Introduction
Nowadays, as a result of the vast development of
therapeutic intervention in lung cancer, including targeted
therapy, thousands of patients have a longer overall survival and
an improved quality of life. Although lung cancer remains the
leading cause of cancer-associated mortality worldwide, the
mortality of the malignant tumor has reduced slightly (1,2). Notably,
metastasis is responsible for as much as 90% of cancer-associated
mortality. Lung cancer exhibits a strong predilection to develop to
bone metastasis, which would bring not only physical torment,
including pain, broken bones and spinal compression, but also the
mental affliction to the patients, which greatly reduce the quality
of life and overall survival of the patients (3,4). Despite
the fact that the medical management of bone metastases in lung
cancer has made great progress, the effective curative clinical
therapy is limited (5). Additionally,
the mechanism regarding bone metastasis remains poorly understood
in the pathogenesis of lung cancer. In order to have a general
understanding of the mechanisms of bone metastasis in lung cancer,
the present review outlined the relative previous studies and
suggested the possible mechanisms involved.
According to accumulating evidence, the bone marrow
is one of the most common places for the lung cancer cells to
metastasize (6). With the bone
organophilic phenomenon, Paget's ‘seed and soil’ hypothesis may
provide certain reasonable explanation (7). However, contrary to the passive course
of the seed planted into the soil, it is an active process for the
lung cancer cells colonize (‘seed’) in the bone marrow (‘soil’),
under the driving effect of multiple molecules, signaling pathways
and cells. Bone metastasis in lung cancer is the completion of a
complex succession of cell-biological events, in which there are
multiple interactions of cancer cells with host cellular and
extracellular microenvironments being involved, as well as
different types of molecules, including cytokines (8), adhesion molecules, hormones (9) and chemokines (10,11). It
was demonstrated that three types of lesions exist in bone
metastases, osteoblast-mediated bone formation, osteoclast-mediated
bone resorption and a mixture of each (12). The osteolytic lesion is predominant in
the bone metastasis of lung cancer. The present review aimed to
introduce the functions of various molecules involved in different
processes of bone metastasis in lung cancer. Considering of the
‘seed and soil’ hypothesis, the present review will begin with the
‘seed’, the lung cancer cell, and discuss its metastatic
potential.
The ‘seed’ - lung cancer stem cells
A large quantity of experimental evidence supports
there being a group of cells in the tumor exhibiting the ability of
self-renewal, multilineage differentiation and superior levels of
malignancy. These cells may be termed cancer stem cells (CSCs)
(13,14). It has been experimentally demonstrated
that the CSCs exist in an unperturbed solid tumor (15). CSCs have been identified in breast
(16), colon (17,18),
pancreatic (19), prostate (20–22) and
brain cancer (23). Additionally,
CSCs have been identified and isolated from lung cancer, and it was
demonstrated that the lung CSCs express tissue-specific cell
surface markers, including cluster of differentiation (CD)133+
(24). In addition, these lung CSCs
are thought to have high drug resistance and tumorigenicity, owing
to tumor regeneration following chemotherapy (25,26). For
this reason, certain scholars have selected the lung CSCs which
expressed tissue-specific cell surface markers, including CD133,
CD117 and nuclear β-catenin, and do not express differentiation
markers, including cytokeratin (CK)8/18. It was revealed that these
CSCs, through an efficient cytokine network production, have high
tumorigenic and metastatic potentials (27).
However, with the exception of intrinsic CSCs, the
neighboring cancer cells may acquire CSC-like characteristics under
the interaction with the active stroma (28). The epithelial-to-mesenchymal
transition (EMT) is a cell-biological program, in which the
epithelial cells change to exhibit the traits of cells
disseminating from primary tumors and seeding metastases (29–31).
Experimental data has revealed that the non-CSCs can be induced to
enter into a CSC-like state via the EMT (32,33).
However, the induced CSCs may well lapse back to a fully epithelial
state without CSC function (34). To
a certain extent, the transformation of the tumor cells may not
completely allow the cells to obtain the function of CSCs,
therefore, they may be in the stage between the epithelial cell and
induced CSCs (35). In order to study
the biology of migrating CSCs, the epithelial-specific and
cell-surface markers involved in the EMT are used to detect the
circulation tumor cells (CTCs) (36–38). Using
a blood filtration approach in patients with lung cancer, Hou et
al (39) observed the CTCs lost
the expression of E-cadherin, while obtained the expressing of the
vimentin, indicating that the CTCs performed a feature of EMT, when
the CSCs or induced CSCs enter into the circulation (39).
Despite this, no definite evidence exists to affirm
that it is the CSCs that launch the distant metastasis. Considering
the ability of self-renewal, multilineage differentiation and
superior levels of malignancy, it is generally thought the CSCs are
the ‘seed’ to plant into the distant ‘soil’. Whether certain lung
CSCs perform the bone organophilic property requires further
investigation.
Escaping from the primary tumor
Tumor cells escaping from the tumor
mass
Prior to the metastases, the tumor cells are tightly
bound to neighboring cells and to the underlying basement membranes
through adheren junctions, tight junctions, desmosomes and
hemi-desmosomes. These tight physical constraints immobilize the
cells effectively as a whole. As the carcinoma progresses, the
tumor cells have to break away from the constraints, preparing for
metastases.
Initially, the intercellular adhesion molecule
changes the features of the adhesion between the tumor cells that
make the tumor cells remove themselves from the tumor cell mass.
Numerous types of adhesion molecules exist, in which the E-cadherin
is a direct mediator of intercellular adhesion. Reduction of
E-cadherin causes the tumor cells to invade and metastasize early
(40). In a meta-analysis of
non-small cell lung cancer (NSCLC), the reduction or lack of
E-cadherin represented the high motility of the tumor cells and
indicated a poor prognosis (41).
It has also been revealed that it is necessary,
although not sufficient, for the EMT to reduce the E-cadherin
function, which enables the detachment and reorganization of
epithelial-cell sheets in tumor invasion and metastasis (42,43). It
was previously observed in A549 cells that transforming growth
factor (TGF)-β1 induces the EMT by upregulating the expression of
mesenchymal markers, including vimentin and Slug, and
downregulating the levels of epithelial markers, including
E-cadherin and cytokeratins (44).
Zeb1 and Snail1 negatively regulate the expression of E-cadherin
(28), and a previous study
demonstrated that Wnt signaling can accelerate bone metastasis in a
lung cancer model via the upregulation of Snail1 and Zeb1, and
down-regulating E-cadherin (45).
Besides E-cadherin, selectins and integrins are
involved in the process of the dissociation of the tumor cells from
the mass. The successful dissociation is the result of the
cooperation of these molecules, thus, more studies are required to
analyze the complex mechanism in the bone metastasis of lung
cancer.
