Introduction
The successful treatment of patients with head and
neck squamous cell carcinoma (HNSCC) depends on the early detection
and the appropriate therapy (1). The
incomplete resection of the primary tumour frequently leads to a
decrease in the survival rate as a consequence of increased risk of
recurrence; therefore, complete surgical resection of the primary
tumour is a crucial prognostic step for HNSCC (2,3).
Molecular markers can be used to establish tumour
free surgical margins and assist in the complete resection of the
tumour (4). The molecular marker,
p53, has been established to predict for recurrence in the surgical
margins (5,6). Mutation of the p53 gene leads to the
pathogenesis of patients with HNSCC exhibiting expression levels
ranging between 50 and 60% of the tumour cells (7,8). A
previous study suggests that immunohistochemical (IHC) staining
with p53 has an advantage over histopathology to identify the
patients at high risk for local recurrence (6). IHC analysis of the expression of p53 may
therefore be a diagnostic of margin status and prognostic in the
clinical management of HNSCC (9).
Overexpression of eukaryotic translation imitation
factor 4E (eIF4E) is involved in the initiation of protein
synthesis (10). However,
overexpression of eIF4E not only induces the transformation and
tumorigenesis, but also initiates metastasis (10). Previous studies have observed 100%
expression of eIF4E in HNSCC (11,12).
Clinically, the overexpression of eIF4E is commonly observed in a
variety of human tumour types, and its overexpression is usually
associated with disease progression, higher tumour recurrence rate
and tumour-associated mortality (13,14). In
previous studies of breast and bladder cancer, the overexpression
of eIF4E protein also correlated with an increased risk of disease
progression and poor prognosis (10,15,16).
Another previous study reported that the overexpression of eIF4E in
tumour free surgical margins correlated with local recurrence in
patients with HNSCC (17).
The 5-year survival rate for HNSCC remains low at
~50% (18,19). A five year survival rate for HNSCC is
significantly lower compared with other cancer types, including
colorectal, cervix and breast (18,20). With
the advent of novel surgical procedures, improved radiotherapy and
concomitant chemotherapy, there is a considerable improvement in
the local rates (6). By contrast, the
survival rates for HNSCC have failed to significantly improve
(21,22) since it is generally believed that
incomplete resection of the primary tumour is the principal reason
of local recurrence and mortality from HNSCC (2).
The present retrospective clinical study aimed to
investigate the prognostic significance of the molecular markers,
p53 and eIF4E, in the histologically tumour free surgical margins
of HNSCC. In addition, the present study was designed to analyse
the association between the expression levels of the p53 and eIF4E
molecular markers with the clinical outcomes, including recurrence
and survival, and also assessed whether eIF4E is more sensitive
compared with p53 in predicting for the risk of recurrence.
Materials and methods
Study design
The present retrospective clinical study was
performed on patients who underwent primary surgical resection for
HNSCC. A total of 48 patients with HNSCC diagnosed at the Royal
Darwin Hospital between 2006 and 2009 were identified. Out of the
48 patients, 24 were selected based on hematoxylin and eosin
(H&E) staining, and hospital records. The present study was
approved by the Human Research Ethics Committee of the Northern
Territory, Department of Health and Menzies School of Health
Research (HR-10-1490).
Selection criteria
The inclusion criteria were as follows: i) The
patient was treated at the Royal Darwin Hospital throughout their
management; ii) The patients underwent surgical resection of a
mucosal oropharyngeal cancer at Royal Darwin Hospital between 2006
and 2009; iii) The hospital reports for the patients were
available; iv) The patient slides were available. The exclusion
criteria were as follows: i) Patients with nodal diseases; ii)
Patients who were lost at follow-up.
Specimen collection
Paraffin-embedded tissue blocks from the surgical
margins were obtained following the surgical resection and final
pathology reports showing that the blocks were histologically free
of tumour. In the present clinical retrospective study, a
pathologist reviewed all the H&E stained sections to confirm
tumour-free surgical margins. Another two 5 µm thick sections were
mounted onto poly-L-lysine-coated slides for IHC staining with
mouse monoclonal anti-human anti-p53 (cat. no. M7001; 1:200; Dako
Australia Pty, Ltd., Campbellfield, VIC, Australia) and rabbit
polyclonal anti-human anti-eIF4E (cat. no. ab47482; 1:500; Abcam,
Cambridge, MA, USA) antibodies. The details of the staining
technique and analysis are described below.
