Introduction
Transcatheter arterial chemoembolization (TACE) is
currently the standard first-line treatment for Barcelona Clinic
Liver Cancer (BCLC) stage B hepatocellular carcinoma (HCC) patients
(1,2).
Chemotherapeutic agents such as doxorubicin
(3), epirubicin (4–6), mitomycin
C (7) and cisplatin (8) are combined in TACE for the treatment of
HCC; however, their treatment efficacy remains unclear.
We previously reported that whole-liver
transcatheter arterial infusion (TAI) with a high concentration of
a fine-powder formulation of cisplatin (DDP-H)
(IA-call®; Nippon Kayaku Co. Ltd., Tokyo, Japan) prior
to radical local treatment inhibited intrahepatic recurrence of HCC
(9), and that whole-liver TAI of
DDP-H administered to HCC patients with a Japan Integrated Staging
(JIS) score of 0–1 improved survival (10).
The aim of the present study was to evaluate whether
epirubicin (Farmorubicin; Pfizer Japan, Tokyo, Japan) or miriplatin
(Miripla; Dainippon Sumitomo Pharma, Osaka, Japan) best contributes
to survival when used as the chemoagent in TACE in combination with
DDP-H whole-liver TAI in patients with primary BCLC-B HCC.
Patients and methods
Patients
This was a single-center, explorative, cohort study
of 55 patients with BCLC-B stage primary treatment-naïve HCC
diagnosed between July, 2004 and March, 2015. The survival time and
predictive factors were analyzed in 55 BCLC-B stage HCC patients
who underwent DDP-H whole-liver TAI in combination with TACE at the
Saiseikai Niigata Daini Hospital. Epirubicin (Farmorubicin; Pfizer
Japan, Tokyo, Japan) was used as the TACE chemoagent in 29
patients, whereas miriplatin (Miripla; Dainippon Sumitomo Pharma,
Osaka, Japan) was used in 26 patients. Epirubicin was selected as
first-line treatment before miriplatin approval (October, 2009),
and miriplatin was selected as first-line treatment thereafter.
HCC was diagnosed by dynamic contrast-enhanced
computed tomography or dynamic contrast-enhanced magnetic resonance
imaging. The inclusion criteria were as follows: BCLC-B, no prior
treatment for HCC, poor candidate for surgery, Eastern Cooperative
Oncology Group performance status 0–2, normal electrocardiographic
findings, normal serum creatinine level and age 20–85 years.
Ethics statement
This study was approved by the Institutional Review
Board of Saiseikai Niigata Daini Hospital and was conducted in
accordance with the principles of the Declaration of Helsinki. All
the patients provided written informed consent.
Treatment
In all the patients, the femoral artery was
punctured using the Seldinger technique and a 5-F introducer was
inserted, followed by insertion of a 5-F catheter. Subsequently, a
3-F microcatheter was advanced into the proper hepatic artery, and
DDP-H (IA-call®, Nippon Kayaku, Co., Ltd.) 65 mg/m2 was
infused through the catheter into the whole liver over 30 min. The
DDP-H solution was prepared by dissolving DDP-H (100 mg/vial) in 70
ml physiological saline warmed to 50°C (cisplatin concentration:
1.43 mg/ml). A 5-hydroxytryptamine-3 receptor antagonist and
corticosteroids were administered during DDP-H infusion to reduce
emesis. To prevent renal toxicity, hydration was performed by
intravenous drip infusion of fluids (500 ml prior to DDP-H infusion
and 1,500 ml following DDP-H infusion), and the patients were
instructed to ingest ≥2,000 ml/day of water beginning the day after
treatment.
Following DDP-H infusion, superselective TACE was
administered to embolize subsectional and/or peripheral nutrient
vessels.
The patients were divided into two groups according
to the anticancer agent used in TACE: A total of 29 patients were
included in the epirubicin group and 26 patients in the miriplatin
group.
