Introduction
Ovarian cancer (OC) is the third most common tumor
of the female genital tract after carcinomas of the cervix and
endometrium and remains the leading cause of gynecological
malignancy-related mortality (1).
In total, 75% of the patients are diagnosed at an advanced stage
and the 5-year survival rate is consequentially poor, since OC is
generally asymptomatic in its early stages and there is currently
no effective screening method (2).
Advances in the resolution of sonography have increased its
accuracy for the differential diagnosis of OC; however, the results
may vary with differences in equipment and among different
operators. With the development of genomics and proteome analysis,
an increasing number of tumor markers have been introduced to aid
the diagnosis of cancer and have become an important and convenient
diagnostic tool. It is difficult to detect OC at its early stages
using conventional methods. Therefore, there is a need for
biomarkers of higher diagnostic accuracy to distinguish malignant
from benign pelvic masses at an early stage and set up an effective
screening program (3).
Carbohydrate antigen 125 (CA125) measurement is
currently considered to be a significant component in the workup of
a patient with an adnexal mass and is the standard biomarker for
detecting OC recurrence and monitoring treatment efficacy. However,
the application of CA125 is compromised by its low specificity,
particularly in premenopausal women, as the CA125 levels may be
elevated above normal in a number of common benign gynecological
conditions and in other malignancies (4). Therefore, considerable efforts are
aimed at identifying novel markers, which are more sensitive and
specific compared to CA125 and may be used in combination with or
instead of CA125 to improve the diagnosis of OC (5,6).
Among a wide spectrum of biomarkers, including
CA125, TPA and TAG72, human epididymis protein 4 (HE4) has been
proposed as a novel tumor marker for OC. Despite the low number of
available studies, HE4 was reported to be used as an aid in the
diagnosis of OC, as it was found to be overexpressed in ovarian
carcinomas but not in ovarian benign diseases, normal ovarian
tissue or low-malignant potential tumors (7,8).
However, previous studies on the roles of HE4 and CA125 in the
differential diagnosis of OC reported controversial and
inconclusive results. Certain published studies and meta-analyses
indicated that HE4 is not superior to CA125 in predicting OC
(9,10). Furthermore, it has not been
determined whether diagnostic performance may be improved by
combining measurements of HE4 and CA125, instead of each marker
used alone. Therefore, we conducted a meta-analysis of the
available evidence on the diagnostic accuracy of HE4 and CA125 by a
stepwise selection of relevant studies, considering only those
studies that evaluated both markers in the same case series. These
data may provide evidence supporting further application of HE4 in
the diagnosis of OC.
Materials and methods
Data sources and search strategy
We followed the Meta-analysis Of Observational
Studies in Epidemiology (12) and
the Cochrane Handbook for Systematic Reviews of Diagnostic Test
Accuracy and conducted this meta-analysis in accordance with the
Preferred Reporting Items for Systematic Reviews and Meta-analyses
guidelines (11). A prespecified
protocol, including data sources, search strategy,
inclusion/exclusion criteria for the articles and methods for
analysis, was developed prior to the initiation of the study. A
systematic review of original articles analyzing the diagnostic
performance of HE4 and CA125 was performed by searching the
Medline, Embase and Cochrane databases. Original and review
articles published between 2008 and 2012 were sought. The search
terms used were as follows: ‘HE4/WAP 4-disulfide core domain 2
(WFDC2)’, ‘CA125’, ‘ovarian carcinoma/ovarian’,
‘sensitivity/specificity/false-negative/false-positive/diagnosis/detection/accuracy’.
All the related publications were evaluated in order to retrieve
the most eligible studies and their reference lists were searched
manually to identify additional relevant publications. The aim of
the search was to identify those articles in which HE4 and CA125
measurements were compared for OC diagnosis in order to provide a
synthesis of evidence for the meta-analysis.
Inclusion/exclusion criteria
The authors evaluated the titles and abstracts of
all the preliminary identified articles in order to assess whether
the study was relevant to the aim of the meta-analysis. The
complete study was evaluated using the following eligibility
criteria for the meta-analysis of the studies: i) The sensitivity
and specificity of HE4 and CA125 for the diagnosis of OC were
provided; ii) the included patients were aged ≥50 years; iii) the
study design included patients with OC and evaluated the
contribution of HE4 and CA125; iv) all the subjects were diagnosed
by a gold standard (pathological examination of biopsied
specimens), newly diagnosed patients with pathologically confirmed
OC were the case group and patients with benign disease or healthy
subjects were the control group; v) the diagnostic parameters were
not of fixed specificity or sensitivity; vi) presence of data on
sensitivity and specificity, or the possibility of deriving such
values from the literature; vii) measurement of serum HE4 and CA125
in OC by ELISA or enzyme immunoassay with a clear cut-off value;
and viii) the investigated population was represented by women with
a gynecological disease suspected of being OC, which was the
intended spectrum of patients to be investigated by circulating
biomarker detection.
The exclusion criteria were as follows: i) Duplicate
publications; ii) case reports; iii) insufficient data to construct
a 2×2 table of the test results; iv) serum/plasma HE4
concentrations were measured to assess OC recurrence, monitor
disease progression or treatment efficacy; v) lack of control
group; and vi) abstracts, reviews, talks and review class
documentations.
Data extraction and quality
assessment
The data extracted from each study included name of
first author, year of publication, country, number of patients,
sensitivity, specificity, cut-off value, study design, patient
selection and reconstructed 2×2 tables. Data were extracted from
each study by two independent authors (Z.S. and B.L.H.). Any
disagreements were resolved by consulting a third author (G.X.).
The authors of the studies were contacted via e-mail in case of
missing information.
The quality of the studies was assessed using a
revised version of the Quality Assessment of Diagnostic Accuracy
Studies (QUADAS-2) tool (13)
(Table I) and the standards for
reporting diagnostic accuracy tool (14). Each item scored ‘yes’, ‘no’ or
‘unclear’ if there was no sufficient information for an accurate
judgment to be made.
 | Table IQUADAS list. |
Table I
QUADAS list.
| Item no. | Description |
|---|
| 1 | Representative
patient spectrum |
| 2 | Clear description of
selection criteria |
| 3 | Acceptable reference
standard |
| 4 | Acceptable delay
between tests |
| 5 | Avoiding partial
verification bias |
| 6 | Sufficient
differential verification bias |
| 7 | Avoiding
incorporation bias |
| 8 | Sufficient
description of index test |
| 9 | Sufficient
description of reference test |
| 10 | Blinded
interpretation of index test results |
| 11 | Blinded
interpretation of index reference results |
| 12 | Availability of
clinical data to the researchers |
| 13 | Reporting of
uninterpretable indeterminate results |
| 14 | Explanation of
withdrawals from study |
Statistical analysis
We used the standard methods recommended for
meta-analyses of diagnostic test evaluations (15). In this meta-analysis, the pooled
sensitivity, pooled specificity, positive likelihood ratio (PLR),
negative likelihood ratio (NLR), diagnostic odds ratio (DOR) and
95% confidence interval (CI) were calculated using the DerSimonian
and Laird (16) method. In
particular, the strength of the indication for the presence of the
disease provided by the positive result of the test was relevant
when PLR>10, moderate when 5<PLR<10 and poor when
2<PLR<5. The strength of the indication for the absence of
the disease provided by the negative result of the test was
relevant when NLR<0.10, modest when 0.10<NLR<0.20 and poor
when 0.20<NLR<0.50 (17).
