Introduction
As a subtype of adenosis, sclerosing adenosis (SA)
is a benign proliferative disease of the breast associated with
disordered acinar, myoepithelial and connective tissue in the
terminal ductal lobular unit. Despite its benign pathological
behavior, SA may mimic in situ and invasive carcinoma
grossly and microscopically (1,2); thus
further investigation is crucial to fully understand this disease
and its imaging characteristics.
Several articles have described the clinical and
imaging characteristics of SA over the past few decades (3–6). As
reported in previous studies (5), a
proportion of patients with SA complained of mastalgia (14.0%),
others were detected with a palpable mass (30.2%), while several
asymptomatic patients were identified through imaging examination,
and the findings on mammography (MG) were mainly
microcalcifications (55.8%), mass (11.6%), asymmetric focal density
(6.9%) and focal architectural distortion (6.9%). However, only few
studies have investigated the imaging characteristics of SA on
ultrasonography (US).
In China, a higher number of individuals seek
periodic breast examination due to the increasing incidence of
breast diseases, and breast US, as a radiation-free modality, is
the most popular screening method due to the advantages of high
safety and low cost. In addition, Eastern women tend to have dense
mammary glands, which may be more clearly displayed on US compared
with MG. The objective of the present study was to focus on the
radiological findings, particularly the US characteristics of SA,
and to determine the correlation of these findings with
histopathological results.
Patients and methods
Patients
A total of 24,239 patients who underwent breast
surgery between July, 2009 and December, 2012 at the Fudan
University Cancer Center (Shanghai, China) were retrospectively
investigated. SA was histopathologically diagnosed in a total of
191 female patients (200 lesions), among whom 145 patients (151
lesions) with SA as the major component were included for clinical
and imaging analysis. In the remaining 46 patients (49 lesions), SA
was an incidental pathological finding following surgery for
malignant tumors (21 lesions in 19 patients) and benign tumors (28
lesions in 27 patients), and these cases were excluded from the
study.
The present study was conducted following approval
by the Institutional Review Board (IRB) of Fudan University
Shanghai Cancer Center. Written informed consent was waived by the
IRB due to the retrospective nature of the study. No study subjects
or cohorts have been previously reported.
US
All 145 patients underwent US prior to surgery. All
the examinations were performed by sonographers experienced in
breast US with a linear array transducer (IU22, Philips, Bothell,
WA, USA; and Mylab 90, Esaote, Genoa, Italy). A final assessment
was made and each case was classified preoperatively based on the
American College of Radiology Breast Imaging Reporting and Data
System (BI-RADS) US lexicon criteria (7,8). The
morphology of the lesions, including size, shape, margin, internal
echo pattern and posterior acoustic feature, was analyzed by two
experienced investigators blinded to the study. Color Doppler US
and power Doppler US were performed to evaluate the vessels within
and surrounding the lesions.
MG
Among the 145 patients (151 lesions), 9 (15 lesions)
underwent MG in other hospitals prior to admission to our center,
and the mammograms of these patients were unavailable for analysis.
The remaining 136 patients underwent MG in our center prior to
surgery; craniocaudal and mediolateral oblique mammograms of both
breasts were obtained using Senographe 2000D equipment (General
Electrics, Detroit, MI, USA). The mammograms for each lesion were
reviewed by three radiologists experienced in breast imaging and
characterized according to mass size, characteristics, morphology
and distribution of microcalcifications.
Correlation with histopathology
All the lesions were surgically excised. The
non-palpable lesions were preoperatively localized by using a
needle-wire system placed under MG (23 lesions in 23 patients) and
US (52 lesions in 48 patients) guidance, and underwent specimen
radiographs and US prints to confirm removal of the lesions.