When TACE was performed with epirubicin, one 50-mg
vial of epirubicin was dissolved in 1–2 ml of non-ionic contrast
agent, and a suspension was prepared by pumping a maximum of 50 mg
of epirubicin and 2–8 ml of lipiodol between 2 syringes 10–20
times. When TACE was performed using miriplatin, one 70-mg vial of
miriplatin was dissolved in 4 ml of lipiodol, and a suspension was
prepared containing a maximum of 140 mg of miriplatin and 8 ml of
lipiodol. In both groups, the amount of suspension used was
determined according to the size of the nodule [the suspension was
administered until it filled the tumor blood vessels to a maximum
of 6 ml (120 mg miriplatin)]. In all procedures, TACE was performed
until stasis of the blood flow in the target artery was observed.
Following TACE, angiograms were taken from multiple perspectives to
confirm that there was no tumor staining or accumulation of
lipiodol in the tumor.
Statistical analysis
The primary endpoint of this study was overall
survival. Background clinical characteristics were compared between
the epirubicin and miriplatin groups by the Wilcoxon rank sum test
or Fisher's exact test. Overall survival was calculated from the
date of therapy initiation to the date of death, and survival
curves were constructed using the Kaplan-Meier method. Overall
survival was compared between the two groups by the log-rank test
and generalized Wilcoxon test. Prognostic factors were identified
by univariate and multivariate analyses using the Cox proportional
hazards model. Values of P<0.05 were considered to indicate
statistically significant differences. Furtheremore, the time to
progression (TTP) was calculated from the date of therapy
initiation to the date of radiological progression. All analyses
were performed using Statistical Analysis System software, version
9.2 (SAS Institute Inc, Cary, NC, USA). Adverse events were
evaluated according to the National Cancer Institute Common
