Introduction
Glioblastoma Multiforme (GBM) is an incurable
primary brain cancer. The incidence of GBM increases with age, with
the highest rates observed in individuals aged 75–84 years
(1). Based on the landmark European
Organization for Research and Treatment of Cancer (EORTC)/National
Cancer Institute of Canada (NCIC) randomized trial published in
2005 by Stupp et al (2), which
demonstrated improved survival when temozolomide (TMZ) was added to
radiotherapy (RT), the current standard of care for newly diagnosed
GBM patients is 6 weeks of RT delivered in 1.8–2.0-Gy daily
fractions to a total dose of 60 Gy, followed by adjuvant TMZ
chemotherapy. However, the Stupp study excluded patients aged
>70 years. Therefore, it has not been rigorously determined
whether such patients benefit from this treatment, as was
demonstrated for younger patients (3). The optimal treatment approach for
elderly GBM patients has not yet been clearly established. In
addition, due to factors such as poorer prognosis associated with
older age and the presence of other comorbidities, elderly GBM
patients may occasionally be treated with alternative therapies
that may be better tolerated (4,5).
The alternative treatment for elderly GBM patients
primarily includes hypofractionated RT (HRT), where larger
fractions of radiation are administered over a shorter period of
time compared with standard RT (6).
In certain cases, concurrent TMZ has been added to HRT, based on
the superior outcomes reported with the Stupp protocol (7,8), although
it has not yet been established that combining HRT with concurrent
TMZ is superior to HRT alone (9).
More recently, TMZ was investigated as a single agent and, based on
certain genetic factors in the tumor, it may also be an effective
treatment option (10–13).
The number of studies investigating population-based
treatment trends for elderly GBM patients is currently limited
(14–16), whereas none have assessed survival
outcomes by treatment method with the associated cost, which may
significantly affect treatment recommendations. In order to
determine treatment patterns, survival outcomes and cost associated
with therapy for elderly GBM patients, we analyzed data from the
Surveillance, Epidemiology and End Results (SEER)-medicare linked
database in a contemporary cohort of elderly patients with GBM.
Materials and methods
SEER-Medicare
In 2011, Medicare covered 40.4 million individuals
aged ≥65 years (2012 Medicare Report); this is ~14% of the total US
population, which essentially includes all US citizens aged >65
years. The SEER-Medicare linked database contains information on
Medicare beneficiaries with cancer. The SEER portion includes
information pertaining to cancer, such as tumor details, first
course of treatment and cause of death, along with patient
demographics. The Medicare portion includes claims of individuals
eligible to Medicare from enrollment until death. Each patient has
a unique identifier that may be used to link the different sets.
SEER-Medicare is available to researchers on a project basis for a
fee.
In this study, we included a SEER-Medicare tailored
cohort containing patients diagnosed with primary brain tumors from
1997 to 2010 and their Medicare claims. The Medicare part of our
data includes the Medicare Provider Analysis and Review (Med PAR)
file, the outpatient claims file, the Physician/Supplier Part B
[National Claims History (NCH)] file, the Durable Medical Equipment
(DME) file and the hospice file.
Cohort selection
From the SEER data, we extracted patients with
histology codes 9440/3, 9441/3 and 9442/3, with concurrent topology
codes C71.0-C71.4. The date of diagnosis was noted. As SEER only
provides the month and year of diagnosis, the day of diagnosis was
imputed to the 15th of the month. All the analyses were adjusted to
account for this 15-day error. Patients who were diagnosed with
other primary tumors within 1 year of diagnosis were excluded. In
order to have a full year look-back for all, the cut-off age for
inclusion was set to ≥66 years.
Only patients with confirmed surgical resection or
biopsy were selected. At this end, patients who, according to the
SEER data, did not undergo surgery, or for whom it was unknown
whether surgery was performed, or the surgery data were missing,
were excluded. In addition, patients for whom, according to the
Medicare data, the surgery or biopsy was not found in the period
from 15 days prior to the diagnosis date to 2 months after surgery,
were also excluded.
Other inclusion/exclusion criteria included no
Health Maintenance Organization (HMO) enrollment from the period
from 1 month prior to diagnosis until death or December 31st, 2010;
having both part A and B during the period from 1 month prior to
diagnosis until death or December 31st, 2010; and having age as the
sole reason for Medicare entitlement. The Medicare claims for the
included patients were extracted.
Patient characteristics and treatment
variables
Patient characteristics were mainly obtained from
SEER. The age at diagnosis and the race and gender of the patients
were taken into consideration. All the included patients underwent
surgery (biopsy or resection) within 2 months of the reported
diagnosis date. Patients who did not undergo RT or chemotherapy
within 90 days of surgery were considered to belong to the surgery
alone group. If a patient received chemotherapy without RT within
this period of 90 days after surgery, that patient was then
considered to belong to the chemotherapy alone group. Similarly,
the RT alone group was defined as undergoing RT without
chemotherapy within 90 days after surgery. The HRT group was
defined as having ≤20 claims of RT within 45 days after the first
post-surgery RT and the standard RT (SRT) group comprised patients
who had ≥20 RT sessions within 45 days after the first post-surgery
radiation. There were 6 groups in total.