Clinical data entry
The anatomical pathology department based at the
Royal Darwin Hospital coded each specimen at the time of
acquisition and the specimen was tracked using that code. A
computerised notebook linked the code numbers to the patient data
and hospital records. In the present study, the pathologists scored
the tumours in the surgical margins in a blinded manner. In
addition, data entry was coded and no inadvertent bias was
presented to the physician during patient follow-up.
IHC staining
The Ventana Benchmark XT machine (Ventana Medical
Systems, Inc., Tucson, AZ, USA) has a programmed p53 and eIF4E IHC
procedure file for processing the tissue samples. Each procedure
file consisted of a specific sequence for buffer rinse, enzyme
inhibitors, blocking serum, antibody detection complexes,
chromogens and counter-stains. These reagents were used according
to the manufacturer's instructions (Ventana Medical Systems,
Inc.).
Following a series of buffer rinses and normal serum
pre-incubation, 100 µl monoclonal mouse p53 antibody (Dako Pty
Ltd., Campbellfield, VIC, Australia) at a dilution of 1:200 and
polyclonal rabbit eIF4E (Abcam, Cambridge, MA, USA) antibody at a
dilution of 1:500 were dispensed per slide. The slides were treated
with streptavidin-enzyme conjugate with diaminobenzidine (Dako Pty
Ltd.).
At the end of the automated staining process, the
slides were washed in hot water with detergent for 3 min to remove
the oily material and were subsequently dehydrated through a series
of ethanol dilutions, followed by treatment in xylene. Finally,
cover slips were placed on each slide and fixed with the permanent
mounting material.
Microscopic examination
All immunohistochemically stained slides were
assessed for the expression levels of p53 and eIF4E protein by
light microscopy by two clinical pathologists in a blinded manner.
The stained slides of tumour margins were evaluated for the protein
expression levels of p53 and eIF4E. The 5% cut-off value was
selected on the basis of the expression levels of p53 and eIF4E in
tumour cells in the basal cell layer of surgical margin (7). Therefore, surgical margins were
considered as positive if IHC staining was observed in >5% of
the cells in the epithelial layer.
These tumour margins were considered as positively
expressing p53 if there was a brown nuclear staining in the basal
cell layer of epithelium. Similarly, surgical margins were
considered as positively expressing eIF4E if there was a reddish
brown peri-nuclear cytoplasmic staining of >5% in the layer of
epithelium.
Statistical analysis
The clinical characteristics of patients with HNSCC
and surgical margins were examined statistically using SAS version
9.3 software (SAS Institute Inc., Cary, NC, USA). The time prior to
recurrence was considered as the period from surgery until the date
of the first documented recurrence among the patients (7). Local recurrence was defined as a
recurrence in the original tumour bed (23). The overall survival time was defined
as the interval between the dates of the beginning of the treatment
(surgery) and mortality, or the last information for censored
observations (24).
Contingency tables and Fisher's exact tests were
used to evaluate the association of p53 and eIF4E in the surgical
margins with age, sex, race, anatomical site, tumour stage, nodal
status, nodal stage, post-operative therapy and histological grade.
The recurrence and survival rate were estimated by the Kaplan-Meier
method and compared using the log-rank test (7,25).
Results
Clinical characteristics of the
patients
The clinical characteristic records of the 24
patients with HNSCC were obtained from the Royal Darwin Hospital
and the Department of Births, Deaths and Marriages (Darwin,
Australia). Those records were reviewed to assess patient survival.
The clinical characteristics included age, sex, race, anatomical
site, tumour stage, nodal status, nodal stage, post-operative
radiation therapy and histological grade. The median age was 60
years (range, 46–81 years). A total of 21 patients were male and
three were female; 4 were indigenous and 20 were non-indigenous.
The median follow-up period was 74 months (range, 1–74 months). Of
the 24 patients, 12 patients succumbed to mortality and 12 patients
remained alive at the end of the present study. The floor of the
mouth was the most common anatomical sub-site (14/24; 58.3%),
followed by the tongue (5/24; 20.8%), lips (3/24; 12.5%) and tonsil
(2/24; 8.4%). No statistically significant association was observed
between the expression of p53 and eIF4E, and the clinical
characteristics of patients with HNSCC (Table I).
 | Table I.Clinical characteristics of 24
patients, according to the expression levels of p53 and eIF4E on
tumour margin. |
Table I.
