Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer type in males, and the ninth most common in females
(1). Although the number of
hemodialysis (HD) patients with HCC remains low, the development of
an effective therapeutic strategy for them is of clinical
importance. It is well known that, as HD poses a risk for hepatitis
C virus (HCV) infection, the HCV-positive rate is increased in HD
patients, while HCV is a major risk factor for HCC (2). Ozer Etik et al (3) reported that the prevalence of anti-HCV
seropositivity among patients receiving maintenance HD in developed
countries ranges from 5–60% (3).
However, the number of patients with diabetes mellitus (DM) has
been increasing and diabetic nephropathy has become the primary
reason for requiring HD (4).
Furthermore, DM has recently been reported to be an independent
risk factor for HCC (5), and thus it
is expected that the number of HD patients with HCC will increase
in the near future.
Hepatic resection (Hx) is a good therapeutic option
for HCC (6,7), while radiofrequency ablation (RFA) has
become the standard low-invasive therapy for small HCC (8,9).
However, there is no consensus regarding which therapeutic option
is better and/or safer for HD patients with small HCC. The aim of
the present study was to retrospectively analyze clinical features,
complications and prognosis of HD patients with small HCC treated
with Hx or RFA.
Materials and methods
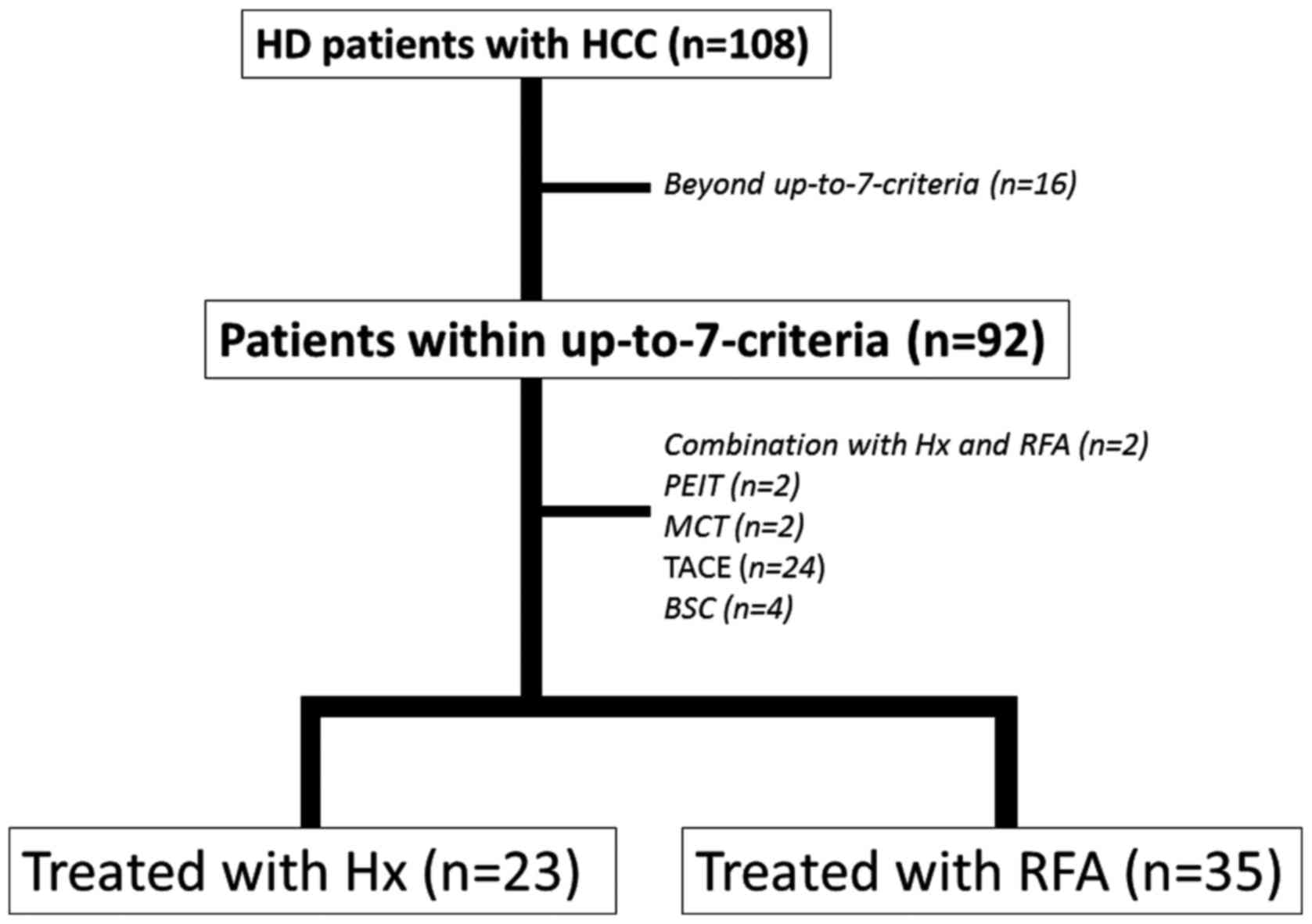
A total of 108 HD patients with naïve HCC who were
treated at one of our 15 institutions (Ehime Prefectural Central
Hospital, Matsuyama; Ogaki Municipal Hospital, Ogaki; Kagawa
Prefectural Central Hospital, Takamatsu; Teine Keijinkai Hospital,
Sapporo; Asahi General Hospital, Asahi; Isesaki Municipal Hospital,
Isesaki; Ehime University Hospital, Toon; Matsuyama Red Cross
Hospital, Matsuyama; Shiritsu Uwajima Hospital, Uwajima; Shiritsu
Ozu Hospital, Ozu; Toyama University Hospital, Toyama; Kagawa
University Hospital, Kagawa; Tokushima University Hospital,
Tokushima; Saiseikai Imabari Hospital, Imabari; Okayama Saiseikai
General Hospital, Okayama, Japan) between January 1988 and December
2014 were enrolled in the present study. Of these, 58 fulfilled the
up-to-7-criteria [7 as the sum of the size of the largest tumor
(cm) and the number of tumors] (10,11), and
were treated with Hx (Hx group, n=23) or RFA (RFA group, n=35) as
the curative therapy. Transcatheter arterial chemoembolization
(TACE) was performed as the palliative treatment in 24 patients.