To analyze the accuracy of US and MG diagnosing the
cases as benign or malignant, the BI-RADS category was compared
with the histopathological results. Categories 1–3 were classified
as the benign group, while categories 4B, 4C and 5 were classified
as the malignant group. Despite a malignant probability of <10%,
category 4A was classified in the malignant group in our study, due
to the indication of clinical intervention. Category 0 was also
included in the malignant group due to the requirement for further
examinations.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 19.0 (SPSS, Chicago, IL, USA). To evaluate
diagnostic performance, receiver operating characteristic (ROC)
curves were analyzed using MedCalc for Windows, version 13.1.2.0
(MedCalc Software, Mariakerke, Belgium). Sensitivity, specificity
and area under the ROC curve (AUC) were calculated. Measurement
data were analyzed with the t-test and count data were analyzed
with the Chi-squared test. A P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
All the 145 patients analyzed were female, with a
mean age ± standard deviation of 46.8±7.8 years (range, 25–71
years). None of the patients had a family history of breast cancer.
In 6 patients (4.1%), SA was found bilaterally; 18 patients (12.4%)
complained of mastalgia and 5 patients (3.4%) complained of nipple
discharge; 74 patients (51.0%) reported awareness of a breast mass,
while 48 patients (33.1%) were asymptomatic.
All the lesions were surgically excised. Of the 151
lesions, 20 (13.4%) were SA with malignant lesions as the minor
component, among which 13 lesions were ductal carcinoma in
situ (DCIS) with bilateral involvement in 1 patient, 4 lesions