Clinical characteristics of 24
patients, according to the expression levels of p53 and eIF4E on
tumour margin.
|
| p53 |
| eIF4E |
|
|---|
|
|
|
|
|
|
|---|
| Clinical
characteristic | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Age, Mean, years | 60 | | | 60 | | 0.2 |
| Gender |
|
|
|
|
|
|
|
Female | 1 | 2 |
| 0 | 3 |
|
| Male | 3 | 1 | 0.64 | 3 | 18 | 1.0 |
| Race |
|
|
|
|
|
|
|
Indigenous | 2 | 2 |
| 0 | 4 |
|
|
Non-Indigenous | 9 | 11 | 0.85 | 3 | 17 | 1.0 |
| Anatomical sites |
|
|
|
|
|
|
| Floor of
mouth | 8 | 6 |
| 1 | 13 |
|
|
Tongue | 1 | 2 |
| 1 | 2 |
|
| Lips | 2 | 3 |
| 1 | 4 |
|
|
Tonsil | 0 | 2 | 0.6 | 0 | 2 | 0.46 |
| Tumour stage |
|
|
|
|
|
|
| T1 | 4 | 7 |
| 1 | 10 |
|
| T2 | 3 | 3 |
| 1 | 5 |
|
| T3 | 0 | 0 |
| 0 | 0 |
|
| T4 | 4 | 3 | 0.67 | 1 | 6 | 0.88 |
| Nodal status |
|
|
|
|
|
|
|
Negative | 8 | 10 |
| 3 | 15 |
|
|
Positive | 3 | 3 | 0.81 | 0 | 6 | 0.54 |
| Nodal stage |
|
|
|
|
|
|
| N0 | 8 | 10 |
| 3 | 15 |
|
| N1 | 2 | 3 |
| 0 | 5 |
|
|
N2b | 1 | 0 | 0.81 | 0 | 1 | 1.0 |
| Post-op XRT |
|
|
|
|
|
|
| No | 5 | 3 |
| 2 | 6 |
|
|
Yes | 6 | 10 | 0.25 | 1 | 15 | 0.2 |
| Histological
grade |
|
|
|
|
|
|
| Poorly
differentiated | 3 | 1 |
| 1 | 3 |
|
|
Moderate differentiated | 2 | 4 | 1 | 5 |
|
|
| Well
differentiated | 7 | 7 | 0.61 | 1 | 13 | 0.5 |
IHC analysis for p53 and eIF4E
Of the 24 patients with histologically negative
margins, 13 (54.2%) patients exhibited p53-positive and 21 (87.5%)
patients exhibited eIF4e-positive, with at least one of the tumour
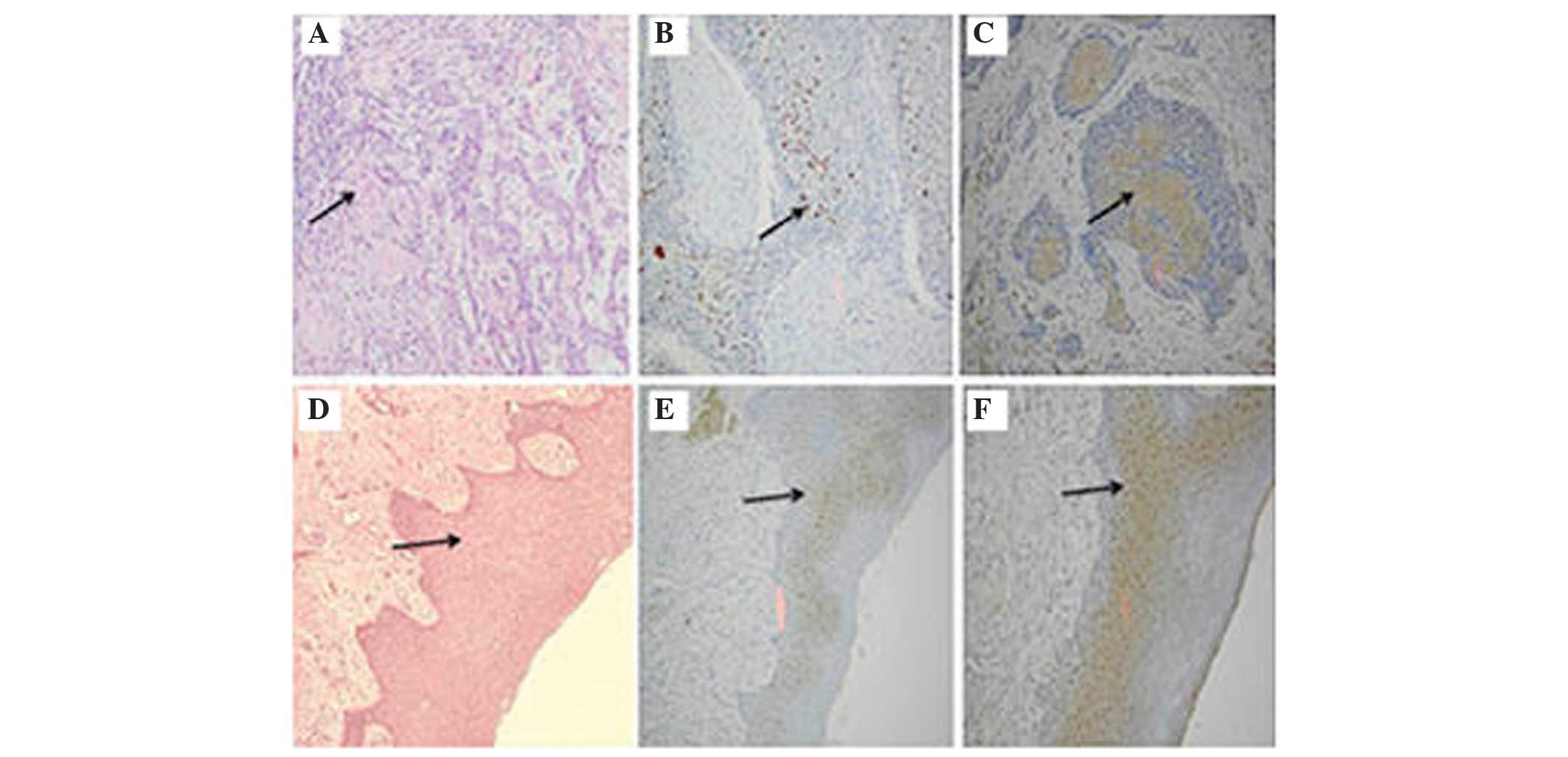
margins positively stained. Fig. 1
demonstrated an HNSCC primary tumour and surgical margins stained
with H&E, p53 and eIF4E markers, respectively. The sections of
solid tumour stained with H&E showed cords and nests of
malignant squamous epithelial cells exhibiting nuclear
