Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies worldwide. The incidence of HCC has increased
in Eastern Asia and Africa over the last several decades and has
also increased in the United States (1). In several countries, this trend is
attributed to hepatitis C virus (HCV) infection and in Japan,
>70% of HCC cases are associated with chronic liver disease with
HCV infection (2). Recent advances
in imaging procedures and surveillance programs for high-risk
patients have led to increased detection of early-stage HCC,
resulting in an increase in the identification of patients in whom
curative treatments, such as hepatic resection and radiofrequency
ablation, may be feasible (3).
However, as HCC frequently recurs even following curative
treatment, prevention of recurrence is required to prolong the
survival of HCC patients with HCV infection. For the prevention of
HCC recurrence following curative treatment, adjuvant interferon
therapy is known to be highly effective and is generally used in
patients with HCV infection (4,5).
However, due to the high rate of non-response and severe adverse
effects, interferon therapy is not universally used in HCV
patients, particularly those who are older or who have a high viral
load, genotype 1 and/or severe fibrosis (6,7).
Therefore, it is crucial to investigate other adjuvant therapies
for the prevention of HCC recurrence following curative treatment
in patients with HCV infection.
In chronic liver disease with HCV infection, the
prevalence of type 2 diabetes mellitus (DM) has been reported to be
higher compared to that associated with other chronic liver
diseases, including hepatitis B virus infection (8). To explain this strong association, in
addition to obesity, hepatic inflammation and fibrosis, Kawaguchi
et al (9) and Shintani
et al (10) suggested that
HCV directly causes hepatic insulin resistance and subsequent
hyperinsulinemia. Moreover, DM with insulin resistance was found to
be a potential risk factor for the development of HCC, as well as
for the recurrence of HCC in patients with HCV infection (11,12).
Thus, these findings led us to hypothesize that the treatment of
type 2 DM associated with insulin resistance significantly affects
the development of HCC in patients with HCV infection.
Pioglitazone is a member of the thiazolidinedione
family and is widely used for the treatment of type 2 DM.
Pioglitazone reduces insulin resistance in the liver and peripheral
tissues by stimulating the peroxisome proliferator-activated
receptor (PPAR)-γ and improves macrovascular outcomes (13). Previous studies reported that
pioglitazone improves insulin resistance in patients with HCV
infection treated with peginterferon and ribavirin (14,15).
In addition, pioglitazone itself was shown to exert
anticarcinogenic activity through the inhibition of DNA synthesis
and cell cycle progression in HCC cell lines and in an animal model
of HCC (16,17). Moreover, pioglitazone was recently
reported to suppress the onset of HCC in a hospital-based
case-control study (18) and a
population-based cohort study (19). Therefore, the aim of this study was
to determine whether pioglitazone decreases the risk of HCC
recurrence following curative treatment in patients with HCV
infection. We also investigated the effect of pioglitazone on type
2 DM due to insulin resistance.
Patients and methods
Patients
This clinical trial was conducted at the Kurume
University School of Medicine. A total of 85 patients who met the
inclusion criteria were enrolled between 2009 and 2011. The
diagnosis of HCC was histologically confirmed by needle biopsy or
based on the findings of typical radiological characteristics on
dynamic computed tomography (CT) and magnetic resonance imaging
(MRI). Pretreatment hepatic function was evaluated using the
Child-Pugh scoring system. The inclusion criteria were i) HCV
infection, ii) diagnosis of HCC with ≤3 tumors, each ≤3 cm, by
imaging studies and iii) HCC treated with curative treatment
(radiofrequency ablation or resection). The exclusion criteria were
i) severe gastrointestinal stasis, ii) severe renal injury
(creatinine >2.0 mg/dl), iii) severe esophageal and/or gastric
varices, iv) HCC with macroscopic vascular invasion or extrahepatic
metastasis, v) poorly differentiated HCC, vi) heart failure, vii)
liver cirrhosis of Child-Pugh grade C and viii) type 1 DM.
The study protocol was approved by the Ethics
Committee of Kurume University and conformed to the guidelines of
the 1975 Declaration of Helsinki. Written informed consent was
obtained from each subject prior to enrolment. This study has been
registered in the University Hospital Medical Information Network
(UMIN) Clinical Trials Registry under the registration number
UMIN000007344.