Terminology Criteria, version 4.0.
Results
Patient characteristics
There were no significant differences in background
clinical characteristics between the epirubicin and miriplatin
groups (Table I). The median
follow-up period for all 55 patients was 14.2 months (range,
2.2–60.5 months).
 | Table I.Clinical background of 55
BCLC-Ba hepatocellular
carcinoma patients. |
Table I.
Clinical background of 55
BCLC-Ba hepatocellular
carcinoma patients.
| Demographic
variables | Epirubicin
(n=29) | Miriplatin
(n=26) | P-value |
|---|
| Gender,
male:female | 24:5 | 22:4 | 0.85 |
| Age, years | 69.10±8.09 |
67.31±8.97 | 0.44 |
| Etiology,
HBV/HCV/non-B, non-C | 5/15/9 | 5/12/9 | 0.91 |
| Total bilirubin,
mg/dl | 0.87±0.34 | 0.77±0.42 | 0.29 |
| Serum albumin,
g/dl | 3.53±0.37 | 3.64±0.54 | 0.42 |
| Prothrombin activity,
% | 84.24±7.262 |
90.89±14.99 | 0.12 |
| ALT, IU/l | 54.56±37.47 | 58.38±42.30 | 0.72 |
| AST, IU/l | 74.03±42.94 | 76.85±103.98 | 0.89 |
| Platelet count,
×104/mm3 | 13.79±7.25 | 21.62±8.53 | 0.16 |
| AFP, ng/ml | 934.95±3,236.65 |
1,604.06±3,775.83 | 0.48 |
| DCP, mAU/ml |
5,104.88±12,687.17 |
7,466.65±15,111.95 | 0.53 |
| Child-Pugh score | 6.41±1.11 | 5.97±0.72 | 0.09 |
Overall survival and TTP
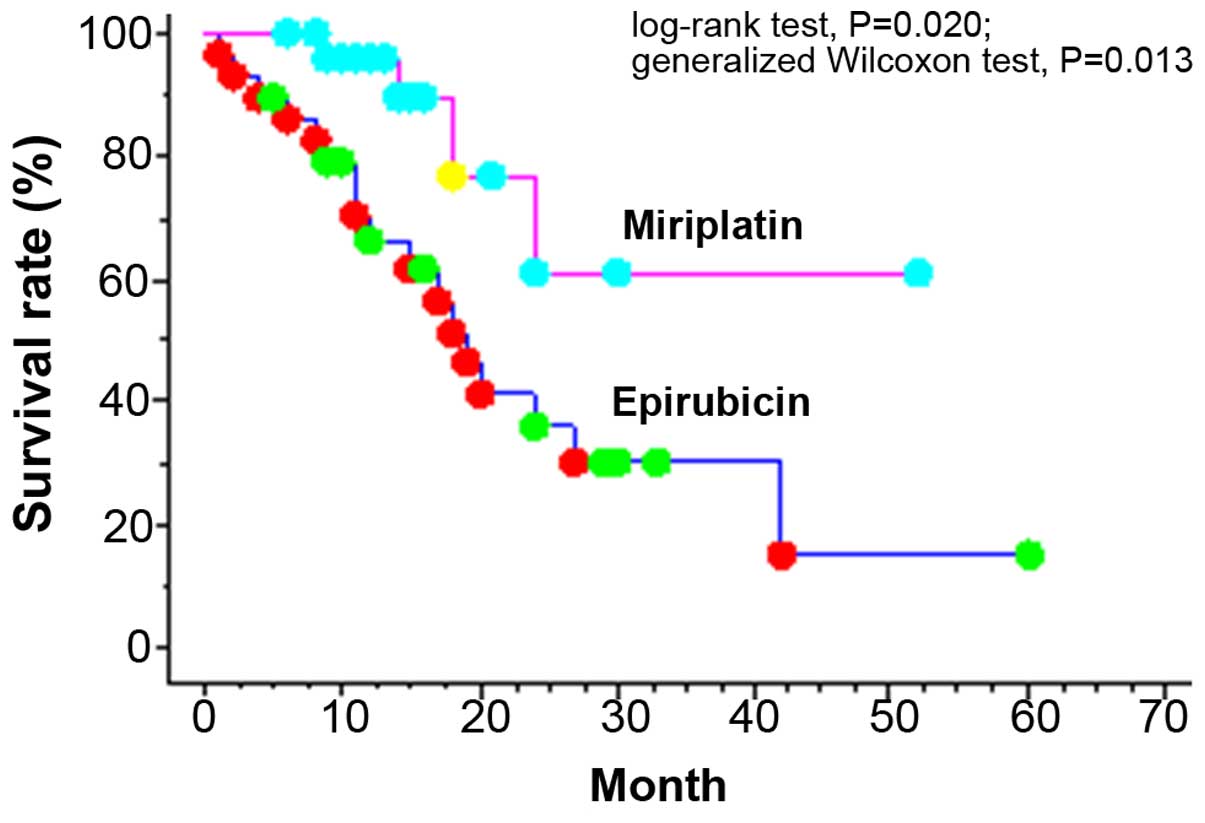
The 1-, 3- and 5-year cumulative survival rates of
BCLC-B patients were 80.0, 40.3 and 23.9%, respectively.
Significant differences were found in the cumulative 1- and 2-year
survival rates, which were 66.4 and 36.0%, respectively, in the
epirubicin group vs. 95.8 and 61.3%, respectively, in the
miriplatin group (log-rank test, P=0.020; generalized Wilcoxon
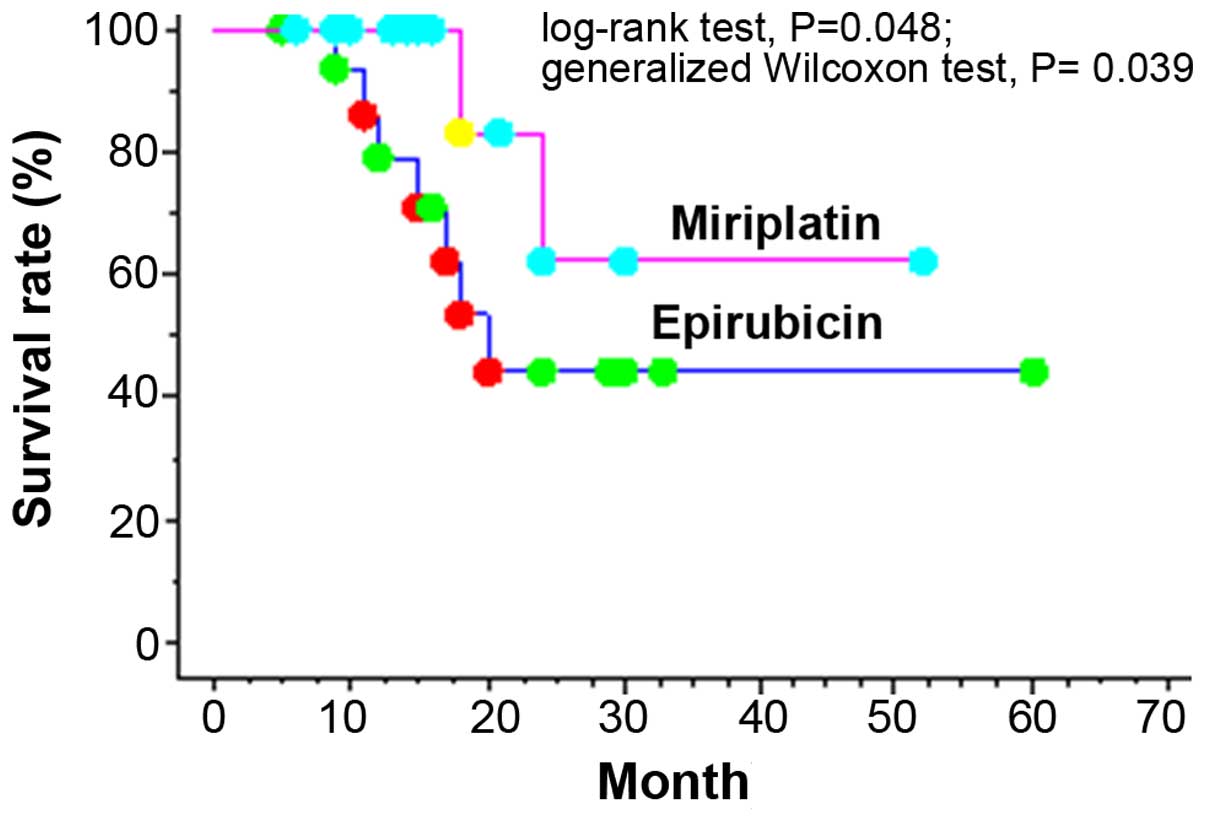
test, P=0.013; Fig. 1). In the Child
A group, significant differences were observed with respect to
cumulative 1- and 2-year survival rates, which were 79.0 and 44.4%,
respectively, in the epirubicin group vs. 100.0 and 62.5%,
respectively, in the miriplatin group (log-rank test, P=0.048;