pleomorphism, hyperchromatism and keratin pearls (Fig. 1A). The identical tumour section was
also stained for p53 and exhibited dark brown staining of the
nucleus of the tumour cells (Fig.
1B). In addition, the identical tissue section was also stained
with eIF4E antibody and exhibited brown peri-nuclear cytoplasmic
staining of the tumour cells in the epithelial layer (Fig. 1C).
Furthermore, Fig. 1D and
E illustrated negative surgical margins with H&E staining
and p53 IHC staining, respectively. However, the identical tumour
margin exhibited positive staining for eIF4E (brown peri-nuclear
cytoplasmic) around the residual cancer cells in the epithelial
layer (Fig. 1F).
Association of p53 and eIF4E
overexpression with clinical outcomes
Of the 24 patients with HNSCC, 7 (29.2%) patients
exhibited tumour recurrence and 12 (50%) patients succumbed to
mortality during the follow-up period. For those patients who
exhibited p53-positive staining, 3/13 patients (23.1%) had local
recurrence and 10 (76.9%) showed no recurrence. In the identical
way, the patients who were eIF4e-positive, 6/21 patients (28.5%)
had a recurrence and 15 (71.5%) showed no recurrence. In addition,
10/11 (90.9%) patients with p53-negative margins and 2/3 patients
(66.6%) with eIF4e-negative margins had no local recurrence.
However, one patient with a recurrence exhibited a p53- and
eIF4e-negative surgical margin. No significant difference was
observed with regards to local recurrence for p53-positive and
p53-negative patients (P=0.88). Similarly, no statistical
significance was observed in the local recurrence in patients
exhibiting eIF4e-positive or eIF4e-negative surgical margins
(P=0.99; Table II).
 | Table II.Local recurrence of 24 patients,
according to the expression levels of p53 and eIF4E on tumour
margin. |
Table II.