were DCIS with microinvasion (DCISM), and 3 lesions were invasive
ductal carcinoma (IDC). A total of 58 lesions (38.4%) were SA with
benign lesions, such as ductal hyperplasia, fibroadenomas and
intraductal papilloma, and the remaining 73 lesions (48.3%) were
pure SA.
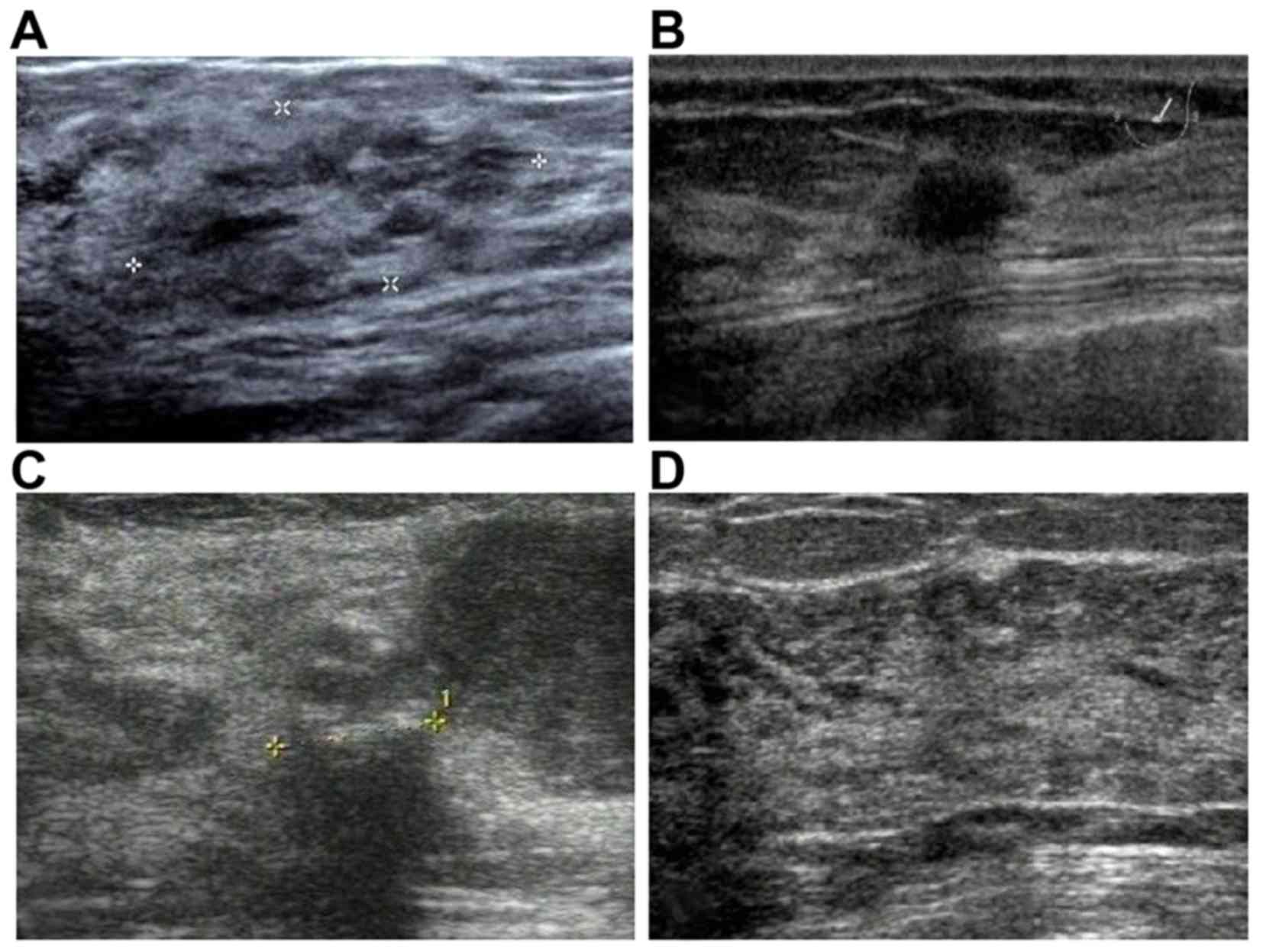
US findings
All 145 patients (151 lesions) underwent US
examination. Apart from the 32 lesions (21.2%) without visible
abnormalities, there were mainly four types of imaging findings:
Heterogeneously echogenic areas (n=14; 9.3%), mass lesions (n=78;
51.7%), masses with calcifications (n=21; 13.9%) and focal acoustic
shadowing (n=6; 4.0%) (Fig. 1). The
US images of these 119 visible lesions were reviewed and the
characteristics were summarized as follows (Table I): 87.4% of the lesions (104/119)
were hypoechoic, 58.0% (69/119) were irregular in shape and 52.1%
(62/119) had an ill-defined margin. Among the mass lesions with or
without calcifications, 90.9% (90/99) were solid, 4.0% (4/99) were
cystic with thickened walls, and 5.1% (5/99) were mixed.
Calcifications were detected in 21 lesions (17.6%), of which 9 were
clustered punctate, 5 were diffusely punctate and 7 were clustered
amorphous. According to the grading system reported by Adler et
al (9), only 9 lesions (7.6%)
were hypervascular. However, none of the characteristics mentioned
above was significantly correlated with pathological type
(P>0.05).
 | Table I.Ultrasonographic findings of 119
solitary lesions in patients with sclerosing adenosis of the
breast. |
Table I.
Ultrasonographic findings of 119
solitary lesions in patients with sclerosing adenosis of the
breast.