Study design and pioglitazone
treatment protocol
Patients who met the inclusion criteria were
prospectively enrolled and were first evaluated for the presence of
type 2 DM. Patients who did not have type 2 DM were assigned to the
control group (no treatment; n=40). For patients with type 2 DM, we
additionally confirmed whether they wished to receive pioglitazone.
Patients who declined administration of pioglitazone were assigned
to the control group (n=18) and patients who consented to receiving
pioglitazone were assigned to the pioglitazone treatment group
(n=27). All the patients in the treatment group were administered
pioglitazone at an initial dose of 30 mg/day following curative
treatment. Treatment discontinuation and dose reduction were based
on toxicity. In cases with symptomatic heart failure, pioglitazone
was permanently discontinued without dose reduction, regardless of
the severity. In cases with facial and/or lower limb edema with
functional impairment or symptomatic ascites, the patients were
first treated with diuretics, such as spironolactone and
furosemide. If symptomatic edema or ascites did not improve despite
the administration of diuretics, pioglitazone was reduced to a dose
of 15 mg/day or interrupted until the symptoms disappeared. When
weight gain ≥5% of baseline occurred, pioglitazone was reduced to a
dose of 15 mg/day. To evaluate the tolerability to pioglitazone,
all the adverse events were recorded.
Diagnosis of type 2 DM and use of
antidiabetic agents
Type 2 DM was classified according to the 2010
American Diabetes Association criteria (20). Patients with fasting plasma glucose
(FPG) ≥126 mg/dl, 2-h plasma glucose ≥200 mg/dl during an oral
glucose tolerance test, or a random plasma glucose ≥200 mg/dl, were
considered to have type 2 DM. Of the 85 patients included in this
study, 45 were diagnosed with type 2 DM. Of the 45 patients with
type 2 DM, no patients had been treated with pioglitazone prior to
study enrolment and 24 patients were prescribed other antidiabetic
agents, including biguanides (n=2), α-glucosidase inhibitors
(n=11), sulfonylureas (n=13) and insulin injections (n=5).
Evaluation of type 2 DM control
In 27 of the 45 patients with type 2 DM,
pioglitazone was administered following curative treatment. To
evaluate the treatment effects of pioglitazone, body mass index
(BMI), FPG, serum insulin level fasting immunoreactive insulin
(FIRI), glycated hemoglobin (HbA1c) level and homeostasis model
assessment for insulin resistance (HOMA-IR) values were measured at
baseline and after 1–3 months. HOMA-IR values were calculated using
the formula FPG (mg/dl) × FIRI (mU/l)/405. In addition, serum
low-molecular-weight (LMW), middle-molecular-weight (MMW),
high-molecular-weight (HMW) and total adiponectin levels were
measured at baseline and after 1–3 months by enzyme-linked
immunosorbent assay (ELISA) using the Human Adiponectin kit for
Total and Multimers (Sekisui Medical Co., Ltd., Tokyo, Japan).
Follow-up and assessment of
recurrence
Following curative treatment, each patient was
closely followed up on a monthly basis. Serum biochemistry tests,
α-fetoprotein (AFP) levels and des-γ-carboxy prothrombin (DCP)
levels were measured and ultrasonography was performed monthly.
Contrast-enhanced dynamic CT was performed every 3 months until 6
months post-treatment and every 6 months thereafter. MRI was
performed as a supplemental examination. Recurrence was diagnosed
based on the combined findings of these assessments (characteristic
appearances typical of HCC). The primary endpoint was
recurrence-free survival, which was defined as the time interval
between study entry and the first recurrence of HCC. Patients who
survived without HCC recurrence were censored at the closing date
of this study, which was December, 2011. The median duration of
follow-up was 521 days (range, 100–961 days).
Statistical analysis
Continuous variables are expressed as median (range)
values. Comparisons between the two groups were performed using the
Mann-Whitney U test for continuous variables and the Chi-square or
Fisher's exact tests for discrete variables. Pioglitazone-induced
metabolic changes were examined by the Wilcoxon's signed-rank test.