Tumor cells breaking away from the
ECM
When the carcinoma cells break-away from the tumor
mass, they have to pass through the extracellular matrix (ECM), a
structural framework consisting of fibrous proteins and
proteoglycans (46). Firstly, the
cells must traverse the basement membrane (BM), a specialized ECM,
and subsequently invade the adjacent stromal compartments. The
proliferation of the tumor forms a microenvironment where the tumor
cells interact with various cell types within the ECM, including
the endothelial cells, tumor-associated macrophages (TAM) and
fibroblasts (47). For instance,
under the stimulation of tumor-derived colony stimulating factor 1,
the TAM not only proliferate, but also produce growth factors,
including fibroblast growth factor, epidermal growth factor
receptor ligands and platelet-derived growth factor (PDGF), and
proteases, including matrix metalloproteinases (MMPs) and the
cathepsins (48).
Various types of proteinases degrade the ECM for
distant metastasis, while the MMPs, including MMP2 and MMP9
(49), are regarded as the major
enzymes to make the ECM. It was revealed that MMP9 and MMP13 are
involved in mediating cell migration and invasion in NSCLC
(50). Additionally, previous
clinical research (51) revealed that
with the expression of MMP13, the carcinoma cells of NSCLC patients
are found more easily in the bone marrow, indicating that MMP13 may
be one of the predictive factors for the patients with NSCLC that
may develop bone metastasis. It has been reported that miRNA
(miR)-29c suppresses the adhesion of lung cancer cells to the ECM
and their metastasis by targeting integrin β1 and MMP2 (52). Currently, in the field of bone
metastasis in lung cancer, the degradation mechanism predominantly
focused on the MMPs, which perform the similar effect in other
cancer types.
Moving in the circulation
Intravasation of the tumor cells
As mentioned above, endothelial cells exist in the
microenvironment within the ECM, which are attracted by the
angiogenic stimuli produced by the tumor cells to migrate (53). Under the synthetic action of several
signaling pathways, including vascular endothelial growth factor
(VEGF), hypoxia-inducible factors and Notch, and several ECM
proteins, the endothelial cells gradually build the new blood
vessels (54), which provide a
highway for the tumor cells to metastasize to distant sites. The
induced blood vessels are generally leaky, with weak cell-cell
junctions, and the tumor cells can enter vasculature through the
crack easily (55).
Beyond the new blood vessels, the tumor cells exit
the ECM and arrive at the intrinsic vessels. Similar to the
degradation of the BM, the tumor cells produce proteases to
break-down the basement membrane outside of the vessels, then with
the motility to adhere to the endothelial cells. Finally, the tumor
cells enter into the vasculature with the ameboid movement.
When the carcinoma cells enter into the circulatory
system, they can be termed circulating tumor cells (CTCs), which
are so rare that they are found at a frequency as low as 1 CTC per
106/7 leukocytes, with much lower numbers in early stage disease
(56). However, numeorus studies have
indicated that CTCs offer the prospect of under-key intermediaries
between the primary site and metastases (57,58).
Although CTCs are extremely rare in the circulation, they can be
detected even prior to the definitive diagnosis of lung cancer, as
well as at other stages (59).
Increasing clinical evidence in lung cancer has shown that the
counts of the CTCs have a significant association with a worse
prognosis (60–62). Additionally, it is popularly
hypothesized that the metastasis has an intimate association with
the circulating tumor microemboli (CTM). As an aggregate of CTCs,
the CTM is either associated with other tumor cells or associated
with fibroblasts, leukocytes, endothelial cells or platelets
(63), which assist with avoiding the
destruction of the immune system in the circulation, as well as to
assist tumor cells extravasation (64). In a previous pilot study, the CTM was
found not only in patients with samll cell lung cancer (SCLC), but
also in NSCLC. In comparison with CTCs, CTM has a higher propensity
to metastasize, and the cardinal feature of EMT has been observed
in the majority of CTM in patients with lung cancer (65). In SCLC, P-selectin can induce the
combination of the SCLC cells and activated platelets, therefore
facilitating the formation of the CTM (66).
According to these previous studies, it may be
hypothesized that when the tumor cells permeate into the vessels,
certain components of the blood combine with the tumor cells and
convoy them to the target organs, which from a dynamic
microenvironment for the tumor cells in the circulation.
Oriented moving in the
circulation
According to the routine, the tumor cells in the
circulation will move with the bloodstream and they will have the
same chance to metastasize throughout the whole body. However, this
may not to be the case. In lung cancer, the tumor cells have a
preference for the bone (67), as
well as the brain, liver and adrenal gland. Previous experimental
research has demonstrated that sites of metastasis are
co-determined by the characteristics of carcinoma cells and the
microenvironment of the target organ (68), the precise molecules and mechanism of
oriented metastasis remain to be explained fully. However, it has
been demonstrated that the target organs can attract the cancer
cells from the primary lesion through chemotactic factors (69). The mutual attraction between the
chemokine and relative receptors serves a vital role in tumor cell
tropism.
C-X-C motif chemokine (CXCL)12 (stromal cell-derived
factor-1) and its receptor C-X-C chemokine receptor (CXCR)4 are
thought to regulate the metastasis of breast cancer, particularly
bone metastasis (70–72). The role of the CXCL12-CXCR4 chemokine
axis has also has been revealed not only in the murine model, but
also in the patients of bone metastasis of lung cancer. The lung
cancer cells expressing CXCR4 are attracted to the bone and other
target organs though the chemotactic gradients, for the
concentration gradients of the CXCL12 exist between the primary
tumor, plasma and organ sites of metastases (73). However, this axis appears to have
effects in both target organs in lung cancer, not just the bone. It
is very contradictory that, when the CXCR4 is genetically
disrupted, the osteoclast activity would be elevated rather than
suppressed, and the tumor cells in the bone proliferate faster
(74). Additionally, it is reported
that the interruption of this chemokine axis may bring destruction
to the immune system or cause metastasis to the other target organs
(75). Therefore, even though the
CXCL12-CXCR4 chemokine axis serves a vital role in guiding the
tumor cells to the target organs, the therapeutic strategies aiming
at this axis may easily cause further issues.
When investigating SCLC, Nakamura et al
(76) found that C-C motif chemokine
22 expressed by the osteoclasts induced the motility and invasion
of the SBC-5 cells through the integration with the C-C motif
receptor 4 expression on the surface of SBC-5 cells. Therefore,
whatever type of lung cancer, the chemokine has a great effect in
the target organs metastasis, and with regards to bone metastasis,
whether there are exclusive factors that induce the oriented move
require further elucidation.
Besides this well-studied ‘classical’ chemokine
axis, several novel chemokines have been recently identified in
tumor bone metastasis. CXCR7 has been uncovered as a second CXCL12
receptor, which performs a similar function to CXCR4 in tumor
development (77). In addition,
CXCL10 has been reported to facilitate the trafficking of
CXCR3-expressing cancer cells to the bone (78). The effect of these novel factors in
lung cancer bone metastasis require further elucidation.