The DOR, as a single indicator measure of the accuracy of a
diagnostic test (18), describes
the odds of positive results in patients with the disease compared
to the results in patients without disease. The present study used
Moses’ linear model to draw a summary receiver operating
characteristic (SROC) curve, which summarized the joint
distribution of sensitivity and specificity. The area under the
SROC curve (AUC) was calculated and an AUC close to 1.0 signified
that the test achieved almost perfect discrimination, while an AUC
close to 0.5 indicated poor discrimination. The AUC was found to be
useful to summarize the curve, but also quite robust to
heterogeneity (19). Heterogeneity
was assessed using the LR I2 index and the χ2
test. The I2 index is a measure of the percentage of
total variation across studies due to heterogeneity beyond chance;
values >50% indicate the presence of heterogeneity (20). A P-value of <0.05 calculated by
the χ2 test was considered to indicate a statistically
significant difference. In case of heterogeneity, the random
effects model was used (21). All
the analyses were performed using the Meta-Analysis of Diagnostic
and Screening Test (Meta-DiSc) program, version 1.4 (Ramon y Cajal
Hospital, Madrid, Spain) and Review Manager (RevMan) version 5 (The
Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen,
Denmark).
Results
Study characteristics
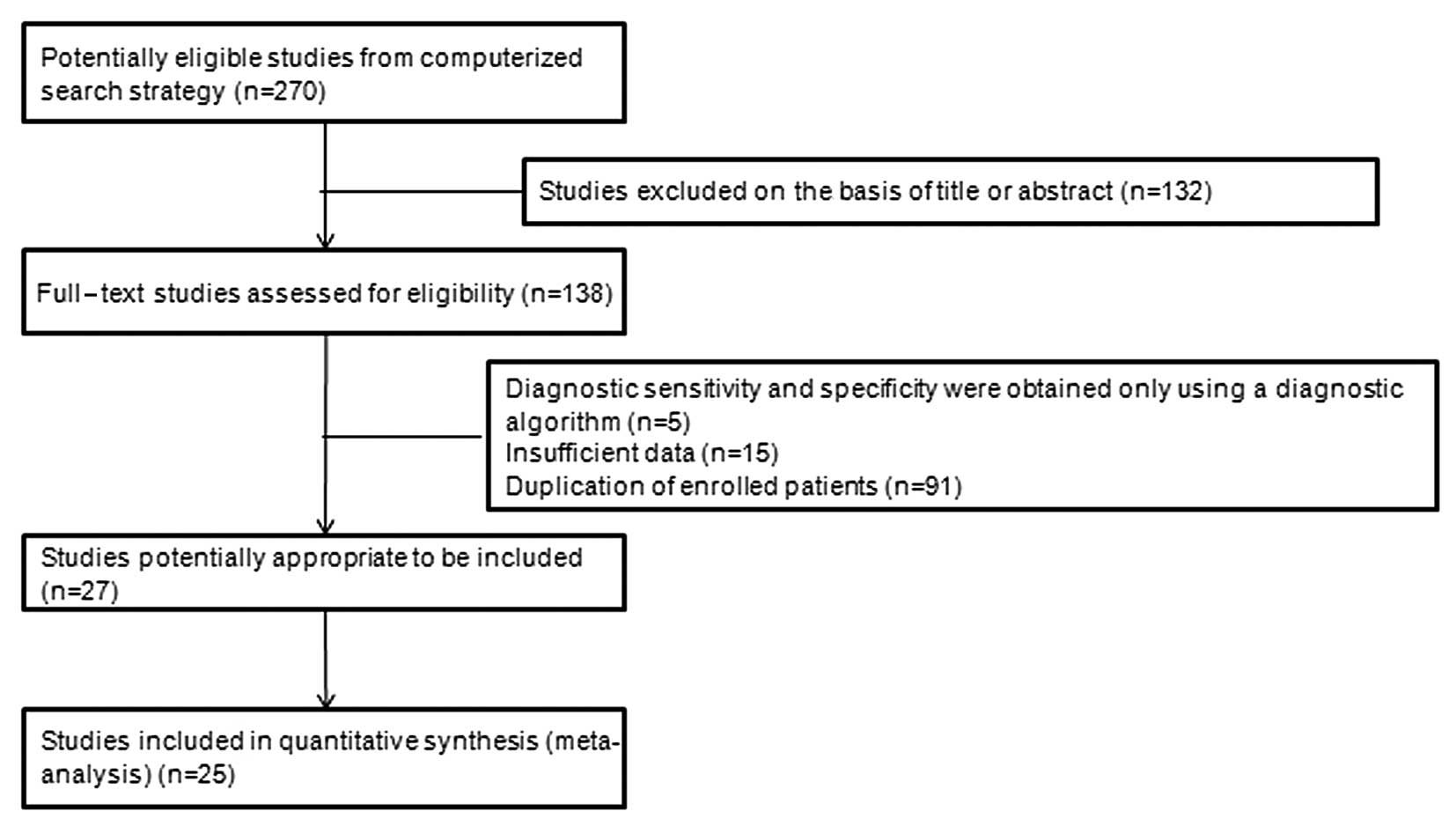
Based on the aforementioned search terms, a total of
270 articles were identified. After scanning the titles and
abstracts, 132 articles were excluded. Subsequently, a further 111
articles were excluded for the following reasons: 5 only used a
diagnostic algorithm, 15 presented insufficient data and 91 had
duplicate patient enrollment. Finally, a total of 25 studies
(2,22–45)
with 4,729 patients fulfilled all the inclusion criteria and were
considered for the analysis (Fig.