Recurrence-free survival was measured by the Kaplan-Meier method
and differences between subgroups were compared with log-rank
tests. To investigate treatment efficacy in preventing HCC
recurrence in the 45 patients with type 2 DM, we applied the Cox
proportional hazards model, including not only the main terms BMI
and group (pioglitazone or control) and their interaction term, but
also knot terms for BMI that serve as inflection points. The Akaike
information criterion (AIC) was used to evaluate these alternate
models (21). The location of
knots was determined objectively, with the minimum AIC among their
prespecified candidates, which were whole-number BMI values from 15
to 30. This model is referred to as a spline model, which we have
previously described in detail (22,23).
Briefly, if the interaction term is selected, the hazard ratio (HR)
between the pioglitazone and control groups has a functional form
of BMI as the output of this model. All the P-values were
two-tailed and P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
SPSS software, version 20 (IBM Corp., Somers, NY, USA) and the R
package version 3.0.1 (http://www.r-project.org/index.html).
Results
Patient characteristics
Patient characteristics are shown in Table I. The baseline characteristics of
the two groups, including age, gender, BMI, aspartate
aminotransferase level, alanine aminotransferase level, platelet
count and Child-Pugh grade, were comparable, with no significant
differences between groups. In addition, no significant differences
in tumor characteristics, including AFP level, DCP level, tumor
size, or tumor number, were observed. All the patients in the
pioglitazone group and 18 (32.0%) patients in the control group had
type 2 DM (P<0.001).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Pioglitazone group
(n=27) | Control group
(n=58) | P-value |
|---|
| Age, years
(range) | 72 (53–84) | 70 (50–86) | 0.832 |
| Gender
(male/female) | 17/10 | 35/23 | 0.818 |
| BMI
(kg/m2) | 22.9
(20.1–30.7) | 23.0
(15.2–29.3) | 0.401 |
| AST (U/1) | 53 (23–137) | 52 (17–118) | 0.257 |
| ALT (U/1) | 44 (18–136) | 45 (14–240) | 0.781 |
| Platelet count
(x109/1) | 107 (46–185) | 111 (37–281) | 0.431 |
| Child-Pugh grade
(A/B) | 22/5 | 45/13 | 0.682 |
| Type 2 diabetes
mellitus (present/absent) | 27/0 | 18/40 | <0.001 |
| FPG (mg/dl) | 117 (89–200) | 96 (76–185) | |
| FIRI (µU/ml) | 11.4
(3.2–31.2) | 10.6
(4.0–30.9) | |
| HOMA-IR | 3.3 (1.1–10.1) | 2.6 (0.9–7.7) | |
| HbA1c (%) | 6.1 (4.7–8.0) | 5.3 (4.5–8.6) | |
| AFP (ng/ml) | 14.8
(2.3–282.1) | 13.4
(2.4–710.4) | 0.393 |
| DCP (mAU/ml) | 21 (11–8,220) | 25 (8–486) | 0.688 |
| Tumor size
(mm) | 16 (12–26) | 17 (10–29) | 0.401 |
| Tumor number
(single/2–3) | 20/7 | 37/21 | 0.348 |
Effect of pioglitazone on prevention of
recurrence
Whole analysis
The recurrence-free survival curves of the 85
patients (whole analysis) are shown in Fig. 1A. The recurrence-free survival
rates at 1 and 2 years were 84.6 and 44.2% respectively, in the
pioglitazone group and 71.9 and 59.1% respectively, in the control
group; none of these differences were statistically significant
(P=0.9089).
Subanalysis of patients with type 2
DM
Log-rank test
The 45 patients with type 2 DM were evaluated in a
subanalysis. The recurrence-free survival curves of these patients
are shown in Fig. 1B. The
recurrence-free survival rates at 1 and 2 years were 84.6 and
44.2%, respectively, in the pioglitazone group and 61.1 and 36.5%,
respectively, in the control group; none of these differences were
statistically significant (P=0.3735).