Local recurrence of 24 patients,
according to the expression levels of p53 and eIF4E on tumour
margin.
|
| p53 |
| eIF4E |
|
|---|
|
|
|
|
|
|
|---|
| Local
recurrence | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Non-recurrence | 10 | 7 |
| 15 | 2 |
|
| Recurrence | 3 | 4 | 0.88 | 6 | 1 | 0.99 |
A total of 12/24 patients (50%) during the follow-up
period and 7/13 p53-positive patients (53.8%) succumbed to
mortality . It was shown that 6 (46.2%) patients who survived
exhibited positive p53 expression in their surgical margins. Of the
21 patients with eIF4e-positive margins, 10 patients succumbed to
mortality and 11 remained alive. No significant association was
observed between the overall survival, and p53-positive and
negative surgical margins (P=0.36). Similarly, no association was
identified between eIF4e-positive and negative margins, and the
overall survival of patients with HNSCC (P=0.64).
Table III compared
the comparative study of recurrence and survival of patients with
HNSCC, with p53 and eIF4E status of surgical margins. The patients
were divided into two groups, the recurrence group and
non-recurrence group. In the recurrence group, one (4.2%) patient
survived with positive p53 and eIF4E surgical margins, and two
(8.3%) patients exhibiting the identical pattern succumbed to
mortality. In the non-recurrence group, 5 (20.8%) patients survived
with positive p53 and eIF4E surgical margins; whereas, 5 (20.8%)
patients succumbed to mortality during the follow-up period.
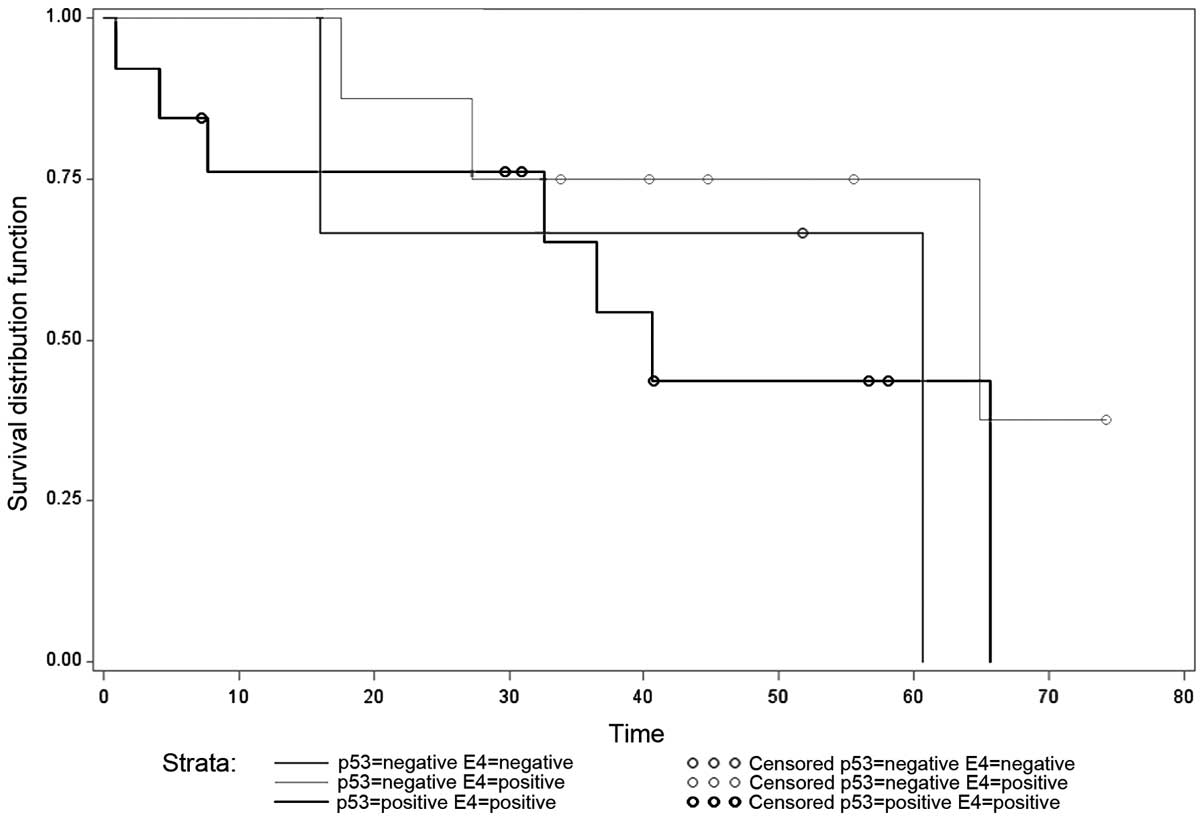
Fig. 2 demonstrated the Kaplan-Meier
curve with three combinations of p53 and eIF4E expression,
including p53-positive and eIF4E-negative, p53-positive and
eIF4E-negative, and p53-positive and eIF4E-positive. No significant
differences were observed between these three different
combinations (P=0.46).