The Medicare Med PAR, outpatient, NCH and DME were
used to search for claims of surgery, RT and chemotherapy. To
search for surgery, we used the international classification of
diseases, 9th revision (ICD-9) codes 01.11–01.14 and 01.18 and
current procedural terminology (CPT)-4 61140 for biopsy; ICD-9
01.24, 01.25, 01.31, 01.32, 01.39, 01.51, 01.53 and 01.59 and CPT-4
61304 were used for resection. To check whether a patient had
undergone RT, we used the CPT codes 77261–77799. In order to define
the fractionation, we counted the number of RT sessions using two
different methods and, for each patient, retained the one that
yielded the highest value. The first method was to use the
radiation delivery codes (CPT-4 codes 77401–77416, 77418, 0197T and
G0174), which account for the treatment component. These delivery
codes are billed for each fraction. The second method was to use
the management code CPT 77427, which accounts for the physician
examination and other services for each RT session. This management
claim is billed for each 5 fractions. The chemotherapy considered
was oral TMZ administration. Oral TMZ was searched for with the CPT
code J8700 and the National Drug Code (NDC) numbers 54868–4141,
54868–5348, 54868–5350, 54868–5354, 54868–5980, 0085–1366,
0085–1381, 0085–1417, 0085–1425, 0085–1430, 0085–1519, 0085–3004,
0093–7599, 0093–7600, 0093–7601, 0093–7602, 0093–7638, 0093–7639,
47335–890, 47335–891, 47335–892, 47335–893, 47335–929, 47335–930,
0741–2641, 1741–2692, 0741–2693, 0741–2694, 0741–2695 and
0741–2696. TMZ was food and drug administration-approved for use in
1999. To account for this fact, we looked at the data for the
diagnosis years 1997–2009 and for the sub-sample of 2000–2009.
Medicare data were also used to evaluate
comorbidities. For each patient, comorbidity was measured as the
mean Charlson index score for all claims within 30 days of the
diagnosis date (17). The Charlson
index was computed using Deyo's adaptation to the ICD-9-CM codes
(18).
Cost data
We only considered Medicare payments for the cost
data analysis. We focused on the cost of treatment, which is the
cost of the initial surgery, the cost of post-surgery RT during the
entire time of survival and the cost of TMZ for this period. We
also considered the total cost, which was the sum of the index
surgery cost, plus all the post-surgery RT and TMZ. All the
payments were inflated to 2014 USD using the medical component of
the consumer price index accessed through the US bureau of Labor
Statistics website (www.bls.gov/cpi).
Statistical analysis
The patient characteristics were compared across
treatment groups using the Chi-square test for gender, race and age
group at diagnosis, whether the patient sussumbed to GBM and
whether the patient sussumbed to other causes. The costs of
treatment were compared using the Wilcoxon Rank Sum test.
Kaplan-Meier curves were used to estimate the survival time from
diagnosis to either death or to the censoring date of December
31st, 2010. The unadjusted comparison of survival time was
performed using the log-rank test. The proportional hazard models
were used to compare the risk of death adjusting for age and
comorbidities. The significance level was set at 0.05.
Results
Patients
Using the search criteria, a total of 3,759 patients
were identified. A total of 53.45% of the patients were male and
46.55% were female; 93.11% of the patients were Caucasian. The
median age for all the patients was 74 years (range, 66–97 years).
Approximately 48% of the patients (n=1,818) were treated using SRT
without TMZ; ~10% (n=386) were treated with SRT plus concurrent
TMZ; ~29% (n=1,094) received no treatment for their tumors
following diagnostic surgery; ~10% (n=390) received HRT without
TMZ; ~1% (n=43) had HRT with TMZ; and <1% (n=28) were treated
with TMZ alone (Table I).
 | Table I.Baseline characteristics and outcomes
for all GBM patients diagnosed between 1997 and 2009. |
Table I.