Extravasation of the tumor cells
When the tumor cells are attracted to the
capillaries of the target organs, they will prepare to pull in to
the organ and dock here. It is generally hypothesized that the
extravasation of the tumor cells is similar with the
trans-endothelial migration of the leukocytes to the inflammation
area, including the pulling over, slowing down, attaching to the
wall, rolling and moving out.
Under the attraction of the P-selectin and
E-selectin of the endothelial cell, the tumor cells must move
slowly, and subsequently adhere to the endothelial cells following
combination with the integrins through the
arginine-glycine-aspartic acid peptide. At last, the tumor
cell-activated platelets induce the opening of the endothelial
barrier, allowing tumor cells to migrate trans-endothelially
(64). Therefore, the tumor cells
will exit from the vessels with ameboid movement. During this
process, the adhesion of the tumor cells to the endothelial cells
is the critical step, and the adhesion molecules serve an important
role in this. Various types of adhesion molecules, including the
CD44 family, integrin family and the selectins. The majority of
these cooperate to take part in the bone metastasis of lung
cancer.
Integrin is a type of cell surface receptor, which
interacts with the ECM and mediates intracellular adhesion. It has
a high expression pattern in the malignant cell surface and its
expression is positively correlated with the motility of the tumor
cells. Li et al (79) observed
that, compared with the SBC-3 cells with low bone metastasis
potential, the SBC-3 with high bone metastasis potential exhibited
a higher expression of the β3-integrine. When the SBC-3 cells were
interrupted with small interfering RNA against β3-integrine, the
adhesion, motility and invasion was significantly reduced (79). CD44 induced the adhesion through the
combination with the hyaluronic acid (HA), collagen and laminin. It
is recognized as a potential cancer stem cell marker (80), and has also been suggested to promote
bone metastasis by enhancing the production of HA, tumorigenicity
and cell motility (81). The
upregulation of the CD44 gene of the human lung squamous cancer
cell HARA was identified, which was co-cultured with the skull of
the newborn mice (82). In a bone
metastasis model of nude mice injected with SBC-5 cells, a
considerable number of osteoclasts expressing CD44, markedly
positive for osteopontin in the stromal tissues of the metastatic
lesion were observed, which implied that the osteopontin may serve
to facilitate osteoclast migration with CD44 (83). This indicated that CD44 not only
served a vital role in the course of the adhesion of lung cancer
cells to the bone, but was also involved in the course of the
osteolysis.
During this process, when the tumor cells move in
the circulation, the tropism movement is the special and unique
feature of the bone metastasis in lung cancer. The chemokine and
associated receptors are the helmsman, leading the tumor cells to
the target organs. Until now, the specific chemokine involved
remains to be elucidated.
Colonizing in the ‘soil’ - bone
According to the anatomy and pathology, the tumor
cells would be arrested in the bone marrow with the bloodstream in
the bone metastasis, then they have to egress from the central
sinus of the bone marrow. Following the attachment to bone
surfaces, the tumor cells induce osteolytic bone destruction in
conjunction within the bone microenvironment, and colonize in the
bone (84). The bone metastasis of
lung cancer predominantly occurs at the spinal bone, ribs, sternum,
and the tips of the long bone where there are special
micro-sinuses. These sinuses provide good access for the tumor
cells to exude from the vessels. The wide-diameter and slow
bloodstream of the sinus are better for the tumor cells to travel
though and adhere to the endothelial cells. The sinus has a special
structure, which is composed of endothelial cells with broad
intercellular space, and the tumor cells can easily egress from the
sinus and then attach to the bone.
Attachment to the bone
The tumor cell attachment and lodgment in the bone
are complex processes, which are actively driven by specific
interactions between tumor and normal cells, and with the ECM
components of the osseous milieu. Prior to the tumor cells to
joining in the osseous milieu, the attachment to the perimyelis is
the first step. Certain adhesion molecules are involved in this
step, including vascular cell adhesion protein-1, α4β1 integrin and
cadherin-11 (84).
Notbaly, MGr1-Ag demonstrates high laminin-binding
activity and it was observed that compared with the expression in
cells without bone-metastatic ability (SBC-3 cell line), MGr1-Ag
was highly expressed in bone-metastatic SCLC cells (SBC-5 cell
line). In addition, MGr1-Ag promotes SCLC cell invasion and bone
metastasis both in vitro and in vivo via the EMT
pathway (85). Discoidin domain
receptor-1 (DDR1) is a collagen receptor highly expressed in
invading tumor cells and mediates tumor cell survival in bone
metastasis (86). Karmele et
al (87) found that disruption of
DDR1 hampers lung cancer cell survival, leading to impaired early
tumor-bone engagement during skeletal homing, and crucially
altering bone colonization. PDGF is secreted from the bone stroma.
Previous studies have found that the disruption of the PDGF
receptor in the bone marrow stroma prevents efficient engagement
required for bone homing and osseous colonization, by altering
heterotypic tumor-stromal and tumor-matrix interactions (88).
Osteoclastic bone destruction
Once the tumor cells land on the bone successfully,
the tumor cells join with the bone cells and began to interact with
the local bone microenvironment with numerous different types of
cells, including osteoclasts, osteoblasts, osteocytes, macrophages
and adipocytes. Under the interaction with several molecules and
signaling pathways, the tumor cells can construct a hospitable
environment for survival and proliferation.
In the pathophysiology of bone metastasis, multiple
evidence has demonstrated that it is the osteoclast that destroys
the bone, rather than the tumor cells (89,90). As
with the bone metastasis of lung cancer, the destruction of the
bone is primarily the soluble osseous, in which the osteoclast is
the arch-criminal. In this osteolytic lesion, the tumor cells
promote osteoclast formation by continuingly secreting
pro-osteoclastogenic factors, including parathyroid hormone-related
protein (PTHrP), receptor activator of nuclear factor-κB (RANK)L
and macrophage colony-stimulating factor. Simultaneously, the
excessive generation of abnormally activated osteoclasts elevate
the bone resorption, in which the growth factors, including TGF-β,
insulin-like growth factors (IGFs), PDGFs and bone morphogenetic
proteins, are released from the bone matrix. As a result of that,
the abundant growth factors provide a rich ‘soil’ for the tumor
cells to proliferate. Additionally, the destruction of the bone
matrix supply provides more room for the tumor cells to expand.
Therefore, this tumor-osteoclast cooperation forms a vicious cycle
in the bone microenvironment, accelerating the bone injury, as well
as the pain (91). Therefore, the
metabolism of the osteoclast is becoming the critical point to
research the development of the osteolytic lesion. Notably,
multiple molecules and signaling pathways are involved in this
process in conjunction with tumor cells, osteoblasts and other cell
types in the bone microenvironment, while the key signaling pathway
is the osteoprotegerin (OPG)/RANK/RANKL axis.
