Introduction
Triple-negative breast cancer (TNBC) has an estrogen
receptor (ER)-negative, progesterone receptor (PR)-negative and
human epidermal growth factor receptor (HER)-2-negative phenotype,
and has an aggressive behavior with early visceral metastasis and
consequently poorer outcomes (1).
Endocrine and HER-2-directed therapy are unsuitable for patients
with TNBC, and chemotherapy remains the mainstay of treatment in
these cases.
Certain studies of neoadjuvant chemotherapy have
suggested that TNBC patients who have a pathological complete
response (pCR) to treatment achieve excellent outcomes (2,3). However,
the majority of patients with TNBC who receive anthracycline and/or
paclitaxel regimens have a lower pCR rate, and for these patients,
there is a high risk of relapse and a sharp decrease in the
survival rate in the first 3–5 years after treatment. (4,5).
Certain experiments have shown that BRCA1-deficient
cells have increased sensitivity to cisplatin (6–8). Cancer
cells with a BRCA1 mutation have a defect in the homologous
recombination-based repair of double-strand DNA breaks and are
sensitive to inter-strand cross-linking agents, such as platinum
salts (7,9,10,11). A high proportion of TNBC patients have
a BRCA1 functional alteration, and 90% of tumors carrying a
BRCA1 mutation are of the TNBC type (12–14).
Preclinical models and several phase II studies have
suggested that platinum-based compounds are active drugs in TNBC,
although there have been no randomized studies to support this
hypothesis. Patients with BRCA1 mutations receiving
cisplatin have pCR rates of 72–83% (15,16).
Therefore, we hypothesized that TNBC may be sensitive to
platinum-based regimens. In the present meta-analysis, data were
extracted and the overall response rate (ORR) was analyzed for TNBC
patients who received a platinum- or non-platinum-based
regimen.
Materials and methods
Literature search strategy
The concept of TNBC was introduced in 2006 (17); therefore, searches of the PubMed
database, the China Knowledge Resource Integrated Database, the
China Science and Technology Journal Database and the WanFang
database were performed using the date limits between January 2006
and June 2014. Studies in Chinese and English were searched. The
keywords used were ‘platinum-based regimen and triple-negative.’
The abstracts of the resulting citations were reviewed, and
full-text manuscripts were retrieved for the potential studies. In
addition, the references of the selected studies were examined for
any additional relevant studies.
Literature search strategy
Studies were included in the meta-analysis if the
number of TNBC patients treated with a platinum- or a
non-platinum-based regimen could be extracted, together with the
related data. Studies with incomplete data on the platinum-based
regimen, ERs and PRs, and HER2 status were excluded.
Data extraction
Based on the search strategies described above,
studies were selected and their eligibility was confirmed by three
independent investigators. The following information was extracted
from each study: Authors' names, year of publication, study type,
the total number of patients and chemotherapy regimens.
Quality evaluation
The collated evidence was evaluated using the
Grading of Recommendations Assessment, Development and Evaluation
(GRADE) working group framework; accordingly, quality was graded as
high, medium, low or extremely low. Randomized controlled trials
were considered to be of a high grade, but the following factors
were also considered: Risk of bias, inconsistency, indirectness,
imprecision and publication bias. Case-control and cohort studies
were considered to be of a medium grade.
Statistical analyses
Meta-analysis was conducted using Review Manager
software (RevMan, version 5.1 for Windows; Cochrane Collaboration,
Oxford, UK). The odds ratio (OR) and 95% confidence interval (95%
CI) were calculated. A χ2 test was used to evaluate
heterogeneity in the data. The fixed-effects model was used for
studies without significant heterogeneity (I2≤50% or
P≥0.1), whereas the random-effects model was used for studies with
significant heterogeneity. Funnel plots were generated using RevMan
to detect publication bias. Quality evaluation was conducted using
GRADEpro software (version 3.6 for Windows; Cochrane
Collaboration). A paired sample t-test was analyzed using SPSS
(version 19; IBM Corp., Armonk, NY, USA).