Baseline characteristics and outcomes
for all GBM patients diagnosed between 1997 and 2009.
|
|
| Groups |
|
|---|
|
|
|
|
|
|---|
| Variables | All patients
(n=3,759) | Surgery, no RT, no
TMZ (n=1,094) | Surgery, HRT, no TMZ
(n=390) | Surgery, SRT, no TMZ
(n=1,818) | Surgery, HRT, TMZ
(n=43) | Surgery, SRT, TMZ
(n=386) | Surgery, no RT, TMZ
(n=28) | P-value |
|---|
| Female gender, n
(%) | 1,750 (46.55) | 548 (50.09) | 190 (48.72) | 814 (44.77) | 18 (41.86) | 167 (43.26) | 13 (46.43) | 0.0597 |
| Race, n (%) |
|
|
|
|
|
|
| 0.0346 |
|
Caucasian | 3,500 (93.11) | 1,000 (91.41) | 361 (92.56) | 1,699 (93.45) | 43 (100.00) | 371 (96.11) | 26 (92.86) |
|
| African
American | 118 (3.14) | 46 (4.20) | 17 (4.36) | 49 (2.70) |
| 6 (1.55) |
|
|
|
Other | 141 (3.75) | 48 (4.39) | 12 (3.08) | 70 (3.85) |
| 9 (2.33) | 2 (7.14) |
|
| Age (years) at dx, n
(%) |
|
|
|
|
|
|
| <0.0001 |
|
66–69 | 818 (21.76) | 144 (13.16) | 60 (15.38) | 486 (26.73) | 7 (16.28) | 115 (29.79) | 6 (21.43) |
|
|
70–74 | 1,133 (30.14) | 257 (23.49) | 106 (27.18) | 614 (33.77) | 12 (27.91) | 136 (35.23) | 8 (28.57) |
|
|
75–79 | 1,038 (27.61) | 333 (30.44) | 125 (32.05) | 469 (25.80) | 17 (39.53) | 87 (22.54) | 7 (25.00) |
|
|
80–84 | 546 (14.53) | 230 (21.02) | 69 (17.69) | 196 (10.78) | 5 (11.63) | 41 (10.62) | 5 (17.86) |
|
|
>85 | 224 (5.96) | 130 (11.88) | 30 (7.69) | 53 (2.92) | 2 (4.65) | 7 (1.81) | 2 (7.14) |
|
| Charlson score |
|
|
|
|
|
|
|
|
| Mean
(SD) | 0.020 (0.03) | 0.03 (0.05) | 0.02 (0.03) | 0.02 (0.03) | 0.02 (0.02) | 0.01 (0.02) | 0.02 (0.02) |
|
| Median
(Q1-Q3) | 0.01 (0.00–0.03) | 0.02 (0.00–0.04) | 0.01 (0.00–0.03) | 0.01 (0.00–0.02) | 0.01 (0.00–0.03) | 0.00 (0.00–0.01) | 0.02 (0.00–0.03) |
|
| Survival months
ESTIMATE |
|
|
|
|
|
|
| <0.0001 |
| Median
(range) | 6 (0–121) | 2 (0–89) | 4 (0–33) | 9 (1–121) | 3 (1–29) | 11 (2–56) | 6 (1–24) |
|
| Post-dx
post-surgery total treatment COSTa, USD |
|
|
|
|
|
|
| <0.0001 |
| Mean
(SD) | 60,380
(46,379) | 38,600
(27,403) | 46,923
(23,095) | 67,180
(43,430) | 61,868
(27,036) | 103,762
(74,680) | 56,979
(42,889) |
|
| Median
(range) | 48,275
(0–452,143) | 33,443
(0–263,292) | 42,834
(852–230,331) | 55,228
(0–383,114) | 63,915
(13,646–132,550) | 78,784
(16,644–452,143) | 48,298
(3,772–195,836) |
|
The median survival for all 3,759 patients was 6
months (range, 0–121 months). Those patients who received no
treatment for their tumors following tissue diagnosis had a median
survival of 2 months (range, 0–89 months). The median survival for
all the treated patients was 8 months (range, 0–121 months)
(P<0.0001). Patients who were treated with SRT plus concurrent
TMZ had a median survival of 11 months (range, 2–56 months) and
those treated with SRT without TMZ had a median survival of 9
months (range, 1–121 months) (P=0.01). The median survival of
patients treated with HRT alone was 4 months (range, 0–33 months).
Patients treated with HRT plus concurrent TMZ had a median survival
of 3 months (range, 1–29 months). Those patients treated with TMZ
alone had a median survival of 6 months (range, 1–24 months). There
was no significant survival difference among the 3 cohorts of
patients treated with HRT alone, HRT plus TMZ (P=0.4344) or TMZ
alone (P=0.3150). However, survival in these 3 patient groups was
statistically superior to that in the group of patients who
received no treatment following surgical diagnosis (P<0.0001).
When comparing the combined cohorts of patients treated with HRT
with or without TMZ (median survival, 4 months; range, 0–33 months)
to those receiving SRT with or without TMZ (median survival, 9
months; range, 1–121 months), superior survival was exhibited by
the SRT cohorts, even when adjusted for age and other comorbidities
[adjusted hazard ratio (AHR)=0.531, 95% confidence interval (CI):
0.477–0.591, P<0.0001) (Table
II).
 | Table II.Adjusted comparison of GBM survival
analysis among different treatment groups. |
Table II.