Spline model analysis
The efficacy of pioglitazone in preventing HCC
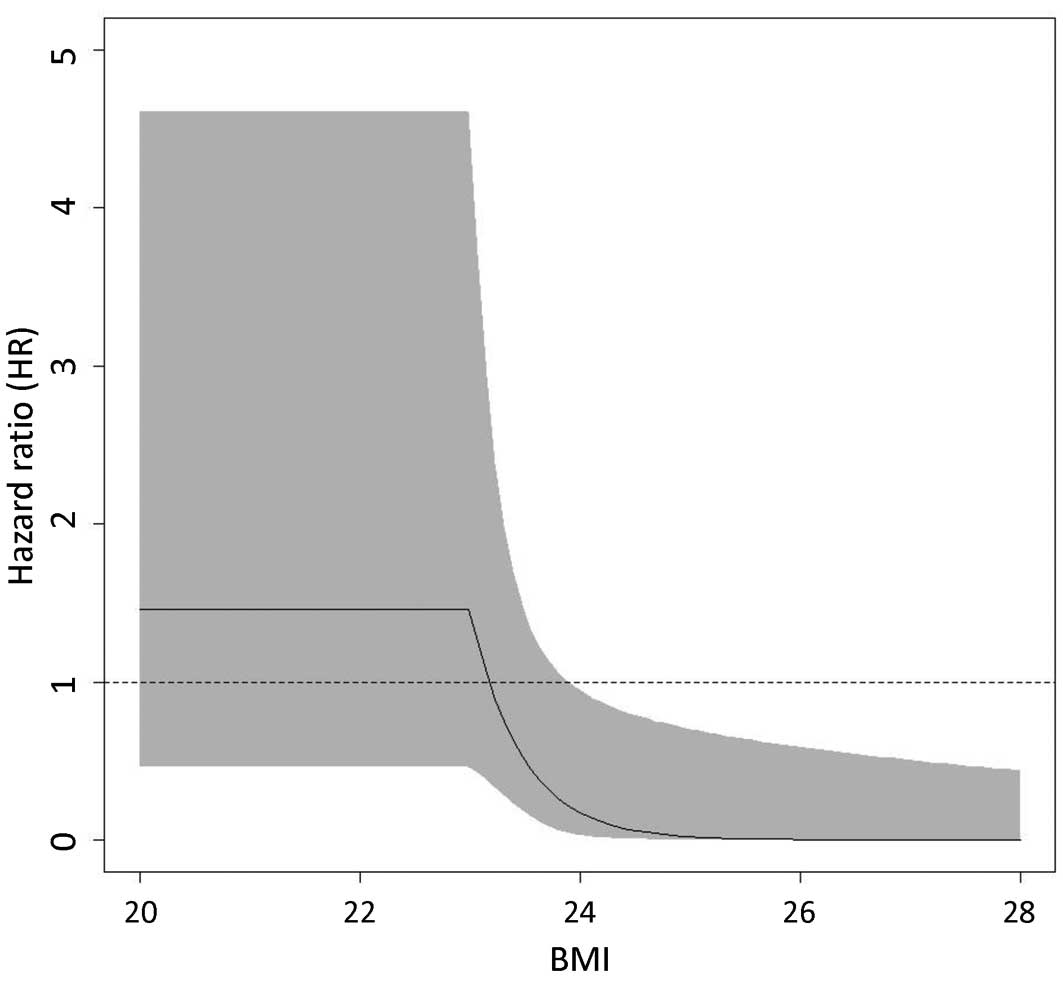
recurrence was evaluated by a spline model. Fig. 2 shows the association between BMI
and HRs with 95% confidence intervals (CIs) for HCC recurrence in
the 45 patients with type 2 DM. In the Cox proportional hazards
model, BMI=23 was selected as the knot location (i.e., interaction
term with group) and indicated a significant dependence of the HR
between the pioglitazone and control groups for BMI ≥23. A
significantly decreased risk of HCC recurrence was observed in the
pioglitazone group for patients with a BMI ≥24 (HR=0.17; 95% CI:
0.03–0.95).