Results
Eligible studies and data summary
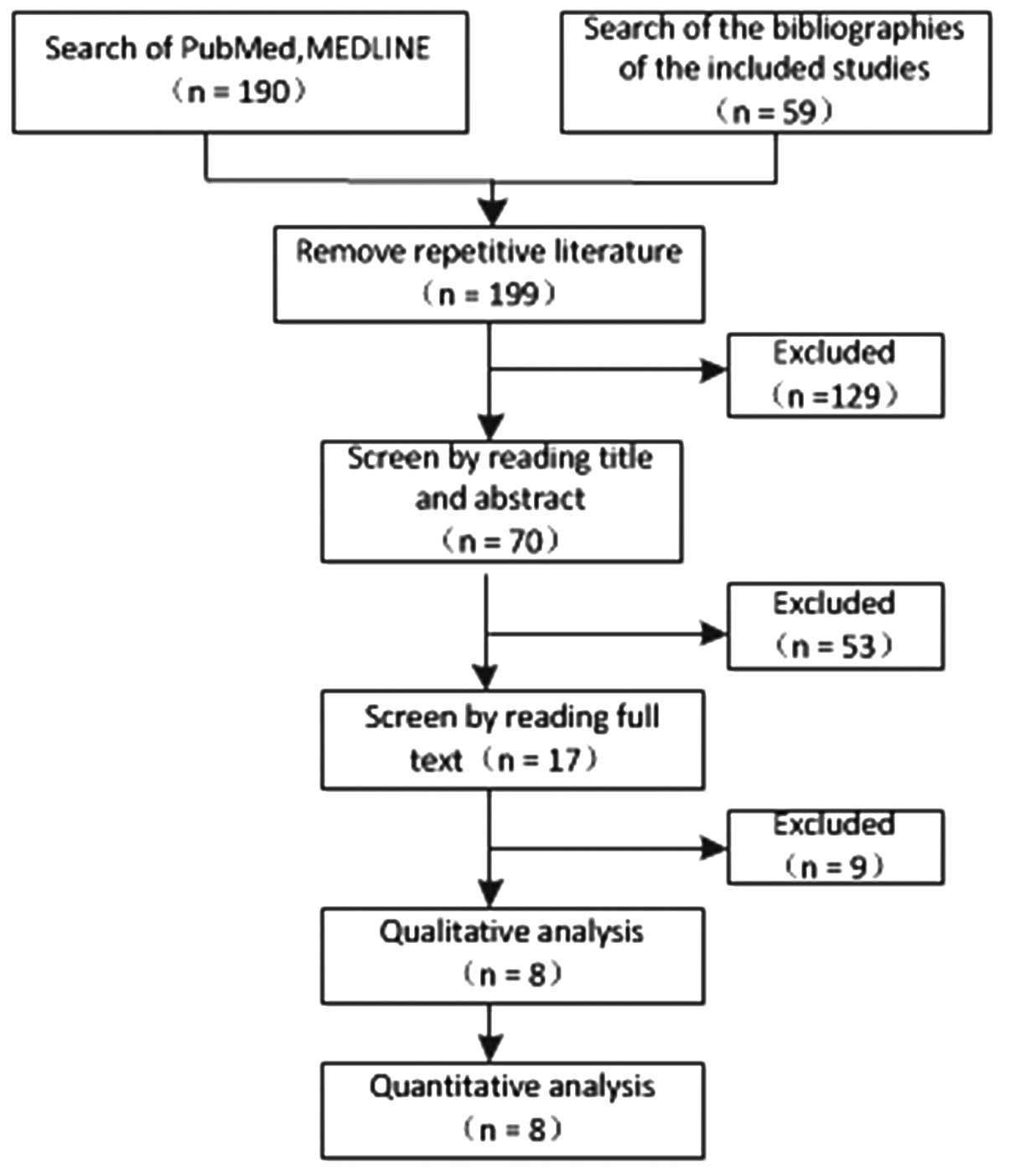
A total of 248 studies were first identified for
evaluation. Based on the criteria described, 8 publications with
1,349 patients were eligible for inclusion in the meta-analysis.
The search process is described in Fig.
1, and more details are provided in Table I.
 | Table I.Characteristics of the eligible
studies. |
Table I.