All the patients had end-stage renal disease (ESRD), which requires
HD or peritoneal dialysis. HCC patients within the up-to-7-criteria
were regarded as having early-stage HCC. After receiving informed
consent for curative treatment from each patient or their family,
Hx or RFA was performed. The first HD session was performed 1 or 2
days after Hx or RFA with nafamostat mesylate (Torii Co., Ltd.,
Tokyo, Japan) instead of heparin.
Surveillance of HCC was mainly performed using
ultrasonography (US), and diagnosis was based on an increasing
course of α-fetoprotein as well as findings obtained by dynamic
computed tomography (12), magnetic
resonance imaging and/or contrast-enhanced US (CEUS) with
perflubutane (Sonazoid®; Daiichi Sankyo Co., Ltd. Tokyo,
Japan) (13). The
tumor-nodes-metastasis (TNM) stage was determined according to the
HCC staging systems of the Union for International Cancer Control,
7th edition (14) and the Liver
Cancer Study Group of Japan (LCSGJ), 5th edition (15). Child-Pugh classification (16) was used for evaluation of hepatic
reserve function affected by liver cirrhosis. HBV and HCV
positivity were determined based on positivity for the hepatitis B
virus surface antigen (HBsAg) and HCsAg, respectively.
RFA was performed from 2000 onwards as a curative
therapy using previously reported methods (9). Selection of therapy (Hx or RFA) was
independently determined by each institution. The physicians tended
to select RFA when the size and number of tumors was small (2.1±0.8
cm and 1.1±0.3, respectively). When the patient or their family
refused curative treatments, TACE was selected. For the TACE
procedure, a microcatheter was inserted into the artery feeding the
tumor in a super-selective manner after conventional hepatic
angiography, followed by a segmental or subsegmental TACE procedure
(17) performed by experienced
radiologists and hepatologists. For embolization, epirubicin
hydrochloride (Farmorubicin®; Pfizer Japan Inc., Tokyo,
Japan) was used throughout January 2010, while miriplatin hydrate
(MIRIPLA®; Sumitomo Dainippon Pharma Co., Ltd., Osaka,
Japan), was used from February 2010, which was injected together
with lipiodol in all cases, after which a gelatin sponge cut into
small fragments (Gelfoam®; Upjohn, Kalamazoo, MI, USA)
were used throughout August 2006, while small gelatin sponge
fragments (Gelpart®; Nippon Kayaku Co., Ltd., Tokyo,
Japan) were used from September 2006.
The study protocol was approved by the Institutional
Ethics Committee of Ehime Prefectural Central Hospital (Matsuyama,
Japan; no. 26–11).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using Welch's t-test for
unpaired data, as well as Fischer's exact test, Mann-Whitney's
U-test or a log-rank test with the Kaplan-Meyer method, as
appropriate. All statistical analyses were performed using SPSS
version 21 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
The average age of all the enrolled patients (91
males, 17 females) was 67.9±8.7 years. The rate of HCV-positivity,
HBV-positivity and HBV + HCV-positivity was 68.5, 8.3 and 2.8%,
respectively, while that of patients negative for HCV and HBV
(nonBnonC) was 20.4%. Among the nonBnonC patients with HCC, DM was
the primary disease in 72.7% (16/22). The average time from the
first HD was 4.6±4.8 years (range, 0.1-27 years) (Table I). The frequency of patients within
the up-to-7 criteria was 85.2% (92/108) and 64 of these underwent
curative therapies; among them, 23 received Hx, 39 received
ablative therapy [RFA, 35; percutaneous ethanol injection therapy
(PEIT), 2; microwave coagulation therapy (MCT), 2] and 2 received
combination therapy with Hx and RFA (Fig. 1).
 | Table I.Clinical features of HD patients with
naïve HCC (n=108). |
Table I.