Effect of pioglitazone on glucose
metabolism and adiponectin levels
The changes in the metabolic variables in the 27
patients treated with pioglitazone are listed in Table II. BMI was significantly increased
following pioglitazone treatment. By contrast, a significant
improvement was observed in the glycemic variables, including FPG,
FIRI, HOMA-IR and HbA1c. In addition, serum total, LMW, MMW and HMW
adiponectin levels were significantly increased following
pioglitazone treatment.
 | Table IIChanges in metabolic variables in 27
patients treated with pioglitazone. |
Table II
Changes in metabolic variables in 27
patients treated with pioglitazone.
| Pioglitazone
treatment | |
|---|
|
| |
|---|
| Variables | Before | After | P-value |
|---|
| Body weight
(kg) |
59.8
(39.6–83.1) |
60.3
(41.3–86.2) |
0.012 |
| FPG (mg/dl) |
117.5
(89.0–200.0) |
110.0
(80.0–159.0) |
0.007 |
| FIRI (µU/ml) |
11.3
(3.2–31.2) |
9.3
(1.8–27.7) |
0.006 |
| HOMA-IR |
3.3 (1.1–10.0) |
2.6
(0.6–7.1) |
0.002 |
| HbA1c (%) |
6.1
(4.7–8.0) |
5.6
(4.4–7.1) |
0.002 |
| Total adiponectin
(µg/ml) |
8.8
(1.5–49.3) |
26.8
(3.1–60.7) |
<0.001 |
| HMW adiponectin
(µg/ml) |
4.3
(0.2–32.4) |
17.3
(0.9–39.1) |
<0.001 |
| MMW adiponectin
(µg/ml) |
1.6
(0.4–9.1) |
4.1
(0.9–12.6) |
<0.001 |
| LMW adiponectin
(µg/ml) |
3.7
(0.9–7.8) |
5.6
(1.4–13.0) |
<0.001 |
Adverse events
Of the 27 patients treated with pioglitazone, 18
(66.7%) experienced ≥1 adverse event. The most common adverse
events were edema and subsequent weight gain, which occurred in 14
(51.8%) and 8 (29.6%) patients, respectively. These events were
largely controlled by dose reduction of pioglitazone and diuretic
treatment; however, 6 patients discontinued pioglitazone due to
uncontrollable edema which inerfere with the activities of daily
living. The median serum albumin levels in patients with and
without edema were 3.4 and 3.9 mg/dl, respectively (P<0.016).
Moreover, the serum albumin level in all patients with edema was
<4.0 mg/dl. Other mild adverse events included increased
appetite and ascites in 3 (11.1%) and 2 (7.4%) patients,
respectively. Two patients developed hypoglycemia, possibly caused
by combination of insulin injections; this was controlled by
reducing the insulin injections. Severe adverse events included
acute heart failure in 1 patient, who discontinued pioglitazone ~6
months after treatment initiation. No patients developed liver
dysfunction or gastrointestinal symptoms.
Discussion
In the whole analysis of this study, no significant
differences in HCC recurrence-free survival rates were observed
between the control and pioglitazone groups. However, a spline
model analysis revealed that pioglitazone significantly decreased
the risk of HCC recurrence in HCV-infected diabetic patients with a
BMI ≥24. Moreover, pioglitazone treatment decreased HOMA-IR values
and increased serum multimeric adiponectin levels in HCV-infected
diabetic patients. Thus, pioglitazone may inhibit HCC recurrence
through the regulation of insulin resistance and adipocytokine
levels in HCV-infected diabetic patients who are overweight.
Pioglitazone, a PPAR-γ agonist, inhibits DNA
synthesis and cell cycle progression, resulting in anticarcinogenic
activity in HCC cell lines and in an animal model of HCC (16,17).
Pioglitazone was recently reported to suppress the onset of HCC in
a hospital-based case-control study (18) and a population-based cohort study
(19). These findings suggest that
pioglitazone may also exert inhibitory effects on HCC recurrence.
However, the present results revealed no significant differences in
HCC recurrence-free survival rates between the control and
pioglitazone groups in the whole analysis. Although the reason for
the discrepancy between previous studies and the present findings
remains unclear, the proportions of patients with HCV infection may
be responsible for the differences between these results. All the
patients included in our study were HCV-positive, whereas the
majority of patients in the previous studies were not infected with
HCV. HCC frequently recurs due to various factors, including
hepatic inflammation, hepatic fibrosis, HCV proteins, aging and
therapeutic procedures in patients with HCV infection (24). Thus, pioglitazone may not have been
sufficiently effective to universally suppress HCC recurrence in
the present study.