Adjusted comparison of GBM survival
analysis among different treatment groups.
| Treatment
groups | Survival months
estimate, median (range) | Adjusted analysis
HR (95% CI) | P-value |
|---|
| Surgery alone
(n=1,094) | 2
(0–89) | Reference |
|
| All other
treatments (n=2,665) |
8 (0–121) | 0.345
(0.320–0.373) | <0.0001 |
| HRT with or without
TMZ (n=433) | 4
(0–33) | Reference |
|
| SRT with or without
TMZ (n=2,204) |
9 (1–121) | 0.531
(0.477–0.591) | <0.0001 |
| HRT without TMZ
(n=390) | 4
(0–33) | Reference |
|
| HRT with TMZ
(n=43) | 3
(1–29) | 0.880
(0.639–1.213) |
0.4344 |
| TMZ alone
(n=28) | 6
(1–24) | 0.821
(0.558–1.207) |
0.3150 |
| SRT without TMZ
(n=1,818) |
9 (1–121) | Reference |
|
| SRT with TMZ
(n=386) | 11 (2–56) | 0.863
(0.770–0.967) |
0.0111 |
| Surgery, no RT, no
TMZ (n=1,094) | 2
(0–89) | Reference |
|
| Surgery, HRT, no
TMZ (n=390) | 4
(0–33) | 0.556
(0.495–0.625) | <0.0001 |
| Surgery, SRT, no
TMZ (n=1,818) |
9 (1–121) | 0.320
(0.295–0.347) | <0.0001 |
| Surgery, HRT, TMZ
(n=43) | 3
(1–29) | 0.484
(0.356–0.660) | <0.0001 |
| Surgery, SRT, TMZ
(n=386) | 11 (2–56) | 0.277
(0.244–0.313) | <0.0001 |
| Surgery, no RT, TMZ
(n=28) | 6
(1–24) | 0.434
(0.298–0.633) | <0.0001 |
Patients were stratified by age into those aged
66–74 years and those aged ≥75 years. In the ≥75 cohort, 38.33%
(693/1,808) of the patients were not treated following diagnostic
surgery, whereas in the 66–74 cohort, only 20.55% (401/1,951) of
the patients received no therapy. In the ≥75 group, the median
overall survival for all the patients was 4 months, compared with 8
months in the younger cohort (P<0.0001). A total of 69% of the
patients in the younger cohort were treated with SRT with or
without TMZ (1,351/1,951) and the median survival was 11 and 10
months, respectively (P<0.01). In the older cohort, 47% of the
patients (853/1,808) were treated with SRT with or without TMZ,
with median survivals of 9 and 7 months, respectively (P=0.27).
The younger patients treated with HRT with or
without TMZ or TMZ alone totaled 10% (199/1,951) of the patients in
this cohort, with median survivals of 5, 5 and 5 months,
respectively. In the older cohort, a total of 14% were treated with
HRT with or without TMZ or TMZ alone (262/1,808), with median
survivals of 3, 4 and 6 months, respectively (Table III).
 | Table III.Comparison of survival months and