generalized Wilcoxon test, P=0.039; Fig.
2). Even in patients with up- to-seven criteria deviations,
significant differences were detected in the cumulative 1- and
2-year survival rates, which were 56.5 and 25.4%, respectively, in
the epirubicin group vs. 93.3 and 40.8%, respectively, in the
miriplatin group.
The median TTP remained significantly longer in the
miriplatin group compared with that in the epirubicin group (9.4
vs. 4.4 months, respectively).
Univariate and multivariate analyses
for factors predicting survival
Univariate analyses demonstrated that Child-Pugh
classification, up-to-seven criteria and the chemoagent were
significantly associated with survival (Table II). Multivariate analysis identified
Child-Pugh classification [B vs. A: hazard ratio (HR) = 0.177, 95%
confidence interval (CI): 0.059–0.534], up-to-seven criteria
(beyond vs. within: HR=0.104, 95% CI: 0.018–0.593) and the use of
miriplatin vs. epirubicin as the chemoagent (HR=4.103, 95% CI:
1.267–13.289) as independent predictors of survival (Table III). Therefore, miriplatin as the
chemoagent was considered to be the most important treatment factor
affecting survival.
 | Table II.Univariate analyses for survival. |
Table II.
Univariate analyses for survival.
|
|
| P-value |
|---|
|
|
|
|
|---|
| Variables | Categories | Log-rank test | Generalized Wilcoxon
test |
|---|
| Gender | Male/female | 0.902 | 0.766 |
| Age, years | ≥70/<70 | 0.169 | 0.354 |
| Child-Pugh class | A/B | 0.030 | 0.014 |
| AFP, ng/ml | ≥400/<400 | 0.373 | 0.224 |
| DCP, mAU/ml | ≥400/<400 | 0.893 | 0.768 |
| Up-to-seven
criteria | Beyond/within | 0.018 | 0.076 |
| Albumin, g/dl | ≥3.5/<3.5 | 0.217 | 0.309 |
| Total bilirubin,
mg/dl | <1.0/≥1.0 | 0.140 | 0.257 |
| Platelet count,
×104/mm3 | ≥10/<10 | 0.417 | 0.671 |
| Chemoagents |
Epirubicin/miriplatin | 0.020 | 0.013 |
 | Table III.Multivariate analyses for
survival. |
Table III.