1). The target population of the study was women in a
preoperative setting or with a known adnexal mass. The
characteristics and results of the included studies are summarized
in Table II. According to the
QUADAS-2 checklist, details on selection, data collection and
enrolment were retrieved. As shown in Table II, the enrolment differed widely
among studies: Sample size and OC prevalence, setting of data
collection, patient characteristics (prevalence of women of
postmenopausal status) and severity of OC (prevalence of late
stage-disease). Each of these points may represent a source of
heterogeneity among studies.
 | Table IICharacteristics of studies included in
the analysis. |
Table II
Characteristics of studies included in
the analysis.
| Author (year) | Location | No. | Cut-off value | Test method | Study design | Patient
enrollment | (Refs.) |
|---|
| Abdel-Azeez et
al (2010) | Egypt | 65 | HE4: 72 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | ND | ND | (22) |
| Anastasi et
al (2010) | Italy | 190 | HE4: 150 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
RIA | P | C | (23) |
| Andersen et
al (2010) | USA | 211 | HE4: upper 95th
percentile of benign groups | HE4: ELISA; CA125:
ELISA | P | C | (24) |
| Chang et al
(2011) | China | 202 | HE4: 150 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | ND | C | (2) |
| Van Gorp et
al (2011) | Belgium | 389 | HE4: 70 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
EIA | P | C | (25) |
| Holcomb et
al (2011) | USA | 229 | HE4: 70 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | C | (26) |
| Jacob et al
(2011) | Switzerland | 160 | HE4: 70 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | ND | (27) |
| Montagnana et
al (2011) | Italy | 104 | HE4: 74.2 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | R | C | (28) |
| Moore et al
(2008) | USA | 233 | HE4: 70 pmol/l | HE4: ELISA; CA125:
RIA | R | C | (29) |
| Nolen et al
(2010) | USA | 790 | HE4: 38.5 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | ND | ND | (30) |
| Park et al
(2012) | Korea | 323 | HE4: 70 pmol/l;
CA125: 35 U/ml | HE4: EIA; CA125:
CLIA | R | C | (31) |
| Dong et al
(2008) | China | 212 | HE4: 70 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | C | (32) |
| Wu et al
(2012) | China | 203 | HE4: 150 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | C | (33) |
| Yang et al
(2010) | China | 86 | HE4: 150 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | C | (34) |
| Huang and Zeng
(2011) | China | 96 | HE4: 150 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | C | (35) |
| Li et al
(2013) | China | 98 | HE4: 70 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | C | (36) |
| Liu et al
(2010) | China | 131 | HE4: 75 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | C | (37) |
| Jiang et al
(2010) | China | 225 | HE4: 46.15 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | R | C | (38) |
| Jing et al
(2011) | China | 100 | HE4: 150 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | C | (39) |
| Yao and Hong
(2012) | China | 95 | HE4: 150 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | C | (40) |
| Yao et al
(2010) | China | 91 | HE4: 70 pmol/l;
CA125: 35 U/ml | HE4: EIA; CA125:
ECLIA | P | C | (41) |
| Wang et al
(2010) | China | 161 | HE4: 70 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | C | (42) |
| Li et al
(2013) | China | 70 | HE4: 70 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | P | C | (43) |
| Ke and Liu
(2010) | China | 84 | HE4: 70 pmol/l;
CA125: 35 U/ml | HE4: EIA; CA125:
ELISA | P | C | (44) |
| Lin et al
(2013) | China | 181 | HE4: 150 pmol/l;
CA125: 35 U/ml | HE4: ELISA; CA125:
ELISA | R | C | (45) |
Approximately 60% of the studies were performed in a
gynecological oncology setting, which suggested a different
assessment and a higher grade of severity for OC. The remaining
studies, including early-stage OCs, were performed in a
gynecological setting. The wide variation in the prevalence of
women of postmenopausal status across the studies was likely to
affect diagnostic performance.
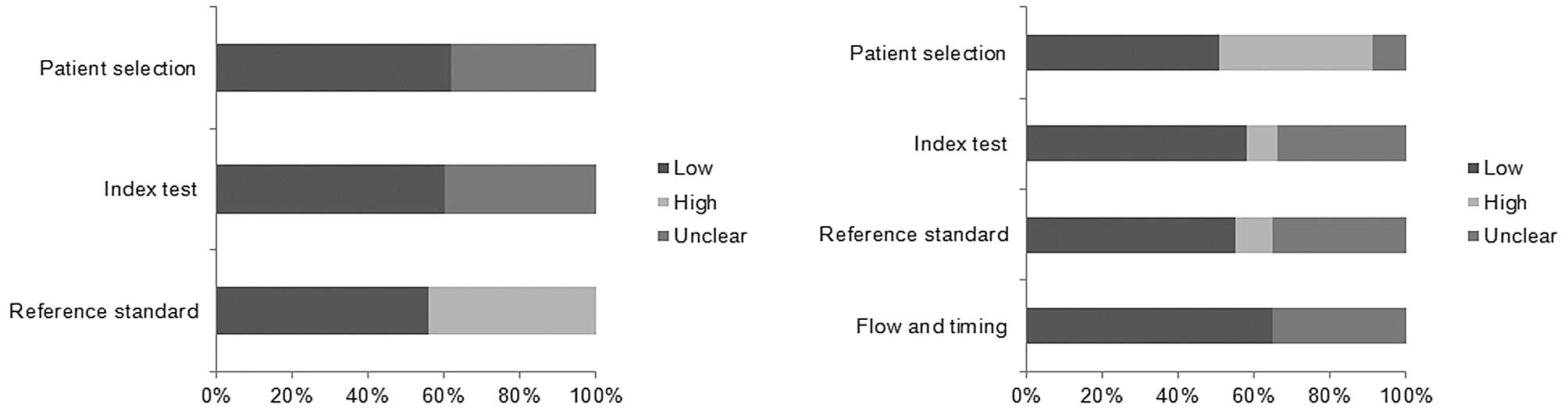
Quality assessment of the included
studies
The methodological quality assessment for the
included studies is shown in Fig.
2. All the studies included in our meta-analysis met on average
10 of the 14 QUADAS criteria, reflecting high quality.