A balance exists between the osteoclastic bone
resorption regulated by the osteoclast, and the osteoblastic bone
formation dominated by the osteoblast in the bone metabolism.
Additionally, it is widely hypothesized that the OPG/RANK/RANKL
axis is the primary factor to regulate this balance. The osteolytic
metastatic lesion is the result of the fact that the osteoclastic
bone resorption supersedes the osteoblastic bone formation,
indicating that the axis is out of balance. It has been previously
reported that the soluble RANKL and OPG in the serum are elevated
in lung cancer patients with bone metastases (92), indicating that the axis serves a role
in the bone metastasis of lung cancer. In samples from patients
with bone metastasis of NSCLC, it was demonstrated that the
upregulation of OPG, RANK and RANKL, and the increase in the ratio
of RANKL/OPG occurred. In vitro, the NSCLC cells with
transfection of RANKL cDNA exhibited higher motility and invasion,
while the ability reduced following the addition of OPG (93). When inhibiting the RANK-RANKL
interaction, the osteolytic lesion induced by the NSCLC cell line
A549 in SCID mice was limited (94).
Notably, an abundance of molecules have been
revealed to take part in the regulation of the axis, including
prostaglandin E, parathyroid hormone, TGF-β, interleukin-1, tumor
necrosis factor-α and PTHrP. Of these, PTHrP has been identified to
serve a vital role in the bone metastasis of breast and prostate
cancer (95,96).
In addition, there is popular belief that PTHrP may
be one of the unique regulatory factors involved in the bone
metastasis of lung cancer (97).
Positive expression of PTHrP in lung cancer indicated a higher
chance for the development of the bone metastasis, and researchers
also revealed that the PTHrP produced by the lung cancer cells may
induce the osteoblasts to express the RANKL, as well as reduce the
generation of OPG, leading to osteoclast maturation and activation
(98). Miki et al (9) repeatedly injected the neutralizing
antibody of PTHrP into the SCLC model mice and found that the bone
metastasis was markedly suppressed (9). It was revealed that a highly bone
metastatic lung squamous cell carcinoma cell line (HARA)
overexpressed PTHrP, and that the treatment of nude mice with
anti-PTHrP antibody inhibited the formation of bone metastasis
(99). The TGF-β can also stimulate
the lung cancer cells to overexpressing PTHrP and if the TGF-β
signaling pathway was suppressed, the PTHrP expression was also
reduced (100). Therefore, it is
clear that the different cytokines can interact or influence each
other, and their complex association constitutes a web to regulate
the bone metastasis microenvironment. Deng et al (82) analyzed the gene expression levels in
the HARA human lung squamous cancer cell line, which exhibits a
high bone metastatic tendency, and demonstrated that besides PTHrP,
ezrin was also expressed at a higher rate in the bone metastasis
lesion (82).
Apart from the osteoclasts, researchers also pay
increasing attention to the macrophages in the tumor
microenvironment, as well as in the bone metastasis (101). Macrophages are derived from myeloid
progenitors and are an important component of the bone marrow. This
cell type can be classified into two main subsets: M1 macrophages,
generally promoting inflammation, and M2 macrophages, typically
suppressing inflammation and assisting tissue repair (102). Within the tumor microenvironment, M1
macrophages have been proposed to be antitumorigenic and kill
cancer cells. By contrast, the M2 macrophages are generally
considered to be pro-tumorigenic by releasing a variety of growth
factors, including fibroblast growth factors and VEGF, to promote
tumor growth and invasion (103).
Hiraoka et al (104) broadly
assessed the effects of monocytes and macrophages in a metastatic
lung cancer cell cardiac injection mouse model. When they depleted
monocytes and macrophages with clodronate-packaged liposomes, they
found that targeting both macrophages and osteoclasts revealed a
more pronounced reduction in the number and size of bone metastatic
lesions compared with the sole osteoclast-targeting agent,
reveromycin A (104).
In the bone microenvironment, besides the
osteoclasts and macrophages, other cells (osteoblasts and
adipocytes), are also in conjunction with the tumor cells to
develop the osteolytic lesion (105), as well as several different
approaches and molecules. Despite of the complex process in the
bone metastasis of lung cancer, numerous researchers are
investigating a specific critical cell or molecule involved in
this. For instance, receptor of activated protein C, histone
deacetylase 4, paired-like homeodomain 1, roundabout axon guidance
receptor homolog 1 and Wnt/T cell factor signaling perform a
negative function in bone metastasis of lung adenocarcinoma
(106–109). Additionally, increasing attention is
being paid to the miR involved in the bone metastasis of lung
cancer. Valencia et al (110)
found that miR-326 was a relevant biomarker representative of
osteolysis in the model of bone metastasis in lung cancer. It was
observed that systemic delivery of miR-192 can decrease osteolytic
lesions in a lung cancer mouse model (111). In SBC-5 cells, the loss of miR335
promoted SCLC metastatic bone lesion by reducing the expression
levels of RANKL and IGF-IR (109).
Conclusions
Compared with the osteolytic bone metastasis in lung
cancer, the osteoblastic bone metastasis is seldom observed
clinically, therfore, relative research is limited. Although the
osteolytic lesion is most frequently observed clinically,
osteoblastic bone metastasis and the mixed bone metastasis occur in
patients with lung cancer (112). In
addition, the bone lesion in the patients with NSCLC is
predominantly osteolytic (105). By
contrast, SCLC is mostly osteoblastic. However, the mouse model of
bone metastasis in lung cancer was used to assess pathological
changes in osteoclstic lesion. Therefore, attention must be paid to
select the proper models to analyze the bone metastasis in lung
cancer.
The process of the bone metastasis in lung cancer
can be divided into three steps: i) Escaping from the primary
tumor; ii) moving in the circulation; iii) colonizing in the bone.
In each of these mechanisms, this process is continuous and
progressive, coordinating and complex. Certain molecules will be
involved in the different steps with different effects. For
instance, when the lung cancer cells leave the primary lesion, the
decrease of the adhesion molecules contribute to its dissociation
with the neighbor cells. Additionally, the tumor cells moving in
the circulation also demand adhesion and dissociation with the
endothelial cells. In addition, when attaching to the bone, the
tumor cells require the assistance of the adhesion molecules.
Therefore, the adhesion and dissociation regulated by the adhesion
molecules perform an essential rold during the entire metastatic
process. Notably, with the exception of the adhesion molecules,
numerous other molecules are cooperating to accomplish each step.