Clinical features of HD patients with
naïve HCC (n=108).
|
Parameter/characteristic | Value |
|---|
| Sex (male/female)
(n) | 91:17 |
| Average age
(years) | 67.9±8.7 |
| Etiology of HCC
(HCV/HBV/HBV + HCV/nonBnonC) (n) | 74:9:3:22 |
| Performance status
(0/1/2/3/4/unknown) (n) | 54:35:4:3:12 |
| Basal disease
causing ESRD (n) | DM, 72; unknown,
26; nephrosclerosis, 3; chronic glomerulonephritis, 3; renal stone,
1; multiple myeloma, 1; IgA nephropathy, 1; rapidly progressive
glomerulonephritis, 1 |
| HD
(machine/peritoneal) (n) | 107:1 |
| Average time from
first HD, years (range) | 4.6±4.8
(0.1-27) |
| Aspartate
aminotransferase (IU/l) | 28.9±20.9 |
| Alanine
aminotransferase (IU/l) | 24.7±24.1 |
| Platelets
(x104 cells/µl) | 13.3±6.1 |
| Total bilirubin
(mg/dl) | 0.49±0.25 |
| Albumin (g/dl) | 3.57±0.53 |
| Prothrombin time
(%) | 90.2±17.4 |
| Child-Pugh
classification (A/B/C) (n) | 89:19:0 |
| Tumor size
(<2/≥2 cm, n), (average, cm) | 27:81
(3.24±2.27) |
| Number of tumors
(single/multiple) (average) | 81:27
(1.45±1.06) |
| Extrahepatic
metastasis, n (%) | 2 (bone),
(1.9%) |
| Portal vein tumor
thrombosis, n (%) | 6 (5.6%) |
| Alpha-fetoprotein
(ng/ml) |
1,683.2±5,879.9 |
| Des-gamma-carboxy
prothrombin (mUA/ml) |
2,706.5±8,308.3 |
| TNM stage (UICC
7th) I/II/III/IV (n) | 77:22:7:2 |
| TNM stage (LCSGJ
5th) I/II/III/IV (n) | 25:57:21:5 |
| Therapeutic method
(Hx/RFA/Hx+RFA/MCT/PEIT/ |
28:35:3:2:2:29:2:1:6 |
|
TACE/RT/chemotherapy/BSC) (n) |
|
Comparison between patients within the up-to-7
criteria treated with Hx and those treated with RFA revealed no
significant differences for most parameters, apart from the
etiology of HCC (P=0.002), platelet count (P=0.013) and prothrombin
time (P=0.042) (Table II). The
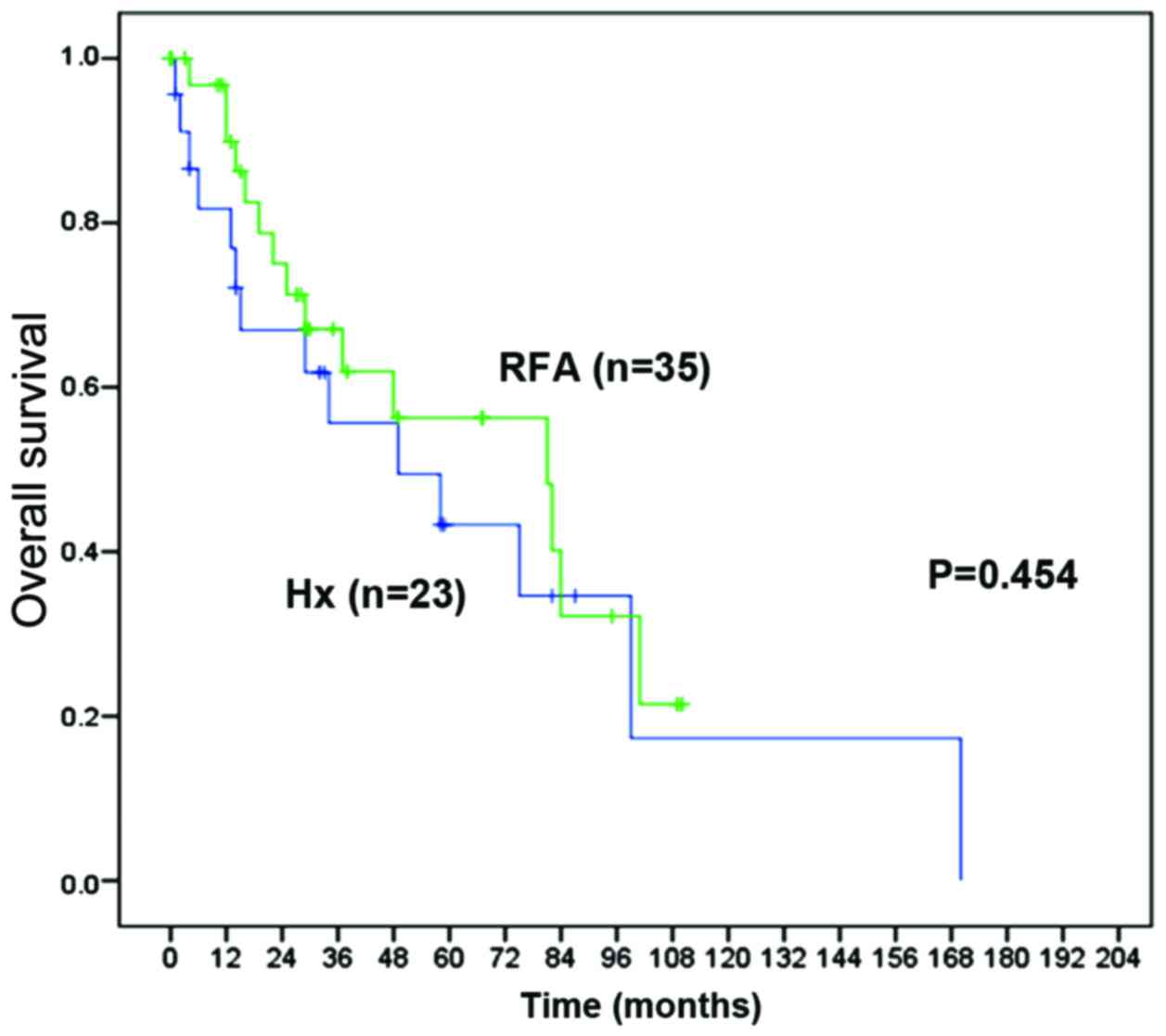
overall survival (OS) rate and disease-free survival (DFS) rate
were not significantly different between the Hx and RFA groups [1-,
3- and 5-year OS rates: 81.7, 55.6 and 43.3% vs. 89.9, 67.1 and
56.3%, respectively; P=0.454 (Fig.