| Characteristics | No. of lesions
(%) | Pearson χ2
(P-value)b |
|---|
| Shape |
| 0.159 (0.690) |
|
Regular | 50 (42.0) |
|
|
Irregular | 69 (58.0) |
|
| Echo pattern |
| 4.877 (0.181) |
|
Hypoechoic | 104 (87.4) |
|
|
Isoechoic | 4 (3.4) |
|
|
Hyperechoic | 2 (1.7) |
|
| Mixed
echoic | 5 (4.3) |
|
|
Echoless | 4 (3.4) |
|
| Margin |
| 0.646 (0.421) |
|
Well-defined | 57 (47.9) |
|
|
Ill-defined | 62 (52.1) |
|
| Calcifications |
| 0.044 (0.833) |
|
Absent | 98 (82.4) |
|
|
Present | 21 (17.6) |
|
| Focal acoustic
shadowing |
| 0.415 (0.519) |
|
Absent | 113 (96.0) |
|
|
Present | 6 (4.0) |
|
|
Vascularityaa |
| 2.132 (0.344) |
| None
(grade 0) | 92 (77.3) |
|
|
Hypovascular (grade I) | 18 (15.1) |
|
|
Hypervascular (grade
II-III) | 9 (7.6%) |
|
Upon comparing BI-RADS US category with
histopathology, the AUC of US distinguishing benign from malignant
lesions was 0.547. The sensitivity, specificity, positive
predictive value and negative predictive value of indicating
clinical intervention (BI-RADS categories 0 and 4A-5) were 44.4,
54.9, 11.8 and 88.0%, respectively. Of the 23 lesions of reasonable
and high suspicion of malignancy (4B-5), only 1 case was finally
diagnosed as malignant, which was SA with IDC as the minor
component. However, 85.3% of category 4A cases were finally
confirmed as benign (Table II). The
false-positive rate, defined as the percentage of lesions with an
indication for clinical intervention (BI-RADS categories 0 and
4A-5) among the pathologically benign lesions, was 45.1% (60/133).
Among false-positive lesions, 12 were accompanied by atypical
ductal hyperplasia, 4 displayed calcium deposition microscopically,
whereas 1 lesion had both. The false-negative rate, defined as the
percentage of lesions indicated as benign (BI-RADS categories 1–3)
among the pathologically malignant lesions, was 55.6% (10/18).
 | Table II.Performance of ultrasonography (US)
and mammography (MG) in sclerosing adenosis of the breast. |
Table II.
Performance of ultrasonography (US)
and mammography (MG) in sclerosing adenosis of the breast.
|
| Histopathology of the
lesions |
|---|
|
|
|
|---|
|
| Benign (n) | Malignant (n) |
|---|
| BI-RADS US (151
lesions) |
|
| 0 | 9 | 2 |
| 1–3 | 73 | 10 |
| 4A | 29 | 5 |
| 4B-5 | 22 | 1 |
|
Total | 133 | 18 |
| BI-RADS MG (136
lesions) |
|
| 0 | 17 | 4 |
| 1–3 | 41 | 4 |
| 4A | 34 | 5 |
| 4B-5 | 26 | 5 |
|
Total | 118 | 18 |
In the 14 lesions with heterogeneously echogenic
areas, apart from 5 lesions classified as BI-RADS US categories
1–3, 4 required further examination (category 0), whereas the
remaining 5 lesions were classified as category 4A-4B, among which
only 2 lesions were finally confirmed as malignant. A total of
78.1% (25/32) of the US-negative lesions were simple SA
histopathologically, without accompanying malignant or benign
lesions.
Focal acoustic shadowing had been described in
previous studies (5,6); ours included 6 lesions (4.0%), with 1
lesion classified as BI-RADS US category 0, 1 as 4A, and 4 as ≥4B,
but only 1 lesion exhibiting focal acoustic shadowing was finally
histopathologically confirmed as SA with malignant lesions.
Although considered as a sign of malignancy, focal acoustic
shadowing was not found to be significantly correlated with
histopathological malignancy (P=0.519).