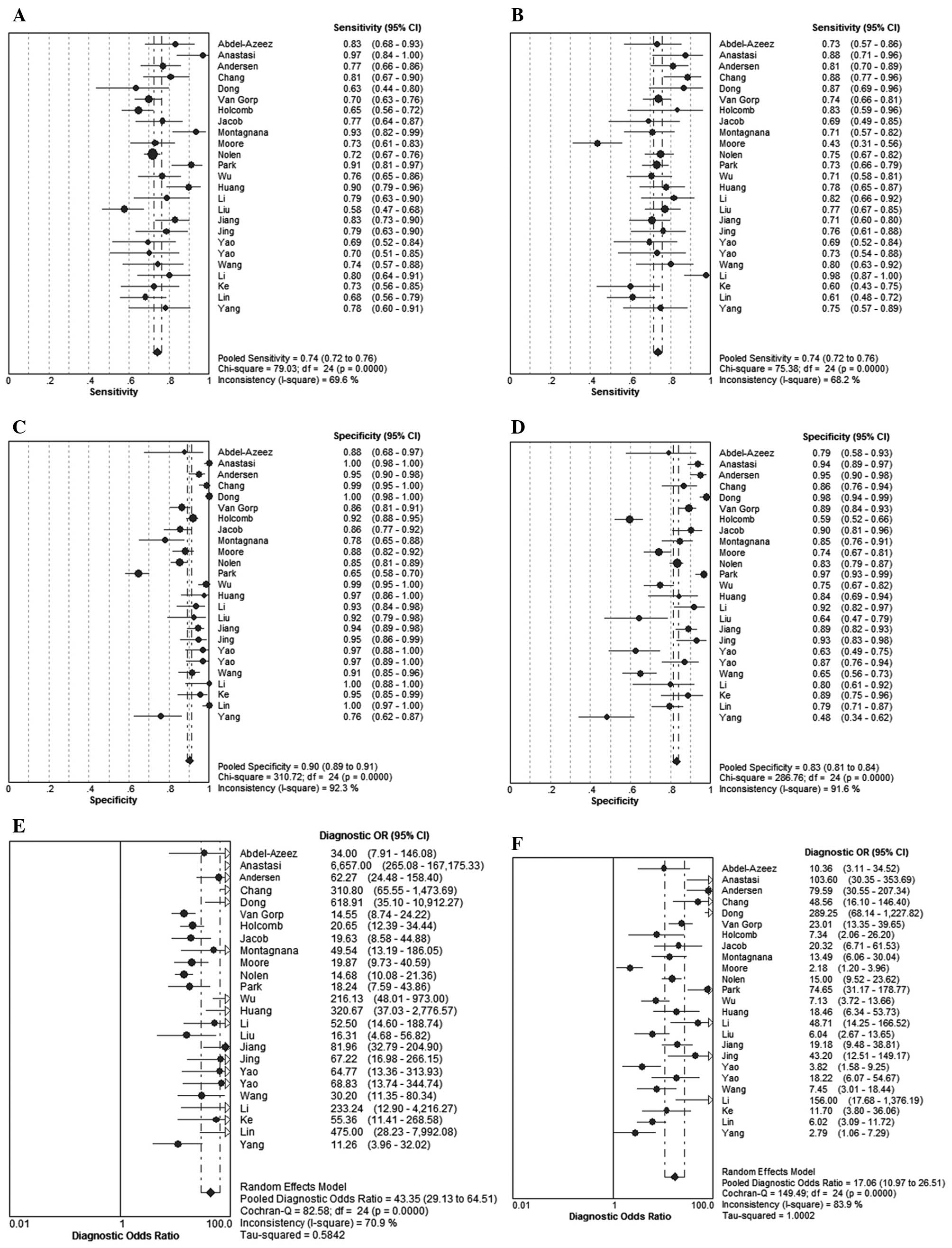
Data synthesis and meta-analysis
The results revealed that the pooled sensitivities
and 95% CIs for HE4 and CA125 were 0.74 (0.72–0.76) and 0.74
(0.72–0.76), respectively. The pooled specificities and respective
95% CIs for HE4 and CA125 were 0.90 (0.89–0.91) and 0.83
(0.81–0.84), respectively. The summary DORs and 95% CIs for HE4 and
CA125 were 43.35 (29.13–64.51) and 17.06 (10.97–26.51),
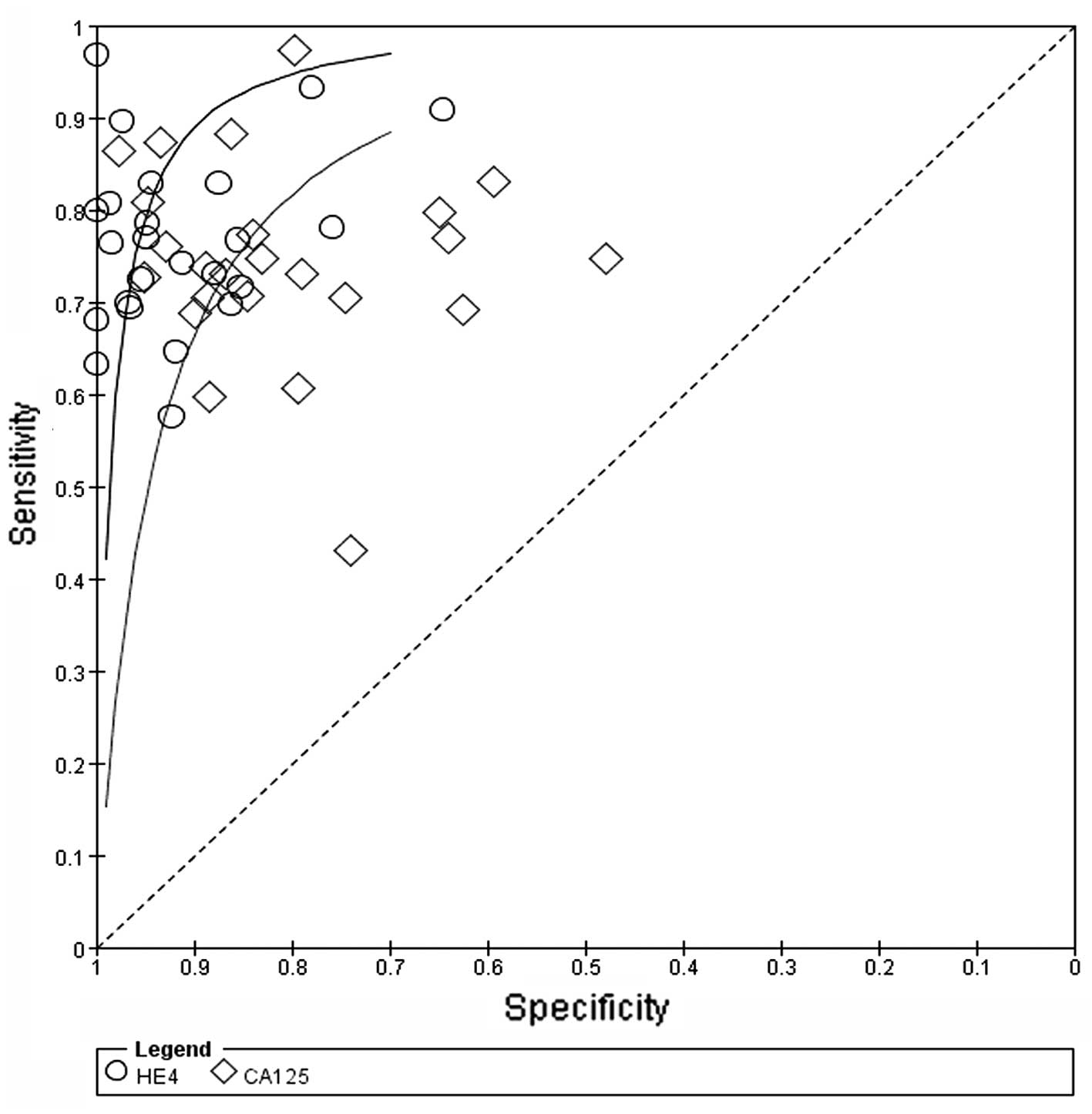
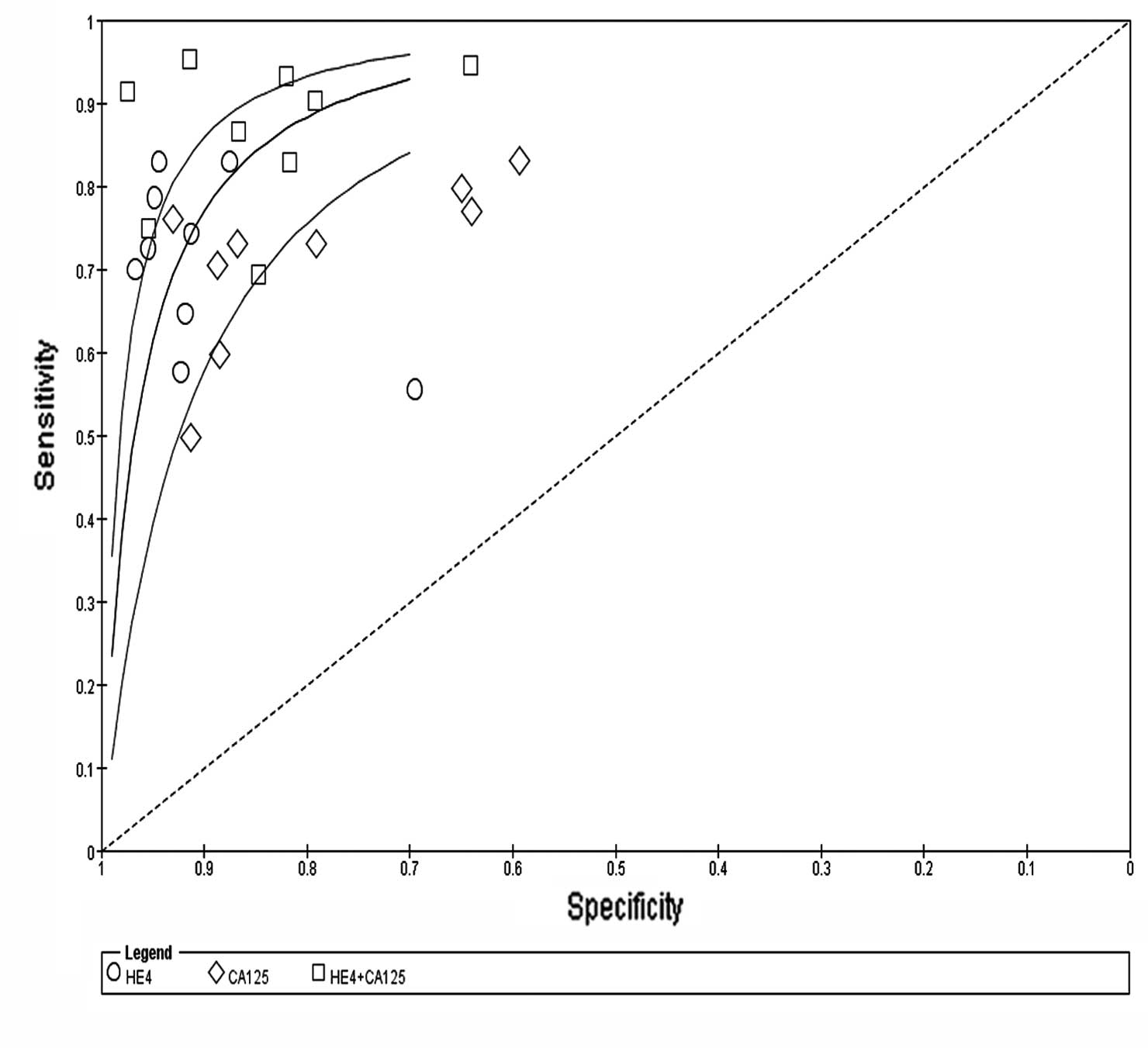
respectively (Fig. 3). The SROC
curve, which illustrates the correlation between sensitivity and
specificity, was obtained using the random effects model to present
the overall summary of HE4 and CA125. The PLRs and respective 95%
CIs for HE4 and CA125 were 10.59 (7.20–15.58) and 4.84 (3.59–6.54),
respectively. The NLRs and 95% CIs for HE4 and CA125 were 0.27
(0.24–0.31) and 0.31 (0.26–0.38), respectively. The AUC for HE4 and