Multivariate analyses for
survival.
| Variables | Categories | HR | 95% CI | P-value |
|---|
| Child-Pugh class | B | 0.177 | 0.059–0.534 | 0.002 |
|
| A | 1 |
|
|
| Up-to-seven
criteria | Beyond | 0.104 | 0.018–0.593 | 0.011 |
|
| Within | 1 |
|
|
| Chemoagents | Miriplatin | 4.103 | 1.267–13.289 | 0.018 |
|
| Epirubicin | 1 |
|
|
Adverse events
Treatment-related adverse events were assessed
according to the National Cancer Institute Common Terminology
Criteria, version 4.0. Adverse events were evaluated as the maximum
change in the grade within 3 months after therapy. Grade 3 or 4
adverse events occurred in both the epirubicin and miriplatin
groups (Table IV). In the epirubicin
group, these events included an elevated aspartate aminotransferase
(AST) level in 13 patients (44.8%), elevated alanine
aminotransferase (ALT) level in 13 (44.8%), thrombocytopenia in 4
(13.7%) and hyperbilirubinemia in 1 patient (3.4%). In the
miriplatin group, these events included an elevated AST level in 5
patients (19.2%), elevated ALT level in 7 (26.9%) and
thrombocytopenia in 1 patient (3.8%). The grade 3 or 4 elevation in
AST and ALT level was transient in all patients and there was no
significant difference in the time to return of the AST and ALT
level to normal between the two groups.
 | Table IV.Adverse events (laboratory data) with
epirubicin and miriplatin used for transcatheter arterial
chemoembolization. |
Table IV.
Adverse events (laboratory data) with
epirubicin and miriplatin used for transcatheter arterial
chemoembolization.
|
| Epirubicin | Miriplatin | Grade 3–4 (%) |
|
|---|
|
|
|
|
|
|
|---|
| Adverse events | n | Grade 1 | Grade 2 | Grade 3 | Grade 4 | n | Grade 1 | Grade 2 | Grade 3 | Grade 4 | EpiI | MPT |
P-valuea |
|---|
|
Thrombocytopenia | 29 | 20 | 5 | 4 | 0 | 26 | 23 | 2 | 1 | 0 | 13.7 |
3.8 | 0.366 |
|
Hyperbilirubinemia | 29 | 23 | 5 | 1 | 0 | 26 | 21 | 5 | 0 | 0 |
3.4 |
0.0 | 1.000 |
|
Hypoalbuminemia | 29 | 16 | 13 | 0 | 0 | 26 | 15 | 11 | 0 | 0 |
0.0 |
0.0 | 1.000 |
| Elevated AST
level | 29 | 6 | 10 | 9 | 4 | 26 | 12 | 9 | 4 | 1 | 44.8 | 19.2 |
0.1775 |
| Elevated ALT
level | 29 | 10 | 6 | 9 | 4 | 26 | 12 | 7 | 6 | 1 | 44.8 | 26.9 | 0.433 |
| Elevated Cre
level | 29 | 28 | 1 | 0 | 0 | 26 | 26 | 0 | 0 | 0 |
0.0 |
0.0 | 1.000 |
Regardless of the use of platinum, there was no
grade 3 or 4 renal dysfunction in either group.
Discussion
The Barcelona Clinic Liver Cancer (BCLC) staging
system serves to estimate life expectancy and link the assessment
of disease extent and patient status with optimal treatment.
TACE has been established for the treatment of HCC
when surgical resection or other local treatment is not indicated
(11,12).
TACE is recommended as the standard treatment for
BCLC-B stage HCC.
The major local effects of TACE include local
retention of anticancer drugs and lipiodol and the ischemic effects
of the embolic material. Various anticancer drugs may be selected,
including doxorubicin (3), epirubicin
(4–6),
mitomycin C (7) and cisplatin
(8).
However, there are institutional differences
regarding the use of these agents, and the superiority of a
specific drug with regard to efficacy has yet to be established.