non-treatment rate following diagnostic surgery across age
groups. |
Table III.
Comparison of survival months and
non-treatment rate following diagnostic surgery across age
groups.
| Variables | Aged 66–74 years
(n=1,951) | Aged ≥75 years
(n=1,808) |
P-valuea |
|---|
| Survival months,
median (range) |
|
|
|
| All
patients |
8 (0–121) | 4
(0–95) | <0.0001 |
|
Surgery, no RT, no TMZ | 2
(0–61) | 2
(0–89) |
<0.0001b |
|
Surgery, HRT, no TMZ | 5
(0–31) | 4
(0–33) | 0.0671 |
|
Surgery, SRT, no TMZ | 10 (1–121) | 7
(1–95) | <0.0001 |
|
Surgery, HRT, TMZ | 5
(1–21) | 3
(1–29) | 0.8314 |
|
Surgery, SRT, TMZ | 11 (2–56) | 9
(2–31) | <0.0001 |
|
Surgery, no RT, TMZ | 5
(1–24) | 6
(1–24) | 0.7289 |
| Non-treatment rate
following diagnostic surgery |
|
|
|
| Number
of non-treated patients (%) | 401 (20.55) | 693 (38.33) | <.0001 |
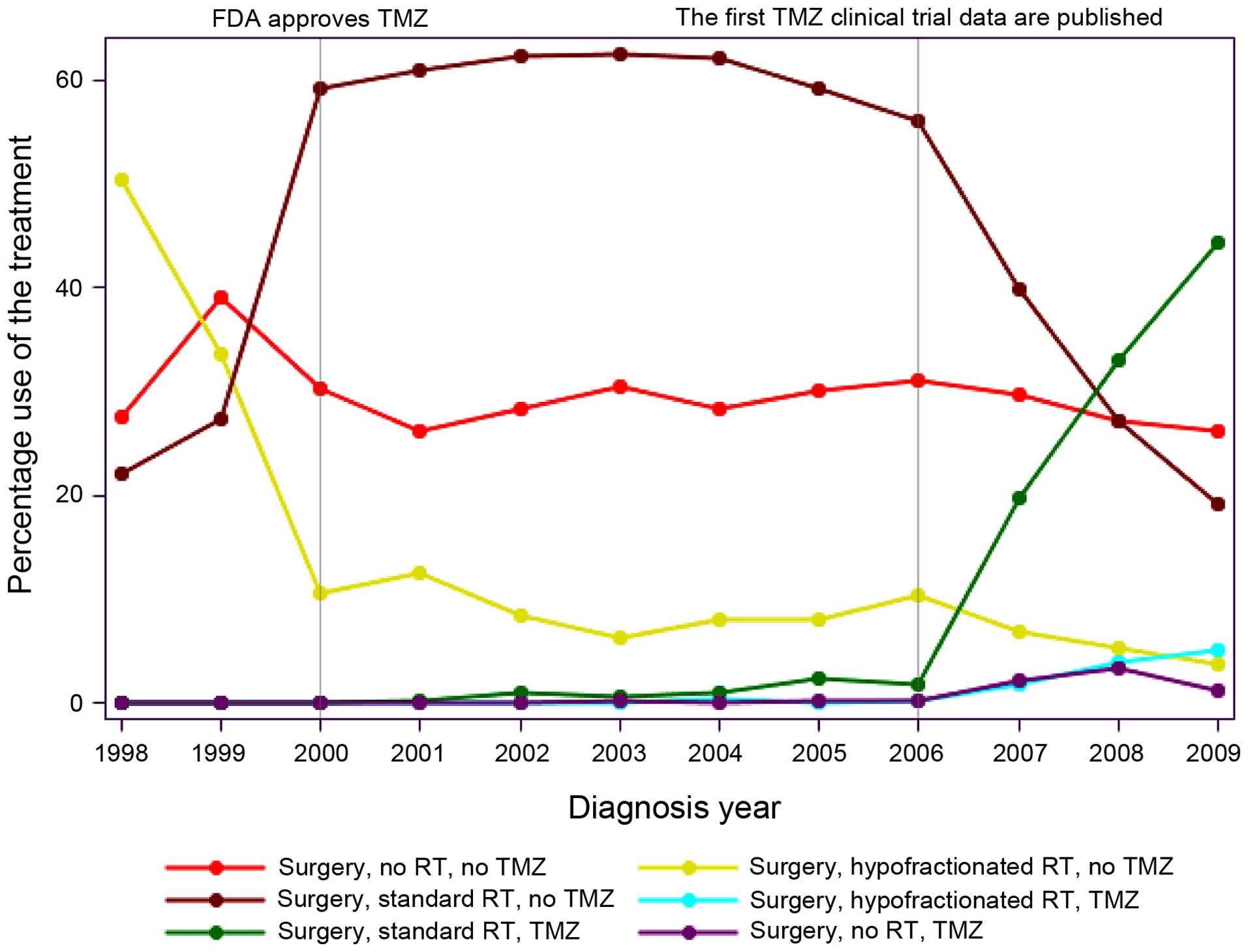
To assess temporal trends, the percentage of use by
treatment type/year for the 6 different treatment groups was
analyzed from 1998 to 2009 (Fig. 1).
Over this time period, the percentage of patients for Egoing any
therapy following surgery remained approximately the same, ranging
between 30 and 40%. From 2005 onwards, with the publication of the
Stupp protocol, there was an increase in the use of SRT plus
concurrent TMZ. Interestingly, prior to 2000, almost 50% of the
patients were treated with HRT, as opposed to SRT. In 2000, this
trend was reversed and by 2009, more elderly GBM patients were
treated with SRT. The use of HRT had decreased to 5% by 2009. The
use of TMZ alone (1–3%, 2007–2009) and HRT plus TMZ (1–6%,
2007–2009) was also low.
The median payer-reported treatment cost following
diagnostic surgery for all the patients was 48,275 USD (range,
0–452,143 USD). Patients who did not receive RT or TMZ as initial
treatment following diagnostic surgery had a median payer-reported
cost of 33,443 USD (range, 0–263,292 USD). For those patients
treated after surgery with SRT plus TMZ, the reported cost was
78,784 USD (range, 16,644–452,143 USD). The cost of SRT without TMZ
was 55,228 USD (range, 0–383,114 USD). The median cost of HRT
without TMZ was 42,834 USD (range, 852–230,331 USD). The cost of
HRT plus TMZ was 63,915 USD (range, 13,646–132,550 USD) and the
cost of TMZ alone was 48,298 USD (range, 3,772–195,836 USD)
(Table I).