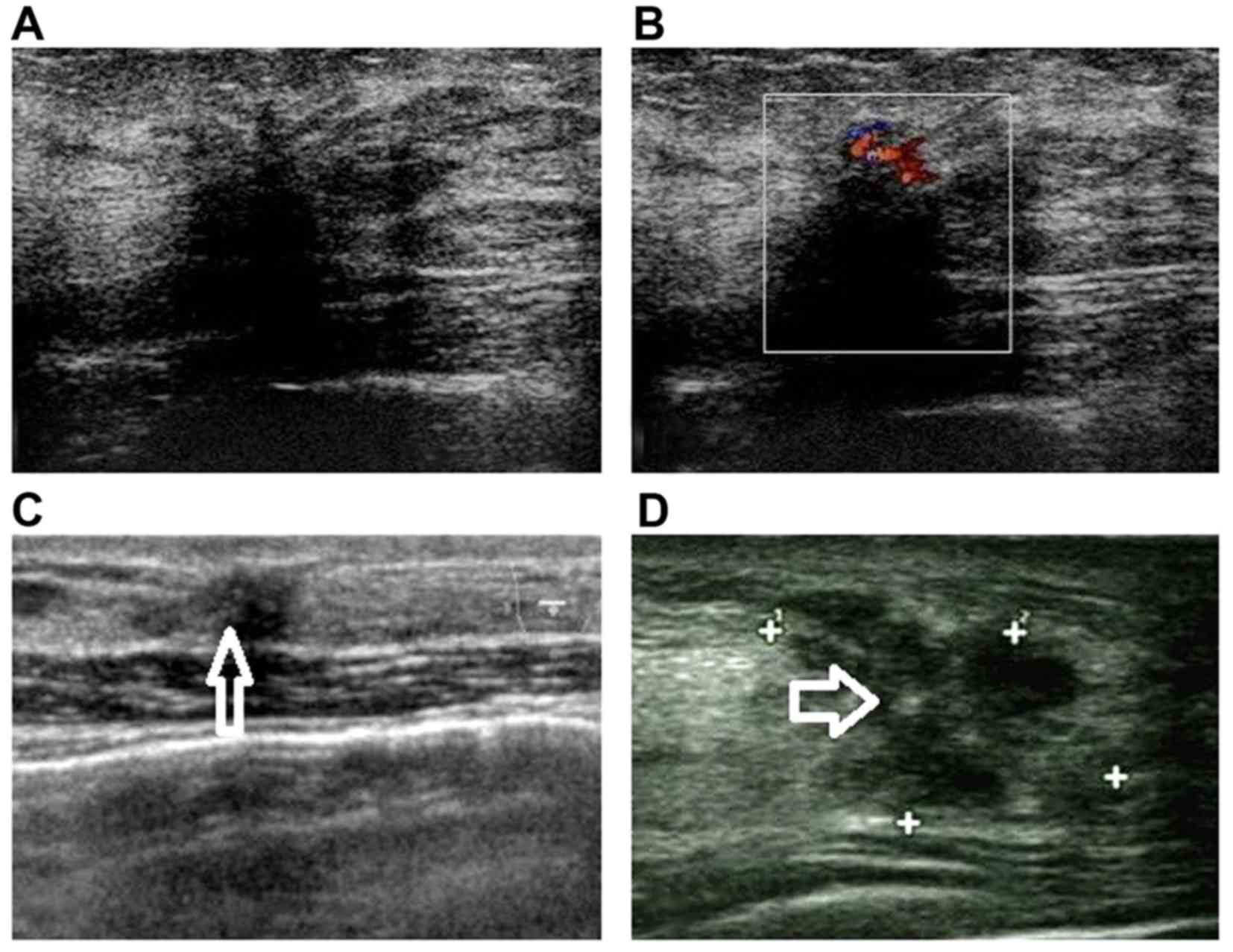
In the 60 lesions that were overestimated by BI-RADS
US category, one or more characteristics of malignancy were found
in US imaging, such as irregular shape in 54 lesions (90.0%),
ill-defined margins in 46 lesions (76.7%), calcifications in 12
lesions (20%), focal acoustic shadowing in 6 lesions (4.0%) and
hypervascularity in 7 lesions (11.7%) (Fig. 2).
MG findings and comparison with
US
A total of 136 patients underwent MG in our center
and abnormalities were detected in 126 patients (92.6%), whereas
the examination was negative in 10 patients (7.4%). There were
mainly four types of imaging findings: Microcalcifications (n=43;
31.6%), masses (n=32; 23.5%), asymmetric focal density (n=20;
14.7%) and focal architectural distortion (n=31; 22.8%).
