Spandidos Publications style
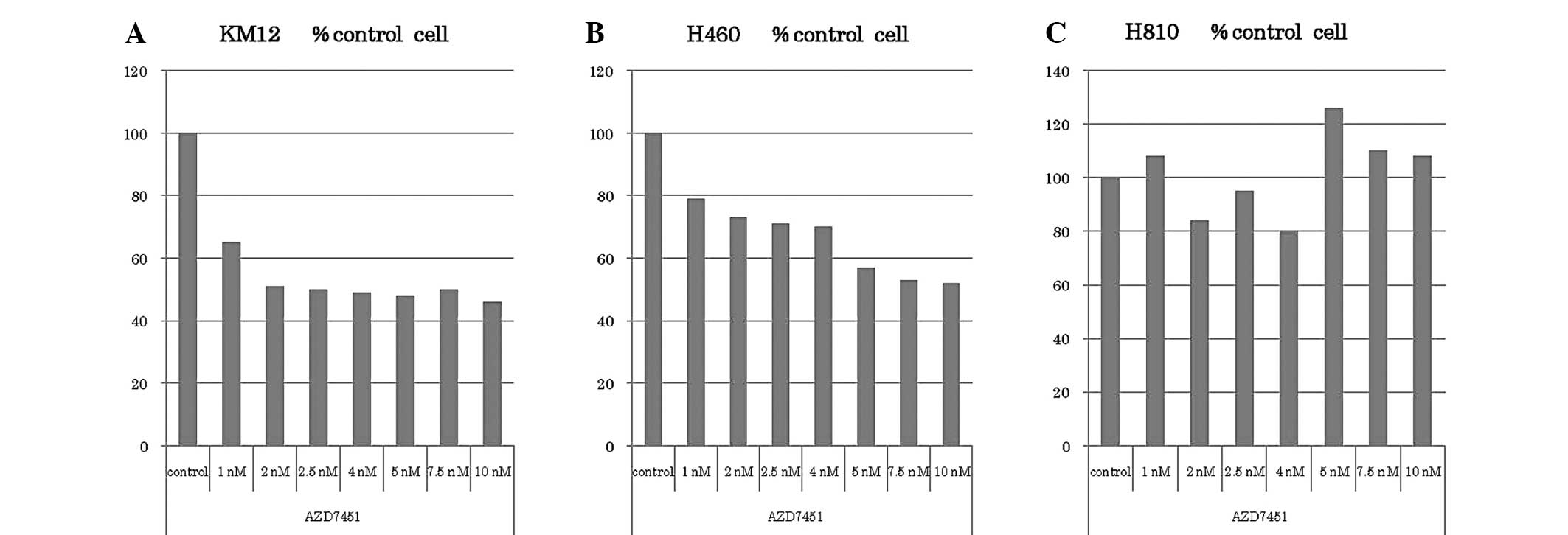
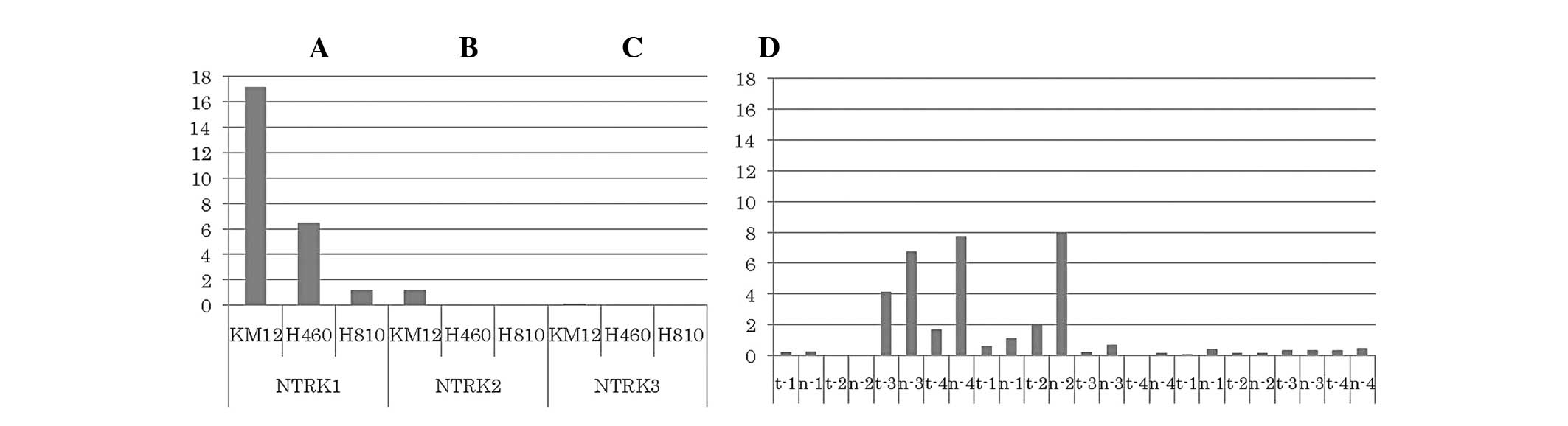
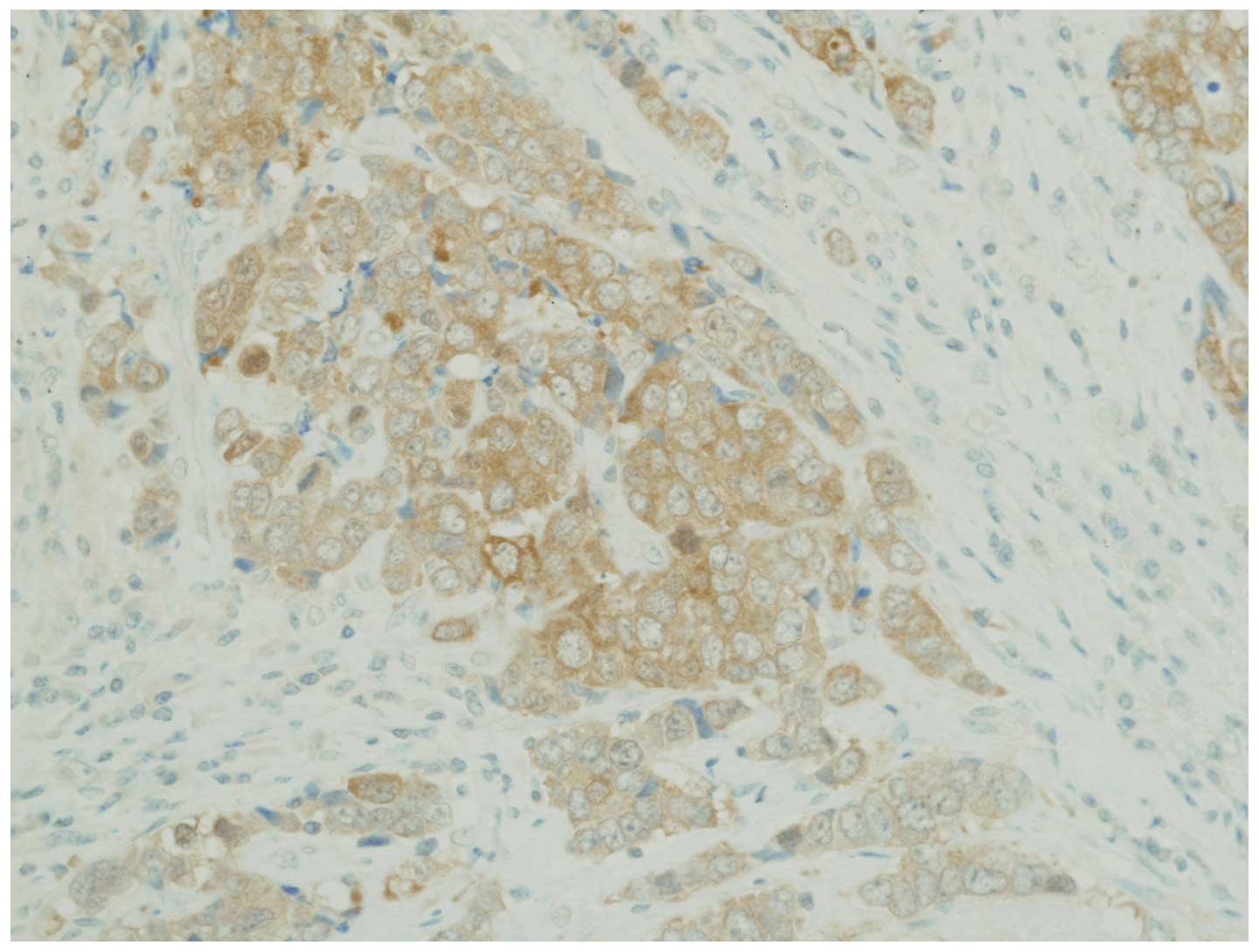
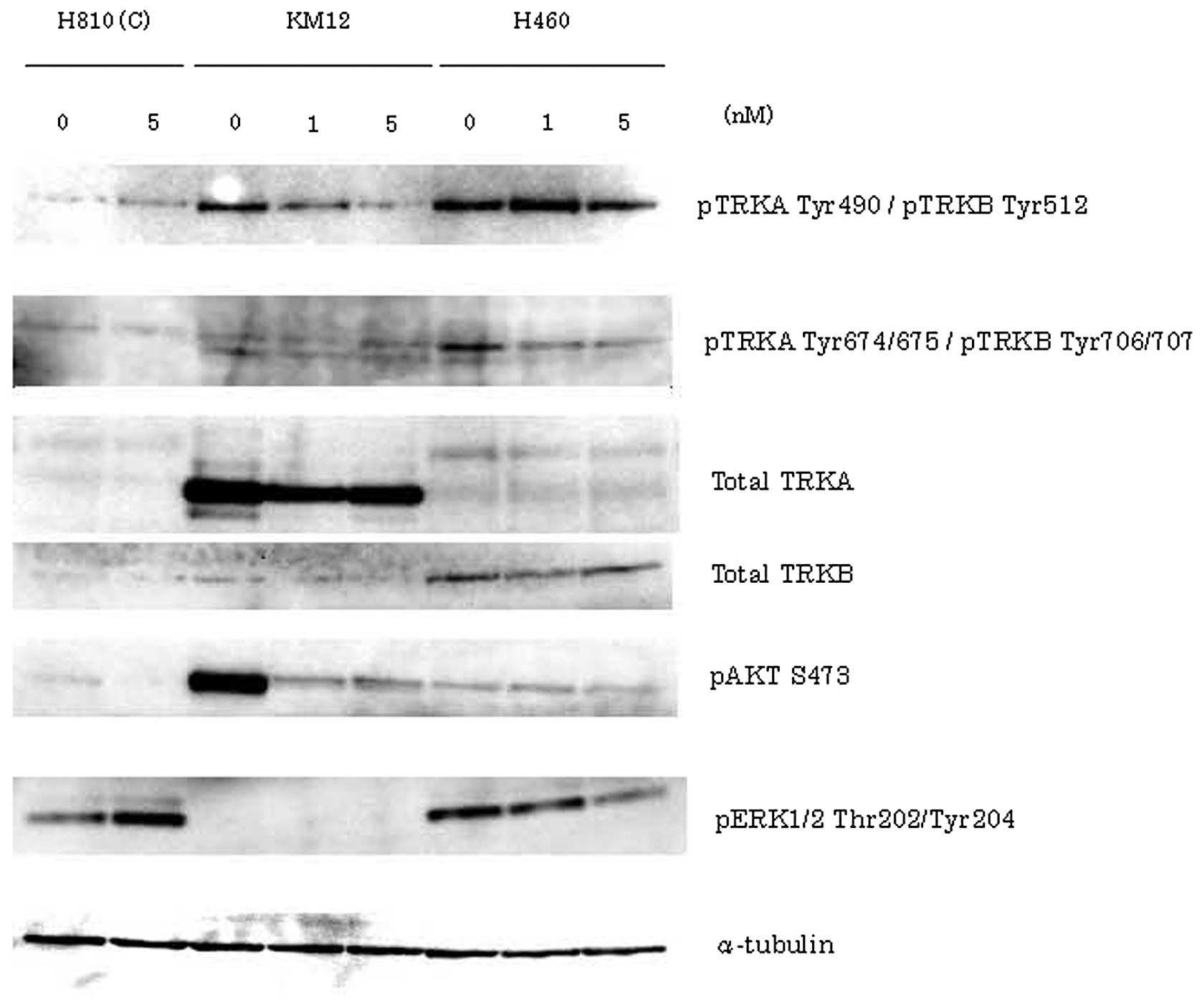
Tatematsu T, Sasaki H, Shimizu S, Okuda K, Shitara M, Hikosaka Y, Moriyama S, Yano M, Brown J, Fujii Y, Fujii Y, et al: Investigation of neurotrophic tyrosine kinase receptor 1 fusions and neurotrophic tyrosine kinase receptor family expression in non‑small‑cell lung cancer and sensitivity to AZD7451 in vitro. Mol Clin Oncol 2: 725-730, 2014.
APA