It is similar for the lung cancer cells to metastasize to other
target organs in the former two steps. Although there are
chemokines leading to the bone metastasis in the early steps, they
reveal no specificity of bone metastasis. Therefore, the most
specific and unique is the third step in bone metastasis of lung
cancer. In this process, under the interaction with tumor cells,
the osteoclast regulates the osteolytic lesion. In this stage,
numerous signaling pathways and molecules are involved. The most
important and critical pathway is the RANK/RANKL axis. This has
been studied in depth in the field of bone metastasis and
diphosphonate treatment targeting this axis has been clinically
applied. However, there is no way to delay or stop the occurrence
of the bone metastasis and the side-effect of diphosphonate,
mandible necrosis, has no effective treatment. In addition, the
exact molecules and mechanism of this axis in bone metastasis of
lung cancer requires more investigation.
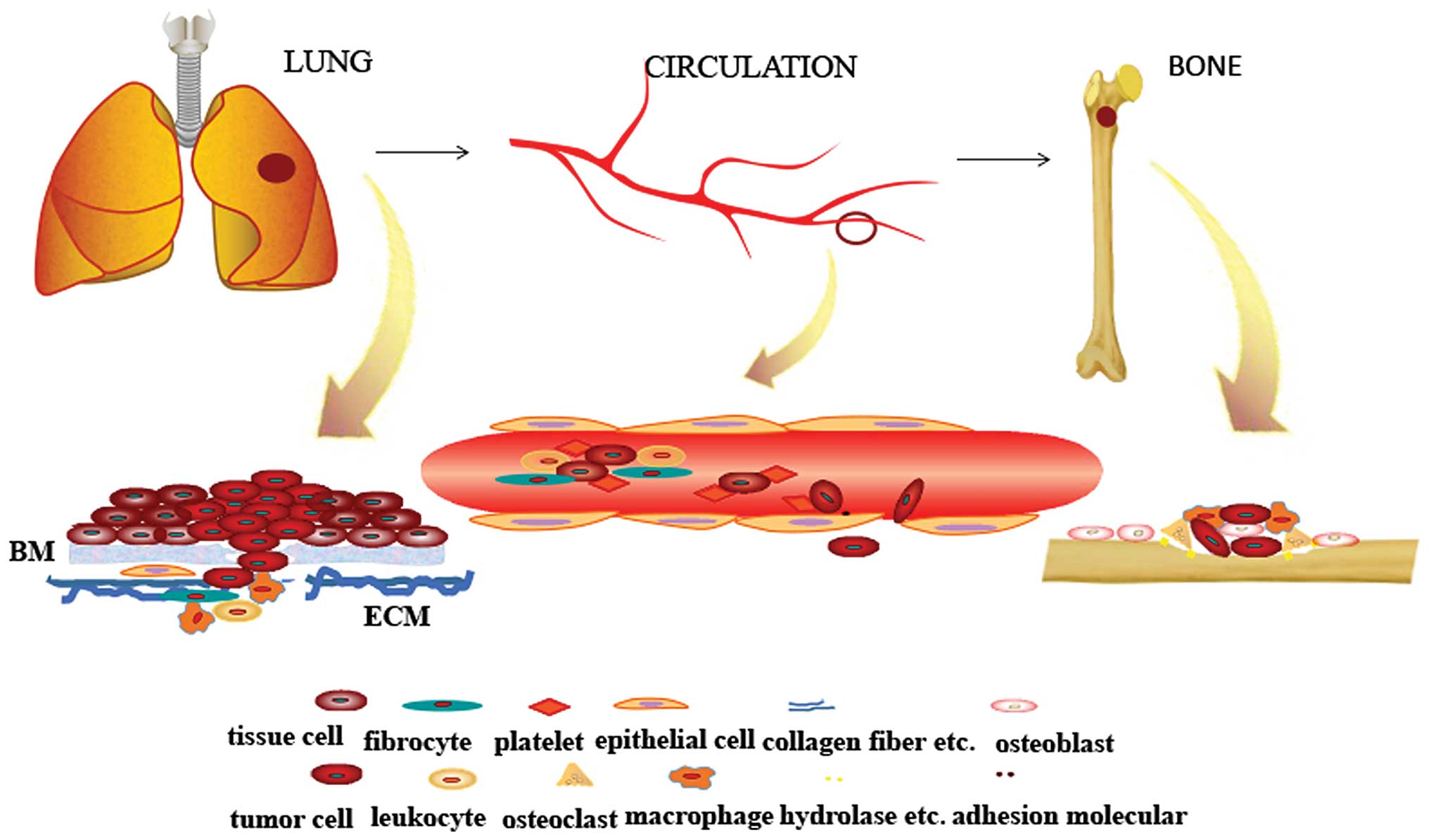
As shown in Fig. 1,
from the primary tumor to the circulation and finally, the settling
down in the bone, the tumor cells are living in a microenvironment
in each site, therefore the tumor cells can generate, invade,
escape from the immune system, and obtain more nutrition and space
to expand. Therefore, the smart ‘seed’ applies the collective
strength from the neighbor cells to trudge to the rich ‘soil’. This
indicates that the enemy is more than the tumor cells in the battle
to fight with the bone metastasis. Thus, more attention should be
paid to the entire microenvironment. Nowadays, with the improvement
of the therapy in lung cancer, patients with lung cancer have a
longer survival time. Therefore, how to prevent the bone metastasis
prior to the late stage is becoming very important. Further
investigations and in depth studies must be performed to determine
how to prevent the bone metastasis in the earlier stages of lung
cancer.
References
|
1
|
Salomaa ER and Walta M: The prognosis of
lung cancer continues to be poor-treatment outcome within the
hospital district of Southwest Finland in 2004 to 2011. Duodecim.
131:69–75. 2015.(In Finnish). PubMed/NCBI
|
|
2
|
GLOBOCAN: Estimated cancer incidence,
mortality and prevalence worldwide in 2012. IARC. 2014.
|
|
3
|
Hirsh V, Major PP, Lipton A, Cook RJ,
Langer CJ, Smith MR, Brown JE and Coleman RE: Zoledronic acid and
survival in patients with metastatic bone disease from lung cancer
and elevated markers of osteoclast activity. J Thorac Oncol.
3:228–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taubenberger AV: In vitro
microenvironments to study breast cancer bone colonisation. Adv
Drug Deliv Rev. 79–80:135–144. 2014. View Article : Google Scholar
|
|
6
|
Hess KR, Varadhachary GR, Taylor SH, Wei
W, Raber MN, Lenzi R and Abbruzzese JL: Metastatic patterns in
adenocarcinoma. Cancer. 106:1624–1633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
8
|
Uy HL, Mundy GR, Boyce BF, Story BM,
Dunstan CR, Yin JJ, Roodman GD and Guise TA: Tumor necrosis factor
enhances parathyroid hormone-related protein-induced hypercalcemia
and bone resorption without inhibiting bone formation in vivo.