Characteristics of the eligible
studies.
| First author
(Ref) | Year | Country | Type | Platinum-based
regimen | Nonplatinum
regimen |
|---|
| Fan (22) | 2013 | China | Phase II clinical
trial | Docetaxel plus
cisplatin for 6 cycles | Docetaxel plus
capecitabine for 6 cycles |
| Bhattacharyya
(7) | 2009 | / | Phase II clinical
trial | Cyclophosphamide plus
methotrexate plus cisplatin | Cyclophosphamide plus
methotrexate |
| Wu (23) | 2012 | China | Retrospective
analysis | TP
(paclitaxel+Platinum), NP (vinorelbine+Platinum), GP
(gemcitabine+Platinum) | AT
(antharcycline+paclitaxel), TX (paclitaxel+capecitabin |
| Alba (18) | 2012 | Spain | Phase II clinical
trial | EC-DCb: EC
(epirubicin plus for 4 cycles) followed by DCb (docetaxel plus
carboplatin AUC 6 for 4 cycles) | EC-D: EC (epirubicin
plus cyclophosphamide for 4 cycles) followed by D (docetaxel for 4
cycles) |
| Villarreal-Garza
(25) | 2014 | Canada | Retrospective
analysis | TP
(paclitaxel+Platinum), NP (vinorelbine+Platinum), GP
(gemcitabine+Platinum) | Not given |
| von Minckwitz
(19) | 2014 | Germany | Phase II clinical
trial | Carboplatin (AUC
1.5–2.0) plus paclitaxel plus non-pegylated liposomal doxorubicin
plus bevacizumab | Paclitaxel plus
non-pegylated liposomal doxorubicin plus bevacizumab |
| Sikov (20) | 2015 | America | Phase II clinical
trial | Carboplatin (AUC 6)
plus paclitaxel plus doxorubicin plus cyclophosphamide for 4 cycles
with or without bevacizumab for 9 cycles | Paclitaxel plus
doxorubicin plus cyclophosphamide for 4 cycles with or without
bevacizumab for 9 cycles |
| Zhang (21) | 2013 | China | Phase II clinical
trial | Paclitaxel plus
carboplatin (AUC 5) for 4-6 cycles | Epirubicin plus
paclitaxel for 4-6 cycles |
pCR rate and ORR of TNBC patients
treated with a platinum- or non-platinum-based regimen
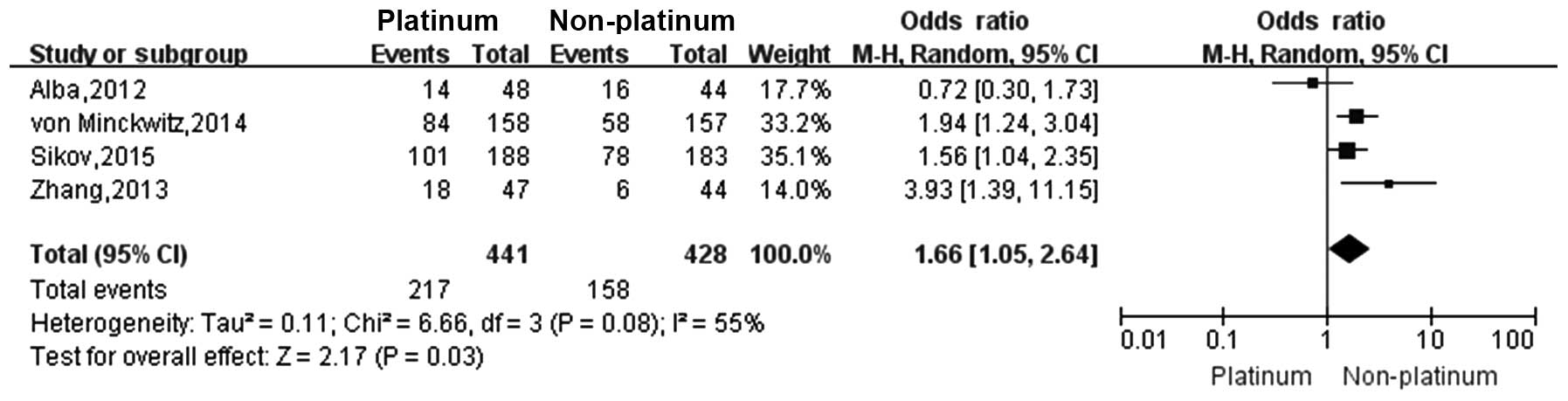
Four studies (18–21)
reported the pCR rate and five studies (18,21–24)
reported the ORR in TNBC patients who were treated with a platinum-
or a non-platinum-based regimen. There was significant
heterogeneity between different study results
(I2>50%, P<0.1), so the random-effects model was
applied for data analysis. The pCR rate in TNBC patients who were
treated with a platinum-based regimen was significantly higher than
that in those treated with a non-platinum-based regimen (49.2 vs.