Tatematsu, T., Sasaki, H., Shimizu, S., Okuda, K., Shitara, M., Hikosaka, Y. ... Fujii, Y. (2014). Investigation of neurotrophic tyrosine kinase receptor 1 fusions and neurotrophic tyrosine kinase receptor family expression in non‑small‑cell lung cancer and sensitivity to AZD7451 in vitro. Molecular and Clinical Oncology, 2, 725-730. https://doi.org/10.3892/mco.2014.318
MLA
Tatematsu, T., Sasaki, H., Shimizu, S., Okuda, K., Shitara, M., Hikosaka, Y., Moriyama, S., Yano, M., Brown, J., Fujii, Y."Investigation of neurotrophic tyrosine kinase receptor 1 fusions and neurotrophic tyrosine kinase receptor family expression in non‑small‑cell lung cancer and sensitivity to AZD7451 in vitro". Molecular and Clinical Oncology 2.5 (2014): 725-730.
Chicago
Tatematsu, T., Sasaki, H., Shimizu, S., Okuda, K., Shitara, M., Hikosaka, Y., Moriyama, S., Yano, M., Brown, J., Fujii, Y."Investigation of neurotrophic tyrosine kinase receptor 1 fusions and neurotrophic tyrosine kinase receptor family expression in non‑small‑cell lung cancer and sensitivity to AZD7451 in vitro". Molecular and Clinical Oncology 2, no. 5 (2014): 725-730. https://doi.org/10.3892/mco.2014.318