Cancer Res. 57:3194–3199. 1997.PubMed/NCBI
|
|
9
|
Miki T, Yano S, Hanibuchi M, Kanematsu T,
Muguruma H and Sone S: Parathyroid hormone-related protein (PTHrP)
is responsible for production of bone metastasis, but not visceral
metastasis, by human small cell lung cancer SBC-5 cells in natural
killer cell-depleted SCID mice. Int J Cancer. 108:511–515. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai Z, Chen Q, Chen J, Lu Y, Xiao G, Wu Z,
Zhou Q and Zhang J: Monocyte chemotactic protein 1 promotes lung
cancer-induced bone resorptive lesions in vivo. Neoplasia.
11:228–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han JH, Choi SJ, Kurihara N, Koide M, Oba
Y and Roodman GD: Macrophage inflammatory protein-1alpha is an
osteoclastogenic factor in myeloma that is independent of receptor
activator of nuclear factor kappaB ligand. Blood. 97:3349–3353.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bogenrieder T and Herlyn M: Axis of evil:
Molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vescovi AL, Galli R and Reynolds BA: Brain
tumour stem cells. Nat Rev Cancer. 6:425–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Driessens G, Beck B, Caauwe A, Simons BD
and Blanpain C: Defining the mode of tumour growth by clonal
analysis. Nature. 488:527–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Todaro M, Perez Alea M, Scopelliti A,
Medema JP and Stassi G: IL-4 mediated drug resistance in colon
cancer stem cells. Cell Cycle. 7:309–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collins AT and Maitland NJ: Prostate
cancer stem cells. Eur J Cancer. 42:1213–1218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
International Stem Cell Initiative.
Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW,
Beighton G, Bello PA, Benvenisty N, Berry LS, et al:
Characterization of human embryonic stem cell lines by the
international stem cell initiative. Nat Biotechnol. 25:803–816.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miki J, Furusato B, Li H, Gu Y, Takahashi
H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S and Rhim JS:
Identification of putative stem cell markers, CD133 and CXCR4, in
hTERT-immortalized primary nonmalignant and malignant tumor-derived
human prostate epithelial cell lines and in prostate cancer
specimens. Cancer Res. 67:3153–3161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J and Song E: let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Levina V, Marrangoni AM, DeMarco R,
Gorelik E and Lokshin AE: Drug-selected human lung cancer stem
cells: Cytokine network, tumorigenic and metastatic properties.
PLoS One. 3:e30772008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lacroix M: Significance, detection and
markers of disseminated breast cancer cells. Endocr Relat Cancer.
13:1033–1067. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pantel K, Brakenhoff RH and Brandt B:
Detection, clinical relevance and specific biological properties of
disseminating tumour cells. Nat Rev Cancer. 8:329–340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mego M, Mani SA and Cristofanilli M:
Molecular mechanisms of metastasis in breast cancer-clinical
applications. Nat Rev Clin Oncol. 7:693–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hou JM, Krebs M, Ward T, Sloane R, Priest
L, Hughes A, Clack G, Ranson M, Blackhall F and Dive C: Circulating
tumor cells as a window on metastasis biology in lung cancer. Am J
Pathol. 178:989–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Perl AK, Wilgenbus P, Dahl U, Semb H and
Christofori G: A causal role for E-cadherin in the transition from
adenoma to carcinoma. Nature. 392:190–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan B, Zhang W, Jiang LY, Qin WX and Wang
X: Reduced E-Cadherin expression is a prognostic biomarker of
non-small cell lung cancer: A meta-analysis based on 2395 subjects.
Int J Clin Exp Med. 7:4352–4356. 2014.PubMed/NCBI
|
|
42
|
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang
SY, Liu CJ, Teng SC and Wu KJ: Direct regulation of TWIST by
HIF-1alpha promotes metastasis. Nat Cell Biol. 10:295–305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sánchez-Tilló E, Lázaro A, Torrent R,
Cuatrecasas M, Vaquero EC, Castells A, Engel P and Postigo A: ZEB1
represses E-cadherin and induces an EMT by recruiting the SWI/SNF
chromatin remodeling protein BRG1. Oncogene. 29:3490–3500. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ko H, Jeon H, Lee D, Choi HK, Kang KS and
Choi KC: Sanguiin H6 suppresses TGF-β induction of the
epithelial-mesenchymal transition and inhibits migration and
invasion in A549 lung cancer. Bioorg Med Chem Lett. 25:5508–5513.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang X, Li L, Huang Q, Xu W, Cai X, Zhang
J, Yan W, Song D, Liu T, Zhou W, et al: Wnt signaling through
Snail1 and Zeb1 regulates bone metastasis in lung cancer. Am J
Cancer Res. 5:748–755. 2015.PubMed/NCBI
|
|
46
|
Gilkes DM, Semenza GL and Wirtz D: Hypoxia
and the extracellular matrix: Drivers of tumour metastasis. Nat Rev
Cancer. 14:430–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Joyce JA: Therapeutic targeting of the
tumor microenvironment. Cancer Cell. 7:513–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: Trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hu T and Lu YR: BCYRN1, a c-MYC-activated
long non-coding RNA, regulates cell metastasis of non-small-cell
lung Cancer. Cancer Cell Int. 15:362015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hsu CP, Shen GH and Ko JL: Matrix
metalloproteinase-13 expression is associated with bone marrow
microinvolvement and prognosis in non-small cell lung cancer. Lung
Cancer. 52:349–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang H, Zhu Y, Zhao M, Wu C, Zhang P, Tang
L, Zhang H, Chen X, Yang Y and Liu G: miRNA-29c suppresses lung
cancer cell adhesion to extracellular matrix and metastasis by
targeting integrin β1 and matrix metalloproteinase2 (MMP2). PloS
One. 8:e701922013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Papetti M and Herman IM: Mechanisms of
normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol.
282:C947–C970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dimova I, Popivanov G and Djonov V:
Angiogenesis in cancer-general pathways and their therapeutic
implications. J BUON. 19:15–21. 2014.PubMed/NCBI
|
|
55
|
Weis SM and Cheresh DA: αV integrins in
angiogenesis and cancer. Cold Spring Harb Perspect Med.
1:a0064782011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Young R, Pailler E, Billiot F, Drusch F,
Barthelemy A, Oulhen M, Besse B, Soria JC, Farace F and Vielh P:
Circulating tumor cells in lung cancer. Acta Cytol. 56:655–660.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
O'Flaherty JD, Gray S, Richard D, Fennell
D, O'Leary JJ, Blackhall FH and O'Byrne KJ: Circulating tumour
cells, their role in metastasis and their clinical utility in lung
cancer. Lung Cancer. 76:19–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Parkinson DR, Dracopoli N, Petty BG,
Compton C, Cristofanilli M, Deisseroth A, Hayes DF, Kapke G, Kumar
P, Lee JSh, et al: Considerations in the development of circulating
tumor cell technology for clinical use. J Transl Med. 10:1382012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tanaka F, Yoneda K, Kondo N, Hashimoto M,
Takuwa T, Matsumoto S, Okumura Y, Rahman S, Tsubota N, Tsujimura T,
et al: Circulating tumor cell as a diagnostic marker in primary
lung cancer. Clin Cancer Res. 15:6980–6986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hou JM, Greystoke A, Lancashire L,
Cummings J, Ward T, Board R, Amir E, Hughes S, Krebs M, Hughes A,
et al: Evaluation of circulating tumor cells and serological cell
death biomarkers in small cell lung cancer patients undergoing
chemotherapy. Am J Pathol. 175:808–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hofman V, Long E, Ilie M, Bonnetaud C,
Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E, Mourad N, Butori C,
et al: Morphological analysis of circulating tumour cells in
patients undergoing surgery for non-small cell lung carcinoma using
the isolation by size of epithelial tumour cell (ISET) method.