36.9%; OR, 1.66; 95% CI, 1.05–2.64; Fig.
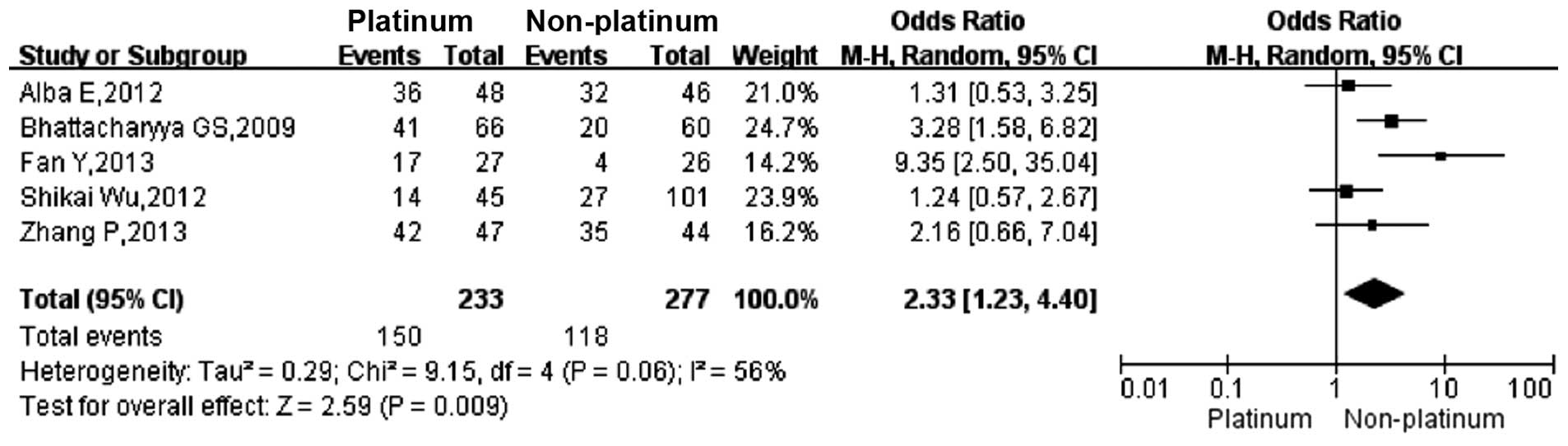
2). The ORR in TNBC patients who were treated with a
platinum-based regimen was significantly higher than that in those
treated with a non-platinum-based regimen (64.3 vs. 42.5%; OR,
2.33; 95% CI, 1.23–4.40; Fig. 3).
Survival rate of TNBC patients treated
with a platinum- or non-platinum-based regimen
Four studies (22–25)
reported that the overall survival (OS) rate was not significantly
different between TNBC patients treated with a platinum-based
regimen and those treated with a non-platinum-based regimen
(P>0.05). So does the disease-free survival (DFS) rate according
to three studies (Table II)
(22–24).
 | Table II.Disease-free survival (DFS) and
overall survival (OS) rates in studies of triple-negative breast
cancer patients who were treated with a platinum- or
non-platinum-based regimen. |
Table II.
Disease-free survival (DFS) and
overall survival (OS) rates in studies of triple-negative breast
cancer patients who were treated with a platinum- or
non-platinum-based regimen.