Discussion
When analyzing the treatment trends for patients in
this study, the majority of the patients (~60%), were treated using
SRT with or without TMZ. However, in this cohort, the majority of
the patients did not receive concurrent TMZ with SRT, due to the
larger number of included patients who were treated prior to the
publication of the Stupp protocol in 2005 (2). From 2005 onwards, the trend for SRT plus
TMZ increased. A total of 29% of the patients received no therapy
following diagnostic surgery, which is consistent with previous
elderly GBM population-based studies in the USA and Europe
(14,16,19). As of
2009, only a small percentage of patients included in this study
were treated using HRT with or without TMZ or with TMZ alone; the
use of HRT declined after 2000, for reasons that remain
unclear.
The median overall survival for the entire cohort of
patients was 6 months. Patients stratified into untreated vs.
treated groups exhibited median survivals of 2 and 8 months,
respectively. These outcomes are consistent with other published
population-based studies and prognostic schemata (14,19,20). Since
the publication of the Stupp protocol EORTC/NCIC randomized trial
adding TMZ to RT, there has been no population-based study directly
comparing the efficacy of SRT to that of SRT plus TMZ for elderly
patients. In this analysis, treatment using SRT with concurrent TMZ
correlated with an improved survival, which was also the trend for
patients aged ≥75 years. This finding is supported by previously
published studies reporting increased overall survival in elderly
GBM patients since the TMZ era, as well as a recently published
meta-analysis of non-randomized studies of elderly GBM patients
treated with RT and concurrent TMZ or RT alone, who also exhibited
improved survival (21–23).
The groups treated using HRT with or without TMZ or
TMZ alone did exhibit statistically significantly improved survival
when compared to patients who received no therapy. There was no
statistically significant survival advantage among these three
treatment methods. However, when compared to patients treated using
SRT with or without TMZ, even when adjusted for age and other
comorbidities, patients treated using these regimens exhibited a
poorer survival, although the extent of surgery and functional
status were not included in the analysis. This is in conflict with
a prospective study published by Roa et al (24) in 2004, which demonstrated equivalent
survival outcomes in elderly GBM patients randomized to receive a
3-week HRT course vs. the standard 6 weeks of RT. Interestingly,
the survival times in that study (5.1 months for SRT and 5.6 months
for HRT) were closer to the survival estimates of the patients in
this analysis who were treated with alternatives to SRT. The
relatively short survivals in the Roa study may be explained by the
limited proportion of enrolled patients (<15%) who had undergone
complete surgical resections, since the extent of surgery has been
shown to positively affect survival (25). In addition, this trial was closed
early due to slow accrual and was not sufficiently powered to
conclude that the two treatments were truly statistically
equivalent.
Further elucidating the role of alternative
therapies, two important randomized phase III trials were published
in 2012 that included comparisons of RT monotherapy vs. TMZ
monotherapy in elderly GBM patients. The first trial was the German
NOA-08, which enrolled high-grade glioma patients aged >65 years
to receive SRT or TMZ (10). The
median age was 72 years and 412 patients were enrolled. The median
overall survival was 9.6 months for RT and 8.6 months for TMZ, with
a P-value of 0.03, which was consistent with the non-inferiority of
TMZ. The second study, the Nordic trial, randomized GBM patients
onto 3 arms between TMZ, HRT and SRT (11). The median age was 70 years and 291
patients were enrolled. The median survival was 8.3 months in the
TMZ group, 7.5 months in the HRT group and 6.0 months in the SRT
group. For patients aged >70 years, TMZ (HR=0.35; P<0.01) and
HRT (HR=0.59; P=0.02) were associated with a significantly longer
survival compared with SRT.
The two aforementioned clinical trials reported
better survival outcomes for patients treated with HRT or TMZ
compared with those observed in this study cohort. A possible
explanation for this discrepancy is that, in this population-based
retrospective analysis, patients treated with these abbreviated
therapies possibly had worse prognostic factors (i.e., extent of
surgery and performance status) compared with the patients enrolled
in the German NOA-08 and Nordic clinical trials. To clarify,
although the extent of surgery was beyond the scope of this
analysis, in 2014 Noorbakhsh et al (25) published a SEER-based population study
of elderly GBM patients, analyzing the extent of resection and the
outcomes. They found that, of the 3,631 patients aged ≥75 years,
only 24.2% had undergone complete resection, 24.1% had undergone
partial resection, 17.2% local excision or biopsy, 32.7% had
received no surgery and 1.8% was unknown. Moreover, the extent of
resection correlated with survival. Patients aged ≥75 years who had
no resection, partial resection or complete resection had median
survivals of 3, 4 and 6 months, respectively. By comparison, in the
Nordic trial, 67% of patients aged ≥70 years had undergone complete
or partial resection and in the German NOA-08 trial, 58% of the
patients treated with TMZ had undergone complete or partial
resection. The patients in these trials also had good overall good
performance status, with a median Karnofsky score of 80, and high
performance scores are also known to be a treatment-independent
positive prognostic factor in brain tumor patients (20). Although our analysis could not include
patient functional status, a common reason for treating elderly GBM
patients with abbreviated therapies is poor function. Therefore,
our population-based analysis may reflect a ‘real-world’ assessment
as to the type of patient these alternative treatment methods are
offered to, as opposed to patients who are enrolled in clinical
trials.