CA125 was 0.8915 and 0.8538, respectively. These findings indicated
that HE4 and CA125 may be useful biomarkers for OC diagnosis and
HE4 appears to be superior to CA125 regarding diagnostic accuracy
in distinguishing OC from other benign gynecological diseases
(Fig. 4).
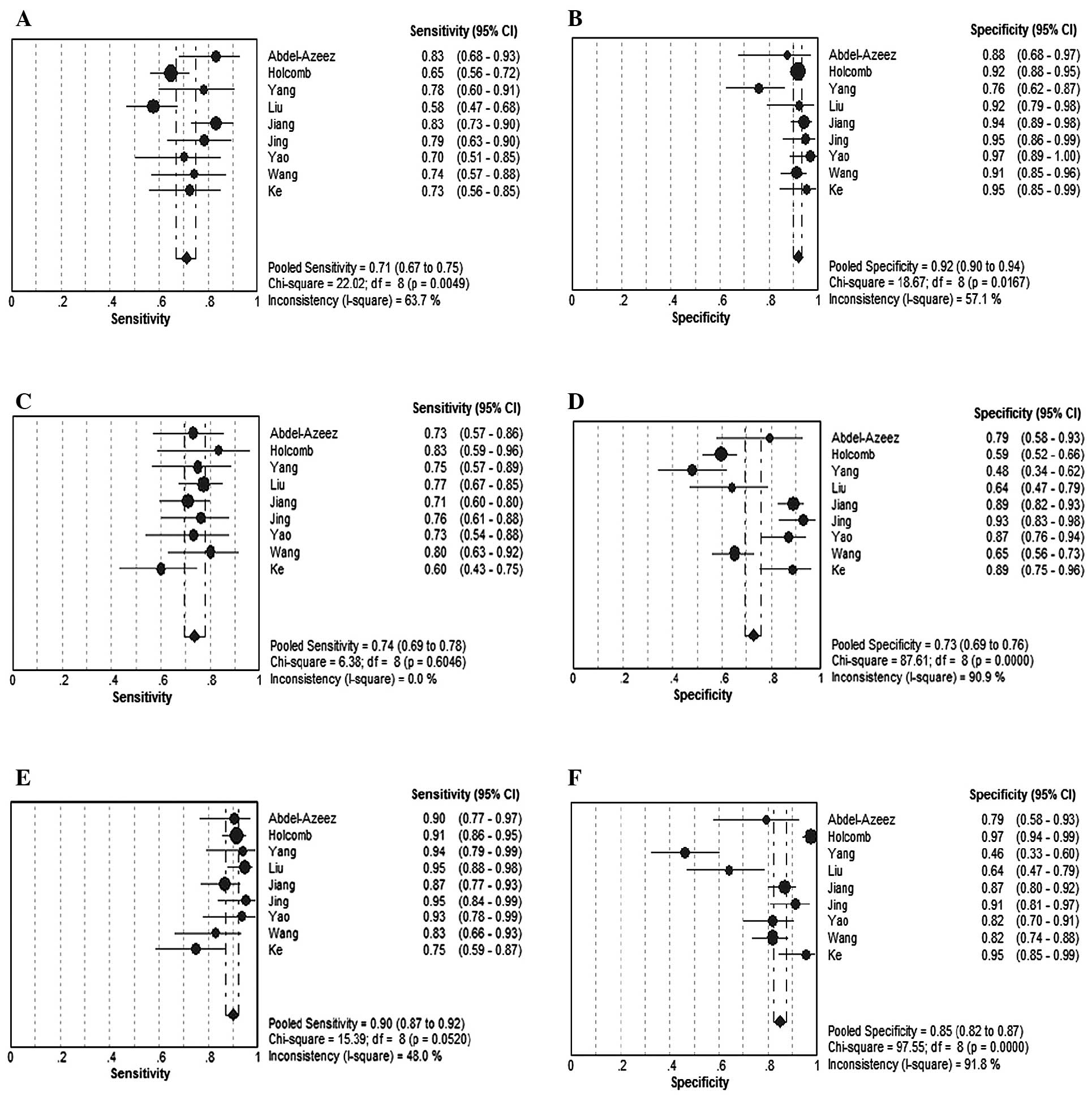
In total, 9 studies investigated the diagnostic
accuracy of HE4 combined with CA125 for the diagnosis of OC. The
data are presented in Fig. 5. The
pooled sensitivity and 95% CIs for HE4, CA125 and HE4+CA125 in this
subgroup were 0.71 (0.67–0.75), 0.74 (0.69–0.78) and 0.90
(0.87–0.92), respectively; the pooled specificity and 95% CIs for
HE4, CA125 and HE4+CA125 were 0.92 (0.90–0.94), 0.73 (0.69–0.76)
and 0.85 (0.82–0.87), respectively. In this subgroup, the
sensitivity of HE4 combined with CA125 was significantly elevated
compared to that of HE4 or CA125 alone (Fig. 6). The pooled DORs and 95% CIs for
HE4, CA125 and HE4+CA125 were 31.83 (19.77–51.26), 10.31