|
| OS, months | P-value | DFS, months | P-value |
|---|
| Platinum-based
regimen | 32.8 | 24.9 | 16.0 | 14.5 | >0.05 | 10.9 | 2.8 | 13.0 | >0.05 |
| Non-platinum-based
regimen | 21.5 | 26.3 | 12.0 | 10.0 |
| 4.8 | 3.0 | 7.0 |
|
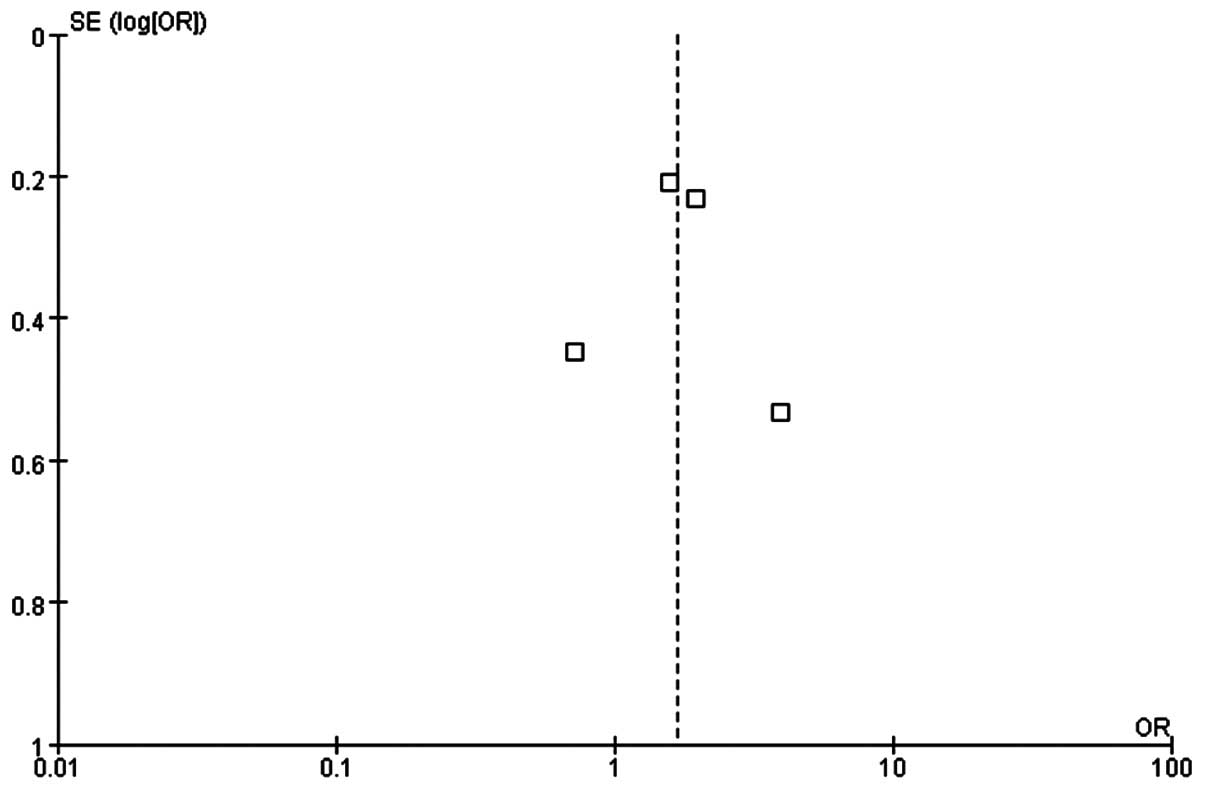
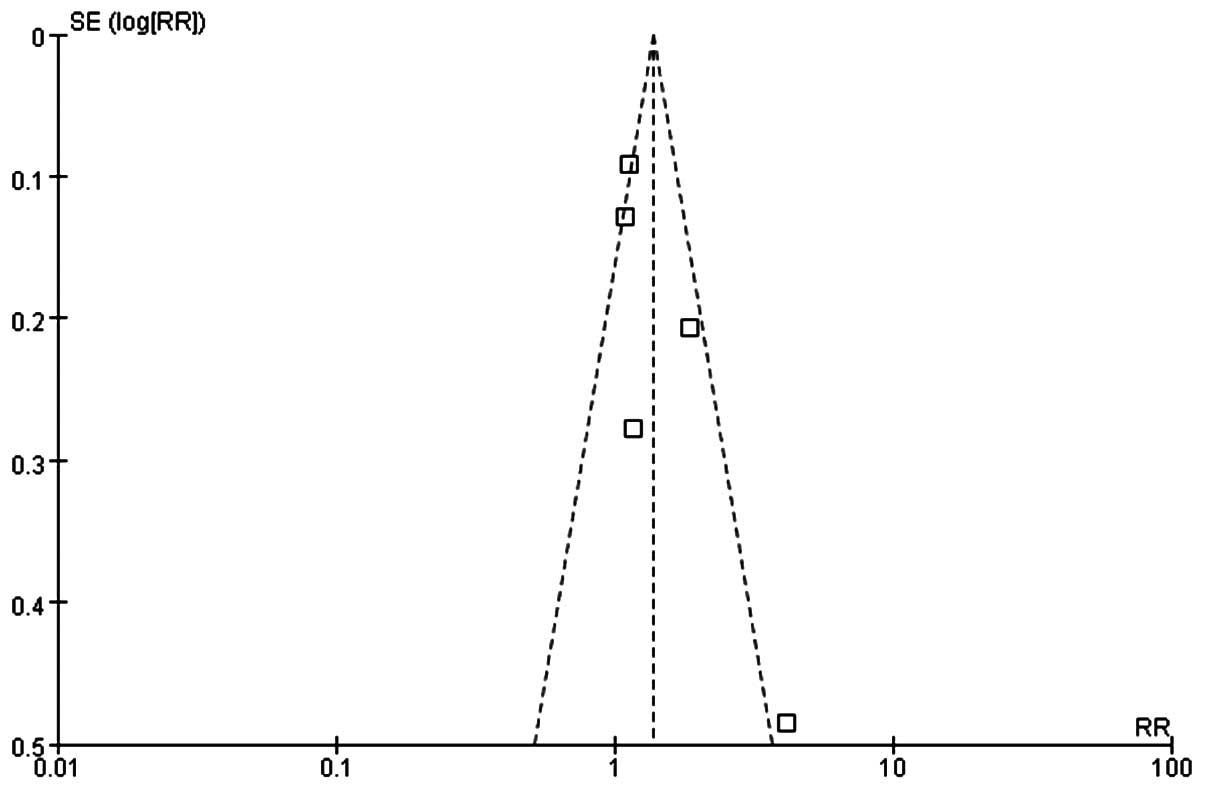
Quality evaluation
The quality of the meta-analysis was evaluated using
the GRADE framework and is shown in Table III. The assessment was considered to
be of moderate quality. Moderate between-study heterogeneity was
present for the risk difference analysis. Funnel plots for risk
ratio and risk difference showed mild asymmetry, indicating certain
publication bias (Figs. 4 and
5).
 | Table III.GRADE framework assessment of
eligible studies. |
Table III.
GRADE framework assessment of
eligible studies.
|
| Design | Quality
assessment |
|
|
|---|
|
|
|
|
|
|
|---|
| Outcome | Experiment | Control | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of eligible
studies | Quality |
|---|
| pCR | Platinum-based
regimen | Non-platinum
regimen | Serious | No serious
inconsistency | No serious
indirectness | No serious
imprecision | 5 | Moderate |
| ORR | Platinum-based
regimen | Platinum-based
regimen | Serious | No serious
inconsistency | No serious
indirectness | No serious
imprecision | 4 | Moderate |
Discussion
The present study showed that TNBC patients treated
with a platinum-based regimen had a higher pCR rate and ORR. There
was no significant increase in the OS and DFS, but due to the few