 | Table III.Recurrence and survival of head and
neck squamous cell carcinoma patients according to the p53 and
eIF4E status of surgical margin. |
Table III.
Recurrence and survival of head and
neck squamous cell carcinoma patients according to the p53 and
eIF4E status of surgical margin.
|
| p53 expression | eIF4E
expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
outcomes | Positive (%) | Negative (%) | Positive (%) | Negative (%) | p53/eIF4e-positive
(%) |
|---|
| Recurrence |
|
|
|
|
|
|
Alive | 1 (14.3) | 2 (28.6) | 3 (42.8) | 0 (0) | 1 (4.2) |
|
Died | 2 (28.6) | 2 (28.6) | 3 (42.8) | 1 (14.1) | 2 (8.3) |
| No recurrence |
|
|
|
|
|
|
Alive | 5 (29.4) | 4 (23.5) | 8 (47.1) | 1 (5.8) | 5 (20.8) |
|
Died | 5 (29.4) | 3 (17.7) | 7 (41.2) | 1 (5.9) | 5 (20.8) |
Discussion
Immunodiagnostic investigation of the overexpression
of p53 and eIF4E in the surgical margin may contribute to early
detection of residual disease. The patients with a tumour received
a more direct and effective treatment therapy to improve the
survival rate. The results from Nathan et al (7) showed the impact of the overexpression of
p53 and eIF4E in the recurrence and survival in HNSCC. The
overexpression of eIF4E is more consistent compared with p53 HNSCC
(4). Therefore, eIF4E is often
considered as a specific tumour marker for HNSCC, and is more
sensitive compared with p53 (26).
The overexpression of eIF4E is more common in HNSCC
compared with p53 and it occurs earlier in the process of
tumorigenesis (7). Mutations of p53
in HNSCC are not only a common event, but also occur comparatively
late in tumour progression (7). In
patients with HNSCC, p53 expression has been observed as a late
event in the carcinogenesis (27).
Therefore, mutations in p53 are less common in pre-invasive cancer
compared with in invasive cancer (28). Li et al (26) reported that the overexpression of p53
was observed in 63/112 patients (56.3%), while that of eIF4E was
observed in 105 (93.8%). Nathan et al (13) identified 22/54 patients (42%) with
p53-positive and 27 (52%) with eIF4E-positive staining in the
surgical margins of patients with HNSCC. In the present
retrospective study, the overexpression of p53 was identified in
13/24 patients (54.2%) and overexpression of eIF4E was identified
in 21 patients (87.5%) with HNSCC. A lower frequency of p53
expression in surgical margins may be attributed to the small
sample size in the present study.
Overexpression of eIF4E in the primary tumours was
not a significant predictor of recurrence (7). It appeared that the majority of solid
tumours require an elevated expression of eIF4E for tumour
progression. However, p53 and eIF4E expression in surgical margins
were often associated with an increase in recurrence rate (7). Nathan et al (13) reported that, 14/52 patients exhibited
recurrence and a significant difference was observed in the
recurrence rate between eIF4E-positive and eIF4E-negative margins
(P=0.003). This previous study reported that 12/14 recurrent
patients (86%) were identified with eIF4e-positive margins.
However, no significant difference was observed between
p53-positive and p53-negative margin recurrence rate (P=0.11)
(13). A previous study showed that
33.3% (9/27) of recurrent patients were p53-positive margins and
eIF4E-positive was 63.6% (14/27), demonstrating a significant
difference in the HNSCC margin (P=0.006) (16).
In the present retrospective study, 6/7 cancer
recurrent patients (85.7%) had eIF4e-positive margins, whereas only
three patients had recurrence (42.8%) with p53-positive margins.