(6.18–17.21) and 53.92 (26.07–111.54), respectively.
Publication bias and heterogeneity
assessment
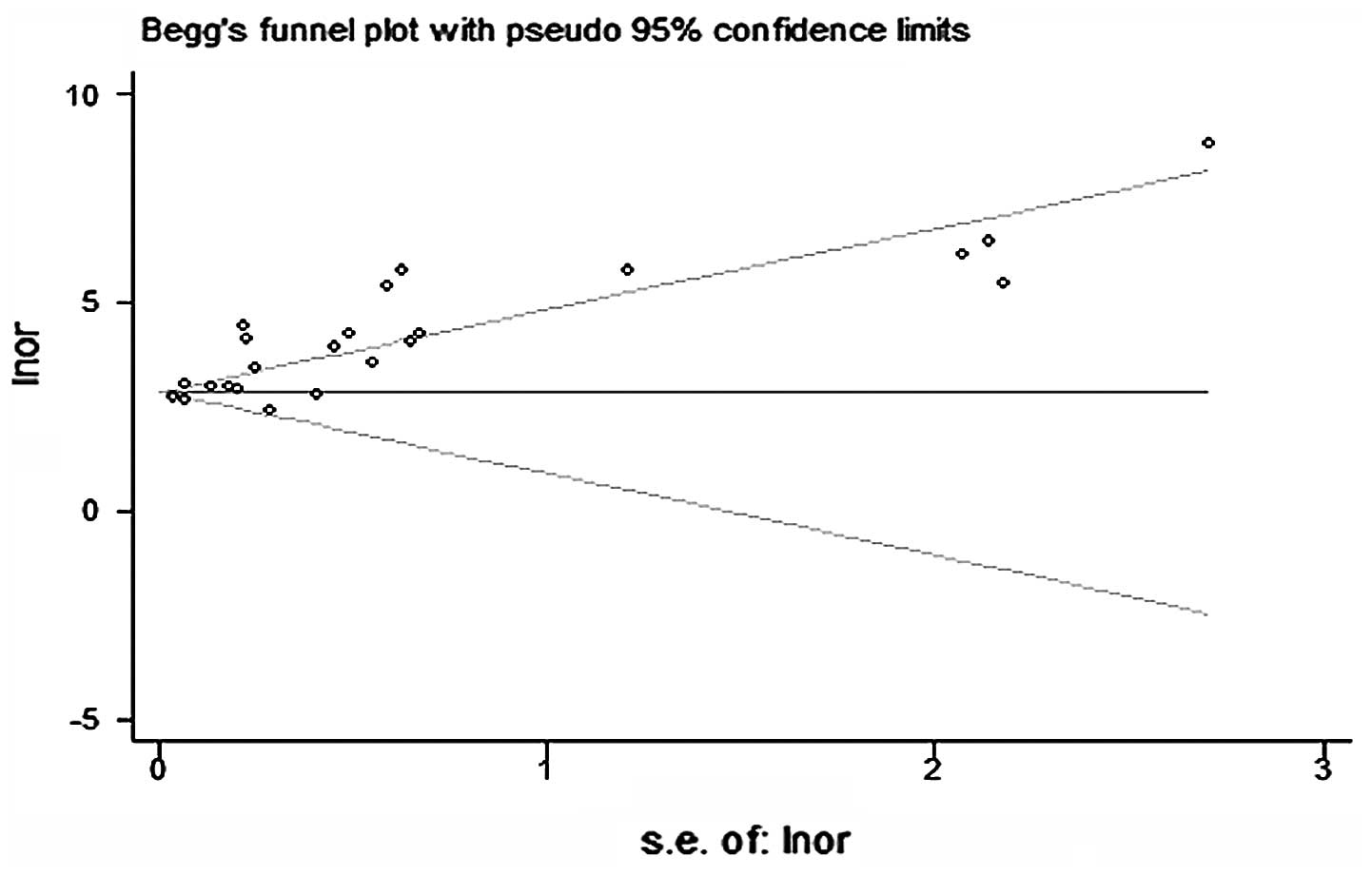
The asymmetry of the funnel plots using Egger’s and
Begg’s tests revealed that there was publication bias among the
included studies (Fig. 7). In
addition, the heterogeneity was significant among the included
studies. A random effects model was used and meta-regression was
used to explain the heterogeneity by investigating the study
characteristics; however, we observed that the differences in race,
cut-off value and study design did not exert a statistically
significant effect on diagnostic accuracy. In total, 22 studies
were filtered (all using ELISA for the HE4 test) and the
I2 of sensitivity for HE4 in these 22 studies was low;
however, the I2 of specificity was not as low as
anticipated. Further studies are required to confirm the role of
cut-off value and race in the diagnostic accuracy for OC.