We also performed a subanalysis of patients with
type 2 DM. A log-rank test revealed no significant difference in
HCC recurrence-free survival rates between the control and
pioglitazone groups. However, the use of a Cox proportional hazards
model, referred to as a spline model (22,23),
revealed that the HR decreased exponentially in association with
increased body weight starting at BMI=23 and this association
became statistically significant at BMI ≥24. These findings
revealed that being overweight is a key factor for
pioglitazone-induced suppression of HCC recurrence. The overweight
state causes insulin resistance and subsequent hyperinsulinemia
(25). Since insulin is a
growth-promoting hormone for HCC (26), we hypothesize that pioglitazone
suppresses HCC recurrence through improvement of insulin
resistance. In support of this hypothesis, we also demonstrated
that pioglitazone significantly decreased HOMA-IR values, as well
as serum insulin levels. An increased HOMA-IR value is a known risk
factor for HCC recurrence (12),
as is the use of antidiabetic agents that cause hyperinsulinemia,
such as exogenous insulin and second-generation sulfonylurea agents
(27), which further supports our
hypothesis. Another mechanism underlying the development of HCC in
overweight patients may be that overweight individuals exhibit
increased expression of the pro-inflammatory cytokines tumor
necrosis factor-α and interleukin-6 in adipose tissue, thereby
causing hepatic inflammation and subsequent development of HCC
(28). These cytokines have also
been demonstrated to be positively associated with the development
of HCC in patients with HCV infection (29). Consequently, pioglitazone treatment
may also suppress HCC recurrence through the downregulation of
these cytokines in adipose tissue.
In this study, we also demonstrated that
pioglitazone significantly increased multimeric adiponectin levels
in HCV-infected diabetic patients. Adiponectin is an adipocytokine
that is inversely correlated with the amount of visceral fat
accumulation, another important risk factor for HCC (30). Adiponectin not only increases
insulin sensitivity, but was also shown to increase the levels of
the tumor suppressor tuberous sclerosis, resulting in a significant
reduction of liver tumorigenesis in an animal HCC model (31). We previously demonstrated that low
serum adiponectin levels are associated with the development of HCC
(32). Taken together, these
findings suggest that the pioglitazone-induced upregulation of
adiponectin may have contributed to the suppression of
hepatocarcinogenesis observed in the present study. Moreover, lower
adiponectin levels were recently reported to be associated with the
malignant potential of HCC and poor prognosis in HCC patients
(31,33). Further studies should focus on the
effects of pioglitazone on the overall survival of HCC
patients.
The most common adverse events in this study were
edema and subsequent weight gain. In particular, severe lower limb
edema may compromise the quality of life of the patients.
Thiazolidinedione-induced edema has been shown to occur through
enhanced sodium-coupled bicarbonate absorption from the renal
proximal tubules via PPAR-γ-dependent non-genomic signaling
(34). In this study, patients
with low serum albumin levels frequently experienced edema. Thus,
physicians must be on the lookout for the development of
pioglitazone-induced edema in patients with advanced liver disease.
Although no patients in this study developed severe liver
dysfunction, troglitazone, a first-generation thiazolidinedione, is
known to cause severe liver failure (35). Troglitazone-induced changes in the
hepatic molecular profiles differ from those caused by pioglitazone
(36). However,
thiazolidinedione-induced liver injury may occur through an
idiosyncratic mechanism (37). In
fact, fatal liver failure associated with pioglitazone has been
previously reported (38).
Therefore, we should also be aware of pioglitazone-induced liver
injury in chronic liver disease with HCV infection.
In conclusion, the results of the present study
demonstrated that pioglitazone did not suppress HCC recurrence in
all patients. However, a spline model analysis revealed that
pioglitazone significantly decreased the risk of HCC recurrence in
HCV-infected diabetic patients with a BMI ≥24. Moreover,
pioglitazone decreased HOMA-IR values and increased serum
multimeric adiponectin levels in HCV-infected diabetic patients.