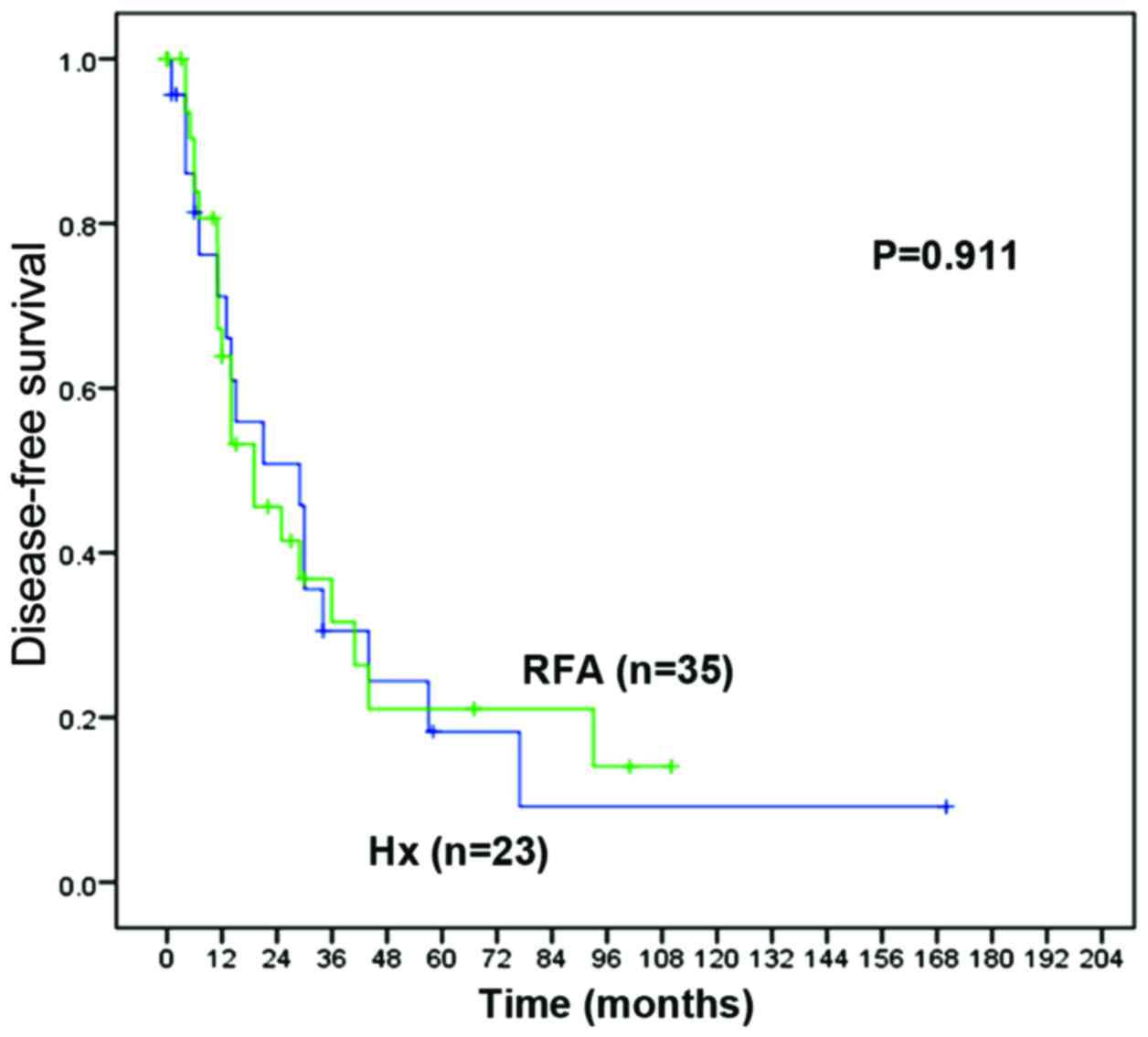
2) and 1-, 3- and 5-year DFS rates: 71.1, 30.5 and 18.3% vs.
63.8, 31.6 and 21.1%, respectively; P=0.911 (Fig. 3)]. Post-procedure complications were
observed in 4 of the 35 patients (11.4%) treated by RFA
(subcapsular hemorrhage in the liver, 2; intraperitoneal bleeding,
1; tardive intrahepatic hematoma 4 days after RFA procedure, 1) and
in 4 of the 23 patients (17.4%) treated by Hx (post-operative
infection, 2; liver failure, 1; pleural effusion, 1). No
significant differences were identified in the frequencies of
complications between the two groups (P=0.700). One patient died
due to postoperative infection within 1 month in the Hx group,
whereas no mortalities occurred in the RFA group (P=0.397,
according to Fischer's exact test).
 | Table II.Comparison of clinical features
between Hx and RFA groups. |
Table II.