When we investigated the effect of the addition of TAI with DDP-H
and carboplatin as pretreatment prior to TACE/radiofrequency
ablation therapy as radical local treatment for stage I/II HCC, we
reported that whole-liver TAI using DDP-H significantly inhibited
intrahepatic recurrence (9). In
addition, in patients with JIS 0–1 stage HCC, survival was
significantly prolonged in the group that received whole-liver TAI
with DDP-H compared with the group that did not undergo infusion,
and the results of the multivariate analyses demonstrated that
performing whole-liver TAI with DDP-H was a factor contributing to
improved survival (10). Kim et
al conducted a retrospective study on the efficacy of arterial
cisplatin infusion following TACE in advanced HCC with hepatic vein
invasion, and reported that, in the group of patients who underwent
arterial cisplatin infusion, survival was significantly prolonged
without increased adverse events, compared with that in patients
who did not undergo infusion (13).
Subgroup analysis demonstrated that cisplatin significantly
prolonged survival in patients without extrahepatic metastasis and
in patients with a diffuse type of tumor. Based on these findings,
arterial infusion of a platinum agent, such as DDP-H, is considered
to play an important role as an add-on therapy to TACE in HCC. In
the BCLC Guideline, TACE is recommended for BCLC intermediate stage
B HCC (1,2).
However, there have been no studies on the efficacy
of the chemoagents used when TACE is performed in addition to DDP-H
arterial infusion to date. In the present study, we evaluated which
chemoagent would be more useful in TACE when used in addition to
DDP-H whole-liver TAI.
Epirubicin has been the gold standard among TACE
agents. The mechanism of action of epirubicin, similar to that of
doxorubicin, is considered to involve DNA intercalation (14). Specifically, as a result of the
insertion of epirubicin molecules between bases of DNA, the
high-order structure of the DNA is modified, which suppresses DNA
polymerase, DNA ligase, topoiomerase I and II, DNA helicase and RNA
polymerase activity, and inhibits DNA replication and RNA
synthesis. A response rate of 15% has been reported with the use of
hepatic arterial infusion therapy using epirubicin for unresectable
HCC (15). However, when administered
as an emulsion with lipiodol, a response rate of 42% has been
reported, indicating that epirubicin is more useful when used in
combination with lipiodol, rather than as a simple arterial
infusion (16).
Miriplatin (cis-[((1R,2R)-1,2-cyclohexanediamine-N,
N')bis(myristato)]-platinum(II)monohydrate; Dainippon Sumitomo
Pharma Co., Ltd., Osaka, Japan) is a novel lipophilic cisplatin
derivative that may be suspended in lipiodol (17–20).
Miriplatin has been developed as a new drug for use in
transcatheter arterial chemotherapy for HCC (21).
Compared with conventionally used water-soluble
anticancer agents, miriplatin has a high affinity for lipiodol,
which is considered to lead to prolonged retention in the tumor and
an increased sustained release effect. Due to its lipophilic
nature, miriplatin has a high degree of affinity for lipiodol, and
when suspended in lipiodol and administered by hepatic arterial
infusion, it is characterized by retention at the tumor site where
the platinum component is released.
The therapeutic efficacy of TACE is based on the
sustained release of the anticancer agent in the tumor and an
ischemic effect due to an embolic agent. Miriplatin has a high
capacity for retention at the tumor site due to its high affinity
for lipiodol and the fact that only a very small amount is lost in
the general circulation. Furthermore, as only a small amount enters
the general circulation, renal toxicity is expected to be low and
the effectiveness of miriplatin is expected to be high.
However, Miyayama et al reported that
superselective TACE using miriplatin resulted in frequent local
recurrence, despite less arterial damage compared with TACE using
epirubicin and mitomycin C (22). It
has also been reported that the local control rate is higher with
epirubicin compared with miriplatin (22). However, the short-term effects of
miriplatin and epirubicin are reported to be comparable (23). In the present study of BCLC-B HCC, in
terms of tumor factors, it was clear that up-to-seven criteria and
hepatic reserve play a role in survival; in terms of tumor
treatment factors, the combined use of DDP-H and miriplatin
contributed to improved survival. We hypothesized that a
synergistic antitumor effect of these platinum-based agents has
been elicited by combining the sustained release nature of
miriplatin and the concentration-dependent property of cisplatin.