Thus, although pioglitazone does not appear to suppress HCC
recurrence universally, it may exert an inhibitory effect on HCC
recurrence in overweight HCV-infected diabetic patients, possibly
through the regulation of insulin resistance and adipocytokine
levels.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kiyosawa K, Umemura T, Ichijo T, et al:
Hepatocellular carcinoma: recent trends in Japan. Gastroenterology.
127 (Suppl 1):S17–S26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noda I, Kitamoto M, Nakahara H, et al:
Regular surveillance by imaging for early detection and better
prognosis of hepatocellular carcinoma in patients infected with
hepatitis C virus. J Gastroenterol. 45:105–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shiratori Y, Shiina S, Teratani T, et al:
Interferon therapy after tumor ablation improves prognosis in
patients with hepatocellular carcinoma associated with hepatitis C
virus. Ann Intern Med. 138:299–306. 2003. View Article : Google Scholar
|
|
5
|
Mazzaferro V, Romito R, Schiavo M, et al:
Prevention of hepatocellular carcinoma recurrence with
alpha-interferon after liver resection in HCV cirrhosis.
Hepatology. 44:1543–1554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iwasaki Y, Ikeda H, Araki Y, et al:
Limitation of combination therapy of interferon and ribavirin for
older patients with chronic hepatitis C. Hepatology. 43:54–63.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernandez-Rodriguez CM, Alonso S, Martinez
SM, et al: Peginterferon plus ribavirin and sustained virological
response in HCV-related cirrhosis: outcomes and factors predicting
response. Am J Gastroenterol. 105:2164–2173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mason AL, Lau JY, Hoang N, et al:
Association of diabetes mellitus and chronic hepatitis C virus
infection. Hepatology. 29:328–333. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawaguchi T, Yoshida T, Harada M, et al:
Hepatitis C virus down-regulates insulin receptor substrates 1 and
2 through up-regulation of suppressor of cytokine signaling 3. Am J
Pathol. 165:1499–1508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shintani Y, Fujie H, Miyoshi H, et al:
Hepatitis C virus infection and diabetes: direct involvement of the
virus in the development of insulin resistance. Gastroenterology.
126:840–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen CL, Yang HI, Yang WS, et al:
Metabolic factors and risk of hepatocellular carcinoma by chronic
hepatitis B/C infection: a follow-up study in Taiwan.
Gastroenterology. 135:111–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imai K, Takai K, Nishigaki Y, et al:
Insulin resistance raises the risk for recurrence of stage I
hepatocellular carcinoma after curative radiofrequency ablation in
hepatitis C virus-positive patients: a prospective, case series
study. Hepatol Res. 40:376–382. 2010. View Article : Google Scholar
|
|
13
|
Miyazaki Y, Mahankali A, Matsuda M, et al:
Improved glycemic control and enhanced insulin sensitivity in type
2 diabetic subjects treated with pioglitazone. Diabetes Care.
24:710–719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khattab M, Emad M, Abdelaleem A, et al:
Pioglitazone improves virological response to peginterferon
alpha-2b/ribavirin combination therapy in hepatitis C genotype 4
patients with insulin resistance. Liver Int. 30:447–454. 2010.
View Article : Google Scholar
|
|
15
|
Harrison SA, Hamzeh FM, Han J, Pandya PK,
Sheikh MY and Vierling JM: Chronic hepatitis C genotype 1 patients
with insulin resistance treated with pioglitazone and peginterferon
alpha-2a plus ribavirin. Hepatology. 56:464–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rumi MA, Sato H, Ishihara S, et al:
Peroxisome proliferator-activated receptor gamma ligand-induced
growth inhibition of human hepatocellular carcinoma. Br J Cancer.