Comparison of clinical features
between Hx and RFA groups.
|
Parameter/characteristic | Hx (n=23) | RFA (n=35) | P-value |
|---|
| Sexa (male:female) (n) | 21:2 | 25:10 | 0.070 |
| Average
ageb (years) | 68.4±9.1 | 66.2±8.8 | 0.359 |
| Etiology of
HCCc (n) | 10:4:1:8 | 29:2:1:3 | 0.002 |
| (HCV/HBV/HBV +
HCV/nonBnonC) (n) |
|
|
|
| Performance
statusc
(0/1/2/3/4/unknown) | 11:10:1:1:0:0 | 16:15:0:1:0:3 | 0.387 |
| Basal disease
causing ESRD (n) | DM, 15; IgA, 1;
chronic glomerulonephritis, 1; nephrosclerosis, 1; unknown, 5 | DM, 25; multiple
myeloma, 1; nephrosclerosis, 1; chronic glomerulonephritis, 1;
unknown, 7 | – |
| HD
(machine/peritoneal) (n) | 23:0 | 35:0 | – |
| Average period
after introducing HDb
(years) | 5.3±5.7 | 5.4±5.7 | 0.953 |
| Aspartate
aminotransferaseb
(IU/l) | 23.0±12.1 | 34.4±30.7 | 0.057 |
| Alanine
aminotransferaseb
(IU/l) | 20.3±10.6 | 30.4±35.2 | 0.123 |
|
Plateletsb (x104 cells/µl) | 15.1±6.3 | 11.2±5.1 | 0.013 |
| Total
bilirubinb (mg/dl) | 0.42±0.22 | 0.56±0.27 | 0.055 |
|
Albuminb
(g/dl) | 3.76±0.51 | 3.63±0.50 | 0.350 |
| Prothrombin
timeb (%) | 96.0±13.0 | 87.4±16.7 | 0.042 |
| Child-Pugh
classificationc (A/B/C)
(n) | 22:1:0 | 28:7:0 | 0.094 |
| Tumor
sizea (<2/≥2 cm, n)
(average, cm) | 4:19 (2.8±1.0) | 14:21
(2.1±0.8) | 0.071 |
| Tumor
numbera
(single/multiple) | 20:3 (1.1±0.3) | 32:3 (1.1±0.3) | 0.612 |
|
Alpha-fetoproteinb (ng/ml) | 681.9±2,110.9 | 831.5±4,147.0 | 0.877 |
| Des-gamma-carboxy
prothrombinb
(mUA/ml) |
2,035.4±6,265.8 |
1,173.7±3,565.4 | 0.522 |
| TNM
stagec (UICC 7th)
I/II/III/IV (n) | 20:3:0:0 | 32:3:0:0 | 0.588 |
| TNM
stagec (LCSGJ 5th)
I/II/III/IV (n) | 3:18:2:0 | 13:19:3:0 | 0.095 |
| JIS
scorec 0/1/2/3 (n) | 3:17:3:0 | 11:17:6:1 | 0.489 |
|
Complicationsa,d
(n) | Postoperative
infection, 2 (1 died within 1 month), liver failure, 1; pleural
effusion, 1d | Intra-hepatic
hematoma after 4 days of RFA, 1; subcapsular hemorrhage of liver,
2; intraperitoneal bleeding, 1 | 0.700 |
| Cause of mortality
(n) | HCC, 2; infection,
3; liver failure, 2; acute subdural hematoma, 1; cardiac failure,
1; arrhythmia, 1; others/unknown, 4 | HCC, 6; infection,
1; liver failure, 1; cerebral hemorrhage, 2; suffocation by
accidental ingestion, 1; acute respiratory distress syndrome, 1;
general prostration, 1; others/unknown, 2 | – |
In HCC patients who fulfilled the up-to-7-criteria,
results of the Japan Integrated Scoring (JIS) system, calculated
based on the Child-Pugh classification and TNM stage according to
the LCSGJ classification, 5th edition (18) did not show any significant
differences between patients treated with curative treatments (Hx
or RFA, n=58) and those who underwent palliative treatment (TACE,
n=24) (P=0.071). The OS rate for those who received curative
treatments revealed a better prognosis compared with those who
received TACE (n=24) [1-, 3- and 5-year OS rates: 90.6, 62.3 and
50.4% vs. 86.5, 45.9 and 0.0%, respectively; P=0.010, according to
the log-rank test (data not shown)].
Discussion
Although the number of HD patients with HCC is low,
such cases are at times encountered in the clinic. A previous study
published in 1996 found that the frequency of HCV-positive HD
patients was as high as 18.9% (409/2,164), while HCC was observed
in only 2.4% (10/409) of HD patients with HCV infection (19). Factors associated with HCC in
HCV-positive HD patients have remained elusive. However, positivity
for DM was considered to have an important role for the occurrence
of HCC in HD patients with HCV infection. In the present study,
diabetic nephropathy was the most common primary disease leading to
the requirement for HD, while Henderson et al (20) noted that HCC was 1.3 times more
likely to occur in HCV-positive HD patients with DM. Furthermore,
the number of DM patients worldwide has been increasing recently.