In a phase I clinical study in which TACE with miriplatin was used
in combination with hepatic arterial infusion chemotherapy using
cisplatin, investigations were conducted at a dose of 65
mg/m2 of cisplatin in combination with ≤120 mg/body of
miriplatin, the maximum dose approved in Japan, and it was reported
that, in terms of safety, this dose was tolerable (24). In the present study, there were no
grade ≥4 adverse events, and no clinically important adverse
events; given that there have been no reports of adverse events
when miriplatin or cisplatin were used in combination with an
embolizing agent, the adverse events in the present study were
within the expected range.
The limitation of this study was the selection of
chemoagent for TACE. There is a lack of high-quality evidence on
the selection of chemolipiodolization agent to date. As no
significant differences in background characteristics were found
between the epirubicin and miriplatin TACE groups, we consider our
analyses to be useful. Although investigations in numerous patients
in various stages of diseases are required in the future, it is
believed that double-platinum therapy with DDP-H and miriplatin for
BCLC-B HCC may be a useful treatment strategy.
References
|
1
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tommasini M, Colombo M, Sangiovanni A,
Orefice S, Bignami P, Doci R and Gennari L: Intrahepatic
doxorubicin in unresectable hepatocellular carcinoma. The
unfavorable role of cirrhosis. Am J Clin Oncol. 9:8–11. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ichida T, Kato M, Hayakawa A, Watanabe M,
Igarashi K, Hata K, Doya Y, Miura M, Sato H and Asakura H:
Treatment of hepatocellular carcinoma with a
CDDP-epirubicin-lipiodol suspension: A pilot
clinico-pharmacological study. Cancer Chemother Pharmacol.
31:(Suppl). S51–S54. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aoyama K, Tsukishiro T, Okada K, Tsuchida
T, Aiba N, Nambu S, Miyabayashi C, Yasuyama T, Higuchi K and
Watanabe A: Evaluation of transcatheter arterial embolization with
epirubicin-lipiodol emulsion for hepatocellular carcinoma. Cancer
Chemother Pharmacol. 31:(Suppl). S55–S59. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colleoni M, Gaion F, Liessi G,
Mastropasqua G and Nelli P: Arterial chemoembolization with
epirubicin in unresectable hepatocellular carcinoma in cirrhosis.
Oncol Rep. 1:1171–1175. 1994.PubMed/NCBI
|
|
7
|
Ohnishi K, Tsuchiya S, Nakayama T, Hiyama
Y, Iwama S, Goto N, Takashi M, Ohtsuki T, Kono K, Nakajima Y, et
al: Arterial chemoembolization of hepatocellular carcinoma with
mitomycin C microcapsules. Radiology. 152:51–55. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasaki Y, Imaoka S, Kasugai H, Fujita M,
Kawamoto S, Ishiguro S, Kojima J, Ishikawa O, Ohigashi H, Furukawa
H, et al: A new approach to chemoembolization therapy for hepatoma
using ethiodized oil, cisplatin, and gelatin sponge. Cancer.
60:1194–1203. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishikawa T, Higuchi K, Kubota T, Seki K,
Honma T, Yoshida T and Kamimura T: Prevention of intrahepatic
distant recurrence by transcatheter arterial infusion chemotherapy
with platinum agents for stage I/II hepatocellular carcinoma.