studies included this may be disregarded. Anthracycline and
paclitaxel are the common non-platinum-based regimens; however,
they result in only a low pCR rate (2,3). Patients
with TNBC who achieved a pCR usually have an improved outcome, and
thus combining a platinum-based regimen in treatment may be of
significant benefit. However, more trials are required to fully
evaluate the survival rate of TNBC patients receiving
platinum-based regimens.
Previous studies have shown that mutations in the
BRCA1 gene are prevalent in TNBC tumors, and certain
preclinical studies showed that TNBC cell lines are more sensitive
to DNA-damaging agents, such as platinum compounds (7,8). There is
also evidence for a dysfunctional BRCA1 pathway in sporadic TNBC
(26). Previously, it has been
reported that cisplatin selectively induces cell death in TNBC
cells through a mechanism involving the p53 family members, p63 and
p73 (27).
Platinum-based chemotherapy appears to be effective
in a high proportion of patients with mutant
BRCA1-associated breast cancers. A pCR rate of 72% following
neoadjuvant cisplatin treatment in 25 patients carrying the
BRCA1 mutation was reported (16), and a high proportion (83%) of females
with BRCA1-associated breast cancer responded to
platinum-based chemotherapy in a study conducted in Poland
(15). Furthermore, TNBC patients
with high-risk features are ~5.6 times more likely to carry a
BRCA1 mutation compared to patients with a non-TNBC tumor,
and approximately two in nine females with TNBC harbor a
BRCA1 mutation (28).