On the other hand, in patients without chronic viral hepatitis, DM
increases the risk of chronic liver disease and HCC, with
incidences of chronic non-alcoholic liver disease in patients with
and without DM of 18.13 and 9.55, respectively, per 10,000
individuals per year, and an incidence of HCC of 2.39 and 0.87,
respectively (21). Furthermore,
that study found that DM is associated with an increased risk of
chronic non-alcoholic liver disease and HCC [hazard ratio (HR),
1.98 and 2.16, respectively]. Noto et al (22) also reported that DM was associated
with an increased risk for HCC (HR, 3.64; 95% confidence interval,
2.61–5.07) in Japanese patients. DM and aging, particularly in
patients with a high fibrosis-4 index, were found to be associated
with an increased risk of HCC in a previous study by our group
(23). In the present study, at
least 72.7% (16/22) of the patients without viral hepatitis had DM.
Cases of HCC among HD patients without chronic viral hepatitis
infection may increase in the near future in association with the
increase in patients with DM and diabetic nephropathy. In the
present study, numerous HCC patients without viral hepatitis had DM
and establishment of a surveillance strategy for HCC in HD patients
with DM nephropathy is therefore required to detect HCC in as early
a stage as possible. Furthermore, screening for HCC should also be
performed in HD patients with chronic hepatitis, particularly those
with HCV. Although interferon and ribavirin treatment is difficult
in HD patients, the effectiveness and safety of HCV-NS5A-inhibitor
(daclatasvir) and protease inhibitor (asunaprevir) combination
therapy for HD patients with HCV has been reported recently
(24,25). Further progression in the development
of direct-acting antiviral drugs will reduce the chronic hepatic
diseases, HCV and HCC, in HD patients.
Although Hx and RFA are routinely performed as
curative treatments for HCC, few studies have compared their
efficacy and safety in HD patients. Generally, HX is performed as a
curative treatment for HCC patients with good hepatic function
(6,26), while treatment with RFA is regarded
as a curative local therapy for small HCC (27). Although Tung et al (28) reported that the prognosis of HD
patients with HCC who were treated with Hx was not significantly
different from that of those who received best supportive care, Hx
has recently been established as an acceptable procedure for HD
patients with HCC (29–32). In patients with good liver function
and early-stage HCC (within up-to-7 criteria), curative treatments
are expected to prolong survival. However, HD has been reported to
be a risk factor in cases that undergo surgical resection due to a
tendency to bleed arising from platelet dysfunction and heparin
usage (33), susceptibility to
infection (34) and impaired wound
healing (35). In the present study,
complications were observed in 17.4% (4/23) of patients in the Hx
group (infection, 2; liver failure, 1; pleural effusion, 1), while
complications occurred in 11.4% (4/35) of patients in the RFA
group, each of which was based on bleeding (subcapsular hemorrhage
in the liver, 2; intraperitoneal bleeding, 1; tardive intrahepatic
hematoma, 1). However, no statistical difference in the
complication rates was observed between the two groups (P=0.700).
The characteristics of the patients who showed complications of
bleeding could not be determined in the present cohort. The rate of
complications associated with curative treatments in patients with
HD is thought to be higher compared with that seen in those without
HD. Few studies have noted that HCC patients with HD had a higher
rate of complications compared with those without HD, although the
rates of post-operative infection after Hx (4.03 vs. 1.17%;
P=0.0175) (31) and bleeding after
RFA (13.3 vs. 0.79%; P=0.0002) (36)
have been reported. When a curative treatment for HCC (Hx or RFA)
is applied in a patient with HD, it is important to keep in mind
that the risk of complications is greater compared with that in
patients without HD; thus, detailed information must be given and
informed consent must be obtained.
Although RFA has been gaining in importance in terms
of prolonging the survival of HCC patients, particularly those with
small tumors, there are few reports on RFA as a treatment for HCC
in patients with HD (37). With
increasing detection of small HCC due to the progression of imaging
modalities [e.g., CEUS with Sonazoid (38)], the clinical significance of RFA in
the treatment of small HCC for improving the prognosis of affected
patients is expected to increase. In the present study, no
significant differences in the OS and DFS rates were observed
between the Hx and RFA groups, and no differences with regard to
the JIS score were obtained (18).
Lee et al (32) reported
that, with regard to predicting the prognosis of HCC, the JIS score
is a more accurate model for patients undergoing HD and is suitable
for comparing the efficacy of therapies. The present study has
demonstrated that the JIS scores and the therapeutic outcome were
not different between the Hx and RFA groups, indicating equally
important roles for the two modalities in HD patients with HCC.
In conclusion, the present study has determined that
there were no significant differences between Hx and RFA with
regard to the therapeutic outcome when applied for the treatment of
HCC in HD patients within the up-to-7-criteria. As the present
study was retrospective in nature and the number of patients was
small, there were several limitations in terms of drawing firm
conclusions. Accumulation of a greater number of cases and further
analysis is therefore required.