The asymmetry of the funnel plots using Egger’s and
Begg’s tests demonstrated that there was publication bias among the
included studies.
Discussion
Due to the lack of sensitive and specific screening
methods, OC is the leading cause of gynecological
malignancy-related mortality in the USA and Western Europe. The
main aim for laboratory biomarkers is to accurately detect OC at an
early stage (46). Compared to
transvaginal ultrasound, OC serum markers are more convenient and
cost-effective. HE4, as a novel serum biomarker for OC, has been
reported as the most promising assistant marker in OC diagnosis.
The WFDC2 gene, which encodes the HE4 protein, was confirmed as
being overexpressed in OC but not in normal tissue (7). HE4 has been suggested to have a
diagnostic sensitivity similar to that of CA125 and an increased
diagnostic specificity in patients with gynecological malignancies
(47).
However, the few available meta-analyses evaluating
these diagnostic values are affected by several limitations. In the
study by Yu et al (48), a
healthy population was enrolled as the control group, resulting in
a possible spurious increase in the efficacy of the biomarker
compared to that from a clinically relevant population. Thus, the
evaluation of the diagnostic performance of the combined
measurements of HE4 and CA125 was not considered.
In this study, we assessed the included studies that
compared HE4 with CA125 in the same population. Our results
demonstrated that women with gynecological disease and increased
concentrations of HE4 or CA125 exhibit a higher risk of malignancy.
The summary DOR of HE4 was higher compared to that of CA125 (29.07
vs. 20.99). In particular, the sensitivity of HE4 was higher
compared to that of CA125 (0.74 vs. 0.73), whereas HE4 exhibited a
higher specificity compared to that of CA125 (0.87 vs. 0.84). The
LR calculation confirmed that HE4 outperforms CA125 in identifying
OC (LR+, 6.92 vs. 5.75), whereas the ability to rule out OC was
quite similar for the two markers and rather poor. A subgroup was
established to assess the two factors and we observed that the
sensitivities of the subgroup exhibited significant homogeneity.
These results further support the hypothesis that HE4 and CA125 may
be useful biomarkers for OC diagnosis and HE4 may replace CA125 as
a standalone biochemical test for OC diagnosis. Futhermore, an
increase in sensitivity was achieved by combining HE4 with CA125. A
meta-regression identified cut-off value and race as the major
sources of heterogeneity. CA125 was the only U.S. Food and Drug
Administration (FDA)-approved biomarker for OC prior to 2008 and
HE4 was approved as a marker of epithelial OC by the FDA in 2008;
therefore, more robust estimates of the diagnostic performance of
HE4 are required.
There were certain limitations to our meta-analysis.
First, there are >4,000 studies on the application of CA125 as a
diagnostic tool for OC, whereas only ~50 studies on HE4 have been
published in PubMed, due to its recent identification during
genomic research. Consequently, there are not enough studies to
accurately evaluate the performance of HE4 in this clinical
setting. Second, our study evaluated the performance of HE4
regardless of the menopausal status, as menopausal status is not
marginal, since higher HE4 concentrations are detectable in
postmenopausal women. Therefore, HE4 may be different, as for OC
histological subtypes and OC International Federation of Gynecology
and Obstetrics stages. Third, the results present with a
significant publication bias for HE4 studies and heterogeneity
among retrieved studies. Finally, the definition of specific
clinical thresholds may be required for pre- and postmenopausal
women.
In conclusion, this meta-analysis provided
encouraging preliminary evidence that the measurement of HE4 may be
superior to CA125 regarding diagnostic performance in OC.
Furthermore, a combination of HE4 and CA125 may achieve increased
diagnostic sensitivity and specificity; however, heterogeneity
requires further investigation. Large-scale long-term studies
should be performed to determine the clinical use of HE4 and CA125
as tumor markers for OC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81101957).
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang X, Ye X, Dong L, et al: Human
epididymis protein 4 (HE4) as a serum tumor biomarker in patients
with ovarian carcinoma. Int J Gynecol Cancer. 21:852–858. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fountain J, Trimble E and Birrer MJ:
Summary and discussion of session recommendations. Gynecol Oncol.
103:S23–S25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buamah P: Benign conditions associated
with raised serum CA-125 concentration. J Surg Oncol. 75:264–265.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yurkovetsky Z, Skates S, Lomakin A, et al:
Development of a multimarker assay for early detection of ovarian
cancer. J Clin Oncol. 28:2159–2166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cree IA: Improved blood tests for cancer
screening: general or specific? BMC Cancer. 11:4992011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drapkin R, von Horsten HH, Lin Y, et al:
Human epididymis protein 4 (HE4) is a secreted glycoprotein that is
overexpressed by serous and endometrioid ovarian carcinomas. Cancer
Res. 65:2162–2169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galgano MT, Hampton GM and Frierson HF Jr:
Comprehensive analysis of HE4 expression in normal and malignant
human tissues. Mod Pathol. 19:847–853. 2006.PubMed/NCBI
|
|
9
|
Partheen K, Kristjansdottir B and
Sundfeldt K: Evaluation of ovarian cancer biomarkers HE4 and CA-125
in women presenting with a suspicious cystic ovarian mass. J
Gynecol Oncol. 22:244–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li F, Tie R, Chang K, et al: Does risk for
ovarian malignancy algorithm excel human epididymis protein 4 and
CA125 in predicting epithelial ovarian cancer: a meta-analysis. BMC
Cancer. 12:2582012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: the PRISMA statement. J Clin Epidemiol.
62:1006–10012. 2009. View Article : Google Scholar
|
|
12
|
Stroup DF, Berlin JA, Morton SC, et al:
Meta-analysis of observational studies in epidemiology: a proposal
for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar
|
|
13
|
Whiting PF, Rutjes AW, Westwood ME, et al:
QUADAS-2: a revised tool for the quality assessment of diagnostic
accuracy studies. Ann Intern Med. 155:529–536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bossuyt PM, Reitsma JB, Bruns DE, et al:
Standards for Reporting of Diagnostic Accuracy: Towards complete
and accurate reporting of studies of diagnostic accuracy: the STARD
initiative. Standards for Reporting of Diagnostic Accuracy. Clin
Chem. 49:1–6. 2003. View
Article : Google Scholar
|
|
15
|
Devillé WL, Buntinx F, Bouter LM, et al:
Conducting systematic reviews of diagnostic studies: didactic
guidelines. BMC Med Res Methodol. 2:92002.PubMed/NCBI
|
|
16
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pepe MS, Feng Z, Janes H, et al: Pivotal
evaluation of the accuracy of a biomarker used for classification
or prediction: standards for study design. J Natl Cancer Inst.