84:1640–1647. 2001. View Article : Google Scholar
|
|
17
|
Borbath I, Leclercq I, Moulin P, Sempoux C
and Horsmans Y: The PPARgamma agonist pioglitazone inhibits early
neoplastic occurrence in the rat liver. Eur J Cancer. 43:1755–1763.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hassan MM, Curley SA, Li D, et al:
Association of diabetes duration and diabetes treatment with the
risk of hepatocellular carcinoma. Cancer. 116:1938–1946. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai SW, Chen PC, Liao KF, Muo CH, Lin CC
and Sung FC: Risk of hepatocellular carcinoma in diabetic patients
and risk reduction associated with anti-diabetic therapy: a
population-based cohort study. Am J Gastroenterol. 107:46–52. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
American Diabetes Association, . Diagnosis
and classification of diabetes mellitus. Diabetes Care. 33 (Suppl
1):S62–S69. 2010. View Article : Google Scholar
|
|
21
|
Akaike H: A new look at the statistical
model identification. IEEE Trans Automat Control. 19:716–723. 1974.
View Article : Google Scholar
|
|
22
|
Kawaguchi A, Yonemoto K, Tanizaki Y,
Kiyohara Y, Yanagawa T and Truong YK: Application of functional
ANOVA models for hazard regression to the Hisayama data. Stat Med.
27:3515–3527. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tonan T, Fujimoto K, Qayyum A, et al:
Quantification of hepatic iron concentration in chronic viral
hepatitis: usefulness of T2-weighted single-shot spin-echo
echo-planar MR imaging. PLoS One. 7:e338682012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishikawa T: Strategy for improving
survival and reducing recurrence of HCV-related hepatocellular
carcinoma. World J Gastroenterol. 19:6127–6130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rizvi AA: Metabolic markers of insulin
resistance in overweight persons. Ann Intern Med. 141:243–244.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saito K, Inoue S, Saito T, et al:
Augmentation effect of postprandial hyperinsulinaemia on growth of
human hepatocellular carcinoma. Gut. 51:100–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh S, Singh PP, Singh AG, Murad MH and
Sanchez W: Anti-diabetic medications and the risk of hepatocellular
cancer: a systematic review and meta-analysis. Am J Gastroenterol.
108:881–892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park EJ, Lee JH, Yu GY, et al: Dietary and
genetic obesity promote liver inflammation and tumorigenesis by
enhancing IL-6 and TNF expression. Cell. 140:197–208. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa H, Maeda S, Yoshida H, et al:
Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic
hepatitis C patients: an analysis based on gender differences. Int
J Cancer. 125:2264–2269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohki T, Tateishi R, Shiina S, et al:
Visceral fat accumulation is an independent risk factor for
hepatocellular carcinoma recurrence after curative treatment in
patients with suspected NASH. Gut. 58:839–844. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saxena NK, Fu PP, Nagalingam A, et al:
Adiponectin modulates C-jun N-terminal kinase and mammalian target
of rapamycin and inhibits hepatocellular carcinoma.
Gastroenterology. 139:1762–1773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fukushima N, Kuromatsu R, Arinaga-Hino T,
et al: Adipocytokine involvement in hepatocellular carcinoma after
sustained response to interferon for chronic hepatitis C. Hepatol
Res. 40:911–922. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sumie S, Kawaguchi T, Kuromatsu R, et al:
Total and high molecular weight adiponectin and hepatocellular
carcinoma with HCV infection. PLoS One. 6:e268402011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Endo Y, Suzuki M, Yamada H, et al:
Thiazolidinediones enhance sodium-coupled bicarbonate absorption
from renal proximal tubules via PPARgamma-dependent nongenomic
signaling. Cell Metab. 13:550–561. 2011. View Article : Google Scholar
|
|
35
|
Kohlroser J, Mathai J, Reichheld J, Banner
BF and Bonkovsky HL: Hepatotoxicity due to troglitazone: report of
two cases and review of adverse events reported to the United
States Food and Drug Administration. Am J Gastroenterol.
95:272–276. 2000. View Article : Google Scholar
|
|
36
|
Guo L, Zhang L, Sun Y, et al: Differences
in hepatotoxicity and gene expression profiles by anti-diabetic
PPAR gamma agonists on rat primary hepatocytes and human HepG2
cells. Mol Divers. 10:349–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaplowitz N: Avoiding idiosyncratic DILI:
two is better than one. Hepatology. 58:15–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chase MP and Yarze JC:
Pioglitazone-associated fulminant hepatic failure. Am J
Gastroenterol. 97:502–503. 2002. View Article : Google Scholar : PubMed/NCBI
|