Acknowledgements
The authors would like to thank Dr Kazuya Kariyama
(Department of Gastroenterology, Okayama Shimin Hospital, Okayama),
Dr Toru Ishikawa (Department of Gastroenterology, Saiseikai Niigata
Daini Hospital, Niigata), Dr Shintaro Takagi (Department of
Gastroenterology, Hiroshima Red Cross Hospital, Hiroshima), Dr Sung
Kwan Bae (Department of Gastroenterology, Hamanomachi Hospital,
Fukuoka), Dr Kazufumi Domen (Department of Gastroenterology,
Chihaya Hospital, Fukuoka), Dr Chikara Ogawa (Department of
Gastroenterology, Takamatsu Red Cross Hospital, Takamatsu), Dr
Noritomo Shimada (Department of Gastroenterology, Ootakanomori
Hospital, Kashiwa), Dr Akihiro Deguchi (Department of
Gastroenterology, Kagawa Rosai Hospital, Marugame), Dr Ryoken
Tanaka (Department of Gastroenterology, Matsuyama Shimin Hospital,
Matsuyama), Dr Hiroaki Miyaoka (Department of Internal Medicine,
Saiseikai Matsuyama Hospital, Matsuyama) and Dr Masamoto Torisu
(Department of Internal Medicine, Saiseikai Saijo Hospital, Saijo)
for their cooperation in searching the records of HD patients with
HCC treated at their institutions.
References
|
1
|
GLOBOCAN 2012 Estimated Cancer Incidence,
Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxDecember
12–2015.
|
|
2
|
Selcuk H, Kanbay M, Korkmaz M, Gur G,
Akcay A, Arslan H, Ozdemir N, Yilmaz U and Boyacioglu S:
Distribution of HCV genotypes in patients with end-stage renal
disease according to type of dialysis treatment. Dig Dis Sci.
51:1420–1425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Etik D Ozer, Ocal S and Boyacioglu AS:
Hepatitis C infection in hemodialysis patients: A review. World J
Hepatol. 7:885–895. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Toole SM, Fan SL, Yaqoob MM and
Chowdhury TA: Managing diabetes in dialysis patients. Postgrad Med
J. 88:160–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Renehen A, Smith U and Kirkman MS: Linking
diabetes and cancer: A consensus on complexity. Lancet.
375:2201–2202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arii S, Yamaoka Y, Futagawa S, Inoue K,
Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K and Yamada
R: Results of surgical and nonsurgical treatment for small-sized
hepatocellular carcinomas: A retrospective and nationwide survey in
Japan. The Liver Cancer Study Group of Japan. Hepatology.
32:1224–1229. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikai I, Itai Y, Okita K, Omata M, Kojiro
M, Kobayashi K, Nakanuma Y, Futagawa S, Makuuchi M and Yamaoka Y:
Report of the 15th follow-up survey of primary liver cancer.
Hepatol Res. 28:21–29. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiina S, Teratani T, Obi S, Sato S,
Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T
and Omata M: A randomized controlled trial of radiofrequency
ablation with ethanol injection for small hepatocellular carcinoma.
Gastroenterology. 129:122–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hiraoka A, Michitaka K, Horiike N, Hidaka
S, Uehara T, Ichikawa S, Hasebe A, Miyamoto Y, Ninomiya T, Sogabe
I, et al: Radiofrequency ablation therapy for hepatocellular
carcinoma in elderly patients. J Gastroenterol Hepatol. 25:403–407.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mazzaferro V, Llovet JM, Miceli R, Bhoori
S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi
GL, et al: Predicting survival after liver transplantation in
patients with hepatocellular carcinoma beyond the Milan criteria: A
retrospective, exploratory analysis. Lancet Oncol. 10:35–43. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Amico F, Schwartz M, Vitale A, Tabrizian
P, Roayaie S, Thung S, Mdel Rio, Martin Guido J, Schiano T and
Cillo U: Predicting recurrence after liver transplantation in
patients with hepatocellular carcinoma exceeding the up-to-seven
criteria. Liver Transpl. 15:1278–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruix J and M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases
Sherman: Management of hepatocellular carcinoma. Hepatology.
42:1208–1236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hiraoka A, Hiasa Y, Onji M and Michitaka
K: New contrast enhanced ultrasonography agent: Impact of Sonazoid
on radiofrequency ablation. J Gastroenterol Hepatol. 26:616–618.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumors. 7th edition. John Wiley
& Sons, Inc.; Hoboken, NJ: 2009
|
|
15
|
Liver Cancer Study Group of Japan, .
General Rules for the Clinical and Pathological Study of Primary
Liver Cancer. 5th edition. Kanehara & Co., Ltd.; Tokyo: pp.