All three recurrent patients with p53 overexpression also exhibited
eIF4E overexpression in the surgical margins. In addition, these
results revealed no significant association between the expression
levels of p53 and eIF4E with local recurrence (P=0.46). The small
numbers of patients with HNSCC in the present study may not be
sufficient to demonstrated the recurrence risk with respect to
expression of p53 and eIF4E on surgical margins.
Liangping et al (16) reported that the 5 year survival rate
in the p53-positive margin (13/67 cases) and p53-negative margin
group (54/67 cases) was 24.62 and 75.69% respectively, with the
difference being significant (P=0.0012). In that same study, the
5-year survival rate with the eIF4e-positive group (22 cases) and
eIF4e-negative group (45 cases) was 43.31 and 77.52%, respectively,
and the results showed significant differences (P=0.0006).
In the present study, 10/12 patients (83.3%) with
eIF4e-positive surgical margins, and seven patients (53.3%) with
p53-positive margins succumbed to mortality. All 7 patients who
succumbed to mortality with p53-positive margins also exhibited
eIF4e-positive surgical margins. Statistically, no significant
difference was observed when the p53-positive and p53-negative
patients were compared for overall survival (P=0.36). Similarly, no
significant differences were observed between eIF4e-positive and
eIF4e-negative patients with HNSCC (P=0.64). Therefore, the present
study observed that patients with eIF4E overexpression had a higher
mortality rate compared with patients with p53 overexpression,
although this difference was not statistically significant.
In the present study, non-significant results were
obtained regarding the association between p53 and eIF4E
overexpression, and the clinical outcomes of the 24 HNSCC patients.
However, the expression of eIF4E appears to be a more marked
prognosticator compared with p53 since the overexpression of eIF4E
was observed in the margins of 6/7 patients who had local
recurrence and p53 was observed in only three patients. Therefore,
a prospective study in the near future with a larger number of
patients with HNSCC would be ideal to analyse the sensitivity and
specificity of eIF4E in the management of HNSCC and to unmask the
contribution of overexpression in association with recurrence and
overall survival.
Acknowledgements
The present clinical study was supported by the
School of Psychological and Clinical Sciences, Charles Darwin
University, Australia. We also want to thank the clinical
pathologists based at the Anatomical Pathology Department at Royal
Darwin Hospital for their assistance, which was vital for the IHC
work in the present study.
References
|
1
|
Rezende TM: deS ouza Freire M and Franco
OL: Head and neck cancer: Proteomic advances and biomarker
achievements. Cancer. 116:4914–4925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Armstrong W, Vokes DE and Maisel RH:
Malignant tumors of the larynx, cummings otolaryngology head &
neck surgery (5th). America Mosby Elsevier. 482–511. 2010.
|
|
3
|
Jalali MM, Heidarzadeh A, Zavarei MJ and
Sarmast H: p53 overexpression impacts on the prognosis of laryngeal
squamous cell carcinomas. Asian Pac J Cancer Prev. 12:1731–1734.
2011.PubMed/NCBI
|
|
4
|
Nathan CO, Franklin S, Abreo FW and Nassar
R: DeB enedetti A and Glass J: Analysis of surgical margins with
the molecular marker eIF4E: A prognostic factor in patients with
head and neck cancer. J Clin Oncol. 17:2909–2914. 1999.PubMed/NCBI
|
|
5
|
Breda A, Konijeti R and Lam JS: Patterns
of recurrence and surveillance strategies for renal cell carcinoma
following surgical resection. Expert Rev Anticancer Ther.
7:847–862. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Houten VM, Leemans CR, Kummer JA,
Dijkstra J, Kuik DJ, van den Brekel MW, Snow GB and Brakenhoff RH:
Molecular diagnosis of surgical margins and local recurrence in
head and neck cancer patients, A prospective study. Clin Cancer
Res. 10:3614–3620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nathan CO, Sanders K, Abreo FW, Nassar R
and Glass J: Correlation of p53 and the proto-oncogene eIF4E in
larynx cancers, Prognostic implications. Cancer Res. 60:3599–3604.
2000.PubMed/NCBI
|
|
8
|
Bradford CR, Kumar B, Bellile E, Lee J,
Taylor J, D'Silva N, Cordell K, Kleer C, Kupfer R, Kumar P, et al:
Biomarkers in advanced larynx cancer. Laryngoscope. 124:179–187.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gasco M and Crook T: The p53 network in
head and neck cancer. Oral Oncol. 39:222–231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Benedetti A and Graff JR: eIF-4E
expression and its role in malignancies and metastases. Oncogene.