Platinum-based neoadjuvant chemotherapy in TNBC patients resulted
in improved short-term efficacy compared to its use in non-TNBC
patients, but it has not yet been demonstrated to improve efficacy
in advanced breast cancer (29).
There are certain relevant ongoing clinical trials,
including a randomized phase III trial comparing the efficacy of
carboplatin to docetaxel for patients with advanced TNBC (30). Another trial is currently underway to
assess the efficacy of platinum-based therapy for metastatic TNBC,
and evaluating the use of p63/p73 as a biomarker of response
(31).
All the patients included in the present
meta-analysis had either newly diagnosed or relapsed disease.
Therefore, it is possible that ambiguity in the actual cancer stage
could have introduced a bias in the data; however, the quality of
these studies was mostly considered to be moderate. In general, the
overall results were reliable despite certain publication bias.
In conclusion, platinum-based chemotherapy in TNBC
patients resulted in an improved short-term efficacy compared to
the non-platinum-based regimen group. Platinum-based therapy is
more effective to triple-negative breast cancer. Future multicenter
randomized controlled trials are required to validate these
findings and to determine whether platinum-based chemotherapy can
extend the survival rate of TNBC patients.
Acknowledgements
The present study is supported by the programme for
the National Natural Science Foundation of China (grant no.
30800278) and Doctoral Fund of Ministry of Education of China
(Youth Scholars) (grant no. 200804861048) to Dr Liao.
References
|
1
|
Reis-Filho JS and Tutt AN: Triple negative
tumours: a critical review. Histopathology. 52:108–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masuda H, Masuda N, Kodama Y, et al:
Predictive factors for the effectiveness of neoadjuvant
chemotherapy and prognosis in triple-negative breast cancer
patients. Cancer Chemother Pharmacol. 67:911–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang HR, Glaspy J, Allison MA, et al:
Differential response of triple-negative breast cancer to a
docetaxel and carboplatin-based neoadjuvant treatment. Cancer.
116:4227–4237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheang MC, Voduc D, Bajdik C, et al:
Basal-like breast cancer defined by five biomarkers has superior
prognostic value than triple-negative phenotype. Clin Cancer Res.
14:1368–1376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dent R, Trudeau M, Pritchard KI, et al:
Triple-negative breast cancer: clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tassone P, Di Martino MT, Ventura M, et
al: Loss of BRCA1 function increases the antitumor activity of
cisplatin against human breast cancer xenografts in vivo. Cancer
Biol Ther. 8:648–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhattacharyya A, Ear US, Koller BH,
Weichselbaum RR and Bishop DK: The breast cancer susceptibility
gene BRCA1 is required for subnuclear assembly of Rad51 and
survival following treatment with the DNA cross-linking agent
cisplatin. J Biol Chem. 275:23899–23903. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quinn JE, Kennedy RD, Mullan PB, et al:
BRCA1 functions as a differential modulator of chemotherapy-induced
apoptosis. Cancer Res. 63:6221–6228. 2003.PubMed/NCBI
|
|
9
|
Kennedy RD, Quinn JE, Mullan PB, Johnston
PG and Harkin DP: The role of BRCA1 in the cellular response to
chemotherapy. J Natl Cancer Inst. 96:1659–1668. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moynahan ME, Chiu JW, Koller BH and Jasin
M: Brca1 controls homology-directed DNA repair. Mol Cell.
4:511–518. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodler E, Korde L and Gralow J: Current
treatment options in triple negative breast cancer. Breast Dis.