Understanding that certain genetic markers may
predict therapeutic response in addition to having prognostic
value, the German and Nordic studies also investigated treatment
response by methylation status of the promoter region of the
O6-methylguanine methyltransferase (MGMT) gene. It was previously
demonstrated that the expression of mgmt in the tumor may inhibit
the response to TMZ and MGMT promoter methylation (which inhibits
expression of MGMT) and is correlated with improved overall
survival in patients treated with TMZ (26). The two studies found that patients
with methylated tumors treated with TMZ had a significantly higher
survival compared with those with unmethylated tumors, as well as
with those who had methylated tumors but were treated using HRT and
not TMZ. The authors concluded that MGMT methylation status should
be routinely checked in elderly GBM patients in order to determine
treatment with TMZ as a single agent vs. HRT.
The use of HRT with concurrent TMZ has been
investigated in a few small trials and has shown possible benefit
(7). In this analysis, there was no
statistically significant difference in outcome compared with those
patients who were treated with TMZ or HRT alone. There is an
ongoing phase III randomized trial (NCIC CTG CE.6), where HRT is
administered with and without TMZ in elderly patients with GBM,
that should answer the question of whether TMZ added concurrently
to HRT improves survival over either of these modalities alone
(9).
When comparing standard treatment cost to
alternatives, SRT and concurrent TMZ had a total median payer
amount of 78,784 USD. The cost of HRT alone was 42,834 USD and that
of TMZ alone 48,298 USD; in the alternative therapies, HRT with
concurrent TMZ was the clear outlier at 63,915 USD.
In this retrospective study, elderly GBM patients
appeared to benefit from standard as well as alternative treatment
schemes, with the addition of TMZ to SRT appearing to improve
survival. In this analysis, elderly GBM patients treated with
alternative regimens did not fare as well as those treated with
standard treatment protocols. However, in light of recent
randomized prospective studies that demonstrated a benefit using
HRT or TMZ alone, this discrepancy is likely due to patient
selection bias. With regard to combination therapy, the results of
NCIC CTG CE.6 will determine whether adding TMZ to HRT is superior
to HRT alone. A positive outcome may justify the use of this more
costly combination.
This study has used a national and comprehensive
database, hence minimizing discrepancies and biases that are
inherent to single-institution and single-provider studies. The
data include cancer characteristics, as well as composite clinical
and healthcare use information through claims for the United States
elderly population. However, this study is not without limitations.
Primarily, the limitations are those inherent to any retrospective
analysis. Furthermore. important factors, such as functional
status, extent of resection and quality of life, were not
available. In addition, the extraction of the cases was performed
with the use of ICD-9-CM codes and NDC number, both of which carry
a risk for miscoding. Our analysis only considered treatment
initiated within the first 90 days of surgery; thus, potential
secondary therapies were not included.
Future studies are required, that include more
recent patient data, particularly in light of the 2012 German and
Nordic trials, which may further alter practice patterns.
The availability of various treatment options also
brings to light the need for geriatric assessment tools. Currently,
physicians use best clinical judgment in making individualized
treatment decisions and chronological age may not be the optimal
marker for susceptibility to the potentially negative effects of
therapy. Such tools may assist treating physicians in better
managing this patient population.
References
|
1
|
Dolecek TA, Pro JM, Stroup NE and Kruchko
C: CBTRUS statistical report: Primary brain and central nervous
system tumors diagnosed in the United States in 2005–2009. Neuro
Oncol. 14:(Suppl 5). v1–v49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, et al:
European Organisation for Research and Treatment of Cancer Brain
Tumor and Radiotherapy Groups; National Cancer Institute of Canada
Clinical Trials Group: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brandes AA, Franceschi E, Toson A,
Benevento F, Scopece L, Mazzocchi V, Bacci A, Agati R, Calbucci F
and Ermani M: Temozolomide concomitant and adjuvant to radiotherapy
in elderly patients with glioblastoma: Correlation with MGMT
promoter methylation status. Cancer. 115:3512–3518. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arvold ND and Reardon DA: Treatment
options and outcomes for glioblastoma in the elderly patient. Clin
Interv Aging. 9:357–367. 2014.PubMed/NCBI
|
|
5
|
Combs SE, Wagner J, Bischof M, Welzel T,
Wagner F, Debus J and Schulz-Ertner D: Postoperative treatment of
primary glioblastoma multiforme with radiation and concomitant
temozolomide in elderly patients. Int J Radiat Oncol Biol Phys.