242009
|
|
16
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsui O, Kadoya M, Yoshikawa J, Gabata T,
Takashima T and Demachi H: Subsegmental transcatheter arterial
embolization for small hepatocellular carcinomas: Local therapeutic
effect and 5-year survival rate. Cancer Chemother Pharmacol. 33
Suppl:S84–S88. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kudo M, Chung H and Osaki Y: Prognostic
scoring system for hepatocellular carcinoma (CLIP score): Its value
and limitations, and a proposal for a new staging system, the Japan
Integrated Staging Score (JIS score). J Gastroenterol. 38:207–215.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakai Y, Izumi N, Tazawa J, Uchihara M,
Akiba T, Marumo F and Sato C: Characteristics of anti-HCV
antibody-positive patients with hepatocellular carcinoma on chronic
hemodialysis: Recommendation of periodic ultrasonography for early
detection. Nephron. 74:386–389. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Henderson WA, Shankar R, Gill JM, Kim KH,
Ghany MG, Skanderson M and Butt AA: Hepatitis C progressing to
hepatocellular carcinoma: The HCV dialysis patient in dilemma. J
Viral Hepat. 17:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Serag HB, Tran T and Everhart JE:
Diabetes increases the risk of chronic liver disease and
hepatocellular carcinoma. Gastroenterology. 126:460–468. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Noto H, Osame K, Sasazaki T and Noda M:
Substantially increased risk of cancers in patients with diabetes
mellitus: A systematic review and meta-analysis of epidemiologic
evidence in Japan. J Diabetes Complications. 24:345–353. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hiraoka A, Ochi M, Matsuda R, Aibiki T,
Okudaira T, Kawamura T, Yamago H, Nakahara H, Suga Y, Azemoto N, et
al: Ultrasonography screening for hepatocellular carcinoma in
Japanese patients with diabetes mellitus. J Diabetes. Sep
8–2015.(Epub ahead of print). PubMed/NCBI
|
|
24
|
Suda G, Kudo M, Nagasaka A, Furuya K,
Yamamoto Y, Kobayashi T, Shinada K, Tateyama M, Konno J, Tsukuda Y,
et al: Efficacy and safety of daclatasvir and asunaprevir
combination therapy in chronic hemodialysis patients with chronic
hepatitis C. J Gastroenterol. Jan 14–2016.(Epub ahead of print).
View Article : Google Scholar
|
|
25
|
Toyoda H, Kumada T, Tada T, Takaguchi K,
Ishikawa T, Tsuji K, Zeniya M, Iio E and Tanaka Y: Safety and
efficacy of dual direct-acting antiviral therapy (daclatasvir and
asunaprevir) for chronic hepatitis C virus genotype 1 infection in
patients on hemodialysis. J Gastroenterol. Feb 12–2016.(Epub ahead
of print). View Article : Google Scholar
|
|
26
|
Ikai I, Arii S, Kojiro M, Ichida T,
Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K
and Yamaoka Y: Reevaluation of prognostic factors for survival
after liver resection in patients with hepatocellular carcinoma in
a Japanese nationwide survey. Cancer. 101:796–802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hiraoka A, Horiike N, Yamashita Y, Koizumi
Y, Doi K, Yamamoto Y, Hasebe A, Ichikawa S, Yano M, Miyamoto Y, et
al: Efficacy of radiofrequency ablation therapy compared to
surgical resection in 164 patients in Japan with single
hepatocellular carcinoma smaller than 3 cm, along with report of
complications. Hepatogastroenterology. 55:2171–2174.
2008.PubMed/NCBI
|
|
28
|
Tung CF, Yang DY, Hu WH, Peng YC, Chow WK
and Chen GH: Characteristics of hepatocellular carcinoma in
hemodialysis patients in hepatitis B endemic area.
Hepatogastroenterology. 50:1564–1568. 2003.PubMed/NCBI
|
|
29
|
Yamagata M, Kanematsu T, Matsumata T,
Nishizaki T, Utsunomiya T, Sugimachi K and Okuda S: Possibility of
hepatic resection in patients on maintenance hemodialysis.
Hepatogastroenterology. 40:249–252. 1993.PubMed/NCBI
|
|
30
|
Orii T, Takayama T, Haga I, Fukumori T and
Amada N: Efficacy of a liver resection for hepatocellular carcinoma
in patients with chronic renal failure. Surg Today. 38:329–334.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yeh CC, Lin JT, Jeng LB, et al: Hepatic
resection for hepatocellular carcinoma patients on hemodialysis for
uremia: A nationwide cohort study. World J Surg. 37:2402–2409.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee YH, Hsu CY, Hsia CY, et al:
Hepatocellular carcinoma in uremic patients: Is there evidence for
an increased risk of mortality? J Gastroenterol Hepatol.
28:348–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaw D and Malhotra D: Platelet dysfunction
and end-stage renal disease. Semin Dial. 19:317–322. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sarnak MJ and Jaber BL: Mortality caused
by sepsis in patients with end-stage renal disease compared with
the general population. Kidney Int. 58:1758–1764. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahonen J and Salmela K: Wound healing and
infections in chronic renal failureSurgery in Renal Failure. Eigler
FW and Jakubowski HD: George Thieme Verlag; New York: pp.
681984
|
|
36
|
Minami Y, Hayaishi S and Kudo M:
Radiofrequency ablation for hepatic malignancies: Is needle tract
cauterization necessary for preventing iatrogenic bleeding? Dig
Dis. 31:480–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kondo Y, Yoshida H, Tomizawa Y, Tateishi
R, Shiina S, Tagawa K and Omata M: Percutaneous radiofrequency
ablation of hepatocellular carcinoma in 14 patients undergoing
regular hemodialysis for end-stage renal disease. AJR Am J
Roentgenol. 193:964–969. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hiraoka A, Ichiryu M, Tazuya N, Ochi H,
Tanabe A, Nakahara H, Hidaka S, Uehara T, Ichikawa S, Hasebe A, et
al: Clinical translation in the treatment of hepatocellular
carcinoma following the introduction of contrast-enhanced
ultrasonography with Sonazoid. Oncol Lett. 1:57–61. 2010.PubMed/NCBI
|