100:1432–1438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Glas AS, Lijmer JG, Prins MH, et al: The
diagnostic odds ratio: a single indicator of test performance. J
Clin Epidemiol. 56:1129–1135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walter SD: Properties of the summary
receiver operating characteristic (SROC) curve for diagnostic test
data. Stat Med. 21:1237–1256. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huedo-Medina TB, Sánchez-Meca J,
Marín-Martínez F and Botella J: Assessing heterogeneity in
meta-analysis: Q statistic or I2index? Psychol Methods.
11:193–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dinnes J, Deeks J, Kirby J and Roderick P:
A methodological review of how heterogeneity has been examined in
systematic reviews of diagnostic test accuracy. Health Technol
Assess. 9:1–113. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abdel-Azeez HA, Labib HA, Sharaf SM and
Refai AN: HE4 and mesothelin: novel biomarkers of ovarian carcinoma
in patients with pelvic masses. Asian Pac J Cancer Prev.
11:111–116. 2010.PubMed/NCBI
|
|
23
|
Anastasi E, Marchei GG, Viggiani V, et al:
HE4: a new potential early biomarker for the recurrence of ovarian
cancer. Tumor Biol. 31:113–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andersen MR, Goff BA, Lowe KA, et al: Use
of a Symptom Index, CA125, and HE4 to predict ovarian cancer.
Gynecol Oncol. 116:378–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Gorp T, Cadron I, Despierre E, et al:
HE4 and CA125 as a diagnostic test in ovarian cancer: prospective
validation of the Risk of Ovarian Malignancy Algorithm. Br J
Cancer. 104:863–870. 2011.PubMed/NCBI
|
|
26
|
Holcomb K, Vucetic Z, Miller MC and Knapp
RC: Human epididymis protein 4 offers superior specificity in the
differentiation of benign and malignant adnexal masses in
premenopausal women. Am J Obstet Gynecol. 205:358.e1–358.e6. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jacob F, Meier M, Caduff R, et al: No
benefit from combining HE4 and CA125 as ovarian tumor markers in a
clinical setting. Gynecol Oncol. 121:487–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Montagnana M, Danese E, Ruzzenente O, et
al: The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating
the risk of epithelial ovarian cancer in women presenting with
pelvic mass: is it really useful? Clin Chem Lab Med. 49:521–525.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moore RG, Brown AK, Miller MC, et al: The
use of multiple novel tumor biomarkers for the detection of ovarian
carcinoma in patients with a pelvic mass. Gynecol Oncol.
108:402–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nolen B, Velikokhatnaya L, Marrangoni A,
et al: Serum biomarker panels for the discrimination of benign from
malignant cases in patients with an adnexal mass. Gynecol Oncol.
117:440–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park Y, Kim Y, Lee EY, et al: Reference
ranges for HE4 and CA125 in a large Asian population by automated
assays and diagnostic performances for ovarian cancer. Int J
Cancer. 130:1136–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong L, Cheng XH, Ye X, et al: The values
of serum human epididymis secretory protein 4 and CA(125) assay in
the diagnosis of ovarian malignancy. Chin J Obstet Gynecol.
43:931–936. 2008.(In Chinese).
|
|
33
|
Wu XW, Fu GY, Wang R and Shi XQ:
Significance of using combined assays of serum human epididymis
secretory protein 4, CA125 and ROMA in the diagnosis of ovarian
malignancy and pelvic mass. J Basic Clin Oncol. 5:252012.
|
|
34
|
Yang C, Song ML, Zhong HB, et al: The
differential diagnostic value of HE4, CA125 and the risk of ovarian
malignancy aligorithm in ovarian tumor. Suzhou Univ J Med Sci.
30:42010.
|
|
35
|
Huang S and Zeng Q: The clinical value of
HE4 and CA125 combined with sB7-H4 in early diagnosis of ovarian
cancer. Hebei Med. 17:4432011.(In Chinese).
|
|
36
|
Li Q, Song X, Wu Q, et al: Clinical
application of combined HE4 and CA125 differentiate malignant
ovarian tumors from ovarian endometriotic cysts. Mod Oncol.
21:22013.
|
|
37
|
Liu G, Wang A, Liu Q, et al: Combined
detection of serum CA125 and human epididymis protein 4 levels in
ovarian cancer. Chin J Clin Lab Sci. 28:119–121. 2010.(In
Chinese).
|
|
38
|
Jiang DL, Sun JM, Cai LL, et al: Changes
and clinical significance of serum HE4 in patients with ovarian
cancer. J Pract Med. 26:142010.
|
|
39
|
Jing XG, Wang GJ, Pei YX, et al:
Diagnostic value of combined measurement of CA125, HE4 and imaging
examination patients with epithelial ovarian cancer. J Third Mil
Med Univ. 33:62011.
|
|
40
|
Yao YX and Hong W: Clinical significance
of detecting serum HE4, CA125 and CA724 levels in diagnoses of
ovarian malignancies. Labeled Immunoassays Clin Med. 19:32012.(In
Chinese).
|
|
41
|
Yao YL, Liu Q and Li XY: The diagnostic
values of combined determination of serum tumor markers HE4, TPS
and CA125 levels in patients with ovarian cancer. J Radioimmunol.
23:42010.(In Chinese).
|
|
42
|
Wang KY, Leng JH, Zheng H and Jiang LH:
Studies on value of combination of human epididymis protein 4 and
CA125 in patients with ovarian cancer. Chin J Health Lab Technol.
20:1139–1140. 2010.
|
|
43
|
Li ZJ, Zheng YQ and Xu XF: Clinic value of
HE4, CA125 combined with risk of ovarian malignancy algorithm
(ROMA) in the diagnosis for ovarian cancer. J Chin Oncol.
19:219–222. 2013.
|
|
44
|
Ke and Liu F: Serum HE4 and CA125 in the
diagnosis of ovarian cancer. Mod Hosp. 10:52010.
|
|
45
|
Lin YY, Chen Y, Hu MH, et al: Significance
of HE4 detection for diagnosis of ovarian cancer as compared with
CA125 in 69 cases. Curr Immun. 33:12013.
|
|
46
|
Moore RG and Bast RC Jr: How do you
distinguish a malignant pelvic mass from a benign pelvic mass?
Imaging, biomarkers, or none of the above. J Clin Oncol.
25:4159–4161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin J, Qin J and Sangvatanakul V: Human
epididymis protein 4 for differential diagnosis between benign
gynecologic disease and ovarian cancer: a systematic review and
meta-analysis. Eur J Obstet Gynecol Reprod Biol. 167:81–85. 2013.
View Article : Google Scholar
|
|
48
|
Yu S, Yang HJ, Xie SQ and Bao YX:
Diagnostic value of HE4 for ovarian cancer: a meta-analysis. Clin
Chem Lab Med. 50:1439–1446. 2012.PubMed/NCBI
|