70:987–992. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minniti G and Enrici RM: Radiation therapy
for older adults with glioblastoma: Radical treatment, palliative
treatment, or no treatment at all? J Neurooncol. 120:225–233. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minniti G, Lanzetta G, Scaringi C,
Caporello P, Salvati M, Arcella A, De Sanctis V, Giangaspero F and
Enrici RM: Phase II study of short-course radiotherapy plus
concomitant and adjuvant temozolomide in elderly patients with
glioblastoma. Int J Radiat Oncol Biol Phys. 83:93–99. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao JQ, Fisher BJ, Bauman GS, Megyesi JF,
Watling CJ and Macdonald DR: Hypofractionated radiotherapy with or
without concurrent temozolomide in elderly patients with
glioblastoma multiforme: A review of ten-year single institutional
experience. J Neurooncol. 107:395–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
PERRY JR, O'Callaghan CJ, Ding K, et al: A
phase III randomized controlled trial of short-course radiotherapy
with or without concomitant and adjuvant temozolomide in elderly
patients with glioblastoma (NCIC CTG CE.6, EORTC 26062–22061, TROG
08.02, NCT 00482677). Neuro O ncol. 16:(Suppl 3). iii462014.
View Article : Google Scholar
|
|
10
|
Wick W, Platten M, Meisner C, et al:
NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German
Cancer Society: Temozolomide chemotherapy alone versus radiotherapy
alone for malignant astrocytoma in the elderly: The NOA-08
randomised, phase 3 trial. Lancet Oncol. 13:707–715. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malmström A, Grønberg BH, Marosi C, et al:
Nordic Clinical Brain Tumour Study Group (NCBTSG): Temozolomide
versus standard 6-week radiotherapy versus hypofractionated
radiotherapy in patients older than 60 years with glioblastoma: The
Nordic randomised, phase 3 trial. Lancet Oncol. 13:916–926. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gállego Pérez-Larraya J, Ducray F, Chinot
O, et al: Temozolomide in elderly patients with newly diagnosed
glioblastoma and poor performance status: An ANOCEF phase II trial.
J Clin Oncol. 29:3050–3055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin AA, Cai S, Dong Y, Zhang LH, Liu BL,
Cheng JX and Zhang X: A meta-analysis of temozolomide versus
radiotherapy in elderly glioblastoma patients. J Neurooncol.
116:315–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iwamoto FM, Reiner AS, Panageas KS, Elkin
EB and Abrey LE: Patterns of care in elderly glioblastoma patients.
Ann Neurol. 64:628–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barnholtz-Sloan JS, Maldonado JL, Williams
VL, Curry WT, Rodkey EA, Barker FG II and Sloan AE: Racial/ethnic
differences in survival among elderly patients with a primary
glioblastoma. J Neurooncol. 85:171–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arrigo RT, Boakye M and Skirboll SL:
Patterns of care and survival for glioblastoma patients in the
Veterans population. J Neurooncol. 106:627–635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deyo RA, Cherkin DC and Ciol MA: Adapting
a clinical comorbidity index for use with ICD-9-CM administrative
databases. J Clin Epidemiol. 45:613–619. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zouaoui S, Darlix A, Fabbro-Peray P, et
al: Oncological patterns of care and outcomes for 265 elderly
patients with newly diagnosed glioblastoma in France. Neurosurg
Rev. 37:415–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scott JG, Bauchet L, Fraum TJ, et al:
Recursive partitioning analysis of prognostic factors for
glioblastoma patients aged 70 years or older. Cancer.
118:5595–5600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Darefsky AS, King JT Jr and Dubrow R:
Adult glioblastoma multiforme survival in the temozolomide era: A
population-based analysis of Surveillance, Epidemiology, and End
Results registries. Cancer. 118:2163–2172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dubrow R, Darefsky AS, Jacobs DI, Park LS,
Rose MG, Laurans MS and King JT Jr: Time trends in glioblastoma
multiforme survival: The role of temozolomide. Neuro Oncol.
15:1750–1761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin AA, Zhang LH, Cheng JX, Dong Y, Liu
BL, Han N and Zhang X: Radiotherapy plus concurrent or sequential
temozolomide for glioblastoma in the elderly: A meta-analysis. PLoS
One. 8:e742422013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roa W, Brasher PM, Bauman G, et al:
Abbreviated course of radiation therapy in older patients with
glioblastoma multiforme: A prospective randomized clinical trial. J
Clin Oncol. 22:1583–1588. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noorbakhsh A, Tang JA, Marcus LP,
McCutcheon B, Gonda DD, Schallhorn CS, Talamini MA, Chang DC,
Carter BS and Chen CC: Gross-total resection outcomes in an elderly
population with glioblastoma: A SEER-based analysis. J Neurosurg.
120:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hegi ME, Diserens AC, Gorlia T, et al:
MGMT gene silencing and benefit from temozolomide in glioblastoma.
N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|