Cytopathology. 23:30–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nieva J, Wendel M, Luttgen MS, Marrinucci
D, Bazhenova L, Kolatkar A, Santala R, Whittenberger B, Burke J,
Torrey M, et al: High-definition imaging of circulating tumor cells
and associated cellular events in non-small cell lung cancer
patients: A longitudinal analysis. Phys Biol. 9:0160042012.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Palumbo JS, Talmage KE, Massari JV, La
Jeunesse CM, Flick MJ, Kombrinck KW, Hu Z, Barney KA and Degen JL:
Tumor cell-associated tissue factor and circulating hemostatic
factors cooperate to increase metastatic potential through natural
killer cell-dependent and-independent mechanisms. Blood.
110:133–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Schumacher D, Strilic B, Sivaraj KK,
Wettschureck N and Offermanns S: Platelet-derived nucleotides
promote tumor-cell transendothelial migration and metastasis via
P2Y 2 receptor. Cancer Cell. 24:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hou JM, Krebs M, Ward T, Sloane R, Priest
L, Hughes A, Clack G, Ranson M, Blackhall F and Dive C: Circulating
tumor cells as a window on metastasis biology in lung cancer. Am J
Pathol. 178:989–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Stone JP and Wagner DD: P-selectin
mediates adhesion of platelets to neuroblastoma and small cell lung
cancer. J Clin Invest. 92:804–813. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Coleman RE: Skeletal complications of
malignancy. Cancer. 80(Suppl 8): S1588–S1594. 1997. View Article : Google Scholar
|
|
68
|
Hart IR and Fidler IJ: Role of organ
selectivity in the determination of metastatic patterns of B16
melanoma. Cancer Res. 40:2281–2287. 1980.PubMed/NCBI
|
|
69
|
Stetler-Stevenson WG and Kleiner DEJ:
Molecular biology of cancer: Invasion and metastases. Cancer:
Principles and Practice of Oncology. 6:123–136. 2001.
|
|
70
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Richert MM, Vaidya KS, Mills CN, Wong D,
Korz W, Hurst DR and Welch DR: Inhibition of CXCR4 by CTCE-9908
inhibits breast cancer metastasis to lung and bone. Oncol Rep.
21:761–767. 2009.PubMed/NCBI
|
|
72
|
Smith MC, Luker KE, Garbow JR, Prior JL,
Jackson E, Piwnica-Worms D and Luker GD: CXCR4 regulates growth of
both primary and metastatic breast cancer. Cancer Res.
64:8604–8612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Phillips RJ, Burdick MD, Lutz M, Belperio
JA, Keane MP and Strieter RM: The stromal derived
factor-1/CXCL12-CXC chemokine receptor 4 biological axis in
non-small cell lung cancer metastases. Am J Respir Crit Care Med.
167:1676–1686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hirbe AC, Rubin J, Uluçkan O, Morgan EA,
Eagleton MC, Prior JL, Piwnica-Worms D and Weilbaecher KN:
Disruption of CXCR4 enhances osteoclastogenesis and tumor growth in
bone. Proc Natl Acad Sci USA. 104:14062–14067. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hirbe AC, Morgan EA and Weilbaecher KN:
The CXCR4/SDF-1 chemokine axis: A potential therapeutic target for
bone metastases? Curr Pharm Des. 16:1284–1290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Nakamura ES, Koizumi K, Kobayashi M,
Saitoh Y, Arita Y, Nakayama T, Sakurai H, Yoshie O and Saiki I:
RANKL-induced CCL22/macrophage-derived chemokine produced from
osteoclasts potentially promotes the bone metastasis of lung cancer
expressing its receptor CCR4. Clin Exp Metastasis. 23:9–18. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Burns JM, Summers BC, Wang Y, Melikian A,
Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ,
et al: A novel chemokine receptor for SDF-1 and I-TAC involved in
cell survival, cell adhesion and tumor development. J Exp Med.
203:2201–2213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lee JH, Kim HN, Kim KO, Jin WJ, Lee S, Kim
HH, Ha H and Lee ZH: CXCL10 promotes osteolytic bone metastasis by
enhancing cancer outgrowth and osteoclastogenesis. Cancer Res.
72:3175–3186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li N, Zhang JP, Guo S, Min J, Liu LL, Su
HC, Feng YM and Zhang HL: Down-regulation of β3-integrin inhibits
bone metastasis of small cell lung cancer. Mol Biol Rep.
39:3029–3035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yin H and Deng J: Advances in lung stem
cells and lung cancer stem cells. Zhongguo Fei Ai Za Zhi.
18:633–639. 2015.(In Chinese). PubMed/NCBI
|
|
81
|
Hiraga T, Ito S and Nakamura H: Cancer
stem-like cell marker CD44 promotes bone metastases by enhancing
tumorigenicity, cell motility and hyaluronan production. Cancer
Res. 73:4112–4122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Deng X, Tannehill-Gregg SH, Nadella MV, He
G, Levine A, Cao Y and Rosol TJ: Parathyroid hormone-related
protein and ezrin are up-regulated in human lung cancer bone
metastases. Clin Exp Metastasis. 24:107–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li M, Amizuka N, Takeuchi K, Freitas PH,
Kawano Y, Hoshino M, Oda K, Nozawa-Inoue K and Maeda T:
Histochemical evidence of osteoclastic degradation of extracellular
matrix in osteolytic metastasis originating from human lung small
carcinoma (SBC-5) cells. Microsc Res Tech. 69:73–83. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yoneda T and Hiraga T: Crosstalk between
cancer cells and bone microenvironment in bone metastasis. Biochem
Biophys Res Commun. 328:679–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang F, Wang Y, Xu M, Dong H, Liu N, Zhou
J, Pang H, Ma N, Zhang N, Pei Y, et al: MGr1-Ag promotes invasion
and bone metastasis of small-cell lung cancer in vitro and in vivo.