Of the 43 microcalcifications, 34 (79.1%) were
clustered, 8 (18.6%) were scattered and 1 (2.3%) was diffuse. The
shapes of microcalcifications were punctate in 29 (67.4%), round in
5 (11.6%), pleomorphic in 7 (16.3%), and amorphous in 2 (4.7%)
cases. On US imaging, 15 (34.9%) of the MG microcalcifications were
invisible, 6 (14.0%) were masses with calcifications and the
remaining were lesions without calcifications, including 4 (9.3%)
heterogeneously echogenic areas and 18 masses (41.9%).
Unlike US imaging, on which masses and masses with
calcificaitons constituted the majority (65.6%), masses were only
found in 32 (23.5%) cases in MG. Of the 6 lesions with focal
acoustic shadowing on US, 2 were negative on MG and 4 exhibited as
asymmetric focal density (Table
III).
 | Table III.Comparison of findings in sclerosing
adenosis of the breast between ultrasonography (US) and mammography
(MG). |
Table III.
Comparison of findings in sclerosing
adenosis of the breast between ultrasonography (US) and mammography
(MG).
|
| MG findings |
|---|
|
|
|
|---|
| US findings | Microcalcifications
(n=43) | Masses (n=32) | Asymmetric focal
density (n=20) | Focal architectural
distortion (n=31) | Negative (n=10) | NAa (n=15) |
|---|
| Heterogeneously
echogenic areas (n=14) | 4 | 0 | 2 | 6 | 1 | 1 |
| Masses (n=78) | 18 | 21 | 8 | 16 | 5 | 10 |
| Masses with
calcifications (n=21) | 6 | 7 | 3 | 2 | 2 | 1 |
| Focal acoustic
shadowing (n=6) | 0 | 0 | 4 | 0 | 2 | 0 |
| Negative
(n=15) | 15 | 4 | 3 | 7 | 0 | 3 |
Comparing BI-RADS MG category with histopathology,
the AUC of MG distinguishing between benign and malignant lesions
was significantly smaller compared with US (0.497 vs. 0.547,
respectively; P=0.036).
Discussion
SA is a benign proliferative disease of the
epithelium and myoepithelium that originates in glandular lobules
and is accompanied by desmoplasia. Although it is a benign
disorder, SA may prove challenging in both clinical and
radiological aspects, as it may mimic malignancy grossly and
microscopically. Furthermore, SA is occasionally complicated by
malignant tumors, such as IDC and DCIS, and certain benign
disorders, such as fibroadenosis, intraductal papilloma and
atypical ductal hyperplasia, which makes differential diagnosis
more difficult prior to surgery.
Although SA was not considered as a precancerous
lesion, a higher risk of breast carcinoma has been reported in
patients with SA (10,11). In a cohort of 13,434 women followed
up for a median of 15.7 years, Visscher et al (12) found that the presence of SA
stratified risk in subsets of women defined by age, involution
status and family history, and that SA is a common proliferative
lesion of the breast that conveys an approximate doubling of breast
cancer risk.
In the present study, malignant lesions were
identifies in 20 of the 151 lesions (13.4%), among which 13 lesions
were DCIS (SA DCIS), 4 were DCISM and 3 were IDC. Among the
patients with SA DCIS, 1 patient was confirmed as synchronous
bilateral breast cancer. In previous studies, Moritani et al
(13) reported 5 cases of
synchronous or metachronous contralateral breast carcinoma
developing among 23 patients with SA DCIS; Yoshida et al
(14) reported a significantly
higher rate of synchronous and metachronous bilateral breast cancer
in SA DCIS compared with non-SA DCIS [9 (38%) of 24 patients vs. 22
(13%) of 174 patients; P<0.01]. A retrospective study in our
center previously demonstrated that the presence of SA was an
independent risk factor for synchronous bilateral breast cancer
(P<0.001) (15). Cancer genuinely
arising in SA may often have biological characteristics of
bilateral breast cancer (16), thus
making it more important to distinguish it through radiological
examinations.
