture, Sports, Science and Technology of
Japan for the financial support of this study (no. 221S0001).
References
|
1.
|
Gemba K, Fujimoto N, Kato K, Aoe K,
Takeshima Y, Inai K and Kishimoto T: National survey of
mesothelioma and asbestos exposure in Japan. Cancer Sci.
103:483–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nakano T: Current therapies for malignant
pleural mesothelioma. Environ Health Prev Med. 13:75–83. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Robinson BW, Musk AW and Lake RA:
Mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Reid A, de Klerk N, Ambrosini G, Olsen N,
Pang SC and Musk AW: The additional risk of mesothelioma in former
workers and residents of Wittenoom with benign pleural disease or
asbestosis. Occup Environ Med. 62:665–669. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sandén A and Järvholm B: A study of
possible predictors of mesothelioma in shipyard workers exposed to
asbestos. J Occup Med. 33:770–773. 1991.PubMed/NCBI
|
|
6.
|
Hino O, Kobayashi E, Nishizawa M, et al:
Renal carcinogenesis in the Eker rat. J Cancer Res Clin Oncol.
121:602–605. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yamashita Y, Yokoyama M, Kobayashi E,
Takai S and Hino O: Mapping and determination of the cDNA sequence
of the Erc gene preferentially expressed in renal cell carcinoma in
the Tsc2 gene mutant (Eker) rat model. Biochem Biophys Res Commun.
275:134–140. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hino O: Multistep renal carcinogenesis in
the Eker (Tsc 2 gene mutant) rat model. Curr Mol Med. 4:807–811.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hassan R, Bera T and Pastan I: Mesothelin:
a new target for immunotherapy. Clin Cancer Res. 10:3937–3942.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shiomi K, Miyamoto H, Segawa T, et al:
Novel ELISA system for detection of N-ERC/mesothelin in the sera of
mesothelioma patients. Cancer Sci. 97:928–932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shiomi K, Hagiwara Y, Sonoue K, et al:
Sensitive and specific new enzyme-linked immunosorbent assay for
N-ERC/mesothelin increases its potential as a useful serum tumor
marker for mesothelioma. Clin Cancer Res. 14:1431–1437. 2008.
View Article : Google Scholar
|
|
12.
|
Imashimizu K, Shiomi K, Maeda M, Aoki N,
Igarashi K, Suzuki F, Koizumi M, Suzuki K and Hino O: Feasibility
of large-scale screening using N-ERC/mesothelin levels in the blood
for the early diagnosis of mesothelioma. Exp Ther Med. 2:409–411.
2011.PubMed/NCBI
|
|
13.
|
Shiomi K, Shiomi S, Ishinaga Y, et al:
Impact of renal failure on the tumor markers of mesothelioma,
N-ERC/mesothelin and osteopontin. Anticancer Res. 31:1427–1430.
2011.PubMed/NCBI
|