32:99–122. 2010.PubMed/NCBI
|
|
12
|
Chacón RD and Costanzo MV: Triple-negative
breast cancer. Breast Cancer Res. 12 Suppl 2:32010. View Article : Google Scholar
|
|
13
|
Andre F and Zielinski CC: Optimal
strategies for the treatment of metastatic triple-negative breast
cancer with currently approved agents. Ann Oncol. 23 Suppl
6:vi46–vi51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Byrski T, Gronwald J, Huzarski T, et al:
Pathologic complete response rates in young women with
brca1-positive breast cancers after neoadjuvant chemotherapy. J
Clin Oncol. 28:375–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Byrski T, Huzarski T, Dent R, et al:
Response to neoadjuvant therapy with cisplatin in BRCA1-positive
breast cancer patients. Breast Cancer Res Treat. 115:359–363. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livasy CA, Perou CM, Karaca G, et al:
Identification of a basal-like subtype of breast ductal carcinoma
in situ. Hum Pathol. 38:197–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alba E, Chacon JI, Lluch A, et al: A
randomized phase II trial of platinum salts in basal-like breast
cancer patients in the neoadjuvant setting. Results from the
GEICAM/2006-03, multicenter study. Breast Cancer Res Treat.
136:487–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
von Minckwitz G, Schneeweiss A, Loibl S,
Salat C, Denkert C, Rezai M, et al: Neoadjuvant carboplatin in
patients with triple-negative and HER2-positive early breast cancer
(geparsixto; GBG 66): a randomised phase 2 trial. Lancet Oncol.
15:747–756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sikov WM, Berry DA, Perou CM, et al:
Impact of the addition of carboplatin and/or bevacizumab to
neoadjuvant once-per-week paclitaxel followed by dose-dense
doxorubicin and cyclophosphamide on pathologic complete response
rates in stage II to III triple-negative breast cancer: CALGB 40603
(alliance). J Clin Oncol. 33:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang P, Yin Y, Xu B, et al: Carboplatin
plus paclitaxel compared with epirubicin plus paclitaxel as
neoadjuvant chemotherapy for triple-negative breast cancer – a
phase ii clinical trial. Cancer Res. 73:P3-14-072013. View Article : Google Scholar
|
|
22
|
Fan Y, Xu BH, Yuan P, et al:
Docetaxel-cisplatin might be superior to docetaxel-capecitabine in
the first-line treatment of metastatic triple-negative breast
cancer. Ann Oncol. 24:1219–1225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Sk, Zhao X, Meng XY, et al: Analysis of
chemotherapeutic efficacies in metastatic triple-negative breast
cancer. Zhonghua Yi Xue Za Zhi. 13:3001–3003. 2012.
|
|
24
|
Bhattacharyya GS, Basu S, Agarwal V, et
al: Single institute phase ii study of weekly cisplatinum and
metronomic dosing of cyclophosphamide and methotrexate in second
line metastatic breast cancer triple-negative. Eur J Cancer (Abstr
41LBA, presented data-ECCO 15-ESMO 34 2009). 7:2009.
|
|
25
|
Villarreal-Garza C, Khalaf D, Bouganim N,
Clemons M, Pena-Curiel O, Baez-Revueltas B, et al: Platinum-based
chemotherapy in triple-negative advanced breast cancer. Breast
Cancer Res Treat. 146:567–572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Turner NC, Reis-Filho JS, Russell AM, et
al: BRCA1 dysfunction in sporadic basal-like breast cancer.
Oncogene. 26:2126–2132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leong CO, Vidnovic N, DeYoung MP, Sgroi D
and Ellisen LW: The p63/p73 network mediates chemosensitivity to
cisplatin in a biologically defined subset of primary breast
cancers. J Clin Invest. 117:1370–1380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tun NM, Villani G, Ong K, Yoe L and Bo ZM:
Risk of having BRCA1 mutation in high-risk women with
triple-negative breast cancer: a meta-analysis. Clin Genet.
85:43–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu M, Mo QG, Wei CY, Qin QH, Huang Z and
He J: Platinum-based chemotherapy in triple-negative breast cancer:
a meta-analysis. Oncol Lett. 5:983–991. 2013.PubMed/NCBI
|
|
30
|
ISRCTN Registry: Triple Negative Trial: a
randomised phase III trial of carboplatin compared to docetaxel for
patients with advanced oestrogen receptor-progesterone
receptor-human epidermal growth factor receptor two-breast cancer.
https://www.isrctn.com/ISRCTN97330959Accessed. July
4–2014
|
|
31
|
ClinicalTrials.gov, . Platinum for
triple-negative metastatic breast cancer and evaluation of p63/p73
as a biomarker of response. https://clinicaltrials.gov/ct2/show/NCT00483223Accessed.
July 4–2014
|