US is generally accepted as an efficient method that
is most commonly used in breast diseases, due to its advantages of
non-invasiveness and convenience; in addition, its clearer imaging
of dense breast tissue compared with MG makes it more popular in
Eastern countries. However, previous studies mostly focused on the
MG findings of SA, while those of US have rarely been discussed
(3–5). The results of MG findings in our study
were almost consistent with previous studies, which revealed mainly
four types of imaging, with microcalcifications as the most common
type. Although MG was more sensitive for discovering abnormalities
compared with US in our study, the AUC of US distinguishing benign
from malignant lesions was larger compared with that of MG (0.547
vs. 0.497, respectively; P=0.036).
In the present study, 151 lesions in 145 patients
were detected by US, and SA was histopathologically confirmed as
the major component. Mass lesions without calcification constituted
the major component among all cases (78 lesions; 51.7%), more
frequently compared with MG (32 lesions; 23.5%), which was
consistent with previous reports (12–44%) (5,6). The
majority of the visible lesions were irregular in shape (58.0%) and
had an ill-defined margin (52.1%), which were probable indications
of malignancy; this may be one of the reasons for the
overestimation of BI-RADS US category in certain cases. Likewise,
hypervascular lesions were all classified as category ≥4,
indicating a malignant tendency to a certain degree. Only few of
the previous studies had described the US characteristics, so we
herein compared our results with those of MG. However, the
morphology of masses detected by MG, including shape and margin,
did not lead to the same conclusion in each study (4–6),
possibly as a result of the small samples, whereas, to the best of
our knowledge, our sample was the largest thus far. However, the
shape and margin of the lesions were not significantly different
among simple SA, SA with malignant lesions and SA with benign
lesions, which may be attributed to the variable pathological
manifestations of SA (1,13,17).
Microcalcifications were found to be one of the
major characteristics of SA on MG (6), with 80% of the cases presenting as
microcalcifications in clusters and 20% being diffusely scattered.
Only 1 case of clustered calcifications without a mass was found
through US in our study, in which SA was the minor component and
DCIS was the major component. Of the 151 lesions with SA as the
major component, masses with calcification were found in 21 (13.9%)
cases on US; the most frequent calcification pattern was clustered
punctate and the remaining were diffusely punctate and clustered
amorphous calcifications. However, the presence of calcifications
was not significantly correlated with histopathology, which may be
attributed to the hyposensitivity of US for identifying
calcifications.
In addition to masses with or without calcification,
there were quite a few cases [14 (9.3%)] presenting as
heterogeneously echogenic areas on US images; focal architectural
distortion of breast tissue without occupying effect was the
characteristic imaging finding. There were also 32 lesions (21.2%)
without visible abnormalities on US, 78.1% of which (25/32) were
simple SA histopathologically, without accompanying malignant or
benign lesions. Thus, simple SA more frequently presented as
negative on US, and patients with this type of SA partly
contributed to the false-negative rate.
The difficulty of diagnosing SA through US also
manifested as the relatively high false-positive rate, which was
45.1% in our study. As mentioned above, certain US characteristics
of malignancy, such as calcifications, irregular shape, indistinct
margins, focal acoustic shadowing and hypervascularity, contributed
to the overestimation of diagnosis (Fig.
2). As reported in studies on fine-needle aspiration cytology
of SA, the most frequent characteristics, although not specific,
were low-to-moderate cellularity, bland epithelial cells that
focally formed cohesive groups/tubules or occasionally discohesive
clusters or individual cells, and fragments of dense fibrous stroma
(18). Some tubules had an angulated
configuration. These histopathological characteristics might
contribute to the malignant signs of SA on imaging examination.
In conclusion, the accuracy of US in diagnosing SA
was limited, although it was significantly higher compared with MG.
Four types of US presentation were also observed in our series and
compared with MG; to the best of our knowledge, the present study
was the first to discuss these findings in detail to date. The high
frequency of malignant signs on US may contribute to the
misdiagnosis of SA. These findings may prove helpful for further
investigation, which should focus on contrast-enhanced US and shear
wave elastography and may improve the accuracy of diagnosis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81371575).
References
|
1
|
Tavassoli FA: Pathology of the Breast.