Cancer. 117:4018–4025. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishikawa T, Kubota T, Abe S, Watanabe Y,
Sugano T, Inoue R, Iwanaga A, Seki K, Honma T and Yoshida T:
Hepatic arterial infusion chemotherapy with cisplatin before
radical local treatment of early hepatocellular carcinoma (JIS
score 0/1) improves survival. Ann Oncol. 25:1379–1384. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamada R, Sato M, Kawabata M, Nakatsuka H,
Nakamura K and Takashima S: Hepatic artery embolization in 120
patients with unresectable hepatoma. Radiology. 148:397–401. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsui O, Kadoya M, Yoshikawa J, Gabata T,
Arai K, Demachi H, Miyayama S, Takashima T, Unoura M and Kogayashi
K: Small hepatocellular carcinoma: Treatment with subsegmental
transcatheter arterial embolization. Radiology. 188:79–83. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HC, Lee JH, Chung JW, Kang B, Yoon JH,
Kim YJ, Lee HS, Jae HJ and Park JH: Transarterial chemoembolization
with additional cisplatin infusion for hepatocellular carcinoma
invading the hepatic vein. J Vasc Interv Radiol. 24:274–283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka M, Yoshida S and Kimura K:
Mechanism of inhibition of DNA polymerases by 4′-epiadriamycin and
4′-O-tetrahydropyranyladriamycin. Gan. 74:829–836. 1983.PubMed/NCBI
|
|
15
|
Nagasue N, Yukaya H, Okamura J, Kuroda C,
Kubo Y, Hirai K, Tanikawa K, Okita K, Ando K and Tamura K:
Intra-arterial administration of epirubicin in the treatment of
non-resectable hepatocellular carcinoma. Epirubicin study group for
hepatocellular carcinoma. Cancer & chemotherapy. 13:2786–2792.
1986.(In Japanese).
|
|
16
|
Yoshikawa M, Saisho H, Ebara M, Iijima T,
Iwama S, Endo F, Kimura M, Shimamura Y, Suzuki Y, Nakano T, et al:
A randomized trial of intrahepatic arterial infusion of
4′-epidoxorubicin with Lipiodol versus 4′-epidoxorubicin alone in
the treatment of hepatocellular carcinoma. Cancer Chemother
Pharmacol. 33:(Suppl). S149–S152. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maeda M, Uchida NA and Sasaki T:
Liposoluble platinum (II) complexes with antitumor activity. Jpn J
Cancer Res. 77:523–525. 1986.PubMed/NCBI
|
|
18
|
Kishimoto S, Ohtani A, Fukuda H, Fukushima
S and Takeuchi Y: Relation between intracellular accumulation and
cytotoxic activity of cis-[((1R, 2R)-1,2-cyclohexanediamine-N,
N')bis(myristato)]platinum(II) suspended in Lipiodol. Biol Pharm
Bull. 26:683–686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanada M, Baba A, Tsutsumishita Y, Noguchi
T, Yamaoka T, Chiba N and Nishikaku F: Intra-hepatic arterial
administration with miriplatin suspended in an oily lymphographic
agent inhibits the growth of tumors implanted in rat livers by
inducing platinum-DNA adducts to form and massive apoptosis. Cancer
Chemother Pharmacol. 64:473–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanada M, Baba A, Tsutsumishita Y, Noguchi
T and Yamaoka T: Intra-hepatic arterial administration with
miriplatin suspended in an oily lymphographic agent inhibits the
growth of human hepatoma cells orthotopically implanted in nude
rats. Cancer Sci. 100:189–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okusaka T, Okada S, Nakanishi T, Fujiyama
S and Kubo Y: Phase II trial of intra-arterial chemotherapy using a
novel lipophilic platinum derivative (SM-11355) in patients with
hepatocellular carcinoma. Invest New Drugs. 22:169–176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyayama S, Yamashiro M, Shibata Y,
Hashimoto M, Yoshida M, Tsuji K, Toshima F and Matsui O: Comparison
of local control effects of superselective transcatheter arterial
chemoembolization using epirubicin plus mitomycin C and miriplatin
for hepatocellular carcinoma. Jpn J Radiol. 30:263–270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Handa T, Imai Y, Sugawara K, Chikayama T,
Nakazawa M, Ando S, Hamaoka K, Inao M, Nakayama N and Mochida S:
Transcatheter arterial chemoembolization for hepatocellular
carcinoma: Comparison of the therapeutic efficacies between
miriplatin and epirubicin. Hepatol Res. 44:1072–1080. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamimura K, Suda T, Tamura Y, Takamura M,
Yokoo T, Igarashi M, Kawai H, Yamagiwa S, Nomoto M and Aoyagi Y:
Phase I study of miriplatin combined with transarterial
chemotherapy using CDDP powder in patients with hepatocellular
carcinoma. BMC Gastroenterol. 12:1272012. View Article : Google Scholar : PubMed/NCBI
|