23:3189–3199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cardesa A and Nadal A: Carcinoma of the
head and neck in the HPV era. Acta Dermatovenerol Alp Pannonica
Adriat. 20:161–173. 2011.PubMed/NCBI
|
|
12
|
Nathan CO, Liu L, Li B, Abreo FW, Nandy I
and De Benedetti A: Detection of the proto-oncogene eIF4E in
surgical margins may predict recurrence in head and neck cancer.
Oncogene. 15:579–584. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nathan CO, Amirghahri N, Rice C, Abreo FW,
Shi R and Stucker FJ: Molecular analysis of surgical margins in
head and neck squamous cell carcinoma patients. Laryngoscope.
112:2129–2140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salehi Z and Mashayekhi F: Expression of
the eukaryotic translation initiation factor 4E (eIF4E) and 4E-BP1
in esophageal cancer. Clin Biochem. 39:404–409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crew JP, Fuggle S, Bicknell R and Cranston
DW: deB enedetti A and Harris AL: Eukaryotic initiation factor-4E
in superficial and muscle invasive bladder cancer and its
correlation with vascular endothelial growth factor expression and
tumour progression. Br J Cancer. 82:161–166. 2000.PubMed/NCBI
|
|
16
|
Liangping X: The prognostic value of
pathological and molecular margins marked by p53 and elF4E in
laryngeal carcinoma. Chinese-German J Clin Oncol. 4:56–60.
2005.
|
|
17
|
Chakraborty S, Mohiyuddin SM, Gopinath KS
and Kumar A: Involvement of TSC genes and differential expression
of other members of the mTOR signaling pathway in oral squamous
cell carcinoma. BMC Cancer. 8:1632008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kokko LL, Hurme S, Maula SM, Alanen K,
Grénman R, Kinnunen I and Ventelä S: Significance of site-specific
prognosis of cancer stem cell marker CD44 in head and neck
squamous-cell carcinoma. Oral Oncol. 47:510–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clark C, Shah S, Herman-Ferdinandez L,
Ekshyyan O, Abreo F, Rong X, McLarty J, Lurie A, Milligan EJ and
Nathan CO: Teasing out the best molecular marker in the AKT/mTOR
pathway in head and neck squamous cell cancer patients.
Laryngoscope. 120:1159–1165. 2010.PubMed/NCBI
|
|
21
|
Partridge M, Costea DE and Huang X: The
changing face of p53 in head and neck cancer. Int J Oral Maxillofac
Surg. 36:1123–1138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chao YK, Chuang WY, Yeh CJ, Chang YS, Wu
YC, Kuo SY, Hsieh MJ and Hsueh C: High phosphorylated 4E-binding
protein 1 expression after chemoradiotherapy is a predictor for
locoregional recurrence and worse survival in esophageal squamous
cell carcinoma patients. J Surg Oncol. 105:288–292. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Waitzberg AF, Nonogaki S, Nishimoto IN,
Kowalski LP, Miguel RE, Brentani RR and Brentani MM: Clinical
significance of c-myc and p53 expression in head and neck squamous
cell carcinomas. Cancer Detect Prev. 28:178–186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song HS, Do YR, Kang SH, Jeong KY and Kim
YS: Prognostic significance of immunohistochemical expression of
p53 gene product in operable breast cancer. Cancer Res Treat.
38:218–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li S and Wei Q: The expression and the
clinical significance of eukaryotic translation initiation factors
4E and p53 in squamous cell carcinoma. Chinese-German J Clin Oncol.
8:286–288. 2009. View Article : Google Scholar
|
|
27
|
Boyle JO, Hakim J, Koch W, van der Riet P,
Hruban RH, Roa RA, Correo R, Eby YJ, Ruppert JM and Sidransky D:
The incidence of p53 mutations increases with progression of head
and neck cancer. Cancer Res. 53:4477–4480. 1993.PubMed/NCBI
|
|
28
|
Pihan GA, Wallace J, Zhou Y and Doxsey SJ:
Centrosome abnormalities and chromosome instability occur together
in pre-invasive carcinomas. Cancer Res. 63:1398–1404.
2003.PubMed/NCBI
|