Oncol Rep. 29:2283–2290. 2013.PubMed/NCBI
|
|
86
|
Alves F, Vogel W, Mossie K, Millauer B,
Höfler H and Ullrich A: Distinct structural characteristics of
discoidin I subfamily receptor tyrosine kinases and complementary
expression in human cancer. Oncogene. 10:609–618. 1995.PubMed/NCBI
|
|
87
|
Valencia K, Ormazábal C, Zandueta C,
Luis-Ravelo D, Antón I, Pajares MJ, Agorreta J, Montuenga LM,
Martínez-Canarias S, Leitinger B and Lecanda F: Inhibition of
collagen receptor discoidin domain receptor-1 (DDR1) reduces cell
survival, homing, and colonization in lung cancer bone metastasis.
Clin Cancer Res. 18:969–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Catena R, Luis-Ravelo D, Antón I, Zandueta
C, Salazar-Colocho P, Larzábal L, Calvo A and Lecanda F: PDGFR
signaling blockade in marrow stroma impairs lung cancer bone
metastasis. Cancer Res. 71:164–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sela J: Bone remodeling in pathological
conditions. A scanning electron microscopic study. Calcif Tissue
Res. 23:229–234. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Boyde A, Maconnachie E, Reid SA, Delling G
and Mundy GR: Scanning electron microscopy in bone pathology:
Review of methods, potential and applications. Scan Electron
Microsc. 1537–1554. 1986.PubMed/NCBI
|
|
91
|
Guise TA: The vicious cycle of bone
metastases. J Musculoskelet Neuronal Interact. 2:570–572.
2002.PubMed/NCBI
|
|
92
|
Karapanagiotou EM, Terpos E, Dilana KD,
Alamara C, Gkiozos I, Polyzos A and Syrigos KN: Serum bone turnover
markers may be involved in the metastatic potential of lung cancer
patients. Med Oncol. 27:332–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Peng X, Guo W, Ren T, Lou Z, Lu X, Zhang
S, Lu Q and Sun Y: Differential expression of the RANKL/RANK/OPG
system is associated with bone metastasis in human non-small cell
lung cancer. PLoS One. 8:e583612013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Feeley BT, Liu NQ, Conduah AH, Krenek L,
Roth K, Dougall WC, Huard J, Dubinett S and Lieberman JR: Mixed
metastatic lung cancer lesions in bone are inhibited by noggin
overexpression and Rank: Fc administration. J Bone Miner Res.
21:1571–1580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bundred NJ, Walker RA, Ratcliffe WA,
Warwick J, Morrison JM and Ratcliffe JG: Parathyroid hormone
related protein and skeletal morbidity in breast cancer. Eur J
Cancer. 28:690–692. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Burton DW, Geller J, Yang M, Jiang P,
Barken I, Hastings RH, Hoffman RM and Deftos LJ: Monitoring of
skeletal progression of prostate cancer by GFP imaging, X-ray and
serum OPG and PTHrP. Prostate. 62:275–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Miki T, Yano S, Hanibuchi M, Kanematsu T,
Muguruma H and Sone S: Parathyroid hormone-related protein (PTHrP)
is responsible for production of bone metastasis, but not visceral
metastasis, by human small cell lung cancer SBC-5 cells in natural
killer cell-depleted SCID mice. Int J Cancer. 108:511–515. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Muguruma H, Yano S, Kakiuchi S, Uehara H,
Kawatani M, Osada H and Sone S: Reveromycin A inhibits osteolytic
bone metastasis of small-cell lung cancer cells, SBC-5, through an
antiosteoclastic activity. Clin Cancer Res. 11:8822–8828. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Iguchi H, Tanaka S, Ozawa Y, Kashiwakuma
T, Kimura T, Hiraga T, Ozawa T and Kono T: An experimental model of
bone metastasis by human lung cancer cells: The role of parathyroid
hormone-related protein in bone metastasis. Cancer Res.
56:4040–4043. 1996.PubMed/NCBI
|
|
100
|
Lorch G, Gilmore JL, Koltz PF, Gonterman
RM, Laughner R, Lewis DA, Konger RL, Nadella KS, Toribio RE, Rosol
TJ and Foley J: Inhibition of epidermal growth factor receptor
signalling reduces hypercalcaemia induced by human lung
squamous-cell carcinoma in athymic mice. Br J Cancer. 97:183–193.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Herroon MK, Rajagurubandara E, Rudy DL,
Chalasani A, Hardaway AL and Podgorski I: Macrophage cathepsin K
promotes prostate tumor progression in bone. Oncogene.
32:1580–1593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lawrence T and Natoli G: Transcriptional
regulation of macrophage polarization: Enabling diversity with
identity. Nat Rev Immunol. 11:750–761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A
and Di W: A high M1/M2 ratio of tumor-associated macrophages is
associated with extended survival in ovarian cancer patients. J
Ovarian Res. 7:192014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hiraoka K, Zenmyo M, Watari K, Iguchi H,
Fotovati A, Kimura YN, Hosoi F, Shoda T, Nagata K, Osada H, et al:
Inhibition of bone and muscle metastases of lung cancer cells by a
decrease in the number of monocytes/macrophages. Cancer Sci.
99:1595–1602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Krzeszinski JY and Wan Y: New therapeutic
targets for cancer bone metastasis. Trends Pharmacol Sci.
36:360–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Antón I, Molina E, Luis-Ravelo D, Zandueta
C, Valencia K, Ormazabal C, Martínez-Canarias S, Perurena N,
Pajares MJ, Agorreta J, et al: Receptor of activated protein C
promotes metastasis and correlates with clinical outcome in lung
adenocarcinoma. Am J Respir Crit Care Med. 186:96–105. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Luis-Ravelo D, Antón I, Zandueta C,
Valencia K, Ormazábal C, Martínez-Canarias S, Guruceaga E, Perurena
N, Vicent S, De Las Rivas J and Lecanda F: A gene signature of bone
metastatic colonization sensitizes for tumor-induced osteolysis and
predicts survival in lung cancer. Oncogene. 33:5090–5099. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Nguyen DX, Chiang AC, Zhang XH, Kim JY,
Kris MG, Ladanyi M, Gerald WL and Massagué J: WNT/TCF signaling
through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis.
Cell. 138:51–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Gong M, Ma J, Guillemette R, Zhou M, Yang
Y, Yang Y, Hock JM and Yu X: miR-335 inhibits small cell lung
cancer bone metastases via IGF-IR and RANKL pathways. Mol Cancer
Res. 12:101–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Valencia K, Martín-Fernández M, Zandueta
C, Ormazábal C, Martínez-Canarias S, Bandrés E, de la Piedra C and
Lecanda F: miR-326 associates with biochemical markers of bone
turnover in lung cancer bone metastasis. Bone. 52:532–539. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ell B and Kang Y: MicroRNAs as regulators
of bone homeostasis and bone metastasis. Bonekey Rep. 3:5492014.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Napoli LD, Hansen HH, Muggia FM and Twigg
HL: The incidence of osseous involvement in lung cancer, with
special reference to the development of osteoblastic changes.
Radiology. 108:17–21. 1973. View Article : Google Scholar : PubMed/NCBI
|