Appleton and Lange; Norwalk, CT: pp. 93–97. 1992
|
|
2
|
Santen RJ and Mansel R: Benign breast
disorders. N Engl J Med. 353:275–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nielsen NS and Nielsen BB: Mammographic
features of sclerosing adenosis presenting as a tumor. Clin Radiol.
37:371–373. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DiPiro PJ, Gulizia JA, Lester SC and Meyer
JE: Mammographic and sonographic appearances of nodular adenosis.
AJR Am J Roentgenol. 175:31–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Günhan-Bilgen I, Memiş A, Ustün EE,
Ozdemir N and Erhan Y: Sclerosing adenosis: Mammographic and
ultrasonographic findings with clinical and histopathological
correlation. Eur J Radiol. 44:232–238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taşkin F, Köseoğlu K, Unsal A, Erkuş M,
Ozbaş S and Karaman C: Sclerosing adenosis of the breast:
Radiologic appearance and efficiency of core needle biopsy. Diagn
Interv Radiol. 17:311–316. 2011.PubMed/NCBI
|
|
7
|
Levy L, Suissa M, Chiche JF, Teman G and
Martin B: BIRADS ultrasonography. Eur J Radiol. 61:202–211. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mercado CL: BI-RADS update. Radiol Clin
North Am. 52:481–487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adler DD, Carson PL, Rubin JM and
Quinn-Reid D: Doppler ultrasound color flow imaging in the study of
breast cancer: Preliminary findings. Ultrasound Med Biol.
16:553–559. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kabat GC, Jones JG, Olson N, Negassa A,
Duggan C, Ginsberg M, Kandel RA, Glass AG and Rohan TE: A
multi-center prospective cohort study of benign breast disease and
risk of subsequent breast cancer. Cancer Causes Control.
21:821–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hartmann LC, Sellers TA, Frost MH, Lingle
WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS,
Hillman DW, et al: Benign breast disease and the risk of breast
cancer. N Engl J Med. 353:229–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Visscher DW, Nassar A, Degnim AC, Frost
MH, Vierkant RA, Frank RD, Tarabishy Y, Radisky DC and Hartmann LC:
Sclerosing adenosis and risk of breast cancer. Breast Cancer Res
Treat. 144:205–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moritani S, Ichihara S, Hasegawa M, Endo
T, Oiwa M, Shiraiwa M, Nishida C, Morita T, Sato Y, Hayashi T, et
al: Topographical, morphological and immunohistochemical
characteristics of carcinoma in situ of the breast involving
sclerosing adenosis. Two distinct topographical patterns and
histological types of carcinoma in situ. Histopathology.
58:835–846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshida A, Hayashi N, Akiyama F, Yamauchi
H, Uruno T, Kikuchi M, Yagata H, Tsugawa K, Suzuki K, Nakamura S
and Tsunoda H: Ductal carcinoma in situ that involves sclerosing
adenosis: High frequency of bilateral breast cancer occurrence.
Clin Breast Cancer. 12:398–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen JJ, Wang Y, Xue JY, Chen Y, Chen YL,
Xiao Q, Yang WT, Shao ZM and Wu J: A clinicopathological study of
early-stage synchronous bilateral breast cancer: A retrospective
evaluation and prospective validation of potential risk factors.
PLoS One. 9:e951852014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogura K, Horii R, Oosako T, Iwase T and
Akiyama F: A clinico-pathological study on cancer in sclerosing
adenosis. Breast Cancer. 21:732–737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fechner RE: Carcinoma in situ involving
sclerosing adenosis. Histopathology. 28:5701996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kundu UR, Guo M, Landon G, Wu Y, Sneige N
and Gong Y: Fine-Needle aspiration cytology of sclerosing adenosis
of the breast: A retrospective review of cytologic features in
conjunction with corresponding histologic features and radiologic
findings. Am J Clin Pathol. 138:96–1028. 2012. View Article : Google Scholar : PubMed/NCBI
|